Abstract
The development of a collateral circulation (arteriogenesis), bypassing an arterial occlusion, is important for tissue survival, but it remains functionally defective. Micro array data of growing collateral vessels, exposed to chronically elevated fluid shear stress (FSS), showed increased transcription of the transient receptor potential cation channel, subfamily V, member 4 (Trpv4). Thus, the aim of this study was to investigate the role of the shear stress sensitive Trpv4 in transmitting this physical stimulus into an active growth response. qRT‐PCR at different time points during the growth of collateral vessels after femoral artery ligature (FAL) in rats showed a strong positive correlation of Trpv4 transcription and the intensity of FSS. An increased protein expression of Trpv4 was localized in the FSS‐sensing endothelium by means of confocal immunohistochemistry. Cultured porcine endothelial cells showed a dose‐dependent expression of Trpv4 and an increased level of Ki67‐positive cells upon treatment with 4α‐Phorbol 12,13‐didecanoate (4αPDD), a specific Trpv4 activator. This was also demonstrated by flow culture experiments. These results were confirmed by in vivo application of 4αPDD in rabbit hind limb circulation via an osmotic mini‐pump after FAL. Trpv4 expression as well as Ki67‐positive staining was significantly increased in collateral vessels. Finally, 4αPDD treatment after FAL led to a 61% (215.5 ml/min/mmHg versus 350 ml/min/mmHg) recovery of conductance when compared with the non‐occluded artery. Cell culture and in vivo studies demonstrate that an FSS‐ or a 4αPDD‐induced activation of Trpv4 leads to an active proliferation of vascular cells and finally triggers collateral growth. Trpv4, a well‐known FSS‐sensitive vasodilator, has hitherto not been implicated in active growth processes of collateral arteries. This new function may lead to new therapeutic strategies for the treatment of arterial occlusive diseases.
Keywords: arteriogenesis, Trpv4, calcium, cardiovascular disease
Introduction
It is known that in patients with ischaemic vascular diseases collateral vessels grow bypassing the occlusion site. This process is termed arteriogenesis. However, the physiological collateral growth is not sufficiently able to compensate the deterioration of blood flow. Recent studies could show that collateral vessels only attain about 40% of the maximal conductance of the artery they had replaced [1, 2].
Thus, a major therapeutic goal is to stimulate the growth of collateral vessels in order to optimize this natural process. However, significant effects for angiogenic growth factors could not be demonstrated in clinical trials, despite some anecdotal evidence [3].
After femoral artery ligature (FAL) in hind limbs of rabbits and pigs with an arterio‐venous anastomosis leading to a chronically elevated fluid shear stress (FSS), it was shown that this physical force distinctively triggers collateral growth [4, 5]. As a consequence, the long‐lasting growth of collateral vessels completely restored (and overshot) the physiological function of the occluded artery. We adapted the high shear stress model to the rat, whose genome is known, and performed a genome‐wide screening, using the Whole Rat Genome Oligo Microarray Kit (Agilent Technologies, Boeblingen, Germany). Among the up‐regulated genes, we focused on genes which were known to be dependent on mechanical stress (e.g. shear stress). We identified the transient receptor potential cation channel, subfamily V, member 4 (Trpv4) as a possible mediator of physical stimuli to intracellular signals. Previous results showed that Trpv4 is activated by changes of osmotic pressure, temperature and changes in FSS, which are exerted to the inner lining of the vascular wall [6, 7]. Trpv4 is known to play an important role in the regulation of vascular tone, and its activation leads to vasodilatation of the vessel wall via Ca2+‐influx [8]. Therefore, we tested the hypothesis whether Trpv4 plays a crucial role in arteriogenesis by transmitting the physical stimulus (FSS) into an active intracellular growth response thereby representing a very early event in vascular remodelling.
Methods
Animal models
The study was performed according to Section 8 of the German Law for the Protection of Animals, which complies with the US National Institutes of Health (NIH) guidelines.
Due to their known genomic background, rats were used for genome‐wide transcriptional profiling of growing collaterals or quantitative real‐time PCR. Because the reliability of hemodynamic measurements is greater in larger animals, collateral conductance CCmax (ml/min/100 mmHg) was assessed in rabbits.
Therefore, the femoral artery was ligated in New Zealand White rabbits (3.0 ± 0.3 kg body weight) and Sprague Dawley rats (275 ± 25 g body weight). They were assigned to one of the following groups (each n= 6): (i) AV‐shunt treatment, (ii) local administration of 4α‐Phorbol‐12,13‐didecanoate (4αPDD) dissolved in EtOH (0.05 mg/kg body weight per day), (iii) Ruthenium Red (RR) dissolved in NaCl (0.5 mg/kg body weight per day) and injected into the collateral circulation via osmotic mini‐pumps, (iv) FAL without AV‐shunt and (v) osmotic mini‐pump solvent‐controls. Six sham‐operated rabbits as well as six rats served as sham controls.
