Abstract
There is a growing interest for the clinical use of platelet derivates in wound dressing. Platelet beneficial effect is attributed to the release of growth factors and other bioactive substances, though mechanisms are mostly unknown. We studied wound‐healing processes of human primary fibroblasts, by exposing cells to a platelet lysate (PL) obtained from blood samples. Crystal violet and tetrazolium salt (MTS) assays showed dose–response increase of cell proliferation and metabolism. In scratch wound and transwell assays, a dose of 20% PL induced a significant increase of wound closure rate at 6 and 24 hrs, and had a strong chemotactic effect. BAPTA‐AM, SB203580 and PD98059 caused 100% inhibition of PL effects, whereas wortmannin reduced to about one third the effect of PL on wound healing and abolished the chemotactic response. Confocal imaging showed the induction by PL of serial Ca2+ oscillations in fibroblasts. Data indicate that cell Ca2+ plays a fundamental role in wound healing even without PL, p38 and ERK1/2 are essential for PL effects but are also activated by wounding per se, PI3K is essential for PL effects and its downstream effector Akt is activated only in the presence of PL. In conclusion, PL stimulates fibroblast wound healing through the activation of cell proliferation and motility with different patterns of involvement of different signalling pathways.
Keywords: cell migration, confocal imaging, platelets, scratch wound, wound dressing
Introduction
The healing of chronic wounds represents a major health burden and drain of resources and there is a growing interest in the development of dressings and skin substitutes for the treatment of these conditions [1]. However, for an appropriate design and use of these tools, an understanding of wound‐healing mechanisms at the cellular and molecular level is needed. Wound healing involves a complex of interactions among epidermal and dermal cells, extracellular matrix, angiogenesis and plasma proteins. This dynamic process is classically, but somewhat artificially, divided into three overlapping phases: inflammation, proliferation and remodelling [2, 3].
Factors impairing wound healing include tissue maceration, ischaemia and infection, although systemic factors as diverse as aging, malnutrition, diabetes and renal disease may also be important. In addition, the reduction in tissue growth factors, an imbalance between proteolytic enzymes and their inhibitors, and the presence of senescent cells seem to be particularly important for the pathogenesis of chronic wounds. Growth factors are essential for the regulation of cellular events involved in wound healing, such as migration of various cell types into the wound, cell proliferation, angiogenesis and the synthesis and degradation of extracellular matrix [4]. Compared with acute wounds, chronic ulcers are known to have reduced levels of these healing‐promoting molecules [5].
Human platelets contain granules of cytokines and growth factors, including fibronectin, fibrinogen, serotonin, platelet‐derived growth factor (PDGF), transforming growth factor‐β, insulin‐like growth factor, epidermal growth factor and fibroblast growth factor [6]. Platelet activation and granule secretion promotes the wound‐healing process, and in clinical trials mixtures of growth factors from activated platelets have proved to be effective in the treatment of chronic wounds [2, 7, 8, 9, 10]. In vitro and in vivo studies, aimed at clarifying the role of platelets in tissue regeneration, have reported that a platelet lysate (PL) accelerates the proliferation of fibroblasts, osteoblasts and periodontal ligament [11, 12].
In the medical field, platelet derivatives and platelet‐released growth factors find numerous applications, including orthodontic practices and dental surgery [13, 14, 15], plastic surgery [16] and the treatment of chronic wounds and ulcers [17, 18]. However, the cellular and molecular mechanisms involved in platelet‐promoted tissue remodelling are either partially understood or unknown [19]. Also, divergent data on the healing ability of different platelet derivates have been reported [20], possibly depending on different methods and devices used in preparations [16], on pH conditions [21] and on the interindividual variability of growth factors in platelet concentrates [16, 20]. This points to an emerging need for the standardization of platelet derivates and for a precise definition of their components and specific roles.
Fibroblasts play a pivotal role in wound repair. Their proliferation, production of collagen and other matrix components, and partial differentiation into myofibroblasts are major processes leading to stable tissue restoration [16, 21]. Some effects of platelet derivates on human primary cultures of fibroblasts have already been studied [22, 23], and it has also been shown that PDGF‐β receptor activation is essential for fibroblast recruitment [24]. We speculated that fibroblasts treated with PL exhibit increased wound‐healing rate due to a more rapid proliferation and enhanced motility. We therefore used a classic in vitro wound‐healing model [25, 26] to study the effects induced on human primary fibroblasts by a PL preparation obtained from platelet‐enriched blood samples. Scratch wound and cell migration assays were carried out, coupled to light microscope image analysis, Western immunoblotting and confocal calcium imaging. Data showed that PL accelerates wound closure through the stimulation of cell proliferation and migration. Such a process is strictly Ca2+ dependent and requires the involvement of p38, ERK1/2 and phosphatidylinositol 3 kinase (PI3K).
Materials and methods
Reagents
SB203580, PD98059, wortmannin and BAPTA‐AM were from Calbiochem (La Jolla, CA, USA); fluo‐3/AM was from Invitrogen (Carlsbad, CA, USA); all other reagents were from Sigma (St. Louis, MO, USA).
