Abstract
Pre‐eclampsia (PE), the major cause of maternal morbidity and mortality, is thought to be caused by shallow invasion of the maternal decidua by extravillous trophoblasts (EVT). Data suggest that a fine balance between the expressions of pro‐ and anti‐invasive factors might regulate EVT invasiveness. Recently, we showed that the expression of the new growth factor endocrine gland‐derived vascular endothelial growth factor (EG‐VEGF) is high in early pregnancy but falls after 11 weeks, suggesting an essential role for this factor in early pregnancy. Using human villous explants and HTR‐8/SVneo, a first trimester extravillous trophoblast cell line, we showed differential expression of EG‐VEGF receptors, PKR1 and PKR2, in the placenta and demonstrated that EG‐VEGF inhibits EVT migration, invasion and tube‐like organisation. EG‐VEGF inhibitory effect on invasion was supported by a decrease in matrix metalloproteinase (MMP)‐2 and MMP‐9 production. Interference with PKR2 expression, using specific siRNAs, reversed the EG‐VEGF‐induced inhibitory effects. Furthermore, we determined EG‐VEGF circulating levels in normal and PE patients. Our results showed that EG‐VEGF levels were highest during the first trimester of pregnancy and decreased thereafter to non‐pregnant levels. More important, EG‐VEGF levels were significantly elevated in PE patients compared with age‐matched controls. These findings identify EG‐VEGF as a novel paracrine regulator of trophoblast invasion. We speculate that a failure to correctly down‐regulate placental expression of EG‐VEGF at the end of the first trimester of pregnancy might lead to PE.
Keywords: EG‐VEGF, prokineticin, pre‐eclampsia, trophoblast invasion
Introduction
Successful human placentation depends on adequate transformation of the uteroplacental vasculature by extravillous trophoblast (EVT) following proliferation, migration and invasion of these cells into the maternal decidua [1, 2]. This process of vascular remodelling rises to a peak by the end of the first trimester and declines rapidly thereafter [3]. At around 10–12 weeks of gestation (wg), cytotrophoblasts (CT) that are present in anchoring villi generate multi‐layered columns of EVTs that colonise the interstitium of the maternal decidua, the inner third of the myometrium and the uterine blood vessels. During pregnancy, the depth of invasion by placental trophoblast cells into the uterine wall is critical and finely controlled. Poor invasion of maternal vessels has been correlated to both pre‐eclampsia (PE), the most common cause of retarded foetal development [4] and foetal growth restriction, whereas an excessive trophoblast invasion is associated with invasive mole, placenta accreta and choriocarcinoma [5]. Consequently, a careful coordination of proliferation, differentiation and invasion of trophoblasts is required during the early stages of placental development. Various growth factors and cytokines, such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), transforming growth factor (TGF)‐α, amphiregulin, IGF‐II and IL‐1β, stimulate trophoblast differentiation towards an invasive phenotype [6, 7, 8]. In contrast, limited data exist regarding possible negative regulators of trophoblast differentiation. Graham and Lala (1991) first demonstrated that TGF‐β, produced primarily by the decidua, inhibits trophoblast invasion through stimulation of tissue inhibitor of matrix metalloproteinases‐1 (MMP‐1) secretion in EVT. Subsequent studies revealed that TGF‐β3 inhibits trophoblast invasion, following interaction with endoglin, a component of the TGF‐β receptor complex [7, 9].
Recently, a new growth factor specifically expressed by endocrine glands was discovered. This factor was named endocrine gland‐derived vascular endothelial growth factor (EG‐VEGF) and is also known as prokineticin‐1 (PK1) [10]. Human EG‐VEGF exerts its functions via two G protein‐coupled receptors (PKRs), termed PKR1 and PKR2 [11]. EG‐VEGF was found to be expressed in the testis, adrenal gland, ovary and placenta [10] and its effects appeared to be restricted to endothelial cells (EC) derived from these endocrine tissues [10].
We recently reported the pattern of expression of placental EG‐VEGF throughout mouse gestation [12] and during early pregnancy in humans [13]. In both species, placental EG‐VEGF exhibited the highest levels of expression early on during pregnancy. Its expression was restricted to the endocrine cells in the placenta, that is, syncytiotrophoblast (ST) in human placenta and trophoblast cells of the labyrinth layer in mouse placenta [12, 13]. Moreover, we showed that EG‐VEGF expression was up‐regulated by hypoxia [13]. In light of these recent findings, we hypothesized that EG‐VEGF might play a role in trophoblast differentiation during the first trimester of human pregnancy, and that its circulating levels might be altered in PE. In the present study, we (i) localised and determined the pattern of expression of PKR1 and PKR2 proteins in human placenta; (ii) investigated the role of EG‐VEGF on EVT proliferation, migration, invasion and tube‐like formation and (iii) measured EG‐VEGF circulating levels in non‐pregnant and pregnant women throughout human gestation. Moreover, EG‐VEGF circulating levels were compared in pre‐eclamptic (PE) patients and gestational age‐matched controls.
Materials and methods
Tissue collection
First trimester human placentas from 7 to 12 wg (defined as 7–12 weeks of amenorrhoea) were obtained from elective terminations of pregnancies. Shortly after collection, the tissue was snap‐frozen in dry ice and stored at –80°C (for protein extraction) or fixed in 4% paraformaldehyde (PFA) at room temperature (for immunohistochemistry). The collection and processing of human placentas was approved by the University Hospital ethics committee, and informed consent was obtained from each patient.