The surgical procedures were carried out under anaesthesia with ketamine hydrochloride (40 mg/kg body weight for rabbits and 100 mg/kg body weight for rats) and xylazine (4 mg/kg for rabbits and 3 mg/kg for rats) administrated i.m. for rabbits and i.p in rats. Buprenorphine (50 μg/kg for rabbits and 20 μg/kg for rats) was used to prevent pain. The surgical interventions to create the AV‐fistula or to implant the osmotic mini‐pumps were performed as described [1, 4, 5].
In order to perform post‐mortem angiographies after 7 days, a gelatine‐based barium sulphate contrast medium was infused into rats as described [9]. Arteriovenous shunt experiments were carried out in rats, in order to isolate total RNA from collateral vessels or to collect tissue for immunohistochemistry after day 1, 3, 5, 7 and 14 (each n= 5).
Seven days after FAL, the capacity of the collateral system was assessed by hemodynamic measurements in rabbits as described [5].
The femoral arteries were also ligated in 12‐week‐old male wild‐type mice (WT, n= 7) and Trpv4 knock‐out mice (Trpv4−/−, n= 11; a kind gift by W. Liedtke, Duke University, USA), both with a CL57BL/6 background. The relative blood flow in the mouse foot was estimated via erythrocyte motion detection, using laser Doppler imager (MLDI 5063 and MLDI Sofware Version 3.01, both from Moor Instruments, Devon, UK) as described previously [10]. In brief, the mice were anaesthetized and kept at 37°C in a climate‐controlled chamber until they reached the steady state. The measurements were performed before and immediately after surgery and on the 3rd, 7th and 14th post‐operative day. Killed mice were used to determine the background level for subsequent subtraction. The right‐ to‐left ratio (R/L ratio) was calculated for each mouse.
Cell culture
Porcine aortic endothelial cells (PAECs) were obtained from swine aortas as previously described [11] and cultured in DMEM, 10% FCS, 20 mM L‐Glutamine, 10 mM sodium‐pyruvat, 10 mM non‐essential amino acids and 10 mM penicillin/streptomycin.
For activation of Trpv4 in cultured PAECs, cells were grown in 6‐well plates to 60% confluence and 4αPDD (7 μM respectively 37 μM) was added to the medium for the duration of 30 min. and 1 hr, respectively. Then medium was replaced, and after 6 hrs cells were harvested for RNA isolation. For immunohistological analysis, PAECs were grown on 8‐well chamber slides (Vol. 0.2–0.5 ml, Nunc, Langenselbold, Germany) and treated with 4αPDD for 1 hr as described above.
To investigate proliferation activity of cultured PAECs, the MTT Cell Proliferation Assay (ATCC®, Manasses, VA, USA) was used. The yellow tetrazolium salt (MTT) is reduced in metabolically active cells to form insoluble purple formazan crystals. The colour can then be quantified by spectrophotometric means. Prior to proliferation tests, cells were serum‐starved for 24 hrs to synchronize the cell cycle. Cells were seeded in 96‐well cell culture plates at an optimum cell density of 40,000 cells/ml and treated with 4αPDD (7 μM and 37 μM, respectively). Absorption corresponding to proliferation activity was photometrically assessed after 24, 48 and 72 hrs at a wavelength of 570 nm in a Tecan Reader Sunrise™ (TECAN Deutschalnd GmbH, Crailsheim, Germany) and analysed with Magellan software version 5.3.
For flow culture experiments, cells were cultured in μ‐Slide I slides (Ibidi, Munich, Germany) under standard conditions (see above). 7 × 105 PAECs were plated on the slide and pre‐incubated under static conditions for 48 hrs until confluence was obtained. Then cells were either kept under static conditions and served as controls or were treated in a flow‐chamber for 4 hrs using a peristaltic pump (Minipuls 3 MP‐8‐Channel, Ismatec Laboratoriumstechnik GmbH, Wertheim‐Mondfeld, Germany), which produced shear stress of 8 dyne/cm2.
RNA isolation and quantitative real‐time PCR (qRT‐PCR)
Total RNA was isolated (RNeasy Mini kit, Qiagen, Hilden, Germany) from collaterals of the rats on day 1, 3, 5, 7 and 14 after AV‐shunt treatment or FAL without AV‐shunt and sham operation (n= 5). After treatment with DNase‐I (Turbo DNAfree, Ambion, Darmstadt, Germany), cDNA was synthesized according to Superscript II reverse transcriptase protocol (Invitrogen, Karlsruhe, Germany), by using 300 ng total RNA and 200 ng random nonamer oligonucleotides (NEB, Ipswich, MA, USA). Gene‐specific RT‐PCR primers were selected using FastPCR software (Institute of Biotechnology, University of Helsinki, Finland). qRT‐PCR was performed in a 25 μl reaction, 96‐well format (1.0 μl cDNA (1:20); 200 nM each primer; 1X IQ SYBR Green Super Mix (BioRad, Munich, Germany), using an iCycler real time PCR system (BioRad, Munich, Germany). Samples were measured in triplicates, with a minimum of two independent experiments. The relative amount of target mRNA normalized to 18 S RNA was calculated as described previously [12].