Cell culture
In the present study, the standards and laws of medical ethics were followed, and patients were treated after informed consent. Human fibroblasts were harvested from skin biopsies of a single patient undergoing plastic surgery. The epidermis was finely minced with scissors, digested with 200 U/mg collagenase (Gibco, Invitrogen Life Technologies, S. Giuliano Milanese, Italy) in DMEM (Dulbecco’s modified Eagle’s medium, Gibco) additioned with 1% antibiotic mixture (Gibco) for 18 hrs at 37°C in a humidified atmosphere with 5% CO2, and washed three times in DMEM. Fibroblasts were then grown in DMEM supplemented with 10% foetal bovine serum (Euroclone, Pero, Italy) under the same conditions as above.
Platelet lysate preparation
Platelet concentrates were obtained from a single volunteer donor, using platelet apheresis collection qualified for clinical use and produced by a discontinuous blood cell separator (MCS 3P, Haemonetics, Mirandola, Italy). The platelet‐rich plasma was automatically separated from other blood components by centrifugation, collected into a sterile disposable bag, and then washed and concentrated to a final density of 2 × 109 cells/ml. To obtain the PL, platelets concentrates were frozen (−80°C) and thawed (37°C) for three consecutives times, the remaining platelet bodies and debris were eliminated by centrifugation (3000 ×g× 10 min.), and the supernatant was stored in aliquots at −80°C until use.
MTS assay
Fibroblasts were seeded on 96‐well plates and grown for 24 hrs prior to experiments. Each well contained about 20,000 cells in a total volume of 100 μl of complete DMEM. Cell metabolic rate was assayed using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐5(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium (MTS) assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega, Milan, Italy). The proliferation rate of incubated cells was determined by a 24‐hr stimulation with PL in complete DMEM. Samples were read in a plate reader (Sirio S, SEAC, Florence, Italy) at 492 nm.
Crystal violet assay
Cell proliferation was assessed by staining cells with crystal violet dye. Briefly, fibroblasts were seeded on 96‐well plates as described for MTS assay and then exposed to PL at various concentrations for 24 hrs. The medium was removed, cells were gently washed once with 1× phosphate buffered saline solution (PBS), stained for 10 min. with 0.5% crystal violet in 145 mmol/l NaCl, 0.5% formal saline, 50% ethanol and washed thrice with water. Crystal violet was eluted from cells with 33% acetic acid and the absorption of the supernatants was measured at 540 nm as described for MTS assay.
Wound scratch assay
To determine the effect of PL on wound healing, a scratch‐wounded fibroblast monolayer model was employed. Briefly, cells were seeded into 12‐well tissue culture plates, cultured to confluence and wounded by scraping with a 0.1–10 μL pipette tip. Following phosphate‐buffered saline washes, cultures were re‐fed with medium supplemented with 20% PL for 6 or 24 hrs. Cells were then fixed (3.7% formaldehyde in PBS for 30 min.), stained with 0.1% toluidine blue at room temperature for 30 min. and digital images were captured using a camera‐equipped, inverted Televal microscope (Carl Zeiss, Inc., Thornwood, NY, USA). Wound width measurements at 6 and 24 hrs were subtracted from wound width at time zero, in order to obtain net wound closure.
Cell migration assay
Chemotaxis assays were performed at 37°C using 24‐well transwells containing a polycarbonate filter with 8‐μm pores (Corning Costar, Acton, MA, USA). Cells were seeded in the upper well at a density of 100,000 cells/well, and after 6 hrs membranes were removed and then fixed and stained as above. The upper side of the membrane was scraped using a cotton swab to remove cells that had attached but not migrated, and the membrane was then mounted onto a microscope slide and stained as above. Chemotaxis was assessed by counting the number of cells that migrated in five independent microscope fields (0.83 mm2).
Calcium measurements
Fibroblasts were plated on cell culture glass‐base dishes (Iwaki Glass, Inc., Tokyo, Japan), allowed to settle overnight, and then loaded in the dark at 4°C for 60 min. with the cell‐permeant fluorescent calcium probe fluo‐3/AM (20 μM) in a loading buffer consisting of (mM) 10 HEPES, 140 NaCl, 10 glucose, 1 MgCl2, 2 CaCl2, 5 KCl, pH 7.4. For Ca2+‐free experiments, Ca2+ was omitted from the loading buffer. After probe loading and washing, cells were examined through confocal time‐lapse analysis at 21°C, using a Zeiss LSM 510 confocal system interfaced with a Zeiss Axiovert 100 M microscope (Carl Zeiss). Excitation was obtained by the 488 nm line of an Ar laser, and emission was collected using a 505–550 bandpass filter. The laser power was reduced to 15% in order to lower probe bleaching. Confocal imaging was performed with a resolution of 512 × 512 pixels at 256 intensity values, with a framing rate of 1 frame/20 sec. Several cells were viewed together through a 20× Plan‐Neofluar Zeiss objective (0.5 numerical aperture). Fluo‐3 fluorescence was measured in digitized images as the average value over defined contours of individual cells, using the region of interest (ROI)‐mean tool of the Zeiss LSM 510 2.01 software. Fluo‐3 calibration was achieved by the equation [27]:
where K d= 400 nM, and F max and F min are maximum and minimum fluorescence intensities, measured after cell treatment with 200 μM A23187 and 2.0 mM EGTA, respectively.
