Abstract
Unrestricted somatic stem cells (USSC) have the potential to differentiate into tissues derived from all three germinal layers and therefore hold promise for use in regenerative therapies. Furthermore, they have haematopoietic stromal activity, a characteristic that may be exploited to enhance haematopoietic engraftment. Both applications may require USSC to be used in an allogeneic, HLA‐mismatched setting. We have therefore studied their in vitro interaction with cellular immunity. USSC showed no allostimulatory activity and caused only minimal inhibition of allogeneic T‐cell responses. However, following pre‐stimulation with IFNγ and TNFα, they inhibited T‐cell proliferation in an indoleamine 2, 3‐dioxygenase‐dependent manner and suppressed graft‐versus‐host type reactions. In addition, USSC inhibited DC maturation and function. This inhibition was overridden by stronger DC maturation signals provided by IL‐1β, IL‐6, PGE2 and TNFα compared to TNFα alone. Pre‐stimulation of USSC with IFNγ and TNFα had a similar effect: Inhibition of DC maturation was no longer observed. Thus, USSC are conditionally immunosuppressive, and IFNγ and TNFα constitute a switch, which regulates their immunological properties. They either suppress T‐cell responses in the presence of both cytokines or in their absence block DC differentiation and function. These activities may contribute to fine‐tuning the immune system especially at sites of tissue damage in order to ensure appropriate differentiation of USSC and subsequent tissue repair. Therapeutically, they may help to protect USSC and possibly their progeny from immune rejection.
Keywords: T cells, dendritic cells, immunosuppression, regenerative medicine, USSC, MSC, IDO, IFNγ, TNFα
Introduction
Non‐haematopoietic stem cells were first described in cord blood (CB) in 2000 [1], and osteogenic, adipogenic and chondrogenic developmental potential of these cells was documented [2, 3]. In addition to these three ‘classical’ mesodermal lineages, indicative of multipotent mesenchymal stem cells (MSC), CB‐derived cells have been shown to differentiate into cells with features of skeletal muscle [4], endothelial cells [5], neural cells [6] and hepatic cells [7]. The potential to generate cell types of mesodermal, ectodermal or endodermal origin resides in a single multipotent stem cell type, which we termed USSC (unrestricted somatic stem cell; [8]). USSC are spindle‐shaped cells, which differentiate to mesodermal osteoblasts, chondroblasts, adipocytes, haematopoietic cells and cardiomyocytes [8], to ectodermal neuronal cells [8, 9] as well as to endodermal hepatic cells [8, 10]. A similar population and multilineage differentiation on a clonal level has been reported by Lee et al. after limiting dilution studies [11], a result which we could confirm recently, using lentiviral marking and integration site analysis (manuscript in preparation).
USSC resemble MSC, particularly those from foetal tissues. Although the exact relationship of the two stem cell types is still unclear, there are immunophenotypic differences [8], and it has been suggested that MSC represent more differentiated progeny of USSC, particularly because USSC can be easily triggered towards the mesodermal/MSC lineage depending on the culture conditions, thereby partially loosing their multipotency [12]. In addition, USSC have longer telomeres than bone marrow (BM)‐derived MSC, indicating a shorter proliferative history [8]. They are karyotypically normal even after extended in vitro expansion and so far, no tumour formation in animal models has been observed [8].
USSC hold promise for use in regenerative medicine, due their wide differentiation and expansion potential, the absence of cytogenetic abnormalities and tumour formation, together with the advantages of using CB as a stem cell source, which include a lower incidence of viral infections compared to adult stem cells and a lack of ethical issues surrounding their use. In a pre‐clinical porcine model, beneficial effects of transplantation on cardiac function after infarction have been reported [13], and there also appears to be a partial recovery of nerve function in a canine spinal injury model [14]. Moreover, improvements have been observed after transplantation into patients suffering from Buerger’s syndrome [15].
Furthermore, USSC have stromal activity and support haematopoiesis in vitro[16] as well as after co‐transplantation with CD34+ cells in NOD/SCID mice [17]. Thus, USSC may also be exploited to improve engraftment after haematopoietic stem cell transplantation.
The use of USSC in regenerative medicine or in haematopoietic stem cell transplantation would require USSC to be active in an allogeneic environment, making them a potential target for immune rejection. Currently, little is known about the immunogenicity of USSC, however MSC [18], in vitro differentiated MSC [19], dermal fibroblasts [20] or potentially most mesenchymal cells [21], all of which represent potential progeny of USSC, show immunomodulating activities. They suppress T‐cell responses [18, 19, 20, 21, 22, 23] as well as NK [24, 25] and B‐cell function [26] and have been shown to interfere with the differentiation, maturation and function of dendritic cells (DC; [24, 27, 28]).
The contribution of cell‐to‐cell contact‐dependent pathways such as the interaction between PD‐1 and its ligands [29] and of soluble factors such as TGFβ[23], HGF [23], HLA‐G [30], PGE2[24], haem oxygenase‐1 [31], nitric oxide [31] and indoleamine 2,3‐dioxygenase (IDO; [32]) have been implicated in immunosuppression. Multiple factors or interactions also act in concert, e.g. IDO, which causes T‐cell cell‐cycle arrest due to tryptophan‐depletion [33], is induced in MSC by IFNγ produced early in the mixed leukocyte reaction (MLR) as a result of initial T‐cell activation [32].
Whether USSC share such immunomodulating properties with MSC and their progeny is currently unknown. In order to investigate these phenomena, we have therefore studied USSC for their influence on T‐cell responses and graft‐versus‐host (GvH) type alloreactions as well as on DC differentiation, maturation and function.
Materials and methods
Cell preparations
The generation and culture of USSC was performed as previously described [8, 12]. In brief, CB was collected after informed consent of the mother and approval from the local Ethics Committee. CB mononuclear cells were plated at 5–7×106 cells/ml in DMEM medium (Lonza, Verviers, Belgium) supplemented with 30% FCS (Lonza or PerBio, Bonn, Germany), 10−7 M dexamethasone (Sigma‐Aldrich, Seelze, Germany), 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L‐glutamine (all from Lonza). After 1 week, medium and non‐adherent cells were removed and fresh medium was added. When adherent clusters formed, cultures were expanded in the absence of dexamethasone, which is only required during the initial culture phase, and passaged when 70–80% confluence was reached. For certain experiments, USSC were stimulated for 6 days with 0–1000 U/ml IFNγ (R&D Systems, Wiebaden, Germany), 100 U/ml TNFα (Cellgenix, Freiburg, Germany), 2000 U/ml IL‐1β (Cellgenix) or 500 U/ml IL‐6 (Cellgenix).
Monocytes and T cells were enriched as previously described [34, 35]. Blood samples were obtained from healthy individuals after informed consent. Briefly, CD14+ monocytes were immunoselected from PBMC using LS separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany). T cells were negatively selected from PBMC by immunodepleting lineage marker positive cells (CD11b (BEAR 1), CD14 (RMO52), CD16 (3G8), CD19 (J4.119), CD34 (QBEND10), CD56 (C218), CD66b (80H3) and HLA‐DR (B8.12.2); all mAb from Beckman‐Coulter, Krefeld, Germany) using LD separation columns (Miltenyi Biotec).
