Abstract
Thyroid‐stimulating hormone receptor (TSHR) consists of a hormone‐binding extracellular subunit and a seven‐transmembrane spanning subunit that interacts with the G proteins Gαs and Gαq. The two subunits, generated by proteolytic cleavage of a single polypeptide chain, are held together by disulphide bridges. The receptor is completely cleaved in thyroid tissue, while in cultured cells (thyrocytes and non‐thyroid cells) the cleaved and uncleaved forms coexist. The reasons for these divergent data are not understood. Here we provide an explanation by showing that cleavage depends on cell–cell contacts. An almost complete cleavage was observed in confluent cells, while in sparse cells most of the receptor was in the uncleaved form. We also show that coupling of TSHR to Gαq (as measured by inositolphosphate generation) is markedly reduced when the receptor is not cleaved. In contrast, coupling to Gαs [as measured by cyclic adenosine 3′,5′‐monophosphate (cAMP) synthesis] is unaffected by cleavage of the receptor. These results suggest that the cell–cell contacts are necessary for cleavage of the receptor, which acts as a regulatory step in inositolphosphate production via phospholipase C activation. The latter observation was confirmed using cells that express the uncleavable mutant TSHR‐Δ50‐NET, for which the TSH‐stimulated inositolphosphate production was completely abolished.
Keywords: thyrotropin receptor cleavage, phospholipase C, adenylate cyclase, L cells, cell–cell contact
Introduction
The main biological function of thyroid‐stimulating hormone (TSH) is to regulate the synthesis and secretion of thyroid hormones from thyroid follicular cells. TSH interacts with a seven‐transmembrane glycosylated G protein‐coupled TSH receptor (TSHR) which in thyrocytes is localized on the basolateral surface [1, 2]. TSHR includes a large hormone‐binding extracellular domain and the seven‐transmembrane spanning domain that interacts with Gαs and Gαq proteins via the intracellular loops and the C‐terminal tail [3]. The TSH‐TSHR/Gαs/adenylate cyclase/cyclic adenosine 3′,5′‐monophosphate (cAMP) regulatory cascade controls thyroid growth and differentiated functions (thyroid hormone secretion, iodide trapping), whereas TSH‐TSHR/Gαq/phospholipase C/diacylglycerol‐IP3 cascade stimulates iodination and thyroid hormone synthesis [4].
TSHR has a high degree of homology with the luteinizing hormone receptor (LHR) and with the follicle‐stimulating hormone receptor, two other receptors for glycoprotein hormones. Although all three newly synthesized receptors are inserted in the plasma membrane as single‐chain molecules, only TSHR undergoes an intramolecular cleavage. The resulting protomers, termed subunits A or α and B or β, are held together by disulphide bridges [1, 5]. In the thyroid tissue the A‐subunit represents the receptor ectodomain, has a molecular weight of 53 kD and is glycosylated. The B‐subunit represents the transmembrane and cytoplasmic regions, has a molecular weight of 34 kD and is not glycosylated [1]. Experiments with primary cultures of human thyrocytes [6, 7] and with the rat thyroid cell line FTRL‐5 [7, 8] confirmed this post‐translational cleavage of TSHR. This maturation is also observed to various extents in transfected cells expressing the recombinant human TSHR, such as L, CHO and K562 cell lines [6, 9, 10, 11, 12]. The A‐subunit is more extensively glycosylated (m.w. ∼60 kD) in L cells stably expressing the human TSHR than in the thyroid [6].
It is generally accepted that the cleaved receptor is the only molecular form in thyroid tissue. In contrast, for thyrocytes in culture variable proportions of TSHR cleavage have been reported. While complete receptor cleavage was observed in homogenates of cultured cells [6], cross‐linking of TSHR with radiolabelled TSH in intact cells revealed that both single‐chain and cleaved receptors coexist on the cell surface [7]. The coexistence on the cell surface of cleaved and intact TSHR was also described for transfected cells expressing the recombinant human receptor [6, 7, 9, 11]. The reasons for these divergent data are not understood.
The enzyme that cleaves TSHR is probably a membrane‐associated protease [13] related to TACE (tumour necrosis factors‐α converting enzyme), a member of the adamalysin family of metzincin metalloproteases [10].
Our hypothesis was that the divergent data could be explained by the dependency of TSHR cleavage on cell–cell contacts. The hypothesis was supported by recent data showing that metalloproteases are highly expressed and activated by homotypic cell–cell contacts, leading to enhanced cleavage and processing of various cell surface components [14, 15].
