Abstract
Neovascularization by endothelial progenitor cells (EPC) for the treatment of ischaemic diseases has been a topic of intense research. The CD34+ cell is often designated as EPC, because it contributes to repair of ischaemic injuries through neovascularization. However, incorporation of CD34+ cells into the neovasculature is limited, suggesting another role which could be paracrine. CD14+ cells can also differentiate into endothelial cells and contribute to neovascularization. However, the low proliferative capacity of CD14+ cell‐derived endothelial cells hampers their use as therapeutic cells. We made the assumption that an interaction between CD34+ and CD14+ cells augments endothelial differentiation of the CD14+ cells. In vitro, the influence of CD34+ cells on the endothelial differentiation capacity of CD14+ cells was investigated. Endothelial differentiation was analysed by expression of endothelial cell markers CD31, CD144, von Willebrand Factor and endothelial Nitric Oxide Synthase. Furthermore, we assessed proliferative capacity and endothelial cell function of the cells in culture. In monocultures, 63% of the CD14+‐derived cells adopted an endothelial cell phenotype, whereas in CD34+/CD14+ co‐cultures 95% of the cells showed endothelial cell differentiation. Proliferation increased up to 12% in the CD34+/CD14+ co‐cultures compared to both monocultures. CD34‐conditioned medium also increased endothelial differentiation of CD14+ cells. This effect was abrogated by hepatocyte growth factor neutralizing antibodies, but not by interleukin‐8 and monocyte chemoattractant protein‐1 neutralizing antibodies. We show that co‐culturing of CD34+ and CD14+ cells results in a proliferating population of functional endothelial cells, which may be suitable for treatment of ischaemic diseases such as myocardial infarction.
Keywords: CD34, CD14, HGF, MCP‐1, IL‐8, endothelial progenitor cell, endothelial cell differentiation, paracrine signalling
Introduction
Coronary artery disease and myocardial infarctions are the major cause of death in the industrialized world (http://www.who.org). Current treatments, such as percutaneous angioplasty and bypass surgery, focus on the relieving of the ischaemia, reducing ongoing tissue damage, rather than on tissue regeneration. This warrants investigation into new strategies for the treatment of ischaemic diseases.
Regenerative medicine aims to maintain, improve or restore tissue function by employing the bodies’ own regenerative mechanisms. An example is cell therapy, which is an experimental strategy for the treatment of ischaemic diseases, such as myocardial infarction. Herein, neovascularization induced by progenitor cells is an essential factor and has been a topic of intense research over the past decade. The rare CD34+ stem cell is often designated as the endothelial progenitor cell (EPC), as it contributes to repair of vascular damage in vivo. However, incorporation of CD34+ cells into the neovasculature is limited [1, 2, 3, 4]. We, and others, have shown that in vivo neovascularization relies on a paracrine function of human CD34+ cells, which involves recruitment of monocytes, rather than actual incorporation [5, 6] among others.
In a mouse model of myocardial infarction, we have shown that ischaemic tissue damage leads to the recruitment of bone marrow derived progenitor cells and monocytes to the damaged site, which is a prerequisite for repair. These bone marrow derived cells also differentiate, to myofibroblasts and thus contribute to wound healing [7]. Furthermore, the recruited monocytes were shown to influence neovascularization and remodelling after myocardial infarction, as depletion of these cells from the circulation resulted in decreased neovascularization and impaired remodelling of the myocardial infarction [8]. In vitro, we have shown that human monocytes, i.e. CD14+ cells, differentiate into functional endothelial cells [9]. As a consequence of this ability, these CD14+ cells are often referred to as ‘early EPC’[10], ‘colony forming unit‐endothelial cell (CFU‐EC)’[11, 12] or ‘endothelial‐like cells’[9, 13]. Thus, differentiated CD14+ cells may contribute to neovascularization in vivo and to remodelling of the infracted area.
These data suggest that both CD34+ cells and CD14+ cells play a key role in the repair and regeneration in cardiovascular and ischaemic diseases. We made the assumption that circulating EPCs, i.e. CD34+ cells, and CD14+ cells interact during neovascularization and that paracrine signalling of CD34+ cells augments endothelial cell differentiation of the CD14+ cells. We examined the interaction between CD34+ cells and CD14+ cells and the effect on endothelial cell differentiation and cell proliferation in vitro. To this end, these cells were co‐cultivated and the expression of endothelial cell markers was evaluated. Additionally, the (paracrine) factors that drive the endothelial cell differentiation and proliferation were investigated. Finally, the functionality of the newly differentiated endothelial cells was assayed in vitro.
Materials and methods
Cell isolation and culture
Peripheral blood mononuclear cells (pbMNC) were isolated from buffy coats from healthy donors (Sanquin, Groningen, the Netherlands) using density gradient centrifugation on lymphoprep (Nycomed Pharma, Roskilde, Norway). CD34+ cells were isolated by magnetic bead separation and further purified by fluorescence‐activated cell sorting (FACS) as described previously [6]. Subsequently, monocytes, i.e. CD14+ cells, were isolated from the remaining MNC‐fraction by magnetic bead separation as described previously [9]. Flow cytometric analysis revealed an average purity of 98.4 ± 1.2% (n= 4) for isolated CD34+ cells and 99.8 ± 0.1% (n= 4) for isolated CD14+ cells.
CD34+ cells and CD14+ cells were cultured either in monoculture or in co‐culture in ratios 1:10, 1:100 and 1:1000 (CD34+ cells in CD14+ cells, respectively). Co‐cultures were derived from one single donor to avoid immunological mismatches. Cells were cultured in fibronectin/gelatin [both 10 μg/ml in phosphate‐buffered saline (PBS)] coated wells, at a density of 50,000/cm2. Culture medium consisted of RPMI1640 supplemented with foetal calf serum (both BioWhittaker, Verviers, Belgium), 5 U/ml heparin (LEO Pharma, Ballerup, Denmark), 0.3 mg/ml L‐glutamine (GIBCO Products, Grand Island, NY, USA) 1% Penicillin/Streptomycin (Sigma, St. Louis, MO, USA), 50 μg/ml endothelial cell growth factor (ECGF, according to Burgess et al.[14]), 10 ng/ml basic fibroblast growth factor (bFGF) and 1 ng/ml vascular endothelial growth factor (VEGF)‐A (both Peprotech, Rockhill, NJ, USA). Culture medium was refreshed every third day.
Colony forming unit‐endothelial cell assays
Isolated CD34+ and CD14+ cells were labelled using carboxyfluorescein diacetate, succinimidyl ester (CFSE) (green) and CM‐DiI (red), respectively (both Molecular Probes, Eugene, OR, USA), according to manufacturer’s instructions. The labelled cells were mixed with the remaining autologous pbMNC‐fraction and cultured as described above. After 7 days, fluorescent microscopic analysis was performed with a Leica DMRXA fluorescent microscope (Leica Microsystems, Wetzlar, Germany) to determine the morphological status of labelled CD34+ and CD14+ cells. After 14 days, spindle‐shaped cells originating from CFU‐ECs were quantified. Part of the CFU‐ECs were fixed using 2% Glutar Aldehyde (Merck, Darmstadt, Germany) at 4°C for 24 hrs, and prepared for transmission electron microscopy. In short, fixed samples were rinsed with a 6.8% sucrose solution (pH 7.4) for 30 min. Samples were post‐fixed using a 0.1 M potassium hexacyanoferrate(II)trihidrate (K4Fe(CN)6·3 H2O; Merck) solution containing 1% osmiumtetroxide (OsO4) at 4°C for 45 min. Next, the samples were dehydrated with ethanol, embedded in EPON 812 (Serva Feinbiochemica, Heidelberg, Germany), and polymerized at 60°C for at least 48 hrs. Ultrathin sections were cut on a Sorvall microtome (Sorvall, Newton, CT, USA) and contrasted with uranyl acetate and lead citrate. The ultrathin sections were analysed in a Phillips 201 transmission electron microscope (Phillips, Eindhoven, The Netherlands) operated at 60 kV.
