Key Points
Question
Does exercise affect the risk of developing cardiovascular diseases in patients with early-stage breast cancer?
Findings
This randomized clinical trial of 100 women with stage I to III breast cancer who were sedentary and with overweight condition or obesity found that the 10-year risk of developing cardiovascular disease, as assessed by the Framingham Risk Score, was significantly lower in patients with early-stage breast cancer who participated in a 16-week exercise intervention.
Meaning
Supervised clinical exercise appeared to be an effective strategy for reducing the 10-year risk of cardiovascular disease in patients with early-stage breast cancer.
Abstract
Importance
The Framingham Risk Score (FRS) is a valid method for predicting the 10-year risk of developing cardiovascular disease. Higher FRS is reported in patients with early-stage breast cancer who are overweight than in healthy, age-matched women, but whether exercise reduces FRS in this patient population is unclear.
Objective
To examine the effects of a 16-week aerobic and resistance exercise intervention on the FRS in women with early-stage breast cancer and with overweight condition or obesity.
Design, Setting, and Participants
This single-center, prospective randomized clinical trial included 100 women with stage I to III breast cancer who were sedentary, with overweight condition or obesity (body mass index of ≥25.0 or body fat of ≥30%), and completed cancer treatment within 6 months prior to enrollment. Participants were randomized to either the usual care or exercise group. Differences in mean changes for outcomes were evaluated using mixed-model repeated-measures analyses. Data were collected from August 1, 2012, through July 1, 2017. Data analysis, which followed the intention-to-treat approach, was performed from May 24 to October 2, 2018.
Interventions
The exercise group underwent supervised aerobic and resistance exercise sessions thrice weekly for 16 weeks.
Main Outcomes and Measures
The FRS was calculated for each participant using preset points for each of the 6 FRS categories: age, systolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, diabetes presence, and smoking status.
Results
In total, 100 women were randomized to either the exercise group (n = 50) or usual care group (n = 50). Of these women, 55 (55%) were of Hispanic white race/ethnicity and the mean (SD) age was 53.5 (10.4) years. The mean (SD) total FRS scores postintervention were 2.0 (1.5) in the exercise group and 13.0 (3.0) in the usual care group. The postintervention FRS was significantly reduced in the exercise group compared with the usual care group (mean, −9.5; 95% CI, −13.0 to −6.0), which corresponds to an 11% (95% CI, −15.0 to −5.0) decrease on the FRS-predicted 10-year risk of developing cardiovascular disease.
Conclusions and Relevance
A 16-week supervised aerobic and resistance exercise intervention appeared to reduce the FRS-predicted 10-year risk of cardiovascular disease in women with early-stage breast cancer with overweight condition or obesity.
Clinical Trial Registration
ClinicalTrials.gov identifier: NCT01140282
This randomized clinical trial examines whether an exercise intervention improves the Framingham Risk Score for cardiovascular disease among women with early-stage breast cancer with overweight condition or obesity.
Introduction
Patients with early-stage breast cancer have approximately double the risk of mortality from cardiovascular disease (CVD) than their age-matched counterparts without a cancer history.1 The Framingham Risk Score (FRS) is a valid method for assessing the 10-year risk of developing CVD.2,3 Developed in 1998 and based on the Framingham Heart Study results that estimated 10-year CVD risk in asymptomatic individuals, the FRS incorporates 6 risk factors: age, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, systolic blood pressure, presence of diabetes, and smoking status.4 Exercise has been shown to reduce CVD risk factors in patients with early-stage breast cancer who are overweight,5 but to date no studies have examined the effects of exercise on the FRS in this population. Our previous trial found statistically significant effects of an exercise intervention on individual components of the FRS.6 In the present study, we report the intervention effects on the FRS, an unplanned composite measure of CVD risk in the study, to provide a more clinically useful summary.
Methods
Study Design
This randomized clinical trial compared an aerobic and resistance exercise intervention with usual care on baseline to 4-month changes in metabolic syndrome. Detailed methods of the trial7 and the primary outcomes related to metabolic syndrome were published previously.6 End points were assessed at baseline, after the intervention, and 3-month follow-up (exercise group only). The trial protocol approved by the University of Southern California Institutional Review Board is available in Supplement 1. Written informed consent was obtained from all participants. No compensation was received.