In the present study we confirm our hypothesis that cell–cell contacts control TSHR cleavage by comparing it under various degrees of intercellular contact of L cells stably expressing human TSHR [6]. We also demonstrate that the TSHR cleavage is required for full activation of phospholipase C (PLC) by TSH.
Materials and methods
Stable cell line cultures
L cells stably expressing the human TSHR (L‐TSHR cells) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen Corporation, Cergy Pontoise, France) containing 10% foetal calf serum (FCS; Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in the presence of 5% CO2 in a humidified atmosphere as previously described [6]. The cells were plated in Petri culture dishes of 10‐cm diameter at various densities (25 × 104, 25 × 105, 50 × 105 and 10 × 106 cells/culture dish) and cultured for 16 or 72 hrs.
Transient transfections
Wild‐type L cells were plated in 12‐well plates at 1.5 × 105/well in DMEM supplemented with 10% FCS. Construction of plasmids for wt‐human TSHR and TSHR‐Δ50‐NET mutant has been reported previously [16]. After 16 hrs 1 μg of DNA (wt‐hTSHR or TSHR‐Δ50‐NET) in FuGENE 6 (Roche, Indianapolis, IN, USA) was added to the culture medium. Sixteen hours later the medium was replaced with DMEM supplemented with 10% FCS. The cells were processed for subsequent tests 48 hrs after transfection. Parallel cultures and transfections were done in 4‐well LabTecII chambers (Nalgene Nunc International, Naperville, IL, USA) to monitor the transfection efficiency by immunocytochemistry using either T5–317, a monoclonal antibody against the extracellular domain (Ec‐Ab) or T3–365, a monoclonal antibody against the endodomain (En‐Ab) of TSHR [1]. Negative controls included transfection with the insert‐less vector pcDNA3.1. Each experiment was done two times with three samples for each experiment.
SDS‐PAGE and Western blotting
Cells were scraped in 4°C PBS supplemented with the metalloprotease inhibitor 1,10‐phenanthroline (10 mM, Sigma‐Aldrich, Sant Quentin, France) and the complete protease inhibitor cocktail (Roche) and centrifuged at 800 g for 10 min. at 4°C. The pellet of cells was resuspended in 50 mM Tris‐HCl, pH 7.5 containing 1.2% Triton X‐100 and protease inhibitors. After centrifugation for 30 min. at 14,000 ×g the supernatant was collected and the total cellular protein were measured by the bicinchoninic acid assay (Pierce Perbio Science, Brebières, France). Samples containing 50 μg of total protein were electrophoresed on a 5–15% gradient SDS‐PAGE under reducing and denaturing conditions as described [6]. Proteins were electrotransferred to nitrocellulose membranes and the molecular species of TSHR were detected by using monoclonal anti‐receptor ectodomain or endodomain antibodies [1] followed by secondary sheep antimouse IgG‐horseradish peroxidase conjugate (Amersham Biosciences, Buckinghamshire, UK). Bound antibodies were visualized using ECL reagents and exposure of Hyperfilm™ ECL (Amersham Biosciences). The intensity of immunoreactive bands was quantified using the image analysis software ImageJ_1.32J (NIH, Bethesda, MD, USA).
Enzymatic deglycosylation of TSHR
Deglycosylation of TSHR was done using H and F glycosidases, as previously described [6]. The reaction products were detected by immunoblotting using the T5–317 antibody. The enzymes were omitted from control experiments.
Functional studies
Sparse cell cultures were obtained by plating 25 × 104 cells in 10‐cm‐diameter Petri dishes. Confluent cell cultures were obtained by plating the same number of cells in each well of 12‐well plates. The cells were analysed 16 hrs after plating. Each experiment was repeated two to seven times with three samples for each experiment.
-
1
Measurements of cAMP – For adenylate cyclase activation bioassays, the culture medium was replaced by DMEM supplemented with 0.5 mM 3‐isobutylmethylxanthine, 1 mg/ml bovine serum albumin (BSA) and 20 mM HEPES, pH 7.3. After 30 min. the cells were incubated for 1 hr at 37°C with 10 mU/ml bovine TSH (Merck Calbiochem, Darmstad, Germany, specific activity 0.7 IU/mg protein). The total (intracellular + medium) cAMP was extracted with perchloric acid (final concentration 0.3 M). Samples, neutralized with a mixture of 0.6 M KHCO3 and 0.72 M KOH, were assayed using the Amersham cAMP kit (Code TRK 432) according to the protocol of the manufacturer (Amersham Biosciences). Experiments with 10 μM forskolin (Sigma‐Aldrich) (a direct stimulator of adenylate cyclase) were used as control experiments.