Western blot analysis
Protein extracts of cultured porcine aortic endothelial cells were harvested 4 hrs after 4αPDD (7 μM) treatment, using the following protein buffer (300 mM saccharose, 1 mM PMSF, 20 mM PIPES, 10 mM EDTA and 50 mM NaH2PO4). Ten μg of denatured protein were electrophoretically separated under reducing conditions on a 4–12% Bis–Tris gel (Invitrogen, Karlsruhe, Germany) and immunoreactive bands were visualized using the Super Signal West Femto Maximum Sensitivity Substrate (Pierce Perbio, Bonn, Germany) and a polyclonal rabbit anti‐rat Trpv4 (1:200, Alomone Labs #ACC‐034, Jerusalem, Israel). Relative amount of proteins was normalized to Pan‐Actin (1:1000; Cell‐Signalling, #4968, Danvers, MA, USA) and was quantified using QuantityOne software (v4.6.3, BioRad, Munich, Germany).
Immunohistochemistry
Immunostaining was performed as described previously [13]. The following antibodies were used: Trpv4 (Alomone Labs Ltd, Jerusalem, Israel), CD31 (Antigenix, New York, NY, USA), FITC‐conjugated goat‐anti‐chicken IgG (Invitrogen, Karlsruhe, Germany), Biotin‐SP‐conjugated donkey anti‐rabbit IgG (Dianova, Hamburg, Germany), Cy2‐conjugated Streptavidin (Biotrend, Cologne, Germany). Actin was stained using Phalloidin TRITC‐labelled (Sigma, Munich, Germany) and nuclei were stained with Draq5 (Alexis Biochemicals, Loerrach, Germany). Ki67 (clone MIB‐1, Dako) was used for proliferation activity. Sections were viewed with a confocal microscope (TCS SP, Leica, Wetzlar, Germany). The quantification of fluorescence intensity was performed with representative images, using ImageJ software (http://rsb.info.nih.gov/ij/). A full range of grey values, from black to peak white (0‐pixel to 255‐pixel intensity level), was set during measurements. The intensity of fluorescence was expressed as arbitrary units AU/μm2.
Statistical analysis
All values are expressed as mean ± S.E.M. Two treatment groups were compared by the unpaired Student’s t‐test. One‐wayanova (GraphPadPrism Software) was performed for maximum collateral conductance. Probability values less than 0.05 were considered to be statistically significant.
Results
Chronically elevated FSS leads to growth of collateral arteries
Typically, the femoral artery is ligated which induces growth of bypassing collateral arteries from pre‐existing arterio‐arteriolar anastomoses. In a specific approach in rats, we chronically increased FSS by creating an arterio‐venous shunt (AV‐shunt) (Fig. 1A). According to the resulting blood pressure conditions, collateral blood flow increases leading to an ongoing elevated increase of FSS. This experimental setting resulted in a marked enhancement of collateral growth as visualized in angiographies (Fig. 1B) and significantly increased the number of visible collaterals from 8.3 ± 0.4 (n= 8) to 16.0 ± 0.7 after 7 days (n= 5) (Fig. 1C).
Figure 1.
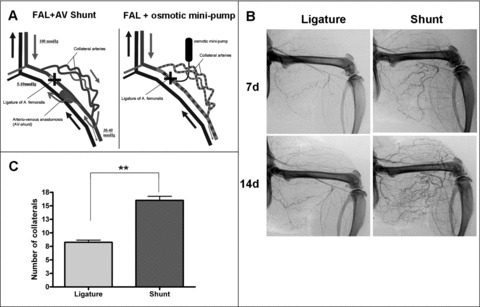
High FSS results in extensive collateral growth in femoral artery ligated rats. (A) Scheme of the arterio‐venous shunt (left) and femoral artery ligature (FAL) with implanted osmotic mini‐pump (Alzet, USA) (right). The AV‐shunt was created between the distal stump of the occluded femoral artery and the accompanying vein. Because of the steep blood pressure gradient along the collateral arteries (arterial pressure at the collateral stem and venous pressure at the reentry), collateral blood flow increases distinctively. Fluid shear stress along the collateral arteries is maximized. (B) Post‐mortem angiograms of rat hind limbs on day 7 and day 14 after indicated treatment. (C) Elevated FSS (shunt) resulted in an increase of size and number of visible collateral arteries after 7 days, which were counted (n= 5). (Values are given in mean ± S.E.M.; **P < 0.01).
Mechano‐sensitive Trpv4 transcription is increased under elevated FSS
Potential FSS‐dependent mRNA transcription was detected for Trpv4 at different time points (1 day, 3 days, 5 days, 7 days and 14 days) after AV‐shunt treatment and compared to FAL without AV‐shunt and sham‐treated animals (Fig. 2A). Trpv4 is transiently elevated 3‐ to 4‐fold until day 5 after FAL without AV‐shunt but declines to sham levels on day 7 (fold change 1.5) and day 14 (fold change 1.4). Compared to sham levels, Trpv4 transcription is significantly up‐regulated (>4.5‐fold) in shunt‐treated collateral arteries of rats and is still increased after 14 days (fold change 4.9).
