Abstract
Objectives
The relationship between prior glycemic status and outcomes in intracerebral hemorrhage (ICH) is not established. We hypothesized that higher hemoglobin (Hb) A1c is associated with worse outcomes in ICH.
Patients and methods
Using the GWTG-Stroke registry, data on patients with ICH between April 1, 2003 and September 30, 2015 were harvested. Patients were divided into four ordinal groups based on HbA1c values of <5.7%, 5.7–6.4%, 6.5–8.0% and >8.0%. Outcomes (mortality, modified Rankin Scale (mRS), home discharge and independent ambulatory status) were analyzed for patients overall and separately for patients with or without history of diabetes using multivariable regression models.
Results
Among 75,455 patients with ICH (with available HbA1c data), patients with lower HbA1c (<5.7%) had higher rates of in-hospital mortality in the entire cohort (15.5%; 3947/25473); as well as those with history of diabetes (19.0%; 542/2852). Among those without history of diabetes, both lower HbA1c (15.1%; 3405/22621) and higher HbA1c (>8.0%), (15.0%; 205/1364) were associated with higher in-hospital mortality. Lower HbA1c was also associated with higher mRS, less chance of going home, and lower likelihood of having independent ambulatory status in patients with prior history of diabetes.
Conclusions
Among patients with no reported history of diabetes, both very low and very high HbA1c were directly associated with higher in-hospital mortality. Only very low HbA1c was associated with higher mortality in known diabetic patients. Further studies are needed to better define the relationship between HbA1c and outcomes, for it may have important implications for care of ICH patients.
Keyword: Neurology
1. Introduction
Intracerebral hemorrhage (ICH) is a disease with high morbidity and mortality [1]. Diabetes mellitus (DM) is an established risk factor for ischemic stroke. However, very few studies have shown that DM and admission hyperglycemia is associated with poor outcome in ICH patients [2, 3]. In many prior studies, diabetes and glycemic status was ascertained only by past medical history [4], or by admission glucose levels which were probably influenced by the acute stress response that accompanies ICH. Hemoglobin (Hb) A1c remains the best screening tool to diagnose prediabetes and diabetes while taking into account the glucose fluctuations in such acute events [5]. However, very few studies have examined the relationship between HbA1c and outcomes in ICH patients [6, 7].
Utilizing HbA1c as the marker of glycemic control and data from the Get with The Guidelines (GWTG) Stroke registry, we hypothesized that poor hyperglycemic control is associated with worst clinical outcomes in ICH patients.
2. Materials and methods
2.1. Data source
GWTG-Stroke is an ongoing, voluntary, continuous registry, and performance improvement initiative in stroke care. It has been collected since 2003 by the Quintiles Company (Cambridge, MA) and analyzed by Duke Clinical Research Institute (DCRI) (Durham, NC). The details of the GWTG-stroke registry data collection and definitions methods have been described before [8, 9, 10]. In brief, the data is de-identified at patient collection level by the participating sites, while following local regulations and privacy laws, with local IRB approval obtained as required. The Duke IRB approved the data analysis by DCRI.
2.2. Primary and secondary outcomes
The primary outcome of interest was in-hospital mortality and secondary outcomes of interest were discharge disposition, ambulatory status and modified Rankin Scale (mRS). We compared outcomes of interest in ICH patients across the different HbA1c groups, stratified by history of diabetes mellitus.
2.3. Study population
The study population was limited to patients diagnosed with ICH in fully participating sites in the GWTG-stroke registry from April 1, 2003 to September 30, 2015. Patients with missing HbA1c, lack of hemorrhage on first CT, missing medical history or discharge disposition were excluded from the analysis.
2.4. Statistical analysis
For the study analysis, the patients with recorded HbA1c values were divided into four ordinal categories: HbA1c less than 5.7%, HbA1c 5.7–6.4%, HbA1c 6.5–8.0% and HbA1c > 8.0%.
