Abstract
The chest radiograph is an essential tool for the diagnosis of several lung diseases in intensive care units (ICU). However, several factors make the interpretation of the chest radiograph difficult including the number of X-rays done daily in ICU, the quality of the chest radiograph, and the lack of a standardized interpretation. To overcome these limitations in the interpretation of chest radiographs, researchers have developed computer-aided diagnosis (CAD) systems. In this review, the authors report the methodology used to develop CAD systems including identification of the region of interest, analysis of these regions, and classification. Currently, only a few CAD systems for chest X-ray interpretation are commercially available. Some promising research is ongoing, but the involvement of the pediatric research community is needed for the development and validation of such CAD systems dedicated to pediatric intensive care.
Keywords: children, intensive care, computer-aided diagnosis, chest radiograph
Introduction
The chest radiograph is an essential tool for the diagnosis of several lung diseases in intensive care units (ICU).1 Its low cost, portability, speed, and its use of a moderate dose of radiation make it the most adequate imaging tool, especially in ICUs. Usually, a chest radiograph showing a disease is followed by a therapeutic intervention or a second examination using a high-resolution modality imaging such as computed tomography (CT) scan to confirm the presence and the location of the disease. Misinterpretation of the chest X-ray makes this tool less effective, as this can lead to delayed diagnosis and treatment, which can potentially have an impact on patient outcome.
Several factors can make the interpretation of the chest radiograph difficult. Many images are produced for each patient in the ICU. The substantial number of images as well as the quality of the chest radiograph itself and the lack of a standardized interpretation for some diseases2 make it difficult for physicians to provide accurate interpretation of the image in a short time to arrive at a timely diagnosis. All these factors lead to interobserver variability for the diagnosis of the chest radiograph and generally result in imprecise and/or delayed diagnosis.2
The development of computer-aided diagnosis (CAD) systems aiming to standardize chest X-ray interpretation is one of the major research areas in medical imaging.3 CAD systems are built to identify radiological abnormalities using specific computerized algorithms. In intensive care, a CAD system can help physicians make diagnoses with more precision and more quickly while showing all the regions of the chest X-ray that may contain abnormalities that may not have been initially detected by the physician. With such a system, the physician rapidly arrives at a final decision and can interact with the CAD system's output to confirm or invalidate the presence of any abnormalities to elaborate a final diagnosis (Fig. 1).
Fig. 1.

Algorithm of a computer-aided diagnosis (CAD) system use in intensive care.
Several CAD systems have been developed to help physicians in chest radiograph diagnosis.4 These CAD interprets radiographs to detect nodules, interstitial diseases (acute respiratory distress syndrome [ARDS], pneumoconiosis, tuberculosis [TB], etc.), and other diseases. In this review, we report the different CAD systems in chest radiographs developed, their clinical relevance, and the challenges that still remain to obtain an accurate CAD system for chest X-ray interpretation.
Commercialized CAD Systems for Chest Radiographs
Few CAD systems for chest radiographs are commercialized. In this section, we present some of the available systems tested and evaluated to show their efficiency in disease detection. Mitsubishi Space Software (Tokyo, Japan) developed a commercial CAD system (EpiSight/XR).5 This CAD system aims to detect lung nodules up to 25 mm in diameter (8–25). The CAD development is detailed in the study of Xu et al.6 Kakeda et al5 evaluated the ability of radiologists to interpret chest radiographs for the detection of lung nodules with and without this commercial CAD system. The observer's performance was evaluated using a receiver operating characteristic (ROC) analysis involving 45 cases with solitary lung nodules and 45 healthy patients. The average value of area under the curve (AUC) improved from 0.924 without the CAD to 0.986 with the commercialized CAD output.5 This commercialized CAD did, however, have some limitations. It had a sensitivity performance of 73% with 3.15 false positive (FP) findings per image which is insufficient for adequate detection5; such a low sensitivity results in undetected lung nodules which are not recognized because they overlap with normal anatomic structures such as ribs and vessels. Li et al7 tested the use of a commercial CAD system (Food and Drug Administration–approved CAD nodule detection program) in 34 patients with missed lung cancer on chest radiographs read by radiologists. This commercial CAD system detected the overlooked cancers in 12 (35%) patients with an average of 5.9 FP marks per image. Thus, this CAD system can identify more lung cancers that are more subtle and that may be missed by radiologists; however, the major limitation is the high number of FP marks due to the resemblance between some normal structures and the nodules in chest radiographs. The authors concluded that further development is needed to improve this CAD system.
White et al8 have studied the potential of using a commercial CAD system (OnGuard 3.0; Riverain Medical) to detect nodules in 114 chest radiographs (89 patients) that were missed on initial interpretation by radiologists. In 47% of the radiographs read and in more than 50% of patients, the CAD successfully detected nodules that had been overlooked at initial interpretation resulting in 3.9 FP per radiograph. However, these two studies7 8 evaluated only the standalone performance of the CAD system and did not involve support of the decisions made by radiologists. Therefore, the final diagnosis retained by radiologists was not reported.
De Boo et al9 evaluated a commercial CAD system (IQQA-Chest; EDDA Technology, Princeton Junction, New Jersey, United States) used as second reader for the detection of small pulmonary nodules on chest radiographs from 113 patients. Six readers with different levels of experience individually evaluated chest radiographs with and without the CAD system as second reader in two separate reading sessions. Sensitivity was improved for inexperienced readers who used the commercial CAD system for detection of small nodules (39 vs. 45%) and unchanged for experienced readers (50 vs. 51%).
The interpretation of a software system developed for automated detection of pulmonary TB (CAD4TB, version 1.08, Diagnostic Image Analysis Group, Nijmegen, the Netherlands) was comparable with the interpretation made by clinical officers in 161 subjects in a Zambian clinic10 among whom 120 had abnormal chest radiographs. The AUCs for CAD4TB and the clinical officers were, respectively, 0.91 and 0.89 to 0.94 in comparison with the radiological reference.