Endothelial cell marker analysis
After 3 weeks of culture, mono‐ and co‐cultured cells were dissociated by accutase treatment (PAA Laboratories, Pashing, Austria). Cells were incubated with either phycocerythrin (PE)‐conjugated mouse antibodies to human (MaH) CD34 (1:10 in 2% bovine serum albumin [BSA]; BD Biosciences, San Jose, CA, USA), PE‐conjugated MaH CD14 (1:10 in 2% BSA; IQ Products, Groningen, The Netherlands), fluorescien (FITC)‐conjugated MaH VEGFR‐2 (1:10 in 2% BSA; R&D Systems, Minneapolis, MN, USA), MaH cMET (1:100 in 2% BSA; R&D Systems) followed by FITC‐conjugated rabbit antibodies to mouse IgG (1:100 in 2% BSA; DakoCytomation, Glostrup, Denmark), PE‐conjugated mouse antibodies to human CD31 (1:10 in 2% BSA/PBS; IQ Products) or with PE‐conjugated MaH CD144 (1:20 in 2% BSA/PBS; R&D Systems) at 4°C for 30 min. Next, cells were washed using PBS to remove excess antibodies and fixed using 2% paraformaldehyde in PBS at room temperature (RT) for 10 min. Thereafter, the cells were permeabilized with 0.01% saponin in PBS. Subsequently, cells were incubated with rabbit antibodies to human (RaH) von Willebrand factor (vWF) (1:50; DakoCytomation) or RaH endothelial cell nitric oxide synthesis (eNOS) (1:50; BD Biosciences). Cells were washed twice with 2% BSA/PBS and incubated with FITC‐conjugated donkey antibody F(ab′)2 fragments to rabbit IgG (1:100 in 2% BSA/PBS; Jackson ImmunoResearch, Suffolk, UK) on ice for 30 min. Cells were washed three times in 2% BSA/PBS and analysed using a FACSCaliber system (BD Biosciences). Isotype‐matched, PE‐conjugated nonsense antibodies and FITC‐conjugated donkey antibody F(ab′)2 fragments to rabbit IgG were used as controls.
Cell proliferation
After 3 weeks, mono‐ and co‐cultured cells were dislodged by accutase treatment. Cytospots were made from 50,000 cells and fixed in acetone at RT for 10 min. and post‐fixed using 2% paraformaldehyde in PBS at RT for 30 min. Fixed cells were permeabilized using 0.5% Triton X‐100 at RT for 10 min. After permeabilization, cells were pre‐incubated with 3% BSA in PBS at RT for 10 min. Next, samples were incubated with MaH Ki67 (1:50; DakoCytomation) at RT for 60 min. After washing and incubation with RedX‐conjugated donkey antibody F(ab′)2 fragments to mouse IgG (1:100; Jackson ImmunoResearch) at RT for 30 min. Samples were mounted in citifluor AP1 (Agar Scientific, Essex, UK) and examined by immunofluorescence microscopy using a Leica DMRXA microscope and Leica Software (Leica Microsystems). RedX‐conjugated donkey antibody F(ab′)2 fragments to mouse IgG were used as controls.
Thrombin generation assays and sprout formation
To assess their endothelial cell function, cultured cells were assayed for their ability to inhibit factor‐3‐dependent thrombin formation. To this end, a Thrombin Generation Assay (HaemoScan, Groningen, The Netherlands) was used. In brief, mono‐ and co‐cultured cells were dissociated by accutase treatment and replated at a density of 50,000 cells/cm2 on fibronectin/gelatin‐coated culture wells subsequently. After overnight culture, the culture medium was removed and replaced by fibrinogen‐depleted human plasma. During this incubation the intrinsic coagulation cascade is activated. Thereafter, the clotting cascade was further activated by addition of a mixture of 30 mM CaCl2 and phospholipids, which results in the generation of thrombin. Samples were taken at regular intervals and added to ice‐cold 25 mM TrisHCl to prevent any further thrombin generation. Finally, the diluted samples were incubated with 3 mM thrombin substrate S2238. Thrombin releases p‐nitroaniline chromophore from the substrate, which results in a change of colour. The change in colour was measured at 405 nm with 540 nm as reference wavelength in a microtitre plate reader (BioRad, Hercules, VA, USA). A calibration curve of known thrombin concentrations was used to quantify the thrombin formation in the experimental samples. Human umbilical vein endothelial cells (HUVEC) and factor‐3 positive vascular smooth muscle cells (VSMC) were used as negative and positive controls, respectively.
The ability to form capillary‐like sprouts was investigated using a Matrigel Sprouting Assay (BD Biosciences). After 21 days of culturing, monocultured and co‐cultured cells were dissociated using accutase and plated on Matrigel (BD Biosciences) coated wells at a density of 250,000 cells/cm2 and cultured in endothelial cell medium for an additional 24 hrs. The formation of capillary‐like structures was analysed using regular light microscopy.
Cytokine and growth factor secretion
At days 0, 3, 7 and 21 of culture, culture medium was sampled. The concentration of cytokines angiopoietin (ANG)‐2, interleukin (IL)‐8, monocyte chemoattractant protein (MCP)‐1 and tumour necrosis factor‐α (TNF‐α) as well as growth factors epidermal growth factor (EGF), bFGF, hepatocyte growth factor (HGF), transforming growth factor‐β (TGF‐β) and VEGFa were determined using multiplex protein analysis by PerBio Sciences (Cramlington, UK).
At day 3, total RNA was isolated from CD34+ cell monocultures or CD14+ cell monocultures using an RNeasy Micro Kit (Qiagen, Valencia, CA, USA) following manufacturer’s instructions. RNA integrity was determined by gel electrophoresis and RNA purity and concentration were determined using Nanodrop equipment (Nanodrop Technologies, Wilmington, NC, USA). Subsequently, 100 ng RNA was reverse transcribed using a First Strand cDNA synthesis Kit (Fermentas UAB, Vilnius, Lithuania). One micro litre cDNA was used for amplification in 384‐well microtitre plates in a TaqMan ABI7900HT cycler (Applied Biosystems, Foster City, CA, USA) in a final reaction volume of 10 μl containing 5 μl TaqMan universal PCR Master Mix (Applied Biosystems) and 0.5 μl primer/probe mix. Applied Biosystems ‘assay on demand’ primer/probe sets were used to detect amplimers of β‐2‐Microglobulin (β2M; Hs99999907_m1), IL‐8 (Hs00174103_m1), MCP‐1 (Hs00234140_m1) and HGF (Hs00300159_m1). Cycle threshold (CT) values for individual reactions were determined using ABI Prism SDS 2.2 data processing software (Applied Biosystems). To determine differences in gene transcripts between CD34+ cells and CD14+ cells CT‐values were normalized against β2M using the ΔCT‐method (ΔCT(gene)= CT(gene)– CT(β2M)). To correct for interassay variance, ΔCT values were normalized against expression levels of an external calibrator (ΔΔCT(gene)=ΔCT(gene)–ΔCT(calibrator)). Fold variance in transcript levels between CD34+ cells and CD14+ cells were calculated as 2−(ΔΔΔCT), where ΔΔΔCT(gene)=ΔΔCT(gene;CD34)–ΔΔCT(gene;CD14). All cDNA samples were amplified in triplicate and RNA controls were included to test for primer binding to genomic DNA. Differences of twofold higher or greater were considered to be biologically relevant.