-
2
Measurements of inositolphosphates (IPs) – Cells were incubated at 37°C with inositol‐free DMEM containing 20 mM HEPES pH 7.4, 1 mg/ml bovine serum albumin (Sigma, BSA‐fraction V) and 2 μCi/ml of myo‐[2‐3H] inositol (Amersham Biosciences, specific activity 17 Ci/mmol). After 16 hrs the cells were washed twice with DMEM and incubated for 30 min. with DMEM containing20 mM HEPES, 20 mM LiCl and 1 mg/ml BSA. Cells were then stimulated with various concentrations (0, 1, 10, 50 and 100 mU/ml) of bovine TSH for 1 hr at 37°C. The accumulation of IPs was stopped with trichloroacetic acid (3% final concentration). After neutralization with sodium acetate, IP levels were determined by anion exchange chromatography using AGR 1‐X8 resin (200–400 mesh, acetate form; BioRad Laboratories, Hercules, CA, USA). After elution of neutral inositol with distilled water the bound IPs were eluted with 1 M trisodium citrate. As positive control for PLC activation we used L cells stimulated with 100 μM ATP (Amersham Biosciences) as previously described [17].
Results
TSHR cleavage depends on cell–cell contacts
The initial experiments were destined to identify the molecular forms of TSHR present in adherent TSHR‐L cells in culture at various densities. The cells have been cultured for 16 hrs in DMEM supplemented with 10% FCS. (Culturing the cells at low serum concentration (0–5%) was shown to lead to defects in TSHR processing, which do not manifest if cells are grown in 10% serum [18].) Total protein extracts were electrophoresed on 5–15% SDS‐PAGE gradient gels under reducing conditions and analysed by immunoblotting with antibodies against the receptor ectodomain (Ec‐Ab). We compared the proportions of mature intact and cleaved TSHR at various increasing cell culture densities. In cells plated at low densities (0.25 × 106 cells/dish) two major bands of 120 and 95 kD (p120 and p95, respectively) and two minor bands, p65 and p52, were detected (Fig. 1A [lane 1]). The sizes of the detected bands are the same as those observed previously in sub‐confluent L cells permanently expressing the receptor: the single‐chain uncleaved receptor with mature carbohydrates (p120), the high‐mannose receptor precursor (p95) and the α (or A) subunit of the cleaved TSHR (p60) [6]. The minor p52 band seems to be the result of proteolytic action of trypsin‐like proteases on mature receptor (our unpublished observations). The small amount of cleaved TSHR (p60) could reflect either a weak constitutive cleavage, independent of cell–cell contacts, or may reflect the fact that even at low culture densities the random distribution of cells bring some of them in contact.
Figure 1.
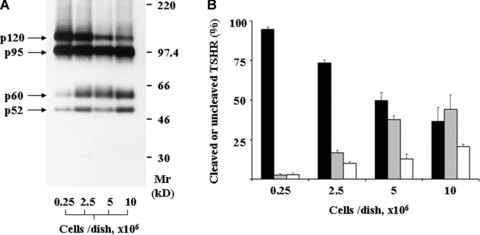
Cleavage of TSHR in stably transfected L cells as function of plating density. (A) Immunoblotting of cell homogenates using the monoclonal antibody against the TSHR ectodomain. (B) Quantitative evaluation of TSHR cleavage based on the intensity of the molecular forms that correspond to the mature TSHR receptor (the p120, p60 and p52 bands in (A). Closed bars: relative intensity (%) of p120 divided by total amount of mature TSHR (total = p120 + p60 + p52). Grey bars: p60/total; Open bars: p52/total. The bars represent the mean ± S.E.M. of triplicate experiments.
The transmembrane B‐subunit of TSHR, of 34 kD, is not detected by the anti‐Ec antibody. In order to confirm that p120 and p60 are the mature forms of TSHR characterized by the presence of coupled complex carbohydrates, detergent extracts of the membrane fraction of TSHR‐L cells have been treated with endoglycosidases F or H, which remove all protein‐coupled sugar moieties, or only high mannose moieties bound to the immature forms. The endoglycosidase H affected only the mobility of p95, indicating that p95 is the immature precursor of TSHR (data not shown). As expected, the endoglycosidase F reduced the molecular weight of all detected molecular species.