These commercial CAD systems have many limitations. The sensitivity observed is frequently low. This implies that several chest X-rays are considered normal when in fact they are not. Low specificity (high FP rate) is also observed and may result in further investigation with unnecessary expenses. Validations of CAD systems are generally done in only one center (the one who developed the CAD) and, as a result, reproducibility in other centers is usually not tested. None of the CAD systems described have been used in ICUs.
The limitations cited earlier support the undertaking of further research in this field to improve performances to use CAD systems in clinic settings as a second reader that might help to make early diagnosis possible.
Methodology to Develop a Computer-Aided Diagnosis in Chest Radiographs
We present in this section the various steps needed to develop a CAD system, the technical characteristics of the CAD systems already developed according to lung disease, and their respective impact on the sensitivity and specificity (Table 1).
Table 1. Characteristics of CAD systems in chest radiographs interpretation reported in the literature.
| Disease | Reference | Database | Commercial | Performance |
|---|---|---|---|---|
| Lung nodules | 5 | 45 patients with lung nodules and 45 healthy patients | Yes | Area under the curve was improved from 0.924 without the CAD to 0.986 with the commercialized CAD |
| 7 | 34 patients | Yes | Overlooked cancer in 12 (35%) patients was detected but with 5.9 FP/image | |
| 8 | 114 chest radiographs (89 patients) | Yes | Overlooked nodules were detected on 47% of radiographs and in more than 50% of patients with 3.9 FP per radiograph | |
| 9 | 113 patients | Yes | The sensitivity of inexperienced readers for the detection of small nodules was improved (39 vs. 45%) and remained unchanged for experienced readers (50 vs. 51%) | |
| 47 | 25 normal and 25 abnormal | No | The value of the AUC has improved from 0.926 to 0.962 | |
| 48 | Training set: 172 TP nodules, 44 TP + 377 FP for the test set. For the template 3,077 FP + 236 TP | No | 44.3% of the FP nodules were removed with reduction of TP (2.3%) | |
| 49 | 924 chest radiographs | No | 70.1% of sensitivity with 5 FP/image | |
| 50 | 106 pairs of PA and lateral views | No | Sensitivity of 70.5%, with 4.9 FP/image for the PA view, sensitivity of 86.9% (6.6 FP/2 images) for PA + lateral view | |
| 51 | Public database: 247 chest radiographs | No | A sensitivity of 0.71 and 0.78 with 1.5 and 2.5 FP/image, respectively | |
| Interstitial lung disease | 52 | 20 radiographs with interstitial infiltrates and 20 normal chest radiographs | No | The radiologists' detection accuracy was improved from an Az of 0.948 to 0.970 |
| Pneumoconiosis | 54 | 300 normal and 125 pneumoconiosis cases | No | Sensitivity of 91.2% at a specificity of 86.3% |
| 55 | 85 normal chest radiographs and 40 with pneumoconiosis | No | Sensitivity, specificity, and accuracy were 0.974 ± 0.018, 0.957 ± 0.021, 0.873 ± 0.024, and 0.929 ± 0.018, respectively | |
| TB | 10 | 161 subjects: 120 had abnormal chest radiographs | Yes | The AUCs for CAD and the clinical officers were, respectively, 0.91 and 0.89–0.94 |
| 59 | 147 images with TB and 241 normal images. 100 normal images and 100 abnormal images with interstitial disease | No | For the diffuse lung disease database, a sensitivity of 0.97 at a specificity of 0.90. For the TB database, a sensitivity of 0.86 at a specificity of 0.50 | |
| 60 | 365 chest radiographs | No | The AUC (Az) is 85% | |
| 61 | The first: 138 chest radiographs (80 normal, 58 TB). The second set: 340 normal, 275 with TB | No | Accuracy of 78.3 for the first set and 84% for the second set | |
| 62 | 50 normal and 45 with TB | No | Sensitivity of 91% at a specificity of 95.4%. | |
| ARDS | 63 | 120 chest radiographs A training database:321 patches Test database: 90 radiographs |
No | Sensitivity of 90.6% and a specificity of 86.5% |
Abbreviations: ARDS, acute respiratory distress syndrome; AUC, area under the ROC curve; CAD, computer-aided diagnosis; FP, false positive; TB, tuberculosis; TP, true positive.
CAD is generally based on extracting the regions of interest (ROI) that may contain abnormalities on a chest X-ray, analyzing these regions to identify the characteristics that distinguish the abnormalities on the radiograph and finally classifying them to decide if the patient has disease or not (Fig. 2). At each of these steps, researchers used a specific methodology.
Fig. 2.
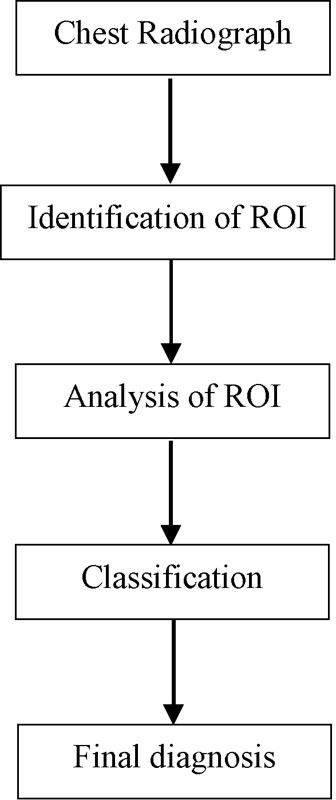
General diagram of the different steps performed by computer-aided diagnosis (CAD) system for chest radiograph interpretation.
Identification of the ROI: A segmentation method is applied to extract only the ROI that may contain abnormalities and to eliminate all normal structures (diaphragm, heart, and sometimes ribs). A good segmentation avoids analyzing regions that do not interest readers and may therefore distort our analysis. Using an automatic segmentation is a good choice, but, for cases where large abnormalities close to the lung walls are present, the detection of anomalies within the lungs is difficult and can result in the elimination of areas of the lung that in fact contain abnormalities. Using an interactive segmentation (semiautomatic) is a good solution for this problem, where the physician can interact with the system and manually correct the lung segmentation. However, this interactive segmentation must be very easy to use for physicians.