Conditioned medium cultures
To assess the influence of paracrine factors produced during co‐cultivation, conditioned medium cultures were performed. Briefly, CD14+ cells were plated in fibronectin/gelatin‐coated wells at a density of 250,000 cells/cm2. Every third day, culture medium was removed from cells in mono‐ or in co‐culture. Conditioned culture medium from different donors was pooled to reduce donor variation and sterilized by filtration through a 0.2‐μm filter. Plated monocytes were cultured in conditioned culture medium supplemented with excess neutralizing goat antibodies to either human HGF, IL‐8, MCP‐1 or mock goat IgG (all 200 ng/ml medium; all R&D Systems). Conditioned medium and antibodies were refreshed every third day. After 21 days of culture, cells were dissociated using accutase and stained for protein expression of CD31, vWF, CD144 and eNOS. Protein expression of these endothelial cell markers was analysed by FACS analysis as described above. Inhibition of endothelial cell differentiation by neutralizing antibodies was calculated as the fraction of differentiation decrease by the neutralizing antibodies in relation to the mock IgGs according to the following equation:
 |
Statistical analysis
Data are presented as mean ± S.E. of the mean. Data in Gaussian distribution are analysed by two‐factor anova followed by Tuckey post hoc analysis. All other data were analysed using Mann‐Whitney tests. Probabilities lower than P < 0.05 were considered to be statistically significant.
Results
Phenotypes of CD34+ cells and CD14+ cells
CD34+ cells and CD14+ cells were isolated by magnetic bead isolation from buffy coats from healthy donors. Subsequently, CD34+ cells were further purified by MoFlo cell sorting and analysed by flow cytometry. The CD34+ cells had an average purity of 98.4 ± 1.2%. The isolated CD34+ cells did not express CD14, but showed slight expression of vascular endothelial growth factor receptor‐2 (VEGF‐R2) (1.6 ± 0.3%). The isolated CD14+ cells had an average purity of 99.8 ± 0.1%. Part of the isolated CD14+ cells co‐expressed of CD34 (0.96 ± 0.18%), KDR (85.6 ± 4.8%), or the HGF receptor c‐MET (88.2 ± 2.8%). Of the adherent CD34+ or CD14+ cells, neither cell fraction showed expression of endothelial cell specific proteins (Table 1).
Table 1.
Protein expression of adherent CD34+ or CD14+ cells (day 1)
| CD34 | CD14 | |
|---|---|---|
| CD14 %‐positive cells | 0.0 | 99.80 ± 0.10% |
| CD34 %‐positive cells | 98.40 ± 1.20% | 0.96 ± 0.18% |
| c‐MET %‐positive cells | ND | 88.21 ± 2.81% |
| VEGF‐R2 %‐positive cells | 1.60 ± 0.30% | 85.60 ± 4.80% |
| CD31 %‐positive cells | 0.0 | 87.28 ± 6.29% |
| vWF %‐positive cells | 0.0 | 0.91 ± 0.37% |
| CD144 %‐positive cells | 0.0 | 1.32 ± 0.66% |
| eNOS %‐positive cells | 0.0 | 1.20 ± 1.04% |
Data are presented as mean ± S.E.M. of at least three independent experiments. ND = Not determined.
Colony forming unit‐endothelial cells
Human pbMNC, containing fluorescently labelled CD34+ cells‐ and CD14+ cells were cultured under angiogenic conditions. Colonies had formed after 7 days of culturing (Fig. 1A). Examination of the CFU‐ECs by immunofluorescence microscopy revealed that these CFU‐ECs consisted mainly of CD14+‐derived cells (Fig. 1B; red cells) and that CD34+‐derived cells (green cell) co‐localized with CD14+‐derived cells in these CFU‐ECs (Fig. 1B). Although CD34+‐derived cells were also found outside the CFU‐ECs, colonies lacking CD34+‐derived cells were almost never observed. Analysis of outgrowth endothelial colonies by transmission electron microscopy also showed co‐localization and cell–cell contacts (arrows) of cells with progenitor cell morphology (Fig. 1C and D; small‐round cells) and cells with endothelial cell morphology (elongated cells). Cultured cells with an endothelial‐like morphology, i.e. spindle‐shaped cells, were quantified after 14 days (Fig. 1E and F). By that time, the CFU‐ECs had disappeared. The frequency of spindle‐shaped cells was significantly higher in the CD14+ monocultures and in all CD34+/CD14+ co‐cultures than in the CD34+ monoculture (20–32%versus 0.5%, respectively, P < 0.01). Spindle‐shaped cells originated mainly from the CD14+ cell fraction (over 85%; Fig. 1D; white bars), whereas the CD34+ cells (black bars) hardly contributed to the total amount of spindle‐shaped cells. The number of spindle‐shaped cells did not differ between the CD14+ monocultures and the CD34+/CD14+ cells in co‐culture.
Figure 1.
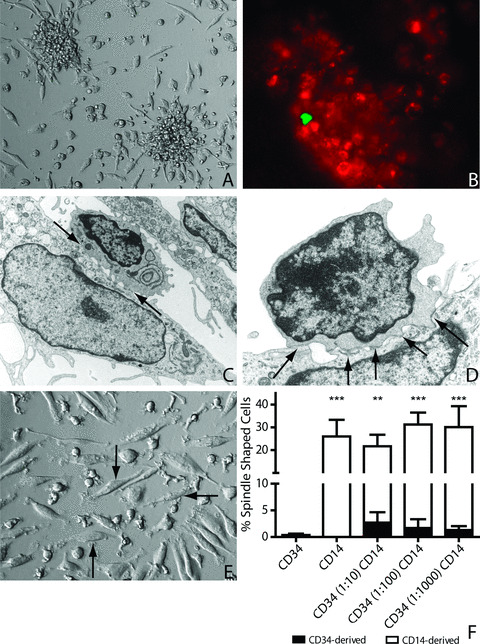
Formation of endothelial outgrowth colonies and cells. Peripheral blood MNC form colony forming unit‐endothelial cells (CFU‐ECs) after angiogenic stimulation (A). Within these colonies, CD34+ cells (green cell) and CD14+ cells (red cells) co‐localize (B). At the sites of endothelial cell formation, cell–cell contacts (arrows) are formed between cells with progenitor cell‐ (small round) and endothelial cell (spindle‐shaped) morphology (C). At day 14, spindle‐shaped cells (D, arrows) originating from these CFU‐ECs are mainly derived from the CD14+ cells (E).
Endothelial cell marker expression
Protein expression of endothelial cell markers was determined by immunofluorescent stainings (Fig. 2A and B) and FACS analysis for CD14, CD31, VEGFR‐2, vWF, CD144 and eNOS (Fig. 2C and D and Table 2). CD34+ cells in monoculture hardly ever expressed endothelial cell markers. Although over 30% of CD34+ cells did express CD31 (Table 2), co‐expression of CD31 and vWF was almost never observed. In fact, only 6–9% of the CD34+ cells showed signs of endothelial cell differentiation by the combined expression of either CD31 and vWF or CD144 and eNOS. In contrast, CD14+ cells in monoculture efficiently differentiated into an endothelial cell phenotype. CD14+ cell‐derived endothelial cells lost their expression of monocyte marker protein CD14, which decreased from 99.80 ± 0.10% (day 1) to 12.53 ± 2.54% (day 21; Table 2). Over 60% of the CD14+ cells had acquired a CD31+vWF+ phenotype and a CD144+eNOS+ phenotype (Fig. 2C). Interestingly, endothelial cell differentiation was significantly increased in all the co‐cultures, where over 85% of cells acquired the CD31+vWF+ phenotype (P < 0.01 versus CD14+ cells) and over 90% of cells acquired the CD144+eNOS+ phenotype (P < 0.001 versus CD14+ cells). Expression of VEGFR‐2 was present on the CD14+ cells at the start of culture and did not change during the mono‐ or co‐culture time (Table 2). Expression of all endothelial cell markers by co‐cultured cells was not different from HUVEC and was similar in all co‐cultures (Fig. 2C).
Figure 2.