Western blotting
Cells were exposed to scratch wound in the presence or absence of 20% PL for 5, 15 and 30 min., and then lysed in Laemmli buffer [28]. Amounts of 40 μg of protein were loaded on gel, subjected to SDS‐PAGE (12% gel) and transferred to a nitrocellulose membrane using a Bio‐Rad Mini Trans Blot electrophoretic transfer unit (Bio‐Rad Laboratories, Hercules, CA, USA). The membranes were blocked for non‐specific protein with 5% non‐fat, dry milk in PBS and then probed at room temperature for 1 hr, or at 4°C overnight, with specific primary antibodies (Cell Signaling Technology, Celbio SpA, Milan, Italy) against p‐ERK1/2 (1:1000), p‐p38 (1:1000), p‐AKT (1:1000, a kind gift of Prof. Stefano Biffo, HSR, Milan, Italy), ERK1/2 (1:1000), p38 (1:1000) and AKT (1:1000, a kind gift of Prof. Biffo). Membranes were then washed three times (10 min. per wash) with PBS additioned with 0.05% Tween‐20, to remove unbound antibodies, and then further incubated with appropriate horseradish‐peroxidase‐conjugated secondary antibodies. Membranes were developed by an ECL kit (Millipore, Billerica, MA, USA), according to the manufacturer’s protocol, digitized with the Quantity One Image Software (ChemiDoc XRS, Bio‐Rad Laboratories) and normalized against proper loading controls. Band intensities were quantified by densitometric analysis using the Adobe Photoshop 7.0.1 software (Adobe Systems Inc., San Jose, CA, USA).
Statistics
Data were analysed by ANOVA and post hoc pairwise comparisons were performed by the Tukey’s test, using the Instat and Prism software package (GraphPad Software, Inc., San Diego, CA, USA). Median and minimum effect concentrations (EC50 and EC05, respectively), and their 95% confidence intervals, were determined by using a downhill logistic dose–response curve.
Results
Cell proliferation and metabolism
Cells were exposed to increasing PL (1–100% v/v in DMEM) concentrations for 24 hrs under normal (10% foetal bovine serum) or serum‐free conditions. The effects of PL on fibroblast proliferation were assessed by the crystal violet assay. In the presence of serum, addition of PL induced a dose‐dependent increase of cell proliferation, up to 142% of control at 100% v/v PL, with an EC50 of 30% v/v (95CI: 24–37). The removal of serum from the culture medium, in the absence of PL, caused a significant decrease of the cell proliferation rate (P < 0.01). However, the addition of PL in the absence of serum, produced a dose‐dependent recovery of cell proliferation rate, up to the levels recorded in the presence of serum, with an EC50 of 13% v/v (95CI: 5–36).
MTS is a commonly used in vitro end‐point based on the ability of mitochondrial dehydrogenase from viable cells to produce the brown soluble formazan. Exposure to PL in the presence of serum determined an increase of mitochondrial metabolism in a dose‐dependent way, with an EC50 of 19% v/v (95CI: 10–34). In the absence of serum, the stimulatory effect of PL on mitochondrial metabolism was even more pronounced, with an EC50 of 8.1% v/v (95CI: 6.7–9.8).
Crystal violet data showed that the PL treatment induced no cytotoxicity on fibroblasts, but it conversely was mitogenic. Such an effect was even more pronounced on cells cultured in a serum‐free medium, and it was in line with the stimulation of mitochondrial metabolism recorded by the MTS assay, both in the presence and absence of serum. Based on these data, we chose to carry out wound‐healing experiments in the presence of serum, because such a condition is more resembling the in vivo situation. In addition, a dose of 20% PL was adopted, which is close to the EC50s derived by crystal violet and MTS assays, in the presence of serum.
Scratch wound repair
Fibroblasts were scratch wounded, incubated in complete medium, with or without 20% PL, and observed 6 or 24 hrs after wounding (Fig. 1). The distance between edges of the injured monolayer was measured by the National Institutes of Health (NIH) ImageJ software in terms of pixel, and wound closure was expressed as the difference between measurements at 0 hrs and at 6 or 24 hrs after wounding (Fig. 1). Cells exposed to PL showed significantly higher wound closure rates at both 6 and 24 hrs with respect to controls (P < 0.01), and moreover wound closure was significantly increased from 6 to 24 hrs (P < 0.01). Consequently, wound closure was almost complete at 24 hrs in the presence of PL, whereas it was far from being complete in the control (Fig. 1).
Figure 1.