Analysis of ROI: To characterize the abnormalities in the ROI, the specifications of the abnormalities are determined and the best features for analysis chosen and tested. Generally, different methods of analysis are combined, tested on a database, and the most accurate is chosen. The main approaches to represent the texture for analysis are divided into three categories, namely, statistical, structural, and spectral.11 To analyze the spatial distribution of gray values, statistical methods compute local features at each point in the image, and then derive a set of statistics based on the distributions of the local features.12 Structural approaches use a set of predefined texture primitives and a set of construction rules to define the way a texture region is constructed.13 Spectral approaches are based on properties of the Fourier spectrum, Gabor, and wavelet based. Their main use is to describe the directionality of periodic or almost periodic two-dimensional patterns in an image.13
Classification: After having extracted the features, a classifier that differentiates a normal and abnormal chest radiographs is selected, tested on a database, and the most accurate is chosen. For the development of CAD systems, supervised machine learning classification techniques are used. The supervised learning consists in first creating a training database which comprises a set of feature vectors that have a corresponding class label. In our case, the training database contains diagnosed chest radiographs or parts of them. The resulting classifier is then used to assign class labels to an instance from the test database, with known features, but unknown class labels.14
Many CAD systems have been developed for diverse lung diseases including lung nodules,15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 interstitial lung disease,30 31 32 33 34 35 36 37 38 and pneumoconiosis.39 40 41 42 These are cited in the study of van Ginneken et al4 and detailed in the following section.
Detection of a Lung Nodule
Lung cancer has become one of the leading causes of mortality in the world.43 The missed rate for radiographic detection for small lung nodules is approximately 30%.4 Early detection of lung cancer can improve its management and prognosis. Many CAD systems have been developed to help doctors interpret chest radiographs and detect lung nodules, especially when the latter are very small and are merged with anatomical structures. These systems are detailed in the study of De Boo et al.44
Li et al45 developed a novel contralateral subtraction technique to identify the ROI that eliminates symmetrical skeletal structures such as ribs and clavicles and enhances asymmetrical opacities (such as lung nodules). This technique consists of correcting the lateral inclination by rotating and shifting the original chest image so that the midline of the thorax is aligned with the vertical centerline of the original chest image. Then, the mirror image is wrapped and subtracted from the original image. Li et al46 developed an improved contralateral subtraction image to assist radiologists in the detection of asymmetrical abnormalities. With contralateral subtraction technique presented by Li et al,45 they observed severe and minor misregistration artifacts in some cases that were caused by the asymmetry of the ribs in the two lungs of the original image. To reduce these artifacts, three image warping techniques (global warping and two iterative warping techniques based on an elastic matching technique) were used. To evaluate the effectiveness of this contralateral subtraction technique in the detection of delicate lung nodules in chest radiographs, Tsukuda et al47 undertook an observer performance study with a ROC analysis. In this study, 25 normal and 25 abnormal chest radiographs, with a subtle lung nodule, were diagnosed by 12 radiologists (10 attending physicians and 2 residents) with and without the contralateral subtraction technique. Use of the contralateral subtraction technique improved the value of the area under the ROC curve from 0.926 to 0.962. However, this technique is just an enhancement step for highlighting lung nodules that does not identify lung nodules on chest radiographs like a full CAD system but can be used as a first step in CAD systems.
Analysis of ROI: Li et al48 developed a CAD system using a template matching technique for nodule detection to significantly reduce the number of FPs. For the training set, 172 true positive (TP) nodules were used and 44 TP + 377 FP nodules were used for the test set. For the template, 3,077 FP + 236 TP nodules were used. A nodule from a test set can be considered as FP and eliminated if its largest cross-correlation value with non-nodule templates is larger than that with nodule templates. Using this technique, 44.3% of the FP nodules were removed with reduction of a very small number of TPs (2.3%).
Shiraishi et al49 developed a CAD system for the detection of various types of lung nodules using a large database that contained a large number of cases which could reflect a real implementation in clinical situations. This database contained 924 chest radiographs divided into two sets that included 459 radiographs for the test base (492 lung nodules) and 465 for the training set (465 lung nodules).
Identification of ROI: Lung fields are first segmented using a ribcage detection technique. Lung nodules are then detected in these regions using an anatomical classification by region of interest (classification into apical, peripheral, hilar, and diaphragm/heart regions) and identified by using the nodule-enhanced image obtained with the average radial-gradient filtering technique.
Analysis of ROI: A total of 71 image features based on geometric, gray-level, background structure, and edge-gradient features and the corresponding locations in the contralateral subtraction image were extracted.49
Classification: A classification into three artificial neural networks (ANNs) is applied to reduce the number of FP nodules. Using the anatomical classification, the average sensitivity for detecting lung nodules was 70.1% with 5.0 FPs per image for testing cases. The performance of this CAD system is not high enough to be used in a clinical setting. The CAD system has the advantage of using a large number of lung nodule cases in its analysis. However, the variation in lung nodule characteristics was very large, making it difficult to compare it to systems with a limited number of lung nodule cases.
Shiraishi et al50 also developed a CAD system for the detection of lung nodules using the lateral and posteroanterior (PA) views of the chest radiograph. They noted that some nodules were detected only on lateral views. In this study, PA and lateral views of 106 chest radiographs (122 lung nodules) were used.
Identification of ROI: In the CAD scheme for lateral views, initial lung nodule candidates were identified by using a nodule enhancement filter based on the edge gradients.
Analysis of ROI: Thirty-four image features were extracted from the original and the nodule-enhanced images.
Classification: For the PA views, the CAD system developed by Shiraishi et al49 was used and resulted in a sensitivity of 70.5%, with 4.9 FP per image. For the lateral views, the rule-based scheme and the ANNs were used for the removal of some FP candidates. The computer performance was evaluated with a leave-one-case-out test method for ANNs. Using the lateral views combined with the PA views improved the sensitivity to 86.9% but increased FPs to 6.6 per two views.50
Campadelli et al51 used the support vector machine (SVM) as a classifier. They presented a fully automated system for detection of lung nodules from digital PA chest radiographs and improved the performance of the CAD system scheme by introducing a new lung segmentation method.
