Abstract
Fibroblast growth factor‐2 (FGF2) is a potent angiogenic growth factor. Here, gene expression profiling of FGF2‐stimulated microvascular endothelial cells revealed, together with a prominent pro‐angiogenic profile, a pro‐inflammatory signature characterized by the up‐regulation of pro‐inflammatory cytokine/chemokines and their receptors, endothelial cell adhesion molecules and members of the eicosanoid pathway. Real‐time quantitative PCR demonstrated early induction of most of the FGF2‐induced, inflammation‐related genes. Accordingly, chick embryo chorioallantoic membrane (CAM) and murine Matrigel plug angiogenesis assays demonstrated a significant monocyte/macrophage infiltrate in the areas of FGF2‐driven neovascularization. Similar results were obtained when the conditioned medium (CM) of FGF2‐stimulated endothelial cells was delivered onto the CAM, suggesting that FGF2‐upregulated chemoattractants mediate the inflammatory response. Importantly, FGF2‐triggered new blood vessel formation was significantly reduced in phosphatidylinositol 3‐kinase‐γ null mice exhibiting defective leucocyte migration or in clodronate liposome‐treated, macrophage‐depleted mice. Furthermore, the viral pan‐chemokine antagonist M3 inhibited the angiogenic and inflammatory responses induced by the CM of FGF2‐stimulated endothelial cells and impaired FGF2‐driven neovascularization in the CAM assay. These findings point to inflammatory chemokines as early mediators of FGF2‐driven angiogenesis and indicate a non‐redundant role for inflammatory cells in the neovascularization process elicited by the growth factor.
Keywords: angiogenesis, chemokines, FGF, inflammation, macrophages
Introduction
Angiogenesis and inflammation are closely integrated processes in a number of physiological and pathological conditions, including wound healing, psoriasis, diabetic retinopathy, rheumatoid arthritis, arteriosclerosis and cancer [1, 2]. Inflammatory cells may produce angiogenic cytokines, growth factors and proteases that contribute to the formation of new vascular structures at the site of inflammation, tissue damage or tumour growth [3]. Conversely, microvascular endothelium activated by a number of cytokines and angiogenic growth factors can express pro‐inflammatory molecules involved in leucocyte recruitment and activation [4, 5]. Strikingly, neovascularization and inflammation share a number of common signalling pathways and molecular mediators, the cyclooxygenase (Cox)/prostaglandin pathway representing a paradigm of this convergence [6]. Also, various chemokines may act both as leucocyte attractants and as angiogenic inducers by acting directly on endothelial cells [7]. Moreover, a number of pro‐inflammatory cytokines [e.g. interleukin‐1α (IL‐1α), IL‐1β, IL‐6, tumour necrosis factor‐α (TNFα), high mobility group box‐1 (HMGB1) and osteopontin (Opn)] may induce blood vessel formation via direct engagement of target endothelial cells or indirectly by inducing leucocytes and/or endothelial cells to produce pro‐angiogenic mediators [8, 9, 10]. Conversely, the angiogenic factors vascular endothelial growth factor (VEGF) and angiopoietin‐1 (Ang‐1) may elicit pro‐inflammatory responses in endothelial cells by up‐regulating the expression of cell adhesion molecules and inflammatory mediators [11, 12].
Fibroblast growth factor 2 (FGF2) is a pleiotropic heparin‐binding factor that promotes growth and differentiation of a broad spectrum of cell types [13]. FGF2 triggers a complex ‘pro‐angiogenic phenotype’ in endothelial cells that recapitulates the neovascularization process and exerts a potent angiogenic response in a variety of in vivo animal models [13]. The angiogenic activity of FGF2 is mediated by its interaction with high‐affinity tyrosine kinase FGF receptors (FGFRs) and low‐affinity heparan sulphate proteoglycans and integrin receptors, leading to the activation of multiple signal transduction pathways, including phospholipase C‐γ, phosphatidylinositol 3‐kinase (PI3K) and mitogen‐activated protein kinases [13, 14].
Elevated levels of FGF2 have been implicated in the pathogenesis of several diseases characterized by a deregulated angiogenic/ inflammatory response, including cancer [13]. Inflammatory cells, including mononuclear phagocytes, T lymphocytes and mast cells, express FGF2. Moreover, FGF2 production and release from endothelial cells are triggered by inflammatory mediators (reviewed in [13]). Conversely, FGF2 may amplify the endothelial cell response to inflammatory stimuli [15] and up‐regulates the expression of Opn[10], Ccl2 chemokine [16] and Cox‐2[17] in endothelial cells. Taken together, these observations point to the existence of a tight cross‐talk between inflammatory and angiogenic responses during FGF2‐driven neovascularization.
Here, we report that FGF2 induces a pro‐inflammatory signature in murine microvascular endothelial cells. Consistently, we provide in vivo evidence about the non‐redundant role of chemokines and infiltrating monocytes/macrophages in FGF2‐driven neovascularization.
Materials and methods
Reagents and cells
Recombinant human FGF2 was purified as previously described [18]. Ketoprofen and hydrocortisone were purchased from Sigma‐Aldrich (Saint Louis, MO, USA). Recombinant M3 protein was expressed in the baculovirus system and purified by affinity chromatography [19].
Murine lung microvascular endothelial cells (1G11 cells) [20] were obtained from A. Vecchi (Istituto Scientifico Humanitas, Rezzato, Milan, Italy) and cultured in Dulbecco modified Eagle medium (DMEM) containing 20% inactivated foetal bovine serum (FBS). Cells were usually starved for 24 hrs with DMEM containing 0.5% FBS before stimulation with FGF2. In all the assays, endotoxin content was lower than 0.06 EU/ml (6 pg/ml), as determined by the Limulus amebocyte lysate method (Cambrex BioSiences, Walkersville, MD, USA).
Affymetrix genechip analysis
Three independent 1G11 cell cultures were stimulated for 10 hrs with 30 ng/ml FGF2 in DMEM supplemented with 0.5% FBS. As a control reference, duplicate samples from non‐treated cells were also analysed. RNA extraction, reverse transcription, cRNA preparation and GeneChip hybridization were performed according to the manufacturer's instructions (http://www.affymetrix.com/support/technical/manual/expression_manual.affx, Affymetrix, Santa Clara, CA, USA). Briefly, total RNA was extracted using Trizol (Invitrogen Life Technologies, Carlsbad, CA, USA) and phase lock gels (Eppendorf, Hamburg, Deutschland) and purified with Rneasy columns (Qiagen, Valencia, CA, USA). Ten micrograms of RNA were then used as a template for double‐stranded cDNA synthesis primed using a T7‐(dT)24 oligonucleotide. Double‐stranded cDNA was then transcribed using T7 RNA polymerase to produce biotin‐labelled cRNA. Resulting cRNA was fragmented and hybridized to Affymetrix GeneChip Murine Genome MOE430A oligonucleotide microarrays.
To define the transcriptional profile modulated by FGF2, raw expression measures at the probe level data were computed using robust multi‐array average. Quantile normalization was performed across all microarrays to achieve the same distribution of signal intensities for each array [21]. Data analysis was then carried out using Genespring 7.3 software (Silicon Genetics, Redwood City, CA, USA). Initial data filtering of genes with a ‘present’ detection call in at least one chip, according to Affymetrix MAS5 algorithm, was applied. Differential expression was assessed by applying a twofold change cut‐off and a Welch‐modified two‐sample t‐test. A false discovery rate of 5% was used as a cut‐off for statistical significance.
FGF2‐regulated genes were annotated by employing the web‐accessible software DAVID [22] (Database for Annotation, Visualization and Integrated Discovery; http://david.abcc.ncifcrf.gov/) and NetAffx Analysis Center (http://www.affymetrix.com/analysis/index.affx), which provides functional genomic annotations for gene ontology (GO), protein domain and biological pathways. Over‐represented signatures, based on GO terms (cellular localization, molecular function and biological process) were identified using statistical Fisher's test (P < 0.05) and the whole MOE430A gene list as the reference list. The complete, minimum information about a microarray experiment (MIAME)‐compliant dataset is available at the public repository ArrayExpress at the EBI (Hinxton, UK) (accession number E‐MEXP‐1467).
Real‐time PCR analysis
Two‐step quantitative RT‐PCR (qRT‐PCR) was employed to validate microarray expression data on a selected list of genes (Table 1). Random‐primed RT was carried out with 50 ng of RNA and High‐Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Reactions lacking enzyme were carried out in tandem for each RNA sample as negative controls. One‐fiftieth of the final RT reaction was used as template in qRT‐PCR reactions containing HPLC‐purified oligonucleotide primers (Thermo Electron, Ulm, Germany) specific for selected genes (the list of oligonucleotide primer sequences utilized in the present work are shown in Table 1). Primers were designed with Primer3 software (http://frodo.wi.mit.edu/cgi‐bin/primer3/primer3_www_slow.cgi) using the following settings: 100–200 bp PCR products, 18–22 mer primers, 60°C melting temperatures. Gene names, accession numbers and forward and reverse primer sequences are listed in Table 1 with the only exception for the Cxcl1 gene that was analysed by using a Gene Expression Assay (Mm00433859_m1) from Applied Biosystems and the manufacturer's TaqMan® PreAmp Master Mix Kit Protocol. Each primer set produced a single product, as determined by melt‐curve analysis. Real‐time PCR was carried out on a iCycler Real‐Time PCR Detection System (BioRad Richmond, CA, USA) using 25‐μl reactions containing iQ SYBR Green Supermix, 150–300 nM forward and reverse primers and 5 μl of cDNA‐diluted template. The PCR cycling profile was as follows: 3 min. at 95°C and 40 cycles for 15 sec. at 95°C, 60°C for 1 min. After PCR amplification, melting curve analysis was performed for each reaction.
Table 1.
Quantitative RT‐PCR: oligonucleotide primer sequences
| Gene | RefSeq | Forward | Reverse | Amplicon |
|---|---|---|---|---|
| Actb | NM_007393 | CGTAAAGACCTCTATGCCAACA | CCACCGATCCACACAGAGA | 161 |
| Ccl2 | NM_011333 | CTTCTGGGCCTGCTGTTCA | CCAGCCTACTCATTGGGATCA | 127 |
| Ccl7 | NM_013654 | CCTGGGAAGCTGTTATCTTCA | TTGGCTCCTAGGTTGGTTTC | 159 |
| Cx3cl1 | NM_009142 | CATGTGCGACAAGATGACC | CTTGGACCCATTTCTCCTTC | 149 |
| Cxcl16 | NM_023158 | CCATTCTTTATCAGGTTCCAGT | CTCGTGTCCGAAGGTGTC | 200 |
| Egr1 | NM_007913 | CCTGACCACAGAGTCCTTTT | ATAGGTGATGGGAGGCAAC | 103 |
| IL6 | NM_031168 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA | 141 |
| Jam2 | NM_023844 | TGCTGGAGAGTATCGCTGT | CTTCTTTATCCTGGCATCGT | 157 |
| Ptgs2 | NM_011198 | GGTGTGAACAATCAAACAAAATG | TAACATGCTTGGGTCAGTCAAT | 160 |
| Vcam1 | NM_011693 | GAACTGATTATCCAAGTCTCTCCA | CCATGTCTCCTGTCTTTGCTT | 157 |
Each PCR reaction was performed in triplicate on one plate and fluorescence data were recorded using iCycler software (BioRad). Relative expression ratios were calculated by use of Pfaffl equation and Relative Expression Software Tool (http://www.gene‐quantification.info). The mRNA expression levels of target genes were normalized to the levels of β‐actin gene, which, according to microarray experiments, behaves as a housekeeping gene under the tested conditions.
Endothelial cell adhesion to FGF2‐coated plastic dishes
Adhesion of 1G11 cells to FGF2‐coated plastic dishes was performed as described [23] with minor modifications. Non‐tissue polystyrene culture plastic 35‐mm dishes were incubated with 100 mM NaHCO3, pH 9.6 (carbonate buffer) containing 5 μg/ml of FGF2. After 16 hrs of incubation at 4°C, the dishes were washed with cold carbonate buffer and DMEM containing 0.5% FBS. Then, serum‐starved 1G11 cells were seeded at 100,000 cells/dish and incubated for 24 hrs in DMEM/0.5% FBS. Cells were dissociated in Trizol for RNA extraction followed by qRT‐PCR. The conditioned medium (CM) from FGF2‐stimulated cells was collected and stored at −20°C until use. For comparison, confluent cultures of 1G11 cells were treated for 24 hrs with 30 ng/ml of free human recombinant FGF2 whereas untreated cells seeded on tissue culture polystyrene plastic plates were used as negative controls.
