Abstract
Osteopontin (OPN) is a secreted, integrin‐binding matrix phosphorylated glycoprotein. OPN has been shown to facilitate the progression and metastasis of malignancies and has prognostic value in several types of cancer, including gastric cancer. However, the functional mechanism of OPN mediated metastatic growth in gastric cancer remains unclear. Here, using multiple in vitro and in vivo models, we report that OPN strongly promoted the progression and metastasis of gastric cancer. Immunohistochemical staining revealed that OPN, matrix metalloproteinase (MMP)9 and hypoxia‐inducible factor (HIF)‐1α have statistically significant different expression patterns between well‐ and poorly differentiated tissue samples (P < 0.05). Correlations existed between OPN and MMP9, and between OPN and HIF‐1α (r 1= 0.872, p 1 < 0.01 and r 2= 0.878, p2 < 0.01). Furthermore, OPN dramatically increased colony formation and invasion of gastric cancer cells in vitro and promoted tumour growth and metastasis in vivo. In addition, OPN potently protected gastric cancer cells from serum depletion‐induced apoptosis. Further study shows that OPN activated phosphoinositide 3‐kinase/Akt survival pathway and up‐regulated HIF‐1αvia binding to αvβ3 integrins in gastric cancer cells. Moreover, we found that OPN could activate MMP9 and up‐regulate MMP2. Taken together, our results suggest that the survival‐promoting function is crucial for OPN to promote the development of gastric cancer, and HIF‐1α and MMP9 may play key roles during this process. Thus, targeting OPN and its related signalling network may develop an effective therapeutic approach for the management of gastric cancer.
Keywords: Akt, gastric cancer, HIF‐1α, MMP9, osteopontin (OPN), survival
Introduction
Osteopontin (OPN), also known as early T‐cell activation‐1, was originally discovered as an inducible marker of transformation of epithelial cells. It is a secreted, integrin‐binding matrix phosphorylated glycoprotein involved in a number of cell functions including cell adhesion and migration, anti‐apoptosis, inflammation, angiogenesis, tissue remodelling and tumour development (see reviews [1, 2, 3, 4, 5]). Recent findings showed that OPN expression correlates with tumour progression in breast [6], lung [7], prostate [8], liver [9], stomach [10], colon [11], cervices [12], ovary [13], and that OPN concentration in the plasma of patients with metastatic disease is significantly higher than that in normal serum [14]. Studies have indicated that OPN has multifunctional properties in promoting cell survival, cell adhesion, and cell migration through its interaction with the integrins and CD44 receptors [4, 5]. The C‐terminal fragment of OPN binds directly to variant isofroms of CD44 [15], which could interact with matrix metalloproteinase (MMPs). MMPs are extracellular matrix degrading enzymes that play a crucial role in embryogenesis, tissue remodelling, inflammation, tumour growth, angiogenesis and metastasis.
OPN binds to integrins and induces various cellular signalling events. Multiple signalling molecules are activated by OPN which may contribute to tumour progression and metastatic behaviour. For example, the phosphorylation and activation of various kinases, such as phosphoinositide 3‐kinase (PI3‐K), nuclear factor inducing kinase, phospholipase C, protein kinase C, mitogen‐activated protein kinase (MAPK) [3], induce the DNA binding and transactivation potential of various transcription factors, including nuclear factor‐κB and activator protein‐1 [16]. Thus, OPN contributes to malignancies through both inhibition of apoptosis and activation of various matrix‐degrading proteases such as urokinase plasminogen activator and MMPs [17], leading to tumour cell motility, and metastatic growth. PI3‐K is a central regulator in OPN‐induce survival, cell motility and invasion. It has been documented that OPN protects cells from apoptosis by activating PI3‐K/Akt pathway in BA/F3 murine pro‐B‐cell lines [18]. OPN induces PI3‐K activity and PI3‐K‐dependent Akt phosphorylation through the αvβ3 integrin‐mediated pathway in breast cancer cells [19].
During the process of tumour development, cancer cells have to overcome a number of stresses [20]. Hypoxia is an inevitable stress that tumour cells have to face during the development of solid tumour. HIF‐1α is induced by exposure of cells to hypoxia or growth factors [21]. Because HIF‐1α undergoes rapid ubiquitination and degradation by proteasomes, it is maintained at a low level in normoxic cells. On the contrary, under hypoxia or treatment with iron chelators, the praline hydroxylases keep inactivated, so the HIF‐1α activity is stable [22]. Studies have shown that activation of some oncogenes increases HIF‐1α activity through activating PI3‐K/Akt, MAPK or other pathways [23, 24]. Although PI3‐K/Akt and HIF pathways share many common features, such as promoting cell survival, angiogenesis and tumour malignance and metastasis, how PI3‐K/Akt regulates HIF activity remains controversial [25, 26].
Gastric cancer is one of the most common malignancies around the world [27]. In China, it remains the most frequently diagnosed cancer and the second‐most common cancer‐related cause of death with a high case fatality [27]. As to gastric cancer treatment, the combination of chemotherapeutic agents is the major strategy. However, these agents at clinically effective doses are often accompanied by severe toxicity. This lack of specificity has stimulated the development of a new strategy that targets the molecular signalling pathways in cancer cells, and thus, tends to be less toxic to normal cells than conventional chemotherapies. Recent findings showed that OPN overexpression contributed to the development of gastric cancer. Two studies using DNA microarrays identified OPN as an overexpressed gene in gastric cancer tissues and in highly metastatic gastric cancer cell lines [28, 29]. Other studies have reported that OPN expression at mRNA and protein level and its plasma concentration were all closely related to invasion and metastasis, indicating that OPN may have the potential to be developed into an effective prognostic factor for gastric cancer [10, 30, 31]. However, the functional significance and molecular mechanisms of OPN contributed to gastric carcinogenesis remain unknown.
Here, using multiple in vitro and in vivo models, we examined the roles of OPN expression in the progression of gastric cancer development, and revealed the cellular and molecular mechanisms by which OPN promotes gastric cancer development. Our study demonstrated that OPN increased the survival of gastric cancer cells through PI3‐K/Akt pathway activation and HIF‐1α up‐regulation via binding to αvβ3 integrins. Furthermore, we found OPN could activate MMP9 and up‐regulate MMP2 to promote metastatic growth in gastric cancer.
