Abstract
During the last decade, interest in the brain natriuretic peptide (BNP) and N-terminal probrain natriuretic peptide (NT-proBNP) in the pediatric population has progressively increased. The aim of this article is to provide an up to date review of evidences regarding the use of BNP/NT-proBNP in pediatrics, with a particular focus on neonatal intensive care and congenital heart disease. The potentialities of the BNP have been demonstrated in multiple settings, particularly: the screening of congenital/acquired heart disease (CHD) versus pulmonary disease; the evaluation of CHD severity (grade of heart failure, degree of left-to-right shunts); the management of children undergoing cardiac surgery; and monitoring premature infants with patent arterial duct. BNP/NT-proBNP values may be considered an easy and relatively low cost additional diagnostic and prognostic tool. Interpretation of BNP values in children requires attention to important factors, including: laboratory methods, the type of cardiac defect, its severity, and the presence of extracardiac conditions. Of these, the hemodynamic characteristic of CHD and physiologic variations of BNP values occurring during the first weeks of life play a major role. The current evidences in favor of BNP use are mainly derived from single-center, nonrandomized studies, and cost-effectiveness analysis are still lacking. As such, despite sufficient evidences supporting the diagnostic and prognostic potentialities of BNP, these findings should be reinforced by multicenter, randomized studies specifically designed to evaluate outcomes and cost-effectiveness. In addition, standard consensus documents/guidelines, that are currently lacking, are warranted for a more systematic use of BNP in the pediatric age.
Keywords: biomarker, natriuretic peptides, BNP, children, neonates, congenital heart disease
Background
The diagnostic and prognostic role of the B-type cardiac natriuretic peptide hormones (BNP and N-terminal probrain natriuretic peptide [NT-proBNP]) in the management of adult patients with both acute and chronic heart failure (HF) is well established.1 2 As recently reviewed, several studies support the use of BNP/NT-proBNP in the pediatric population also. Despite crescent evidence supporting the use of BNP in pediatrics no guidelines or expert consensus recommendations exist for their routine use in different clinical setting.3 4 5 6 The aim of this opinion article is to provide an up to date of evidences regarding the use of BNP/NT-proBNP in pediatrics, with a particular focus on the congenital heart disease (CHD).
In the hospital, BNP and NT-proBNP may be helpful in multiple settings including: (1) screening for CHD and acquired heart disease (including the differential diagnosis of pulmonary versus cardiac causes of dyspnea); (2) the evaluation of CHD severity (the grade of heart failure, the degree of left-to-right shunts); (3) the management of children undergoing cardiac surgery for correction/palliation of CHD.
Characteristics and the Pathophysiological Role of Natriuretic Peptides
In the first part of this article, the most important biochemical characteristics and the pathophysiological role of natriuretic peptides will be summarized.
Biochemical Characteristics of B-Type Cardiac Natriuretic Peptide System
Cardiac natriuretic peptides, including atrial natriuretic peptide (ANP) and BNP are synthesized and secreted mainly by cardiomyocytes.7 8 9 ANP is preferentially produced in atria, while BNP is preferentially synthesized in ventricles, and particularly in patients with chronic cardiac diseases.7 8 Synthesis and secretion of those two peptides may be differently regulated in atrial versus ventricular myocytes and, probably, during neonatal versus adult life.7 8
The human BNP gene encodes for a pre-proBNP molecule with 134 amino acids, which including a signal peptide of 26 amino acids. BNP is cleaved out of a prohormone molecule of 108 amino acids, the proBNP1–108 (usually indicated as proBNP). Before being secreted from cardiomyocytes into the bloodstream, proBNP is split by proteolytic enzymes (such as corin and/or furin) into two peptides: (1) the biologically inactive NH2-terminal peptide fragment proBNP1–76 (indicated as NT-proBNP), and (2) the COOH-terminal peptide fragment, proBNP77–108, which is the biologically active peptide of 32 amino acids, usually indicated as BNP.7 8 These data suggest that the peptides BNP and NT-proBNP can be produced from the intact precursor proBNP also in blood circulation through enzymatic cleavage by plasma proteases (such as corin).10 11 12 13
Measurement Challenges and Kinetics
The active hormone BNP has shorter plasma half-life compared with NT-proBNP, and consequently has lower plasma concentration.14 15 16 More specifically, studies have estimated that BNP has a plasma half-life of approximately 15 to 20 minutes, while that of NT-proBNP is more than 60 minutes in healthy subjects.14 15 16 Furthermore, there appears to be significant within individual variability in BNP and NT-proBNP concentrations, ranging from as little as 30% to as much as 70% both in healthy subjects and patients with heart failure 14 15 16 For these reasons, only variations in serial measurements of circulating levels of BNP and NT-proBNP higher than 30% should be considered to have clinical relevance in the follow-up of patients with heart failure.17 18 From an analytical chemistry point of view, the marked heterogeneity of the B-type natriuretic peptides that are circulating in human blood may explain the large differences seen in the results reported for the different immunoassay methods that are considered specific to the biologically active peptide hormone BNP. On the other hand, one sees greater consistency in the results observed using the various immunoassay techniques for the NT-proBNP, perhaps because there is less heterogeneity in the circulating forms of that biologically inactive peptide.19 20 21 22 For example, a recent study, using standardized protocols and quality control techniques, demonstrated that the IRMA method (Shionogi's Diagnostic Division, Tokyo, Japan), the ADVIA method for the Centaur platform (Siemens Health Care Diagnostics, Tarrytown, New York, United States) and the ST AIA-PACK method for the AIA platform (TOSOH Corporation, Tokyo, Japan) yielded significantly lower (as great as 50%) BNP levels compared with other immunoassays, such as the POCT Triage method (Alere Diagnostics Livermore, California, United States), the BNP Triage Biosite for Access and UniCell DxI platforms (Beckman Coulter Diagnostics Brea, California, United States), the MEIA method for the AxSYM platform (Abbotts Diagnostics Chicago, United States), and the chemiluminescent microparticle immunoassay for ARCHITECT platform (Abbotts Diagnostics).22 Luckenbill et al23 reported that many of these systematic differences between the various BNP immunoassay systems could be due to cross-reactivity between the glycosylated and nonglycosylated forms of the precursor proBNP. Altogether these data suggest that clinicians should be cautious when comparing results obtained from different laboratories using different BNP immunoassay methods.