Chemotaxis assay
Human monocytes were obtained from buffy coats of healthy blood donors by Ficoll (Lympholite‐H, Cederlane Labs, Hornly, Canada) and Percoll (GE Healthcare, Little Chalfont, UK) gradients. Chemotaxis was assayed in 96 well‐plates (Neuro Probe, Inc., Gaithersburg, MD, USA) containing a polycarbonate filter with 5‐μm pores. Briefly, monocytes were resuspended in RPMI‐1640 medium containing 1% serum, and then loaded onto inserts at 5 × 103 cells/50 μl for each well. Thirty microlitres of RPMI‐1640 medium containing the chemoattractants at the indicated concentrations were placed in the bottom compartment. After 1.5 hrs of incubation at 37°C with 5% CO2, non‐migrating cells were scraped from the upper surface of the filter. Migrating cells on the lower surface were fixed with methanol, stained with Diff‐Quik (Baxter Healthcare, Miami, FL, USA) and their number was determined by counting five microscopic fields per well at ×250 magnification. For inhibitory assays, cells and media were pre‐incubated for 30 min. with the indicated concentrations of the pan‐chemokine inhibitor M3 before loading onto Transwell inserts (Sigma‐Aldrich). Each sample was tested in triplicate.
Matrigel plug angiogenesis assay
Liquid Matrigel (10 mg/ml; 0.5 ml/mouse) was mixed at 4°C with FGF2 (1.0 μg/ml) and injected subcutaneously into the flank of 6‐week‐old C57BL/6 mice (Charles River, Calco, Italy). Matrigel with PBS alone was used as negative control. Alternatively, 5‐week‐old 129sv WT and 129sv PI3Kγ−/− mice [24] were used. On day 7 after implantation, mice were killed and plugs were removed, embedded in Tissue Tec OCT (Sigma‐Aldrich), snap‐frozen in liquid nitrogen‐cooled isopentane and stored at −80°C.
Macrophage depletion
Six‐week‐old C57BL/6 mice were injected intraperitoneally with clodronate liposomes (Clodro‐lip) or PBS liposomes (PBS‐lip) every 4 days (initial dose 1.5 mg/20 g mouse, then every fourth day 0.8 mg/20 g mouse) for 4 weeks as described [25]. During the fourth week of treatment, mice were used for the FGF2‐Matrigel assay as described above and killed by cervical dislocation 3 days after the last clodronate liposome injection.
Immunofluorescence analysis
Eight micrometres frozen sections of Matrigel plugs were fixed in ice‐cold acetone. After blocking with 10% goat serum in Tris‐buffered saline, sections were stained with rat IgG2b monoclonal antibodies raised against mouse CD45, mouse F4/80 or mouse CD11b (all at 1/100 dilution, Serotec, Martinsried/Planegg, Germany), followed by incubation with FITC‐conjugated goat anti‐rat IgG antibody (1/100 dilution, Santa Cruz, Biotechnology, Santa Cruz, CA, USA). Alternatively, to evaluate microvessel density, sections were incubated with rat IgG2a antimouse CD31 monoclonal antibody followed by incubation with biotinylated mouse anti‐rat IgG1/2a antibody (both at 1/100 dilution, BD Pharmingen, San Diego, CA, USA) and Texas red avidin (1/800 dilution, Vector Laboratories, Inc., Burlingame, CA, USA). Nuclei were counterstained by 4′,6‐diamidino‐2‐phenylindole (Sigma‐Aldrich). For imaging analysis, CD31 immunostaining was performed on F4/80‐pre‐stained sections. Images were acquired using an epifluorescence microscope (Zeiss, Inc., Jena, Germany) equipped with an Olympus N547 digital camera (Olympus, Hamburg, Germany) at ×200 magnification.
Imaging and statistical analysis
Experimental groups included at least five mice. The Matrigel regions containing the most intense CD31+ areas of neovascularization (‘hotspots’) were chosen for quantification. Five hotspots per Matrigel section and two sections per Matrigel plug were analysed. The ImagePro Plus analysis system was used to measure CD31+ and F4/80+ areas in each hotspot. Statistical analysis was performed with two‐tailed Student's t‐test. Differences were considered statistically significant at P < 0.05.
Chicken embryo chorioallantoic membrane (CAM) assay
Alginate beads (5 μl) containing the sample under test were placed on top of the CAM of fertilized White Leghorn chicken eggs at day 11 of incubation [26]. After 72 hrs, blood microvessels entering the implants within the focal plane of the CAM were counted in ovo at ×5 magnification using a STEMI SR stereomicroscope equipped with an objective f equal to 100 mm with adapter ring 475070 (Zeiss). Then, the CAMs were cut, fixed with 4% paraformaldehyde and stained May Grünwald‐Giemsa to visualize the inflammatory infiltrate. The experiments were repeated at least twice with 7–10 eggs per group.
Results
Transcriptional profiling of FGF2‐stimulated murine microvascular endothelial cells reveals a pro‐inflammatory signature
To assess the effect of FGF2 on the transcriptional profile of microvascular endothelium, confluent monolayers of mouse lung capillary endothelial 1G11 cells were stimulated for 10 hrs with 30 ng/ml FGF2 in low‐serum culture medium. The transcriptional profile was then determined by microarray analysis using Affymetrix MOE4303A genechips (consisting of 22,690 probe sets, corresponding to approximately 15,000 genes) and compared to that of unstimulated cells.
FGF2 treatment exerts a significant impact on the microvascular endothelial cell transcriptome. Indeed, 239 FGF2‐modulated genes were identified by combining twofold change filtering with statistical significance analysis. Among these genes, 146 transcripts were up‐regulated following FGF2 stimulation whereas 93 genes were down‐regulated. Most of the FGF2‐modulated transcripts correspond to annotated genes whereas 14 genes were unidentified or hypothetical (a comprehensive list of all the differentially expressed genes is provided in Table 2).
Table 2.
FGF2‐regulated genes in microvascular 1G11 endothelial cells
| FGF2‐upregulated genes (fold change > 2; P < 0.05) | ||||
|---|---|---|---|---|
| Fold change | Gene symbol | Gene name | Unigene ID | Affymetrix ID |
| 21.5 | Mmp13 | Matrix metallopeptidase 13 | Mm.5022 | 1417256_at |
| 18.5 | Ptgs2 | Prostaglandin‐endoperoxide synthase 2 | Mm.292547 | 1417262_at |
| 14.3 | 1417263_at | |||
| 16.4 | Sprr1a | Small proline‐rich protein 1A | Mm.331191 | 1449133_at |
| 13.4 | Prl2c2 | Prolactin family 2, subfamily c, member 2 | Mm.88796 | 1427760_s_at |
| 11.1 | Spp1 | Secreted phosphoprotein 1 | Mm.288474 | 1449254_at |
| 8.4 | Hbegf | Heparin‐binding EGF‐like growth factor | Mm.289681 | 1418350_at |
| 7.1 | 1418349_at | |||
| 8.1 | Ereg | Epiregulin | Mm.4791 | 1419431_at |
| 7.8 | Sgk | Serum/glucocorticoid regulated kinase | Mm.28405 | 1416041_at |
| 7.7 | Hmga2 | High mobility group AT‐hook 2 | Mm.157190 | 1450780_s_at |
| 7.3 | 1422851_at | |||
| 5.8 | 1450781_at | |||
| 7.0 | Prkg2 | Protein kinase, cGMP‐dependent, type II | Mm.263002 | 1435162_at |
| 6.0 | 1600029D21Rik | RIKEN cDNA 1600029D21 gene | Mm.29959 | 1423933_a_at |
| 6.0 | Cd44 | CD44 antigen | Mm.423621 | 1452483_a_at |
| 4.9 | 1423760_at | |||
| 6.0 | Errfi1 | ERBB receptor feedback inhibitor 1 | Mm.318841 | 1416129_at |
| 2.6 | 1419816_s_at | |||
| 5.7 | 1810011O10Rik | RIKEN cDNA 1810011O10 gene | Mm.25775 | 1451415_at |
| 5.6 | Serpinb2 | Serine (or cysteine) peptidase inhibitor, clade B, member 2 | Mm.271870 | 1419082_at |
| 5.5 | Egr2 | Early growth response 2 | Mm.290421 | 1427683_a_at |
| 4.9 | 1427682_at | |||
| 5.0 | Ivl | Involucrin | Mm.207365 | 1422222_at |
| 4.9 | Tnfrsf23 | Tumour necrosis factor receptor superfamily, member 23 | Mm.290780 | 1422101_at |
| 4.8 | Ptger4 | Prostaglandin E receptor 4 (subtype EP4) | Mm.18509 | 1424208_at |
| 4.1 | 1421073_a_at | |||
| 4.2 | Fosl1 | Fos‐like antigen 1 | Mm.6215 | 1417487_at |
| 3.2 | 1417488_at | |||
| 4.2 | Tnfrsf22 | Tumour necrosis factor receptor superfamily, member 22 | Mm.261384 | 1422039_at |
| 3.1 | 1422038_a_at | |||
| 2.1 | 1426095_a_at | |||
| 4.1 | Mgp | Matrix Gla protein | Mm.243085 | 1448416_at |
| 4.0 | Edg1 | Endothelial differentiation sphingolipid G‐protein‐coupled receptor 1 | Mm.982 | 1423571_at |
| 3.8 | Hmga1 | High mobility group AT‐hook 1 | Mm.4438 | 1416184_s_at |
| 3.8 | Il6 | Interleukin 6 | Mm.1019 | 1450297_at |
| 3.8 | Arhgap6 | Rho GTPase activating protein 6 | Mm.441810 | 1451867_×_at |
| 2.7 | 1456333_a_at | |||
| 2.6 | 1417704_a_at | |||
| 3.6 | Ier2 | Immediate early response 2 | Mm.399 | 1416442_at |
| 3.6 | Metrnl | Meteorin, glial cell differentiation regulator‐like | Mm.153566 | 1424356_a_at |
| 3.5 | Egr1 | Early growth response 1 | Mm.181959 | 1417065_at |
| 3.5 | Ccl2 | Chemokine (C‐C motif) ligand 2 | Mm.290320 | 1420380_at |
| 3.5 | Gfpt2 | Glutamine fructose‐6‐phosphate transaminase 2 | Mm.24402 | 1418753_at |
| 3.4 | Ccrn4l | CCR4 carbon catabolite repression 4‐like (S. Cerevisiae) | Mm.86541 | 1425837_a_at |
| 3.4 | Pvr | Poliovirus receptor | Mm.227506 | 1450295_s_at |
| 3.3 | 1423905_at | |||
| 2.8 | 1423903_at | |||
| 2.7 | 1451160_s_at | |||
| 3.3 | Myc | Myelocytomatosis oncogene | Mm.2444 | 1424942_a_at |
| 3.2 | A030007L17Rik | RIKEN cDNA A030007L17 gene | Mm.294708 | 1435695_a_at |
| 3.2 | Plaur | Plasminogen activator, urokinase receptor | Mm.1359 | 1452521_a_at |