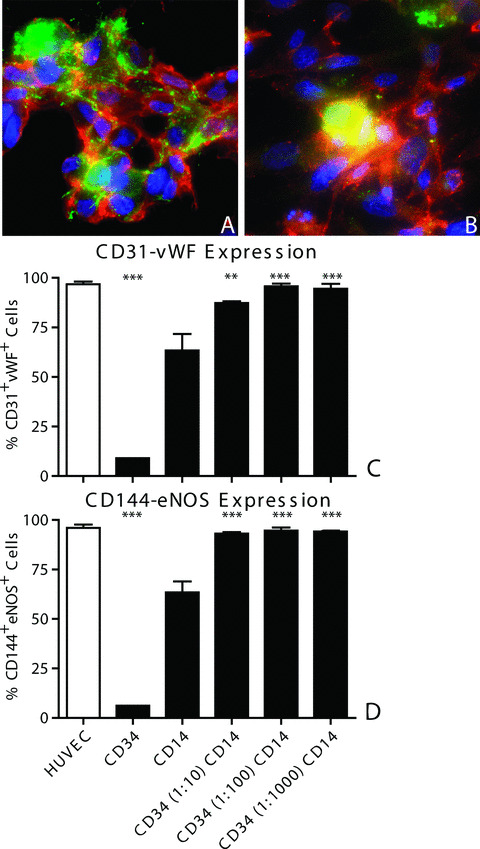
Endothelial cell differentiation of CD14+ cells. Monocultured and co‐cultured CD14+ cells expressed CD31, vWF, CD144 and eNOS after 21 days in culture. Immunofluorescent stainings show co‐expression of CD31 and vWF (A) as well as CD144 and eNOS (B) by cells in CD34 (1:100) CD14 co‐culture. FACS analysis showed that co‐cultured cells had increased percentages of cells that had acquired the CD31+vWF+ (C) and the CD144+eNOS+ phenotype (D). **=P < 0.01 versus CD14, ***=P < 0.001 versus CD14
Table 2.
Protein expression of endothelial cell specific markers (day 21)
| HUVEC | CD34* | CD14 | CD34 (1:10) CD14 | CD34 (1:100) CD14 | CD34 (1:1000) CD14 | |
|---|---|---|---|---|---|---|
| CD14 %‐positive cells | ND | ND | 12.53 ± 2.54 | ND | ND | ND |
| VEGF‐R2 %‐positive cells | 88.27 ± 1.16 | ND | 94.00 ± 3.62 | 87.27 ± 2.72 | 88.74 ± 3.16 | 83.49 ± 3.94c |
| (rMFI) | (100±12.16) | (91.86 ± 12.60) | (102.01 ± 1.31) | (105.61 ± 2.31) | (89.99 ± 12.42) | |
| CD31 %‐positive cells | 98.96 ± 0.81# | 34.28 | 63.84 ± 8.28† | 90.01 ± 0.78# | 95.10 ± 2.36# | 96.39 ± 1.21# |
| (rMFI) | (100 ± 53.04) | (362.47) | (145.61 ± 66.41) | (177.37 ± 13.38) | (195.51 ± 36.74) | (197.87 ± 8.85) |
| vWF %‐positive cells | 98.30 ± 0.37# | 9.07 | 65.29 ± 7.09# | 92.25 ± 0.02# | 89.70 ± 8.51 | 95.87 ± 1.77# |
| (rMFI) | (100 ± 38.67) | (93.83) | (93.48 ± 0.17) | (56.07 ± 5.08) | (75.00 ± 39.15) | (64.72 ± 5.23) |
| CD144 %‐positive cells | 98.04 ± 0.11 | 8.45 | 78.96 ± 2.51 | 94.99 ± 1.50 | 93.20 ± 9.76 | 91.65 ± 4.40 |
| (rMFI) | (100 ± 13.36) | (320.45) | (219.33 ± 84.60) | (114.33 ± 17.44) | (126.05 ± 23.76) | (112.19 ± 16.62) |
| eNOS %‐positive cells | 99.22 ± 0.56# | 6.43 | 61.24 ± 3.89# | 95.25 ± 1.09# | 84.08 ± 10.80# | 82.93 ± 12.12# |
| (rMFI) | (100 ± 18.16) | (182.71) | (137.32 ± 28.79) | (98.46 ± 9.06) | (108.50 ± 19.64) | (92.07 ± 8.81) |
Data are presented as mean ± S.E.M. of at least three independent experiments.
Relative mean fluorescent intensity (rMFI) was calculated as follows: rMFI = (MFIsample/MFIHUVEC)*100%.
ND = not determined.
*n= 1; † P < 0.05 versus HUVEC and # P < 0.05 versus CD14.
Cell proliferation
Proliferation of cultured cells was assessed by staining of nuclear antigen Ki67 at day 21 (Fig. 3A and B). In the monocultures containing either CD34+ cells or CD14+ cells, no or limited proliferation was observed (Fig. 3C). CD34+ monocultures did not proliferate at all, and only 1.13 ± 0.24% of the CD14+ cells in monoculture stained positive for Ki67. The co‐cultured cells in the ratio of CD34 (1:100) CD14 and CD34 (1:1000) CD14 showed significantly higher proliferation rates (10.70 ± 1.02% and 9.20 ± 1.14%, respectively, P < 0.01) than the CD14+ cells in monoculture. The co‐culture of CD34 (1:10) CD14 also showed higher proliferation rates (6.01 ± 1.37%, P < 0.05) than the CD14+ cells in monoculture did, but proliferation in the CD34 (1:10) CD14 co‐culture was lower than in the other two co‐cultures (P < 0.05). At no time, proliferation was observed in the monocultures (data not shown).
Figure 3.
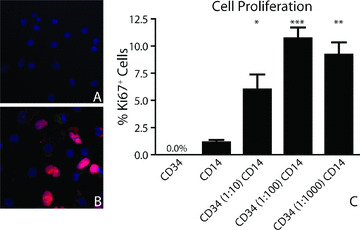
Cell proliferation of CD14+ cells. Cell proliferation was almost never observed in monocultured CD14+ cells (A), but was observed in co‐cultured cells (CD34 (1:100) CD14; B). *=P < 0.05 versus CD14, **=P < 0.01 versus CD14, and ***=P < 0.001 versus CD14.
Endothelial cell function
To assess the functionality of the cultured cells, their ability to prevent factor 3‐dependent thrombin formation was determined (Fig. 4A). The formation of thrombin by HUVEC was 73.5 ± 9.5 mU/ml, whereas thrombogenic VSMC showed a 10‐fold increase in thrombin formation (733.6 ± 203.8 mU/ml, P < 0.01 versus HUVEC). Co‐cultured CD34+ cells and CD14+ cells produced lower amounts of thrombin than VSMC (85–116 mU/ml, P < 0.01). Moreover, thrombin generation by co‐cultured cells was not different from HUVEC. Although CD14+‐derived endothelial cells in monoculture tended to generate more thrombin than HUVEC (175.5 ± 36.3 mU/ml, P < 0.10), no significant difference was found. Furthermore, the thrombin generation by CD14+ cells in monoculture was not different from the thrombin generation observed by the co‐cultured cells.
Figure 4.
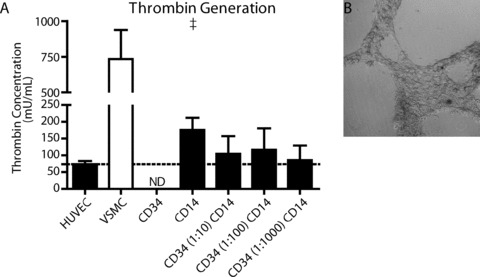
Endothelial cell function of differentiated CD14+ cells. Endothelial cell function was determined as the ability to inhibit thrombin formation in vitro and the ability to form capillary‐like sprouts. Human umbilical vein endothelial cells (HUVEC) are able to inhibit the factor‐3 dependant formation of thrombin, whereas factor‐3 positive smooth muscle cells show high amounts of thrombin formed. Differentiated endothelial cells derived from CD14+ cells or CD34+/CD14+ co‐cultures both inhibited thrombin formation (A). After 3 weeks in mono‐ or co‐culture, CD14+ derived endothelial cells had the ability to form capillary‐like sprouts on Matrigel (B). †=P < 0.10 versus HUVEC.