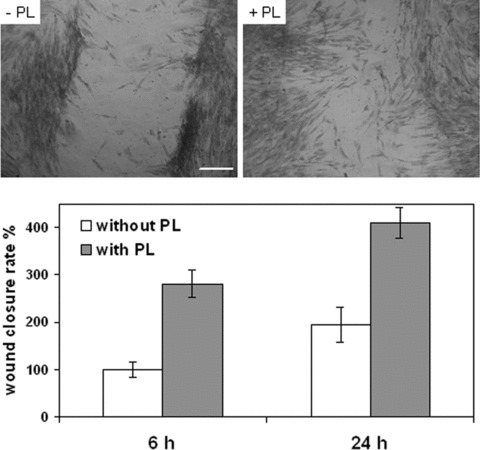
Effects of platelet lysate (PL) exposure on scratch wound healing of confluent fibroblasts. Cells cultured in 12‐well plates were mechanically scratched with a sterile 0.1–10‐μl pipet tip, and then maintained for 6 or 24 hrs at 37°C in the presence or absence of 20% PL. Upper panel. Phase contrast micrographs of scratched fibroblasts that were fixed and stained with blue‐toluidine 24 hrs after scratching. Scale bar: 300 μm. Lower panel. Each bar indicates mean ± S.D. (n= 8) of wound closure rates expressed as the difference between wound width at 0 hrs and at 6 or 24 hrs. The mean of controls at 6 hrs was set at 100%. All pairwise comparisons yield significant differences according to the Tukey’s test (P < 0.01).
To investigate the role played by signalling pathways in the effect of PL on wound closure, we performed scratch wound experiments using PD98059 (ERK inhibitor, 10 μM), SB203580 (p38 inhibitor, 20 μM), wortmannin (PI3K inhibitor, 500 nM) and the cell‐permeant, calcium chelator BAPTA‐AM (30 μM). The vehicle alone (DMSO, dimethyl sulfoxide, 0.1%) was also used. Confluent fibroblasts were scratched in the absence or presence of each inhibitor, with or without PL, and wound closure was then measured 24 hrs after wounding, as described above (Fig. 2). In the absence of PL, inhibitors did not alter wound closure rate respect to control, with the exception of BAPTA, which induced a significant reduction of wound closure (P < 0.01). Conversely, PD98059, SB203580 and BAPTA completely abolished the increase of wound closure rate induced by PL (P < 0.01), whereas wortmannin reduced the effect of PL, but at a significantly lower degree with respect to the other drugs (P < 0.01). The effect of DMSO alone (0.1%) was also checked, finding no significant variation respect to wound healing in the absence of DMSO, either in the presence or absence of PL (not shown).
Figure 2.
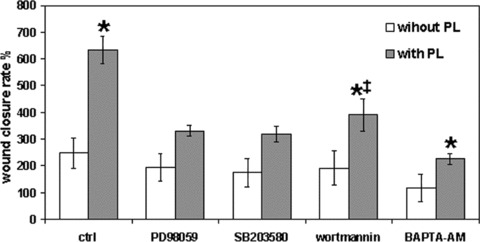
Effect of different inhibitors on scratch wound repair of confluent fibroblasts. Data were recorded 24 hrs after wound scratching of cells exposed or not to platelet lysate (PL), in the presence or absence of various drugs. Each bar represents mean ± S.D. (n= 6) of wound closure rates expressed as in Fig. 1. The mean of controls without PL was set at 100%. Only the results of the most relevant pairwise comparisons in the Tukey’s test are shown: *= significantly different from control without PL (P < 0.01); ‡= significantly different from control without PL and from control with PL (P < 0.01).
Cell chemotactic response
In order to assess whether PL can influence cell migration rate, we used a chemotaxis assay performed with 8‐μm polycarbonate filters (Fig. 3). In this set of experiments, the inhibitors used in scratch wound assays (except for BAPTA‐AM) were also used. In the presence of PL and in the absence of inhibitors, the number of migrating cells was significantly increased respect to control (P < 0.01). By contrast, all inhibitors totally abrogated the effect of PL on cell migration (P < 0.01). The vehicle alone caused no variations with respect to control (not shown).
Figure 3.
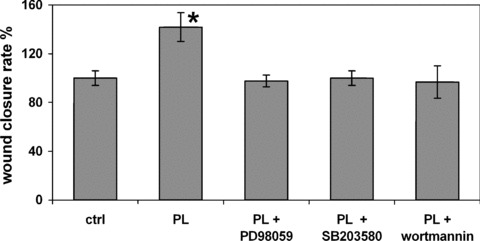
Effects of platelet lysate (PL) and of different inhibitors on fibroblast cell migration evaluated by the transwell assay (see section ‘Materials and methods’). Each bar represents mean ± S.D. of the numbers of migrating cells in five different microscope fields. The mean of controls was set at 100%. *= significantly different from control (P < 0.01) according to the Tukey’s test.
Calcium signalling
Confocal Ca2+ imaging of confluent fibroblasts allowed the estimation of an average basal level of cytosolic free Ca2+ of 72 ± 30 nM (n= 30 individual cells in three different experiments). Under control conditions, some cells showed spontaneous Ca2+ oscillations, peaking at about 300–700 nM and lasting for about 60 sec. After addition of 20% PL, the frequency of Ca2+ oscillations was dramatically increased, so that in some cases they tended to transform into prolonged transients. This kind of activity lasted for about 200–300 sec., and thereafter cells tended to re‐establish normal Ca2+ baseline (Fig. 4). In the absence of extracellular Ca2+, smaller Ca2+ oscillations occurred in a few cells, but after PL addition almost all cells showed series of Ca2+ oscillations peaking at about 150–250 nM and lasting for about 800 sec. (Fig. 4).
Figure 4.