| 3.2 | Dusp6 | Dual specificity phosphatase 6 | Mm.1791 | 1415834_at |
| 3.2 | Hk2 | Hexokinase 2 | Mm.255848 | 1422612_at |
| 3.2 | Slc2a1 | Solute carrier family 2 (facilitated glucose transporter), member 1 | Mm.21002 | 1426599_a_at |
| 3.1 | 1426600_at | |||
| 2.8 | 1434773_a_at | |||
| 3.1 | Ccl7 | Chemokine (C‐C motif) ligand 7 | Mm.341574 | 1421228_at |
| 3.1 | Serpine1 | Serine (or cysteine) peptidase inhibitor, clade E, member 1 | Mm.250422 | 1419149_at |
| 3.1 | Gch1 | GTP cyclohydrolase 1 | Mm.10651 | 1420499_at |
| 3.1 | 1429692_s_at | |||
| 3.1 | Vcam1 | Vascular cell adhesion molecule 1 | Mm.76649 | 1451314_a_at |
| 2.6 | 1415989_at | |||
| 3.1 | Mmd | Monocyte to macrophage differentiation associated | Mm.277518 | 1423489_at |
| 2.6 | 1423488_ats | |||
| 3.1 | Pdgfb | Platelet‐derived growth factor, B polypeptide | Mm.144089 | 1450414_at |
| 2.1 | 1450413_at | |||
| 3.0 | Slc4a7 | Solute carrier family 4, sodium bicarbonate cotransporter, member 7 | Mm.258893 | 1438673_at |
| 3.0 | Slit2 | Slit homolog 2 (Drosophila) | Mm.289739 | 1424659_at |
| 2.9 | Junb | Jun‐B oncogene | Mm.1167 | 1415899_at |
| 2.9 | Nr4a1 | Nuclear receptor subfamily 4, group A, member 1 | Mm.119 | 1416505_at |
| 2.9 | Ell2 | Elongation factor RNA polymerase II 2 | Mm.21288 | 1450744_at |
| 2.9 | Tnfrsf12a | Tumour necrosis factor receptor superfamily, member 12a | Mm.28518 | 1418571_at |
| 2.9 | 1418572_×_at | |||
| 2.9 | Rgs2 | Regulator of G‐protein signalling 2 | Mm.28262 | 1419248_at |
| 2.6 | 1419247_at | |||
| 2.9 | Ankrd1 | Ankyrin repeat domain 1 (cardiac muscle) | Mm.10279 | 1420991_at |
| 2.3 | 1420992_at | |||
| 2.9 | Sox9 | SRY‐box containing gene 9 | Mm.286407 | 1424950_at |
| 2.1 | 1451538_at | |||
| 2.8 | F3 | Coagulation factor III | Mm.273188 | 1417408_at |
| 2.8 | Grem1 | Gremlin 1 | Mm.458492 | 1425357_a_at |
| 2.8 | Ifrd1 | Interferon‐related developmental regulator 1 | Mm.168 | 1416067_at |
| 2.7 | Antxr2 | Anthrax toxin receptor 2 | Mm.24842 | 1426708_at |
| 2.7 | Areg | Amphiregulin | Mm.8039 | 1421134_at |
| 2.7 | Nmt2 | N‐myristoyltransferase 2 | Mm.65021 | 1423581_at |
| 2.7 | Runx1 | Runt‐related transcription factor 1 | Mm.4081 | 1422864_at |
| 2.4 | 1422865_at | |||
| 2.7 | Thbs1 | Thrombospondin 1 | Mm.4159 | 1450377_at |
| 2.3 | 1460302_at | |||
| 2.7 | Lrig1 | Leucine‐rich repeats and immunoglobulin‐like domains 1 | Mm.245210 | 1449893_a_at |
| 2.3 | 1434210_s_at | |||
| 2.6 | Car8 | Carbonic anhydrase 8 | Mm.119320 | 1427482_a_at |
| 2.6 | Clcf1 | cardiotrophin‐like cytokine factor 1 | Mm.347919 | 1437270_a_at |
| 2.6 | Tnnt2 | Troponin T2, cardiac | Mm.247470 | 1418726_a_at |
| 2.4 | 1424967_×_at | |||
| 2.6 | Steap1 | Six transmembrane epithelial antigen of the prostate 1 | Mm.85429 | 1424938_at |
| 2.3 | 1451532_s_at | |||
| 2.5 | Map3k6 | Mitogen‐activated protein kinase kinase kinase 6 | Mm.36640 | 1449901_a_at |
| 2.5 | Fxyd5 | FXYD domain‐containing ion transport regulator 5 | Mm.1870 | 1418296_at |
| 2.5 | Timp1 | Tissue inhibitor of metalloproteinase 1 | Mm.8245 | 1460227_at |
| 2.5 | Baiap2l1 | BAI1‐associated protein 2‐like 1 | Mm.18814 | 1424951_at |
| 2.5 | Adam19 | A disintegrin and metallopeptidase domain 19 (meltrin β | Mm.89940 | 1418403_at |
| 2.4 | 1418402_at | |||
| 2.5 | 1200016E24Rik | RIKEN cDNA 1200016E24 gene | Mm.332931 | 1435138_s_at |
| 2.3 | 1453237_s_at | |||
| 2.5 | Ero1l | ERO1‐like (S. cerevisiae) | Mm.387108 | 1419030_at |
| 2.1 | 1419029_at | |||
| 2.2 | 1449324_at | |||
| 2.4 | Tec | Cytoplasmic tyrosine kinase, Dscr28C related (Drosophila) | Mm.319581 | 1460204_at |
| 2.4 | Rhoj | RAS homolog gene family, member J | Mm.27467 | 1418892_at |
| 2.4 | Lyve1 | Lymphatic vessel endothelial hyaluronan receptor 1 | Mm.396078 | 1429379_at |
| 2.4 | Slc20a2 | Solute carrier family 20, member 2 | Mm.323901 | 1434235_at |
| 2.4 | Slc25a37 | Solute carrier family 25, member 37 | Mm.293635 | 1417750_a_at |
| 2.4 | Wisp1 | WNT1 inducible signalling pathway protein 1 | Mm.10222 | 1448594_at |
| 2.3 | 1448593_at | |||
| 2.4 | Cp | Ceruloplasmin | Mm.13787 | 1455393_at |
| 2.1 | 1417495_×_at | |||
| 2.1 | 1417496_at | |||
| 2.0 | 1448734_at | |||
| 2.4 | Tes | Testis‐derived transcript | Mm.436548 | 1460378_a_at |
| 2.1 | 1424246_a_at | |||
| 2.3 | Calcrl | Calcitonin receptor like | Mm.75467 | 1425814_a_at |
| 2.3 | Dfna5h | Deafness, autosomal dominant 5 homolog (human) | Mm.248361 | 1421534_at |
| 2.3 | Serpina3n | Serine (or cysteine) peptidase inhibitor, clade A, member 3N | Mm.22650 | 1419100_at |
| 2.3 | Tnfaip8 | Tumour necrosis factor, ·‐induced protein 8 | Mm.27740 | 1416950_at |
| 2.3 | Fgf2 | Fibroblast growth factor 2 | Mm.457975 | 1449826_a_at |
| 2.3 | Lif | Leukaemia inhibitory factor | Mm.4964 | 1421207_at |
| 2.3 | Plk2 | Polo‐like kinase 2 (Drosophila) | Mm.380 | 1427005_at |
| 2.3 | Ptpre | Protein tyrosine phosphatase, receptor type, E | Mm.945 | 1418540_a_at |
| 2.3 | Il1rap | Interleukin 1 receptor accessory protein | Mm.253424 | 1421844_at |
| 2.3 | Ctgf | Connective tissue growth factor | Mm.393058 | 1416953_at |
| 2.3 | Phlda1 | Pleckstrin homology‐like domain, family A, member 1 | Mm.3117 | 1418835_at |
| 2.3 | Oaf | OAF homolog (Drosophila) | Mm.246479 | 1424086_at |
| 2.3 | Jam2 | Junction adhesion molecule 2 | Mm.41758 | 1419288_at |
| 2.3 | 1431416_a_at | |||
| 2.1 | 1449408_at | |||
| 2.3 | Slc12a2 | Solute carrier family 12, member 2 | Mm.399997 | 1417622_at |
| 2.2 | 1417623_at | |||
| 2.3 | Hsd17b7 | Hydroxysteroid (17‐β) dehydrogenase 7 | Mm.12882 | 1417871_at |
| 2.2 | 1448865_at | |||
| 2.3 | Tnfaip2 | Tumour necrosis factor, α‐induced protein 2 | Mm.255332 | 1438855_×_at |
| 2.1 | 1416273_at | |||
| 2.2 | Por | P450 (cytochrome) oxidoreductase | Mm.3863 | 1416933_at |
| 2.2 | Nfil3 | Nuclear factor, interleukin 3, regulated | Mm.136604 | 1418932_at |
| 2.2 | Tgfb1 | Transforming growth factor, β1 | Mm.248380 | 1420653_at |
| 2.2 | Trib2 | Tribbles homolog 2 (Drosophila) | Mm.266679 | 1426641_at |
| 2.2 | Fli1 | Friend leukaemia integration 1 | Mm.258908 | 1433512_at |
| 2.2 | Etv4 | Ets variant gene 4 (E1A enhancer binding protein, E1AF) | Mm.5025 | 1423232_at |
| 2.2 | Btg1 | B‐cell translocation gene 1, anti‐proliferative | Mm.272183 | 1426083_a_at |
| 2.2 | Tgfa | Transforming growth factor α | Mm.137222 | 1421943_at |
| 2.2 | Rai14 | Retinoic acid induced 14 | Mm.212395 | 1417401_at |
| 2.2 | Osmr | Oncostatin M receptor | Mm.10760 | 1418674_at |
| 2.1 | 1418675_at | |||
| 2.2 | Rasa1 | RAS p21 protein activator 1 | Mm.259653 | 1426477_at |
| 2.0 | 1426476_at | |||
| 2.2 | Gprc5b | G protein‐coupled receptor, family C, group 5, member B | Mm.103439 | 1451411_at |
| 2.0 | 1424613_at | |||
| 2.1 | Hmgcr | 3‐hydroxy‐3‐methylglutaryl‐Coenzyme A reductase | Mm.316652 | 1427229_at |
| 2.1 | Grwd1 | Glutamate‐rich WD repeat containing 1 | Mm.274847 | 1455841_s_at |
| 2.1 | Lrrc8c | Leucine‐rich repeat containing 8 family, member C | Mm.319847 | 1423614_at |
| 2.1 | Chst11 | Carbohydrate sulfotransferase 11 | Mm.360747 | 1450509_at |
| 2.1 | Fos | FBJ osteosarcoma oncogene | Mm.246513 | 1423100_at |
| 2.1 | Tnfsf9 | Tumour necrosis factor (ligand) superfamily, member 9 | Mm.41171 | 1422924_at |
| 2.1 | Run×2 | Runt‐related transcription factor 2 | Mm.391013 | 1424704_at |
| 2.1 | Cyb561 | Cytochrome b‐561 | Mm.149403 | 1417507_at |
| 2.1 | Mpp6 | Membrane protein, palmitoylated 6 (MAGUK p55 subfamily member 6) | Mm.41288 | 1449348_at |
| 2.1 | B4galt6 | UDP‐Gal: βGlcNAc β 1,4‐galactosyltransferase, polypeptide 6 | Mm.398181 | 1460329_at |
| 2.1 | Spred1 | Sprouty protein with EVH‐1 domain 1, related sequence | Mm.392726 | 1423161_s_at |
| 2.1 | Efnb2 | Ephrin B2 | Mm.209813 | 1449548_at |
| 2.1 | 1419639_at | |||
| 2.0 | 1419638_at | |||
| 2.1 | Enc1 | Ectodermal‐neural cortex 1 | Mm.241073 | 1450061_at |
| 2.1 | 1420965_a_at | |||
| 2.0 | Snai1 | Snail homolog 1 (Drosophila) | Mm.2093 | 1448742_at |
| 2.0 | Maff | v‐maf musculoaponeurotic fibrosarcoma oncogene family, protein F (avian) | Mm.86646 | 1418936_at |
| 2.0 | Rab20 | RAB20, member RAS oncogene family | Mm.390014 | 1438097_at |
| 2.0 | Chka | Choline kinase α | Mm.225505 | 1450264_a_at |
| 2.0 | Mthfd2 | Methylenetetrahydrofolate dehydrogenase (NAD+ dependent), methenyltetrahydrofolate cyclohydrolase | Mm.443 | 1419254_at |
| 2.0 | Slc20a1 | Solute carrier family 20, member 1 | Mm.457995 | 1448568_a_at |
| 2.0 | 2010002N04Rik | RIKEN cDNA 2010002N04 gene | Mm.273197 | 1423306_at |
| 2.0 | Slco3a1 | Solute carrier organic anion transporter family, member 3a1 | Mm.425467 | 1418030_at |
| 2.0 | C×3cl1 | Chemokine (C‐X3‐C motif) ligand 1 | Mm.103711 | 1415803_at |
| 2.0 | Flnb | filamin, β | Mm.28095 | 1426750_at |
| 2.0 | 1810054D07Rik | RIKEN cDNA 1810054D07 gene | Mm.5540 | 1440192_at |
| 2.0 | Ifi204 | Interferon activated gene 204 | Mm.442561 | 1419603_at |
| 2.0 | Itga5 | Integrin α 5 (fibronectin receptor α) | Mm.16234 | 1423267_s_at |
| 2.0 | Klf10 | Kruppel‐like factor 10 | Mm.4292 | 1416029_at |