Cells from CD14+ monocultures or CD34+/CD14+ co‐cultures were plated on Matrigel‐coated wells plates in order to investigate their ability to form capillary like structures. Freshly isolated CD14+ cells did not form any capillary‐like structures on Matrigel (data not shown). Both monocultured CD14+ cells as well as co‐cultured CD34+/CD14+ cells formed branched structures on Matrigel (Fig. 4B). There was no difference found in the number of branches between CD14+ cell monocultures or CD34+/CD14+ co‐cultures. Nor did addition of HGF potentiate the formation of capillary‐like structures.
Cytokine and growth factor secretion
Culture medium from CD34+ cells and CD14+ cells in monoculture and the CD34 (1:100) CD14 co‐culture were analysed for the presence of EGF, bFGF, HGF, TGF‐β1 and VEGF‐A, as well as for the cytokines ANG‐2, IL‐8, MCP‐1 and TNF‐α. Basic FGF (189.9 ± 28.8 pg/ml), VEGF‐α (271.0 ± 40.2 pg/ml) were present in the culture medium. Background levels of TGF‐β1 (2.38 ± 0.13 ng/ml) and TNF‐α (28.3 ± 9.8 pg/ml) were detected whereas EGF was not detected. Although the levels of these growth factors and cytokines varied slightly, no significant changes were detected in their protein levels during the 21‐day culture period (see online data supplement). ANG‐2 was also not present in the culture medium, but a concentration of 33.8 ± 8.4 pg/ml was detected in the CD34 (1:100) CD14 co‐culture at day 3. At this time, concentration of ANG‐2 was higher in the co‐culture than in both monocultures (P < 0.001), where ANG‐2 protein was not detected. Although ANG‐2 protein was detected in the CD34+ and CD14+ monocultures after 7 days (6.7 ± 6.7 pg/ml and 3.7 ± 3.7 pg/ml, respectively), ANG‐2 concentration in the CD34 (1:100) CD14 co‐culture tended to be higher throughout the 21‐day culture period (P < 0.10; see online data supplement).
Background levels of HGF (40.3 ± 5.8 pg/ml), IL‐8 (289.9 ± 174.9 pg/ml) and MCP‐1 (105.4 ± 55.2 pg/ml) were detected at the start of culture. In contrast to the growth factors and cytokines described above, significant increases in the concentrations of these factors were observed (Fig. 5). The concentration of HGF increased most rapidly in the CD34+ monoculture, where HGF concentration reached 11,202 ± 5421 pg/ml (≈270‐fold increase) at day 3 (Fig. 5A). By then, the concentration of HGF protein in the CD34+ monoculture was significantly higher than in the CD14+ monoculture (405.9 ± 68.4 pg/ml, P < 0.05) and the CD34 (1:100) CD14 co‐culture (408.7 ± 73.5 pg/ml, P < 0.05). Concentrations of HGF protein in the CD14+ monoculture and in the CD34 (1:100) CD14 co‐culture also increased during culture. HGF concentration reached a maximum of over 10,000 pg/ml in all cultures at day 21.
Figure 5.
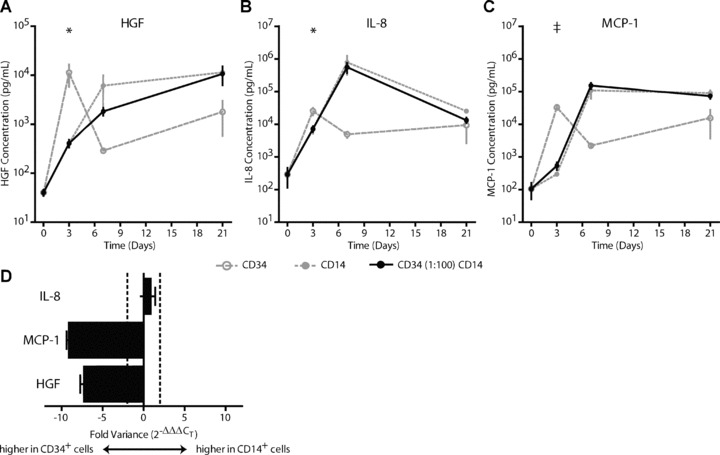
Growth factor and cytokine secretion. Production and secretion of hepatocyte growth factor (HGF), IL‐8 and MCP‐1 by cells in monoculture or in CD34 (1:100) CD14 co‐culture. CD34+ cells showed a more rapid increase in protein concentrations of HGF (A), IL‐8 (B) and MCP‐1 (C) compared to CD14+ monocultures or CD34 (1:100) CD14 co‐culture. Gene transcript analysis for IL‐8, MCP‐1 and HGF showed higher transcript availability for MCP‐1 and HGF in the CD34+ cell cultures. No difference in transcript availability was found for IL‐8 between the CD34+ cell and CD14+ cell monocultures. †=P < 0.10 versus CD14 and CD34 (1:100) CD14, *=P < 0.05 versus CD14 and CD34 (1:100) CD14.
Concentrations of IL‐8 and MCP‐1 also showed a more rapid increase in the CD34+ monoculture compared to the CD14+ monoculture and the CD34 (1:100) CD14 co‐culture. At day 3, the concentration of IL‐8 in the CD34+ monoculture was 25,277 ± 6474 pg/ml (≈240‐fold increase). Increases in IL‐8 concentrations were also observed in the CD14+ cell monocultures and in the CD34 (1:100) CD14 co‐cultures (Fig. 5B). Although these increases started later than in the CD34+ monocultures, concentrations of IL‐8 increased even further. At day 7, concentrations of IL‐8 did not differ between CD14+ monocultures and CD34 (1:100) CD14 co‐cultures and reached concentrations of 788,455 ± 460,545 pg/ml (≈2700‐fold increase). The MCP‐1 protein concentration in CD34+ monocultures was 32,853 ± 5549 pg/ml (≈310‐fold increase) at day 3 and tended to be higher than MCP‐1 concentrations in the CD14+ monocultures (294.1 ± 34.0 pg/ml, P < 0.10) and CD34 (1:100) CD14 co‐cultures (537.8 ± 140.6, P < 0.10, Fig. 5C). Similar to IL‐8 protein concentration, the MCP‐1 concentration in the CD14+ monocultures and the CD34 (1:100) CD14 co‐cultures increased more slowly, but reached higher levels (108,211 ± 47,400 pg/ml and 152,132 ± 32,371 pg/ml, respectively). Concentrations of MCP‐1 remained high in all cultures throughout the 21‐day culture period, but did not differ between the cultures at day 21.
To examine if differences in protein concentrations of HGF, IL‐8 and MCP‐1 were related to differences in gene expression between CD34+ cells and CD14+ cells in monoculture, we performed transcriptome analysis (Fig. 5D). At day 3 of culture, CD34+ cells in monoculture had a higher gene transcript level for both MCP‐1 (9‐fold) and HGF (7‐fold) compared to CD14+ cells in monoculture, but there was no biologically relevant difference in gene expression of IL‐8 (<2‐fold).