Materials and methods
Materials
Human recombinant OPN protein and rabbit anti‐phospho‐Akt (Ser 473) antibody were purchased from R&D System (Abingdon, UK). Akt specific inhibitor (1L‐6‐hydroxymethyl‐chiro‐inositol 2‐(R)‐2‐O‐methyl‐3‐O‐octadecylcarbonate), and the PI3‐K inhibitor LY2940002 were purchased from Calbiochem (San Diego, CA, USA). Soft agar was purchased from Merck (Darmstadt, Germany), Mouse anti‐Flag, anti‐Akt, anti‐β‐actin and Goat anti‐OPN were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Mouse anti‐HIF‐1α and anti‐rabbit IgG, antimouse IgG and anti‐goat IgG antibodies were purchased from Santa Cruz (Santa Cruz, CA, USA). Surgical specimens of gastric cancer and normal tissues were selected randomly from Division of Tumor Pathology, Zhongshan Hospital (Xiamen, China). Tissue procurement was approved by the institutional review board of Xiamen University. In vivo animal experiments with female Balb/c nude mice were carried out in the Cancer Research Center, Xiamen University Medical College, China.
Immunohistochemical staining
The tissue sections of the primary gastric cancers, including the well‐ and poorly differentiated gastric cancer tissues, and the matched non‐cancer gastric tissues were fixed in 10% formaldehyde and embedded in paraffin. Sections were then cut and immunohistochemical staining was performed as described previously [31]. Briefly, Unmasking of antigens was performed by microwave heating of sections in citrate buffer (pH 6.0). Sections were incubated overnight at 4°C with the primary antibodies and were then incubated with the secondary antibodies for 30 min. at room temperature. After rinsing, the sections were incubated with DAB, counterstained with haematoxylin, dehydrated, and then mounted. As a negative control, the primary antibody was replaced with normal mouse IgG.
Cell culture and generation of OPN‐producing gastric cancer cells
The human gastric cancer cell line SGC7901 was obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences in Shanghai, and was cultured as previously described [32]. The full open reading frame of human OPN cDNA (a kind gift from Dr. Xiao‐Fan Wang, Duke University Medical Center) was cloned into pcDNA3.1 mammalian expression vector (Invitrogen, Carlsbad, CA, USA) with a Flag tag at C‐terminus of OPN protein. Cell transfections were performed with FuGene 6 reagent (Roche Diagnostics, GmbH, Mannheim, Germany) according to the manufacturer’s instruction. The OPN/pcDNA3.1 plasmid or the vector control was introduced into SGC7901 gastric cancer cells, and the stable cell clones were obtained by 200 μg/ml hygromycin B (Amresco, Solon, OH, USA) selection in the culture medium. Three OPN‐expressing clones and control clone were chosen for the subsequent experiments.
Cell survival assay
The cell viability was measured by 3‐[4,5‐ dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide (MTT, Sigma Chemical Co.) method as previously described [33]. The other method for cell survival assay was done as follows, the cell numbers were determined after trypan blue staining of viable cells in parallel plates. The experiment was repeated at least three times and triplicate assays were performed for each group and data were presented as mean ± S.D.
Soft‐agar growth
Cells (1 × 103 cells/dish) were plated in 35‐mm‐diameter dishes with a top layer of 0.3% agar and a bottom layer of 0.6% agar in medium. A total of 0.3 ml of medium was supplemented every 3 days and the dishes were examined under microscope. After 2 weeks, the number of cell clusters (≥50 cells per cluster) per dish was counted, and cell clusters were photographed. The results were quantified from three independent experiments. Colony formation efficiency was calculated as follows: number of clusters/number of plated cells × 100%. Assays were performed in triplicate for each group of cells and data were presented as mean ± S.D.
Cell invasion assay
Cell invasion assay was performed with Matrigel™‐coated invasion chamber according to the standard procedure [34]. Briefly, the cell suspension was added to the upper chamber of the Matrigel™ coated pre‐hydrate polycarbonate membrane filter. The lower chamber was filled with fronectin‐contained condition medium, which acted as chemoattractant. Cells were added to the upper chamber, and then incubated for 24 hrs. The non‐invaded cells from the upper side of the filter were scraped and removed using moist cotton swab. The invaded cells in the reverse side of the filter were fixed with methanol and stained with haematoxylin and eosin. The invaded cells on the filter were counted under an inverted microscope. Data were presented as mean ± S.D. of three separate experiments to obtain the statistical results.
Zymography analysis
The gelatinolytic activity was measured as described previously [35]. Briefly, to check the effect of OPN on MMP2 and MMP9 expression and activation, the equal OPN‐expressing cells were incubated in serum‐free medium or the equal parental cells were treated with 400 ng/ml OPN in serum‐free medium, and all the cells were incubated for 24 hrs. The equal condition medium was colleted by centrifugation, and loaded onto zymographic SDS‐PAGE containing gelatin (0.1% (w/v)). The gels were washed and incubated in incubation buffer for 48 hrs. The gels were stained with Coomassie Blue and destained. The zones of gelatinolytic activity were shown by negative staining.
Western blot analysis
Western blot was performed as described before [33, 36]. After harvesting, the experimental cells were washed and lysed in lysis buffer. The total lysate was mixed with 4= loading buffer, and pre‐heated at 95°C for 10 min. The samples were then loaded onto SDS‐PAGE. The proteins were transferred onto a PVDF membrane by semi‐dry transfer system (Bio‐Rad, Hercules, CA, USA). The membrane was blocked in 5% milk, and then incubated 1 hr or overnight at 4°C with primary antibody. After hybridization with primary antibody, the membrane was washed and incubated with HRP‐labelled secondary antibody. Final detection was performed with ECL™ (enhanced chemiluminescence) Western blotting reagents (Amersham, Pharmacia Biottech, San Francisco, CA, USA). The blot was then stripped at 50°C for 30 min. The membranes were re‐blotted for detection of other proteins after extensive wash.
Xenograft assays in nude mice
In vivo tumourigenesis experiments were conducted in nude mice. Two OPN‐overexpressing clones (nos. O3 and O16), control vector and Parental cells were used. Eighteen female Balb/c nude mice, aged 8 weeks were randomly divided into three groups. Two groups were injected via subcutaneous injection with the equal cells of OPN‐expressing SGC7901 and the control clone Vec/SGC7901 (2 = 106/mice) in the left and the right flanks respectively. In separate experiments, the SGC7901 cells were pre‐treated with 400 ng/ml OPN for 24 hrs, and injected into the left flanks. Meanwhile, the same number of untreated cells was injected into the right flanks as control. 400 ng/ml OPN was again injected into the tumour sites twice a week for up to 5 weeks.