Identification of ROI: Given that lung nodules may be found in the parts of the lungs hidden behind the heart, the spine, and the diaphragm, the investigators performed an accurate segmentation of the lung field area that included those parts. For this, an initial outline of lung borders was contoured by the first derivatives of Gaussian filters in four different orientations. Then, an edge tracking procedure was used to find a continuous external lung contour using the Laplacian of Gaussian operator at three different scales. Finally, the edges were integrated with the initial outline to produce the final lung segmentation. Next, to select potential nodules which often appear in the chest image as circular regions of various sizes, with the highest gray levels at the center surrounded by a much darker ring, the segmented area was processed with a simple multiscale method that enhanced the visibility of the nodules, and an extraction scheme was then applied.51
Analysis of ROI: A total of 160 features were calculated and a feature selection technique was applied to select the best features.
Classification: The system was tested on a public database that included 247 chest radiographs which made comparison with other systems possible; there were 154 patient images containing lung nodules and 93 with no disease. For the classification, polynomial and Gaussian SVMs were used. The authors reported a sensitivity of 0.71 and 0.78 with 1.5 and 2.5 FP/image, respectively. When sensitivity increased to 0.85, they observed 4.5 FP/image. For the highest sensitivity values (0.92 and 1.0), 7 to 8 FP/image were observed.51
Detection and Characterization of Parenchymal/Diffuse Lung Diseases
Detection of diffuse lung disease in chest radiographs is one of the most difficult problems, hence the necessity of CAD system to help detect these abnormalities. Katsuragawa et al developed a CAD system that detects interstitial lung disease.52
Identification of ROI: The authors developed an algorithm to eliminate the posterior ribs by first selecting a large number of squares (ROI) in the chest radiograph and then eliminating those that contain sharp rib edges, using edge-gradient analysis to extract intercostal regions.
Analysis of ROI: Subsequently, they used the variation of root mean square and the first moment of the Fourier spectrum as features extracted from small automatically selected intercostal regions.
Classification: The features measured were compared with an empirically determined threshold level to classify the ROI as normal or abnormal. To show the usefulness of this CAD system, they have undertaken an observer performance study to evaluate the effect of the CAD system on radiologists' interpretation of interstitial diseases. Twenty radiographs with interstitial infiltrates and 20 normal chest radiographs were used.52 Sixteen radiologists interpreted chest radiographs with and without the CAD system output. The ROC curves indicated that the detection accuracy of radiologists using the CAD system output significantly improved from an Az (the area under the ROC curve) of 0.948 to 0.970.
For discussion of parenchymal lung diseases, we present in this section pneumoconiosis, TB, and ARDS.
Pneumoconiosis
In the 1970s, texture analysis was applied in chest radiographs to detect pneumoconiosis using the Fourier spectrum.53 More recently, fully automated CAD schemes for pneumoconiosis have been elaborated.54
Identification of ROI: The system used an active shape method (ASM) for segmenting lung fields which were then subdivided into six non-overlapping regions.
Analysis of ROI: Histogram and co-occurrence matrices were used to obtain a full feature vector of 427 features and the selected feature vector consisting of 20 selected features for each region.54
Classification: Both the training and the test sets had 300 normal and 125 cases of pneumoconiosis. To detect pneumoconiosis, a SVM classifier was used. The evaluation resulted in a sensitivity of 91.2% at a specificity of 86.3%. Zhu et al55 developed a CAD system for pneumoconiosis using other features based on wavelet transform. In this CAD system, chest radiographs with stage III pneumoconiosis were excluded to make it more suitable for clinical practice. It is of note that stage III disease is generally considered fairly easy to diagnose for radiologists. However, almost all of the CAD systems were built and tested using chest X-ray with stage III pneumoconiosis. This system used a database containing 85 normal chest radiographs and 40 with pneumoconiosis.
Identification of ROI: Lung fields were first segmented by combining the traditional Otsu-threshold method with a morphological reconstruction, and then subdivided into six non-overlapping regions. If the lungs were not segmented perfectly (up to 10% of all images), the corrected lung contours were drawn manually by radiologists.55
Analysis of ROI: Twenty-two wavelet-based energy texture features were calculated exclusively from each region.
Classification: A SVM was used to classify an individual region of a healthy subject or a patient with pneumoconiosis. The classification of chest radiographs resulted in an AUC of 0.974 ± 0.018, a sensitivity of 0.957 ± 0.021, a specificity of 0.873 ± 0.024, and an accuracy of 0.929 ± 0.018. These CAD systems developed for the detection of pneumoconiosis54 55 have a good performance, but their impact on physician interpretation in a clinical setting has not been prospectively studied.
Tuberculosis
TB remains the second leading cause of death from infectious disease worldwide, after human immunodeficiency virus. In 2013, there were 1.5 million TB deaths among 9.0 million TB cases.56 Many CAD systems developed to help doctors detect TB disease are detailed in two survey articles.57 58 The first CAD system for TB was developed by van Ginneken et al59 who also developed a CAD system for interstitial disease. They used two databases to develop the CAD: (1) the TB database consisted of 147 images with textural abnormalities and 241 normal images; (2) the interstitial disease database included 100 normal images and 100 abnormal images with interstitial disease.
Identification of ROI: Lungs were first segmented using an ASM.
Analysis of ROI: To detect TB and interstitial disease, the features used included the moments of responses to a multiscale filter bank.
Classification: A KNN (K-nearest neighbor) classifier was then used to classify the regions and the chest radiograph. For the diffuse lung disease database, sensitivity and specificity were 0.97 and 0.90, respectively. For the TB database, the classification had a sensitivity of 0.86 at a specificity of 0.50. The low specificity was due to lung segmentation failure on some TB images including analysis of regions outside the contours of the lungs.
To decrease FP responses and improve specificity, Hogeweg et al60 combined a texture-based abnormality detection system (textural abnormality detection and shape abnormality detection) with a normal detection system.
Identification of ROI: The normal detection system consisted of a segmentation system for the chest radiograph based on pixel classification to detect the lung fields and clavicles.