BNP Response to Acute and Chronic Stimulation
BNP-NT-proBNP response is different in acute and chronic conditions. In fact normal ventricular myocardium may produce only a limited amount of BNP in response to an acute stimulation (i.e., acute cardiac failure), probably via a constitutive secretory pathway. In contrast, prolonged stimulation (i.e., chronic heart failure) leads to upregulation in transcription, translation, and secretion of additional amounts through complex interactions of the myocardium with the neurohormonal and immunological systems.7 8
Wall distension is generally considered the main mechanical stimulus for BNP production by ventricular tissue both in acute and in chronic conditions. Several studies, however, indicate that BNP production/secretion may be differently regulated in normal compared with diseased ventricular myocardium. Some experimental and clinical studies have also indicated that ventricular hypertrophy (and especially the concomitant presence of fibrosis), myocardial ischemia, and perhaps hypoxia, per se, could all stimulate BNP production.7 8 24 25 26 27 28 29 30 31 32
Interpreting BNP Values in Neonates, Infants, and Children
Multiple factors may affect BNP/NT-proBNP plasma concentrations values in the pediatric age.3 4 5 6 First BNP values need to evaluated according to patient age and the laboratory-specific range for normality.3 4 5 6 Second BNP values greatly vary according the underlying cardiac disease, with some patterns of response in different groups of CHD.3 4 5 6 Finally, BNP/NT-proBNP concentrations may be affected by several physiological or clinical conditions outside of the cardiovascular system.3 4 5 6 In particular, in the neonatal period a series of factors may influence BNP/NT-proBNP plasmatic values. Higher BNP/NT-proBNP values have been reported in infants with the following characteristics: mothers with type 1 diabetes, prematurity, intrauterine growth retardation, cesarean section following uterine contraction, and antenatal stress conditions.33 34 35 36 Moreover, newborn twins usually show higher plasma peptide concentrations than singletons.
References Values in the Pediatric Age
In children, plasma concentrations of natriuretic peptides in children need to be evaluated according to age-specific range of normality.3 5 16 37 38 39 40 41 42 43 44 45 46 47 48 In fact, in normal subjects, BNP/NT-proBNP plasma concentrations greatly vary during the somatic growth, with greatest variations observed during the neonatal age.3 5 16 37 38 39 40 41 42 43 44 45 46 47 48 On average, plasma BNP/NT-proBNP concentrations are highest during the first 4 days of life and then rapidly fall during the first week, with a further slower progressive reduction throughout the first month of life (Table 1 and Fig. 1). After the first month of life, BNP/NT-proBNP concentrations remain steady, without any significant change up to 12 years of age. There are generally no gender-related differences in BNP/NT-proBNP values in children up to the adolescent period.7 8 37 38 41 42 49 Throughout adolescence, fertile girls show progressively higher circulating BNP levels than boys, probably due to a direct action of sex steroid hormones on production of the cardiac natriuretic hormones by cardiomyocytes.7 8 49 50 51 52
Table 1. BNP values (ng/L), measured in 253 healthy newborns and infants in the authors laboratory with the BNP triage biosite for access platform (by Beckman Coulter, Inc., Fullerton, CA). Values have been grouped into four age groups from birth to 12 years of life.
| Groups (time periods from birth) |
Number of individuals | Mean ± SD | Median | Range | 97.5th percentile | p Value |
|---|---|---|---|---|---|---|
| 0–2 d | 68 | 280.3 ± 167.5 | 243.5 | 41–866 | 758.7 | < 0.0001a |
| 3–30 d | 75 | 136.1 ± 149.3 | 75 | 10–763 | 741.4 | < 0.0001b |
| 1–12 mo | 46 | 20.3 ± 10.7 | 19 | 5–45 | 43.9 | |
| 1–12 y | 64 | 15.7 ± 8.9 | 13 | 4–46 | 39.8 | |
| All groups (0–12 y) | 253 | 123.4 ± 160.1 | 38 | 4–866 | 622.0 |
Note: Range: minimum and maximum values.
Significantly higher than all the other next time period values.
Significantly higher than the values observed throughout the next time periods (i.e., 2–12 mo and 2–12 y).
Fig. 1.
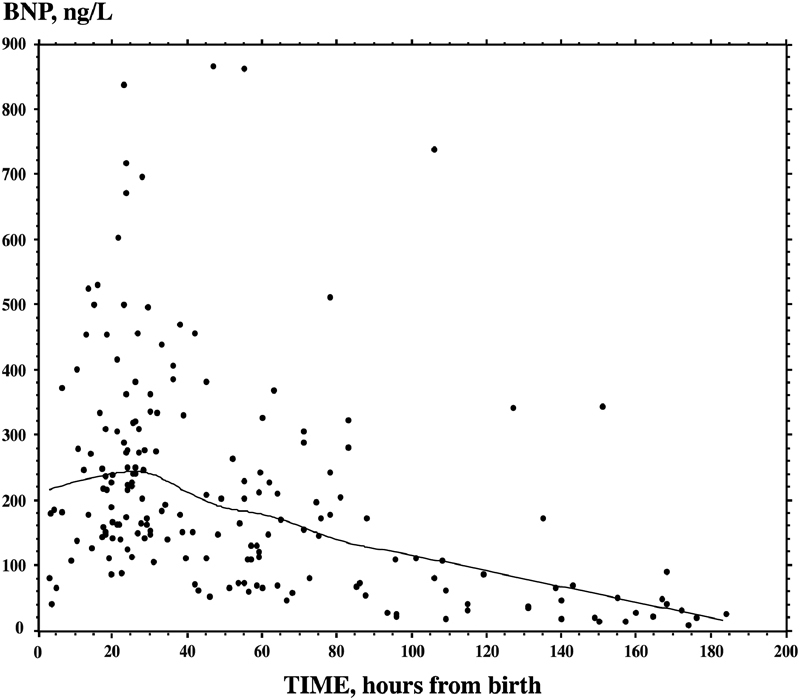
Normal BNP values in healthy newborns throughout the first days of life. Plasma BNP values were measured in 188 healthy newborns throughout the first days of life. Plasma BNP concentrations are very high during the first 4 days of life, then values fall rapidly during the first week with a further slower progressive reduction throughout the first month of life. Plasma BNP was measured in the authors laboratory with the automated access platform (Triage BNP reagents, Access Immunoassay Systems, REF 98200, Beckman Coulter, Inc., Fullerton, CA). The trend was indicated by a continuous line, assessed by smooth spline analysis (data modified from Cantinotti et al6). BNP, brain natriuretic peptide.
Variation in BNP Concentrations Type and Severity of Cardiac Illness
BNP levels greatly vary according to the type of CHD and with disease severity. As expected complex CHD (i.e., transposition of the great arteries, univentricular heart, etc.) showed, on average, higher BNP concentrations compared with simple defects.3 53 54 55 56
BNP concentrations, however, are more related to the type of CHD than to disease severity. In particular, BNP is higher in CHD where the left rather than the right ventricle is mainly involved. Higher BNP concentrations have been observed in CHD characterized by left ventricular volume overload (i.e., defects characterized by left-to-right shunt, such as ventricular septal defect, patent ductus arteriosus, truncus arteriosus, atrial and atrioventricular septal defects) as compared with those with right ventricular volume (i.e., atrial septal defects, anomalous pulmonary venous return, etc.) or pressure overload (i.e., tetralogy of fallot, pulmonary stenosis).3 5 47 54 57 58 Furthermore, BNP values are higher in diseases characterized by left ventricular pressure overload (i.e., aortic stenosis or coarctation) than in CHD with right ventricular pressure overload (i.e., pulmonary stenosis, tetralogy of Fallot). As an example, children with uncorrected pulmonary stenosis or tetralogy of Fallot usually show BNP values only slightly higher than normal children.3 5
Clinical Applications of BNP
The next session will detail application of BNP in specific clinical settings.