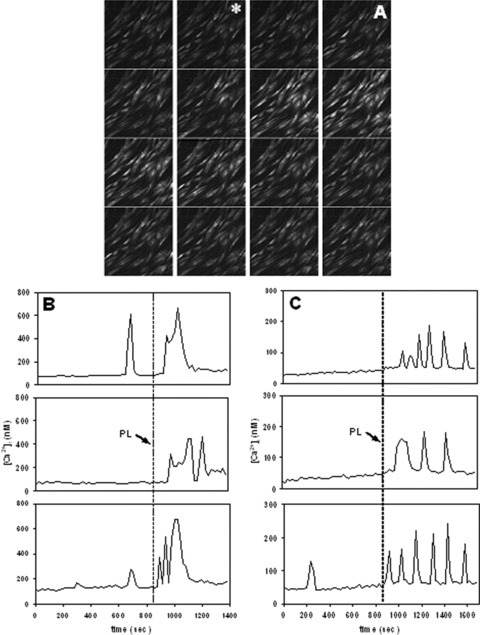
Confocal Ca2+ imaging of fibroblasts loaded with the fluo‐3 probe and exposed to platelet lysate (PL). (A): time lapse confocal images (field size, 450 × 450 μm), acquired at 30‐sec. intervals, showing that PL induces intracellular Ca2+ rise in a number of cells (*= start of exposure). Lower panels: representative tracks of intracellular Ca2+ variations recorded in individual fibroblasts before and after addition of 20% PL, (B) in the presence of external Ca2+ and (C) in the absence of external Ca2+.
Protein phosphorylation signalling
In order to explore the role of phosphorylation cascades in wound repair, the activation of p38 and ERK1/2 MAP kinases, and of the PI3K‐dependent kinase Akt, were examined by Western blotting at 5, 15 and 30 min. after wounding. These analyses were carried out by using antibodies against the two mitogen‐activated protein kinases (MAPKs) and Akt and against their phosphorylated forms. In the presence of PL, the rates of phosphorylation of MAP kinases were significantly more rapid than in the absence of PL, protein phosphorylation reached a plateau at 5 min. (ERK1/2) or at 15 min. (p38) after wounding (Fig. 5). In contrast, without PL these levels of phosphorylation were reached after 30 min. Akt also showed a prompt phosphorylation in the presence of PL, but conversely to the MAP kinases, no Akt activation was detected without PL (Fig. 5).
Figure 5.
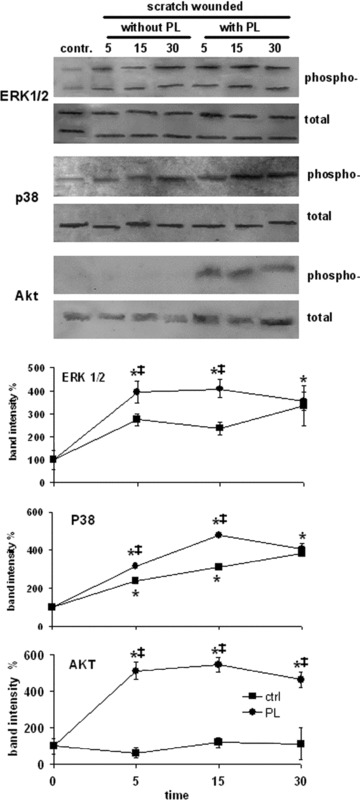
Effect of platelet lysate (PL) on the activation of ERK1/2, p38 and Akt after wound scratching. Upper panels. Total cell lysates (40 μg protein/lane) obtained from wounded fibroblasts were separated on 12% SDS‐PAGE, transferred to a nitrocellulose membrane, labelled with anti‐phospho‐ERK1/2, ‐p38 or ‐Akt, and then stripped and reprobed with anti‐ERK1/2, ‐p38 or ‐Akt as internal controls. Lower panels. Time courses of MAPK and Akt activation within the first 30 min. after wounding. Data are means ± S.D. (n= 3) of the ratio between the optical densities of the bands of the phosphorylated form and the bands of the corresponding total protein recorded on the same membrane. The means of controls were set at 100%. The results of the most relevant pairwise comparisons in the Tukey’s test are shown: *= significantly different from control at time t= 0 (P < 0.01); ‡= significantly different from control at the same time interval (P < 0.01).
Discussion
Our scratch wound data showed beyond reasonable doubt that PL induces a marked increase of the wound repair capabilities of fibroblasts by stimulating cell proliferation and migration. Crystal violet and MTS assays highlighted a mitogenic effect of PL, thus confirming previous data on fibroblast proliferation induced by platelets or PDGF [23, 29, 30]. The cell migration assay showed a chemoattractant activity of PL on fibroblasts, thus being in agreement with chemotactic responses to platelet derivates of retinal glial cells [31], foetal rat cells [11], mature osteoblasts [32] and also with the finding that PDGF promotes fibroblast migration [33, 34].