At higher cell density (2.5 × 106 cells/dish), when cells are still sub‐confluent, the pattern is similar to that at 0.25 × 106 cells/dish. However, increases in the amounts of p60 and p52 and a decrease of p120 are noticeable (Fig. 1A [lane 2] and B). In contrast, at 5 × 106 or 10 × 106 cells/dish, when extensive cell–cell contacts occur, p120 becomes a minor band and the intensities of p60 and p52 are higher (Fig. 1A [lanes 3 and 4] and B). There is a clear tendency for an increased cleavage of TSHR at high densities of plated cells (37.6 ± 2.4% at 5 × 106 cells/culture dish versus 2.5 ± 0.8% at 0.25 × 106 cells/culture dish, P < 0.1 × 10−9, grey bars in Fig. 1B).
Interestingly, when TSHR‐L cells were plated at high density and cultured for 72 hrs in order to obtain confluent cells with stable cell–cell contacts, the cleavage of mature TSHR was almost complete (97.7 ± 0.5% using the antibody against the extracellular domain and 96.1 ± 1.1% using the antibody against the intracellular domain) (Fig. 2). The intensity of the TSHR bands, detected with either the anti‐receptor ectodomain (Ec‐Ab) or the anti‐receptor endodomain (En‐Ab) antibodies, indicated that in confluent cells the majority (>90%) of cleaved mature TSHR consists of A‐subunit (p60) and B‐subunit (p34). About 10% of the cleaved receptor ectodomain is represented by the p52 protein.
Figure 2.
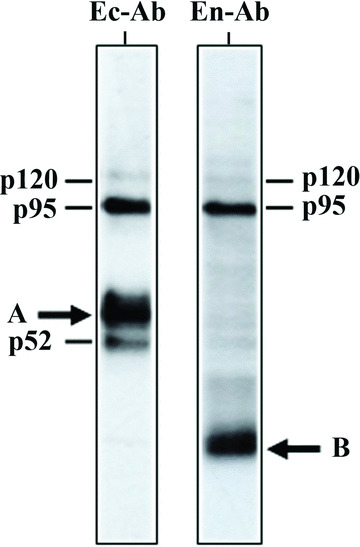
Cleavage of TSHR in confluent stably transfected L cells: Western blotting with monoclonal antibodies against either receptor ectodomain (Ec‐Ab) or receptor endodomain (En‐Ab). Heavy arrows: the extracellular and transmembrane subunits of cleaved TSHR (A and B, respectively). (The other bands are the same as in Fig. 1: p120 – mature uncleaved TSHR; p95 – the immature high‐mannose form of the receptor; p52 – degradation product generated by trypsin‐like protease.)
The effect of the cell–cell contact on TSHR proteolysis could be most directly explained if cleavage of TSHR is performed by a protease anchored to the membrane of the cells adjacent to the cell on which TSHR is exposed. An alternative explanation is that TSHR cleavage is performed by a soluble protease, which is released from cells after they reach confluency. In order to evaluate the latter possibility we collected culture medium from confluent L cells, applied it to sparse TSHR‐L cells for 4 hrs and then assessed the cleavage of TSHR by immunoblotting. The addition of the medium did not produce any cleavage of TSHR.
TSHR cleavage influences PLC response to TSH stimulation
The above data indicate that only the cleaved mature TSHR subunits A and B are present in confluent cells. In contrast, only single‐chain, uncleaved receptors were detected in sparse cell cultures. These data raised the possibility that TSH signalling in cells containing only the uncleaved TSHR species differs from that in cells containing the cleaved receptor. TSH signalling is known to occur via two branches, one via adenylate cyclase and the other via PLC. Therefore, we compared in the activation by hormone of both adenylate cyclase as assessed by cAMP synthesis, and of PLC determined by inositolphosphate production, in sparse and confluent TSHR‐L cells. As illustrated in Fig. 3A, both cleaved and uncleaved receptors stimulated similarly the cAMP cascade. In contrast, only the cleaved TSHR fully activated the phospholipase C cascade (Fig. 3B).
Figure 3.