Role of BNP in Management of Patent Ductus Arteriosus
Multiple studies have been conducted to test the clinical relevance of BNP/NT-proBNP assay in the management of neonatal patent arterial duct (PDA).59 60 61 62 63 64 As expected BNP/NT-proBNP values correlated with magnitude of shunt, pulmonary artery pressure, pulmonary vascular resistance, and end-diastolic volume.54 57 59 60 61 62 63 More importantly, BNP values after the first 3 days of life were able to discriminate the patients requiring PDA closure.59 60 61 62
Role of BNP in Diagnosing Presence and Severity of Congenital Heart Disease
BNP appears to have value for differentiating between pulmonary and cardiac causes of respiratory distress (i.e., tachypnea, work of breathing) in neonates.3 4 5 6 Several studies have demonstrated that BNP shows a good diagnostic accuracy (AUC [area under the curve] values from ROC [receiver operating characteristic] analysis from 0.75 to 0.97) in differentiating between children with and without CHD, when appropriate cut-off values are used.3 5 6 Similar to pulsoximetry, BNP has been proposed as a potential neonatal screening tool for CHD.3 5 65 66 67 The use of pulsoximetry for the screening of CHD in neonatal and pediatric age has been recently encouraged by multiple sources.65 66 BNP may have additional value for children with lesions where reliance solely on pulsoximetry may result in false-negative results for some potentially life-threatening conditions (i.e., aortic coarctation and left-to-right shunts). In the setting where CHD can initially be subtle due to the PDA, elevated BNP may help raise suspicion for CHD. The recent availability of point-of-care test (POCT) methods allows for measurement of BNP in blood samples collected by venipuncture of the heel or fingertip with BNP values comparable to those measured with an automated platform.5 6 The use of POCT may allow easier and less invasive BNP measurement in neonates, even in ambulatory setting, thus facilitating the use of BNP as a screening toll for the diagnosis of CHD.
In the few first hours of life, when there are rapid physiologic variation in cardiac hemodynamics, it may be difficult to understand the severity of the disease.68 69 70 BNP/NT-proBNP values are higher in patients with these CHD than in normal subjects, and the biomarkers levels usually correlate with lesion severity, including oxygen saturation in patients with cyanotic defects 3 5. Unfortunately, the available data are limited and at times contrasting. In fact, no correlation was found between BNP values and some functional or structural parameters, such as hemodynamic gradients or degree of hypertrophy in patients with CHD characterized by left and right heart obstruction.57 Further studies are advised to better clarify the role of BNP in immediate evaluation of lesion type and severity CHD.
BNP in Children Undergoing Cardiac Surgery for Correction/Palliation of CHD
There are sufficient evidences on the prognostic role of BNP-NT-proBNP in children undergoing cardiac surgery.71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 As reported by multiple authors. BNP levels (especially those measured preoperatively) were independently associated with important parameters of outcome, including the duration of mechanical ventilation, intensive care unit stay, need for inotropic support, and low cardiac output syndrome.71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 Of interest two different pattern of response have been observed in neonates and older children. In older children, biomarker values usually increase compared with preoperative values after surgery showing a peak at 12 to 24 hours postsurgery followed by a decrease thereafter76 77 78 82 (Fig. 2B). On the contrary, neonates on average show very high preoperative values of BNP/NT-proBNP with a progressive decrease of biomarkers levels immediately after surgery81 82 (Fig. 2A). This opposite pattern of BNP kinetics after surgery is probably the result of multiple factors including: the higher severity of CHD with neonatal presentation, the complexity of neonatal surgical procedures.90 In addition, the maturational variations in BNP response during the first weeks of life need also to be taken into account.69 70 Usually biomarker values tend to progressively decrease after the initial peak reached within the first 24 hours postsurgery. If biomarker levels do not significantly decrease or even further increases some day after surgery, it may suggest some complications and/or only partial correction of CHD.81 82 Of interest, at all the ages, BNP values at discharge after surgery remained on average higher than those found in normal subjects, thus indicating a slow recovery toward a “normal” hemodynamic balance, even in well corrected defects.3 5 81 82
Fig. 2.

Postoperative BNP trends in neonates (age < 30 days, n = 72) (A) and in children (age ≥ 30 days, n = 115) (B). The results are expressed as boxes with five horizontal lines, displaying the 10th, 25th, 50th (median), 75th, and 90th percentiles of the variable. All values above the 90th percentile and below the 10th percentile (outliers) are plotted separately (as circles). The p levels of statistical significance compared with the presurgery BNP values by the Fisher PLSD test after repeated measures ANOVA, are also indicated in the figure. Log-transformed BNP values are used for the statistical analysis. Plasma BNP was measured in the authors laboratory with the automated access platform (Triage BNP reagents, Access Immunoassay Systems, by Beckman Coulter, Inc., Fullerton, CA). ANOVA, analysis of variance; BNP, brain natriuretic peptide; PLSD, protected least significant difference.
Role of BNP as a Diagnostic Marker for Cardiac Disease
Available literature shows that BNP values can be significantly augmented in cardiac disease outside of congenital heart disease and the neonatal period. For example, BNP is known to be elevated in children with different types of cardiomyopaties92 93 94 95 96 97 98 99 100 101 102 including dilated,92 95 left ventricular noncompaction,92 Duchenne muscular dystrophy,92 inflammatory,92 102 ischemic,92 oncologic,92 98 101 and mitochondrial100 cardiomyopathy. Differences emerged among different cardiomyopathies with dilated cardiomyopathy showing on average higher BNP values than with hypertrophic and restrictive forms.95 Utility of BNP and NT-proBNP was also proven for the early detection of the left ventricular impairment in children with doxorubicin-induced cardiomyopathy,96 97 98 99 100 101 as well as in those with iron-overload cardiomyopathy in β thalassemia major.100
The usefulness of BNP assay has also been evaluating in the tested in the screening for and differential diagnosis of acute and chronic cardiac and respiratory diseases outside of the immediate neonatal period.103 104 105 106 107 108 Unfortunately, the possibility of performing a systematic review and meta-analysis to synthesize the current data are hampered by significant heterogeneity in important study characteristics, including: the limited sample size (with several studies < 50), heterogeneity in methods employed for BNP and NT-proBNP measurement, the use of different cut-off values103 104 105 106 107 108 and the large differences in the clinical severity of CHD patients enrolled. Nonetheless, evaluation of individual study results suggests significant potential value for BNP. For example, in a cohort of 49 children with acute respiratory distress Koulouri et al105 reported a BNP cut-off value of 40 pg/mL for differentiating HF from respiratory disease (sensitivity 91%; specificity 77%). As another example, a prospective study conducted by Maher et al106 on 33 pediatric patients with newly diagnosed congenital or acquired heart disease and 70 pediatric patients with respiratory and infection complaints, demonstrated that BNP levels were significantly higher in patients with cardiac (mean 3,290 ± 1,609 ng/L; range > 521 − 5,000 ng/L) compared with noncardiac disease (mean 17.4 ± 20 ng/L; range < 5 − 174 ng/L) disease group.106 Similar results were obtained by Cohen et al107 who measured NT-proBNP values and demonstrated that values were significantly higher for 17 infants with HF (median: 18,452 ng/L; range: 5,375–99,700 ng/L) than for 18 infants with acute lung disease (median: 311 ng/L; range: 76–1,341 ng/L). Finally, in the only prospective blinded study, conducted on over 100 pediatric patients presenting to the acute care setting, Law et al108 reported two different BNP cut-off values to differentiating cardiac versus pulmonary disease: one for neonates 0 to 7 days of age (170 ng/L with sensitivity of 94% and specificity 73%), and the other for older children (41 ng/L with sensitivity of 87% and specificity 70%).