In other experiments, OPN‐expressing SGC7901 (nos. O3 and O16) (6×106/mice) and the same number of the control Vec/SGC7901 were injected into nude mice by intraportal vein injection. Four nude mice were used in each set of experiments. All nude mice were killed 5 weeks after injection and examined for the growth of subcutaneous tumours and micrometastases formation.
Results
Correlations of OPN, MMP9 and HIF‐1α expression in human gastric cancer tissues
To assess the correlation of OPN, MMP9 and HIF‐1α expression in human primary gastric cancer, we examined a series of matched tissue sections, including well‐differentiated gastric cancer, poorly differentiated gastric cancer, and the adjacent normal gastric tissues from the same patients by immunohistochemical analysis. The anti‐OPN, anti‐MMP9 and anti‐HIF‐1α antibodies were shown to specifically recognize the corresponding proteins (Fig. 1A–C, middle and right panels). The immunohistochemical staining was performed on 32 cases of primary gastric cancer tissues, including 20 cases of well differentiated, and 12 cases of poorly differentiated tumour tissues, as well as 32 cases of matched non‐cancer gastric tissues. As shown in Fig. 1, the immunostaining analyses indicated that high levels of OPN, MMP9 and HIF‐1α were present in areas containing cancer cells of the primary gastric tumours, and more intense protein staining was seen in the poorly differentiated tumour tissues (Fig. 1A–C, right panels). In contrast, OPN, MMP9 and HIF‐1α were hardly detectable in all matched normal gastric tissues. Overall, in the total 32 cases of primary gastric cancer tissues include both well‐ and poorly differentiated samples, 21 (65.6%) cases showed
Figure 1.
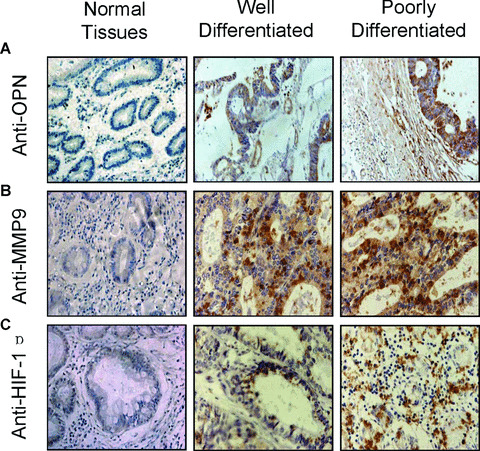
Immunohistochemical staining of OPN, MMP9 and HIF‐1α in human gastric cancer tissues. The tissue sections of the primary gastric cancers, including the well‐ and poorly differentiated gastric cancer tissues, and the matched non‐cancer gastric tissues, were immunostained with anti‐OPN (A), anti‐MMP9 (B) and anti‐HIF‐1α (C) antibodies respectively. The positive staining for OPN, MMP9 or HIF‐1α proteins were shown in brown colour. All sections were counterstained with haematoxylin showing in blue colour (×100).
OPN1 expression, 26 (81.3%) cases showed MMP9+ expression, and 21 (65.6%) cases showed HIF‐1α+ expression (Table 1). Moreover, all cases of poorly differentiated tumour tissues showed OPN‐, MMP9‐ and HIF‐1α+ expression, which presented statistically significant differences compared to the well‐differentiated gastric cancer (P < 0.01, Table 1).
Table 1.
Correlations analysis of OPN, MMP9 and HIF‐1α expression in human gastric cancer tissues
| Proteins | Cases | r‐value | P‐value | ||
|---|---|---|---|---|---|
| Total | (+) | (−) | |||
| OPN | 32 | 21 | 11 | − | − |
| MMP9 | 32 | 26 (19*) | 6 | 0.872 | P < 0.01 |
| OPN | 32 | 21 | 11 | − | − |
| HIF‐1α | 32 | 21 (18**) | 11 | 0.878 | P < 0.01 |
*Indicates the co‐expression cases of OPN and MMP9; **indicates the co‐expression cases of OPN and HIF‐1α.
Furthermore, the statistical analysis was shown in Table 1, there was a correlation between OPN and MMP9, since the majority of cases (19 cases) with MMP9+ expression (26 cases) also expressed high OPN levels (r= 0.872, P < 0.01). Another correlation was also found between OPN and HIF‐1α, since high HIF‐1α expression occurred predominantly (65.6%) in association with high levels of OPN (r= 0.878, P < 0.01). Taken together, these observations indicate that OPN expression was associated with MMP9 and HIF‐1α expression.
Overexpression of OPN promoted gastric cancer cellular survival
To further assess the functional significance of elevated OPN expression during the development of gastric cancer, we investigated whether stable overexpression of OPN in a human gastric cancer cell line could alter cellular survival in either nomoxia or hypoxia condition in vitro. We selected a human gastric cancer cell line SGC7901, which expresses a very low level of endogenous OPN (Fig. 2A, middle band). A C‐terminal Flag‐tagged OPN expression construct was transfected into the SGC7901 cells. Three stable clones (nos. O3, O16 and O19) and vector‐transfected controls (V) were verified with specific antibodies against Flag‐tag and OPN (Fig. 2A).
Figure 2.
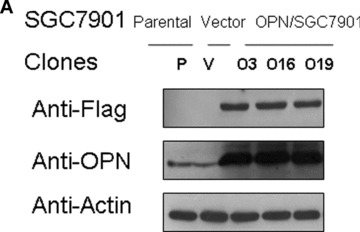
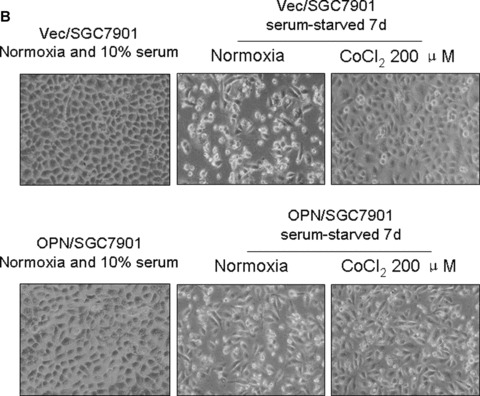
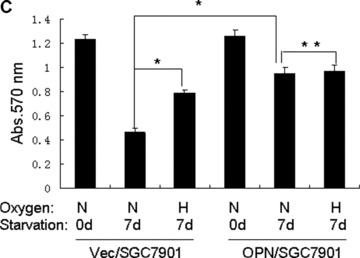
Overexpression of OPN promoted gastric cancer SGC7901 cellular survival in serum depleted condition. (A): Characterization of OPN‐overexpressing SGC7901 clones and vector control clone. Equivalent number of parental SGC7901 cells (P), one vector control SGC7901 clone (V) and three OPN‐producing SGC7901 clones (nos. O3, O19 and O16) were seeded in culture medium in 100 mm culture dishes. Twenty‐four hours later, cells were washed with PBS and undergone starvation in 4 ml serum‐free medium for another 48 hrs. Then the conditional media from the culture were subjected to Western blot assay using anti‐OPN and anti‐Flag antibodies. Levels of β‐actin were monitored as loading control. (B) and (C): Effects of serum starvation and hypoxia on OPN/SGC7901 cell viability. The Vec/SGC7901 and OPN/SGC7901 cells were serum‐starved for one week in the presence or absence of 200 μM CoCl2. Cells were observed and the cell micrographs were taken in random microscopic fields (×100), and the cell numbers were counted by MTT assay. *P < 0.05 and **P > 0.05 (SPSS 11.0).