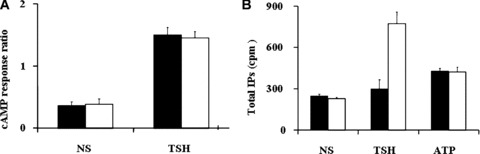
Functional effects of TSHR cleavage. (A) TSH‐stimulated cAMP synthesis. The cAMP response (vertical axis) was normalized by dividing the cAMP amount induced by TSH to the cAMP induced by forskolin (Fk), a direct activator of adenylate cyclase. (B) Hormone‐induced inositolphosphate (IP) accumulation in L cells stably expressing TSHR. Closed bars: sparse cells; open bars: confluent cells. NS: non‐stimulated cells.
In order to verify that PLC signalling is not perturbed in sparse cells for reasons independent of TSHR cleavage, we verified if PLC signalling can be activated by an alternative stimulus known to work in L cells – ATP binding to the adenosine receptor sparse [17]. As shown in Fig. 3B, ATP activates PLC in sparse L cells. ATP induced similar amounts of inositolphosphate in both confluent and isolated TSHR‐L cells. As PLC is known to be weakly activated by ATP, the fact that we detected the activation indicates that the pathway is fully functional. Both TSH and ATP stimulation work via the same Gαq protein, indicating that the defective signalling by the uncleaved TSHR occurs at the level of the interaction between the receptor and the G‐protein. Similarly, LH activation of PLC, which occurs via the LHR and the same Gαq protein, is functional in sparse L cells transfected with LHR (data not shown).
Noncleavable TSHR‐Δ50‐NET receptor mutant does not stimulate phospholipase C in response to thyrotropin stimulation
The above data suggested that TSHR cleavage strongly enhances PLC activation. To confirm this observation, we examined the hormone‐induced production of IPs in L cells transiently expressing the TSHR‐Δ50‐NET receptor, an engineered mutant of TSHR that cannot be cleaved into A and B subunits [16]. Binding studies done by the Rapaport group [19] demonstrated that there is no difference between the affinities for the hormone of the cleaved versus the uncleaved TSHR mutant. L cells transiently expressing the wild‐type human TSHR were used as control. Western blot experiments using anti‐receptor ectodomain antibodies confirmed that the non‐cleaved mutant receptor TSHR‐Δ50‐NET (p115) was the only molecular species expressed in the cells (Fig. 4A, lane 2).
Figure 4.
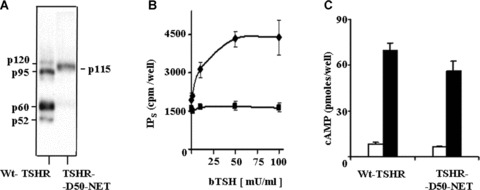
Functional assays of the non‐cleavable TSHR‐Δ50‐NET receptor mutant transiently expressed in L cells. (A) Western blot experiments using anti‐receptor ectodomain antibodies; (B) [3H]‐inositolphosphates (IPs) accumulation in cells as function of TSH concentration (squares – mutant TSHR; diamonds – wtTSHR), (C) Accumulation of cAMP in response to TSH stimulation. Closed bars: cells stimulated with 10 mU/ml TSH; open bars: non‐stimulated cells.
In contrast, both cleaved and uncleaved receptors (p60 and p120, respectively) were detected in control TSHR‐L cells. The uncleaved TSHR‐Δ50‐NET mutant displayed a slightly decreased (P= 0.01, t‐test, 2 tails) cAMP production after stimulation with TSH (Fig. 4C). In contrast, no IPs accumulation was induced by the ligand over a large range of concentrations (Fig. 4B).
Discussion
In normal thyroid tissue the thyrocytes are arranged into continuous monolayers surrounding the glandular lumen. The cells are characterized by a definitive polarity in the arrangement of their surface domains (apical plasma membrane, tight junctions, basolateral plasma membrane) [20]. In contrast, in cultured thyrocytes the cell polarity depends on the extent of cell–cell contacts varying from no polarity in sparse cells to a high polarity in confluent cells, characterized by complete separation of the apical from the basolateral compartment. In the latter case, the accessibility of TSH to the basolateral TSHR is possible only if the cells are seeded on porous inserts [21]. Human thyrocytes express very low concentrations of the receptor [1], comparable to the non‐specific binding of hormone to filters and plastics [22]. Structural and functional receptor studies performed so far circumvented this technical difficulty by using either thyroid slices [23] or sub‐confluent thyrocytes [7]. According to our data presented above, the sub‐confluent cultures do not represent an accurate model for the situation in vivo, due to the different cleavage pattern of the receptor, which in turn affects, as shown, the signalling process.