Role of BNP in the Evaluation of Noncardiac Disease Severity
BNP may also be of prognostic value children with systemic disease and/or single organ defects and no cardiac defects. In particular, BNP has been investigated in two small studies (27 and 11 infants, respectively) evaluating neonates with bronchopulmonary dysplasia.91 109 BNP was elevated in infants with bronchopulmonary dysplasia, and correlated with the severity of respiratory disease.91 109 Similarly, BNP values at 1 day after birth predicted outcome in 27 newborns with congenital diaphragmatic hernia.110 A prognostic role of BNP has been demonstrated furthermore how in a group of 340 patients (including neonates, infants, children, and adults) BNP and procalcitonin concentration were higher in nonsurvivors than in survivors.111
Limitations of Current Research
Despite crescent evidences on its utility, BNP use in the pediatric age remain limited and fragmentary. This is partly due to limitations of current studies and lack of guidelines/expert consensus recommendations regulating the use of BNP beyond research purpose. Limitations of current studies mainly pertain to small sample size for many works (with only few large scale-multicenter studies) and the lack of randomized clinical trials specifically designed to evaluate the cost-effectiveness of BNP/NT-proBNP use.3 4 5 6 112 Criteria to evaluate the clinical utility of a biomarker (including calibration, discrimination, and reclassification tests) have been recently revised113 114 115 and only a few of the studies performed so far have respected these requirements.90 On the whole, pediatric clinical studies on BNP are only a very small portion of the total number (< 10%) of scientific literature of this cardiac biomarker. A series of factors may condition and limit clinical research in pediatric age, especially in neonates. The rarity of CHD (less than 1% of birth per year) makes it challenging to enroll a sufficiently sized cohort within a single-center study. Furthermore, when dealing with neonates and children there are methodological and ethical considerations that may represent a major limitation (i.e., the difficulties to perform blood samples, neonatal compliance/collaboration, etc.).6 Of interest, the use of new POCT methods, which use less invasive samples (such as capillary blood specimens) may help to overcome a few of these problems especially in neonates and infants.
Summary
Crescent evidence support the use of BNP/NT-proBNP as an additional tool in the integrated screening, diagnosis, management, and follow-up of children with CHD or other potential cardiac lesions.3 4 5 6 112 In particular, the prognostic role of BNP in children undergoing cardiac surgery has been sufficiently proved. Utility of BNP in the management of neonates with PDA has also been established. Many evidences support the use of BNP as an additional marker in disease severity classifications in children with established CHD, as well in the screening of CHD with neonatal presentation, and in the differential diagnosis of respiratory distress of cardiac versus pulmonary etiology. Most of these evidences, however, derive from single center, using multivariable model analysis, while randomized clinical trials designed to prove the cost-effectiveness of a routine use of BNP/NT-proBNP in neonatal intensive care unit are lacking. The use of new POCT methods, which use less invasive samples (such as capillary blood specimens) may contribute to a more widespread use of BNP assay especially in neonates and infants, as well to the development of screening programs for CHD including this biomarker.
For a more systematic use of BNP in neonatal intensive care unit there is a need of wide, randomized, multicenter studies to reinforce current evidence and fully evaluate cost-effectiveness of biomarker use in daily practice.
Footnotes
Funding None. Conflict of Interest None.
References
- 1.Thygesen K, Mair J, Mueller C. et al. Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J. 2012;33(16):2001–2006. doi: 10.1093/eurheartj/ehq509. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C W, Jessup M, Bozkurt B. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Cantinotti M, Giovannini S, Murzi B, Clerico A. Diagnostic, prognostic and therapeutic relevance of B-type natriuretic hormone and related peptides in children with congenital heart diseases. Clin Chem Lab Med. 2011;49(4):567–580. doi: 10.1515/CCLM.2011.106. [DOI] [PubMed] [Google Scholar]
- 4.Cantinotti M, Walters H L, Crocetti M, Marotta M, Murzi B, Clerico A. BNP in children with congenital cardiac disease: is there now sufficient evidence for its routine use? Cardiol Young. 2015;25(3):424–437. doi: 10.1017/S1047951114002133. [DOI] [PubMed] [Google Scholar]
- 5.Eindhoven J A, van den Bosch A E, Jansen P R, Boersma E, Roos-Hesselink J W. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol. 2012;60(21):2140–2149. doi: 10.1016/j.jacc.2012.02.092. [DOI] [PubMed] [Google Scholar]
- 6.Cantinotti M, Law Y, Vittorini S. et al. The potential and limitations of plasma BNP measurement in the diagnosis, prognosis, and management of children with heart failure due to congenital cardiac disease: an update. Heart Fail Rev. 2014;19(6):727–742. doi: 10.1007/s10741-014-9422-2. [DOI] [PubMed] [Google Scholar]
- 7.Clerico A, Recchia F A, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol Heart Circ Physiol. 2006;290(1):H17–H29. doi: 10.1152/ajpheart.00684.2005. [DOI] [PubMed] [Google Scholar]
- 8.Clerico A, Giannoni A, Vittorini S, Passino C. Thirty years of the heart as an endocrine organ: physiological role and clinical utility of cardiac natriuretic hormones. Am J Physiol Heart Circ Physiol. 2011;301(1):H12–H20. doi: 10.1152/ajpheart.00226.2011. [DOI] [PubMed] [Google Scholar]
- 9.Goetze J P. Biochemistry of pro-B-type natriuretic peptide-derived peptides: the endocrine heart revisited. Clin Chem. 2004;50(9):1503–1510. doi: 10.1373/clinchem.2004.034272. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, Wu S, Wang W. et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286(12):10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knappe S, Wu F, Masikat M R, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J Biol Chem. 2003;278(52):52363–52370. doi: 10.1074/jbc.M309991200. [DOI] [PubMed] [Google Scholar]
- 12.Dong N, Chen S, Yang J. et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3(2):207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenov A G, Seferian K R, Tamm N N. et al. Human pro-B-type natriuretic peptide is processed in the circulation in a rat model. Clin Chem. 2011;57(6):883–890. doi: 10.1373/clinchem.2010.161125. [DOI] [PubMed] [Google Scholar]