Figure 2.
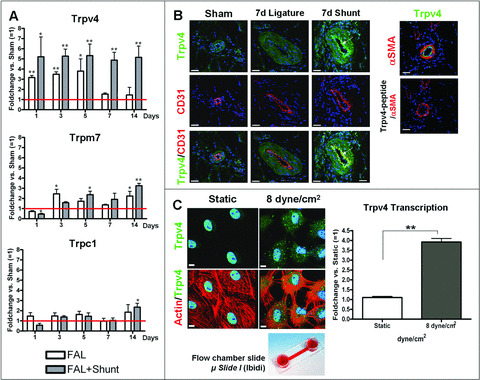
FSS‐dependent Trpv4 expression in vivo and in cultured cells. (A) Time course of mRNA transcription of Trpv4, Trpm7 and Trpc1 in rat collaterals after FAL or FAL+shunt treatment using qRT‐PCR (n= 5). Relative numbers of transcripts were measured in fold changes (FC) versus mRNA transcription of untreated control collaterals (= 1; sham, red line). Note that, after FAL without AV‐shunt, Trpv4 transcription drops to sham levels after 5 days. Trpv4 in shunt‐treated rats is constantly up‐regulated after FAL. (Values are given in mean ± S.E.M.; *P < 0.05, **P < 0.01 significant versus sham). (B) Immunofluorescence confocal microscopy of rat collateral vessels was performed using antibodies against Trpv4 (green) and the endothelial marker CD31 (red) 7 days after the indicated treatments. Double labelling demonstrated unchanged staining of the media but highly increased staining of the endothelium of shunt collaterals (arrows). Right side: Immunofluorescence confocal microscopy from serial sections of M. quadriceps of rats after ligature with AV‐shunt treatment. Sections were stained with Trpv4 (green) and αSMA (red). In the right panel, an immunoabsorption, using Trpv4 peptide which was added before immunoprecipitation, was performed in order to test antibody specificity. Nuclei were counterstained with Draq5 (blue). Scale bars: 40 μm. (C) Confocal laser microscopy of cultured PAECs under flow conditions (8 dyne/cm2/4 hrs) in μ‐Slides I (Ibidi). Cells were double‐labelled with Phalloidin (red) and antibody against Trpv4 (green). Nuclei were counterstained with Draq5 (blue). Scale bars: 40 μm. These results were confirmed by qRT‐PCR of total RNA isolated from these cells (n= 6). (Values are given in mean ± S.E.M.; **P < 0.01).
In addition, we investigated the transcriptional profile of the shear stress responsive transient receptor potential cation channel, subfamily M, member 7 (Trpm7) and transient receptor potential cation channel, subfamily C, member 1 (Trpc1) in rats (Fig. 2A). Even though, compared to controls, transcription of Trpm7 was elevated (up to 3.25‐fold) in collaterals of rat after femoral artery ligation, there was no significant difference between simple FAL and FAL with shunt. Compared with sham controls, Trpc1 also showed a significant change of transcription level in collateral arteries 14 days after ligature, but, similar to Trpm7, there was no difference of transcriptional levels between FAL and FAL combined with shunt.
After immunostaining small pre‐existing sham‐treated arterioles as well as collaterals 7 days after FAL without AV‐shunt, a basal Trpv4 expression in ECs (counterstained with CD31) and SMCs is observed (Fig. 2B). In shunt‐treated collateral arteries, Trpv4 levels in the media are unchanged, but a highly increased expression of Trpv4 restricted to the endothelium reflects an FSS‐dependent up‐regulation (Fig. 2B). A Trpv4 peptide addition prior to an anti‐Trpv4 AB abolished the binding of the AB and demonstrated specificity (Fig. 2B).
These observations were confirmed in sheared PAECs (8 dyne/cm2 for 4 hrs). A qRT‐PCR of total RNA from these cells demonstrated a shear stress‐dependent, 3.9‐fold (n= 6; P < 0.01) increase of Trpv4 trancription, when compared to static controls (no FSS). Confocal laser images of PAECs showed a strong increase of Trpv4‐positive staining after applying FSS (Fig. 3C).
Figure 3.