Blood sample collection
An analysis of circulating EG‐VEGF levels in PE patients versus age‐matched control women was performed using serum samples that had been collected to conduct a study on protein Z in patients with pregnancy complications [14]. In brief, the study was conducted over 2 years (2002–2003) to evaluate protein Z concentration and pregnancy complications. After informed consent, patients presenting with PE and healthy pregnant women were included. The study was approved by the local ethics committee (Hôpital de la Conception, Marseille, France). PE was defined as a diastolic arterial blood pressure greater than 90 mmHg and a systolic blood pressure greater than 140 mmHg, associated with proteinuria (more than 300 mg/24 hrs). The blood pressures were taken at admission and at the inclusion day, after at least half an hour of bed rest (Table 1). From this study, we were able to collect 24 serum samples of PE patients and 29 serum samples from normal pregnancies. Based on their gestational age, the patients were divided into two groups, second and third trimesters. In the second trimester, 9 normal women and 9 PE patients were analysed; in the third trimester 20 normal women and 15 PE patients were analysed.
Table 1.
Clinical characteristics of normal and PE patients
| Normal pregnancies (n= 29) | Pre‐eclamptic pregnancies (n= 24) | P | |
|---|---|---|---|
| Age (years) | 31 (22–40) | 29 (23–40) | n.s. |
| Gestational age at sampling (weeks) | 31 (22–39) | 30.8 (24.0–38.3) | n.s. |
| Systolic blood pressure (mmHg) | 110 (90–130) | 160 (130–200) | <0.0001* |
| Diastolic blood pressure (mmHg) | 80 (50–90) | 90 (80–110) | <0.0001* |
| Proteinuria (g/24 hrs) | – | 2.7 (0.48–3.10) |
* Indicates P < 0.05.
With the approval of our local ethics committee, extra blood samples were collected from 18 volunteers: 9 non‐pregnant and 9 pregnant women at the first trimester of pregnancy.
EG‐VEGF ELISA
EG‐VEGF was measured by ELISA (PeproTech, Neuilly‐Sur‐Seine, France). Two separate standard curves were constructed to allow accurate readings of samples at upper and lower ranges of the assays. All samples were in the linear range of the standard curves. The intra‐assay coefficient of variation (CV) was 6.7% and the interassay CV was 8.1%. The detection limit of the assay was 16 pg/ml.
Immunohistochemistry
Placental tissues of 9–10 wg (n= 6) were collected and fixed for 24 hrs at 4°C in 4% (v/v) PFA, embedded in paraffin and processed as previously described [13]. Immunoreactive PKR1 and PKR2 were detected using in‐house rabbit polyclonal antibodies. For immunohistochemical detection, anti‐PKR1 and anti‐PKR2 antibodies were incubated with the tissue sections for 18 hrs at 4°C and used at final concentrations of 0.56 μg/ml and 0.72 μg/ml, respectively. Control sections were treated with anti‐PKR1 and anti‐PKR2 antibodies that had been pre‐absorbed overnight at 4°C with the appropriate pre‐immune serum.
Western blotting analysis
Frozen placental samples of 7–12 wg (n= 14) were homogenised in RIPA lysis buffer (50 mM Tris‐HCl [pH 7.5], 150 mM NaCl, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 1% Triton X‐100, 1 mM PMSF, 5 μg/ml leupeptin and 5 μg/ml aprotinin) and processed as previously described [13]. Protein extracts were electrophoretically separated on 0.1% sodium dodecyl sulphate–12% polyacrylamide gels and electrically transferred onto 0.45‐μm nitrocellulose membranes. The membranes were blotted with rabbit antibodies against PKR‐1 or PKR‐2, both used at a final concentration of 0.84 μg/ml. To standardise for sample loading, the blots were stripped and re‐probed with an anti‐Gβ antibody (Transduction Laboratories, Lexington, KY, USA) as an internal control for protein loading.
Cell culture
Extravillous trophoblast cell culture
The HTR‐8/SVneo cells were produced by immortalisation of HTR‐8‐8 cells, an EVT cell line, with SV40 virus [15]. In the present study, cells between 40 and 50 passages were used and grown in RPMI 1640 supplemented with 5% foetal bovine serum (FBS), penicillin/streptomycin and amphotericin B (Invitrogen, Cergy Pontoise, France). The cells were maintained at 37°C in an atmosphere of 5% CO2 in air.
Human villous explant cultures
Villous explant cultures were established from first trimester human placentas (10–12 wg, n= 6), as described previously [16, 17]. Small fragments of placental villi (15–20 mg wet weight) were teased apart and placed on a transparent Biopore membrane of 12‐mm diameter (Millicell‐CM culture dish inserts with a pore size of 0.4 μm; Millipore Corp., Bedford, MA). The inserts were pre‐coated with 0.2 ml undiluted Matrigel (Becton‐Dickinson, Le Pont de Claix, France), polymerised at 37°C for 30 min. and transferred in a 24‐well culture dish. Explants were cultured in DMEM‐Ham's F‐12 (DMEM/F12; Invitrogen, Cergy Pontoise, France) supplemented with 100 μg/ml streptomycin, 100 U/ml penicillin and 0.25 μg/ml ascorbic acid, pH 7.4. After 24 hrs of culture, the medium was changed and explants were incubated in the absence or presence of recombinant EG‐VEGF, 50 ng/ml (Tebu, Tebu‐Bio, France). Fresh EG‐VEGF was added to the treated explants every other day. Fifty nanograms per millilitre EG‐VEGF corresponds to 5 nM, which is approximately two times the EC50 (2.7 nM) of EG‐VEGF for its receptors [11, 18]. Villous explants were kept in culture for up to 6 days. Flattening of the distal end of the villous tips, their adherence to Matrigel and the appearance of EVT breaking through from the tips were used as markers of morphological integrity and trophoblast differentiation. EVT cell outgrowth and migration were consistently monitored and quantified using the ratio of EVT outgrowths/villous tip, where the numerator, EVT outgrowths, represents the number of EVT columns sprouting from the villous tips plus the number of cell islands (groups of cells) invading the Matrigel. The denominator represents the total number of villous tips in a single explant. In general, there are three to six such villous tips per explant. Explants (from a single placenta) were used in triplicate for each time‐point of treatment. For statistical analysis, the n value represents the number of placentas (not explants). EVT outgrowth from the distal end of the villous tips and their migration into the surrounding matrix were observed for up to 6 days in culture. All incubations were performed in normoxia. The viability of the explants was assessed by measuring human chorionic gonadotropin (hCG) production rates in the culture medium collected at the time of medium change every 48 hrs. hCG was measured by ELISA‐FβHCG (Cis Bio International, Gif‐sur Yvette, France).