- 14.Clerico A. Pathophysiological and clinical relevance of circulating levels of cardiac natriuretic hormones: are they merely markers of cardiac disease? Clin Chem Lab Med. 2002;40(8):752–760. doi: 10.1515/CCLM.2002.129. [DOI] [PubMed] [Google Scholar]
- 15.Clerico A, Zucchelli G C, Pilo A, Emdin M. Clinical relevance of biological variation of B-type natriuretic peptide. Clin Chem. 2005;51(5):925–926. doi: 10.1373/clinchem.2004.046615. [DOI] [PubMed] [Google Scholar]
- 16.Clerico A, Carlo Zucchelli G, Pilo A, Passino C, Emdin M. Clinical relevance of biological variation: the lesson of brain natriuretic peptide (BNP) and NT-proBNP assay. Clin Chem Lab Med. 2006;44(4):366–378. doi: 10.1515/CCLM.2006.063. [DOI] [PubMed] [Google Scholar]
- 17.Clerico A, Fontana M, Ripoli A, Emdin M. Clinical relevance of BNP measurement in the follow-up of patients with chronic heart failure. Adv Clin Chem. 2009;48:163–179. doi: 10.1016/s0065-2423(09)48007-7. [DOI] [PubMed] [Google Scholar]
- 18.Januzzi J L Troughton R Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide measurements are useful in heart failure management Circulation 20131274500–507., discussion 508 [DOI] [PubMed] [Google Scholar]
- 19.Rawlins M L, Owen W E, Roberts W L. Performance characteristics of four automated natriuretic peptide assays. Am J Clin Pathol. 2005;123(3):439–445. doi: 10.1309/PDJ2-RMM8-0FVR-DH7W. [DOI] [PubMed] [Google Scholar]
- 20.Prontera C, Zaninotto M, Giovannini S. et al. Proficiency testing project for brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP (NT-proBNP) immunoassays: the CardioOrmocheck study. Clin Chem Lab Med. 2009;47(6):762–768. doi: 10.1515/CCLM.2009.153. [DOI] [PubMed] [Google Scholar]
- 21.Clerico A, Zaninotto M, Prontera C. et al. State of the art of BNP and NT-proBNP immunoassays: the CardioOrmoCheck study. Clin Chim Acta. 2012;414:112–119. doi: 10.1016/j.cca.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Franzini M, Masotti S, Prontera C. et al. Systematic differences between BNP immunoassays: comparison of methods using standard protocols and quality control materials. Clin Chim Acta. 2013;424:287–291. doi: 10.1016/j.cca.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Luckenbill K N, Christenson R H, Jaffe A S. et al. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem. 2008;54(3):619–621. doi: 10.1373/clinchem.2007.097998. [DOI] [PubMed] [Google Scholar]
- 24.Sakata Y, Yamamoto K, Masuyama T. et al. Ventricular production of natriuretic peptides and ventricular structural remodeling in hypertensive heart failure. J Hypertens. 2001;19(10):1905–1912. doi: 10.1097/00004872-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Saito Y, Kuwahara K. et al. Angiotensin II-induced ventricular hypertrophy and extracellular signal-regulated kinase activation are suppressed in mice overexpressing brain natriuretic peptide in circulation. Hypertens Res. 2003;26(10):847–853. doi: 10.1291/hypres.26.847. [DOI] [PubMed] [Google Scholar]
- 26.Walther T, Klostermann K, Heringer-Walther S, Schultheiss H P, Tschöpe C, Stepan H. Fibrosis rather than blood pressure determines cardiac BNP expression in mice. Regul Pept. 2003;116(1–3):95–100. doi: 10.1016/j.regpep.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Tóth M Vuorinen K H Vuolteenaho O et al. Hypoxia stimulates release of ANP and BNP from perfused rat ventricular myocardium Am J Physiol 1994266(4 Pt 2):H1572–H1580. [DOI] [PubMed] [Google Scholar]
- 28.Baxter G F. Natriuretic peptides and myocardial ischaemia. Basic Res Cardiol. 2004;99(2):90–93. doi: 10.1007/s00395-004-0458-7. [DOI] [PubMed] [Google Scholar]
- 29.Jernberg T, James S, Lindahl B. et al. Natriuretic peptides in unstable coronary artery disease. Eur Heart J. 2004;25(17):1486–1493. doi: 10.1016/j.ehj.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Goetze J P, Gore A, Møller C H, Steinbrüchel D A, Rehfeld J F, Nielsen L B. Acute myocardial hypoxia increases BNP gene expression. FASEB J. 2004;18(15):1928–1930. doi: 10.1096/fj.03-1336fje. [DOI] [PubMed] [Google Scholar]
- 31.Casals G, Ros J, Sionis A, Davidson M M, Morales-Ruiz M, Jiménez W. Hypoxia induces B-type natriuretic peptide release in cell lines derived from human cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;297(2):H550–H555. doi: 10.1152/ajpheart.00250.2009. [DOI] [PubMed] [Google Scholar]
- 32.Chun Y S Hyun J Y Kwak Y G et al. Hypoxic activation of the atrial natriuretic peptide gene promoter through direct and indirect actions of hypoxia-inducible factor-1 Biochem J 2003370(Pt 1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammerer-Lercher A, Puschendorf B, Sommer R. et al. Natriuretic peptides correlate between newborn twins but not between twins and their mothers. Clin Chim Acta. 2007;377(1–2):279–280. doi: 10.1016/j.cca.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Halse K G, Lindegaard M L, Goetze J P, Damm P, Mathiesen E R, Nielsen L B. Increased plasma pro-B-type natriuretic peptide in infants of women with type 1 diabetes. Clin Chem. 2005;51(12):2296–2302. doi: 10.1373/clinchem.2005.056077. [DOI] [PubMed] [Google Scholar]
- 35.Nybo M, Nielsen L B, Nielsen S J. et al. Discordant expression of pro-B-type and pro-C-type natriuretic peptide in newborn infants of mothers with type 1 diabetes. Regul Pept. 2007;141(1–3):135–139. doi: 10.1016/j.regpep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 36.Kanbe T, Maeno Y, Fujino H. et al. Brain-type natriuretic peptide at birth reflects foetal maturation and antenatal stress. Acta Paediatr. 2009;98(9):1421–1425. doi: 10.1111/j.1651-2227.2009.01357.x. [DOI] [PubMed] [Google Scholar]
- 37.Nir A, Lindinger A, Rauh M. et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol. 2009;30(1):3–8. doi: 10.1007/s00246-008-9258-4. [DOI] [PubMed] [Google Scholar]
- 38.Mir T S, Flato M, Falkenberg J. et al. Plasma concentrations of N-terminal brain natriuretic peptide in healthy children, adolescents, and young adults: effect of age and gender. Pediatr Cardiol. 2006;27(1):73–77. doi: 10.1007/s00246-005-1022-4. [DOI] [PubMed] [Google Scholar]
- 39.Mansoub S, Chan M K, Adeli K. Gap analysis of pediatric reference intervals for risk biomarkers of cardiovascular disease and the metabolic syndrome. Clin Biochem. 2006;39(6):569–587. doi: 10.1016/j.clinbiochem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89(8):875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mir T S, Laux R, Hellwege H H. et al. Plasma concentrations of aminoterminal pro atrial natriuretic peptide and aminoterminal pro brain natriuretic peptide in healthy neonates: marked and rapid increase after birth. Pediatrics. 2003;112(4):896–899. doi: 10.1542/peds.112.4.896. [DOI] [PubMed] [Google Scholar]