Flow cytometry and monoclonal antibodies
To determine antigen‐uptake activity of DC, immature DC were pre‐incubated at 4°C (negative controls) or 37°C for 15 min. Then, graded doses of FITC‐labelled BSA (0.01–100μg/ml; Molecular Probes/Invitrogen, Karlsruhe, Germany) were added and after 1 hr of incubation at 4°C or 37°C, cells were washed, fixed with formaldehyde and FITC mean fluorescence determined by flow cytometry as described [34]. The following mAb were used for immunostainings: PE‐conjugated CD1a (BL 6), CD3 (UCHT1), CD11b (BEAR 1), CD11c (BU15), CD14 (RMO52), CD50 (HP2/19), CD54 (84H10), CD83 (HB15a) and FITC‐conjugated CD11a (25.3.1), CD18 (7E 4), CD25 (B1.49.9), CD49e (SAM1), CD58 (AICD58), CD80 (MAB104), CD83 (HB15a), HLA‐ABC (B9.12.1) and HLA‐DR (Immu‐357) specific mAb from Beckman‐Coulter, PE‐conjugated CD49d (L25.3), HLA‐DR (G46–6) and FITC‐conjugated CD40 (5C3), CD45 (2D1), CD49b (AK7) and CD86 (FUN‐1) specific mAb from BD Biosciences (Heidelberg, Germany).
Generation of dendritic cells
Differentiation and maturation of DC from monocytes was performed following a protocol adapted from Zhou et al. [36]. CD14+ monocytes were cultured in 24‐well plates (Greiner, Nürtingen, Germany) at 1×106 cells/ml in serum‐free CellGroDC medium (Cellgenix) supplemented with 1000 U/ml GM‐CSF (Leukine, Berlex, Richmond, CA, USA) and 1000 U/ml IL‐4 (Cellgenix). In certain experiments, GM‐CSF was replaced by 10 ng/ml IL‐3 (CellSystems, St. Katharien, Germany). After 3 and 6 days, half of the medium was replaced. To induce DC maturation, 1000 U/ml TNFα, GM‐CSF and IL‐4 were added on day 6 and cultures continued for 3 days. For certain DC maturation experiments, IL‐3 + IL‐4 + TNFα or GM‐CSF + IL‐4 + TNFα+ 2000 U/ml IL‐1β+ 500 U/ml IL‐6 + 1 μg/ml PGE2 (Minprostin, Pfitzer, Brussels, Belgium) were used.
The influence of USSC on DC differentiation and maturation was studied in direct co‐cultures or in transwell cultures (0.4 μm pore size transwell chambers; BD Biosciences). USSC (0–5×104 cells per well) or IFNγ+TNFα pre‐stimulated USSC (4 days) were plated in 24‐well plates, cultured overnight and irradiated (15 Gy). After an additional culture period of 16 hrs, monocytes were added and DC differentiation and maturation was induced. In certain experiments, anti‐TGFβ(1, 2, 3) mAb (5 μg/ml; 1D11; R&D Systems) was added to co‐cultures, to neutralize TGFβ activity, or USSC conditioned medium (30%) was used instead of cells.
Proliferative T‐cell alloresponses
USSC allostimulatory activity was determined by co‐culturing unstimulated, IFNγ (1000 U/ml) or IFNγ+TNFα (1000 U/ml and 100 U/ml) stimulated (4 days), irradiated USSC with 1×105 allogeneic PBMC (USSC:responder ratio = 1:3.3) in 96‐well plates (Greiner) in X‐Vivo 15 medium (Lonza) and after 6 days, BrdU‐incorporation was measured (ELISA‐kit; Roche Diagnostics, Mannheim, Germany) [34, 35]. To determine whether USSC affect T‐cell alloresponses, PBMC of two allogeneic donors (1×105 cells each) were co‐cultured on an established layer of irradiated USSC (unstimulated or stimulated with IFNγ (0–1000 U/ml) ± 100 U/ml TNFα for 4 days prior to the mixed leukocyte reaction (MLR)) in 96‐well plates. PBMC were added in 20 μl of tryptophan‐free RPMI‐1640 medium (PAN Biotech, Aidenbach, Germany). After 6 days of co‐culture, BrdU‐incorporation was determined. In certain experiments, at the onset of MLR, cultures were supplemented with 20 μg/ml tryptophan or 100 U/ml IL‐2 (Proleukin, Chiron, Ratingen, Germany).
To evaluate the allostimulatory activity of DC, 0–5000 mature DC/well were co‐cultured with 1×105 allogeneic T cells in 96‐well plates and after 6 days, BrdU‐incorporation was measured.
Determination of kynurenine production
IDO activity was detected by determining the IDO‐catalysed tryptophan‐to‐kynurenine degradation. Kynurenine in culture supernatants was measured as described by Däubener et al. [37]. In brief, trichloric acid (Merck, Darmstadt, Germany) was added to culture conditioned media, and N‐formyl‐kynurenine hydrolysed to L‐kynurenine by incubation at 50°C for 30 min. After sedimentation of proteins for 10 min. at 500 g, supernatants were transferred to flat‐bottom 96‐well plates and Ehrlich’s reagent (12 mg/ml dimethylaminobenzaldehyde (Sigma‐Aldrich) in 96% acetic acid (Sigma‐Aldrich)) was added, resulting in formation of a yellow azo dye. Absorption at 492 nm was measured and concentrations of kynurenine determined using standard curves. In certain experiments, USSC were cultured in the presence of IFNγ (0–1000 U/ml) ± 100 U/ml TNFα for 3 days in tryptophan supplemented medium (50 μg/ml tryptophan) before determination of kynurenine production.
The human skin explant model
The human skin explant assay was performed as previously described using skin biopsies obtained following informed consent and approval from the local Research Ethics Committee and the Newcastle NHS Trust R&D department [38, 39]. Briefly, responder PBMC were primed in a one‐way MLR, using irradiated PBMC from the skin explant donor as stimulators and HLA‐mismatched responder PBMC. At day 7 of MLR, standard 4‐mm punch skin biopsies were obtained (autologous to stimulator PBMC). Dissected skin sections were co‐cultured with MLR‐primed responder cells. After 3 days of co‐culture, skin sections were formalin fixed, paraffin embedded and stained with haematoxylin and eosin. Based on the severity of histopathological changes, skin GvH type alloreactivity was defined as grade I‐IV according to the Lerner grading system [40]. Evaluation was performed blinded by at least two independent observers. Histopathological changes in the skin were scored based on assessment of the whole tissue section. Skin sections cultured in medium alone were used as background controls. To evaluate the effect of USSC on the responder cell alloreactivity as measured in the skin explant model, irradiated USSC (20 Gy), unstimulated or stimulated for 4 days with IFNγ+ TNFα were added into the primary MLR (day 0) at a responder‐to‐USSC ratio of 10:1. Parallel experiments performed in the absence of USSC were used as positive controls.
Statistics
If not stated otherwise, all data are presented as mean ± S.E.M. Statistical analysis was performed with Graph Pad Prism Software Version 3.0 (GraphPad, San Diego, CA, USA). Statistical significance was evaluated with the Student’s paired ratio t‐test.