Analysis of ROI: Gaussian derivative filtered images were calculated at different scales and in different directions and used as features for this system. The detection of textural abnormalities was based on texture analysis of small circular image patches (radius = 32 pixels). Features were based on the moments of intensity distributions of Gaussian derivative filtered images sampled in a patch. Shape abnormality detection was used when large abnormalities close to the lung walls were present because these make segmentation of the lung fields difficult and result in exclusion of lung abnormalities. In such situations, the changed shape of the detected lung fields can be used to detect abnormal images. The results of the three systems were combined to give the final diagnosis.
Classification: A database containing 365 images was used. The training database consisted of 216 images (110 normal, 106 abnormal). The test database included 149 images (69 normal, 80 abnormal). The area under the ROC curve (Az) was improved to 0.85 by combining the three systems.
More recent work undertaken by Jaeger et al aimed to assess efficacy of the system's performance by comparing its ability to detect TB in PA chest radiographs with that of radiologists.61
Identification of ROI: Lung fields were first segmented using a graph cut segmentation method.
Analysis of ROI: A set of texture and shape features were then computed for these lung fields.
Classification: Finally, a classification was applied that permitted a distinction between normal and abnormal chest radiographs using SVM. Two datasets were used. The first set contained 138 chest radiographs, among which 80 were normal and 58 were abnormal with TB. The second set contained 340 normal chest radiographs and 275 abnormal radiographs with TB. Classification accuracy rates of 78.3 and 84% were obtained with the first and second set, respectively. For the first set, system performance was compared with the performance of radiologists. The radiologists' performance was better (accuracy of 82%, and FP half of the CAD system).
In contrast with the CAD systems cited earlier for TB detection, which used an automatic segmentation method, Tan et al62 developed a CAD system for detection of TB disease using an interactive lung field segmentation.
Identification of ROI: A semiautomated segmentation (user-guided snake algorithm) was adopted to ensure better detection of the lung field and less distortion of the texture analysis.
Analysis of ROI: In this study, texture features were based on a first-order textural statistical approach (mean, variance, third moment, and entropy) that calculated an intensity histogram of the segmented lungs.
Classification: A decision tree–based classifier was used to differentiate normal and abnormal radiographs. Fifty normal and 45 TB PA chest radiographs were used. The authors observed a sensitivity of 91% and a specificity of 95.4%. This CAD system had a good performance using only the histogram features and the global diagnosis (database created for the right and left lungs), but the use of an interactive segmentation led to an accurate analysis. However, this CAD system was not compared with radiologist's interpretation to show its efficacy in improving the final diagnosis.
Acute Respiratory Distress Syndrome
The ARDS is an acute inflammatory disease of the lung associated with severe hypoxemia. Our group developed the first CAD system for detection of ARDS in chest radiographs from children.63
Identification of ROI: Our method consists of automatically extracting intercostal patches from chest radiographs belonging to the test database using a semiautomatic segmentation method of the ribs.
Analysis of ROI: Statistical and spectral features were computed from each patch. Then a method of feature transformation was applied using the linear discriminant analysis.
Classification: A training database of 321 patches was classified by an expert into two classes qualified as normal and abnormal patches. Patches belonging to the test database were then classified using the SVM classifier. Finally, the rate of abnormal patches was calculated for each quadrant to ascertain whether the chest radiograph was an ARDS case (bilateral infiltrate of a least one quadrant on each side). The method was evaluated on the chest radiographs of 90 patients among whom 53 presented ARDS. The sensitivity and specificity of the CAD system were 90.6 and 86.5%, respectively. To study the performance of this CAD system, we compared interobserver variability for radiological ARDS diagnosis between two intensivists and the CAD system. Interobserver variability was moderate between the two intensivists (kappa: 0.55). The CAD system was able to significantly improve the kappa score either alone or as a second reader (0.77 and 0.79–0.86, respectively) and reached a good agreement level.64
Chest X-Ray Databases Construction for the Development and Validation of CAD Systems
The current gold standard used to develop and validate CAD systems for chest X-ray interpretation is usually a database that includes chest X-rays and their radiological diagnosis. This implies that the database must be carefully constructed to include the following features: (1) The number of abnormal and normal chest X-rays, and the range of abnormalities included, is important. The higher the number and diversity of radiological presentations of disease cases, the better the CAD system assessment will be.49 (2) The design of chest X-ray diagnosis is crucial. Databases are built by physicians who manually identify anomalies on chest radiographs. There is a variability in the interpretation of chest X-rays between physicians,64 thus the importance of undertaking this step with precision and concordance of diagnosis between several physicians. The number and the experience of physicians who participated in the chest X-ray interpretation are important. The quality of the database can also be improved with the use of other imaging to confirm the diagnosis. For example, the use of other high-quality image modalities (such as tomodensitometry) or chest radiograph follow-up to confirm the presence of abnormal regions improves the accuracy of the diagnosis. (3) The image definition can interfere with CAD system interpretation. The higher the definition, the better the interpretation can be.
In the literature, various databases have been used for validation of CAD systems. Some databases were created using chest X-ray interpretation obtained from a consensus among several radiologists or physicians which subsequently had no diagnosis verification54 62 63 or were verified by CT scans or chest radiograph follow-up.6 48 49 50 51 59 For detection of lung nodules, databases were created by identifying all the nodules on each chest radiograph. However, for other lung diseases, lungs were divided into several regions and specific databases were created for each region: 6 regions,54 55 41 regions,59 or small circular image patches.60 Some databases only included lungs without ribs areas to avoid interpretation bias.52 63 Others used the global diagnosis and created a database for the right and left lungs.61 62
For external validation (outside the center that developed the CAD system), the CAD system can be tested using a public database. There are, however, only a few databases that are public.65 Studies of Schilham et al,66 Hardie et al,67 and Campadelli et al51 that tested CAD systems using a public database for lung nodules detection obtained a sensitivity of 67% (4 FP/image), 78.1% (4 FP/image), and 78% (2.5 FP/image), respectively. Given that the same database was used, it was also easier to assess which CAD system had the highest sensitivity and which should be tested first in clinical trials.