Conditioned medium cultures
To assess the effects of paracrine factors, CD14+ cells were cultured in the presence of conditioned culture media. Thereafter, expression of endothelial cell markers CD31, vWF, CD144 and eNOS was assessed by FACS analysis. Endothelial cell differentiation of monocultured CD14+ cells was approximately 63% (Fig. 6A and B) and did not change by addition of conditioned medium from CD14+ cells. In fact, the co‐expression of CD144 and eNOS was decreased to approximately 40% (P < 0.01; Fig. 6B). In contrast to the CD14+ cells conditioned medium, exposure to conditioned media from either CD34+ cells in monoculture, or CD34+/CD14+ co‐cultures, significantly increased endothelial cell differentiation by CD14+ cells (Fig. 6A and B). Co‐expression of endothelial cell markers CD31 and vWF increased from 63% for CD14+ cells in monoculture to over 95% in all the CD34+ cells‐derived conditioned media (Fig. 6A; P < 0.05). Similarly, the co‐expression of endothelial cell markers CD144 and eNOS increased from 63% for CD14+ cells in monoculture to over 95% in all the CD34+ cells‐derived conditioned media (Fig. 6B; P < 0.001).
Figure 6.
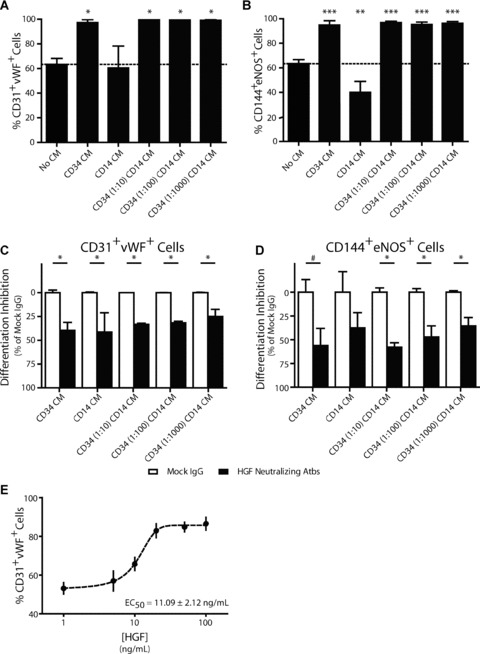
CD34+ cell‐derived hepatocyte growth factor (HGF) augments endothelial cell differentiation of CD14+ cells. Culturing CD14+ cells in CD34‐, or CD34+/CD14+ conditioned media resulted in increased co‐expression of CD31 and vWF (A) and CD144 and eNOS (B) compared to CD14+ monocultures or CD14+ conditioned medium. Increase in endothelial marker expression was partially inhibited by neutralizing antibodies to HGF (C: CD31+vWF+ and D: CD144+eNOS+). Addition of recombinant HGF to CD14+ monocultures increased endothelial marker expression in a dose‐dependent manner (E). †=P < 0.10 versus CD14, *=P < 0.05 versus CD14, **=P < 0.01 versus CD14, and ***=P < 0.001 versus CD14.
Neutralizing antibodies to HGF, IL‐8 and MCP‐1 were added to the conditioned medium in order to determine their influence on the increased endothelial cell differentiation. Neutralizing antibodies to HGF reduced the effect of the conditioned culture media by 30–60% (Fig. 6C and D). Co‐expression of endothelial cell markers CD31 and vWF was reduced by over 30% (P < 0.05) both in the CD34+ and in the CD14+ cells conditioned medium (Fig. 6C). In contrast, HGF neutralizing antibodies added to CD14+ cell conditioned medium did not decrease co‐expression of CD144 and eNOS (P= 0.24). Co‐expression of CD144 and eNOS, however, was reduced by approximately 50% when HGF neutralizing antibodies were added to CD34+/CD14+ cell conditioned media (P < 0.05; Fig. 6D). A trend for decreased co‐expression of CD144 and eNOS was found when HGF neutralizing antibodies were added to CD34+ cells conditioned media (P= 0.057), but did not reach statistical significance. Addition of neutralizing antibodies to either IL‐8 or MCP‐1 did not alter the co‐expression of CD31 and vWF and CD144 and eNOS (see online data supplement).
Addition of recombinant human HGF to CD14+ cells in monoculture increased endothelial cell differentiation of CD14+ cells, as determined by co‐expression of CD31 and vWF (Fig. 6E). The increase in endothelial cell differentiation caused by the addition of HGF was dose‐dependent and had an EC50 of 11.09 ± 2.12 ng/ml.
Discussion
Current gold standard cardiovascular therapies, such as percutaneous angioplasty and stenting, focus on the prevention of acute and ongoing tissue damage by relieve of the ischaemia, rather than on tissue regeneration. Therefore, tissue regeneration by stem cell therapy is a promising new treatment strategy to treat ischaemic diseases, such as myocardial infarction. However, cell therapy strategies that use peripheral blood derived progenitor cells as a source of EPCs have been hampered by low cell numbers, lack of cell proliferation and the absence of cellular contribution to the neovasculature. In this study, we have shown that (1) culturing human pbMNC under angiogenic conditions the formation of CFU‐ECs in which CD34+‐derived cells and CD14+‐derived cells co‐localize, (2) the interaction between CD34+‐derived cells and CD14+‐derived cells results in increased endothelial cell differentiation of the CD14+‐derived cells in a paracrine and HGF‐dependent manner and (3) results in the generation of a functional and proliferating endothelial cell population in vitro.
Although the CD34+ stem cell has been regarded as the archetype EPC, its cellular incorporation into the neovasculature is hardly observed [2, 3, 4]. In fact, there is a growing body of literature showing that endothelial‐like cells of monocytic (i.e. CD14+) origin influence neovascularization [6, 15, 16, 17, 18, 19]. We have previously shown that monocyte depletion results in reduced neovascularization of ischaemic areas after myocardial infarction [8]. Furthermore, data from both animal experiments and clinical trials suggest that the CD34+ cell augments neovascularization and endothelial cell differentiation in a paracrine manner, rather than by incorporation into the neovasculature [4, 5, 6, 20, 21, 22, 23, 24]. In addition, Bonello et al. reported increased numbers of circulating CD14+ cells after angioplasty‐induced endothelial cell damage [25]. These circulating CD14+ cells may be mobilized by increased levels of HGF after angioplasty [26] and help to repair the injured endothelium [16, 27] by mechanisms described here.
Here we aimed to investigate the interaction between CD34+ cells and CD14+ cells in vitro. We have shown that the culture of pbMNC results in a co‐localization of CD34+‐derived cells and CD14+‐derived cells (Fig. 1). Similarly, Yoon et al. showed that early endothelial cell outgrowth colonies comprised of CD14+ monocytic cells and CD14− cells [19]. As shown in Fig. 1, we postulate that the CD14− cells observed by Yoon et al., are in fact CD34+‐derived cells. Complementary, Rohde et al. in a recent study showed that interaction of CD14+ cells with T lymphocytes was accountable for the formation of these CFU‐ECs in vitro[28]. However, the influence of T lymphocytes on endothelial cell differentiation was not investigated. This suggests that T cells, in combination with CD14+ cells and CD34+ cells form a microenvironment, i.e. the CFU‐ECs, in which differentiation of primitive CD14+ cells towards an endothelial cell lineage is instructed.
In agreement with our finding that co‐cultivation of CD34+ cells and CD14+ cells results in increased endothelial cell differentiation in vitro (Fig. 2), Yoon et al. showed increased endothelial cell differentiation in vitro and increased progenitor cell incorporation into the neovasculature when a mixture of CD14+ cells and CD34+ cells were co‐injected into ischaemic hind limbs of nude mice [19]. Similarly, in vivo co‐localization of CD14+ cells and CD34+ cells at sites of active neovascularization and endothelial cell differentiation was reported by Anghelina et al. and Moldovan et al. [15, 29, 30].
Co‐cultivation did not only result in increased expression of endothelial cell markers by CD14+ cells, but also in gain of endothelial cell functionality. In concurrence with the work of Smadja et al. who reported that umbilical cord EPC have anticoagulant effects [31], we show that CD14+ cell‐derived endothelial cells actively inhibit thrombin formation in an in vitro coagulation assay (Fig. 4) and thus can influence haemostasis.