Results
Immunophenotypic analysis of USSC
The expression of immunorelevant USSC surface molecules was analysed by flow cytometry for 11 independent USSC cell lines (Fig. 1; representative results). USSC expressed the adhesion molecules CD49b, CD49e and CD58 as well as HLA‐class I. CD54 identified positive and negative subsets but varied widely among the USSC cell lines tested (range, 3.2–83.1% CD54+). Surface expression of the adhesion and co‐stimulatory molecules CD11a, CD11b, CD11c, CD18, CD27, CD40, CD49d, CD50, CD80 and CD86 as well as that of HLA‐DR were absent. Intracellular HLA‐DR+ compartments were not observed (data not shown). For six USSC cell lines, early (P4‐P6) and late (P8‐P10) passages were analysed. No changes in immunophenotype were observed (data not shown).
Figure 1.
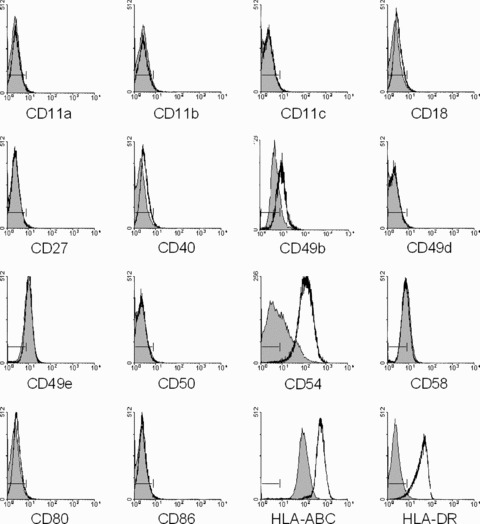
Immunophenotype of USSC. Expression of the molecules indicated on unstimulated (grey histograms) and stimulated USSC (1000 U/ml IFNγ; 6 days – open histograms) was analysed by flow cytometry. Staining with isotype controls is indicated (|––|).
The immunophenotype of USSC was also evaluated under conditions that elicit immunological activity in a variety of cell types, i.e. after stimulation with IFNγ (Fig. 1), IFNγ+TNFα or other pro‐inflammatory signals. Incubation with IFNγ resulted in induction of HLA‐DR as well as in up‐regulation of HLA‐class I and CD54. After stimulation, a CD54− subset was no longer observed. In 4 out of 7 USSC cell lines, CD49b was slightly up‐regulated; expression of the remaining molecules was not altered after IFNγ stimulation. Comparable changes in immunophenotype were observed after stimulation of USSC with IFNγ+TNFα, whereas stimulation with IL‐1β or IL‐6 had no effect (data not shown). Thus, even under pro‐inflammatory conditions, USSC reveal a limited expression of immunorelevant molecules.
Activity of USSC in an allogeneic mixed leukocyte reaction
When USSC were used as stimulator cells in an MLR (Fig. 2A), neither unstimulated nor IFNγ or IFNγ+TNFα pre‐stimulated USSC induced a proliferative response by allogeneic T cells (n= 10). Early or late passages of USSC did not alter these results (data not shown).
Figure 2.
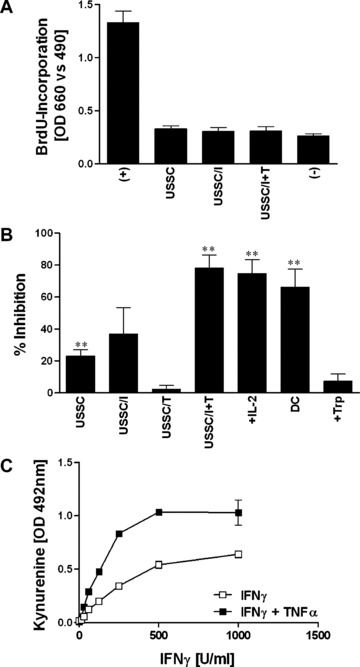
Activity of USSC in an allogeneic mixed leukocyte reaction and kynurenine production. Thirty thousand irradiated USSC, either unstimulated (USSC) or pre‐stimulated with IFNγ (1000 U/ml; 4 days – USSC/I), TNFα (100 U/ml; 4 days USSC/T) or IFNγ+TNFα (1000 U/ml and 100 U/ml, respectively; 4 days – USSC/I+T) were co‐cultured with 105 allogeneic PBMC (A) or as third party with an allogeneic two‐way MLR containing 105 PBMC each of two unrelated donors (B). In certain experiments, at the onset of USSC/MLR co‐cultures (B), IL‐2 (100 U/ml –+IL‐2) or tryptophan (20 μg/ml –+Trp) were added or mature DC served as stimulator cells (DC). After 6 days, BrdU‐incorporation was determined. Allogeneic MLRs without addition of USSC (A) served as positive controls (+) and were set to 100% to calculate percentage inhibition (B). BrdU‐incorporation of responder PBMC only, served as negative controls (–). Assays were performed as triplicates. Results are shown as mean ± S.E.M. for 10 (A) or ≥3 (B) independent experiments. Statistical significance is indicated (**P≤ 0.01). (C) Kynurenine production as a consequence of IDO activity of USSC cultured for 3 days in medium supplemented with tryptophan (50 μg/ml) in the presence of varying doses of IFNγ (0–1000 U/ml) with and without the addition of 100 U/ml TNFα was measured in culture supernatants. Data are presented as mean ± S.E.M. (n= 3) of optical densities at 492 nm.
In order to assess whether USSC modulate an alloresponse, they were co‐cultured as third party cells with PBMC responder and stimulator cells in an allogeneic MLR (Fig. 2B). Unstimulated, IFNγ‐ or TNFα‐ pre‐stimulated USSC caused either no or limited inhibition of BrdU‐incorporation (23.0 ± 4.0%; n= 19, 37.0 ± 16.6%; n= 8 and 2.3 ± 2.3% inhibition; n= 3, respectively). However, after pre‐stimulation with IFNγ together with TNFα, the USSC significantly suppressed T‐cell proliferation (78.1 ± 8.2% inhibition; n= 11, P < 0.001).
This inhibition was independent on IL‐2, because addition of IL‐2 to the MLR in the presence of IFNγ+TNFα pre‐stimulated USSC did not restore T‐cell proliferation (74.7 ± 8.6% inhibition; n= 6; Fig. 2B). Similarly, the use of mature dendritic cells as stimulators also failed to prevent inhibition (73.8 ± 10.3% inhibition; n= 5; Fig. 2B). In contrast, when tryptophan was added to USSC/IFNγ+TNFα– MLR co‐cultures, T‐cell proliferation was restored (5.7 ± 8.9% inhibition; n= 5; Fig. 2B), indicating the involvement of an IDO‐mediated depletion of tryptophan in USSC‐induced T‐cell suppression. This was further supported by the detection of kynurenine, the catabolite of tryptophan, in culture supernatants derived from IFNγ+TNFα pre‐stimulated USSC at levels similar to the pre‐culture tryptophan level in the X‐vivo 15 medium (13.7 ± 3.4 μg/ml kynurenine and 13.6 μg/ml tryptophan, respectively), which suggests that all tryptophan in the X‐vivo 15 medium had been catabolized to kynurenine by IDO. No kynurenine was detected in culture supernatants of unstimulated USSC.
IDO‐activity in USSC was dependent on IFNγ dose, and TNFα acted synergistically with IFNγ in inducing IDO (Fig. 2C). Unstimulated or USSC stimulated with TNFα alone showed no or only very limited IDO‐activity.
Therefore, USSC inhibit T‐cell proliferation via an IDO‐dependent mechanism, which is initiated by pre‐incubation with IFNγ+TNFα.