Conclusion
Chest X-rays are commonly used in PICUs with known interobserver interpretation variability. The use of CAD systems can potentially decrease the interobserver variability, but there are currently no commercially available CAD systems to help in the interpretation of chest X-rays in pediatric intensive care. More research is needed to identify, analyze, and classify radiological abnormalities and to build public databases that can be used for validation of these systems.
Acknowledgments
The authors thank Dr. Marisa Tucci for her contribution to the quality of the English in this article.
References
- 1.Rubinowitz A N, Siegel M D, Tocino I. Thoracic imaging in the ICU. Crit Care Clin. 2007;23(3):539–573. doi: 10.1016/j.ccc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Angoulvant F, Llor J, Alberti C. et al. Inter-observer variability in chest radiograph reading for diagnosing acute lung injury in children. Pediatr Pulmonol. 2008;43(10):987–991. doi: 10.1002/ppul.20890. [DOI] [PubMed] [Google Scholar]
- 3.Doi K. Computer-aided diagnosis in medical imaging: historical review, current status and future potential. Comput Med Imaging Graph. 2007;31(4–5):198–211. doi: 10.1016/j.compmedimag.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Ginneken B, ter Haar Romeny B M, Viergever M A. Computer-aided diagnosis in chest radiography: a survey. IEEE Trans Med Imaging. 2001;20(12):1228–1241. doi: 10.1109/42.974918. [DOI] [PubMed] [Google Scholar]
- 5.Kakeda S, Moriya J, Sato H. et al. Improved detection of lung nodules on chest radiographs using a commercial computer-aided diagnosis system. AJR Am J Roentgenol. 2004;182(2):505–510. doi: 10.2214/ajr.182.2.1820505. [DOI] [PubMed] [Google Scholar]
- 6.Xu X W, Doi K, Kobayashi T, MacMahon H, Giger M L. Development of an improved CAD scheme for automated detection of lung nodules in digital chest images. Med Phys. 1997;24(9):1395–1403. doi: 10.1118/1.598028. [DOI] [PubMed] [Google Scholar]
- 7.Li F, Engelmann R, Metz C E, Doi K, MacMahon H. Lung cancers missed on chest radiographs: results obtained with a commercial computer-aided detection program. Radiology. 2008;246(1):273–280. doi: 10.1148/radiol.2461061848. [DOI] [PubMed] [Google Scholar]
- 8.White C S, Flukinger T, Jeudy J, Chen J J. Use of a computer-aided detection system to detect missed lung cancer at chest radiography. Radiology. 2009;252(1):273–281. doi: 10.1148/radiol.2522081319. [DOI] [PubMed] [Google Scholar]
- 9.De Boo D W, Uffmann M, Weber M. et al. Computer-aided detection of small pulmonary nodules in chest radiographs: an observer study. Acad Radiol. 2011;18(12):1507–1514. doi: 10.1016/j.acra.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Maduskar P, Muyoyeta M, Ayles H, Hogeweg L, Peters-Bax L, van Ginneken B. Detection of tuberculosis using digital chest radiography: automated reading vs. interpretation by clinical officers. Int J Tuberc Lung Dis. 2013;17(12):1613–1620. doi: 10.5588/ijtld.13.0325. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan A P. Hoboken, NJ: John Wiley & Sons, Inc.; 2011. Image Representation, Analysis, and Classification; pp. 265–309. [Google Scholar]
- 12.Ojala T Pietikäinen M Texture Classification, Machine Vision and Media Processing Unit University of Oulu, Finland. Available at http://homepages.inf.ed.ac.uk/rbf/CVonline/LOCAL_COPIES/OJALA1/ texclas.htm. Accessed July 6, 2015
- 13.Srinivasan G N Shobha G Statistical texture analysis In: Proceedings of World Academy of Science, Engineering and Technology 2008361264–1269. [Google Scholar]
- 14.Kotsiantis S B, Zaharakis I, Pintelas P. Supervised machine learning: a review of classification techniques. Artif Intell Rev. 2007;2006(26):159–190. [Google Scholar]
- 15.Ballard D, Sklansky J. A ladder-structured decision tree for recognizing tumors in chest radiographs. IEEE Trans Comput. 1976;C-20:503–513. [Google Scholar]
- 16.Sklansky J Petkovic D Two-resolution detection of lung tumors in chest radiographs Berlin, Germany: Springer-Verlag; 18984:365–378. [Google Scholar]
- 17.Lampeter W A, Wandtke J C. Computerized search of chest radiographs for nodules. Invest Radiol. 1986;21(5):384–390. doi: 10.1097/00004424-198605000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Giger M L, Doi K, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography. 3. Automated detection of nodules in peripheral lung fields. Med Phys. 1988;15(2):158–166. doi: 10.1118/1.596247. [DOI] [PubMed] [Google Scholar]
- 19.Giger M L, Doi K, MacMahon H, Metz C E, Yin F F. Pulmonary nodules: computer-aided detection in digital chest images. Radiographics. 1990;10(1):41–51. doi: 10.1148/radiographics.10.1.2296696. [DOI] [PubMed] [Google Scholar]
- 20.Cox G S, Hoare F J, de Jager G. Experiments in lung cancer nodule detection using texture analysis and neural network classifiers. Third South African Workshop on Pattern Recognition. 1992;3:1–7. [Google Scholar]
- 21.Matsumoto T, Yoshimura H, Doi K. et al. Image feature analysis of false-positive diagnoses produced by automated detection of lung nodules. Invest Radiol. 1992;27(8):587–597. doi: 10.1097/00004424-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y C, Doi K, Giger M L, Metz C E, Zhang W. Reduction of false positives in computerized detection of lung nodules in chest radiographs using artificial neural networks, discriminant analysis, and a rule-based scheme. J Digit Imaging. 1994;7(4):196–207. doi: 10.1007/BF03168540. [DOI] [PubMed] [Google Scholar]