Cytosolic free Ca2+ is essential in regulating cell proliferation and motility [35], and its role in wound healing has been suggested by the finding of wound‐induced Ca2+ waves observed in epithelial cells [36]. Moreover, extracellular Ca2+ levels have been shown to play a central role in tissue wound repair [37]. In our experiments, a fundamental role of cell Ca2+ in wound healing was strikingly shown by the use of BAPTA‐AM. This intracellular Ca2+ chelator was able to completely abolish the effect of PL, and moreover, it also affected the wound closure process induced by scratch wound alone, i.e. in the absence of PL. Confocal Ca2+ imaging showed a marked stimulation of fibroblast Ca2+ signalling by PL, which assumed the form of a series of Ca2+ oscillations, thus possibly involving Ca2+ release from IP3‐sensitive stores. The occurrence of intracellular Ca2+ release was confirmed by experiments in Ca2+‐free medium. The occurrence of larger Ca2+ responses to PL in the presence of extracellular Ca2+ suggests a role of this latter in modulating fibroblast wound healing. In summary, our data on cell Ca2+ concurred to indicate that this basic regulator of cytoskeleton, cell motility and proliferation is essential for the progress of fibroblast wound healing, either in the presence or absence of the stimulatory action of PL.
Besides the basic role of Ca2+, we observed different functional roles in phosphorylation‐dependent cascades. In various cell types, it has been found that ERK1/2 and p38 MAP kinases become activated by tissue wounding [38, 39]. Moreover, in murine fibroblasts, PDGF leads to the activation of ERK1/2 [40], and promotes the migration and proliferation of mouse hepatic myofibroblasts through the activation of p38 and ERK1/2, respectively [41]. Similar mechanisms have been found in corneal wound healing [42]. In our experiments, Western blot showed that although p38 and ERK1/2 are activated by scratch wounding, PL significantly enhances their activation. Also, the use of specific inhibitors indicated an essential role of these MAP kinases in the action of PL, although in the absence of PL these inhibitors did not significantly affect basal wound‐healing rate, in contrast to what observed for the Ca2+ chelator BAPTA. Hence, p38 and ERK1/2 are undoubtedly essential for PL‐induced fibroblast wound healing, but they does not seem to play a fundamental role in basal wound closure.
A still different pattern of involvement was found for the PI3K pathway. It has been reported that this kinase plays a role in the wound healing of corneal epithelial [43, 44], CaCo2 [45] and retinal pigment epithelial cells [46]. In fibroblasts, PDGF leads to actin reorganization, chemotaxis and cell proliferation, which originate from the activation of multiple signalling cascades, also including PI3K [41]. In primary fibroblasts, a role of the PI3K‐dependent Jun N‐terminal kinase in PDGF‐stimulated chemotaxis has been reported, while Akt is also activated in response to PDGF in a strictly PI3K‐dependent manner [41]. Our experiments showed a role of PI3K in PL‐driven wound healing, but not in basal wound healing, and moreover Akt activation was detected only in the presence of PL. The strictly PL‐dependent involvement of PI3K/Akt could possibly explain the highly beneficial long‐term effects of platelet derivatives in wound dressing, suggesting that tissue lesions occurring in certain diseases, namely diabetes, in which cells are shown to be less responsive to growth factors, might take great advantage from the PL treatment.
This study has demonstrated that PL can activate dermal cells involved in wound healing and has provided a characterization of this process, thus bringing scientific support to clinical applications of platelet derivates. These kinds of studies could put the basis for the development of a wide panel of techniques concerning the problem of biomaterial functionalization. Scaffolds used in tissue engineering are frequently driven by hydrophobic materials that lower cell affinity and permeation by nutrient fluids, thus rendering problematic the formation of cell‐scaffold composites [47, 48]. The design of biomimetic materials attempts to make scaffolds capable of eliciting specific cellular responses and directing new tissue formation. Growth factors are preferentially used in this field [49, 50, 51], but platelets have also started to be employed [52]. The implementation of platelet derivates in wound‐healing‐oriented biomaterials could offer interesting solutions, both on the clinical and the commercial ground, because it allows the use of patient‐autologous blood, thus preventing cost inflation and immunorejection problems.
Acknowledgements
We are grateful to Valeria Balbo, Elena Cattana and Simona Martinotti for PL preparation and fibroblast culture. This work was supported by a grant from Ricerca Scientifica Applicata (project n. A102), Regione Piemonte, Italy, 2004, and by grants from the University of Piemonte Orientale ‘Amedeo Avogadro’. E.R. is recipient of a research fellowship from the University of Piemonte Orientale.