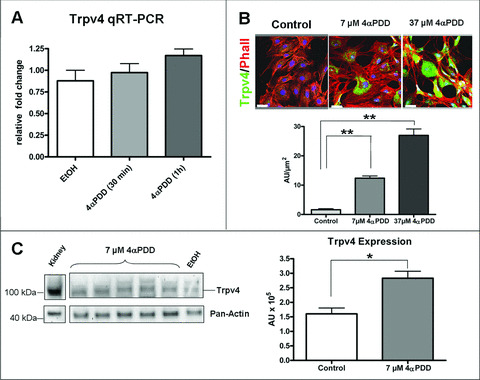
Increased expression of Trpv4 after 4αPDD treatment of cultured porcine endothelial cells. (A) Transcriptional levels of Trpv4 were not increased significantly after treatment of porcine endothelial cells (PAECs) with 37 μM 4αPDD. (B) Cultures of PAECs were double‐labelled with Phalloidin (red) and antibody against Trpv4 (green). 4αPDD treatment stimulates Trpv4 expression in a dose‐dependent manner. Fluorescence intensity given in arbitrary units (AU)/μm2 was quantified from representative images (n= 6) using ImageJ software (http://rsb.info.nih.gov/ij/). Nuclei were counterstained with Draq5 (blue). Scale bars: 40 μm. (C) Western blot analysis of cultured PAECs treated with 4αPDD showed increased expression of Trpv4 protein. Trpv4 expression was compared to solvent controls (EtOH) and protein extracts from kidney (pig), and normalized to Pan‐Actin. Relative protein levels were quantified using QuantityOne software (BioRad) (n= 5). (Values are given in mean ± S.E.M.; *P < 0.05)
4αPDD treatment leads to increased transcription of Trpv4 in vitro
In order to confirm the observation that activation of Trpv4 leads to a self‐induced transcriptional increase, we performed cell culture experiments using cultured porcine endothelial cells (PAECs). The cells were treated with 4αPDD (37 μM), a commonly known Trpv4‐activator. qRT‐PCR demonstrated an insignificant increase of Trpv4 transcription (0.97 ± 0.1 after 30 min.; 1.17 ± 0.07 after 1 hr) compared to solvent controls (0.75 ± 0.12) (Fig. 3A). However, immunohistochemistry, using Trpv4‐specific antibodies, revealed a significant dose‐dependent increase of Trpv4 protein in PAECs (Fig. 3B). After treatment of PAECs with 7 μM 4αPDD for 1 hr, the amount of fluorescence signal increased by 7.5‐fold (12.4 ± 0.7 AU versus 1.6 ± 0.4 AU). Compared to controls, signal strength in cells treated with 37 μM 4αPDD increased 16.9‐fold (27.0 ± 2.1 AU versus 1.6 ± 0.4 AU). These results were also confirmed by Western blot analysis. After treatment with 7 μM 4αPDD, protein levels of Trpv4 were significantly increased (2.8 ± 0.2 × 105 AU) compared to solvent (EtOH) treated controls (1.6 ± 0.2 × 105 AU) (Fig. 3C).
Trpv4 activation promotes active proliferation of vascular cells
Osmo‐ and mechanosensitive Ca2+‐permeable Trpv4 has previously been proposed as a regulator of vasomotion [6, 14]. We could demonstrate that the activation of Trpv4 leads to an elevated level of Ki67‐positive cells in 4αPDD‐treated cultured ECs. After treatment, the ratio of positive/negative Ki67 nuclei increased from 0.80 ± 0.07 in solvent controls to 1.43 ± 0.12 (P < 0.01) (Fig. 4A). This indicates that Trpv4 is not only involved in vasomotion but also in active proliferation of endothelial cells.
Figure 4.
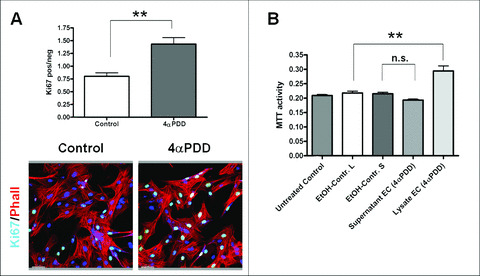
Activation of Trpv4 using 4αPDD leads to increased proliferation of cultured porcine endothelial cells. (A) Representative pictures of Ki‐67 stained PAECs. Cultured PAECs were double‐labelled with Phalloidin (red) and Ki‐67 (green). Nuclei of representative visual fields (n= 10) were counted in order to quantify the relation of Ki‐67‐positive versus negative cells. 4αPDD significantly stimulates proliferation in cultured ECs. Nuclei were counterstained with Draq5 (blue). Scale bars: 50 μm. (Values are given in mean ± S.E.M.; **P < 0.01). (B) Proliferative activity of PSMCs measured with the MTT cell proliferation assay (ATCC). PSMCs were treated with supernatant or cell‐lysate of PAECs, which were incubated for 3 hrs with 4αPDD in order to activate Trpv4. Note that only the cell‐lysate (L) of treated PAECs‐induced proliferative activity of PSMCs; the supernatant (S) showed no effect. (EtOH‐Control L and S: cell‐lysate and supernatant of solvent controls; **P < 0.01; values are given in mean ± S.E.M.; n.s. not significant)
An MTT cell proliferation assay showed no significant increase of proliferative activity of SMCs after treatment with supernatant of Trpv4‐activated ECs compared controls (0.19 ± 0.004 versus 0.21 ± 0.005; n= 24) (Fig. 4B). However, by using the cell lysat of the same cells, a significant increase of proliferative activity could be observed (0.29 ± 0.017 versus 0.21 ± 0.006; n= 24) compared to solvent controls.
Trpv4 modulation in vivo
In order to address the functional implications of Trpv4 to arteriogenesis in vivo, and to confirm the observation that Trpv4 is involved in an active vascular growth response after elevated FSS, we modulated channel activity in an FAL model without AV‐shunt in rabbits, using 4αPDD, a noted specific Trpv4 channel opener.