Modulation of skin GvHR by USSC
To further elucidate the immunosuppressive activity of USSC, their effect on the development of skin GvH‐type alloreactivity was evaluated using a human skin explant assay, which has been shown previously to be predictive for GvHD in human bone marrow transplantation [38].
Responder cells from the primary MLR, set‐up in the presence or absence of TNFα+ IFNγ stimulated or unstimulated USSC, were co‐cultured with skin explants autologous to the stimulator cells. GvH‐like alloreactivity (GvHR) in the skin was graded (grades I‐IV) according to histopathological changes. Unstimulated USSC did not inhibit the GvHR, because in 5/6 experiments the severity of tissue damage was comparable to that observed in positive control assays set‐up the absence of USSC (GvHR grade III, Figs. 3A and 3B, respectively). A mild down‐regulation of skin GvHR from grade III to grade II was observed in only 1/6 experiments. When IFNγ+TNFα pre‐stimulated USSC were used histopathological changes were reduced to negative control levels in all experiments (grade I/0, Figs. 3C and 3D, respectively). The skin explant assay results confirm that USSC cause potent T‐cell immunosuppression, but only when pre‐stimulated with IFNγ+TNFα.
Figure 3.
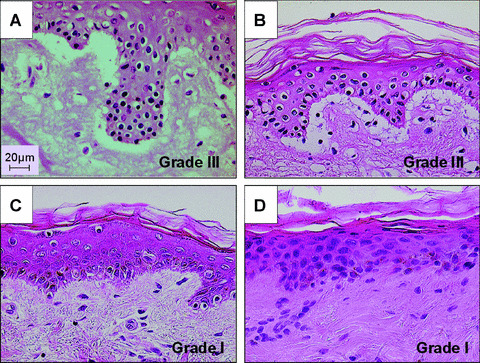
Modulation of skin GvH type alloreactivity by USSC. Irradiated unstimulated (A) or IFNγ+TNFα pre‐stimulated USSC (C) were co‐cultured with an allogeneic MLR using irradiated PBMC of the skin donor as stimulator cells and PBMC of an unrelated, HLA‐mismatched donor as responders (responder‐to‐USSC ratio = 10:1). MLR‐primed responder cells were added to skin biopsies and co‐cultured for an additional 3 days before histopathological evaluation. Skin explants co‐cultured with responder cells primed in the absence of USSC or with medium alone served as positive and negative controls, respectively (B and D).
Interference of USSC with DC differentiation, maturation and function
To determine whether USSC affect antigen‐presenting cell activity, their influence on DC differentiation, maturation and function was studied. DC were generated from monocytes in the presence or absence of USSC with a two‐step culture protocol using GM‐CSF and IL‐4 in the initial differentiation phase and GM‐CSF, IL‐4 and TNFα in the subsequent maturation phase.
Within the first 6 days, monocytes differentiated to immature DC as indicated by down‐regulation of CD14. There was no significant difference between cultures performed in the absence or presence of USSC in cellular yield (48.4 ± 4.3% and 45.5 ± 5.2% recovery; n= 8, respectively) and the frequencies of residual CD14+ (41.2 ± 11.5% and 55.3 ± 8.0% CD14+; n= 8, respectively) or CD1a+ cells (37.5 ± 7.6% and 25.0 ± 7.9% CD1a+; n= 8, respectively). There was also no difference in the density of expression of CD40, CD80, CD86 and HLA‐ABC (data not shown). However, HLA‐DR expression was significantly reduced in density on DC co‐cultured with USSC (mean fluorescence intensities of 6401 ± 566 versus 9583 ± 982; n= 6, P= 0.018, respectively).
The functional competence of the immature DC was evaluated by determining antigen‐uptake activity (Fig. 4A, representative experiment of 14). Co‐culture‐derived immature DC demonstrated potent pinocytosis activity, similar to that observed in control cultures. At a concentration of 10 μg/ml BSA‐FITC, there was no difference between immature DC generated in the presence or absence of 30,000 USSC (mean fluorescence 3477 ± 848 and 3577 ± 988, n= 14, respectively).
Figure 4.
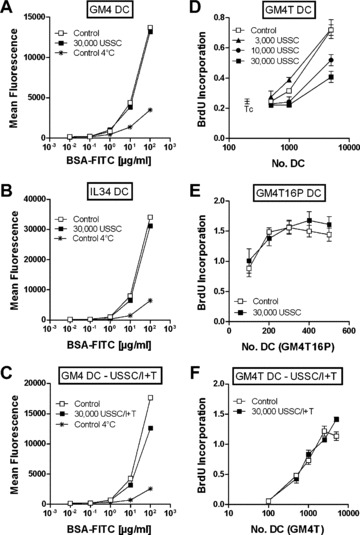
Functional competence of immature and mature monocyte‐derived dendritic cells generated in the presence of USSC. DC were generated in the absence (control) or presence of USSC either unstimulated (A, B, D, E) or pre‐stimulated with IFNγ+TNFα (C, F– USSC/I+T, 4 days 1000 U/ml IFNγ+100 U/ml TNFα). On day 6, immature DC generated either with GM‐CSF+IL‐4 (GM4 DC –A, C) or IL‐3+IL‐4 (IL34 DC –B) were harvested and incubated with BSA‐FITC at the concentrations indicated at 37°C or 4°C (negative controls) for 1 hr. FITC mean fluorescence intensities were determined by flow cytometry. Mature DC were harvested after maturation with GM‐CSF, IL‐4 and TNFα (D, F– GM4T) or GM‐CSF, IL‐4, TNFα, IL‐1β, IL‐6 and PGE2 (E– GM4T16P) on day 9, and graded doses of DC (0–5000) were cocultured with 105allogeneic T cells. After 6 days, BrdU‐incorporation was determined. Results are shown as mean ± S.E.M. of triplicates. BrdU‐incorporation of T cells alone served as control.
When IL‐3 was used instead of GM‐CSF to induce monocyte to DC differentiation (n= 3), similar results were obtained. There was no difference in the frequencies of CD14+ or CD1a+ cells, either in cell yield (data not shown) or in pinocytic activity (Fig. 4B, representative experiment of 3) between cultures performed in the presence or absence of USSC. These results suggest that USSC do not significantly affect monocyte differentiation into immature DC.
Mature DC were analysed after 3 days of maturation with GM‐CSF, IL‐4 and TNFα. The presence of USSC in co‐cultures inhibited the maturation of DC in a dose‐dependent manner as indicated by suppression of CD83 up‐regulation (Fig. 5A). At 30,000 USSC, the frequency of CD83+ cells was 50.6 ± 4.9% compared to 79.2 ± 2.3% in control cultures in the absence of USSC (n= 12; P < 0.001; Fig. 5B). This inhibition was contact‐dependent. In transwell cultures (n= 6) or in the presence of USSC‐conditioned medium (n= 3), no suppression of CD83 up‐regulation was observed (Fig. 5C). In agreement with this observation, neutralization of TGFβ, a factor, which is produced by USSC [16] and may account for suppression of DC maturation, did not restore CD83 expression (Fig. 5C). In control cultures, 77.8 ± 0.4% of cells expressed CD83 compared to 37.6 ± 4.8% and 33.3 ± 3.5% of cells generated in the presence of 30,000 USSC with or without TGFβ neutralizing antibody, respectively (n= 6).