| 2.0 | Ier3 | Immediate early response 3 | Mm.25613 | 1419647_a_at |
| 2.0 | Spred2 | Sprouty‐related, EVH1 domain containing 2 | Mm.266627 | 1434403_at |
| 2.0 | Cyp51 | Cytochrome P450, family 51 | Mm.140158 | 1422533_at |
| 2.0 | Ier5 | Immediate early response 5 | Mm.12246 | 1417612_at |
| 2.0 | Eea1 | Early endosome antigen 1 | Mm.210035 | 1438045_at |
| 2.0 | Cdca7 | Cell division cycle associated 7 | Mm.270676 | 1428069_at |
| 2.0 | Stc2 | Stanniocalcin 2 | Mm.32506 | 1449484_at |
| 2.0 | Acsl4 | Acyl‐CoA synthetase long‐chain family member 4 | Mm.391337 | 1433531_at |
| 2.0 | Cxcl16 | Chemokine (C‐X‐C motif) ligand 16 | Mm.441411 | 1418718_at |
| 2.0 | 1449195_s_at | |||
| −5.7 | Mettl7a | Methyltransferase like 7A | Mm.220975 | 1434150_a_at |
| −5.0 | 1434151_at | |||
| −4.9 | 1454858_×_at | |||
| −4.2 | 1421184_a_at | |||
| −5.1 | Akr1c14 | Aldo‐keto reductase family 1, member C14 | Mm.26838 | 1418979_at |
| −4.4 | Dbp | D site albumin promoter binding protein | Mm.378235 | 1438211_s_at |
| −2.7 | 1418174_at | |||
| −3.8 | Sord | Sorbitol dehydrogenase | Mm.371580 | 1426584_a_at |
| −2.5 | 1438183_×_at | |||
| −3.5 | Sesn1 | Sestrin 1 | Mm.139418 | 1454699_at |
| −3.3 | 1438931_s_at | |||
| −3.0 | 1433711_s_at | |||
| −3.3 | Sncaip | Synuclein, α interacting protein (synphilin) | Mm.292168 | 1423499_at |
| −3.1 | — | Transcribed locus | Mm.391736 | 1455582_at |
| −3.1 | Rab40b | Rab40b, member RAS oncogene family | Mm.281639 | 1436566_at |
| −3.1 | Angptl7 | Angiopoietin‐like 7 | Mm.388929 | 1451478_at |
| −3.3 | Tgm2 | Transglutaminase 2, C polypeptide | Mm.330731 | 1455900_×_at |
| −3.1 | 1417500_a_at | |||
| −3.1 | 1433428_×_at | |||
| −3.1 | 1437277_×_at | |||
| −2.7 | 1426004_a_at | |||
| −3.0 | Ptplad2 | Protein tyrosine phosphatase‐like A domain containing 2 | Mm.386788 | 1450967_at |
| −3.0 | Mapre2 | Microtubule‐associated protein, RP/EB family, member 2 | Mm.132237 | 1451989_a_at |
| −3.0 | Unc119 | Unc‐119 homolog (C. elegans) | Mm.284811 | 1418123_at |
| −3.0 | AW548124 | Expressed sequence AW548124 | Mm.311974 | 1454838_s_at |
| −2.4 | 1460411_s_at | |||
| −2.9 | Rasl11b | RAS‐like, family 11, member B | Mm.293316 | 1423854_a_at |
| −2.9 | D0H4S114 | DNA segment, human D4S114 | Mm.407415 | 1436736_×_at |
| −2.8 | 1450839_at | |||
| −2.8 | Bnc1 | Basonuclin 1 | Mm.243802 | 1424890_at |
| −2.8 | Sfrp2 | Secreted frizzled‐related protein 2 | Mm.19155 | 1448201_at |
| −2.8 | Ogn | Osteoglycin | Mm.4258 | 1419663_at |
| −2.5 | 1419662_at | |||
| −2.7 | Antxr1 | Anthrax toxin receptor 1 | Mm.232525 | 1451446_at |
| −2.7 | Trp53inp1 | Transformation‐related protein 53 inducible nuclear protein 1 | Mm.393018 | 1416926_at |
| −2.3 | 1416927_at | |||
| −2.6 | Slc1a6 | Solute carrier family 1 (high‐affinity aspartate/glutamate transporter), member 6 | Mm.6257 | 1418933_at |
| −2.6 | Fhl1 | Four and a half LIM domains 1 | Mm.3126 | 1417872_at |
| −2.6 | Pkia | Protein kinase inhibitor, α | Mm.3193 | 1420858_at |
| −2.6 | 1420859_at | |||
| −2.6 | Ddit4l | DNA‐damage‐inducible transcript 4‐like | Mm.250841 | 1451751_at |
| −2.4 | 1439332_at | |||
| −2.6 | Vldlr | Very low density lipoprotein receptor | Mm.4141 | 1417900_a_at |
| −2.3 | 1434465_×_at | |||
| −2.6 | Fzd2 | Frizzled homolog 2 (Drosophila) | Mm.36416 | 1418534_at |
| −2.1 | 1418532_at | |||
| −2.1 | 1418533_s_at | |||
| −2.5 | Pdlim3 | PDZ and LIM domain 3 | Mm.282900 | 1449178_at |
| −2.5 | Dhrs3 | Dehydrogenase/reductase (SDR family) member 3 | Mm.14063 | 1448390_a_at |
| −2.5 | Ephx2 | Epoxide hydrolase 2, cytoplasmic | Mm.15295 | 1448499_a_at |
| −2.5 | Gab1 | Growth factor receptor bound protein 2‐associated protein 1 | Mm.277409 | 1417694_at |
| −2.3 | 1417693_a_at | |||
| −2.4 | Zfp521 | Zinc finger protein 521 | Mm.40325 | 1451332_at |
| −2.4 | Sspn | Sarcospan | Mm.49689 | 1417644_at |
| −2.4 | Oplah | 5‐oxoprolinase (ATP‐hydrolysing) | Mm.322738 | 1424359_at |
| −2.4 | Mcam | Melanoma cell adhesion molecule | Mm.275003 | 1416357_a_at |
| −2.4 | Figf | C‐Fos induced growth factor | Mm.297978 | 1438954_×_at |
| 2.3 | 1438953_at | |||
| −2.3 | Npr3 | Natriuretic peptide receptor 3 | Mm.25259 | 1435184_at |
| −2.3 | Mmp11 | Matrix metallopeptidase 11 | Mm.4561 | 1417234_at |
| −2.3 | Tek | Endothelial‐specific receptor tyrosine kinase | Mm.14313 | 1418788_at |
| −2.3 | Wdr6 | WD repeat domain 6 | Mm.335454 | 1415770_at |
| −2.3 | 1455940_×_at | |||
| −2.2 | Adamts5 | A disintegrin‐like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 5 (aggrecanase‐2) | Mm.112933 | 1422561_at |
| −2.2 | Pdgfra | Platelet‐derived growth factor receptor, α polypeptide | Mm.221403 | 1421917_at |
| −2.2 | Appl2 | Adaptor protein, phosphotyrosine interaction, PH domain and leucine zipper containing 2 | Mm.282985 | 1426743_at |
| −2.2 | Stard10 | START domain containing 10 | Mm.28896 | 1448956_at |
| −2.2 | Fgf18 | Fibroblast growth factor 18 | Mm.339812 | 1449545_at |
| −2.2 | Apbb1 | Amyloid, (A4) precursor protein‐binding, family B, member 1 | Mm.38469 | 1423893_×_at |
| −2.2 | Palm | Paralemmin | Mm.34650 | 1423967_at |
| −2.2 | Slc25a23 | Solute carrier family 25 (mitochondrial carrier; phosphate carrier), member 23 | Mm.23720 | 1419045_at |
| −2.2 | Igf1 | Insulin‐like growth factor 1 | Mm.268521 | 1437401_at |
| −2.2 | Serpinb9 | Serine (or cysteine) peptidase inhibitor, clade B, member 9 | Mm.272569 | 1422601_at |
| −2.2 | Gamt | Guanidinoacetate methyltransferase | Mm.7329 | 1422558_at |
| −2.2 | Klhl13 | Kelch‐like 13 (Drosophila) | Mm.224306 | 1448269_a_at |
| −2.0 | 1416242_at | |||
| −2.2 | Acaa2 | Acetyl‐Coenzyme A acyltransferase 2 (mitochondrial 3‐oxoacyl‐Coenzyme A thiolase) | Mm.245724 | 1455061_a_at |
| −2.0 | 1428146_s_at | |||
| −2.1 | Slc39a8 | Solute carrier family 39 (metal ion transporter), member 8 | Mm.30239 | 1416832_at |
| −2.1 | Dzip1 | DAZ interacting protein 1 | Mm.87456 | 1452792_at |
| −2.1 | Aldh1a7 | Aldehyde dehydrogenase family 1, subfamily A7 | Mm.14609 | 1418601_at |
| −2.1 | 2310002J21Rik | RIKEN cDNA 2310002J21 gene | Mm.375091 | 1456393_at |
| −2.1 | Pmp22 | Peripheral myelin protein | Mm.1237 | 1417133_at |
| −2.1 | Ifit3 | Interferon‐induced protein with tetratricopeptide repeats 3 | Mm.426079 | 1449025_at |
| −2.1 | Tsc22d3 | TSC22 domain family 3 | Mm.22216 | 1425281_a_at |
| −2.1 | 6720475J19Rik | RIKEN cDNA 6720475J19 gene | Mm.273536 | 1423072_at |
| −2.1 | Rtn1 | Reticulon 1 | Mm.221275 | 1429761_at |
| −2.1 | Pdcd4 | Programmed cell death 4 | Mm.1605 | 1418840_at |
| −2.1 | Cables1 | Cdk5 and Abl enzyme substrate 1 | Mm.40717 | 1422477_at |
| −2.1 | Reck | Reversion‐inducing cysteine‐rich protein with kazal motifs | Mm.331573 | 1450784_at |
| −2.1 | Gsta3 | Glutathione S‐transferase, α3 | Mm.394593 | 1423436_at |
| −2.1 | Myl7 | Myosin, light polypeptide 7, regulatory | Mm.46514 | 1449071_at |
| −2.1 | Cd200 | Cd200 antigen | Mm.245851 | 1448788_at |
| −2.1 | 1200015N20Rik | RIKEN cDNA 1200015N20 gene | Mm.19825 | 1448557_at |
| −2.1 | Ptprd | Protein tyrosine phosphatase, receptor type, D | Mm.184021 | 1429052_at |
| −2.1 | Dcxr | Dicarbonyl L‐xylulose reductase | Mm.231091 | 1419456_at |
| −2.1 | Gadd45b | Growth arrest and DNA‐damage‐inducible 45 β | Mm.1360 | 1450971_at |
| −2.1 | 1449773_s_at | |||
| −2.0 | Atp6v0e2 | ATPase, H+ transporting, lysosomal V0 subunit E2 | Mm.458098 | 1448211_at |
| −2.0 | 4930570C03Rik | RIKEN cDNA 4930570C03 gene | Mm.28955 | 1450410_a_at |
| −2.0 | Sorbs1 | Sorbin and SH3 domain containing 1 | Mm.210815 | 1436737_a_at |
| −2.0 | Add3 | Adducin 3 (γ) | Mm.426080 | 1423298_at |
| −2.0 | Hadh | Hydroxyacyl‐Coenzyme A dehydrogenase | Mm.260164 | 1460184_at |
| −2.0 | Oprl1 | Opioid receptor‐like 1 | Mm.285075 | 1450486_a_at |
| −2.0 | Armcx3 | Armadillo repeat containing, X‐linked 3 | Mm.67949 | 1460359_at |
| −2.0 | Klhl24 | Kelch‐like 24 (Drosophila) | Mm.392914 | 1451793_at |
| −2.0 | — | Transcribed locus | Mm.275414 | 1454967_at |
| −2.0 | Zfp810 | Zinc finger protein 810 | Mm.306038 | 1451566_at |
| −2.0 | Pdk2 | Pyruvate dehydrogenase kinase, isoenzyme 2 | Mm.29768 | 1448825_at |
| −2.0 | Abhd14b | Abhydrolase domain containing 14b | Mm.335427 | 1451326_at |
| −2.0 | Mxd4 | Max dimerization protein 4 | Mm.391777 | 1434378_a_at |
| −2.0 | Marcksl1 | MARCKS‐like 1 | Mm.424974 | 1437226_x_at |
| −2.0 | Bckdha | Branched chain ketoacid dehydrogenase E1, α polypeptide | Mm.25848 | 1416647_at |
| −2.0 | Stk17b | Serine/threonine kinase 17b (apoptosis‐inducing) | Mm.25559 | 1450997_at |
| −2.0 | Spag5 | Sperm associated antigen 5 | Mm.24250 | 1433893_s_at |
| −2.0 | Aldoc | Aldolase 3, C isoform | Mm.7729 | 1451461_a_at |
| −2.0 | Tuft1 | Tuftelin 1 | Mm.10214 | 1416689_at |
| −2.0 | Tmem9 | Transmembrane protein 9 | Mm.41773 | 1419557_a_at |
| −2.0 | Calml4 | Calmodulin‐like 4 | Mm.440576 | 1424713_at |
The fold change, the official gene symbol and name, the Unigene cluster and the Affymetrix probe set ID number are shown. Note that various genes are interrogated by more than one probe set in the Affymetrix MOE430A genechip.