Objectives
We report exploratory outcomes from an unplanned analysis of the FRS. We hypothesized that compared with usual care, an aerobic and resistance exercise intervention would reduce the FRS in patients with early-stage breast cancer who are sedentary and with overweight condition or obesity.
Patients
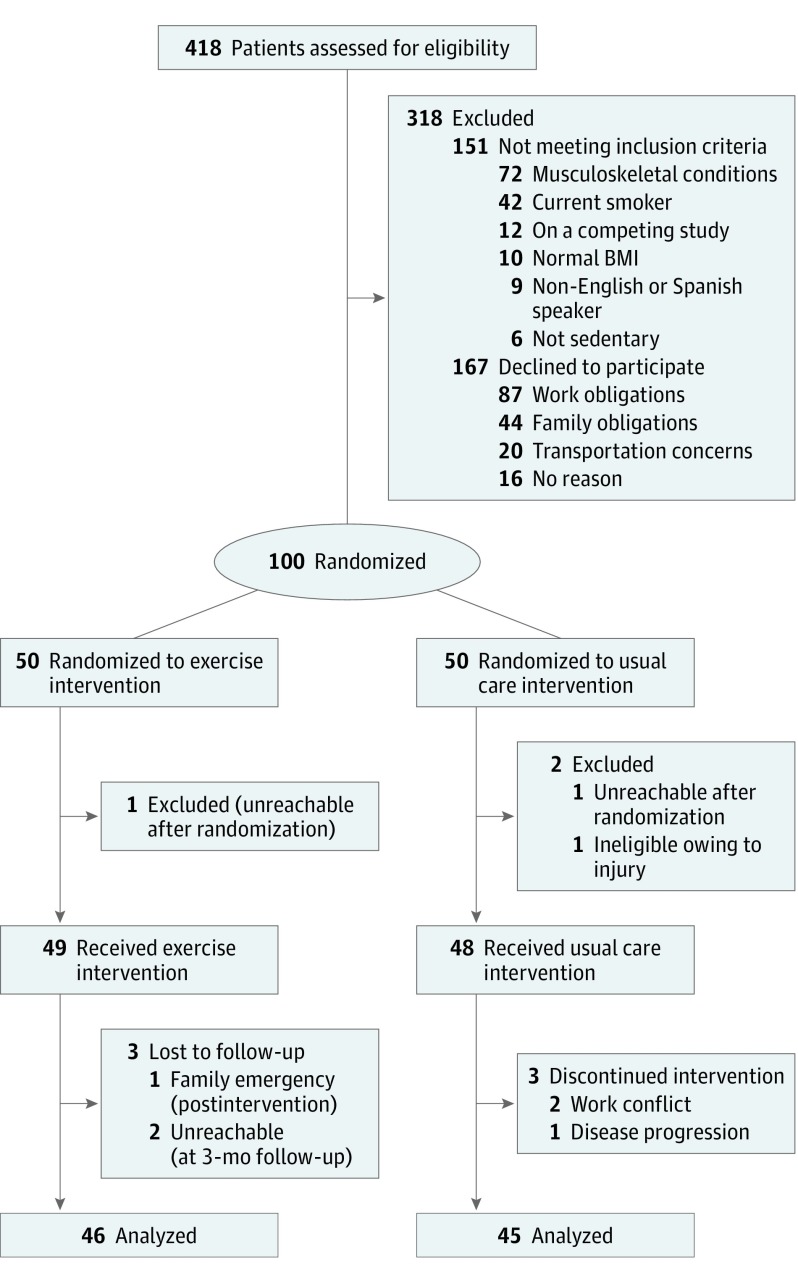
Eligible women with breast cancer were recruited between August 1, 2012, and December 31, 2016 (Figure). These women had less than 6 months of posttreatment for stage I to stage III breast cancer; were nonsmokers and sedentary (<60 minutes of structured exercise per week); and had a body mass index of 25.0 or higher (calculated as weight in kilograms divided by height in meters squared), or body fat of greater than 30%, and a waist circumference of more than 88 cm.
Figure. Patient Flow Diagram.
BMI indicates body mass index; EXE, exercise; UC, usual care.