RNA isolation and reverse transcriptase polymerase chain reaction (PCR) analysis
Total RNA was extracted from EVT cells using a rapid RNA isolation system (RNeasy; Qiagen, Courtaboeuf, France). Reverse transcription was performed on 1 μg total RNA with Superscript II‐RnaseH reverse transcriptase (Invitrogen, Cergy Pontoise, France) under conditions recommended by the manufacturer.
Real‐time (RT)‐PCR analysis
PKR1 and PKR2 mRNAs and 18S rRNA expression were quantified by RT‐PCR using a Light Cycler apparatus (Roche Diagnostics, Meylan, France). The PCR was performed using the primers shown in Table 2 and SYBR green PCR core reagents (Light Cycler‐FastStart Master SYBR Green I; Roche Diagnostics). The PCR conditions were: step 1, 94°C for 10 min.; step 2, 45 cycles consisting of 95°C for 15 sec., temperature indicated in Table 1 for 5 sec. and 72°C for 10 sec. The results were normalised to 18S rRNA expression levels. To assess linearity and efficiency of PCR amplification, standard curves for all transcripts were generated using serial dilutions of cDNA. The RealQuant analysis software (Roche Diagnostics) was used to quantify relative levels of expression.
Table 2.
Primers used for real‐time (RT)‐PCR
| Gene | Forward primer | Reverse primer | T(°C) for RT |
|---|---|---|---|
| PKR1 | 5′‐GTCCTCGTCATTGTCAAGAGCC‐3′ | 5′‐AAACACGGTGGGGAAGTCG‐3′ | 58 |
| PKR2 | 5′‐CATCCCATCGCCTTACTTTGC‐3′ | 5′‐CTTTTCCTTCACGAACACAGTGG‐3′ | 60 |
| 18S | 5′‐TTGTTGTTTTCGGAACTGAGGC‐3′ | 5‐GGCAAATGCTTTCGCTCTGGTC‐3′ | 59 |
RNA interference
The expression of PKR2 mRNA was inhibited by transfection of siRNAs. Briefly, one day after plating, EVT cells were transfected with or without 10–50 nM siRNA duplexes for PKR2 gene, using siPORT lipid reagent (Ambion, Austin, TX, USA). siRNA duplexes (21‐nucleotide) were purchased from Ambion. PKR2 siRNA oligonucleotide templates were the following (5′‐3′): antisense: GGCUUCUUACAAUGGCGGUtt; sense: ACCGCCAUUGUAAGAAGCCtt. Scrambled siRNA duplexes of these targeting sequences served as non‐specific control siRNA. Typically, cells were analysed for the loss of PKR2 mRNA and protein expression 48 hrs after transfection.
Assessment of HTR‐8 migration
A wound healing assay was performed for the effect of EG‐VEGF on HTR‐8 cell motility (n= 3). The EG‐VEGF effect was also examined in cells tansfected with siRNA for PKR2 (10 nM for 48 hrs). The cells were seeded in complete medium (RPMI 5% FBS) at a density of 2 × 105 cells/well into six‐well plates. At confluence, the complete medium was replaced by a serum‐free medium, and the cells were scratched with a sterile tip to create an artificial wound and allowed to heal for the next 24 hrs. Photographs were taken at regular time intervals (0, 12 and 24 hrs). The size of the wound was measured on the photographs from three separate experiments. The closing of the wound was analysed using Scion Image software (version 4.0.2; Scion Corp., Carl Zeiss, Sartrouville, France). The results are presented as percentage of wound closure after 24 hrs of treatment.
Measurement of extravillous trophoblast tube‐like formation
Transfected and non‐transfected HTR‐8 cells with siRNA for PKR2 were seeded in the serum‐free medium at a density of 106/ml on 900 μl Matrigel in glass‐bottom dishes covered by a membrane permeable to gas (Barloworld Scientific, France). The cells were allowed to settle for 4 hrs before the treatment with EG‐VEGF (50 ng/ml) or FGF‐2 (20 ng/ml), and then placed inside a video microscopy platform equipped with a device enabling regulation of temperature and CO2 level. Time lapse of Z series images (Z = 30) were collected with an inverted motorised microscope (Axiovert 200M; Zeiss) controlled by MetaMorph software (Universal Imaging, Downingtown, PA, USA). Phase‐contrast images were acquired with a CoolSnap HQ charge‐coupled device camera (Roper Scientific, Trenton, NJ, USA) every 10 min. for at least 24 hrs with an acquisition time of 100 msec. under low illumination to avoid cell damage. For each Z series images, the best focus was chosen before the reconstitution of the video clip. At least 12 fields were examined per well; each experimental condition was tested in triplicate.
Measurement of MMP‐2 and MMP‐9 secretion in conditioned media (CM)
To determine the effect of EG‐VEGF on MMP‐2 and MMP‐9 secretion, we measured their levels of production in CM collected from villous explants and HTR‐8 cell cultures (n= 6 and n= 4, respectively). Villous explants and HTR‐8 cells were cultured on Matrigel in the absence or presence of EG‐VEGF (50 ng/ml) for 48 hrs and 24 hrs, respectively. We used commercially available ELISA kits (BD Biosciences, Pont de Claix, France) to measure MMP production in the CM. All measurements of secreted MMP‐2 and MMP‐9 were reported to the protein content of the explants or cell.
Statistical analysis
Statistical comparisons were made using the one‐way anova analysis and tested for homogeneity of variance and normality (P < 0.05). Student's t‐test was also used when appropriate. Data are presented as the mean ± standard error (SE) or mean ± standard deviation (SD). Calculations were performed using SigmaStat (Jandel Scientific Software, SanRafael, CA, USA).