- 23.Lo S B, Lou S A, Lin J S, Freedman M T, Chien M V, Mun S K. Artificial convolution neural network techniques and applications for lung nodule detection. IEEE Trans Med Imaging. 1995;14(4):711–718. doi: 10.1109/42.476112. [DOI] [PubMed] [Google Scholar]
- 24.Lin J S, Lo S B, Hasegawa A, Freedman M T, Mun S K. Reduction of false positives in lung nodule detection using a two-level neural classification. IEEE Trans Med Imaging. 1996;15(2):206–217. doi: 10.1109/42.491422. [DOI] [PubMed] [Google Scholar]
- 25.Floyd C E Jr, Patz E F Jr, Lo J Y, Vittitoe N F, Stambaugh L E. Diffuse nodular lung disease on chest radiographs: a pilot study of characterization by fractal dimension. AJR Am J Roentgenol. 1996;167(5):1185–1187. doi: 10.2214/ajr.167.5.8911177. [DOI] [PubMed] [Google Scholar]
- 26.Vittitoe N F, Baker J A, Floyd C E Jr. Fractal texture analysis in computer-aided diagnosis of solitary pulmonary nodules. Acad Radiol. 1997;4(2):96–101. doi: 10.1016/s1076-6332(97)80005-0. [DOI] [PubMed] [Google Scholar]
- 27.Carreira M J, Cabello D, Penedo M G, Mosquera A. Computer-aided diagnoses: automatic detection of lung nodules. Med Phys. 1998;25(10):1998–2006. doi: 10.1118/1.598388. [DOI] [PubMed] [Google Scholar]
- 28.Penedo M G, Carreira M J, Mosquera A, Cabello D. Computer-aided diagnosis: a neural-network-based approach to lung nodule detection. IEEE Trans Med Imaging. 1998;17(6):872–880. doi: 10.1109/42.746620. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Yoshida H, Engelmann R. et al. Computerized analysis of the likelihood of malignancy in solitary pulmonary nodules with use of artificial neural networks. Radiology. 2000;214(3):823–830. doi: 10.1148/radiology.214.3.r00mr22823. [DOI] [PubMed] [Google Scholar]
- 30.Tully R J, Conners R W, Harlow C A, Lodwick G S. Towards computer analysis of pulmonary infiltration. Invest Radiol. 1978;13(4):298–305. doi: 10.1097/00004424-197807000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Katsuragawa S, Doi K, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography: detection and characterization of interstitial lung disease in digital chest radiographs. Med Phys. 1988;15(3):311–319. doi: 10.1118/1.596224. [DOI] [PubMed] [Google Scholar]
- 32.Katsuragawa S, Doi K, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography: classification of normal and abnormal lungs with interstitial disease in chest images. Med Phys. 1989;16(1):38–44. doi: 10.1118/1.596412. [DOI] [PubMed] [Google Scholar]
- 33.Kido S, Ikezoe J, Naito H, Masuike M, Tamura S, Kozuka T. An image analyzing system for interstitial lung abnormalities in chest radiography. Detection and classification by Laplacian-Gaussian filtering and linear opacity judgment. Invest Radiol. 1994;29(2):172–177. doi: 10.1097/00004424-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Kido S, Ikezoe J, Naito H, Tamura S, Machi S. Fractal analysis of interstitial lung abnormalities in chest radiography. Radiographics. 1995;15(6):1457–1464. doi: 10.1148/radiographics.15.6.8577968. [DOI] [PubMed] [Google Scholar]
- 35.Morishita J, Doi K, Katsuragawa S, Monnier-Cholley L, MacMahon H. Computer-aided diagnosis for interstitial infiltrates in chest radiographs: optical-density dependence of texture measures. Med Phys. 1995;22(9):1515–1522. doi: 10.1118/1.597419. [DOI] [PubMed] [Google Scholar]
- 36.Katsuragawa S, Doi K, MacMahon H, Monnier-Cholley L, Ishida T, Kobayashi T. Classification of normal and abnormal lungs with interstitial diseases by rule-based method and artificial neural networks. J Digit Imaging. 1997;10(3):108–114. doi: 10.1007/BF03168597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishida T, Katsuragawa S, Ashizawa K, MacMahon H, Doi K. Application of artificial neural networks for quantitative analysis of image data in chest radiographs for detection of interstitial lung disease. J Digit Imaging. 1998;11(4):182–192. doi: 10.1007/BF03178081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monnier-Cholley L, MacMahon H, Katsuragawa S, Morishita J, Ishida T, Doi K. Computer-aided diagnosis for detection of interstitial opacities on chest radiographs. AJR Am J Roentgenol. 1998;171(6):1651–1656. doi: 10.2214/ajr.171.6.9843307. [DOI] [PubMed] [Google Scholar]
- 39.Kruger R, Thompson W, Turner A. Computer diagnosis of pneumoconiosis. IEEE Trans Syst Man Cybern. 1974;SMC-4:40–49. [Google Scholar]
- 40.Hall E L, Crawford W O Jr, Roberts F E. Computer classification of pneumoconiosis from radiographs of coal workers. IEEE Trans Biomed Eng. 1975;22(6):518–527. doi: 10.1109/tbme.1975.324475. [DOI] [PubMed] [Google Scholar]
- 41.Jagoe J R, Paton K A. Reading chest radiographs for pneumoconiosis by computer. Br J Ind Med. 1975;32(4):267–272. doi: 10.1136/oem.32.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsuragawa S, Doi K, MacMahon H, Nakamori N, Sasaki Y, Fennessy J J. Quantitative computer-aided analysis of lung texture in chest radiographs. Radiographics. 1990;10(2):257–269. doi: 10.1148/radiographics.10.2.2326513. [DOI] [PubMed] [Google Scholar]
- 43.Jemal A, Center M M, DeSantis C, Ward E M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 44.De Boo D W, Prokop M, Uffmann M, van Ginneken B, Schaefer-Prokop C M. Computer-aided detection (CAD) of lung nodules and small tumours on chest radiographs. Eur J Radiol. 2009;72(2):218–225. doi: 10.1016/j.ejrad.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Katsuragawa S, Ishida T. et al. Contralateral subtraction: a novel technique for detection of asymmetric abnormalities on digital chest radiographs. Med Phys. 2000;27(1):47–55. doi: 10.1118/1.598856. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Katsuragawa S, Doi K. Improved contralateral subtraction images by use of elastic matching technique. Med Phys. 2000;27(8):1934–1942. doi: 10.1118/1.1287112. [DOI] [PubMed] [Google Scholar]