To gather biological information about the FGF2‐regulated transcriptional profile, we performed data mining on the web‐based database DAVID [22] that provides functional genomic annotations according to GO.
The most over‐represented GO terms, based on their statistical significance, were ‘extracellular space’ (cellular component), ‘receptor binding/growth factor activity’ (molecular function) and ‘blood vessel morphogenesis/angiogenesis’ (biological process) (Table 3). Accordingly, data mining on published research literature revealed a clear trend towards the process of new blood vessel formation (Table 4). Indeed, several FGF2‐upregulated genes encode for angiogenesis‐promoting extracellular factors and cytokines, including Fgf2 itself, heparin‐binding EGF‐like growth factor (Hb‐egf), prolactin family 2, subfamily c, member 2/Proliferin (Prl2c2/Plf), slit homolog‐2 (Slit2), transforming growth factor‐b1 (Tgfb1) and Tgfa, ephrin‐B2 (EfnB2), Drm/gremlin‐1, Ccl2, C×3cl1, C×cl16, platelet‐derived growth factor‐b (Pdgf‐b), IL‐6 and connective tissue growth factor (Ctgf). Angiogenesis‐related genes were also found in the categories of membrane receptors [anthrax toxin receptor‐2 (Antxr2), calcitonin receptor‐like receptor (Calcrl), integrin α5 (Itg5), endothelial differentiation sphingolipid G‐protein‐coupled receptor‐1 (Edg1), lymphatic vessel endothelial hyaluronan receptor 1 (Lyve1), prostaglandin E receptor 4 (Ptger4), urokinase‐type plasminogen activator receptor (Plaur), coagulation factor III (F3)], transcriptional regulators [(early growth response 1 (Egr1), runt‐related transcription factor (Runx) 1 and 2, ankyrin repeat domain 1 (Ankrd1)], cell adhesion molecules [CD44 antigen], proteases and their inhibitors [matrix metallopeptidase 13 (Mmp13), serpine1, serpinb2, tissue inhibitor of metalloproteinase 1 (Timp1)] (Table 4).
Table 3.
Over‐represented GO terms for FGF2‐upregulated genes in murine microvascular 1G11 cells
| GO term | Number of genes | % | Statistical significance |
|---|---|---|---|
| Molecular function | |||
| Receptor binding | 34 | 12.1 | 1.0E‐07 |
| Growth factor activity | 18 | 6.4 | 1.1E‐07 |
| Cytokine activity | 19 | 6.8 | 2.5E‐06 |
| Cellular component | |||
| Extracellular space | 69 | 24.6 | 2.5E‐04 |
| Extracellular region | 71 | 25.4 | 5.8E‐04 |
| Basolateral plasma membrane | 6 | 2.1 | 6.0E‐03 |
| Extracellular matrix | 10 | 3.6 | 5.2E‐02 |
| Biological process | |||
| Blood vessel morphogenesis | 19 | 6.8 | 2.2E‐09 |
| Angiogenesis | 16 | 5.7 | 4.5E‐08 |
| Ossification | 13 | 4.6 | 2.0E‐07 |
| Bone mineralization | 8 | 2.9 | 3.8E‐05 |
| Regulation of bone remodelling | 7 | 2.5 | 2.7E‐05 |
| Inflammatory response | 12 | 4.3 | 4.4E‐04 |
FGF2‐upregulated genes (see Table 2 for the detailed list of these genes) were classified in terms of their associated GO molecular functions, cellular components and biological processes. The most over‐represented GO terms, the number and percentage of genes belonging to each category, and the statistical significance are shown.
Table 4.
FGF2‐upregulated genes in murine microvascular 1G11 endothelial cells related to angiogenesis, bone formation, and inflammation
| Growth factors, cytokines and chemokines | Symbol | Biological Process | Fold change |
|---|---|---|---|
| Chemokine (C‐C motif) ligand 7 | Ccl7 | I | 3.1 |
| Chemokine (C‐X3‐C motif) ligand 1 | Cx3cl1 | A/I | 2.0 |
| Chemokine (C‐X‐C motif) ligand 1 | Cxcl1 | A/I | 2.0 |
| Chemokine (C‐X‐C motif) ligand 16 | Cxcl16 | A/I | 2.0 |
| Connective tissue growth factor | Ctgf | A/B | 2.3 |
| Ephrin B2 | Efnb2 | A/B | 2.1 |
| Fibroblast growth factor 2 | Fgf2 | A/B/I | 2.3 |
| Gremlin 1 | Grem1 | A | 3.1 |
| Heparin‐binding EGF‐like growth factor | Hbegf | A | 8.4 |
| Interleukin 6 | Il6 | A/B/I | 3.8 |
| Leukemia inhibitory factor | Lif | B/I | 2.3 |
| Platelet‐derived growth factor, B polypeptide | Pdgfb | A | 3.2 |
| Prolactin family 2, subfamily c, member 2/Proliferin | Prl2c2/Pfl | A | 13.5 |
| Secreted phosphoprotein 1/osteopontin | Spp1/Opn | A/B/I | 12.2 |
| Slit homolog 2 (Drosophila) | Slit2 | A/I | 3.0 |
| Thrombospondin 1 | Thbs1 | A/B/I | 2.7 |
| Transforming growth factorα | Tgfa | A | 2.2 |
| Transforming growth factor, β1 | Tgfb1 | A/B/I | 2.2 |
| Tumor necrosis factor, α‐induced protein 2 | Tnfaip2 | A | 2.3 |
| Membrane receptors and adhesion molecules | Symbol | Biological Process | Fold change |
|---|---|---|---|
| Anthrax toxin receptor 2 | Antxr2 | A | 2.7 |
| Calcitonin receptor‐like | Calcrl | A/B | 2.3 |
| CD44 antigen | Cd44 | A/I | 6.1 |
| Coagulation factor III | F3/Tf | A/I | 2.9 |
| Endothelial differentiation sphingolipid G‐protein‐coupled receptor 1 | Edg1 | A | 4.1 |
| Integrin α5 (fibronectin receptor α) | Itga5 | A | 2.0 |
| Interleukin 1 receptor accessory protein | Il1rap | I | 2.3 |
| Junction adhesion molecule 2 | Jam2 | I | 2.3 |
| Lymphatic vessel endothelial hyaluronan receptor 1 | Lyve1 | A | 2.4 |
| Oncostatin M receptor | Osmr | A/B/I | 2.2 |
| Plasminogen activator, urokinase receptor | Plaur | A/B/I | 3.2 |
| Prostaglandin E receptor 4 (subtype EP4) | Ptger4 | A/B/I | 4.9 |
| Tumor necrosis factor receptor superfamily, member 12a | Tnfrsf12a | A/B/I | 2.9 |
| Vascular cell adhesion molecule 1 | Vcam1 | I | 3.1 |
| Transcriptional regulators | Symbol | Biological Process | Fold change |
|---|---|---|---|
| Early growth response 1 | Egr1 | A | 3.4 |
| Early growth response 2 | Egr2 | B | 5.6 |
| Runt‐related transcription factor 1 | Runx1 | A/B | 2.8 |
| Runt‐related transcription factor 2 | Runx2 | A/B | 2.1 |
| Ankyrin repeat domain 1 (cardiac muscle) | Ankrd1 | A | 3.6 |
| Others | Symbol | Biological Process | Fold change |
|---|---|---|---|
| Matrix Gla protein | Mgp | B | 4.1 |
| Matrix metallopeptidase 13 | Mmp13 | A/B | 22.1 |
| Prostaglandin‐endoperoxide synthase 2 | Ptgs2/Cox‐2 | A/B/I | 18.5 |
| Serine (or cysteine) peptidase inhibitor, clade E, member 1 | Serpine1 | A/I | 3.0 |
| Serine (or cysteine) peptidase inhibitor, clade B, member 2 | Serpinb2 | A | 5.6 |
| Tissue inhibitor of metalloproteinase 1 | Timp1 | A/I | 2.5 |
Selected FGF2‐upregulated genes (fold change > 2, P < 0.05, see Table 2) were classified in terms of their association with angiogenesis (A), bone formation (B) and inflammation (I) processes, according to the GO database and bibliographic searching.
A second enriched biological category within the FGF2‐modulated gene list was related to the bone formation process (GO terms ‘ossification’, ‘bone mineralization’, ‘regulation of bone remodelling’) (Table 3), a process in which FGF2 has been shown to play a relevant role [27, 28]. Genes of this functional cluster include master regulators of osteoblast development and function as Tgfb1 and the transcriptional factor Runx2. Other genes related to bone remodelling were the prostaglandin receptor Ptger4, the transcriptional factor Egr2, the protease Mmp13 and downstream TGFb1/Run×2 target genes like the bone matrix components Opn and Mgp (Table 4).
Interestingly, a third prominent group of FGF2‐induced genes was related to the inflammatory response (Table 3). As shown in Table 4, FGF2 up‐regulates the expression of a number of chemokines involved in the recruitment of different inflammatory cells such as monocytes/macrophages (Ccl2, Ccl7, C×3cl1, Opn), neutrophils (C×cl1), NK cells (C×3cl1) and T lymphocytes (C×cl16, C×3cl1). Also, FGF2‐induced genes include inflammatory cytokines [IL‐6, leukaemia inhibitory factor (Lif), Opn] and cytokine receptors [oncostatin M receptor (Osmr), tumour necrosis factor receptor superfamily member 12a (Tnfrsf12a)/Tweak‐receptor, interleukin 1 receptor accessory protein (IL1rap)], cell adhesion molecules related to leucocyte recruitment and transendothelial migration [vascular cell adhesion molecule 1 (Vcam1), junctional adhesion molecule 2 (JAM2)], as well as key inflammatory mediators like the cyclooxygenase Ptgs2/Co×‐2 and the prostaglandin E2 receptor Ptger4.
It must be pointed out that, given the tight interplay among angiogenesis, bone formation and inflammation, the role exerted by various genes mentioned above (e.g. Opn) is not limited to a single biological process (Table 4).
Real‐time PCR analysis of FGF2 up‐regulated genes
The qRT‐PCR was used to confirm the up‐regulation of a number of selected inflammation‐related genes (IL‐6, Ccl2, Ccl7, C×3cl1, C×cl1, C×cl16, Egr1, Jam2, Ptgs2/ Co×‐2, Vcam1) in FGF2‐treated endothelial 1G11 cells. Time‐course analysis demonstrated the up‐regulation of all the genes examined (Fig. 1), showing an early up‐regulation for most of them (1 hr after treatment), thus indicating that the induction of a pro‐inflammatory signature represents an early event in FGF2‐driven endothelial cell activation. Also, dose–response experiments showed that the selected genes Ccl2, Ccl7 and Ptgs2/Co×‐2 were all significantly up‐regulated in 1G11 cells when tested at FGF2 concentrations ranging from 1.0 to 30 ng/ml. Moreover, qRT‐PCR analysis confirmed the up‐regulation of the inflammation‐related genes Ccl2, Ccl7, C×3cl1 and Ptgs2/Co×‐2 in FGF2‐stimulated murine brain microvascular endothelial 10027 cells [29], supporting the notion that the induction of a pro‐inflammatory signature represents a general feature of the FGF2‐mediated response in endothelium.