- 42.Nir A, Bar-Oz B, Perles Z, Brooks R, Korach A, Rein A J. N-terminal pro-B-type natriuretic peptide: reference plasma levels from birth to adolescence. Elevated levels at birth and in infants and children with heart diseases. Acta Paediatr. 2004;93(5):603–607. doi: 10.1111/j.1651-2227.2004.tb02984.x. [DOI] [PubMed] [Google Scholar]
- 43.Kunii Y, Kamada M, Ohtsuki S. et al. Plasma brain natriuretic peptide and the evaluation of volume overload in infants and children with congenital heart disease. Acta Med Okayama. 2003;57(4):191–197. doi: 10.18926/AMO/32809. [DOI] [PubMed] [Google Scholar]
- 44.Rauh M, Koch A. Plasma N-terminal pro-B-type natriuretic peptide concentrations in a control population of infants and children. Clin Chem. 2003;49(9):1563–1564. doi: 10.1373/49.9.1563. [DOI] [PubMed] [Google Scholar]
- 45.Schwachtgen L, Herrmann M, Georg T, Schwarz P, Marx N, Lindinger A. Reference values of NT-proBNP serum concentrations in the umbilical cord blood and in healthy neonates and children. Z Kardiol. 2005;94(6):399–404. doi: 10.1007/s00392-005-0246-x. [DOI] [PubMed] [Google Scholar]
- 46.Albers S, Mir T S, Haddad M, Läer S. N-Terminal pro-brain natriuretic peptide: normal ranges in the pediatric population including method comparison and interlaboratory variability. Clin Chem Lab Med. 2006;44(1):80–85. doi: 10.1515/CCLM.2006.016. [DOI] [PubMed] [Google Scholar]
- 47.Cantinotti M, Storti S, Parri M S, Murzi M, Clerico A. Reference values for plasma B-type natriuretic peptide in the first days of life. Clin Chem. 2009;55(7):1438–1440. doi: 10.1373/clinchem.2009.126847. [DOI] [PubMed] [Google Scholar]
- 48.Soldin S J, Soldin O P, Boyajian A J, Taskier M S. Pediatric brain natriuretic peptide and N-terminal pro-brain natriuretic peptide reference intervals. Clin Chim Acta. 2006;366(1–2):304–308. doi: 10.1016/j.cca.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantinotti M, Passino C, Storti S, Ripoli A, Zyw L, Clerico A. Clinical relevance of time course of BNP levels in neonates with congenital heart diseases. Clin Chim Acta. 2011;412(23–24):2300–2304. doi: 10.1016/j.cca.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 50.Chang A Y, Abdullah S M, Jain T. et al. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol. 2007;49(1):109–116. doi: 10.1016/j.jacc.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 51.Saenger A K, Dalenberg D A, Bryant S C, Grebe S K, Jaffe A S. Pediatric brain natriuretic peptide concentrations vary with age and sex and appear to be modulated by testosterone. Clin Chem. 2009;55(10):1869–1875. doi: 10.1373/clinchem.2009.123778. [DOI] [PubMed] [Google Scholar]
- 52.Cantinotti M, Storti S, Ripoli A. et al. Diagnostic accuracy of B-type natriuretic hormone for congenital heart disease in the first month of life. Clin Chem Lab Med. 2010;48(9):1333–1338. doi: 10.1515/CCLM.2010.251. [DOI] [PubMed] [Google Scholar]
- 53.Cantinotti M, Storti S, Parri M S, Prontera C, Murzi B, Clerico A. Reference intervals for brain natriuretic peptide in healthy newborns and infants measured with an automated immunoassay platform. Clin Chem Lab Med. 2010;48(5):697–700. doi: 10.1515/CCLM.2010.129. [DOI] [PubMed] [Google Scholar]
- 54.Holmgren D, Westerlind A, Lundberg P A, Wåhlander H. Increased plasma levels of natriuretic peptide type B and A in children with congenital heart defects with left compared with right ventricular volume overload or pressure overload. Clin Physiol Funct Imaging. 2005;25(5):263–269. doi: 10.1111/j.1475-097X.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 55.Koch A M, Zink S, Singer H, Dittrich S. B-type natriuretic peptide levels in patients with functionally univentricular hearts after total cavopulmonary connection. Eur J Heart Fail. 2008;10(1):60–62. doi: 10.1016/j.ejheart.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Holmgren D, Westerlind A, Berggren H, Lundberg P A, Wåhlander H. Increased natriuretic peptide type B level after the second palliative step in children with univentricular hearts with right ventricular morphology but not left ventricular morphology. Pediatr Cardiol. 2008;29(4):786–792. doi: 10.1007/s00246-008-9201-8. [DOI] [PubMed] [Google Scholar]
- 57.Koch A, Zink S, Singer H. B-type natriuretic peptide in paediatric patients with congenital heart disease. Eur Heart J. 2006;27(7):861–866. doi: 10.1093/eurheartj/ehi773. [DOI] [PubMed] [Google Scholar]
- 58.Cowley C G, Bradley J D, Shaddy R E. B-type natriuretic peptide levels in congenital heart disease. Pediatr Cardiol. 2004;25(4):336–340. doi: 10.1007/s00246-003-0461-z. [DOI] [PubMed] [Google Scholar]
- 59.Czernik C, Lemmer J, Metze B, Koehne P S, Mueller C, Obladen M. B-type natriuretic peptide to predict ductus intervention in infants <28 weeks. Pediatr Res. 2008;64(3):286–290. doi: 10.1203/PDR.0b013e3181799594. [DOI] [PubMed] [Google Scholar]
- 60.Farombi-Oghuvbu I, Matthews T, Mayne P D, Guerin H, Corcoran J D. N-terminal pro-B-type natriuretic peptide: a measure of significant patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2008;93(4):F257–F260. doi: 10.1136/adc.2007.120691. [DOI] [PubMed] [Google Scholar]
- 61.Eerola A, Jokinen E, Boldt T, Pihkala J. The influence of percutaneous closure of patent ductus arteriosus on left ventricular size and function: a prospective study using two- and three-dimensional echocardiography and measurements of serum natriuretic peptides. J Am Coll Cardiol. 2006;47(5):1060–1066. doi: 10.1016/j.jacc.2005.09.067. [DOI] [PubMed] [Google Scholar]
- 62.Flynn P A, da Graca R L, Auld P A, Nesin M, Kleinman C S. The use of a bedside assay for plasma B-type natriuretic peptide as a biomarker in the management of patent ductus arteriosus in premature neonates. J Pediatr. 2005;147(1):38–42. doi: 10.1016/j.jpeds.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 63.Tosse V, Pillekamp F, Verde P. et al. Urinary NT-proBNP, NGAL, and H-FABP may predict hemodynamic relevance of patent ductus arteriosus in very low birth weight infants. Neonatology. 2012;101(4):260–266. doi: 10.1159/000334826. [DOI] [PubMed] [Google Scholar]
- 64.Kalra V K, DeBari V A, Zauk A, Kataria P, Myridakis D, Kiblawi F. Point-of-care testing for B-type natriuretic peptide in premature neonates with patent ductus arteriosus. Ann Clin Lab Sci. 2011;41(2):131–137. [PubMed] [Google Scholar]
- 65.Zhao Q M, Ma X J, Ge X L. et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014;384(9945):747–754. doi: 10.1016/S0140-6736(14)60198-7. [DOI] [PubMed] [Google Scholar]