Besides increased differentiation of functional endothelial cells, we show that co‐cultivation of CD34+ cells and CD14+ cells results in increased proliferation of the CD14+ cells (Fig. 3). In agreement with other studies, we found that monocultured CD14+ cells proliferate only scarcely [9, 13, 32, 33, 34]. However, when cultured in the presence of CD34+ cells, proliferation increased to approximately 10% at week 3 indicating differentiation of long‐lived CD14+ cell‐derived endothelial cells. Although higher levels of proliferation by CD14+ cells in pre‐differentiation cultures have been described [35, 36, 37], only few reports describe the differentiation of a functional and proliferating endothelial cell population originating from CD14+ cells. Our finding that functional and proliferating endothelial cells can be differentiated from CD14+ cells, corroborates the findings of Elsheikh et al. and Romagnani et al.[38, 39].
To assess whether the increase in endothelial cell differentiation and proliferation depends on cell–cell adhesions, we performed differentiation experiments using conditioned culture media from CD34+ and CD14+ monocultures. Although cell–cell adhesions were identified between cells with an endothelial cell‐ and with progenitor cell‐morphology (Fig. 1), CD34+ cells conditioned culture media displayed a similar increase in endothelial cell differentiation as seen in CD34+/CD14+ cells in co‐culture (comparison Figs 2 and 6). Therefore, we conclude that CD34+ cells influenced the differentiation of CD14+ cells in a paracrine manner. This paracrine effect of CD34+ cells has been suggested previously. More precise, we and others have described the secretion of pro‐angiogenic factors, such as MCP‐1, IL‐8, VEGF and bFGF [4, 5, 6, 20, 21, 22, 23, 24], by CD34+ cells both in vitro and in vivo, rather than endothelial cell differentiation and incorporation into the neovasculature by that same cell type.
Analysis of conditioned culture media revealed large increases in secreted IL‐8, MCP‐1 and HGF during a 3‐week culture period. Notably, the gene transcripts of these factors were present at a higher level in CD34+ cells than in CD14+ cells, and the extracellular concentrations of these pro‐angiogenic factors increased faster in the CD34+ cells culture media than in CD14+ cell conditioned media or the CD34+/CD14+ co‐culture media (Fig. 5). This suggests that these factors are actively taken up by the CD14+ cells and used during downstream signalling cascades. Therefore, we investigated the effects of these factors on endothelial cell differentiation by CD14+ cells using neutralizing antibodies against IL‐8, MCP‐1 and HGF. IL‐8 and MCP‐1 have been described as angiogenic cytokines. In this context, IL‐8 and MCP‐1 were associated with the recruitment and migration of CD14+ cells [6, 29, 40, 41, 42, 43], CD14+ cell adhesion [16], growth factor secretion [44, 45, 46] and endothelial cell proliferation [47]. Although IL‐8 and MCP‐1 are involved in the induction of angiogenesis and cell recruitment, we did not observe inhibition of endothelial cell differentiation by IL‐8 nor MCP‐1 neutralizing antibodies in vitro (see online data supplement). This suggests that IL‐8 and MCP‐1 act at a different level on CD14+ cells during neovascularization, other than endothelial cell differentiation. In contrast, neutralizing antibodies to HGF did decrease endothelial cell differentiation by CD14+ cells and addition of HGF to CD14+ cells in monoculture did increased endothelial cell differentiation of these cells in a dose‐dependent manner (Fig. 6). Our observation in vitro appears to be supported by in vivo data of Cai et al. who showed that HGF is an obligatory molecule for revascularization of ischaemic tissue [48]. Furthermore, Bordoni et al. have shown that hepatocyte‐conditioned medium was able to influence endothelial cell differentiation by EPC in vitro[49]. These findings support our finding that indeed HGF is involved in endothelial cell differentiation by CD14+ cells.
In summary, we show that co‐cultivation of CD34+ cells and CD14+ cells results in increased differentiation of human CD14+ cells towards functional and proliferating endothelial cells in vitro. The increased differentiation and proliferation were dependent on paracrine signalling through HGF, rather than on cell–cell contacts. Taken together, the combination of CD14+ cells and CD34+ cells yields a proliferating population of endothelial cells that may be a suitable cell source that can be applied as cell therapeutic for treatment of cardiovascular diseases, and can augment therapeutic neovascularization in various disease models.
Acknowledgements
The authors acknowledge Dr. Wim van Oeveren and Mr. Geert Kors for expert technical assistance. This research was supported by a grant from the ‘de Drie Lichten’ Foundation, Leinden, The Netherlands, and an institutional grant from Medtronic Bakken Research Center, Maastricht, The Netherlands.
References
- 1. Hristov M, Erl W, Weber P. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol . 2003; 23: 1185–9. [DOI] [PubMed] [Google Scholar]
- 2. Jackson K, Majka S, Wang H, et al . Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest . 2001; 107: 1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawamoto A, Asahara T, Losordo DW. Transplantation of endothelial progenitor cells for therapeutic neovascularization. Cardiovasc Radiat Med . 2002; 3: 221–5. [DOI] [PubMed] [Google Scholar]
- 4. Ma N, Stamm C, Kaminski A, et al . Human cord blood cells induce angiogenesis following myocardial infarction in NOD/Scid‐mice. Cardiovasc Res . 2005; 66: 45–54. [DOI] [PubMed] [Google Scholar]
- 5. Popa ER, Harmsen MC, Tio RA, et al . Circulating CD34+ progenitor cells modulate host angiogenesis and inflammation in vivo . J Mol Cell Cardiol . 2006; 41: 86–96. [DOI] [PubMed] [Google Scholar]
- 6. van der Strate BWA, Popa ER, Schipper M, et al . Circulating human CD34+ progenitor cells modulate neovascularization and inflammation in a nude mouse model. J Mol Cell Cardiol . 2007; 42: 1086–97. [DOI] [PubMed] [Google Scholar]
- 7. van Amerongen MJ, Bou‐Gharios G, Popa E, et al . Bone marrow‐derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol . 2007; 214: 377–86. [DOI] [PubMed] [Google Scholar]
- 8. van Amerongen MJ, Harmsen MC, van RN, et al . Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol . 2007; 170: 818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krenning G, Dankers P, Jovanovic D, et al . Efficient differentiation of CD14+ monocytic cells into endothelial cells on degradable biomaterials. Biomaterials . 2007; 28: 1470–9. [DOI] [PubMed] [Google Scholar]
- 10. Hur J, Yoon C, Kim H, et al . Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol . 2004; 24: 288–93. [DOI] [PubMed] [Google Scholar]
- 11. Rohde E, Malischnik C, Thaler D, et al . Blood monocytes mimic endothelial progenitor cells. Stem Cells . 2006; 24: 357–67. [DOI] [PubMed] [Google Scholar]
- 12. Yoder M, Mead L, Prater D, et al . Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood . 2007; 109: 1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez Pujol B, Lucibello FC, Gehling UM, et al . Endothelial‐like cells derived from human CD14 positive monocytes. Differentiation . 2000; 65: 287–300. [DOI] [PubMed] [Google Scholar]
- 14. Burgess WH, Mehlman T, Friesel R, et al . Multiple forms of endothelial cell growth factor. Rapid isolation and biological and chemical characterization. J Biol Chem . 1985; 260: 11389–92. [PubMed] [Google Scholar]