We next examined whether OPN overexpression and CoCl2‐induced hypoxia could affect gastric cancer cell survival in serum‐depleted condition. As shown in Fig. 2B and C, results from three independent experiments revealed that hypoxia promoted SGC7901 cells are indeed more resistant to serum starvation in comparison with control (P < 0.05). Similarly, OPN‐producing SGC7901 cells were also shown to prevent serum depletion‐induced apoptosis (Fig. 2B and C). These observations suggest that OPN could act to promote cellular survival for gastric cancer cells by mimicking the effect of hypoxia.
OPN activated PI3‐K/Akt pathway and up‐regulated HIF‐1αvia the αvβ3 integrins
To further explore the molecular mechanisms by which OPN promotes cellular survival, we examined the potential effect of OPN on the activity of Akt and HIF‐1α. We found that OPN activated PI3‐K/Akt pathway and increased the cellular level of HIF‐1α protein. As shown in Fig. 3A and B, Ser 473 phosphorylation, which indicates the activation of Akt pathway, was readily detected and HIF‐1α was up‐regulated in SGC7901 cells overexpressing OPN under either normoxic conditions or CoCl2‐induced hypoxia conditions. To define whether the impact of OPN on Akt pathway activation and HIF‐1α up‐regulation is a primary effect or secondary to other cellular effects induced by OPN expression, we examined the effect of exogenous human recombinant OPN on Akt activation and HIF‐1α expression in the parental SGC7901 cells, and the similar results were observed (Fig. 3C).
Figure 3.
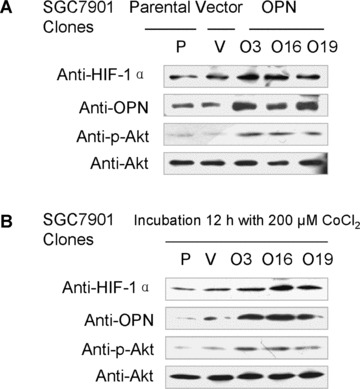
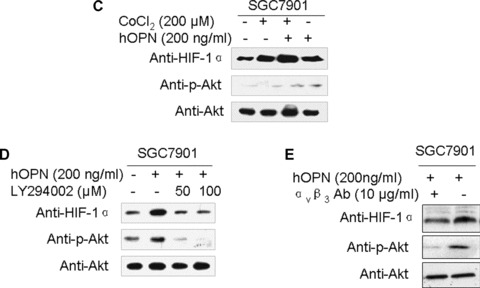
OPN activated PI3‐K/Akt pathway and up‐regulated HIF‐1αvia the αvβ3 integrins in a hypoxia‐independent manner. A and B: Overexpression of OPN activated Akt pathway and up‐regulated HIF‐1α under normoxic conditions and hypoxia conditions induced by CoCl2 respectively. All samples were lysed for HIF‐1α, OPN, p‐Akt (Ser 473) and Akt detection by Western blot analysis. (C): Exogenous OPN up‐regulated the cellular level of HIF‐1α and activated Akt pathway under normoxic conditions. (D): PI3‐K/Akt pathway inhibition attenuated OPN‐mediated HIF‐1α up‐regulation. (E): HIF‐1α expression and Akt phosphorylation on Ser 473 were blocked by an antibody against the αvβ3 integrins.
To elucidate the possible role of the PI3‐K/Akt pathway on OPN‐mediated HIF‐1α up‐regulation under normoxic conditions, we used PI3‐K inhibitor LY2940002 to block the PI3‐K activity and examined its effect on the OPN‐mediated HIF‐1α up‐regulation. As shown in Fig. 3D, LY2940002 inhibits OPN‐induced Akt phosphorylation and HIF‐1α up‐regulation dramatically, suggesting that PI3‐K and Akt activity are required for OPN to enhance HIF‐1α expression. To further determine whether the αvβ3 integrins are involved in the regulation of OPN in gastric cancer, we pre‐incubated the SGC7901 cells with antibody against the αvβ3 integrins and measured the OPN‐induced Ser473 phosphorylation of Akt and expression of HIF‐1α. As shown in Fig. 3E, antibody blockade of OPN and αvβ3 integrins interaction reduces the activation of Akt and HIF‐1α expression, indicating that OPN up‐regulates HIF‐1αvia Akt pathway through interaction with the αvβ3 integrins.
OPN potently promoted gastric cancer cells growth in vitro and in vivo
To further assess the biological significance of elevated OPN expression during the development of gastric cancer, we investigated whether stable overexpression of OPN in human gastric cancer cells or introduction of exogenous human recombinant OPN could alter the tumour growth in vitro and in vivo. Firstly, we examined whether OPN overexpression could affect the colony formation efficiency in soft agar. The colony formation efficiency in soft agar indicates the ability of cells to grow and is correlative with the tumour malignant degree [37]. As shown in Fig. 4A, colony formation analysis indicated that the clone size of SGC7901 cells transfected with vector construct is smaller than that of OPN overexpressing SGC7901 cells. The colony formation efficiency was notably different between vector control and OPN/ SGC7901 clones (P < 0.01). To further investigate the impact of OPN on tumour growth in vivo, we injected the gastric cancer cells into the nude mice to assess the potential differences in tumour growth between the OPN‐overexpression cells and the vector control cells. As shown in Fig. 4B, OPN overexpression appeared to significantly enhance the subcutaneous tumour growth over the controls and the average ratio of tumour weight between OPN‐expressing clones and vector clone was more than twofold (P < 0.05). In separate experiments, the SGC7901 cells were pre‐treated with exogenous human recombinant OPN, and were then injected into the nude mice. Exogenous OPN was repeatedly injected into the tumour sites twice a week for up to 5 weeks, and the similar results were observed (Fig. 4C). Taken together, OPN overexpression or introduction of exogenous human recombinant OPN could enhance gastric tumour growth in vivo.