Trpv4 activation by infusing 4αPDD proximal to the FAL using an osmotic mini‐pump (Fig. 1A) resulted in an increased Trpv4‐protein expression. This was confirmed by immunostaining of collateral arteries of rats 7 days after surgical treatment (Fig. 5A), when compared to collateral arteries treated with Ruthenium Red (RR, a TRPV‐blocker).
Figure 5.
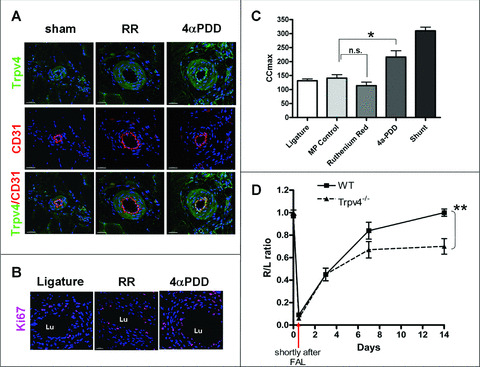
In vivo activation of Trpv4 after femoral artery ligature (A) Immunofluorescence confocal microscopy of collateral vessels of rats on day 7 after the indicated Trpv4 modulation, using antibodies against Trpv4 (green) and endothelial marker CD31 (red). 4αPDD treatment increased Trpv4 expression in the endothelium compared to Ruthenium Red (RR) treatment. Nuclei were counterstained with Draq5 (blue). Scale bars: 30 μm. (B) Collateral vessels on day 7 after indicated treatment in rats. Sections were labelled with Ki67 (red) in combination with the nuclear dye Draq5 (blue). Increased proliferation activity was observed after Trpv4 activation by 4αPDD, compared to channel blockage by RR or after simple ligature. Nuclei were counterstained with Draq5 (blue). Scale bars: 30 μm. (Lu = vessel lumen) (C) Maximum collateral conductance (CCmax) in rabbit hind limbs after indicated treatment. 4αPDD significantly increased CCmax compared to mini‐pump solvent controls (MP control) and reaches 72.6% of the conductance of a shunt‐treated rabbit. RR showed a trend in CCmax reduction. (Values are given in mean ± S.E.M.; *P < 0.05; **P < 0.01; n.s. not significant). (D) Blood flow measurements in Trpv4−/− mice after FAL. A 97% blood flow recovery in wild‐type mice (WT, n= 7) was attained after day 14. Perfusion was still impaired in mice lacking Trpv4 (Trpv4−/−, n= 11) after day 14 and showed only a 70% recovery, compared to the hind limb with non‐ligated femoral arteries. (Values are given in mean ± S.E.M.; **P < 0.05).
In addition, a Ki67 staining of collateral arteries showed an increased proliferative activity of vascular cells after Trpv4 activation, compared to FAL without AV‐shunt or RR‐treated rabbits (Fig. 5B).
Finally, 4αPDD treatment (0.05 mg/kg body weight per day) of rabbits increased maximum collateral conductance to 215.9 ± 23 ml/min/100 mmHg versus control 140.8 ± 12.5 ml/min/100 mmHg (Fig. 5C). This corresponds to a 61% recovery of blood flow compared to a non‐occluded femoral artery (345.8 ± 14 ml/min/100 mmHg). RR treatment (0.5 mg/kg body weight per day) resulted in a trend to reduce collateral flow in comparison to FAL without AV‐shunt.
Arteriogenesis is impaired in mice lacking Trpv4
A quantification of tissue perfusion in the distal hind limbs of femoral artery ligature showed impaired reperfusion in Trpv4−/− mice, when compared to controls (Fig. 5D). In both Trpv4−/− as well as WT mice with the CL57BL/6 background, the right to left ratio decreased immediately after occlusion from 1.00 ± 0.03 to 0.06 ± to 0.02 in Trpv4−/− versus 0.97 ± 0.02 to 0.09 ± 0.01 in controls. A continuous, of up to 97% recovery of blood flow was observed during the entire observation period of 2 weeks in control animals, whereas Trpv4−/− did not further improve 70% of blood flow recovery after 14 days.
Discussion
It is known that, among the physical forces that control the size of the arterial tree, FSS is the most important one [15, 16, 17, 18, 19]. We have recently developed a new animal model, in which FSS was maximized by creating an arterio‐venous shunt between the distal stump of the occluded femoral artery and the accompanying vein. Collateral artery growth was indeed maximally stimulated in this model and provided evidence for the first time that the collateral circulation is not restricted to previously reported levels of adaptation [5], neither anatomically nor functionally.
Given the importance of collateral remodelling triggered by FSS and due to its known mechanosensitive features [14, 20], we studied Trpv4. The vanilloid subfamily of Transient Receptor Potential (TRPV) cation channels has been implicated into a broad range of fundamentally physiological functions [21]. At first described by Strotmann et al. [22], Liedtke et al. [23] and Nilius et al. [24], Trpv4 is known to be a transducer of mechanical, osmotic and thermal stimuli [14, 20, 25, 26, 27]. In endothelial cells of the vasculature, Trpv4 is assigned an important function in regulating vasomotion [6] and, upon dysfunction, is involved in several vascular and cardiovascular pathologies [7, 28].