- 66.Thangaratinam S, Brown K, Zamora J, Khan K S, Ewer A K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379(9835):2459–2464. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 67.Cantinotti M, Vittorini S, Storti S. et al. Diagnostic accuracy and clinical relevance of brain natriuretic peptide assay in pediatric patients with congenital heart diseases. J Cardiovasc Med (Hagerstown) 2009;10(9):706–713. doi: 10.2459/JCM.0b013e32832c15fb. [DOI] [PubMed] [Google Scholar]
- 68.Cantinotti M, Assanta N, Murzi B, Lopez L. Controversies in the definition and management of insignificant left-to-right shunts. Heart. 2014;100(3):200–205. doi: 10.1136/heartjnl-2013-304372. [DOI] [PubMed] [Google Scholar]
- 69.Cantinotti M. Current pediatric nomograms are only one source of error for quantification in pediatric echocardiography: what to expect from future research. J Am Soc Echocardiogr. 2013;26(8):919. doi: 10.1016/j.echo.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 70.Cantinotti M, Scalese M, Murzi B. et al. Echocardiographic nomograms for chamber diameters and areas in Caucasian children. J Am Soc Echocardiogr. 2014;27(12):1279–9200. doi: 10.1016/j.echo.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Berry J G, Askovich B, Shaddy R E, Hawkins J A, Cowley C G. Prognostic value of B-type natriuretic peptide in surgical palliation of children with single-ventricle congenital heart disease. Pediatr Cardiol. 2008;29(1):70–75. doi: 10.1007/s00246-007-9012-3. [DOI] [PubMed] [Google Scholar]
- 72.Hsu J H, Keller R L, Chikovani O. et al. B-type natriuretic peptide levels predict outcome after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2007;134(4):939–945. doi: 10.1016/j.jtcvs.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 73.Walsh R, Boyer C, LaCorte J. et al. N-terminal B-type natriuretic peptide levels in pediatric patients with congestive heart failure undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2008;135(1):98–105. doi: 10.1016/j.jtcvs.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Shih C Y, Sapru A, Oishi P. et al. Alterations in plasma B-type natriuretic peptide levels after repair of congenital heart defects: a potential perioperative marker. J Thorac Cardiovasc Surg. 2006;131(3):632–638. doi: 10.1016/j.jtcvs.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 75.Cannesson M Bionda C Gostoli B et al. Time course and prognostic value of plasma B-type natriuretic peptide concentration in neonates undergoing the arterial switch operation Anesth Analg 200710451059–1065. tables of contents [DOI] [PubMed] [Google Scholar]
- 76.Mir T S, Haun C, Lilje C, Läer S, Weil J. Utility of N-terminal brain natriuretic peptide plasma concentrations in comparison to lactate and troponin in children with congenital heart disease following open-heart surgery. Pediatr Cardiol. 2006;27(2):209–216. doi: 10.1007/s00246-005-1152-8. [DOI] [PubMed] [Google Scholar]
- 77.Koch A, Kitzsteiner T, Zink S, Cesnjevar R, Singer H. Impact of cardiac surgery on plasma levels of B-type natriuretic peptide in children with congenital heart disease. Int J Cardiol. 2007;114(3):339–344. doi: 10.1016/j.ijcard.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Gessler P, Knirsch W, Schmitt B, Rousson V, von Eckardstein A. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in children with congenital heart defects and open-heart surgery. J Pediatr. 2006;148(3):372–376. doi: 10.1016/j.jpeds.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 79.Niedner M F, Foley J L, Riffenburgh R H, Bichell D P, Peterson B M, Rodarte A. B-type natriuretic peptide: perioperative patterns in congenital heart disease. Congenit Heart Dis. 2010;5(3):243–255. doi: 10.1111/j.1747-0803.2010.00396.x. [DOI] [PubMed] [Google Scholar]
- 80.Amirnovin R, Keller R L, Herrera C. et al. B-type natriuretic peptide levels predict outcomes in infants undergoing cardiac surgery in a lesion-dependent fashion. J Thorac Cardiovasc Surg. 2013;145(5):1279–1287. doi: 10.1016/j.jtcvs.2012.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cantinotti M, Clerico A, Iervasi G. Age- and disease-related variations in B-type natriuretic peptide response after pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2013;145(5):1415–1416. doi: 10.1016/j.jtcvs.2012.12.086. [DOI] [PubMed] [Google Scholar]
- 82.Cantinotti M, Lorenzoni V, Storti S. et al. Thyroid and brain natriuretic Peptide response in children undergoing cardiac surgery for congenital heart disease- age-related variations and prognostic value. Circ J. 2013;77(1):188–197. doi: 10.1253/circj.cj-12-0834. [DOI] [PubMed] [Google Scholar]
- 83.Lindblade C L, Chun D S, Darragh R K, Caldwell R L, Murphy D J, Schamberger M S. Value of plasma B-type natriuretic peptide as a marker for rejection in pediatric heart transplant recipients. Am J Cardiol. 2005;95(7):909–911. doi: 10.1016/j.amjcard.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 84.Hammerer-Lercher A, Mair J, Antretter H. et al. B-type natriuretic peptide as a marker of allograft rejection after heart transplantation. J Heart Lung Transplant. 2005;24(9):1444. doi: 10.1016/j.healun.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 85.Lan Y T, Chang R K, Alejos J C, Burch C, Wetzel G T. B-type natriuretic peptide in children after cardiac transplantation. J Heart Lung Transplant. 2004;23(5):558–563. doi: 10.1016/S1053-2498(03)00306-1. [DOI] [PubMed] [Google Scholar]
- 86.Ationu A, Burch M, Singer D, Littleton P, Carter N. Cardiac transplantation affects ventricular expression of brain natriuretic peptide. Cardiovasc Res. 1993;27(2):188–191. doi: 10.1093/cvr/27.2.188. [DOI] [PubMed] [Google Scholar]
- 87.Claudius I, Lan Y T, Chang R K, Wetzel G T, Alejos J. Usefulness of B-type natriuretic peptide as a noninvasive screening tool for cardiac allograft pathology in pediatric heart transplant recipients. Am J Cardiol. 2003;92(11):1368–1370. doi: 10.1016/j.amjcard.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 88.Cantinotti M, Storti S, Lorenzoni V. et al. The combined use of neutrophil gelatinase-associated lipocalin and brain natriuretic peptide improves risk stratification in pediatric cardiac surgery. Clin Chem Lab Med. 2012;50(11):2009–2017. doi: 10.1515/cclm-2012-0125. [DOI] [PubMed] [Google Scholar]
- 89.Koch A M, Zink S, Singer H, Dittrich S. B-type natriuretic peptide levels in patients with functionally univentricular hearts after total cavopulmonary connection. Eur J Heart Fail. 2008;10(1):60–62. doi: 10.1016/j.ejheart.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 90.Cantinotti M, Giordano R, Scalese M. et al. Prognostic role of BNP in children undergoing surgery for congenital heart disease: analysis of prediction models incorporating standard risk factors. Clin Chem Lab Med. 2015;53(11):1839–1846. doi: 10.1515/cclm-2014-1084. [DOI] [PubMed] [Google Scholar]