Figure 1.
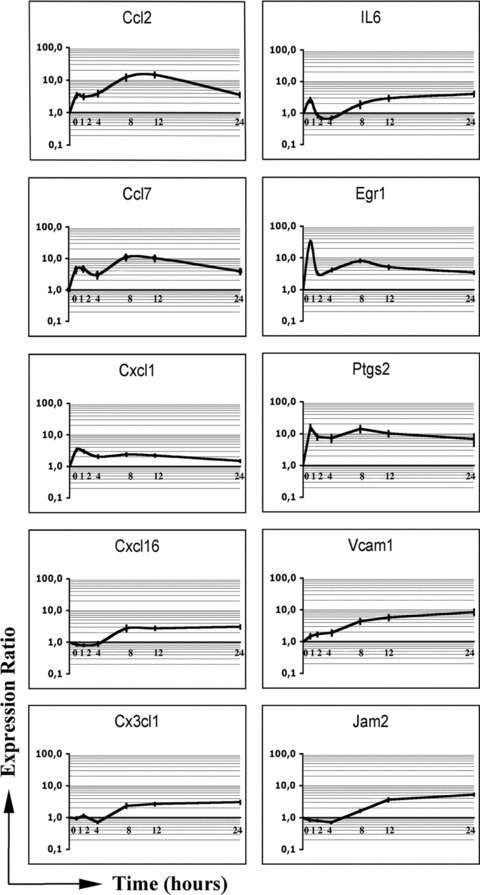
qRT‐PCR time course analysis of selected inflammation‐related genes in FGF2‐stimulated endothelial cells. Serum starved 1G11 endothelial cells were stimulated with 30 ng/ml of FGF2 for 0, 1, 2, 4, 8, 12 and 24 hrs. Total RNA from each time‐point was reverse transcribed to cDNA and analysed by qRT‐PCR. Data (mean values ± SD, n= 3) represent the expression ratio of each target gene relative to the untreated control. Expression levels were normalized to β‐actin gene.
FGF2 induces inflammatory cell recruitment in the areas of neovascularization
The above results led us to investigate the presence of FGF2‐triggered inflammatory cues in two different in vivo models of angiogenesis, the chick embryo CAM assay and the murine Matrigel plug assay.
As shown in Fig. 2, an alginate pellet containing FGF2 triggered a potent angiogenic response when applied on the top of the chick embryo CAM. May Grünwald‐Giemsa staining of the FGF2‐treated CAMs revealed the presence of an inflammatory cell infiltrate in the stroma among the newly formed blood vessels (Fig. 2). However, the lack of specific antibodies and the early stage of development of the chick embryos did not allow a characterization of the inflammatory cells infiltrating the CAM. Next, the anti‐inflammatory drugs hydrocortisone and ketoprofen were used to assess the overall impact of the inflammatory response on FGF2‐induced angiogenesis in the CAM assay. As shown in Fig. 2, both drugs were able to inhibit the angiogenic response triggered by FGF2, thus implicating inflammatory cells/mediators in FGF2‐dependent neovascularization.
Figure 2.
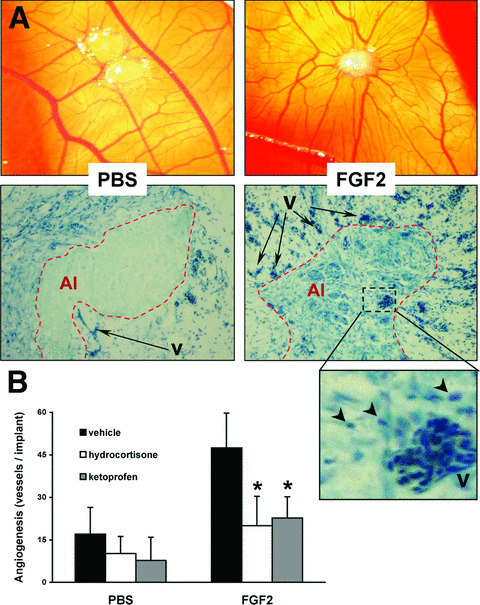
FGF2 induces angiogenic and inflammatory responses in the chick embryo CAM assay. (A) Alginate beads containing vehicle (PBS) or 150 ng of FGF2 (FGF2) were implanted on top of chick embryo CAMs at day 11 of development. After 3 days, CAMs were assessed for new vessel formation (upper panels) using a stereomicroscope (original magnification, ×5) and for inflammatory cell infiltration (lower panels) by May Grünwald‐Giemsa staining of paraffin‐embedded sections (original magnification, ×40). Note the strong presence of inflammatory cells (arrowheads) in the areas of FGF2‐induced neovascularization, as shown in the enlarged lower right panel. Alginate implant (AI, dotted line), vessels (v). (B) Alginate implants containing vehicle or 150 ng of FGF2 were assessed for their angiogenic capacity in the absence or presence of 50 μg of hydrocortisone or 50 μg of ketoprofen. Data (7–10 eggs per group) represent the number of vessels converging towards the alginate implant and are expressed as mean ± SD. *, statistically different from the ‘FGF2 plus vehicle’ group, P < 0.05.
In a second set of experiments, FGF2‐embedded Matrigel plugs were implanted subcutaneously in mice and examined hystologically at day 7 after implantation (Fig. 3). Haematoxylin/eosin staining revealed the presence of numerous blood vessels and of an abundant cellular infiltrate in FGF2‐embedded pellets when compared to PBS‐embedded control implants. Immunofluorescence analysis confirmed the presence of a potent neovascular response in FGF2‐embedded plugs, as shown by the presence of numerous CD31+ endothelial cells, which was accompanied by a consistent CD45+ leucocyte infiltrate. The characterization of the leucocyte subsets revealed that the inflammatory cell infiltrate consists mainly of CD11b+ monocytes and F4/80+ macrophages. Only rare Gr‐1+ neutrophils and CD8+ or CD4+ T‐lymphocytes and no CD19+ B‐lymphocytes, NK1.1+ natural killer or CD11c+ dendritic cells were instead detectable (Fig. 3 and Table 5). A time‐course analysis of the cellular populations infiltrating the FGF2‐embedded Matrigel plugs revealed that monocytes/macrophages are already detectable within the plug at day 2 after implantation whereas a significant CD31+ neovascular response becomes evident on day 4 (data not shown). Thus, macrophage recruitment precedes neovascularization in FGF2‐driven angiogenesis.
Figure 3.
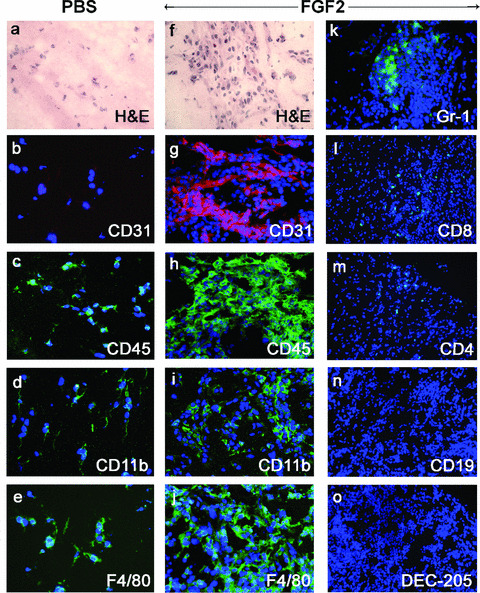
Inflammatory cells infiltrate the areas of FGF2‐induced neovascularization in Matrigel plugs. Matrigel pellets containing PBS or 150 ng of FGF2 were implanted subcutaneously in mice and examined at day 7 by haematoxylin and eosin staining and immunofluorescence analysis with antibodies specific to the indicated antigens. Note the presence in the implanted FGF2‐Matrigel plugs of numerous CD31+ endothelial cells and of CD45+ infiltrating leucocytes, mainly consisting of CD11b+ monocytes and F4/80+ macrophages. Only scarce Gr‐1+ neutrophils, CD8+ and CD4+ T‐lymphocytes and no CD19+ B‐lymphocytes, NK1.1+ natural killer or CD11c+ dendritic cells are instead detectable. Nuclei are shown by DAPI counterstaining. Original magnification: a–j, ×200; k–o, ×100.
Table 5.
Immunoistochemical characterization of the inflammatory infiltrate in FGF2‐Matrigel plugs
| Treatment | CD31+ | CD45+ | CD11b+ | F4/80+ | GR‐1+ | CD4+ | CD8+ |
|---|---|---|---|---|---|---|---|
| PBS | 1074 ± 748 | 7682 ± 3220 | 7002 ± 601 | 2316 ± 186 | 61 ± 63 | 0 | 0 |
| FGF2 | 12,228 ± 2024 | 37,467 ± 5900 | 13,469 ± 2372 | 17,401 ± 7187 | 82 ± 115 | 154 ± 169 | 654 ± 834 |
Eight μm frozen sections of Matrigel plugs containing PBS or FGF2 (five plugs per group) were immunostained for the indicated antigens. Then, the corresponding immunoreactive areas were analysed in five microscopic fields per Matrigel section (two sections per Matrigel plug) using the ImagePro Plus software. Analysis was performed on plugs on day 7 after implantation. Data (mean ± SD) are expressed as μm2 of immunoreactive area per microscopic field (0.38 mm2).
Impairment of macrophage recruitment reduces FGF2‐induced angiogenesis
The above observations support the notion that inflammatory cells are relevant to FGF2‐dependent neovascularization. To further investigate this hypothesis, we evaluated the capacity of FGF2 to trigger an angiogenic response in the Matrigel plug assay under conditions that impair the recruitment of inflammatory cells.
Proper migration of leucocytes to chemotactic agonists in inflammatory sites is dependent on PI3Kγ activity [24]. To investigate the potential role of infiltrating inflammatory cells in the modulation of FGF2‐dependent angiogenesis, FGF2‐embedded Matrigel plugs were implanted subcutaneously in PI3Kγ−/− mice and examined by immunostaining at day 7 after implantation. Computerized image analysis of the immunofluorescence signals demonstrated a significant reduction of the F4/80+ cell infiltrate (–60%) and, importantly, of CD31+ neovessels (–40%) in PI3Kγ−/− mice when compared to wild‐type control animals (Fig. 4).
Figure 4.
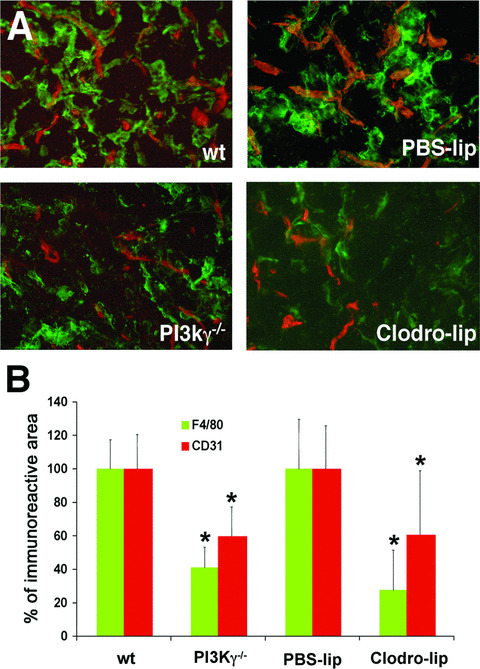
Defective macrophage recruitment impairs FGF2‐induced angiogenesis. (A) Immunohistochemical analysis of FGF2‐Matrigel pellets from wild‐type (wt) or PI3Kγ−/− SV129 mice and from C57Bl/6 mice that underwent PBS‐lips or Clodro‐lips pre‐treatment. Matrigel sections were double‐stained with anti‐CD31 (red) and anti‐F4/80 (green) monoclonal antibodies. Original magnification, ×200. (B) Quantitative analysis of infiltrating CD31+ endothelial cells and F4/80+ macrophages in FGF2‐Matrigel plugs on day 7 after implantation. Data (mean ± SD) represent the percentage of CD31+ (red bars) or F4/80+ (green bars) immunopositive areas of Matrigel sections from PI3Kγ−/− or Clodro‐lip‐treated mice relative to their respective wt and PBS‐lip‐treated controls. *, P < 0.05.