Figure 5.
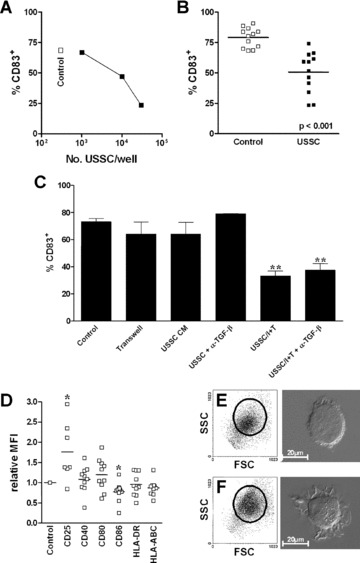
Influence of USSC on maturation of monocyte‐derived dendritic cells. CD14+ monocytes (2×106) were cultured in the absence (Control, F) or presence of irradiated USSC at the cell numbers indicated (A) or 30,000 cells (B, C, D, E) for 6 days with GM‐CSF and IL‐4, followed by a 3‐day maturation period in the presence of GM‐CSF, IL‐4 and TNFα. After a total of 9 days of co‐culture, the frequency of CD83+ cells (A, B, C), the normalized relative expression (mean fluorescence of control cells was set to 1) of the molecules indicated (D) and forward (FSC)/side scatter (SSC) characteristics of cells (E, F) were determined by flow cytometry. Morphology was evaluated by differential interference contrast microscopy (E, F). Horizontal lines (B, D) indicate mean values. In certain experiments (C), USSC and DC in co‐cultures were separated by a membrane (Transwell), USSC conditioned medium (30%) was used instead of cells (USSC CM) or the activity of TGFβ was neutralized with a monoclonal antibody (+α‐TGF‐β; 5 μg/ml α‐TGF‐β(1, 2, 3)). Statistical significance is indicated (*–P≤ 0.05; **–P≤ 0.01).
When the density of co‐stimulatory and HLA‐molecules was analysed, CD86 was significantly reduced (0.77 ± 0.08‐fold; n= 10; P= 0.002) and CD25 elevated (1.76 ± 0.27‐fold; n= 7; P= 0.032) on co‐culture‐derived mature DC, whereas no differences in the expression levels of CD40, CD80, HLA‐ABC and HLA‐DR were detected (Fig. 5D). In transwell cultures, expression of all of these molecules was not affected by the presence of USSC during DC differentiation/maturation (n≥ 5; data not shown), further supporting a contact‐dependent mechanism for USSC‐mediated inhibition of DC maturation.
Consistent with a lower degree of maturation of DC generated in the presence of USSC, such cells did not reveal the typical increase in size and granularity and mature DC morphology (Fig. 5E) as observed in control cultures (Fig. 5F).
When IL‐3 was used instead of GM‐CSF throughout the culture (n= 3), the frequency of mature, CD83+ DC was also reduced after USSC co‐culture (P= 0.020; Fig. 6A). In contrast, increasing the signal for DC maturation by adding IL‐1β, IL‐6 and PGE2 to the maturation cocktail (n= 3), reversed the USSC‐induced inhibition. Consequently there was no difference in the frequency of CD83+, mature DC (Fig. 6B) or in the density of HLA‐ and co‐stimulatory molecule expression (data not shown) between cultures performed in the presence or absence of USSC.
Figure 6.
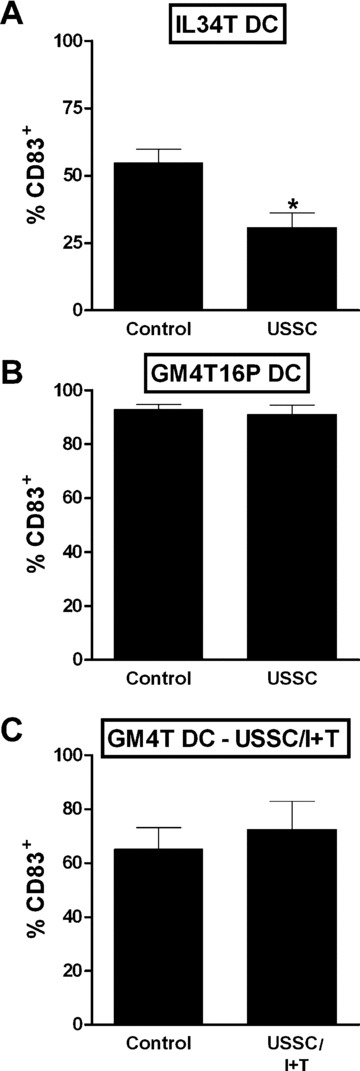
Generation of mature monocyte‐derived DC: Impact of maturation protocol and pre‐stimulation of USSC. Monocyte‐derived, mature DC were generated in the absence (Control) or presence of 30,000 irradiated, unstimulated USSC (A, B) or USSC that had been pre‐stimulated with IFNγ+TNFα (USSC/I+T –C) using the cytokine combinations IL‐3, IL‐4 and TNFα (IL34T DC –A), GM‐CSF, IL‐4, TNFα, IL‐1β, IL‐6 and PGE2 (GM4T16P DC –B) or GM‐CSF, IL‐4 and TNFα (GM4T DC –C). The frequency of CD83+ cells was determined by flow‐cytometry. Data are shown as mean ± S.E.M. for n= 3. Statistical significance (*–P≤ 0.05) is indicated.
Functionally, mature DC generated in the presence of USSC with the cytokine combination GM‐CSF, IL‐4 and TNFα revealed a USSC‐dose‐dependent reduction in allostimulatory activity (Fig. 4D, representative experiment of 12), consistent with their more immature immunophenotype and morphology (Fig. 5). In contrast, DC which had been matured with an increased maturation stimulus, GM‐CSF, IL‐4, TNFα, IL‐1β, IL‐6 and PGE2, revealed no difference in allostimulatory activity, irrespective of whether they had been generated in the presence or absence of USSC (Fig. 4E, representative experiment of 3), consistent with what had been observed immunophenotypically (Fig. 6B). Thus, USSC suppress DC maturation and function but this suppression can be overwritten by these stronger cytokine maturation stimuli.
When USSC pre‐stimulated with TNFα+IFNγ were co‐cultured with developing DC, on day 6 of culture, there was no difference in the frequencies of CD14+ or CD1a+ cells and cellular yield (data not shown). Pinocytic activity of immature DC was comparable or slightly reduced after USSC co‐culture (Fig. 4C, representative experiment of 3). After maturation of DC with TNFα, there was also no difference in the frequency of CD83+ mature DC (65.1 ± 8.0% and 72.5 ± 10.4%; n= 3, for controls and USSC/I+T co‐cultures, respectively; Fig. 6C), in the density of expression of co‐stimulatory and HLA‐molecules (data not shown) as well as the allostimulatory activity of DC (Fig. 4F, representative experiment of 3) generated in the absence or presence of TNFα+IFNγ pre‐stimulated USSC. Therefore, in contrast to unstimulated USSC that were shown to be weak inhibitors of T‐cell proliferation and to suppress DC maturation and function, TNFα+IFNγ pre‐stimulated USSC inhibited T‐cell proliferation but did not suppress the generation of functionally active, mature DC.