Results
Maternal serum EG‐VEGF throughout human pregnancy
We compared EG‐VEGF circulating levels in non‐pregnant and pregnant women at the first, second and third trimesters of pregnancy. Circulating EG‐VEGF was detectable in all the sera tested; however, its levels were significantly different between non‐pregnant and pregnant women at all gestational ages examined (Fig. 1). In non‐pregnant women, EG‐VEGF levels were around 50 pg/ml. This value increased five‐fold during the first trimester of pregnancy (250 pg/ml), and then significantly decreased during the second and third trimesters (70 pg/ml).
Figure 1.
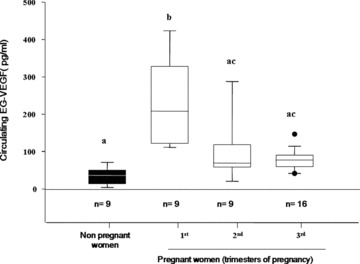
EG‐VEGF serum levels in non‐pregnant and pregnant women at the first, second and third trimesters. A total of 42 serum samples were analysed. EG‐VEGF contents were measured by ELISA. Box plot demonstrates 10th, 25th, 50th, 75th and 90th percentiles. (*P < 0.05, by ANOVA followed by Dunn's method). Values overwritten with different letters are significantly different from each other.
PKR1 and PKR2 protein expression in human placenta during the first trimester of pregnancy
Western blot analysis
The pattern of PKR1 and PKR2 protein expression was analysed by Western blot on protein extracts from placental tissue homogenates. Figure 2A shows a representative Western blot of PKR1 and PKR2 expression in placental extracts from 7 to 12 wg. PKR1 immunoreactivity (Ir‐PKR1) was present since 7 wg, peaked at 10 wg and then gradually decreased. In contrast to PKR1, PKR2 protein showed stable expression throughout the first trimester of pregnancy. Quantification of western blotting analysis revealed an apparent increase in PKR1 expression levels between the 9th and 10th wg (Fig. 2B). No change in PKR2 expression was observed throughout the first trimester of pregnancy (Fig. 2C).
Figure 2.
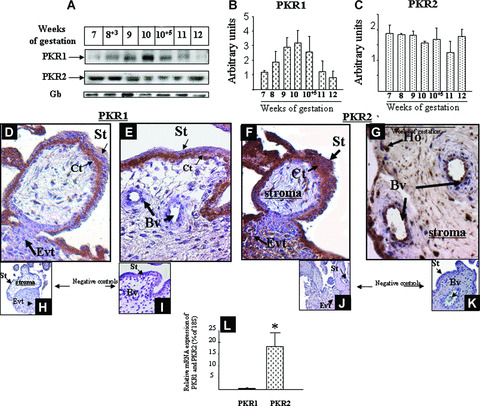
PKR1 and PKR2 protein expression in placental villi during the first trimester of pregnancy. (A) The panel shows a representative Western blot analysis of PKR1 and PKR2 in placental tissues from 7 to 12 wg. For each gestational age, two placentas were examined. Panels B and C show histograms of the mean relative OD of PKR1 and PKR2 protein signals normalised to protein Gβ, respectively. Data are the mean ± SD. Panels F and G show placental column and chorionic villi at 10 wg immunostained with anti‐PKR1 and anti‐PKR2, respectively. The undersized photographs in panels H–K show tissues section incubated with pre‐immune sera for either PKR1 or PKR2. Cytotrophoblast (Ct), Hobfauer cells (Ho), Extravillous trophoblast (EVT) syncytiotrophoblast (St), Blood vessels (Bv). Panel L shows a comparison of PKR1 and PKR2 mRNAs in HTR‐8 cells (*P < 0.05).
Immunohistochemistry
Placentas of 9–10 wg were used to immunolocalise PKR1 and PKR2 proteins in human placental villi. Ir‐PKR1 was mainly observed in the CT. Weak immunoreactivity was also observed in the basal area of the ST and in the EVT forming the anchoring villi. No staining was observed in placental EC (Fig. 2D and E). Ir‐PKR2 was strong in the ST layer, EVT, EC and Hofbauer cells (Fig. 2F and G). Weak staining was also observed in CT. No staining was observed when pre‐immune sera were used as negative controls (undersized pictures in Fig. 2H–K).
Differential expression of PKR1 and PKR2 in EVT
We compared the levels of PKR1 and PKR2 mRNA expression by HTR‐8 cells using quantitative RT‐PCR. Figure 2L shows the mRNA levels of PKR1 and PKR2 in HTR‐8 cells. There was 15 times more PKR2 than PKR1 transcripts. In a previous study, we have reported that isolated CT expressed 80 times more PKR1 than PKR2 transcripts [13]. These results demonstrate that PKR1 is preferentially expressed by CT cells, whereas PKR2 is preferentially expressed by EVT and ST.
Effects of EG‐VEGF on extravillous trophoblast migration, invasiveness and tube‐like formation
Given the important role of EVT in human placentation during the first trimester of pregnancy, we investigated the effect of EG‐VEGF on their migration, invasion and tube‐like formation.
EG‐VEGF effect on HTR‐8 migration
EVT cell migration is an important step in the establishment of the foeto‐maternal circulation. We examined the effect of EG‐VEGF on the migration of HTR‐8 cells. Figure 3A shows representative photographs of HTR‐8 monolayers at 0 hr and 24 hrs after their wounding with a pipette tip and subsequent incubation in the absence or presence of EG‐VEGF (10, 25 and 50 ng/ml). In prior experiments, we have shown that EVT cells preferentially express the type 2 receptor of EG‐VEGF, PKR2. To test that EG‐VEGF effect on HTR‐8 migration was mediated by its PKR2 receptor, we examined the effect of EG‐VEGF on HTR‐8 cells in which PKR2 mRNA was silenced by specific siRNA. At 24 hrs of culture, the wound was almost closed in the control condition but not in the EG‐VEGF condition. PKR2 mRNA silencing completely reversed EG‐VEGF effect, suggesting that EG‐VEGF effect in HTR cells passes via PKR2 receptor activation. Quantification of the three experiments showed that treatment with EG‐VEGF significantly delayed wound healing of HTR‐8 cells. The percentage of wound closure is shown in Fig. 3B. The closure of the wound reached 70% in the control condition versus only 40% in the EG‐VEGF condition.