In a second set of experiments, FGF2‐induced angiogenesis was evaluated after macrophage depletion following intraperitoneal pre‐treatment with clodronate liposomes (Clodro‐lip) [30]. Again, immunofluorescence analysis demonstrated a 72% reduction of the F4/80+ macrophage infiltrate and a 40% decrease of the CD31+ areas of neovascularization in FGF2‐embedded Matrigel plugs implanted in Clodro‐lip‐treated animals when compared to control animals injected with PBS‐containing liposomes (PBS‐lip) (Fig. 4). Taken together, these results point to a role for pro‐inflammatory macrophages in FGF2‐induced angiogenesis in vivo.
The conditioned medium from FGF2‐stimulated microvascular cells is chemotactic for monocytes and promotes chemokine‐dependent angiogenesis in vivo
Gene expression data indicate that FGF2 up‐regulates the production of various chemokines that may serve to recruit monocytes in the neovascularized areas. To test this hypothesis, we evaluated the ability of the CM from FGF2‐stimulated microvascular 1G11 cells to induce monocyte chemotaxis in vitro. To avoid the possibility that exogenously added FGF2 may interfere with the biological activity of the CM from FGF2‐treated cells, 1G11 cells were seeded on FGF2 immobilized to plastic dishes where it retains a full biological activity [31]. Following a 24‐hr stimulation, cells extracts and CM were analysed for gene expression and monocyte chemotactic activity, respectively. The concentration of human FGF2 in the CM was typically lower than 1.0 ng/ml, as assessed by ELISA. As controls, 1G11 cells cultured for 24 hrs on non‐coated dishes or stimulated with soluble FGF2 (30 ng/ml) were analysed in parallel. As shown in Fig. 5, both immobilized and free FGF2 induced the up‐regulation of selected chemotactic factors, thus confirming the ability of substratum‐bound FGF2 to activate endothelial cells.
Figure 5.
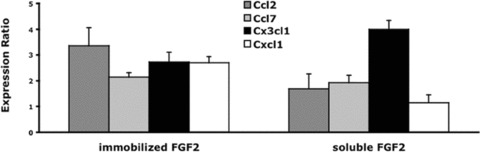
qRT‐PCR analysis of selected chemokines in endothelial cells stimulated by immobilized or soluble FGF2. Microvascular 1G11 cells were incubated for 24 hrs on FGF2‐coated dishes (immobilized FGF2) or stimulated for 24 hrs with 30 ng/ml of FGF2 (soluble FGF2) onto non‐coated dishes. Total RNA was reverse transcribed to cDNA and analysed by real‐time PCR. Data are presented as the expression ratio of each target gene relative to an untreated control. Expression levels were normalized to β‐actin gene. The bars show mean values ± SD (n= 3).
On this basis, the CM from control and FGF2‐stimulated 1G11 cells were tested in a Boyden chamber assay for the capacity to induce a chemotactic response in freshly isolated human monocytes (Fig. 6A). The CM from FGF2‐stimulated 1G11 cells exerted a dose‐dependent chemotactic response whereas the CM from control cells was ineffective. Importantly, the chemotactic response was inhibited in a dose‐dependent manner when the CM from FGF2‐stimulated 1G11 cells was pre‐incubated with the pan‐chemokine inhibitor M3 (Fig. 6B), a murine gammaherpesvirus 68 protein antagonist for human and mouse CC, CXC and CX3C chemokines [19]. At variance, M3 did not affect the capacity of FGF2 to trigger in vitro endothelial cell proliferation and sprouting (data not shown). Taken together, these observations indicate that FGF2‐activated endothelium expresses and secretes biologically active chemokine(s) that represent a chemotactic stimulus for human monocytes.
Figure 6.
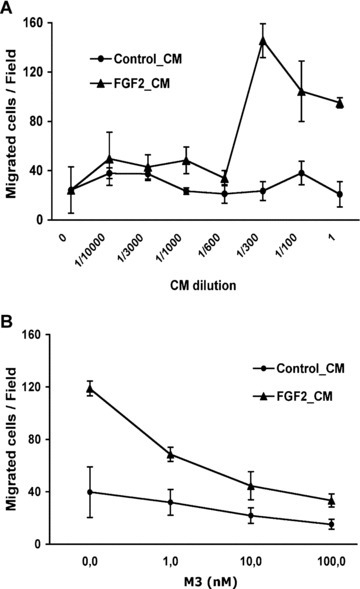
The conditioned medium (CM) from FGF2‐stimulated microvascular endothelial cells is chemotactic to monocytes. (A) The migration of human monocytes towards different dilutions of the CM from non‐stimulated (Control_CM) and FGF2‐stimulated (FGF2_CM) 1G11 endothelial cells was quantified as described in ‘Materials and methods’. Data are expressed as the mean number of migrated cells per field. (B) Inhibition of the chemotactic response of 1G11 endothelial cells towards FGF2‐CM by different concentrations of the pan‐chemokine inhibitor M3. Chemotaxis was tested at the dose of FGF2_CM that induced the maximal chemotactic responses (1/300 dilution) and compared to the equivalent dilution of Control_CM. Data are expressed as the number of migrated cells per field.
Next, the CM from FGF2‐stimulated microvascular 1G11 cells was investigated for the capacity to induce neovascularization in vivo in the chick embryo CAM assay. As shown in Fig. 7A, the CM from FGF2‐stimulated endothelial cells exerted a potent angiogenic response that was significantly reduced in the presence of the pan‐chemokine inhibitor M3. Accordingly, the intense cellular infiltrate observed in the areas of neovascularization induced by the CM from FGF2‐stimulated cells was almost abolished in the presence of M3 (Fig. 7B). In keeping with these observations, the pan‐chemokine inhibitor M3 induced a significant inhibitory effect on neovascularization induced by recombinant FGF2, without affecting the basal levels of vascularization of the CAM (Fig. 8). Taken together, these findings demonstrate a relevant role for pro‐inflammatory chemokines in FGF2‐driven angiogenesis.
Figure 7.
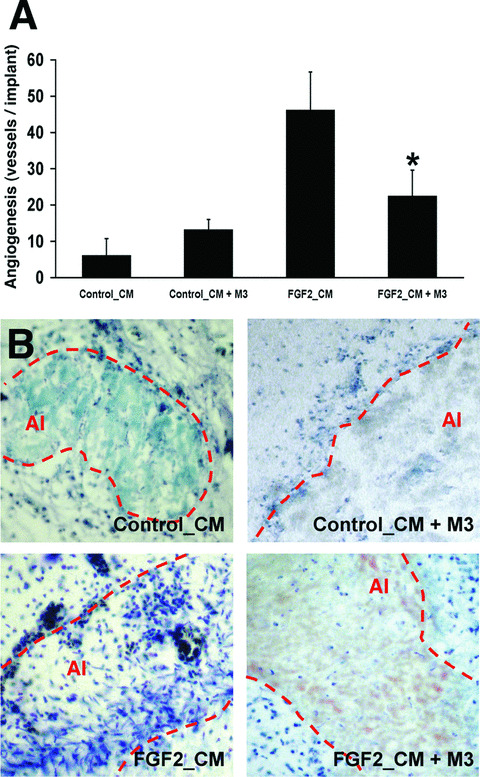
The conditioned medium (CM) from FGF2‐stimulated microvascular endothelial cells promotes chemokine‐dependent angiogenesis in vivo. (A) Chick embryo CAM assay was performed with the CM from non‐stimulated (Control_CM) and FGF2‐stimulated (FGF2_CM) 1G11 endothelial cells in the absence or presence of 75 ng of the pan‐chemokine inhibitor M3. Data are expressed as the mean ± SD of the number of vessels invading the alginate area (*, statistically different from the ‘FGF2_CM’ group, P < 0.05). (B) Representative histological sections of CAMs from the different experimental groups (May Grünwald‐Giemsa staining). Note that FGF2_CM induces neovascularization and a strong inflammatory cell infiltrate within the alginate implant (AI, dotted line), both greatly reduced in the presence of M3.
Figure 8.
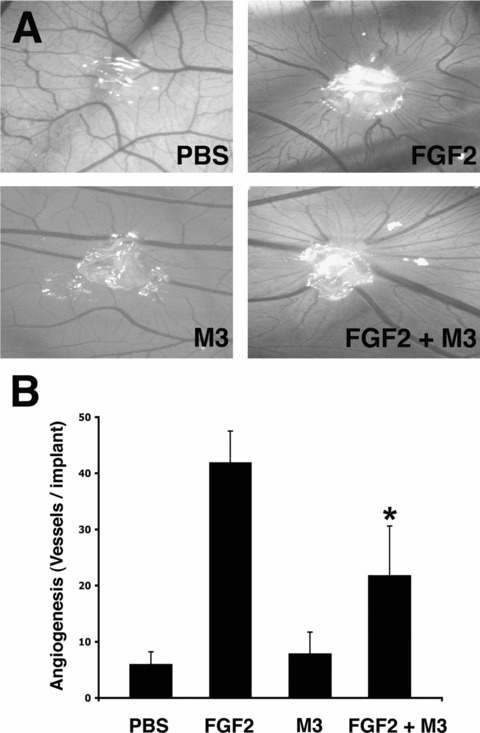
Pan‐chemokine inhibitor M3 impairs FGF2‐induced angiogenesis. CAMs were implanted at day 11 of development with alginate beads containing vehicle (PBS), 150 ng of FGF2, 75 ng of M3 or 150 ng of FGF2 added with 75 ng of M3. After 3 days, CAMs were photographed (A, original magnification ×5) and angiogenesis was quantified by counting the number of microvessels (mean ± SD) invading the alginate area (B). Note the significant reduction (*, P < 0.05) in the number of newly‐formed microvessels converging towards the FGF2 implant in the presence of the M3 inhibitor.
Discussion
Scattered experimental evidence pointed to a role for inflammatory mediators and leucocytes in mediating the neovascularization process triggered by the angiogenic growth factor FGF2 [10, 13, 15, 16, 17, 32]. In the present study, transcriptome analysis demonstrates that FGF2 activates a complex pro‐inflammatory signature in murine microvascular endothelial cells. Accordingly, we provide evidence that FGF2‐induced chemokines and infiltrating monocytes/macrophages are non‐redundant mediators of the neovascularization process elicited by the growth factor. Indeed, FGF2‐triggered angiogenesis is significantly reduced in the CAM assay by mechanistically distinct steroidal (hydrocortisone) and non‐steroidal (ketoprofen) anti‐inflammatory drugs and by the pan‐chemokine inhibitor M3. Also, FGF2 elicits a decreased angiogenic response in PI3Kγ−/− mice exhibiting defective leucocyte migration and in clodronate‐pre‐treated, macrophage‐depleted animals.
Monocytes/macrophages are active players in pathological angiogenesis [33, 34, 35]. They often precede, temporally and spatially, new vessel formation by altering the microenvironment, thus promoting vascular sprouting [36] and the recruitment of endothelial cell precursors [32]. Accordingly, we have observed that the early recruitment of mononuclear phagocytes (within 2–3 days after implantation) precedes blood vessel formation in FGF2‐driven angiogenesis in the Matrigel plug assay. The depletion of monocytes/macrophages reduces also neovascularization driven by VEGF [37], placental growth factor (PIGF) [38] and IL‐1β[39]. Thus, mononuclear phagocytes play a pivotal role in the angiogenesis process driven by various angiogenic growth factors, including FGF2.
Our observations indicate that FGF2‐driven angiogenesis is, at least in part, chemokine‐dependent. Chemotactic factors produced by FGF2‐stimulated endothelium may recruit mononuclear phagocytes that, in turn, will amplify the angiogenic response by releasing monocyte‐derived pro‐angiogenic cytokines. Also, FGF2‐induced chemoattractants may play a direct role in neovascularization by interacting with specific chemokine receptors expressed on endothelial cells [7]. Among them, the FGF2‐induced chemokines Ccl2, C×cl1, C×cl16 and C×c3l1 could act as enhancers of the neovascularization process elicited by the growth factor.