Our data suggest that cleavage of TSH receptor depends on cell–cell contacts. This observation reconciles the opposing views concerning the cleavage extent of TSHR in both native thyroid and non‐thyroid transfected cells [24]. We show that nearly all mature TSHRs are in the cleaved form in confluent cells, thus explaining the results reported for thyroid tissue in vivo[1, 8, 23] and for confluent thyrocytes in vitro[6]. Our data show also that in sparse cells the majority of the receptor is uncleaved, while in sub‐confluent cells the cleaved and uncleaved TSHR coexist in comparable amounts. The latter situation is valid for most studies done with sub‐confluent thyrocytes and transfected cells [24].
Previously we have shown that in sparse and sub‐confluent cells TSHR is highly concentrated at the sites of close contact between the extracellular matrix and the cells, particularly at the leading edge of lamellipodia [25]. As TSHR is cleaved by an unidentified a disintegrin and metalloprotease (ADAM) family member [10], it is relevant to notice that most ADAMs are concentrated also at the leading edge of lamellipodia [26]. However, in spite of this potential co‐localization, TSHR is not cleaved in sparse cells. A potential explanation is that cleavage is not executed by enzymes harboured in the same cell membrane, contrary to what has been documented so far for most ADAMs [27]. An alternative possibility is that TSHR is cleaved by an ADAM member localized in the same cell membrane, but whose expression or insertion in the plasma membrane or activation is triggered by cell–cell contact. This possibility is supported by data showing that in fibroblasts the expression and activation of metalloproteases MMP‐1, MMP‐10 and MT1‐MMP is enhanced by direct cell–cell contacts [15]. We excluded the involvement of a soluble metalloprotease by showing that incubation of sparse cells with collected culture medium from confluent non‐transfected L cells did not produce any cleavage of TSHR.
In sub‐confluent cells, high concentrations of TSH increase the extent of receptor cleavage [28]. This observation can be explained by the fact that the high concentrations of TSH are also known to increase the number of cell–cell contacts due to enhanced cell spreading [25], further supporting the role of cell–cell contacts in TSHR cleavage.
Experiments with cleavable and non‐cleavable mutant constructs demonstrated that TSHR cleavage is not required for high‐affinity TSH binding, it is unrelated to the constitutive ligand‐independent activity of the receptor and does not influence the cAMP response to hormone stimulation [19, 29].
Another pathway activated by TSHR involves production of IP3 and diacylglycerol via PLC. This is the first study that investigates the effect of TSHR cleavage on this pathway. The IPs measurement method used by us detects all IPs, but IP1, IP2, IP4 and IP5 are present in L‐TSHR cells in negligible amounts [25]. As a consequence, the IP variations shown in Figs 3B and 4B reflect variations of IP3, and therefore indicate that the cell–cell contact dependent cleavage of TSHR is necessary for full activation of phospholipase C. In contrast, concentration dependence and kinetic studies for the effect of TSH on cAMP have shown that TSHR cleavage is not necessary for the adenylate cyclase pathway [19, 29]. Ciullo et al.[30] showed also that cleaved two‐chain TSHR activates the cAMP cascade. (Their experimental system consisted of confluent cells, in which most likely no uncleaved receptor was present, so that no valid conclusion can be drawn about the activation ability of the non‐cleaved receptor.)
The mechanism by which the TSHR cleavage affects Gq signalling is not known. TSHR cleavage leads to the loss of a 50 residues segment of the extracellular domain [9, 10, 16], while Gq binds to intracellular loops of TSHR [31, 32]. The Gq binding sites could be inaccessible for Gq in the uncleaved TSHR, and after TSHR cleavage and loss of the 50 residues segment could become exposed due to a conformational change. We attempted to detect the difference in the association with Gq of cleaved and uncleaved TSHR by immunoprecipitation. We used either anti‐TSHR or anti‐Gq as immunoprepitating antibodies, but could not detect any signal. The most likley explanation is that interaction between Gq and TSHR is too weak [3] and rapid to be reproducibly detected by immunoprecipitation.
Although in the adult thyroid in vivo the thyrocytes are confluent and as a consequence TSHR is fully cleaved, a pathological situation exists – in which cleavage is perturbed: in Graves’ disease auto‐antibodies against the cleavage region inhibit this process [33].