Figure 3.
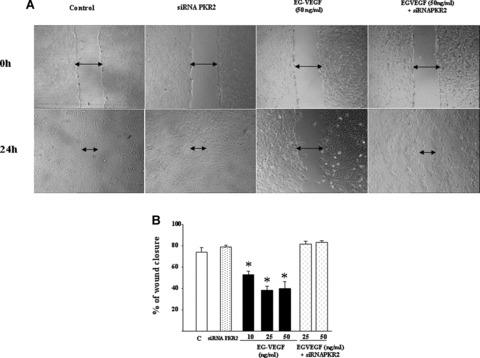
Effect of EG‐VEGF on the migration of HTR‐8 cells. Panel A shows photographs of HTR‐8 monolayer at 0 hr and 24 hrs after the wounding at 0 hr in the control, PKR2 siRNA, EG‐VEGF (50 ng/ml) and EG‐VEGF plus PKR2 siRNA conditions. Panel B shows the percentage of wound closure 24 hrs after the treatment with EG‐VEGF (10, 25 or 50 ng/ml) in the absence or presence of PKR2 siRNA (*P < 0.05).
EG‐VEGF effect on EVT invasion
We investigated the effects of EG‐VEGF on EVT outgrowth and invasion in the villous explant system. It was particularly relevant to study EG‐VEGF effects on trophoblast differentiation towards an invasive phenotype in a system in which the placental villous tissue architecture is maintained. Placental villous explants in culture preserve the topology of intact villi and closely mimic the formation of anchoring villi occurring in vivo by the end of the first trimester of pregnancy [16, 17]. The viability of the explants, as measured by the rates of production of hCG, remained relatively constant for up to 6 days (hCG: day 3: 600 IU/1 g wet weight; day 6: 590 IU/1 g wet weight; a mean of six separate experiments performed in triplicate). Figure 4A shows representative photographs of placental villous tips at day 3 of culture in the absence or presence of EG‐VEGF (50 ng/ml). In the control condition, there was an obvious outgrowth of EVT from the distal end of the villous tip and migration into Matrigel (Fig. 4B). In contrast, in the presence of EG‐VEGF, there was almost no outgrowth or migration of EVT into Matrigel. The graph in Fig. 4C reports the quantification of EVT outgrowth and migration (ratio of EVT/villous tip) in six independent experiments at day 1 and day 3 in the absence or presence of EG‐VEGF. There was a significant increase in EVT outgrowth from day 1 to day 3 of culture, indicating the natural behaviour of these explants in culture. EG‐VEGF treatment decreased this process in statistically significant manner.
Figure 4.
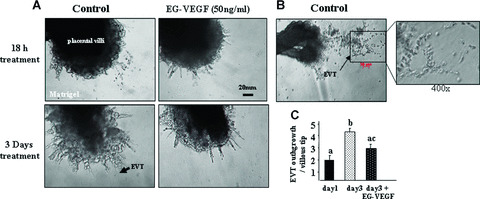
Effect of EG‐VEGF on the invasion of EVT cell in situ. Panel A shows EG‐VEGF effect on EVT invasion in villous explants’ culture. EG‐VEGF (50 ng/ml) treatment started 24 hrs after the launch of the culture. Panel B shows increased budding and outgrowth of EVT from the distal end of the villous tips in the control condition at day 3 of culture. Panel C shows quantification of EVT outgrowths per villous tip in the absence or presence of EG‐VEGF in six independent experiments performed in triplicate (*P < 0.05). Data represent the mean ± SEM. Values overwritten with different letters are significantly different from each other.
EG‐VEGF effect on HTR‐8 cells organisation in tube‐like structures
EVT invasion is accompanied by endovascular differentiation, wherein EVT finally replaces EC in uterine vessels. Previous reports in the literature have shown that primary CT and HTR‐8 cells show typical morphology of EC in the way that EVT can self‐organise as networks of tube‐like structures when grown on Matrigel [19]. Using time‐lapse microscopy, we investigated the effect of EG‐VEGF on tube‐like formation by HTR‐8 cells during 24 hrs, with photographs taken every 10 min. Figure 5A shows representative photographs of HTR‐8 cells at 0 h, 6 hrs, 12 hrs, 18 hrs and 24 hrs of culture on Matrigel and under different treatments. In the control condition, HTR‐8 cells start to organise into tube‐like structures by 6 hrs. By 24 hrs of culture, the whole cells were organised in a network of tubular structures. EG‐VEGF treatment delayed this process, with organisation starting only at 18 hrs of culture. To control the responsiveness of HTR‐8 cells, we examined the effect of a potent angiogenic factor, FGF‐2. As expected, FGF‐2 induced a rapid organisation of HTR‐8 cells. Treatment with PKR2 siRNA reversed the EG‐VEGF effect. To quantify the effect of EG‐VEGF on HTR‐8 organisation, we determined the number of capillary networks formed under the different conditions. The graph in Fig. 5B shows that EG‐VEGF significantly decreased the number of tube‐like structures. This effect was reversed by the siRNA treatment. FGF‐2, in contrast, significantly increased this process. Figure 5C shows the percentage of area occupied by the cells in control, control plus PKR2 siRNA, EG‐VEGF, FGF‐2 and EG‐VEGF plus PKR2 siRNA during 24 hrs. A video clip of tube‐like structure formation has been realised for these conditions (see Video Clip 1 in the Supporting Information).
Figure 5.