Statistical Analyses
The sample size was based on changes in insulin level with a 16-week exercise intervention among survivors of breast cancer. Enrollment of 100 women provided 80% statistical power (α = .05) to detect a difference in mean (SD) insulin level of 2.6 (4.0) mU/mL, assuming a 20% dropout rate using a 2-group, 2-tailed t test. A 1-sided P = .05 was used to indicate statistical significance. Within-group differences in mean change for individual outcomes measured at 16 weeks were evaluated using general linear models repeated-measures analyses of variance, and between-group differences were evaluated with a mixed-model repeated-measures analyses. A priori covariates with potential associations with the outcome of interest (eg, type of treatment, type of surgical procedure, medication use [eg, antihypertensive, hyperglycemia], body mass index, caloric intake, diet quality, and macronutrient distribution) were explored in models, but none modified the results. Analyses were performed using SAS, version 9.4 (SAS Institute, Inc). Data were collected from August 1, 2012, through July 1, 2017. Data analysis, which followed the intention-to-treat approach, was performed from May 24 to October 2, 2018.
Framingham Risk Score
The FRS (1998 version)4 was calculated using female-specific validated methodology (eTable 1 in Supplement 2). For each participant, female-specific preset points for each of the 6 FRS categories were assessed: age, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, presence of diabetes, and smoking status. Age, diabetes, and smoking history were assessed at baseline to calculate the FRS. Ten-year risk of CVD was estimated by the FRS; therefore, we report 2 outcome terms: FRS and FRS-predicted 10-year risk of CVD. Resting blood pressure was measured with an automated sphygmomanometer (Welch Allyn). Serum biomarkers were analyzed from fasting blood samples, which were stored at –80 °C until a batch analysis at study completion.
Exercise Intervention
The intervention was based on the American College of Sports Medicine/American Cancer Society exercise guidelines for cancer survivors (≥150 minutes of aerobic exercise and 2-3 days of resistance exercise training per week). Women participated in thrice weekly supervised one-on-one exercise sessions for 16 weeks. Days 1 and 3 included resistance and aerobic exercise of approximately 80 minutes. Day 2 included approximately 50 minutes of aerobic exercise (65%-80% of maximum heart rate). Aerobic exercise included treadmill walking, machine rowing, or cycling. Resistance-exercise sessions included leg presses, leg flexions or extensions, chest presses, seated rows, bicep curls, and tricep pulldowns performed at 60% to 80% 1-repetition maximum.
Results
Patient Characteristics
In total, 418 women were assessed for eligibility, and 100 were randomized to either the exercise group (n = 50) or usual care group (n = 50). Two participants in the exercise group were lost to follow-up, and 5 participants in the usual care group did not complete the study. Baseline characteristics were similar between groups (eTable 2 in Supplement 2). Among these women, 55 (55%) were of Hispanic white race/ethnicity, the mean (SD) age was 53.5 (10.4) years, the mean (SD) time from diagnosis was 6.2 (2.1) months, the mean (SD) body mass index was 33.5 (5.5). The exercise group adherence rate was 95%.
FRS Variables
The Table displays 4 of the 6 FRS variables by between-group and within-group differences after the intervention. The mean (SD) total FRS scores postintervention were 2.0 (1.5) in the exercise group and 13.0 (3.0) in the usual care group. Total FRS was statistically significantly reduced (mean, −9.5; 95% CI, −13.0 to −6.0) in the exercise group compared with usual care. The reduction in the FRS experienced by the exercise group corresponds to a statistically significant reduction in the FRS-predicted 10-year CVD risk (mean, −11.0; 95% CI, −15.0 to −5.0). The FRS increased by 1 point in the usual care group over the 16 weeks (mean [SD], 12.0 [2.0] to 13.0 [3.0]; P = .49). The statistically significant reduction in FRS was maintained during the 3-month follow-up in the exercise group compared with baseline (mean, −10%; 95% CI, −15.5 to −4.2).