Discussion
USSC were shown to lack expression of immunorelevant adhesion and co‐stimulatory molecules even after stimulation with IFNγ, IFNγ+TNFα or other pro‐inflammatory factors. Similar results have been reported for BM‐derived MSC [19, 41, 42]. Like USSC they lack expression of CD11a, CD11b, CD11c, CD18, CD40, and CD49d, whereas CD49d, CD49e and CD58 are expressed. In common with MSC from BM [19, 41], foetal liver [43] and placenta [44] as well as in vitro differentiated MSC [19] and dermal, synovial and lung fibroblasts [20, 21], USSC are positive for HLA‐class I, lack expression of HLA‐class II, CD80 and CD86 and respond to stimulation with IFNγ with up‐regulation of the HLA molecules.
Consistent with the absence of relevant co‐stimulatory and adhesion molecules on USSC, neither unstimulated nor IFNγ or IFNγ+TNFα stimulated USSC showed allostimulatory activity in an MLR. Lack of allostimulatory activity in primary as well as secondary MLR [45], even in the presence of CD28 co‐stimulation [41] or after IFNγ stimulation [19, 41], has also been described for MSC derived from BM [19, 41, 45] and foetal liver [43, 46] as well as for their in vitro differentiated progeny [19, 43]. However, MSC appear to be able to take up antigens [47] and after stimulation with IFNγ, to induce T‐cell responses to recall antigens such as tetanus toxoid [47]. Furthermore, IFNγ stimulated ovalbumin (OVA)‐loaded MSC from mouse and human BM induce antigen‐specific responses of OVA‐specific transgenic T cells and T‐cell hybridomas, respectively, and cause rejection of OVA‐expressing tumour cells [48]. In contrast, Prevosto et al. reported the generation of regulatory T cells after short‐term co‐culture of MSC and PBMC [49], whereas others did not observe such cells [25]. Chan et al. suggested a critical role for IFNγ and that in the initial phase of responses, when IFNγ concentrations are still low, MSC can act as APC, but when IFNγ concentrations increase, they become immunosuppressive [47]. Neither variation of IFNγ dose nor different stimulator‐to‐effector cell ratios (data not shown) resulted in APC activity of USSC.
When the effect of USSC, as third party cells, in an MLR was analysed, only a minor suppressive activity was observed, which was not enhanced further by stimulation of the cells with IFNγ alone. In contrast, unstimulated BM‐derived MSC [19, 23, 32, 41, 47], in vitro differentiated MSC [19] and fibroblasts [20, 21] demonstrate a substantial inhibitory activity of alloresponses, which can be further enhanced by pre‐stimulation with IFNγ alone. USSC in this present study caused nearly complete inhibition of T‐cell responses in the MLR as well as reduction of GvH‐type alloreactivity in the skin explant assay after pre‐stimulation with IFNγ+TNFα. Foetal liver‐derived MSC also require pre‐stimulation, however, IFNγ alone is sufficient for significant inhibition of T‐cell responses [43]. Thus, immunosuppression by USSC is conditional, dependent on TNFα and IFNγ and appears to be regulated differently compared to MSC and more differentiated mesenchymal cells.
Although still controversial, immunosuppression by MSC may be mediated at least partially by soluble factors and contribution of TGFβ[23, 50], HGF [23], HLA‐G [30], PGE2[24], haem oxygenase‐1 [31], nitric oxide [31] and IDO [25, 32, 51] has been suggested. In this study, IDO was identified as the main factor causing suppression of T‐cell responses by IFNγ+TNFα pre‐stimulated USSC. Exogenous IL‐2 or mature DC as stimulator cells did not overcome immunosuppression, but T‐cell proliferation was restored nearly completely by tryptophan supplementation. This argues for a tryptophan‐depletion‐dependent cell cycle arrest of T cells [33] rather than a direct inhibitory effect of the catabolite kynurenine as suggested by others [51]. Inhibitory activity of USSC coincided with IDO activity in the cells. It was dependent on IFNγ dose and enhanced significantly by the synergistic activity of TNFα. Importantly, MSC also appear not to be immunosuppressive per se but to require an initial activating signal provided by other cells such as T cells [25, 32], NK cells [25] and monocytes [50] or cytokines including IFNγ[32, 51] and IL‐1β[50]. Thus,depending on the nature of this signal, different mechanisms of inhibition or even no inhibitory activity at all may result.
Indeed, differences in the immunosuppressive activity of MSC have been described [47, 52] and in vivo models reveal conflicting results. Several studies confirmed immunosuppression in vivo. MSC infusion prolonged skin and heart allograft survival [22, 53]. Transplantation of MSC in experimental mouse autoimmune encephalomyelitis ameliorated the disease [54] and could improve symptoms in collagen‐induced arthritis [55]. Moreover, immunosuppressive properties of MSC have already been exploited successfully in the clinical setting for the treatment of GvHD [56]. In contrast, other studies could not document an immunosuppressive activity of MSC in vivo with allograft survival remaining unchanged or rejection enhanced [57, 58]. Symptoms have also been shown to worsen in mouse collagen‐induced arthritis [59] or GvHD after allogeneic transplantation in mice post MSC therapy [60]. The reasons for these differences are currently not well understood, but a certain type of pre‐conditioning either in vitro (e.g. by stimulation with IFNγ+TNFα) or by in vivo conditions (e.g. pro‐inflammatory conditions) may be required for the immunosuppressive activity of MSC to fully develop. Such differences in in vivo conditions may account for the observation by Tisato et al, that CB‐derived MSC can be used to prevent but not treat GvHD [61]. For USSC, irrespective of the moderate inhibition detected in vitro in the MLR assays for unstimulated cells, only following pre‐stimulation with IFNγ+TNFα, reduction of GvHR‐like symptoms in the ex vivo skin explant model was observed.
Besides directly affecting T‐cell responses, USSC also interfered with dendritic cell activity. Differentiation of monocytes to DC in vitro was not affected by the presence of USSC and the resulting immature DC resembled both phenotypically and functionally DC generated in control cultures. However, TNFα‐induced development of CD83+ fully mature DC was impaired in the presence of USSC. Interestingly, despite a reduced expression of CD83 and CD86, an altered morphology and a significantly reduced allostimulatory potential of the cells, maturation was not inhibited completely since expression of HLA‐class II as well as that of CD40 and CD80 was normal. Thus, DC appear to be arrested in an aberrant intermediate maturation state. This defect in maturation could not be restored by neutralizing TGFβ, and results from transwell cultures, as well as the use of USSC conditioned medium, suggested that the interference was cell‐to‐cell contact mediated and not due to soluble factors, although involvement of local high concentrations of soluble factors cannot be excluded entirely.
Altered morphology, phenotype and impaired functional activity have also been reported for monocyte‐derived DC generated in the presence of MSC [27, 28, 62], whereas dermal fibroblasts showed no such activity [20]. Differentiation, i.e. commitment of monocytes to the DC lineage, as well as maturation was inhibited. Cells remained CD14+, failed to up‐regulate typical DC differentiation/maturation‐associated surface molecules such as CD1a, CD40, CD80, CD83 and CD86, and developed a macrophage‐like morphology. Furthermore, endocytic and allostimulatory activities of the cells were reduced [28, 62, 63]. Beyth et al. have shown an aberrant maturation of DC as well and documented that this block could be partially overridden by maturation inducing stimuli such as LPS or CD40 cross‐linking [64]. Inhibition of DC maturation by USSC was dependent on the strength of maturation signals, with stronger signals abrogating inhibition. When TNFα, IL‐1β, IL‐6 and PGE2 instead of TNFα alone were used to induce DC maturation, no differences in phenotype and function of DC were observed between cultures performed in the presence or absence of USSC. However, the microenvironment, e.g. the presence of pro‐inflammatory conditions or microbial products, also overrides the USSC‐induced blockade of DC maturation. When USSC were pre‐stimulated with IFNγ+TNFα, the same factors, which elicited potent suppression of T‐cell responses, they failed to inhibit DC maturation and function.