We found that mRNA transcription of this Ca2+ channel was constantly up‐regulated in FSS‐induced collaterals after AV‐shunt treatment. In contrast, after FAL without AV‐shunt, Trpv4 transcriptional levels are transiently up‐regulated until day 5 and drop to control levels after day 7. The discovery that Trpv4 expression is FSS‐dependent was confirmed in sheared endothelial cells, which showed increased levels of Trpv4 RNA and protein levels after flow treatment of 8 dyne/cm2, when compared to static controls. Elevated protein expression was localized in the FSS‐sensing endothelium. Surprisingly, our experiments also show that chronic stimulation (up to 14 days) of Trpv4 is followed by active proliferation of vascular cells. This was confirmed by 4αPDD treatment of cultured endothelial cells and its application to rats after FAL without AV‐shunt, which showed a significant increase of Ki67‐positive vascular cells. It is intriguing to speculate whether the positive feedback of Trpv4 expression leads to an increased responsiveness to FSS, which at a certain level pushes the cells beyond the point of vasodilatation and leads to active growth of the vasculature. The observation that increased cytosolic Ca2+‐levels can stimulate proliferation of vascular cells via calcium/calmodulin‐dependent kinases (CamK) has already been shown by others [29, 30, 31]. The fact that the supernatant of 4αPDD‐treated PAECs did not induce proliferative activity of PSMCs points to a mechanism independent from cytokine release into the surrounding medium (Fig. 4B).
Finally, pharmacological activation of Trpv4 by 4αPDD showed a strong increase of collateral blood flow. Ruthenium Red, a known TRPV‐channel blocker, showed a weak reduction of collateral growth, but not a complete inhibition, which we had expected. This might reflect the complexity of underlying processes and points to molecular pathways, which are able to compensate the lack of Trpv4‐activity. Nevertheless, FAL in Trpv4−/− showed a significant decrease of blood flow recovery in mice.
Downstream signalling of Trpv4‐triggered collateral growth has to be further elucidated. However, it is known that, upon channel activation, three Ca2+‐dependent mechanisms are considered to play a role in transducing Trpv4 signalling across the internal elastic lamina in order to trigger vasodilatation of the smooth muscle cell layer: (1) the endothelial‐derived hyperpolarization factor (EDHF) via opening of Ca2+‐activated K+‐channels, which represents an electrochemical stimulus [32]. (2) Prostacyclin (PGI2) synthesis by cyclooxigenase‐1, which requires Ca2+‐dependent release of arachnidonic acid [14, 33]. And (3) nitric oxide (NO) derived from the Ca2+/calmodulin‐dependent activated endothelial nitric oxide synthase (eNOS) [34]. In the context of arteriogenesis, it is known that eNOS‐antagonsists L‐NAME and L‐NNA show a strong anti‐arteriogenic response [5, 35]. Therefore, one can speculate that Trpv4‐induced growth of smooth muscle cells, a crucial process during collateral formation, acts through NO because known NO inhibitors impair arteriogenesis [5].
In conclusion, we herewith point out the important role of Ca2+ homeostasis in collateral remodelling during arteriogenesis. Furthermore, we propose a novel initial mediator for FSS‐induced arteriogenesis with a new function for the endothelial‐expressed and shear stress‐regulated Trpv4. Trpv4 activation triggers collateral growth and remodelling. As an endothelial membrane bound protein, it is highly suitable for the development of new therapeutic concepts in order to cure peripheral and cardiovascular disease.
Acknowledgements
The authors acknowledge their gratitude to Uta Eule and Brigitte Matzke for their expert technical assistance. This work was in part supported by Servier, the Kerckhoff Foundation and the Kühl Foundation.