The study population was further split into diabetics (prior history of diabetes mellitus and/or documented use of anti-diabetic medications on admission) and non-diabetics (no prior history of diabetes mellitus/no prior use of anti-diabetic medications). This non-diabetic group had potential to include patients who were undiagnosed diabetes mellitus. However, this group was also unique because none of these patients were exposed to any diabetic medications and hence their HbA1c values were true estimates of their overall glycemic status. Hence, patients with HbA1c ranging between 5.7-6.4% were considered true prediabetes patients.
To compare patient characteristics, and outcomes of interest, categorical variables were presented as counts and proportions, and differences between groups were tested using Kruskal-Wallis test. Continuous variables were presented as medians with interquartile ranges (IQR), and difference between groups was tested based on the Spearman rank correlation coefficient. Statistically significant increasing or decreasing trend in the distribution of the row variables across HbA1c groups in the ICH population were indicated by p-values less than 0.05.
Unadjusted and adjusted multivariable logistic regression models with generalized estimating equations (GEE) were used to assess the associations between HbA1c groups and the outcomes of interests, after accounting for within-hospital clustering. Diabetes history and its interaction with HbA1c groups were included in both unadjusted and adjusted models. Adjusted models also accounted for the potential confounding covariates, including demographics (age, gender, race), medical history (Atrial fib/flutter, previous stroke/TIA, CAD/prior MI, heart failure, carotid stenosis, diabetes, PVD, hypertension, dyslipidemia, smoking), arrival via EMS, on-hour arrival, and hospital characteristics (region, teaching status, number of beds, annual ICH volume, rural location, primary stroke center, comprehensive stroke center) (Supplemental Table I). For any patient variables with missing rate lower than 20%, multiple imputations with 25 imputed datasets were used (Supplemental Table II). Linearity of continuous covariates was assessed and transformations or splines were applied when appropriate. Odds ratios (OR) for each binary outcome were reported, with normal range HbA1c (value less than 5.7%) as the reference group. If the interaction p-value is less than 0.05, it suggests that the association between HbA1c groups and the outcome differs significantly by diabetes history. Other p-values less than 0.05 indicate the OR is significantly different from 1. Since many patients had missing NIHSS on admission, a subset analysis was performed on patients who had admission NIHSS score available. All tests were 2-tailed with a level of statistical significance of p < 0.05. Statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc, Cary, NC).
3. Results
3.1. Characteristics of study population
Among 372,822 ICH patients from 2,007 sites, 287,653 patients were excluded due to missing HbA1c data, 6,908 patients were excluded due to lack of hemorrhage on first scan, 2,755 patients were excluded due to being transferred out, leaving against medical advice (AMA), lacking discharge disposition or otherwise unable to determine and 51 patients were excluded due to missing medical history. Our final study population was 75,455 patients from 1,336 sites.
When compared to patients with missing HbA1c values, the study population had lower initial NIHSS score, in-hospital mortality and mRS at discharge but significantly higher median blood glucose (Table 1).
Table 1.
Baseline characteristics by Hba1c missing status.