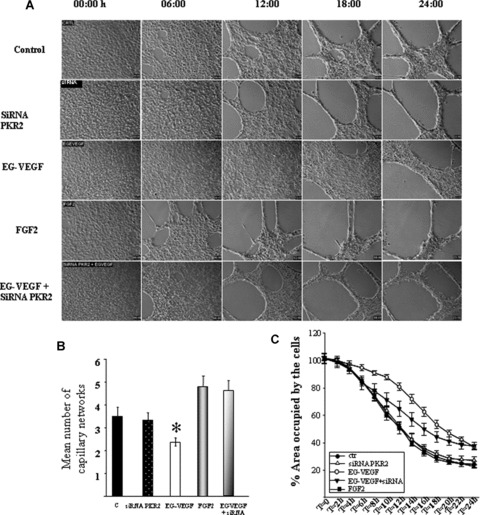
Effect of EG‐VEGF on HTR‐8 tube‐like formation. Panel A shows photographs of HTR‐8 cells cultured on Matrigel at 6, 12, 18 and 24 hrs in the absence or the presence of EG‐VEGF (50 ng/ml), FGF‐2 (20 ng/ml) or EG‐VEGF plus siRNA PKR2. Note that EG‐VEGF delayed HTR‐8 cells organisation in tube‐like structures compared with the control, FGF‐2 and EG‐VEGF plus siRNA conditions. Panel B shows quantification of the number of tube‐like structures formed after 24 hrs under the four conditions. Panel C shows measurement of the percentage area occupied by the cells under the control, control plus siRNA, EG‐VEGF, FGF‐2 and EG‐VEGF plus siRNA conditions during 24 hrs. *P < 0.05.
Effect of EG‐VEGF on trophoblast cell protease secretion
A critical element of decidualisation is remodelling of extracellular matrix (ECM) by MMPs. Cultured EVT express high levels of MMP‐2 and MMP‐9 [20, 21]. We measured MMP‐2 and MMP‐9 production in culture media collected from HTR‐8 cultures and villous explants cultures after treatment with EG‐VEGF (50 ng/ml). Figure 6A and B shows MMP‐2 and MMP‐9 production in CM collected from HTR‐8 cells and villous explants cultures. In both cultures, MMP‐2 was more abundant than MMP‐9, and EG‐VEGF treatment significantly decreased the production of both enzymes.
Figure 6.
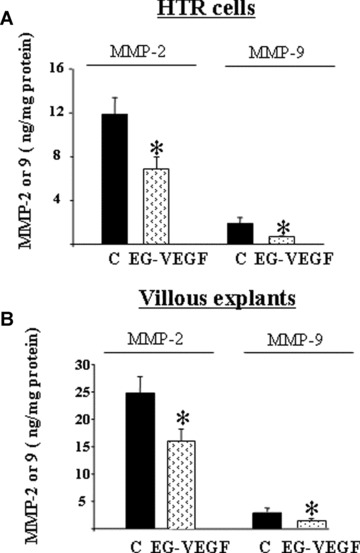
Effect of EG‐VEGF on MMP‐2 and MMP‐9 production. Panels A and B show effects of EG‐VEGF on total MMP‐2 and MMP‐9 production assessed by ELISA of conditioned media collected from HTR‐8 cells for 24 hrs (A) or villous explants cultured for 48 hrs on Matrigel (B) in the absence or presence of EG‐VEGF (50 ng/ml). Data represent the mean ± SEM (*P < 0.05).
Second‐ and third‐trimester levels of EG‐VEGF in normal and pre‐eclamptic pregnancy
The combination of our previous data showing that EG‐VEGF was abundantly expressed in human placenta during the first trimester of pregnancy, and that its expression was up‐regulated by hypoxia, a phenomenon that is believed to be central to most PE pregnancies, and our present results showing that EG‐VEGF is a new factor that regulates EVT invasion suggested to us that EG‐VEGF circulating levels might be altered in PE. Figure 7 shows EG‐VEGF levels in normal and PE pregnancies in the second and third trimesters. There was a trend towards an increase in the second‐trimester serum EG‐VEGF levels in PE patient as compared with controls, but the difference did not reach significance. However, in the third trimester, EG‐VEGF levels were significantly higher in the PE group compared with controls (P < 0.05).
Figure 7.
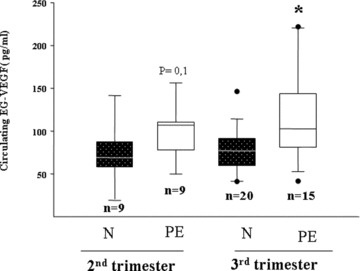
EG‐VEGF serum levels in normal and PE patient at the second and third trimesters of pregnancy. A total of 53 serum samples were analysed. EG‐VEGF contents were measured by ELISA in sera from normal (N) women and pre‐eclamptic (PE) patients. Box plot demonstrates 10th, 25th, 50th, 75th and 90th percentiles. (*P < 0.05, by ANOVA followed by Dunn's method).
Discussion
Here we report for the first time the circulating levels of the new factor EG‐VEGF in non‐pregnant and pregnant women throughout normal and abnormal gestation and show that EG‐VEGF levels are elevated in the pathology of PE. Moreover, we demonstrate that EG‐VEGF is a negative regulator of human EVT invasion. This statement is based on two key observations: first, the demonstration that EG‐VEGF inhibits EVT migration and invasion in cell and villous explant culture systems; second, the demonstration that EG‐VEGF inhibits EVT organisation into tube‐like structures. These data strongly suggest that EG‐VEGF acts as an inhibitor of trophoblast differentiation towards an invasive phenotype and are consistent with a model of normal placentation in which down‐regulation of EG‐VEGF expression at around 10 wg promotes differentiation of EVT. It is known that, during early pregnancy, the placenta is hypoxic and that hypoxia is central to most PE pregnancies. We have previously shown that the expression of EG‐VEGF is up‐regulated by hypoxia [13]. Our present findings that EG‐VEGF regulates trophoblast invasion and that its levels remain elevated in women with PE suggests that hypoxia may in fact play a role in the increased production of this factor in women who develop PE.