References
- 1. Horch RE, Kopp J, Kneser U, et al . Tissue engineering of cultured skin substitutes. J Cell Mol Med . 2005; 9: 592–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knighton DR, Ciresi KF, Fiegel VD, et al . Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet‐derived wound healing factors (PDWHF). Ann Surg . 1986; 204: 322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin P. Wound healing – aiming for perfect skin regeneration. Science . 1997; 276: 75–81. [DOI] [PubMed] [Google Scholar]
- 4. Declair V. The importance of growth factors in wound healing. Ostomy Wound Manage . 1999; 45: 64–8 [PubMed] [Google Scholar]
- 5. Higley HR, Ksander GA, Gerhardt CO, et al . Extravasation of macromolecules and possible trapping of transforming growth factor‐beta in venous ulceration. Br J Dermatol . 1995; 132: 79–85. [DOI] [PubMed] [Google Scholar]
- 6. Ledent E, Berlin G. Inadequate white cell reduction by bedside filtration of red cell concentrates. Transfusion . 1994; 34: 765–8. [DOI] [PubMed] [Google Scholar]
- 7. Knighton DR, Ciresi K, Fiegel VD, et al . Stimulation of repair in chronic, nonhealing, cutaneous ulcers using platelet‐derived wound healing formula. Surg Gynecol Obstet . 1990; 170: 56–60. [PubMed] [Google Scholar]
- 8. Steed DL, Goslen JB, Holloway GA, et al . Randomized prospective double‐blind trial in healing chronic diabetic foot ulcers. CT‐102 activated platelet supernatant, topical versus placebo. Diabetes Care . 1992; 15: 1598–604. [DOI] [PubMed] [Google Scholar]
- 9. Wadhwa M, Seghatchian MJ, Lubenko A, et al . Cytokine levels in platelet concentrates: quantitation by bioassays and immunoassays. Br J Haematol . 1996; 93: 225–34. [DOI] [PubMed] [Google Scholar]
- 10. Zimmermann R, Jakubietz R, Jakubietz M, et al . Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion . 2001; 41: 1217–24. [DOI] [PubMed] [Google Scholar]
- 11. Soffer E, Ouhayoun JP, Anagnostou F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod . 2003; 95: 521–8. [DOI] [PubMed] [Google Scholar]
- 12. Okuda K, Kawase T, Momose M, et al . Platelet‐rich plasma contains high levels of platelet‐derived growth factor and transforming growth factor‐beta and modulates the proliferation of periodontally related cells in vitro . J Periodontol . 2003; 74: 849–57. [DOI] [PubMed] [Google Scholar]
- 13. Sanchez AR, Sheridan PJ, Kupp LI. Is platelet‐rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants . 2003; 18: 93–103. [PubMed] [Google Scholar]
- 14. Sanchez AR, Eckert SE, Sheridan PJ, et al . Influence of platelet‐rich plasma added to xenogeneic bone grafts on bone mineral density associated with dental implants. Int J Oral Maxillofac Implants . 2005; 20: 526–32. [PubMed] [Google Scholar]
- 15. Ito K, Yamada Y, Naiki T, et al . Simultaneous implant placement and bone regeneration around dental implants using tissue‐engineered bone with fibrin glue, mesenchymal stem cells and platelet‐rich plasma. Clin Oral Implants Res . 2006; 17: 579–86. [DOI] [PubMed] [Google Scholar]
- 16. Eppley BL, Pietrzak WS, Blanton M. Platelet‐rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg . 2006; 118: 147–59. [DOI] [PubMed] [Google Scholar]
- 17. Bennett NT, Schultz GS. Growth factors and wound healing: part II. Role in normal and chronic wound healing. Am J Surg . 1993; 166: 74–81. [DOI] [PubMed] [Google Scholar]
- 18. Mazzucco L, Medici D, Serra M, et al . The use of autologous platelet gel to treat difficult‐to‐heal wounds: a pilot study. Transfusion . 2004; 44: 1013–8. [DOI] [PubMed] [Google Scholar]
- 19. Debus ES, Schmidt K, Ziegler UE, et al . The role of growth factors in wound healing. Zentralbl Chir . 2000; 1: 49–55. [PubMed] [Google Scholar]
- 20. Borzini P, Mazzucco L. Platelet gels and releasates. Curr Opin Hematol . 2005; 12: 473–9. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Kalen A, Risto O, et al . Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen . 2002; 10: 336–40. [DOI] [PubMed] [Google Scholar]
- 22. Cenni E, Ciapetti G, Pagani S, et al . Effects of activated platelet concentrates on human primary cultures of fibroblasts and osteoblasts. J Periodontol . 2005; 76: 323–8. [DOI] [PubMed] [Google Scholar]
- 23. Krasna M, Domanovic D, Tomsic A, et al . Platelet gel stimulates proliferation of human dermal fibroblasts in vitro . Acta Dermatovenerol Alp Panonica Adriat . 2007; 16: 105–10. [PubMed] [Google Scholar]
- 24. Rajkumar VS, Shiwen X, Bostrom M, et al . Platelet‐derived growth factor‐beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol . 2006; 169: 2254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lampugnani MG. Cell migration into a wounded area in vitro . Methods Mol Biol . 1999; 96: 177–82. [DOI] [PubMed] [Google Scholar]
- 26. Huang C, Rajfur Z, Borchers C, et al . JNK phosphorylates paxillin and regulates cell migration. Nature . 2003; 424: 219–23. [DOI] [PubMed] [Google Scholar]
- 27. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem . 1985; 260: 3440–50. [PubMed] [Google Scholar]