- 91.Joseph L, Nir A, Hammerman C, Goldberg S, Ben Shalom E, Picard E. N-terminal pro-B-type natriuretic peptide as a marker of bronchopulmonary dysplasia in premature infants. Am J Perinatol. 2010;27(5):381–386. doi: 10.1055/s-0029-1243312. [DOI] [PubMed] [Google Scholar]
- 92.Price J F, Thomas A K, Grenier M. et al. B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation. 2006;114(10):1063–1069. doi: 10.1161/CIRCULATIONAHA.105.608869. [DOI] [PubMed] [Google Scholar]
- 93.Kaski J P, Tomé-Esteban M T, Mead-Regan S. et al. B-type natriuretic peptide predicts disease severity in children with hypertrophic cardiomyopathy. Heart. 2008;94(10):1307–1311. doi: 10.1136/hrt.2007.126748. [DOI] [PubMed] [Google Scholar]
- 94.Sanjeev S, Pettersen M, Lua J, Thomas R, Shankaran S, L'Ecuyer T. Role of plasma B-type natriuretic peptide in screening for hemodynamically significant patent ductus arteriosus in preterm neonates. J Perinatol. 2005;25(11):709–713. doi: 10.1038/sj.jp.7211383. [DOI] [PubMed] [Google Scholar]
- 95.Mir T S, Marohn S, Läer S, Eiselt M, Grollmus O, Weil J. Plasma concentrations of N-terminal pro-brain natriuretic peptide in control children from the neonatal to adolescent period and in children with congestive heart failure. Pediatrics. 2002;110(6):e76. doi: 10.1542/peds.110.6.e76. [DOI] [PubMed] [Google Scholar]
- 96.Knirsch W, Häusermann E, Fasnacht M, Hersberger M, Gessler P, Bauersfeld U. Plasma B-type natriuretic peptide levels in children with heart disease. Acta Paediatr. 2011;100(9):1213–1216. doi: 10.1111/j.1651-2227.2011.02258.x. [DOI] [PubMed] [Google Scholar]
- 97.Hongkan W, Soongswang J, Veerakul G. et al. N-terminal pro brain natriuretic peptide and cardiac function in doxorubicin administered pediatric patients. J Med Assoc Thai. 2009;92(11):1450–1457. [PubMed] [Google Scholar]
- 98.Soker M, Kervancioglu M. Plasma concentrations of NT-pro-BNP and cardiac troponin-I in relation to doxorubicin-induced cardiomyopathy and cardiac function in childhood malignancy. Saudi Med J. 2005;26(8):1197–1202. [PubMed] [Google Scholar]
- 99.Hayakawa H, Komada Y, Hirayama M, Hori H, Ito M, Sakurai M. Plasma levels of natriuretic peptides in relation to doxorubicin-induced cardiotoxicity and cardiac function in children with cancer. Med Pediatr Oncol. 2001;37(1):4–9. doi: 10.1002/mpo.1155. [DOI] [PubMed] [Google Scholar]
- 100.Cheema A N, Phil M, Khan D A, Tuyyab F. Early detection of cardiac dysfunction by BNP in beta-thalassaemia major patients. Acta Cardiol. 2012;67(3):331–335. doi: 10.1080/ac.67.3.2160723. [DOI] [PubMed] [Google Scholar]
- 101.Aggarwal S, Pettersen M D, Bhambhani K, Gurczynski J, Thomas R, L'Ecuyer T. B-type natriuretic peptide as a marker for cardiac dysfunction in anthracycline-treated children. Pediatr Blood Cancer. 2007;49(6):812–816. doi: 10.1002/pbc.21100. [DOI] [PubMed] [Google Scholar]
- 102.Takeuchi D, Saji T, Takatsuki S, Fujiwara M. Abnormal tissue doppler images are associated with elevated plasma brain natriuretic peptide and increased oxidative stress in acute Kawasaki disease. Circ J. 2007;71(3):357–362. doi: 10.1253/circj.71.357. [DOI] [PubMed] [Google Scholar]
- 103.Davlouros P A, Karatza A A, Xanthopoulou I. et al. Diagnostic role of plasma BNP levels in neonates with signs of congenital heart disease. Int J Cardiol. 2011;147(1):42–46. doi: 10.1016/j.ijcard.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 104.Ko H K, Lee J H, Choi B M. et al. Utility of the rapid B-type natriuretic peptide assay for detection of cardiovascular problems in newborn infants with respiratory difficulties. Neonatology. 2008;94(1):16–21. doi: 10.1159/000112584. [DOI] [PubMed] [Google Scholar]
- 105.Koulouri S, Acherman R J, Wong P C, Chan L S, Lewis A B. Utility of B-type natriuretic peptide in differentiating congestive heart failure from lung disease in pediatric patients with respiratory distress. Pediatr Cardiol. 2004;25(4):341–346. doi: 10.1007/s00246-003-0578-0. [DOI] [PubMed] [Google Scholar]
- 106.Maher K O, Reed H, Cuadrado A. et al. B-type natriuretic peptide in the emergency diagnosis of critical heart disease in children. Pediatrics. 2008;121(6):e1484–e1488. doi: 10.1542/peds.2007-1856. [DOI] [PubMed] [Google Scholar]
- 107.Cohen S, Springer C, Avital A. et al. Amino-terminal pro-brain-type natriuretic peptide: heart or lung disease in pediatric respiratory distress? Pediatrics. 2005;115(5):1347–1350. doi: 10.1542/peds.2004-1429. [DOI] [PubMed] [Google Scholar]
- 108.Law Y M, Hoyer A W, Reller M D, Silberbach M. Accuracy of plasma B-type natriuretic peptide to diagnose significant cardiovascular disease in children: the Better Not Pout Children! Study. J Am Coll Cardiol. 2009;54(15):1467–1475. doi: 10.1016/j.jacc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 109.Kalra V K, Aggarwal S, Arora P, Natarajan G. B-type natriuretic peptide levels in preterm neonates with bronchopulmonary dysplasia: a marker of severity? Pediatr Pulmonol. 2014;49(11):1106–1111. doi: 10.1002/ppul.22942. [DOI] [PubMed] [Google Scholar]
- 110.Steurer M A, Moon-Grady A J, Fineman J R. et al. B-type natriuretic peptide: prognostic marker in congenital diaphragmatic hernia. Pediatr Res. 2014;76(6):549–554. doi: 10.1038/pr.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hur M, Kim H, Lee S. et al. Diagnostic and prognostic utilities of multimarkers approach using procalcitonin, B-type natriuretic peptide, and neutrophil gelatinase-associated lipocalin in critically ill patients with suspected sepsis. BMC Infect Dis. 2014;14:224. doi: 10.1186/1471-2334-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cantinotti M, Clerico A, Murzi M, Vittorini S, Emdin M. Clinical relevance of measurement of brain natriuretic peptide and N-terminal pro-brain natriuretic peptide in pediatric cardiology. Clin Chim Acta. 2008;390(1–2):12–22. doi: 10.1016/j.cca.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 113.Pletcher M J, Pignone M. Evaluating the clinical utility of a biomarker: a review of methods for estimating health impact. Circulation. 2011;123(10):1116–1124. doi: 10.1161/CIRCULATIONAHA.110.943860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang T J. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123(5):551–565. doi: 10.1161/CIRCULATIONAHA.109.912568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hlatky M A, Greenland P, Arnett D K. et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119(17):2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]