Thus, IFNγ and TNFα constitute a switch, which regulates the immunological properties of USSC. In the presence of both cytokines during an ongoing immune effector response in peripheral tissues, USSC migrating to sites of tissue damage may dampen or regulate such responses, but at the same time may allow continuation of recruitment of mature DC to the lymph nodes feeding the response as long as the pro‐inflammatory or microbial conditions persist. A decrease in these stimulating conditions ultimately will cause a reduction of the T‐cell effector response and, thereby, also in local IFNγ and TNFα levels. USSC may contribute to normalizing the immunological environment by turning off DC activity. This well‐balanced process may contribute to the stable differentiation of USSC at sites of tissue repair by preventing the presence of immune effector cells or of cytokines produced in the course of immune responses, which could interfere with the required differentiation pathways.
Acknowledgements
This work has been supported by the Germany JosÈ Carreras Leukemia Foundation, EU‐Grant QLRT‐2001‐01918, by BMBF (National Ministry for Education and Research, Germany) and One NorthEast, Regional Development Agency. The authors would like to thank all Gynecology and Obstetric Departments participating in the Düsseldorf cord blood banking program and Nathalie Walter, Aurèlie Lefort and Mahtab Maleki for excellent technical assistance.
References
- 1. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol . 2000; 109: 235–42. [DOI] [PubMed] [Google Scholar]
- 2. Bieback K, Kern S, Kluter H, et al . Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells . 2004; 22: 625–34. [DOI] [PubMed] [Google Scholar]
- 3. Yang SE, Ha CW, Jung M, et al . Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy . 2004; 6: 476–86. [DOI] [PubMed] [Google Scholar]
- 4. Gang EJ, Jeong JA, Hong SH, et al . Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells . 2004; 22: 617–24. [DOI] [PubMed] [Google Scholar]
- 5. Gang E, Jeong J, Han S, et al . In vitro endothelial potential of human UC blood‐derived mesenchymal stem cells. Cytotherapy . 2006; 8: 215–27. [DOI] [PubMed] [Google Scholar]
- 6. Goodwin HS, Bicknese AR, Chien SN, et al . Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant . 2001; 7: 581–8. [DOI] [PubMed] [Google Scholar]
- 7. Hong SH, Gang EJ, Jeong JA, et al . In vitro differentiation of human umbilical cord blood‐derived mesenchymal stem cells into hepatocyte‐like cells. Biochem Biophys Res Commun . 2005; 330: 1153–61. [DOI] [PubMed] [Google Scholar]
- 8. Kogler G, Sensken S, Airey JA, et al . A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med . 2004; 200: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greschat S, Schira J, Küry P, et al . Unrestricted somatic stem cells from human umbilical cord blood can be differentiated into neurons with a dopamineric phenotype. Stem Cells Dev . 2008; 17: 221–32. [DOI] [PubMed] [Google Scholar]
- 10. Sensken S, Waclawczyk S, Knaupp AS, et al . In vitro differentiation of human cord blood‐derived unrestricted somatic stem cells towards an endodermal pathway. Cytotherapy . 2007; 9: 362–78. [DOI] [PubMed] [Google Scholar]
- 11. Lee OK, Kuo TK, Chen WM, et al . Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood . 2004; 103: 1669–75. [DOI] [PubMed] [Google Scholar]
- 12. Kogler G, Sensken S, Wernet P. Comparative generation and characterization of pluripotent unrestricted somatic stem cells with mesenchymal stem cells from human cord blood. Exp Hematol . 2006; 34: 1589–95. [DOI] [PubMed] [Google Scholar]
- 13. Kim BO, Tian H, Prasongsukarn K, et al . Cell transplantation improves ventricular function after a myocardial infarction: a preclinical study of human unrestricted somatic stem cells in a porcine model. Circulation . 2005; 112: I96–104. [DOI] [PubMed] [Google Scholar]
- 14. Lim JH, Byeon YE, Ryu HH, et al . Transplantation of canine umbilical cord blood‐derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci . 2007; 8: 275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim SW, Han H, Chae GT, et al . Successful stem cell therapy using umbilical cord blood‐derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells . 2006; 24: 1620–6. [DOI] [PubMed] [Google Scholar]
- 16. Kogler G, Radke TF, Lefort A, et al . Cytokine production and hematopoiesis supporting activity of cord blood‐derived unrestricted somatic stem cells. Exp Hematol . 2005; 33: 573–83. [DOI] [PubMed] [Google Scholar]
- 17. Chan SL, Choi M, Wnendt S, et al . Enhanced in vivo homing of uncultured and selectively amplified cord blood CD34+ cells by cotransplantation with cord blood‐derived unrestricted somatic stem cells. Stem Cells . 2007; 25: 529–36. [DOI] [PubMed] [Google Scholar]
- 18. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood . 2007; 110: 3499–506. [DOI] [PubMed] [Google Scholar]
- 19. Le Blanc K, Tammik C, Rosendahl K, et al . HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol . 2003; 31: 890–6. [DOI] [PubMed] [Google Scholar]
- 20. Haniffa MA, Wang XN, Holtick U, et al . Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol . 2007; 179: 1595–604. [DOI] [PubMed] [Google Scholar]
- 21. Jones S, Horwood N, Cope A, et al . The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol . 2007; 179: 2824–31. [DOI] [PubMed] [Google Scholar]
- 22. Bartholomew A, Sturgeon C, Siatskas M, et al . Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo . Exp Hematol . 2002; 30: 42–8. [DOI] [PubMed] [Google Scholar]
- 23. Di Nicola M, Carlo‐Stella C, Magni M, et al . Human bone marrow stromal cells suppress T‐lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood . 2002; 99: 3838–43. [DOI] [PubMed] [Google Scholar]
- 24. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood . 2005; 105: 1815–22. [DOI] [PubMed] [Google Scholar]
- 25. Krampera M, Cosmi L, Angeli R, et al . Role for IFN‐{gamma} in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells . 2006; 24: 386–98. [DOI] [PubMed] [Google Scholar]
- 26. Corcione A, Benvenuto F, Ferretti E, et al . Human mesenchymal stem cells modulate B‐cell functions. Blood . 2006; 107: 367–72. [DOI] [PubMed] [Google Scholar]
- 27. Zhang W, Ge W, Li C, et al . Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte‐derived dendritic cells. Stem Cells Dev . 2004; 13: 263–71. [DOI] [PubMed] [Google Scholar]
- 28. Jiang XX, Zhang Y, Liu B, et al . Human mesenchymal stem cells inhibit differentiation and function of monocyte‐derived dendritic cells. Blood . 2005; 105: 4120–6. [DOI] [PubMed] [Google Scholar]
- 29. Augello A, Tasso R, Negrini SM, et al . Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol . 2005; 35: 1482–90. [DOI] [PubMed] [Google Scholar]