Figure 4.
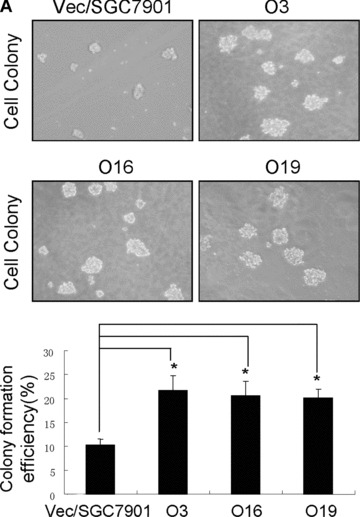
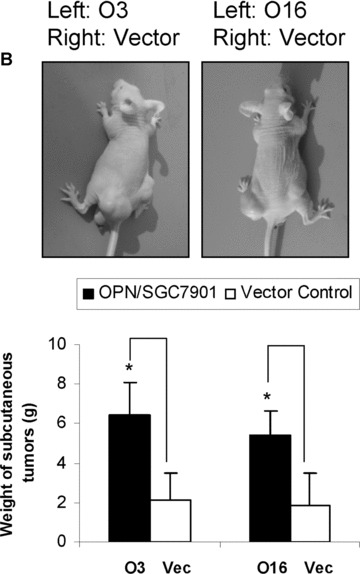
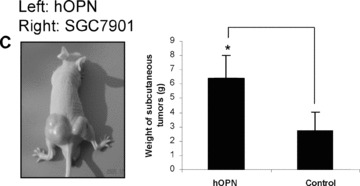
OPN promoted the colony formation in vitro and enhanced gastric cancer growth in vivo. A: Overexpression of OPN increased the colony formation in vitro. The micrographs cell colonies were taken in random microscopic fields, and the efficiency of clone formation was measured as described in ‘Materials and methods’, and the results were obtained from three independent experiments. *P < 0.01 compared with vector control (SPSS 11.0). (B) and (C): OPN promoted gastric cancer growth in vivo. Two OPN‐overexpressing clones (nos. O3 and O16), vector control clone (B) and the SGC7901 cells treated in the presence or absence of exogenous OPN (C) were injected into Balb/c nude mice via subcutaneous injection. After 35 days, the mice were killed and analysed for tumour formation. *P < 0.05 compared with vector control (SPSS 11.0).
Overexpression of OPN in gastric cancer cells activated pro‐MMP9 to promote tumour metastasis
To examine how OPN regulates MMPs and promotes gastric cancer metastasis, we evaluated whether stable overexpression of OPN in human gastric cancer cells or introduction of exogenous human recombinant OPN could alter the in vitro tumour cells invasion and in vivo tumour metastasis. In vitro cell invasion assay was performed with Matrigel™‐coated invasion chamber as described in ‘Materials and methods’. As shown in Fig. 5A, the significant difference in invasiveness was observed between OPN/SGC7901 clones and vector control (P < 0.01). We next examined whether OPN overexpression could affect the metastatic potential and growth of the cancer cells in vivo. Equal numbers of cells (6 × 106/mice) of two OPN‐producing gastric cancer clones or the vector controls were introduced into the nude mice via intrasplenic injection. Five weeks after the inoculation of tumour cells, the animals were killed for the examination of metastatic growth. Strikingly, tumour metastases were detected in the intestine and lung of the survived mice with injection of the OPN‐producing SGC7901 cells (Fig. 5B and C). In contrast, no visually observable metastatic tumours were found in the intestine and the lung of the control mice injected with the vector control cells (Fig. 5B and C). Furthermore, we examined OPN and MMP9 expression on the sections of those metastatic tumours by using immunohistochemical staining. As shown in Fig. 5D and E with the representative staining pattern of OPN and MMP9 expression, the metastatic tumours of the intestine and lung tissues derived from the OPN‐producing gastric cancer cells have much more intensely expression pattern of MMP9 than that of normal intestine and lung tissues. These results suggest that overexpression of OPN in gastric cancer cells strongly promoted tumour metastatic growth in vivo, supporting a critical role for OPN in the late stage of tumour progression.
Figure 5.
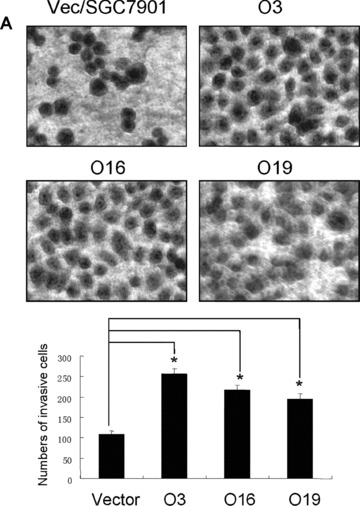
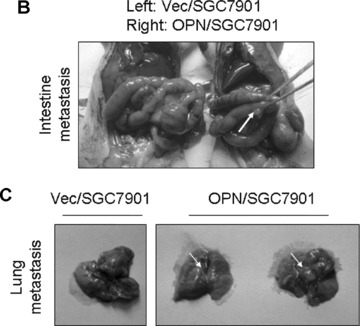
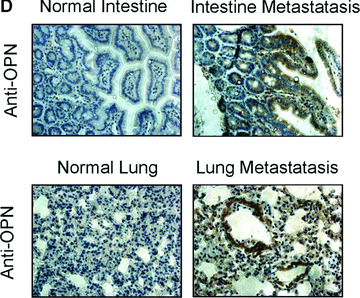
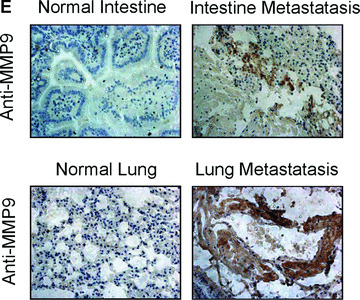
Overexpression of OPN in gastric cancer cells promoted cell invasion and tumour metastasis. (A): Overexpression of OPN increased cell invasion in vitro. The micrographs invasive cells by haematoxylin and eosin stained were taken in random microscopic fields (×400), and the numbers of invasive cells, which obtained from three independent experiments with three OPN‐overexpressing clones and one vector control clone, were counted. *P < 0.01 compared with vector control (SPSS 11.0). (B) and (C): Typical photographs of OPN‐promoted tumour metastasis in the intestine and lung via intrasplenic injection as xenografts in nude mice. (D) and (E): Expression of OPN and MMP9 in the tumour metastasis of the intestine and lung tissues in nude mice by immunohistochemical analysis. The positive staining for OPN and MMP9 proteins were shown in brown colour. All sections were counterstained with haematoxylin showing a blue colour (×100).