The data presented in this study apply only to TSHR expressing cell lines and cannot be extrapolated with certitude to the situation in vivo. A situation which apparently contradicts the necessity of TSHR cleavage for the induction of PLC occurs in dog thyrocytes [3] and FRTL‐5 cells [34]. These cells do not show an IP response to PLC activation by TSH when grown to confluency in culture dishes, although the receptor is cleaved [8]. The probable explanation for the lack of response is, however, not the lack of activity of the cleaved receptor, but the fact that in this system TSH cannot get in contact with the receptor. TSH present in the culture medium can access only the apical surface of the cells, while the receptor is localized only on the basolateral side, and the two compartments are isolated by the tight junctions formed among the confluent cells, as shown for human thyroid cells in similar culture conditions [2].
In summary, our results suggest that the cell–cell contacts are necessary for cleavage of TSHR, which acts as a regulatory step in inositolphosphate production via PLC activation.
Acknowledgements
The excellent technical assistance of Sylvette Reposo is gratefully acknowledged. This project was supported by the Institut National de la Santé et de la Recherche Médicale – France.
References
- 1. Loosfelt H, Pichon C, Jolivet A, et al. Two‐subunit structure of the human thyrotropin receptor. Proc Natl Acad Sci USA. 1992; 89: 3765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baratti‐Elbaz C, Ghinea N, Lahuna O, et al. Internalization and recycling pathways of the thyrotropin receptor. Mol Endocrinol. 1999; 13: 1751–65. [DOI] [PubMed] [Google Scholar]
- 3. Allgeier A, Laugwitz KL, Van Sande J, et al. Multiple G‐protein coupling of the dog thyrotropin receptor. Mol Cell Endocrinol. 1997; 127: 81–90. [DOI] [PubMed] [Google Scholar]
- 4. Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992; 13: 596–611. [DOI] [PubMed] [Google Scholar]
- 5. Kajita Y, Rickards CR, Buckland PR, et al. Analysis of thyrotropin receptors by photoaffinity labelling. Orientation of receptor subunits in the cell membrane. Biochem J. 1985; 227: 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Misrahi M, Ghinea N, Sar S, et al. Processing of the precursors of the human thyroid‐stimulating hormone receptor in various eukaryotic cells (human thyrocytes, transfected L cells and baculovirus‐infected insect cells). Eur J Biochem. 1994; 222: 711–9. [DOI] [PubMed] [Google Scholar]
- 7. Chen CR, Chazenbalk GD, Wawrowsky KA, et al. Evidence that human thyroid cells express uncleaved, single‐chain thyrotropin receptors on their surface. Endocrinology. 2006; 147: 3107–13. [DOI] [PubMed] [Google Scholar]
- 8. Furmaniak J, Hashim FA, Buckland PR, et al. Photoaffinity labelling of the TSH receptor on FRTL5 cells. FEBS Lett. 1987; 215: 316–22. [DOI] [PubMed] [Google Scholar]
- 9. Chazenbalk GD, Rapoport B. Cleavage of the thyrotropin receptor does not occur at a classical subtilisin‐related proprotein convertase endoproteolytic site. J Biol Chem. 1994; 269: 32209–13. [PubMed] [Google Scholar]
- 10. de Bernard S, Misrahi M, Huet JC, et al. Sequential cleavage and excision of a segment of the thyrotropin receptor ectodomain. J Biol Chem. 1999; 274: 101–7. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka K, Chazenbalk GD, McLachlan SM, et al. Subunit structure of thyrotropin receptors expressed on the cell surface. J Biol Chem. 1999; 274: 33979–84. [DOI] [PubMed] [Google Scholar]
- 12. Siffroi‐Fernandez S, Costagliola S, Paumel S, et al. Role of complex asparagine‐linked oligosaccharides in the expression of a functional thyrotropin receptor. Biochem J. 2001; 354: 331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Latrofa F, Chazenbalk GD, McLachlan SM, et al. Evidence that the thyrotropin receptor protease is membrane‐associated and is not within lipid rafts. Thyroid. 2004; 14: 801–5. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki S, Sato M, Senoo H, et al. Direct cell‐cell interaction enhances pro‐MMP‐2 production and activation in co‐culture of laryngeal cancer cells and fibroblasts: involvement of EMMPRIN and MT1‐MMP. Exp Cell Res. 2004; 293: 259–66. [DOI] [PubMed] [Google Scholar]