- 47.Tsukuda S, Heshiki A, Katsuragawa S, Li Q, MacMahon H, Doi K. Detection of lung nodules on digital chest radiographs: potential usefulness of a new contralateral subtraction technique. Radiology. 2002;223(1):199–203. doi: 10.1148/radiol.2231010344. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Katsuragawa S, Doi K. Computer-aided diagnostic scheme for lung nodule detection in digital chest radiographs by use of a multiple-template matching technique. Med Phys. 2001;28(10):2070–2076. doi: 10.1118/1.1406517. [DOI] [PubMed] [Google Scholar]
- 49.Shiraishi J, Li Q, Suzuki K, Engelmann R, Doi K. Computer-aided diagnostic scheme for the detection of lung nodules on chest radiographs: localized search method based on anatomical classification. Med Phys. 2006;33(7):2642–2653. doi: 10.1118/1.2208739. [DOI] [PubMed] [Google Scholar]
- 50.Shiraishi J, Li F, Doi K. Computer-aided diagnosis for improved detection of lung nodules by use of posterior-anterior and lateral chest radiographs. Acad Radiol. 2007;14(1):28–37. doi: 10.1016/j.acra.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campadelli P, Casiraghi E, Artioli D. A fully automated method for lung nodule detection from postero-anterior chest radiographs. IEEE Trans Med Imaging. 2006;25(12):1588–1603. doi: 10.1109/tmi.2006.884198. [DOI] [PubMed] [Google Scholar]
- 52.Katsuragawa S, Doi K. Computer-aided diagnosis in chest radiography. Comput Med Imaging Graph. 2007;31(4–5):212–223. doi: 10.1016/j.compmedimag.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Revesz G, Kundel H L. Notes: Feasibility of classifying disseminated pulmonary diseases based on their Fourier spectra. Invest Radiol. 1973;8(5):345–349. doi: 10.1097/00004424-197309000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Yu P, Xu H, Zhu Y, Yang C, Sun X, Zhao J. An automatic computer-aided detection scheme for pneumoconiosis on digital chest radiographs. J Digit Imaging. 2011;24(3):382–393. doi: 10.1007/s10278-010-9276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu B, Luo W, Li B. et al. The development and evaluation of a computerized diagnosis scheme for pneumoconiosis on digital chest radiographs. Biomed Eng Online. 2014;13(1):141–154. doi: 10.1186/1475-925X-13-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geneva: World Health Organization; 2014. WHO Global Tuberculosis Report 2014. [Google Scholar]
- 57.Jaeger S, Karargyris A, Candemir S. et al. Automatic screening for tuberculosis in chest radiographs: a survey. Quant Imaging Med Surg. 2013;3(2):89–99. doi: 10.3978/j.issn.2223-4292.2013.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naing N, Yan W, Yusof H, Akramin A. Computer-aided tuberculosis detection in chest radiographs: a review. Electrical & Computer Engineering: An International Journal. 2014;3(4):1–14. [Google Scholar]
- 59.van Ginneken B, Katsuragawa S, ter Haar Romeny B M, Doi K, Viergever M A. Automatic detection of abnormalities in chest radiographs using local texture analysis. IEEE Trans Med Imaging. 2002;21(2):139–149. doi: 10.1109/42.993132. [DOI] [PubMed] [Google Scholar]
- 60.Hogeweg L Mol C de Jong P A Dawson R Ayles H van Ginneken B Fusion of local and global detection systems to detect tuberculosis in chest radiographs In: Medical Image Computing and Computer-Assisted Intervention–MICCAI Heidelberg, Berlin: Springer; 2010650–657. [DOI] [PubMed] [Google Scholar]
- 61.Jaeger S, Karargyris A, Candemir S. et al. Automatic tuberculosis screening using chest radiographs. IEEE Trans Med Imaging. 2014;33(2):233–245. doi: 10.1109/TMI.2013.2284099. [DOI] [PubMed] [Google Scholar]
- 62.Tan J H, Acharya U R, Tan C, Abraham K T, Lim C M. Computer-assisted diagnosis of tuberculosis: a first order statistical approach to chest radiograph. J Med Syst. 2012;36(5):2751–2759. doi: 10.1007/s10916-011-9751-9. [DOI] [PubMed] [Google Scholar]
- 63.Zaglam N, Jouvet P, Flechelles O, Emeriaud G, Cheriet F. Computer-aided diagnosis system for the acute respiratory distress syndrome from chest radiographs. Comput Biol Med. 2014;52:41–48. doi: 10.1016/j.compbiomed.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Zaglam N, Essouri S, Fléchelles O, Emeriaud G, Cheriet F, Jouvet P. Inter-observer variability for radiography in pediatric acute respiratory distress syndrome and improvement with a computer-aided diagnosis system. Austin J Emerg Critical Care Med J. 2015;2(3):1020–1024. [Google Scholar]
- 65.Shiraishi J, Katsuragawa S, Ikezoe J. et al. Development of a digital image database for chest radiographs with and without a lung nodule: receiver operating characteristic analysis of radiologists' detection of pulmonary nodules. AJR Am J Roentgenol. 2000;174(1):71–74. doi: 10.2214/ajr.174.1.1740071. [DOI] [PubMed] [Google Scholar]
- 66.Schilham A M, van Ginneken B, Loog M. A computer-aided diagnosis system for detection of lung nodules in chest radiographs with an evaluation on a public database. Med Image Anal. 2006;10(2):247–258. doi: 10.1016/j.media.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Hardie R C, Rogers S K, Wilson T, Rogers A. Performance analysis of a new computer aided detection system for identifying lung nodules on chest radiographs. Med Image Anal. 2008;12(3):240–258. doi: 10.1016/j.media.2007.10.004. [DOI] [PubMed] [Google Scholar]