Table. Comparison of FRS Variables Between Exercise and Usual Care Groupsa.
| Variable | Baseline, Mean (SD) | Postintervention | Postintervention Between-Group Difference | ||
|---|---|---|---|---|---|
| Mean (SD) | P Valueb | Mean (95% CI) | P Valuec | ||
| SBP, mm Hg | |||||
| Exercise | 132.9 (13.0) | 120.7 (9.5) | .001 | −13.7 (−16.5 to −8.7) | .001 |
| Usual care | 133.7 (9.7) | 135.9 (9.8) | .22 | ||
| FRS preset point for SBPd | |||||
| Exercise | 0.0 (2.0) | −3.0 (2.0) | <.001 | −3.0 (−5.0 to −1.0) | .002 |
| Usual care | 0.0 (2.0) | 0.0 (2.0) | >.99 | ||
| HDL-C, mg/dL | |||||
| Exercise | 43.1 (6.6) | 64.7 (7.8) | .001 | 24.4 (27.9 to 17.9) | .001 |
| Usual care | 41.0 (4.3) | 39.9 (4.0) | .45 | ||
| FRS preset point for HDL-Cd | |||||
| Exercise | 2.0 (1.0) | −2.0 (1.5) | <.001 | 4.0 (0.5 to 6.0) | <.001 |
| Usual care | 2.0 (1.0) | 2.0 (2.0) | .97 | ||
| LDL-C, mg/dL | |||||
| Exercise | 167.9 (19.7) | 119.3 (12.1) | <.001 | −48.6 (−61.2 to −27.6) | .001 |
| Usual care | 172.4 (20.3) | 178.3 (21.7) | .59 | ||
| FRS preset point for LDL-Cd | |||||
| Exercise | 2.0 (1.0) | 0 (1.0) | .002 | −2.0 (−4.5 to −0.5) | .001 |
| Usual care | 2.0 (1.0) | 2 (1.0) | .98 | ||
| Diagnosis of diabetes, No. (%) | |||||
| Exercise | 20 (40) | 10 (20) | <.001 | −10.0 (−18.2 to −6.4) | <.001 |
| Usual care | 22 (44) | 24 (53) | .45 | ||
| FRS preset point for diabetesd | |||||
| Exercise | 2.0 (1.5) | 1.0 (0.5) | .001 | −1.0 (−2.5 to −0.5) | .003 |
| Usual care | 2.0 (1.0) | 3.0 (1.0) | .21 | ||
| Total FRS | |||||
| Exercise | 12.0 (2.0) | 2.0 (1.5) | <.001 | −9.5 (−13.0 to −6.0) | <.001 |
| Usual care | 12.0 (2.0) | 13.0 (3.0) | .67 | ||
| FRS-predicted 10-y risk, % | |||||
| Exercise | 13.0 (3.0) | 2.0 (0.5) | <.001 | −11.0 (−15.0 to −5.0) | <.001 |
| Usual care | 13.0 (3.0) | 13.0 (3.0) | .97 | ||
Abbreviations: FRS, Framingham Risk Score; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure.
Two of the 6 groups (age and smoking status) did not apply in this comparison.
P value for repeated-measures analysis of variance comparing changes in the exercise group and in the usual care group from baseline to postintervention.
P value for mixed-model analysis comparing changes between the exercise and usual care group from baseline to postintervention.
Assigned preset point for the respective variable based on calculating the FRS to assess FRS-predicted 10-year cardiovascular disease risk.
Discussion
The supervised 16-week aerobic and resistance exercise intervention designed to improve metabolic syndrome as the primary end point was associated with clinically significant reductions in the FRS, an exploratory composite measure of CVD risk, among patients with early-stage breast cancer who were sedentary and with overweight condition or obesity. To our knowledge, this study is the first randomized clinical trial of exercise training in this patient population to demonstrate the benefits of an exercise intervention on risk as assessed by the FRS.
This trial is concordant with 2 previous studies that tested a 12-week high-intensity, aerobic treadmill intervention against usual care. In a trial of 40 healthy women (from 40 to 55 years of age), the FRS decreased by 1.5%,8 and in 63 survivors of testicular cancer, the FRS decreased by 0.6%.9 These reductions in the FRS correspond to a decrease of 1% in the FRS-predicted 10-year risk of CVD that is less than the 11% reduction we observed in the present study. The finding of large, exercise-induced effects on the FRS may be the result of the physiologic advantage of the combined resistance and aerobic exercise or the higher-risk sample with obesity. Aerobic exercise increases lipoprotein lipase activity, the enzyme responsible for hydrolyzing triglycerides,10 and favorably increases blood lipid uptake, whereas resistance exercise increases insulin receptor substrate-1 expression and improves insulin signaling in skeletal muscle.11,12 It is plausible that the combined exercise intervention resulted in a synergy between these intersecting pathways.