- 15. Sirén V, Salmenperä P, Kankuri E, et al. Cell‐cell contact activation of fibroblasts increases the expression of matrixmetallo‐proteinases. Ann Med. 2006; 38: 212–20. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka K, Chazenbalk GD, McLachlan SM, et al. Thyrotropin receptor cleavage at site 1 does not involve a specific amino acid motif but instead depends on the presence of the unique, 50 amino acid insertion. J Biol Chem. 1998; 273: 1959–63. [DOI] [PubMed] [Google Scholar]
- 17. Grierson J, Meldolesi J. Shear stress‐induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J Biol Chem. 1995; 270: 4451–6. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka K, Chazenbalk GD, McLachlan SM, et al. The shed thyrotropin receptor is primarily a carboxyl terminal truncated form of the A subunit, not the entire A subunit. Mol Cell Endocrinol. 1999; 150: 113–9. [DOI] [PubMed] [Google Scholar]
- 19. Chazenbalk GD, Tanaka K, McLachlan SM, et al. On the functional importance of thyrotropin receptor intramolecular cleavage. Endocrinology. 1999; 140: 4516–20. [DOI] [PubMed] [Google Scholar]
- 20. Gershon MD, Nunez EA. The thyroid gland. In: Weiss L, editor. Cell and tissue biology. A text book of histology. Baltimore , Munich : Urban and Schwarzenberg; 1983. pp. 1009–20. [Google Scholar]
- 21. Nilsson M, Husmark J, Nilsson B, et al. Primary culture of human thyrocytes in Transwell bicameral chamber: thyrotropin promotes polarization and epithelial barrier function. Eur J Endocrinol. 1996; 135: 469–80. [DOI] [PubMed] [Google Scholar]
- 22. Chazenbalk GD, Nagayama Y, Kaufman KD, et al. The functional expression of recombinant human thyrotropin receptors in nonthyroidal eukaryotic cells provides evidence that homologous desensitization to thyrotropin stimulation requires a cell‐specific factor. Endocrinology. 1990; 127: 1240–4. [DOI] [PubMed] [Google Scholar]
- 23. van Sande J, Dequanter D, Lothaire P, et al. Thyrotropin stimulates the generation of inositol 1,4,5‐trisphosphate in human thyroid cells. J Clin Endocrinol Metab. 2006; 91: 1099–107. [DOI] [PubMed] [Google Scholar]
- 24. Rapoport B, McLachlan SM. The thyrotropin receptor in Graves’ disease. Thyroid. 2007; 17: 911–22. [DOI] [PubMed] [Google Scholar]
- 25. Ghinea N, Baratti‐Elbaz C, de Jesus‐Lucas A, et al. TSH receptor interaction with the extracellular matrix: role on constitutive activity and sensitivity to hormonal stimulation. Mol Endocrinol. 2002; 16: 912–23. [DOI] [PubMed] [Google Scholar]
- 26. van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006; 26: 716–28. [DOI] [PubMed] [Google Scholar]
- 27. Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005; 6: 32–43. [DOI] [PubMed] [Google Scholar]
- 28. Ando T, Latif R, Pritsker A, et al. A monoclonal thyroid‐stimulating antibody. J Clin Invest. 2002; 110: 1667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russo D, Chazenbalk GD, Nagayama Y, et al. A new structural model for the thyrotropin (TSH) receptor, as determined by covalent cross‐linking of TSH tothe recombinant receptor in intactcells: evidence for a single polypeptide chain. Mol Endocrinol. 1991; 5: 1607–12. [DOI] [PubMed] [Google Scholar]
- 30. Ciullo I, Latif R, Graves P, et al. Functional assessment of the thyrotropin receptor‐beta subunit. Endocrinology. 2003; 144: 3176–81. [DOI] [PubMed] [Google Scholar]
- 31. Neumann S, Krause G, Claus M, et al. Structural determinants for G protein activation and selectivity in the second intracellular loop of the thyrotropin receptor. Endocrinology. 2005; 146: 477–85. [DOI] [PubMed] [Google Scholar]
- 32. Claus M, Neumann S, Kleinau G, et al. Structural determinants for G protein activation and specificity in the third intracellular loop of the thyroid‐stimulating hormone receptor. J Mol Med. 2006; 84: 943–54. [DOI] [PubMed] [Google Scholar]
- 33. Ando T, Latif R, Davies TF. Antibody‐induced modulation of TSH receptor posttranslational processing. J Endocrinol. 2007; 195: 179–86. [DOI] [PubMed] [Google Scholar]
- 34. Singh J, Hunt P, Eggo MC, et al. Thyroid‐stimulating hormone rapidly stimulates inositol polyphosphate formation in FRTL‐5 thyrocytes without activating phosphoinositidase C. Biochem J. 1996; 316: 175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