- 15. Anghelina M, Krishnan P, Moldovan L, et al . Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev . 2004; 13: 665–76. [DOI] [PubMed] [Google Scholar]
- 16. Fujiyama S, Amano K, Uehira K, et al . Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein‐1‐dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res . 2003; 93: 980–9. [DOI] [PubMed] [Google Scholar]
- 17. Harraz M, Jiao C, Hanlon HD. CD34− blood derived human endothelial cell progenitors. Stem Cells . 2001; 19: 304–12. [DOI] [PubMed] [Google Scholar]
- 18. Sharifi BG, Zeng Z, Wang L, et al . Pleiotrophin induces transdifferentiation of monocytes into functional endothelial cells. Arterioscler Thromb Vasc Biol . 2006; 26: 1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon C, Hur J, Park K, et al . Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation . 2005; 112: 1618–27. [DOI] [PubMed] [Google Scholar]
- 20. Ziegelhoeffer T, Fernandez B, Kostin S, et al . Bone marrow‐derived cells do not incorporate into the adult growing vasculature. Circ Res . 2004; 94: 230–8. [DOI] [PubMed] [Google Scholar]
- 21. Heil M, Ziegelhoeffer T, Mees B, et al . A different outlook on the role of bone marrow stem cells in vascular growth – bone marrow delivers software not hardware. Circ Res . 2004; 94: 573–4. [DOI] [PubMed] [Google Scholar]
- 22. Umland O, Heine H, Miehe M, et al . Induction of various immune modulatory molecules in CD34+ hematopoietic cells. J Leukoc Biol . 2004; 75: 671–9. [DOI] [PubMed] [Google Scholar]
- 23. Bautz F, Rafii S, Kanz L, et al . Expression and secretion of vascular endothelial growth factor‐A by cytokine‐stimulated hematopoietic progenitor cells: possible role in the hematopoietic microenvironment. Exp Hematol . 2000; 28: 700–6. [DOI] [PubMed] [Google Scholar]
- 24. Majka M, Janowska‐Wieczorek A, Ratajczak J, et al . Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood . 2001; 97: 3075–85. [DOI] [PubMed] [Google Scholar]
- 25. Bonello L, Basire A, Sabatier F, et al . Endothelial injury induced by coronary angioplasty triggers mobilization of endothelial progenitor cells in patients with stable coronary artery disease. J Thromb Haemost . 2006; 4: 979–81. [DOI] [PubMed] [Google Scholar]
- 26. Susen S, Sautiere K, Mouquet F, et al . Serum hepatocyte growth factor levels predict long‐term clinical outcome after percutaneous coronary revascularization. Eur Heart J . 2005; 26: 2387–95. [DOI] [PubMed] [Google Scholar]
- 27. Woywodt A, Erdbruegger U, Haubitz M. Circulating endothelial cells and endothelial progenitor cells after angioplasty: news from the endothelial rescue squad. J Thromb Haemost . 2006; 4: 976–8. [DOI] [PubMed] [Google Scholar]
- 28. Rohde E, Bartmann C, Schallmoser K, et al . Immune cells mimic the morphology of endothelial progenitor colonies in vitro . Stem Cells . 2007; 25: 1746–52. [DOI] [PubMed] [Google Scholar]
- 29. Moldovan NI, Goldschmidt‐Clermont PJ, Parker‐Thornburg J, et al . Contribution of monocytes/macrophages to compensatory neovascularization – the drilling of metalloelastase‐positive tunnels in ischemic myocardium. Circ Res . 2000; 87: 378–84. [DOI] [PubMed] [Google Scholar]
- 30. Anghelina M, Krishnan P, Moldovan L, et al . Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol . 2006; 168: 529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smadja DM, Basire A, Amelot A, et al . Thrombin bound to a fibrin clot confers angiogenic and hemostatic properties on endothelial progenitor cells. J Cell Mol Med . 2008; 12: 975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duan HX, Cheng LM, Jian W, et al . Angiogenic potential difference between two types of endothelial progenitor cells from human umbilical cord blood. Cell Biol Int . 2006; 30: 1018–27. [DOI] [PubMed] [Google Scholar]
- 33. Zhang SJ, Zhang H, Wei YJ, et al . Adult endothelial progenitor cells from human peripheral blood maintain monocyte/ macrophage function throughout in vitro culture. Cell Res . 2006; 16: 577–84. [DOI] [PubMed] [Google Scholar]
- 34. Rehman J, Li JL, Orschell CM, et al . Peripheral blood “endothelial progenitor cells” are derived from monocyte/ macrophages and secrete angiogenic growth factors. Circulation . 2003; 107: 1164–9. [DOI] [PubMed] [Google Scholar]
- 35. Fernandez Pujol B, Lucibello FC, Zuzarte M, et al . Dendritic cells derived from peripheral monocytes express endothelial markers and in the presence of angiogenic growth factors differentiate into endothelial‐like cells. Eur J Cell Biol . 2001; 80: 99–110. [DOI] [PubMed] [Google Scholar]
- 36. Zhang R, Yang H, Li M, et al . Acceleration of endothelial‐like cell differentiation from CD14+ monocytes in vitro . Exp Hematol . 2005; 33: 1554–63. [DOI] [PubMed] [Google Scholar]
- 37. Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte‐derived subset acts as pluripotent stem cells. Proc Natl Acad Sci USA . 2003; 100: 2426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elsheikh E, Uzunel M, He Z, et al . Only a specific subset of human peripheral‐blood monocytes has endothelial‐like functional capacity. Blood . 2005; 106: 2347–55. [DOI] [PubMed] [Google Scholar]
- 39. Romagnani P, Annunziato F, Liotta F, et al . CD14+ CD34(Low) cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res . 2005; 97: 314–22. [DOI] [PubMed] [Google Scholar]
- 40. Sparmann A, Bar‐Sagi D. Ras‐induced interleukin‐8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell . 2004; 6: 447–58. [DOI] [PubMed] [Google Scholar]
- 41. Anghelina M, Moldovan L, Zabuawala T, et al . A subpopulation of peritoneal macrophages form capillarylike lumens and branching patterns in vitro . J Cell Mol Med . 2006; 10: 708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charo I, Taubman M. Chemokines in the pathogenesis of vascular disease. Circ Res . 2004; 95: 858–66. [DOI] [PubMed] [Google Scholar]
- 43. Kuroda T, Kitadai Y, Tanaka S, et al . Monocyte chemoattractant protein‐1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res . 2005; 11: 7629–36. [DOI] [PubMed] [Google Scholar]
- 44. Hong K, Ryu J, Han K. Monocyte chemoattractant protein‐1‐induced angiogenesis is mediated by vascular endothelial growth factor‐A. Blood . 2005; 105: 1405–7. [DOI] [PubMed] [Google Scholar]
- 45. Niiyama H, Kai H, Yamamoto T, et al . Roles of endogenous monocyte chemoattractant protein‐1 in ischemia‐induced neovascularization. J Am Coll Cardiol . 2004; 44: 661–6. [DOI] [PubMed] [Google Scholar]
- 46. Yamada M, Kim S, Egashira K, et al . Molecular mechanism and role of endothelial monocyte chemoattractant protein‐1 induction by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol . 2003; 23: 1996–2001. [DOI] [PubMed] [Google Scholar]
- 47. Li A, Varney M, Valasek J, et al . Autocrine role of interleukin‐8 in induction of endothelial cell proliferation, survival, migration and mmp‐2 production and angiogenesis. Angiogenesis . 2005; 8: 63–71. [DOI] [PubMed] [Google Scholar]
- 48. Cai L, Johnstone BH, Cook TG, et al . Suppression of hepatocyte growth factor production impairs the ability of adipose‐derived stem cells to promote ischemic tissue revascularization. Stem Cells . 2007; 25: 3234–43. [DOI] [PubMed] [Google Scholar]
- 49. Bordoni V, Alonzi T, Zanetta L, et al . Hepatocyte‐conditioned medium sustains endothelial differentiation of human hematopoietic‐endothelial progenitors. Hepatology . 2007; 45: 1218–28. [DOI] [PubMed] [Google Scholar]