Limitations
The FRS calculated for the study sample may not be generalizable to a national cohort with breast cancer diagnosis in the United States because we excluded individuals who smoked, as required by the primary study design. In addition, the FRS may overestimate or underestimate the FRS-predicted 10-year risk of CVD in Hispanic populations.13,14 Furthermore, other well-known risk factors, such as inflammatory markers, blood glucose level, coronary calcium score, or hemoglobin A1c, were not incorporated into the FRS calculation, and currently no updated scoring system of the FRS is available to take these factors into account. A more comprehensive index would improve the precision of assessing future CVD risk in patients with early-stage breast cancer.
Conclusions
A 16-week resistance and aerobic exercise intervention appeared to decrease the FRS, which was used as a surrogate measure of the 10-year risk of CVD, by decreasing low-density lipoprotein cholesterol, systolic blood pressure, and the presence of diabetes and increasing high-density lipoprotein cholesterol. Participation in a combined approach of resistance and aerobic exercise during cancer survival may improve the FRS-predicted 10-year risk of CVD in patients with early-stage breast cancer.
Trial Protocol
eTable 1. Female Specific Pre-set Cut Points of Six Variables in FRS and FRS-predicted 10-year Risk (%)
eTable 2. Patient Characteristics
References
- 1.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology. 2016;27(1):6-13. doi: 10.1097/EDE.0000000000000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh LG, Welborn TA, Dhaliwal SS. Independent external validation of cardiovascular disease mortality in women utilising Framingham and SCORE risk models: a mortality follow-up study. BMC Womens Health. 2014;14:118. doi: 10.1186/1472-6874-14-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barroso LC, Muro EC, Herrera ND, Ochoa GF, Hueros JI, Buitrago F. Performance of the Framingham and SCORE cardiovascular risk prediction functions in a non-diabetic population of a Spanish health care centre: a validation study. Scand J Prim Health Care. 2010;28(4):242-248. doi: 10.3109/02813432.2010.518407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837-1847. doi: 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 5.Thomas GA, Alvarez-Reeves M, Lu L, Yu H, Irwin ML. Effect of exercise on metabolic syndrome variables in breast cancer survivors. Int J Endocrinol. 2013;2013:168797. doi: 10.1155/2013/168797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, et al. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J Clin Oncol. 2018;36(9):875-883. doi: 10.1200/JCO.2017.75.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieli-Conwright CM, Mortimer JE, Schroeder ET, et al. Randomized controlled trial to evaluate the effects of combined progressive exercise on metabolic syndrome in breast cancer survivors: rationale, design, and methods. BMC Cancer. 2014;14:238. doi: 10.1186/1471-2407-14-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin-Shokravi F, Rajabi R, Ziaee N. Exercise Effects on Risk of Cardiovascular Disease among Iranian Women. Asian J Sports Med. 2011;2(1):37-43. doi: 10.5812/asjsm.34826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams SC, DeLorey DS, Davenport MH, et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: A phase 2 randomized controlled trial. Cancer. 2017;123(20):4057-4065. doi: 10.1002/cncr.30859 [DOI] [PubMed] [Google Scholar]
- 10.Miyashita M, Eto M, Sasai H, Tsujimoto T, Nomata Y, Tanaka K. Twelve-week jogging training increases pre-heparin serum lipoprotein lipase concentrations in overweight/obese middle-aged men. J Atheroscler Thromb. 2010;17(1):21-29. doi: 10.5551/jat.2337 [DOI] [PubMed] [Google Scholar]
- 11.O’Neill HM. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab J. 2013;37(1):1-21. doi: 10.4093/dmj.2013.37.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16(1):132. doi: 10.1186/s12944-017-0515-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes-Bergoderi M, Thomas RJ, Albuquerque FN, et al. Validity of cardiovascular risk prediction models in Latin America and among Hispanics in the United States of America: a systematic review. Rev Panam Salud Publica. 2012;32(2):131-139. doi: 10.1590/S1020-49892012000800007 [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180-187. doi: 10.1001/jama.286.2.180 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Female Specific Pre-set Cut Points of Six Variables in FRS and FRS-predicted 10-year Risk (%)
eTable 2. Patient Characteristics