The capacity of the pan‐chemokine inhibitor M3 [19] to inhibit angiogenesis triggered by FGF2 or by the CM from FGF2‐stimulated endothelial cells is of interest. This gammaherpesvirus 68‐derived protein prevents chemokine‐mediated signal transduction and leucocyte recruitment induced by a number of chemokines and may have therapeutic potential in inflammatory conditions [40]. Our findings suggest that M3 protein may represent the basis for the design of novel angiogenesis inhibitors with therapeutic implications in angiogenesis‐dependent pathological conditions, including tumour growth and metastasis.
Taken together, our findings support the notion that monocytes/macrophages and inflammation‐related gene products actively participate in the angiogenic process elicited by FGF2 as part of a complex cascade of cellular and molecular events triggered by the growth factor on microvascular endothelium. Indeed, FGF2 up‐regulates also the expression of a variety of angiogenic growth factors in endothelial cells, including FGF2 itself (Table 4). This suggests that FGF2 is able to activate an autocrine loop of amplification of the angiogenic response that, together with the paracrine activity exerted by endothelium‐derived cytokines/chemokines on inflammatory cells, will contribute to the modulation of the neovascularization process triggered by the growth factor.
Like FGF2, also VEGF is known to up‐regulate the expression of pro‐inflammatory mediators in endothelial cells [41, 42, 43]. In our experiments, the induction of inflammation‐related genes by FGF2 represents an early event, most of these genes being up‐regulated 1 hr after treatment (see Fig. 1). This precedes the limited increase of VEGF expression induced by FGF2 in 1G11 cells that reaches a maximal twofold up‐regulation at 24 hrs after stimulation (data not shown). This observation appears to rule out the possibility that the pro‐inflammatory signature triggered by FGF2 in endothelial cells may represent an indirect, VEGF‐mediated effect. On the other hand, in parallel with a significant monocyte/macrophage infiltrate, we have observed a sixfold increase of VEGF mRNA levels in FGF2‐Matrigel plugs when compared to control implants (data not shown). Further experiments are required to fully dissect the complex cross‐talk between FGF2 and VEGF during angiogenesis (reviewed in [13]).
FGF2 expression is augmented at sites of chronic inflammation, tissue injury and in human cancer [13]. Our observations suggest that FGF2 released after tissue damage may contribute to the host defence responses by activating pro‐angiogenic and pro‐inflammatory signatures in endothelium that, by acting in concert, will lead to neovessel formation and monocyte/macrophage engagement. Accordingly, Fgf2‐null mice exhibit delayed wound repair [44] and neutralizing anti‐FGF2 antibodies inhibit angiogenesis and formation of granulation tissue in a rat model of wound healing [45]. Conversely, local application of FGF2 effectively improves wound repair [46], the healing process being accompanied by mononuclear cell infiltrate recruitment [47]. On the other hand, long‐term stimulation by FGF2 inhibits monocyte/macrophages adhesion to endothelium and the chemotactic response to various chemokines [48], suggesting that the pro‐ or anti‐inflammatory activity of FGF2 may be contextual (discussed in [13]).
In conclusion, our findings point to inflammatory chemokines as important early mediators of FGF2‐driven angiogenesis and indicate a relevant role for inflammatory cells in the neovascularization process elicited by the growth factor. Conversely, FGF2 may exert important functions at sites of inflammation and/or tissue injury not only by inducing neovascularization but also by contributing to the activation of innate immune responses.
Acknowledgements
This work was supported by grants from Istituto Superiore di Sanità (Oncotechnological Program), Ministero dell’Istruzione, Università e Ricerca (Centro di Eccellenza per l’Innovazione Diagnostica e Terapeutica, Cofin projects), Associazione Italiana Ricerca sul Cancro, Fondazione Berlucchi, NOBEL Project Cariplo, and Integrated European Commission Project STROMA to M.P. G.A. was supported by a Marie Curie Fellowship from the European Community Quality of Life Programme. A.A. was funded by the Wellcome Trust.
References
- 1. Jackson JR, Seed MP, Kircher CH, et al. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997; 11: 457–65. [PubMed] [Google Scholar]
- 2. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000; 407: 249–57. [DOI] [PubMed] [Google Scholar]
- 3. Zijlstra A, Seandel M, Kupriyanova TA, et al. Proangiogenic role of neutrophil‐like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2006; 107: 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007; 7: 803–15. [DOI] [PubMed] [Google Scholar]
- 5. Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007; 178: 6017–22. [DOI] [PubMed] [Google Scholar]
- 6. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999; 18: 7908–16. [DOI] [PubMed] [Google Scholar]
- 7. Romagnani P, Lasagni L, Annunziato F, et al. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004; 25: 201–9. [DOI] [PubMed] [Google Scholar]
- 8. Voronov E, Shouval DS, Krelin Y, et al. IL‐1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003; 100: 2645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naldini A, Leali D, Pucci A, et al. Cutting edge: IL‐1beta mediates the proangiogenic activity of osteopontin‐activated human monocytes. J Immunol. 2006; 177: 4267–70. [DOI] [PubMed] [Google Scholar]
- 10. Leali D, Dell’Era P, Stabile H, et al. Osteopontin (Eta‐1) and fibroblast growth factor‐2 cross‐talk in angiogenesis. J Immunol. 2003; 171: 1085–93. [DOI] [PubMed] [Google Scholar]
- 11. Aplin AC, Gelati M, Fogel E, et al. Angiopoietin‐1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol Genomics. 2006; 27: 20–8. [DOI] [PubMed] [Google Scholar]
- 12. Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007; 13: 2825–30. [DOI] [PubMed] [Google Scholar]
- 13. Presta M, Dell’Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005; 16: 159–78. [DOI] [PubMed] [Google Scholar]
- 14. Cross MJ, Claesson‐Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001; 22: 201–7. [DOI] [PubMed] [Google Scholar]
- 15. Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF‐2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol. 2006; 168: 835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wempe F, Lindner V, Augustin HG. Basic fibroblast growth factor (bFGF) regulates the expression of the CC chemokine monocyte chemoattractant protein‐1 (MCP‐1) in autocrine‐activated endothelial cells. Arterioscler Thromb Vasc Biol. 1997; 17: 2471–8. [DOI] [PubMed] [Google Scholar]
- 17. Kage K, Fujita N, Oh‐hara T, et al. Basic fibroblast growth factor induces cyclooxygenase‐2 expression in endothelial cells derived from bone. Biochem Biophys Res Commun. 1999; 254: 259–63. [DOI] [PubMed] [Google Scholar]
- 18. Gualandris A, Urbinati C, Rusnati M, et al. Interaction of high‐molecular‐weight basic fibroblast growth factor with endothelium: biological activity and intracellular fate of human recombinant M(r) 24,000 bFGF. J Cell Physiol. 1994; 161: 149–59. [DOI] [PubMed] [Google Scholar]
- 19. Parry CM, Simas JP, Smith VP, et al. A broad spectrum secreted chemokine binding protein encoded by a herpesvirus. J Exp Med. 2000; 191: 573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong QG, Bernasconi S, Lostaglio S, et al. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997; 17: 1599–604. [DOI] [PubMed] [Google Scholar]
- 21. Irizarry RA, Bolstad BM, Collin F, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003; 31: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dennis G Jr, Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003; 4: P3. [PubMed] [Google Scholar]
- 23. Rusnati M, Tanghetti E, Dell’Era P, et al. alphavbeta3 integrin mediates the cell‐adhesive capacity and biological activity of basic fibroblast growth factor (FGF‐2) in cultured endothelial cells. Mol Biol Cell. 1997; 8: 2449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein‐coupled phosphoinositide 3‐kinase gamma in inflammation. Science. 2000; 287: 1049–53. [DOI] [PubMed] [Google Scholar]
- 25. Zeisberger SM, Odermatt B, Marty C, et al. Clodronate‐liposome‐mediated depletion of tumour‐associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006; 95: 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knoll A, Schmidt S, Chapman M, et al. A comparison of two controlled‐release delivery systems for the delivery of amiloride to control angiogenesis. Microvasc Res. 1999; 58: 1–9. [DOI] [PubMed] [Google Scholar]
- 27. Montero A, Okada Y, Tomita M, et al. Disruption of the fibroblast growth factor‐2 gene results in decreased bone mass and bone formation. J Clin Invest. 2000; 105: 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naganawa T, Xiao L, Abogunde E, et al. In vivo and in vitro comparison of the effects of FGF‐2 null and haplo‐insufficiency on bone formation in mice. Biochem Biophys Res Commun. 2006; 339: 490–8. [DOI] [PubMed] [Google Scholar]
- 29. Bastaki M, Nelli EE, Dell’Era P, et al. Basic fibroblast growth factor‐induced angiogenic phenotype in mouse endothelium. A study of aortic and microvascular endothelial cell lines. Arterioscler Thromb Vasc Biol. 1997; 17: 454–64. [DOI] [PubMed] [Google Scholar]
- 30. Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994; 174: 83–93. [DOI] [PubMed] [Google Scholar]
- 31. Tanghetti E, Ria R, Dell’Era P, et al. Biological activity of substrate‐bound basic fibroblast growth factor (FGF2):s recruitment of FGF receptor‐1 in endothelial cell adhesion contacts. Oncogene. 2002; 21: 3889–97. [DOI] [PubMed] [Google Scholar]
- 32. Anghelina M, Krishnan P, Moldovan L, et al. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006; 168: 529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dirkx AE, Oude Egbrink MG, Wagstaff J, et al. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006; 80: 1183–96. [DOI] [PubMed] [Google Scholar]
- 34. Sunderkotter C, Steinbrink K, Goebeler M, et al. Macrophages and angiogenesis. J Leukoc Biol. 1994; 55: 410–22. [DOI] [PubMed] [Google Scholar]
- 35. Rehman J, Li J, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/ macrophages and secrete angiogenic growth factors. Circulation. 2003; 107: 1164–9. [DOI] [PubMed] [Google Scholar]
- 36. Moldovan NI, Goldschmidt‐Clermont PJ, Parker‐Thornburg J, et al. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase‐positive tunnels in ischemic myocardium. Circ Res. 2000; 87: 378–84. [DOI] [PubMed] [Google Scholar]
- 37. Cursiefen C, Chen L, Borges LP, et al. VEGF‐A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004; 113: 1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pipp F, Heil M, Issbrucker K, et al. VEGFR‐1‐selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte‐mediated mechanism. Circ Res. 2003; 92: 378–85. [DOI] [PubMed] [Google Scholar]
- 39. Nakao S, Kuwano T, Tsutsumi‐Miyahara C, et al. Infiltration of COX‐2‐expressing macrophages is a prerequisite for IL‐1 beta‐induced neovascularization and tumor growth. J Clin Invest. 2005; 115: 2979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fallon PG, Alcami A. Pathogen‐derived immunomodulatory molecules: future immunotherapeutics Trends Immunol. 2006; 27: 470–6. [DOI] [PubMed] [Google Scholar]
- 41. Reinders ME, Sho M, Izawa A, et al. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. 2003; 112: 1655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim I, Moon SO, Kim SH, et al. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), and E‐selectin through nuclear factor‐kappa B activation in endothelial cells. J Biol Chem. 2001; 276: 7614–20. [DOI] [PubMed] [Google Scholar]
- 43. Abe M, Sato Y. cDNA microarray analysis of the gene expression profile of VEGF‐activated human umbilical vein endothelial cells. Angiogenesis. 2001; 4: 289–98. [DOI] [PubMed] [Google Scholar]
- 44. Ortega S, Ittmann M, Tsang SH, et al. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA. 1998; 95: 5672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Broadley KN, Aquino AM, Woodward SC, et al. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989; 61: 571–5. [PubMed] [Google Scholar]
- 46. Tsuboi R, Rifkin DB. Recombinant basic fibroblast growth factor stimulates wound healing in healing‐impaired db/db mice. J Exp Med. 1990; 172: 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ribatti D, Nico B, Vacca A, et al. Endogenous and exogenous fibroblast growth factor‐2 modulate wound healing in the chick embryo chorioallantoic membrane. Angiogenesis. 1999; 3: 89–95. [DOI] [PubMed] [Google Scholar]
- 48. Zhang H, Issekutz AC. Down‐modulation of monocyte transendothelial migration and endothelial adhesion molecule expression by fibroblast growth factor: reversal by the anti‐angiogenic agent SU6668. Am J Pathol. 2002; 160: 2219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