Previous reports indicated that MMPs, including MMP9 and MMP2, which are activated by OPN in vitro, play a key role in cancer progression [17, 36]. To determine whether MMP9 and MMP2 are involved in the mediation of OPN action in gastric cancer progression, we next examined the potential effect of OPN on the activity of MMP9 and MMP2 using zymography and Western blot analysis. The equal numbers of OPN‐expressing cells were incubated in serum‐free medium or the equal parental cells were treated with exogenous human recombinant OPN in serum‐free medium. The conditioned media were collected, and the gelatinolytic activity of MMP9 and MMP2 were measured by zymography. Increased levels of MMP2 expression and MMP9 activation (92 kD pro‐ and 86 kD active forms) were observed in OPN‐expressing cells or parental cells treated with exogenous human recombinant OPN, respectively (Fig. 6A and B). In contrast, almost no active MMP9‐specific bands or pro‐MMP2 bands were detected in the control cells. OPN‐expressing cells and control cells were incubated in serum‐free medium and lysed for Western blot analysis, and similar results were observed and shown in Fig. 6C and D.
Figure 6.
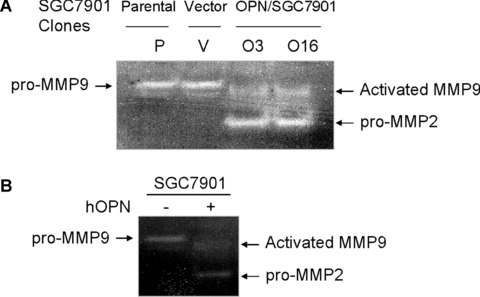
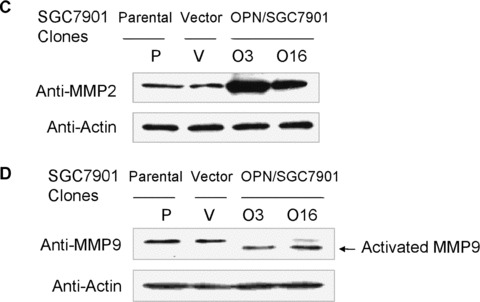
OPN activated pro‐MMP9 and up‐regulated MMP2 in gastric cancer cells. (A) and (B): Overexpression of OPN and exogenous human recombinant OPN protein activated pro‐MMP9 and up‐regulated MMP2 in gastric cancer cells by zymography analysis as described in Materials and Methods. (C) and (D): Overexpression of OPN up‐regulated MMP2 and activated MMP9 in gastric cancer cells shown by Western blot analysis.
Discussion
Cancer cells commonly resist cell death either through disruption of apoptotic processes or activation of survival signals. Within a growing tumour mass, the sequential acquisition of a number of genetic and epigenetic alterations during tumour progression also enable cancer cells to gain the ability to escape from apoptosis, induce angiogenesis and metastasize to distant organs [20, 38]. We have previously demonstrated that a secreted protein called periostin which is overexpressed in colon cancers can activate the Akt signalling pathway through the avb3 integrins to augment the survivals of both cancer cells and endothelial cells, and dramatically enhance metastatic growth of colon cancers [20]. OPN is another secreted protein that plays an important role in the progression of tumour development [1, 3, 5]. Our results suggest that the underlying mechanism of OPN‐mediated promotion of tumour development is largely associated with the ability of Akt activation to enhance cell survival under stresses. In this regard, acquired expression of OPN and similar types of proteins may enable tumour cells to thrive in the hypoxic microenvironment. The discovery of a link between OPN, HIF‐1α and the PI3‐K/Akt signalling pathway may provide a molecular explanation for the pro‐survival activity of OPN in the context of tumour progression.
The inner microenvironment of solid tumour is commonly hypoxic. How cancer cells adapt to the hypoxic condition in a solid tumour is critical for tumour progression. Currently, intratumoural hypoxia has been increasingly considered as a critical microenvironmental factor which promotes tumour aggressiveness and metastasis. Stabilization of the HIF‐1α transcription complex can promote tumour progression and metastasis, leading to treatment failure and mortality in various human cancers [22, 39, 40]. Here we found that OPN could also increase the expression of HIF‐1α, which may be another mechanism by which OPN stimulates the metastatic growth of gastric cancer in vivo. Therefore, the discovery of OPN‐mediated HIF‐1α up‐regulation may provide a molecular explanation for the pro‐survival activity of OPN.
In addition to playing a critical role in the tumourigenicity, OPN appears to be involved in metastasis, as a recent report showed that a significant increase of OPN expression in patients with metastatic tumours [5]. The ability for cancer cells to survive and grow in the distant organ is critical for the successful development of metastases. Here, we observed that tumour metastases were detected in the intestine and lung of the survived mice with injection of the OPN‐producing SGC7901 cells via intrasplenic injection. In contrast, no visually observable metastatic tumours were found in the survived mice with injection of the OPN‐producing SGC7901 cells via subcutaneous injection. Our results indicate that the presence of OPN did not significantly affect the initial formation of micrometastases in the animal models, but that it did stimulate metastatic growth and development at later stage by promoting cancer cell survival. Obviously, OPN is intimately involved in the critical steps of tumour metastatic process. OPN could be a potential target for the development of novel treatment for metastatic gastric cancers.