- 30. Nasef A, Mathieu N, Chapel A, et al . Immunosuppressive effects of mesenchymal stem cells: involvement of HLA‐G. Transplantation . 2007; 84: 231–7. [DOI] [PubMed] [Google Scholar]
- 31. Chabannes D, Hill M, Merieau E, et al . A role for heme oxygenase‐1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood . 2007; 110: 3691–4. [DOI] [PubMed] [Google Scholar]
- 32. Meisel R, Zibert A, Laryea M, et al . Human bone marrow stromal cells inhibit allogeneic T‐cell responses by indoleamine 2,3‐dioxygenase‐mediated tryptophan degradation. Blood . 2004; 103: 4619–21. [DOI] [PubMed] [Google Scholar]
- 33. Munn DH, Shafizadeh E, Attwood JT, et al . Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med . 1999; 189: 1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorg RV, Andres S, Kögler G, et al . Phenotypic and functional comparison of monocytes from cord blood and granulocyte colony‐stimulating factor‐mobilized apheresis products. Exp Hematol . 2001; 29: 1289–94. [DOI] [PubMed] [Google Scholar]
- 35. Sorg RV, Ozcan Z, Brefort T, et al . Clinical‐scale generation of dendritic cells in a closed system. J Immunother . 2003; 26: 374–83. [DOI] [PubMed] [Google Scholar]
- 36. Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA . 1996; 93: 2588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daubener W, Wanagat N, Pilz K, et al . A new, simple, bioassay for human IFN‐gamma. J Immunol Methods . 1994; 168: 39–47. [DOI] [PubMed] [Google Scholar]
- 38. Sviland L, Dickinson AM, Carey PJ, et al . An in vitro predictive test for clinical graft‐versus‐host disease in allogeneic bone marrow transplant recipients. Bone Marrow Transplant . 1990; 5: 105–9. [PubMed] [Google Scholar]
- 39. Dickinson AM, Sviland L, Carey P, et al . Skin explant culture as a model for cutaneous graft‐versus‐host disease in humans. Bone Marrow Transplant . 1988; 3: 323–9. [PubMed] [Google Scholar]
- 40. Lerner KG, Kao GF, Storb R, et al . Histopathology of graft‐vs.‐host reaction (GvHR) in human recipients of marrow from HL‐A‐matched sibling donors. Transplant Proc . 1974; 6: 367–71. [PubMed] [Google Scholar]
- 41. Tse WT, Pendleton JD, Beyer WM, et al . Suppression of allogeneic T‐cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation . 2003; 75: 389–97. [DOI] [PubMed] [Google Scholar]
- 42. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol . 2000; 28: 875–84. [DOI] [PubMed] [Google Scholar]
- 43. Gotherstrom C, Ringden O, Tammik C, et al . Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol . 2004; 190: 239–45. [DOI] [PubMed] [Google Scholar]
- 44. Li CD, Zhang WY, Li HL, et al . Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res . 2005; 15: 539–47. [DOI] [PubMed] [Google Scholar]
- 45. Krampera M, Glennie S, Dyson J, et al . Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen‐specific T cells to their cognate peptide. Blood . 2003; 101: 3722–9. [DOI] [PubMed] [Google Scholar]
- 46. Gotherstrom C, Ringden O, Westgren M, et al . Immunomodulatory effects of human foetal liver‐derived mesenchymal stem cells. Bone Marrow Transplant . 2003; 32: 265–72. [DOI] [PubMed] [Google Scholar]
- 47. Chan JL, Tang KC, Patel AP, et al . Antigen‐presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon‐gamma. Blood . 2006; 107: 4817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stagg J, Pommey S, Eliopoulos N, et al . Interferon‐gamma‐stimulated marrow stromal cells: a new type of nonhematopoietic antigen‐presenting cell. Blood . 2006; 107: 2570–7. [DOI] [PubMed] [Google Scholar]
- 49. Prevosto C, Zancolli M, Canevali P, et al . Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell‐lymphocyte interaction. Haematologica . 2007; 92: 881–8. [DOI] [PubMed] [Google Scholar]
- 50. Groh ME, Maitra B, Szekely E, et al . Human mesenchymal stem cells require monocyte‐mediated activation to suppress alloreactive T cells. Exp Hematol . 2005; 33: 928–34. [DOI] [PubMed] [Google Scholar]
- 51. Ryan JM, Barry F, Murphy JM, et al . Interferon‐gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol . 2007; 149: 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Le Blanc K, Tammik L, Sundberg B, et al . Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol . 2003; 57: 11–20. [DOI] [PubMed] [Google Scholar]
- 53. Zhou HP, Yi DH, Yu SQ, et al . Administration of donor‐derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc . 2006; 38: 3046–51. [DOI] [PubMed] [Google Scholar]
- 54. Zappia E, Casazza S, Pedemonte E, et al . Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T‐cell anergy. Blood . 2005; 106: 1755–61. [DOI] [PubMed] [Google Scholar]
- 55. Augello A, Tasso R, Negrini SM, et al . Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen‐induced arthritis. Arthritis Rheum . 2007; 56: 1175–86. [DOI] [PubMed] [Google Scholar]
- 56. Le Blanc K, Frassoni F, Ball L, et al . Mesenchymal stem cells for treatment of steroid‐resistant, severe, acute graft‐versus‐host disease: a phase II study. Lancet . 2008; 371: 1579–86. [DOI] [PubMed] [Google Scholar]
- 57. Eliopoulos N, Stagg J, Lejeune L, et al . Allogeneic marrow stromal cells are immune rejected by MHC class I‐ and class II‐mismatched recipient mice. Blood . 2005; 106: 4057–65. [DOI] [PubMed] [Google Scholar]
- 58. Inoue S, Popp FC, Koehl GE, et al . Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplan-tation . 2006; 81: 1589–95. [DOI] [PubMed] [Google Scholar]
- 59. Djouad F, Fritz V, Apparailly F, et al . Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen‐induced arthritis. Arthritis Rheum . 2005; 52: 1595–603. [DOI] [PubMed] [Google Scholar]
- 60. Sudres M, Norol F, Trenado A, et al . Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft‐versus‐host disease in mice. J Immunol . 2006; 176: 7761–7. [DOI] [PubMed] [Google Scholar]
- 61. Tisato V, Naresh K, Girdlestone J, et al . Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft‐versus‐host disease. Leukemia . 2007; 21: 1992–9. [DOI] [PubMed] [Google Scholar]
- 62. Nauta AJ, Kruisselbrink AB, Lurvink E, et al . Mesenchymal stem cells inhibit generation and function of both CD34+‐derived and monocyte‐derived dendritic cells. J Immunol . 2006; 177: 2080–7. [DOI] [PubMed] [Google Scholar]
- 63. Djouad F, Charbonnier LM, Bouffi C, et al . Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin‐6‐dependent mechanism. Stem Cells . 2007; 25: 2025–32. [DOI] [PubMed] [Google Scholar]
- 64. Beyth S, Borovsky Z, Mevorach D, et al . Human mesenchymal stem cells alter antigen‐presenting cell maturation and induce T‐cell unresponsiveness. Blood . 2005; 105: 2214–9. [DOI] [PubMed] [Google Scholar]