In this study, we investigated the putative role of EG‐VEGF in human pregnancy. First, we determined the pattern of expression of EG‐VEGF receptors PKR1 and PKR2. Interestingly, these receptors exhibited differential patterns of expression, with PKR1 being mainly expressed in the CT layer within the placental villi, whereas PKR2 was mainly expressed in ST, EC and EVT. PKR1 and PKR2 expression in different cell types suggests that EG‐VEGF might have differential effects on undifferentiated cells (CT) through PKR1 activation and on differentiated cells (ST, EC and EVT) through PKR2. Differential expression of the mRNAs of PKR1 and PKR2 in different cells types has also been reported in the ovary and mouse placenta [12, 22].
We demonstrated that EG‐VEGF inhibits EVT migration and invasion. These effects have been confirmed in the villous explant culture system. The inhibition of EVT outgrowth and invasion into Matrigel in the villous explant system is a strong demonstration of the inhibitory effect of EG‐VEGF on EVT invasion by the end of the first trimester of human pregnancy.
Trophoblast invasion in vivo is accompanied by remodelling of ECM by MMPs. We demonstrated that EG‐VEGF inhibits the production and activity of MMP‐2 and MMP‐9, both in HTR‐8 and in villous explants cultures. MMP‐2 and MMP‐9 are the most important MMP enzymes produced by the CT during the first trimester of pregnancy, and their involvement in the success of EVT functions, such as migration and invasion, has been well documented [20, 21]. The inhibitory effect of EG‐VEGF on MMP production suggests that EG‐VEGF regulates the main enzymes involved in the remodelling of the decidua. Other factors such as tissue inhibitor of matrix metalloprotease 1 (TIMP‐1) and plasminogen activator inhibitor (PAI‐1) have been reported to be up‐regulated in response to the anti‐invasive action of TGF‐β and decorin [23, 24]. We could not detect any change in the protein levels of TIMP‐1 protein in response to EG‐VEGF (data not shown). These data suggest that EG‐VEGF may employ different mechanisms from those described for TGF‐β and decorin to exert its anti‐invasive actions.
Trophoblast differentiation and, especially, pseudovasculogenesis during early pregnancy are crucial for proper functioning of the maternal–foetal interface [25, 26]. It has been reported previously that EC and EVT form tube‐like structures when cultured on Matrigel [27, 28]. This phenomenon seems to be specific to EVT cells, since TCL1, another EVT cell line [29], but not cytotrophoblast cells isolated from the third trimester or the JEG3 cells, a choriocarcinoma cell line, exhibited this organisation. We show here that EG‐VEGF delayed the organisation of HTR‐8 cells into tube‐like structures and decreased the number of capillary networks as compared with control and FGF‐2 conditions. These data demonstrate that EG‐VEGF effects are not restricted to an inhibition of EVT migration and invasion but also prevent morphogenesis into capillary‐like structures.
Trophoblast invasion, characteristic of normal human placentation, is dependent on an intricate balance between positive and negative regulators. Our data suggest that EG‐VEGF is a critical negative regulator of this system and places it among cytokines that negatively control migration and invasion. This raises the possibility that inappropriate expression or function of EG‐VEGF might contribute to major complications of pregnancy, such as PE or choriocarcinoma, which are associated with abnormal trophoblast invasion and placental development.
Under physiological conditions, EG‐VEGF might prevent premature EVT invasion. In contrast, sustained EG‐VEGF expression beyond 10–12 wg might cause an inhibition of EVT colonisation of maternal decidua, which might consequently lead to a failure in maternal spiral arteries remodelling. The high circulating levels observed in PE patient suggest that EG‐VEGF deregulation might be tightly associated with this pathology. However, at this time, we cannot conclude whether EG‐VEGF higher levels are a cause or consequence of PE development. A recent study by Chung et al. reported no change in the expression of EG‐VEGF mRNA in placentas from PE patients [30]. In this study, the authors analysed a cohort of PE patients, and did not examine second and third trimester PE samples separately. Therefore, further studies are definitely needed to conclude whether placental EG‐VEGF expression is also altered in PE.
In conclusion, we report here a physiological role for EG‐VEGF in human placentation. High EG‐VEGF circulating levels during the first trimester of pregnancy, its up‐regulation by hypoxia, its important effects in the control of EVT differentiation and its elevated circulating levels in PE altogether suggest that we should rank this peptide among important physiological regulators of human placentation and potential markers of PE. Future studies will address this hypothesis in a large cohort of PE patients.
Supporting information
Fig. S1 Effect of siRNA treatment on PKR2 expression in HTR‐8 cells. Cells were analysed for the loss of PKR2 mRNA and protein expression 48 hrs after transfection using RT‐PCR (A) and immunoblotting (B), respectively. Scrambled (Scr) siRNA served as non‐specific control siRNAs. *P < 0.05.
Video Clip 1 Attached is a video of HTR‐8 organisation in tube‐like structures in the absence or presence of EG‐VEGF or EG‐VEGF plus siRNA for PKR2. Note that the HTR‐8 organisation was significantly delayed compared with the control and siRNA conditions.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Acknowledgements
We thank Dr. Florence Bretelle (Conception Hospital, Marseille, France) for providing us serum samples from pre‐eclamptic patients and age‐matched controls. This work was supported by INSERM (U878), the Commissariat à l’Energie Atomique (DSV/iRTSV/LAPV) and the Programme National de Recherche en Reproduction et Endocrinologie (grant 2006 to Nadia Alfaidy).