References
- 1. Ito WD, Arras M, Winkler B, et al . Monocyte chemotactic protein‐1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997; 80: 829–37. [DOI] [PubMed] [Google Scholar]
- 2. Lazarous DF, Shou M, Scheinowitz M, et al . Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996; 94: 1074–82. [DOI] [PubMed] [Google Scholar]
- 3. Unger EF. Experimental evaluation of coronary collateral development. Cardiovasc Res. 2001; 49: 497–506. [DOI] [PubMed] [Google Scholar]
- 4. Pipp F, Boehm S, Cai WJ, et al . Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004; 24: 1664–8. [DOI] [PubMed] [Google Scholar]
- 5. Eitenmuller I, Volger O, Kluge A, et al . The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006; 99: 656–62. [DOI] [PubMed] [Google Scholar]
- 6. Hartmannsgruber V, Heyken WT, Kacik M, et al . Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE . 2007; 2: e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwan HY, Huang Y, Yao X. Cyclic nucleotides and Ca2+ influx pathways in vascular endothelial cells. Clin Hemorheol Microcirc. 2007; 37: 63–70. [PubMed] [Google Scholar]
- 8. Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle Endothelium. 2003; 10: 5–15. [DOI] [PubMed] [Google Scholar]
- 9. Hoefer IE, van Royen N, Buschmann IR, et al . Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001; 49: 609–17. [DOI] [PubMed] [Google Scholar]
- 10. Scholz D, Ziegelhoeffer T, Helisch A, et al . Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002; 34: 775–87. [DOI] [PubMed] [Google Scholar]
- 11. Chesterman CN, Ager A, Gordon JL. Regulation of prostaglandin production and ectoenzyme activities in cultured aortic endothelial cells. J Cell Physiol. 1983; 116: 45–50. [DOI] [PubMed] [Google Scholar]
- 12. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res . 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodriguez M, Cai WJ, Kostin S, et al . Ischemia depletes dystrophin and inhibits protein synthesis in the canine heart: mechanisms of myocardial ischemic injury. J Mol Cell Cardiol. 2005; 38: 723–33. [DOI] [PubMed] [Google Scholar]
- 14. Kohler R, Heyken WT, Heinau P, et al . Evidence for a functional role of endothelial transient receptor potential V4 in shear stress‐induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006; 26: 1495–502. [DOI] [PubMed] [Google Scholar]
- 15. Rodbard S. Vascular caliber. Cardiology. 1975; 60: 4–49. [DOI] [PubMed] [Google Scholar]
- 16. Langille BL. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993; 21: S11–7. [DOI] [PubMed] [Google Scholar]
- 17. Holtz J, Forstermann U, Pohl U, et al . Flow‐dependent, endothelium‐mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharmacol. 1984; 6: 1161–9. [PubMed] [Google Scholar]
- 18. Tronc F, Wassef M, Esposito B, et al . Role of NO in flow‐induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996; 16: 1256–62. [DOI] [PubMed] [Google Scholar]
- 19. Ben Driss A, Benessiano J, Poitevin P, et al . Arterial expansive remodeling induced by high flow rates. Am J Physiol . 1997; 272: H851–8. [DOI] [PubMed] [Google Scholar]
- 20. Liedtke W, Tobin DM, Bargmann CI, et al . Mammalian TRPV4 (VR‐OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003; 100: 14531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des. 2008; 14: 18–31. [DOI] [PubMed] [Google Scholar]
- 22. Strotmann R, Harteneck C, Nunnenmacher K, et al . OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000; 2: 695–702. [DOI] [PubMed] [Google Scholar]
- 23. Liedtke W, Choe Y, Marti‐Renom MA, et al . Vanilloid receptor‐related osmotically activated channel (VR‐OAC), a candidate vertebrate osmoreceptor. Cell. 2000; 103: 525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nilius B, Prenen J, Wissenbach U, et al . Differential activation of the volume‐sensitive cation channel TRP12 (OTRPC4) and volume‐regulated anion currents in HEK‐293 cells. Pflugers Arch. 2001; 443: 227–33. [DOI] [PubMed] [Google Scholar]
- 25. Gao X, Wu L, O’Neil RG. Temperature‐modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C‐dependent and ‐independent pathways. J Biol Chem. 2003; 278: 27129–37. [DOI] [PubMed] [Google Scholar]
- 26. Liedtke W. Transient receptor potential vanilloid channels functioning in transduction of osmotic stimuli. J Endocrinol. 2006; 191: 515–23. [DOI] [PubMed] [Google Scholar]
- 27. Alessandri‐Haber N, Yeh JJ, Boyd AE, et al . Hypotonicity induces TRPV4‐mediated nociception in rat. Neuron. 2003; 39: 497–511. [DOI] [PubMed] [Google Scholar]
- 28. Inoue R, Hai L, Honda A. Pathophysiological implications of transient receptor potential channels in vascular function. Curr Opin Nephrol Hypertens. 2008; 17: 193–8. [DOI] [PubMed] [Google Scholar]
- 29. Whitaker M, Larman MG. Calcium and mitosis. Semin Cell Dev Biol. 2001; 12: 53–8. [DOI] [PubMed] [Google Scholar]
- 30. Munaron L. Intracellular calcium, endothelial cells and angiogenesis. Recent Patents Anticancer Drug Discov. 2006; 1: 105–19. [DOI] [PubMed] [Google Scholar]
- 31. Cribbs LL. T‐type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium. 2006; 40: 221–30. [DOI] [PubMed] [Google Scholar]
- 32. Busse R, Edwards G, Feletou M, et al . EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002; 23: 374–80. [DOI] [PubMed] [Google Scholar]
- 33. Eichler I, Wibawa J, Grgic I, et al . Selective blockade of endothelial Ca2+‐activated small‐ and intermediate‐conductance K+‐channels suppresses EDHF‐mediated vasodilation. Br J Pharmacol. 2003; 138: 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin‐requiring enzyme. Proc Natl Acad Sci USA. 1990; 87: 682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai WJ, Kocsis E, Luo X, et al . Expression of endothelial nitric oxide synthase in the vascular wall during arteriogenesis. Mol Cell Biochem. 2004; 264: 193–200. [DOI] [PubMed] [Google Scholar]