| Variable | HbA1c missing |
HbA1c not missing |
P-value |
|---|---|---|---|
| N = 213,220 | N = 75,455 | ||
| Patient demographics | |||
| Age | 72 (58–82) | 67 (56–78) | <.001 |
| Gender, Female | 50.09 | 44.88 | <.001 |
| Race/Ethnicity | |||
| White | 68.29 | 57.68 | <.001 |
| Black | 15.21 | 22.73 | |
| Measurements | |||
| Weight | 90 (69–141) | 91 (72–139) | <.001 |
| BMI | 25.9 (22.6–30.1) | 27.5 (23.8–32.3) | <.001 |
| HbA1C (0–20), % | NA | 5.9 (5.5–6.7) | - |
| SBP (50–250), mm Hg | 160 (139–186) | 163 (143–189) | <.001 |
| DBP (20–200), mm Hg | 85 (72–100) | 88 (74–104) | <.001 |
| Blood glucose (20–800), mg/dL | 129 (106–165) | 134 (109–177) | <.001 |
| Medical history | |||
| Atrial fibrillation/flutter | 16.26 | 12.88 | <.001 |
| CAD/Prior MI | 19.54 | 18.45 | <.001 |
| Carotid stenosis | 1.83 | 1.74 | 0.112 |
| Diabetes mellitus | 20.72 | 36.24 | <.001 |
| Peripheral vascular disease (PVD) | 3.27 | 3.01 | <.001 |
| Hypertension | 71.77 | 77.82 | <.001 |
| Smoker | 13.21 | 15.49 | <.001 |
| Dyslipidemia | 29.86 | 32.71 | <.001 |
| Heart failure | 5.34 | 5.68 | <.001 |
| Prior stroke/TIA | 24.61 | 23.87 | <.001 |
| Chronic renal insufficiency | 2.03 | 2.99 | <.001 |
| Medications prior to admission | |||
| Antiplatelets | 36.18 | 35.52 | 0.005 |
| Anticoagulants | 20.86 | 15.87 | <.001 |
| Cholesterol-reducers | 32.01 | 33.85 | <.001 |
| Diabetic medications | 15.26 | 26.60 | <.001 |
| Arrival information | |||
| Arrival mode: EMS | 59.87 | 49.59 | <.001 |
| On-hour arrival (non-holiday, weekday, 7a-6p) | 40.52 | 38.83 | <.001 |
| Ambulatory status at admission | |||
| Able to ambulate independently | 17.63 | 20.40 | <.001 |
| With assistance from person | 13.78 | 17.76 | |
| Unable to ambulate | 68.58 | 61.85 | |
| NIHSS recorded | 40.52 | 57.77 | <.001 |
| Initial NIHSS score (0–42) | 10 (3–21) | 8 (3–17) | <.001 |
| Hospital characteristics | |||
| Primary stroke center | 56.65 | 58.27 | <.001 |
| Comprehensive stroke center | 17.91 | 23.33 | <.001 |
| Academic/Teaching hospital | 69.79 | 75.49 | <.001 |
| Rural Location | 2.61 | 1.52 | <.001 |
| Region | |||
| West | 20.45 | 19.24 | <.001 |
| South | 35.25 | 37.73 | |
| Midwest | 19.58 | 20.73 | |
| Northeast | 24.72 | 22.29 | |
| Number of Beds | 425 (301–612) | 474 (342–706) | <.001 |
| Annual Volume of ICH Admissions | 53 (29–89) | 63 (38–106) | <.001 |
Table 2 demonstrated the baseline characteristics of the included patients. Prior history of diabetes was present in 36.2% of the overall cohort with increasing trend across the HBA1c groups. Given the large size of the cohort, we detected many statistically significant differences in the data, but these differences might not be clinically meaningful (Table 2).
Table 2.
Comparison of baseline patient characteristics across Hba1c Groups.