References
- 1. Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science . 1994; 266: 1508–18. [DOI] [PubMed] [Google Scholar]
- 2. Strickland S, Richards WG. Invasion of the trophoblasts. Cell . 1992; 71: 355–7. [DOI] [PubMed] [Google Scholar]
- 3. Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci . 1991; 99: 681–92. [DOI] [PubMed] [Google Scholar]
- 4. Sibai B, Dekker G, Kupferminc M. Pre‐eclampsia. Lancet . 2005; 365: 785–99. [DOI] [PubMed] [Google Scholar]
- 5. Wells M. The pathology of gestational trophoblastic disease: recent advances. Pathology . 2007; 39: 88–96. [DOI] [PubMed] [Google Scholar]
- 6. Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol . 1999; 128: 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF‐beta, IGF‐II, and IGFBP‐1. Exp Cell Res . 1995; 217: 419–27. [DOI] [PubMed] [Google Scholar]
- 8. Lysiak JJ, Han VK, Lala PK. Localization of transforming growth factor alpha in the human placenta and decidua: role in trophoblast growth. Biol Reprod . 1993; 49: 885–94. [DOI] [PubMed] [Google Scholar]
- 9. Caniggia I, Taylor CV, Ritchie JW, et al . Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology . 1997; 138: 4977–88. [DOI] [PubMed] [Google Scholar]
- 10. LeCouter J, Kowalski J, Foster J, et al . Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature . 2001; 412: 877–84. [DOI] [PubMed] [Google Scholar]
- 11. Lin DC, Bullock CM, Ehlert FJ, et al . Identification and molecular characterization of two closely related G protein‐coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem . 2002; 277: 19276–80. [DOI] [PubMed] [Google Scholar]
- 12. Hoffmann P, Feige JJ, Alfaidy N. Placental expression of EG‐VEGF and its receptors PKR1 (prokineticin receptor‐1) and PKR2 throughout mouse gestation. Placenta . 2007; 28: 1049–58. [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland‐derived vascular endothelial growth factor/prokineticin‐1 and its receptors in human placenta during early pregnancy. Endocrinology . 2006; 147: 1675–84. [DOI] [PubMed] [Google Scholar]
- 14. Bretelle F, Arnoux D, Shojai R, et al . Protein Z in patients with pregnancy complications. Am J Obstet Gynecol . 2005; 193: 1698–702. [DOI] [PubMed] [Google Scholar]
- 15. Graham CH, Hawley TS, Hawley RG, et al . Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res . 1993; 206: 204–11. [DOI] [PubMed] [Google Scholar]
- 16. Caniggia I, Grisaru‐Gravnosky S, Kuliszewsky M, et al . Inhibition of TGF‐beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest . 1999; 103: 1641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Genbacev O, Jensen KD, Powlin SS, et al . In vitro differentiation and ultrastructure of human extravillous trophoblast (EVT) cells. Placenta . 1993; 14: 463–75. [DOI] [PubMed] [Google Scholar]
- 18. Maldonado‐Perez D, Evans J, Denison F, et al . Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab . 2007; 18: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dokras A, Gardner LM, Seftor EA, et al . Regulation of human cytotrophoblast morphogenesis by hepatocyte growth factor/scatter factor. Biol Reprod . 2001; 65: 1278–88. [DOI] [PubMed] [Google Scholar]
- 20. Bischof P, Meisser A, Campana A, et al . Effects of decidua‐conditioned medium and insulin‐like growth factor binding protein‐1 on trophoblastic matrix metalloproteinases and their inhibitors. Placenta . 1998; 19: 457–64. [DOI] [PubMed] [Google Scholar]
- 21. Fisher SJ, Leitch MS, Kantor MS, et al . Degradation of extracellular matrix by the trophoblastic cells of first‐trimester human placentas. J Cell Biochem . 1985; 27: 31–41. [DOI] [PubMed] [Google Scholar]
- 22. Kisliouk T, Levy N, Hurwitz A, et al . Presence and regulation of endocrine gland vascular endothelial growth factor/prokineticin‐1 and its receptors in ovarian cells. J Clin Endocrinol Metab . 2003; 88: 3700–7. [DOI] [PubMed] [Google Scholar]
- 23. Graham CH. Effect of transforming growth factor‐beta on the plasminogen activator system in cultured first trimester human cytotrophoblasts. Placenta . 1997; 18: 137–43. [DOI] [PubMed] [Google Scholar]
- 24. Graham CH, Lysiak JJ, McCrae KR, et al . Localization of transforming growth factor‐beta at the human fetal‐maternal interface: role in trophoblast growth and differentiation. Biol Reprod . 1992; 46: 561–72. [DOI] [PubMed] [Google Scholar]
- 25. Red‐Horse K, Zhou Y, Genbacev O, et al . Trophoblast differentiation during embryo implantation and formation of the maternal‐fetal interface. J Clin Invest . 2004; 114: 744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou Y, Fisher SJ, Janatpour M, et al . Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion J Clin Invest . 1997; 99: 2139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong YL, Reddy DM, Green KE, et al . Calcitonin gene‐related peptide (CALCA) is a proangiogenic growth factor in the human placental development. Biol Reprod . 2007; 76: 892–9. [DOI] [PubMed] [Google Scholar]
- 28. Kilburn BA, Wang J, Duniec‐Dmuchowski ZM, et al . Extracellular matrix composition and hypoxia regulate the expression of HLA‐G and integrins in a human trophoblast cell line. Biol Reprod . 2000; 62: 739–47. [DOI] [PubMed] [Google Scholar]
- 29. Fukushima K, Miyamoto S, Tsukimori K, et al . Tumor necrosis factor and vascular endothelial growth factor induce endothelial integrin repertories, regulating endovascular differentiation and apoptosis in a human extravillous trophoblast cell line. Biol Reprod . 2005; 73: 172–9. [DOI] [PubMed] [Google Scholar]
- 30. Chung JY, Song Y, Wang Y, et al . Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived‐VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab . 2004; 89: 2484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Effect of siRNA treatment on PKR2 expression in HTR‐8 cells. Cells were analysed for the loss of PKR2 mRNA and protein expression 48 hrs after transfection using RT‐PCR (A) and immunoblotting (B), respectively. Scrambled (Scr) siRNA served as non‐specific control siRNAs. *P < 0.05.
Video Clip 1 Attached is a video of HTR‐8 organisation in tube‐like structures in the absence or presence of EG‐VEGF or EG‐VEGF plus siRNA for PKR2. Note that the HTR‐8 organisation was significantly delayed compared with the control and siRNA conditions.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item