Previous studies indicated that OPN binds to a variety of cell surface receptors including integrins αvβ1, αvβ3, αvβ5 and CD44 [15, 41, 42]. OPN functions are primarily executed through a division of labour between integrin αvβ3 and CD44 [43, 44, 45]. Recently, correlative studies suggest that αvβ3 may be particularly associated with increased tumour aggressiveness [46, 47]. However, the relative contribution of these integrins to gastric cancer cell malignancy remains uncertain. To gain further insight into the role of OPN in gastric cancer cell survival, we pre‐incubated the SGC7901 cells with antibody against the αvβ3 integrins and measured the phosphorylation of Akt on Ser 473 and the expression of HIF‐1α induced by the presence of OPN. The results demonstrated that, without binding to the αvβ3 receptor, OPN can neither activate the PI3‐K/Akt kinase cascade nor increase the expression of HIF‐1α. The in vitro results indicate that HIF‐1α up‐regulation via PI3‐K/Akt pathway by OPN is mediated primarily through the αvβ3 integrins (Fig. 7). Moreover, we observed that OPN could activate MMP9 and up‐regulate MMP2 in our gastric cancer model. MMPs, including MMP9 and MMP2, which are activated by OPN in vitro[17, 36], play a key role in cancer progression. Indeed, the link we found in gastric cancer model between MMP9 and OPN may provide the biological basis in the biological diagnosis and prognosis of gastric cancer.
Figure 7.
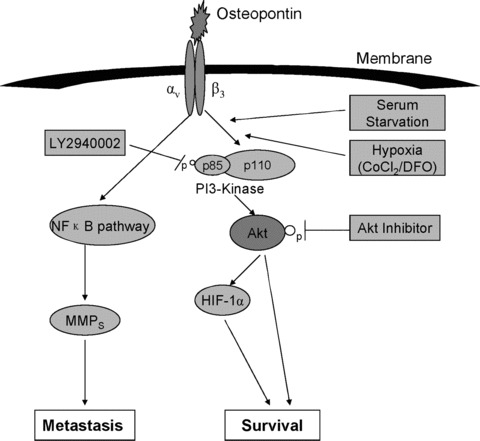
Molecular mechanisms of OPN promoted gastric cancer metastatic growth through MMPs activation and Akt‐mediated HIF‐1α up‐regulation. Binding of OPN to αvβ3 integrin receptor induces phosphorylation and activation of PI3‐K, which subsequently activates Akt through phosphorylation. HIF‐1α is then up‐regulated, thus promotes cell survival. In addition, recent studies showed that OPN promoted cell motility and invasion through nuclear factor‐κB pathways [21, 42]. Here, OPN regulated pro‐MMP9 activation and up‐regulated MMP2 in gastric cancer cells, thus regulated cell motility, invasion and tumour growth.
In conclusion, the present studies demonstrate that acquired expression of OPN may enable gastric cancer cells to thrive under stress conditions by activating PI3‐K/Akt survival pathway and up‐regulating cellular level of HIF‐1αvia binding to αvβ3 integrins. Moreover, OPN could activate MMP9 and up‐regulate MMP2 to promote metastatic growth in gastric cancer. These findings indicate that OPN is closely associated with the progression of gastric cancer and may be considered as a useful molecular target for the therapeutics of gastric cancer.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 30871242, 30400239, 30570935), the Program for New Century Excellent Talents in Xiamen University (to O.G.) and the ‘985 project’ of Xiamen University (to T.H.).
References
- 1. Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001; 1552: 61–85. [DOI] [PubMed] [Google Scholar]
- 2. Rittling SR, Chambers AF. Role of osteopontin in tumor progression. Br J Cancer. 2004; 90: 1877–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006; 16: 79–87. [DOI] [PubMed] [Google Scholar]
- 4. Chakraborty G, Jain S, Behera R, et al. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr Mol Med. 2006; 6: 819–30. [DOI] [PubMed] [Google Scholar]
- 5. Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008; 27: 103–18. [DOI] [PubMed] [Google Scholar]
- 6. Tuck AB, O’Malley FP, Singhal H, et al. Osteopontin expression in a group of lymph node negative breast cancer patients. Int J Cancer. 1998; 79: 502–8. [DOI] [PubMed] [Google Scholar]
- 7. Chambers AF, Wilson SM, Kerkvliet N, et al. Osteopontin expression in lung cancer. Lung Cancer. 1996; 15: 311–23. [DOI] [PubMed] [Google Scholar]
- 8. Khodavirdi AC, Song Z, Yang S, et al. Increased expression of osteopontin contributes to the progression of prostate cancer. Cancer Res. 2006; 66: 883–8. [DOI] [PubMed] [Google Scholar]
- 9. Pan HW, Ou YH, Peng SY, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003; 98: 119–27. [DOI] [PubMed] [Google Scholar]
- 10. Dai N, Bao Q, Lu A, Li J. Protein expression of osteopontin in tumor tissues is an independent prognostic indicator in gastric cancer. Oncology. 2007; 72: 89–96. [DOI] [PubMed] [Google Scholar]
- 11. Agrawal D, Chen T, Irby R, et al. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002; 94: 513–21. [DOI] [PubMed] [Google Scholar]
- 12. Sakaguchi H, Fujimoto J, Hong BL, et al. Clinical implications of osteopontin in metastatic lesions of uterine cervical cancers. Cancer Lett. 2007; 247: 98–102. [DOI] [PubMed] [Google Scholar]
- 13. Kim JH, Skates SJ, Uede T, et al. Osteopontin as a potential diagnostic biomarker for ovarian cancer. JAMA. 2002; 287: 1671–9. [DOI] [PubMed] [Google Scholar]
- 14. Fedarko NS, Jain A, Karadag A, et al. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001; 7: 4060–6. [PubMed] [Google Scholar]
- 15. Weber GF, Ashkar S, Glimcher MJ, et al. Receptor‐ligand interaction between CD44 and osteopontin (Eta‐1). Science. 1996; 271: 509–12. [DOI] [PubMed] [Google Scholar]
- 16. Das R, Mahabeleshwar GH, Kundu GC. Osteopontin induces AP‐1 mediated secretion of urokinase type plasminogen activator through c‐Src dependent epidermal growth factor receptor transactivation in breast cancer cells. J Biol Chem. 2004; 279: 11051–64. [DOI] [PubMed] [Google Scholar]
- 17. Rangaswami H, Bulbule A, Kundu GC. Nuclear factor inducing kinase plays a crucial role in osteopontin induced MAPK/IkBa kinase dependent nuclear factor‐ kB mediated promatrixmetalloproteinase‐9 activation. J Biol Chem. 2004; 279: 38921–35. [DOI] [PubMed] [Google Scholar]
- 18. Lin YH, Yang‐Yen HF. The osteopontin‐CD44 survival signal involves activation of the phosphatidylinositol 3‐kinase/Akt signaling pathway. J Biol Chem. 2001; 276: 46024–30. [DOI] [PubMed] [Google Scholar]