| Variable | HbA1c <5.7% (N = 25473) | HbA1c 5.7–6.5% (N = 26815) | HbA1c 6.5–8.0% (N = 14317) | (HbA1c >8.0% (N = 8850) | P-value |
|---|---|---|---|---|---|
| Age | 64 (53–77) | 70 (58–80) | 69 (59–78) | 62 (54–72) | <.0001 |
| Female | 11434 (44.9) | 12681 (47.3) | 6117 (42.7) | 3629 (41.0) | <.0001 |
| Ethnicity | |||||
| White | 15422 (60.6) | 15758 (58.8) | 8079 (56.4) | 4243 (48.0) | <.0001 |
| Black | 5786 (22.7) | 5946 (22.2) | 3126 (21.8) | 2289 (25.9) | |
| Weight | 85 (68–130) | 90 (71–136) | 97 (77–148) | 101 (79–158) | <.0001 |
| BMI | 26.0 (22.7–30.2) | 27.4 (23.8–32.0) | 29.2 (25.3–34.3) | 30.0 (25.8–35.3) | <.0001 |
| HbA1C (0–20), % | 5.3 (5.1–5.5) | 6.0 (5.8–6.2) | 7.0 (6.7–7.4) | 9.5 (8.6–10.9) | <.0001 |
| SBP (50–250), mm Hg | 161 (141–188) | 164 (144–189) | 164 (144–190) | 168 (146–195) | <.0001 |
| DBP (20–200), mm Hg | 88 (75–105) | 88 (74–103) | 86 (73–102) | 90 (76–106) | 0.0026 |
| Blood glucose (20–800), mg/dL | 118 (102–143) | 127 (108–156) | 164 (132–210) | 254 (187–328) | <.0001 |
| Diabetes mellitus | 2852 (11.2) | 6761 (25.2) | 10248 (71.6) | 7486 (84.6) | <.0001 |
| Hypertension | 18033 (70.8) | 20871 (77.8) | 12226 (85.4) | 7591 (85.8) | <.0001 |
| Chronic renal insufficiency | 768 (3.0) | 587 (2.2) | 534 (3.7) | 366 (4.1) | <.0001 |
| Antiplatelet | 6106 (28.8) | 7662 (36.0) | 4986 (45.3) | 2711 (39.1) | <.0001 |
| Anticoagulants | 2302 (11.9) | 3341 (17.3) | 2045 (21.2) | 933 (15.3) | <.0001 |
| Cholesterol-reducers | 5981 (23.5) | 9248 (34.5) | 6693 (46.8) | 3603 (40.7) | <.0001 |
| Anti-Diabetic | 1514 (6.0) | 4508 (17.0) | 8082 (57.0) | 5759 (65.7) | <.0001 |
| PreICH ambulatory status | |||||
| Able to ambulate independently | 3354 (20.5) | 3361 (20.1) | 1745 (20.0) | 1196 (21.8) | <.0001 |
| With assistance from person | 2708 (16.5) | 3008 (17.9) | 1624 (18.6) | 1066 (19.4) | |
| Unable to ambulate | 10320 (63.0) | 10393 (62.0) | 5343 (61.3) | 3223 (58.8) | |
| Initial NIHSS score (0–42) | 8 (3–17) | 8 (3–17) | 7 (2–16) | 7 (2–17) | <.0001 |
HbA1c: Hemoglobin A1c, BMI: Basic Metabolic Index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, NIHSS: National Institute of Health Stroke Scale.
3.2. Hemoglobin A1c and overall clinical outcome
Overall, patients with HbA1c >8% had 13.5% mortality as compared to patients with HbA1c 6.5–8.0% (13.6%), HbA1c 5.7–6.5% (14%), and HbA1c<5.7% (15%). A similar trend was observed in discharge disposition - higher hemoglobin A1c >8.0% was associated with higher rates of discharge to home and/or independent ambulatory status when compared to other HbA1c groups (Table 3).
Table 3.
Comparison of outcomes in ICH patients across Hba1c Groups.