- 28. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature . 1970; 227: 680–5. [DOI] [PubMed] [Google Scholar]
- 29. Vasquez R, Marien BJ, Gram C, et al . Proliferative capacity of venous ulcer wound fibroblasts in the presence of platelet‐derived growth factor. Vasc Endovascular Surg . 2004; 38: 355–60. [DOI] [PubMed] [Google Scholar]
- 30. Mirabet V, Solves P, Minana MD, et al . Human platelet lysate enhances the proliferative activity of cultured human fibroblast‐like cells from different tissues. Cell Tissue Bank . 2008; 9: 1–10. [DOI] [PubMed] [Google Scholar]
- 31. Castelnovo L, Dosquet C, Gaudric A, et al . Human platelet suspension stimulates porcine retinal glial proliferation and migration in vitro . Invest Ophthalmol Vis Sci . 2000; 41: 601–9. [PubMed] [Google Scholar]
- 32. Celotti F, Colciago A, Negri‐Cesi P, et al . Effect of platelet‐rich plasma on migration and proliferation of SaOS‐2 osteoblasts: role of platelet‐derived growth factor and transforming growth factor‐beta. Wound Repair Regen . 2006; 14: 195–202. [DOI] [PubMed] [Google Scholar]
- 33. Seppa H, Grotendorst G, Seppa S, et al . Platelet‐derived growth factor in chemotactic for fibroblasts. J Cell Biol . 1982; 92: 584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu J, Moon A, Kim HR. Both platelet‐derived growth factor receptor (PDGFR)‐alpha and PDGFR‐beta promote murine fibroblast cell migration. Biochem Biophys Res Commun . 2001; 282: 697–700. [DOI] [PubMed] [Google Scholar]
- 35. Cheng HP, Wei S, Wei LP, et al . Calcium signaling in physiology and pathophysiology. Acta Pharmacol Sin . 2006; 27: 767–72. [DOI] [PubMed] [Google Scholar]
- 36. Tournier JM, Maouche K, Coraux C, et al . alpha3alpha5beta2‐Nicotinic acetylcholine receptor contributes to the wound repair of the respiratory epithelium by modulating intracellular calcium in migrating cells. Am J Pathol . 2006; 168: 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lansdown AB. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen . 2002; 10: 271–85. [DOI] [PubMed] [Google Scholar]
- 38. Cole J, Tsou R, Wallace K, et al . Early gene expression profile of human skin to injury using high‐density cDNA microarrays. Wound Repair Regen . 2001; 9: 360–70. [DOI] [PubMed] [Google Scholar]
- 39. Leiper LJ, Walczysko P, Kucerova R, et al . The roles of calcium signaling and ERK1/2 phosphorylation in a Pax6+/‐ mouse model of epithelial wound‐healing delay. BMC Biol . 2006; 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li J, Kim YN, Bertics PJ. Platelet‐derived growth factor‐stimulated migration of murine fibroblasts is associated with epidermal growth factor receptor expression and tyrosine phosphorylation. J Biol Chem . 2000, 275; 2951–8. [DOI] [PubMed] [Google Scholar]
- 41. Tangkijvanich P, Santiskulvong C, Melton AC, et al . p38 MAP kinase mediates platelet‐derived growth factor‐stimulated migration of hepatic myofibroblasts. J Cell Physiol . 2002; 191: 351–61. [DOI] [PubMed] [Google Scholar]
- 42. Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross‐talk activation between MAP kinase cascades. J Biol Chem . 2003; 278: 21989–97. [DOI] [PubMed] [Google Scholar]
- 43. Xu KP, Riggs A, Ding Y, et al . Role of ErbB2 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci . 2004; 45: 4277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci . 2007; 48: 636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buffin‐Meyer B, Crassous PA, Delage C, et al . EGF receptor transactivation and PI3‐kinase mediate stimulation of ERK by alpha(2A)‐adrenoreceptor in intestinal epithelial cells: a role in wound healing. Eur J Pharmacol . 2007; 574: 85–93. [DOI] [PubMed] [Google Scholar]
- 46. Miura Y, Yanagihara N, Imamura H, et al . Hepatocyte growth factor stimulates proliferation and migration during wound healing of retinal pigment epithelial cells in vitro . Jpn J Ophthalmol . 2003; 47: 268–75. [DOI] [PubMed] [Google Scholar]
- 47. Whang K, Goldstick TK, Healy KE. A biodegradable polymer scaffold for delivery of osteotropic factors. Biomaterials . 2000; 21: 2545–51. [DOI] [PubMed] [Google Scholar]
- 48. Hillmann G, Steinkamp‐Zucht A, Geurtsen W, et al . Culture of primary human gingival fibroblasts on biodegradable membranes. Biomaterials . 2002, 23; 1461–9. [DOI] [PubMed] [Google Scholar]
- 49. Bowen‐Pope DF, Malpass TW, Foster DM, et al . Platelet‐derived growth factor in vivo: levels, activity, and rate of clearance. Blood . 1984; 64: 458–69. [PubMed] [Google Scholar]
- 50. Boccafoschi F, Habermehl J, Vesentini S, et al . Biological performances of collagen‐based scaffolds for vascular tissue engineering. Biomaterials . 2005; 26: 7410–7. [DOI] [PubMed] [Google Scholar]
- 51. Lin H, Chen B, Sun W, et al . The effect of collagen‐targeting platelet‐derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials . 2006; 27: 5708–14. [DOI] [PubMed] [Google Scholar]
- 52. Chang T, Liu Q, Marino V, et al . Attachment of periodontal fibroblasts to barrier membranes coated with platelet‐rich plasma. Aust Dent J . 2007; 52: 227–33. [DOI] [PubMed] [Google Scholar]