- 19. Das R, Mahabeleshwar GH, Kundu GC. Osteopontin stimulates cell motility and nuclear factor‐kB mediated secretion of urokinase type plasminogen activator through phosphotidylinositol 3‐kinase/Akt signaling pathways in breast cancer cells. J Biol Chem. 2003; 278: 28593–606. [DOI] [PubMed] [Google Scholar]
- 20. Bao S, Ouyang GL, Bai XF, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004; 5: 329–39. [DOI] [PubMed] [Google Scholar]
- 21. Déry MAC, Michaud MD, Richard DE. Hypoxia‐inducible factor 1: regulation by hypoxic and non‐hypoxic activators. Int J Biochem Cell Biol. 2005; 37: 535–40. [DOI] [PubMed] [Google Scholar]
- 22. Harris AL. Hypoxia: a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2: 38–47. [DOI] [PubMed] [Google Scholar]
- 23. Dimova EY, Kietzmann T. The MAPK pathway and HIF‐1 are involved in the induction of the human PAI‐1 gene expression by insulin in the human hepatoma cell line HepG2. Ann N Y Acad Sci. 2006; 1090: 355–67. [DOI] [PubMed] [Google Scholar]
- 24. Zhong H, Chiles D, Feldser D, et al. Modulation of hypoxia‐inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol‐3kinase/PTEN/ AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000; 60: 1541–5. [PubMed] [Google Scholar]
- 25. Alvarez‐Tajado M, Alfranca A, Aragones J, et al. Lake of evidence for the involvement of the phosphoinositide 3‐kinase/Akt pathway in the activation of hypoxia‐inducible factors by low oxygen tension. J Biol Chem. 2002; 277: 13508–17. [DOI] [PubMed] [Google Scholar]
- 26. Arsham AM, Plas DR, Thompson CB, et al. phosphoinositide 3‐kinase/Akt signaling is neither required for hypoxic stablilization of HIF‐1α nor sufficient for HIF‐1‐dependent target transcription. J Biol Chem. 2002; 277: 15162–70. [DOI] [PubMed] [Google Scholar]
- 27. Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics 2002. CA Cancer J Clin. 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 28. Lee S, Baek M, Yang H, et al. Identification of genes differentially expressed between gastric cancers and normal gastric mucosa with cDNA microarrays. Cancer Lett. 2002; 184: 197–206. [DOI] [PubMed] [Google Scholar]
- 29. Fukui R, Nishimori H, Hata F, et al. Metastases‐related genes in the classification of liver and peritoneal metastasis in human gastric cancer. J Surg Res. 2005; 129: 94–100. [DOI] [PubMed] [Google Scholar]
- 30. Wu CY, Wu MS, Chiang EP, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2006; 56: 782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higashiyama M, Ito T, Tanaka E, et al. Prognostic significance of osteopontin expression in human gastric carcinoma. Ann Surg Oncol. 2007; 14: 3419–27. [DOI] [PubMed] [Google Scholar]
- 32. Liang J, Pan Y, Zhang D, et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J. 2007; 21: 2247–56. [DOI] [PubMed] [Google Scholar]
- 33. Song G, Mao YB, Cai QF, et al. Curcumin induces human HT‐29 colon adenocarcinoma cell apoptosis via activating p53 and regulating apoptosis‐related proteins. Braz J Med Biol Res. 2005; 38: 1791–8. [DOI] [PubMed] [Google Scholar]
- 34. Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 987; 47: 3239–45. [PubMed] [Google Scholar]
- 35. Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase‐2 through nuclear factor‐kappa B‐mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001; 276: 44926–35. [DOI] [PubMed] [Google Scholar]
- 36. Ming YL, Song G, Chen LH, et al. Anti‐proliferation and apoptosis induced by a novel intestinal metabolite of ginseng saponin in human hepatocellular carcinoma cells. Cell Biol Int. 2007; 31: 1265–73. [DOI] [PubMed] [Google Scholar]
- 37. Zhang GX, He B, Weber GF. Growth factor signaling induces metastasis gene in transformed cells: molecular connection between Akt kinase and osteopontin in breast cancer. Mol Cell Biol. 2003; 23: 6507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cavallaro U, Christofori G. Molecular mechanisms of tumor angiogenesis and tumor progression. J Neurooncol. 2000; 50: 63–70. [DOI] [PubMed] [Google Scholar]
- 39. Weidemann A, Johnson RS. Biology of HIF‐1alpha. Cell Death Differ. 2008; 15: 621–7. [DOI] [PubMed] [Google Scholar]
- 40. Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF‐1alpha promotes metastasis. Nat Cell Biol. 2008; 10: 295–305. [DOI] [PubMed] [Google Scholar]
- 41. Miyauchi A, Alvarez J, Greenfield EM, et al. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991; 266: 20369–74. [PubMed] [Google Scholar]
- 42. Hu DD, Lin EC, Kovach NL, et al. A biochemical characterization of the binding of osteopontin to integrins αvβ1 and αvβ5 . J Biol Chem. 1995; 270: 26232–8. [DOI] [PubMed] [Google Scholar]
- 43. Takayama S, Ishii S, Ikeda T, et al. The relationship between bone metastasis from human breast cancer and integrin alphavbeta3 expression. Anticancer Res. 2005; 25: 79–83. [PubMed] [Google Scholar]
- 44. Marroquin CE, Downey L, Guo H, Kuo PC. Osteopontin increases CD44 expression and cell adhesion in RAW 264.7 murine leukemia cells. Immunol Lett. 2004; 95: 109–12. [DOI] [PubMed] [Google Scholar]
- 45. Lee JL, Wang MJ, Sudhir PR, et al. Osteopontin promotes integrin activation through outside‐in and inside‐out mechanisms: OPN‐CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007; 67: 2089–97. [DOI] [PubMed] [Google Scholar]
- 46. Bauer K, Mierke C, Behrens J. Expression profiling reveals genes associated with transendothelial migration of tumor cells: a functional role for alphavbeta3 integrin. Int J Cancer. 2007; 121: 1910–8. [DOI] [PubMed] [Google Scholar]
- 47. Cui R, Takahashi F, Ohashi R, et al. Abrogation of the interaction between osteopontin and alphavbeta3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer. 2007; 57: 302–10. [DOI] [PubMed] [Google Scholar]