| Variable | Prior diabetes | (HbA1c <5.7%) (N = 25473) | (HbA1c 5.7–6.5%) (N = 26815) | (HbA1c 6.5–8.0%) (N = 14317) | (HbA1c >8.0%) (N = 8850) |
|---|---|---|---|---|---|
| In-hospital mortality | All | 3947 (15.5) | 3762 (14.0) | 1950 (13.6) | 1199 (13.5) |
| Yes | 542 (19) | 1016 (15.03) | 1399 (13.65) | 994 (13.28) | |
| No | 3405 (15.05) | 2746 (13.69) | 551 (13.54) | 205 (15.03) | |
| Discharged home | All | 7353 (34.2) | 7256 (31.5) | 3960 (32.0) | 2845 (37.2) |
| Yes | 644 (27.88) | 1664 (28.96) | 2751 (31.09) | 2417 (37.23) | |
| No | 6709 (34.91) | 5592 (32.31) | 1209 (34.37) | 428 (36.93) | |
| Discharge independent ambulatory status | All | 6958 (29.1) | 6995 (27.9) | 3756 (28.0) | 2599 (31.3) |
| Yes | 577 (21.78) | 1574 (24.95) | 2627 (27.27) | 2198 (31.41) | |
| No | 6381 (30.06) | 5421 (28.89) | 1129 (29.69) | 401 (30.99) | |
| Discharge mRS | All | 4 (3–6) | 4 (3–5) | 4 (3–5) | 4 (3–6) |
| Yes | 5 (4–6) | 4 (3–6) | 4 (3–5) | 4 (3–5) | |
| No | 4 (3–5) | 4 (3–5) | 4 (3–6) | 4 (3–6) |
3.3. Clinical outcomes in diabetics (prior history of DM or use of anti-diabetic medications on admission)
When we analyzed patients with prior medical history of diabetes, patients with HbA1c >8% had 13.5% mortality as compared to patients with HbA1c 6.5–8.0% (13.3%), HbA1c 5.7–6.5% (15%), and HbA1c<5.7% (19%). Higher HbA1c >8% was also associated with higher rates of discharge to home and/or independent ambulatory status and lower scores on modified Rankin Scale (mRS) at discharge when compared to other HbA1c groups (Table 3).
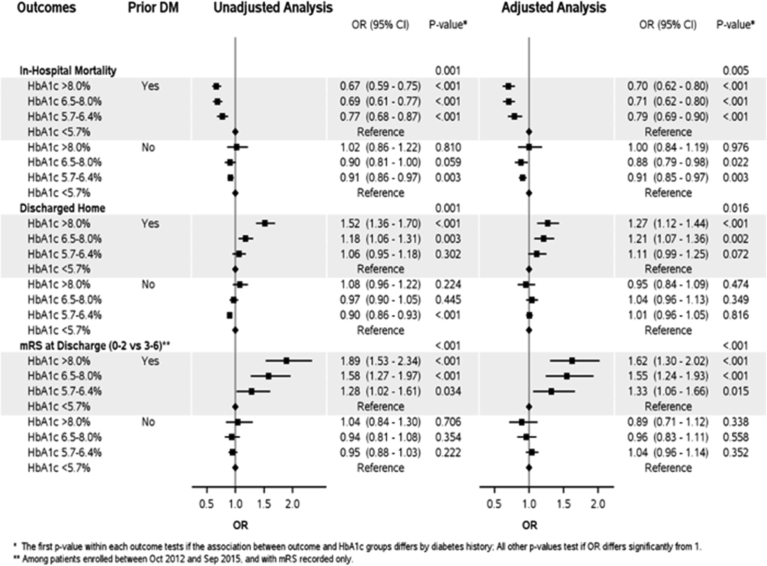
When patients with abnormal HbA1c and prior history of DM were compared to patients with reference HbA1c <5.7% in a multivariate model, there was an overall lower in-hospital mortality, increased chances of going home and lower mRS, across all other ranges of HbA1c (Table 4 and Fig. 1).
Table 4.
Adjusted associations between Hba1c Groups and outcomes without adjusting for NIHSS.
| Outcomes |
Prior diabetes | HbA1c groups | Adjusted |
|
|---|---|---|---|---|
| Dichotomized | OR (95% CI) | P-value | ||
| In-hospital mortality | Interaction p-value (3df) | 0.0048 | ||
| Yes | HbA1c >8.0% | 0.70 (0.62–0.80) | <.0001 | |
| HbA1c 6.5–8.0% | 0.71 (0.62–0.80) | <.0001 | ||
| HbA1c 5.7–6.5% | 0.79 (0.69–0.90) | 0.0004 | ||
| HbA1c <5.7% | Reference | - | ||
| No | HbA1c >8.0% | 1.00 (0.84–1.19) | 0.9755 | |
| HbA1c 6.5–8.0% | 0.88 (0.79–0.98) | 0.0222 | ||
| HbA1c 5.7–6.5% | 0.91 (0.85–0.97) | 0.0027 | ||
| HbA1c <5.7% | Reference | - | ||
| Discharged home | Interaction p-value (3df) | 0.0161 | ||
| Yes | HbA1c >8.0% | 1.27 (1.12–1.44) | 0.0002 | |
| HbA1c 6.5–8.0% | 1.21 (1.07–1.36) | 0.0016 | ||
| HbA1c 5.7–6.5% | 1.11 (0.99–1.25) | 0.0718 | ||
| HbA1c <5.7% | Reference | - | ||
| No | HbA1c >8.0% | 0.95 (0.84–1.09) | 0.4744 | |
| HbA1c 6.5–8.0% | 1.04 (0.96–1.13) | 0.3492 | ||
| HbA1c 5.7–6.5% | 1.01 (0.96–1.05) | 0.8164 | ||
| HbA1c <5.7% | Reference | - | ||
| Modified Rankin scale at discharge (0–2 vs 3–6) | Interaction p-value (3df) | 0.0003 | ||
| Yes | HbA1c >8.0% | 1.62 (1.30–2.02) | <.0001 | |
| HbA1c 6.5–8.0% | 1.55 (1.24–1.93) | 0.0001 | ||
| HbA1c 5.7–6.5% | 1.33 (1.06–1.66) | 0.0149 | ||
| HbA1c <5.7% | Reference | - | ||
| No | HbA1c >8.0% | 0.89 (0.71–1.12) | 0.3378 | |
| HbA1c 6.5–8.0% | 0.96 (0.83–1.11) | 0.5576 | ||
| HbA1c 5.7–6.5% | 1.04 (0.96–1.14) | 0.3522 | ||
| HbA1c <5.7% | Reference | - | ||
Fig. 1.
Forest plot depicting unadjusted and adjusted outcomes across the dichotomized groups when compared to reference (Hba1c < 5.7%).
When we performed a subset analysis in patients with available NIHSS and adjusted for admission severity, there continued to be lower mortality associated with higher hemoglobin A1c values in diabetic population (Table 5).
Table 5.
Adjusted associations between Hba1c Groups and outcomes adjusted for NIHSS among NIHSS non-missing population only.
| Outcomes |
Prior diabetes | HbA1c groups | Adjusted |
|
|---|---|---|---|---|
| Dichotomized | OR (95% CI) | P-value | ||
| In-hospital mortality | Interaction p-value (3df) | 0.1151 | ||
| Yes | HbA1c >8.0% | 0.78 (0.65–0.93) | 0.0070 | |
| HbA1c 6.5–8.0% | 0.73 (0.62–0.87) | 0.0004 | ||
| HbA1c 5.7–6.5% | 0.72 (0.60–0.87) | 0.0006 | ||
| HbA1c <5.7% | Reference | - | ||
| No | HbA1c >8.0% | 0.97 (0.75–1.26) | 0.8463 | |
| HbA1c 6.5–8.0% | 0.88 (0.74–1.05) | 0.1548 | ||
| HbA1c 5.7–6.5% | 0.92 (0.84–1.01) | 0.0690 | ||
| HbA1c <5.7% | Reference | - | ||
| Discharged home | Interaction p-value (3df) | 0.6352 | ||
| Yes | HbA1c >8.0% | 1.04 (0.86–1.25) | 0.6928 | |
| HbA1c 6.5–8.0% | 1.05 (0.88–1.25) | 0.5969 | ||
| HbA1c 5.7–6.5% | 1.00 (0.83–1.19) | 0.9629 | ||
| HbA1c <5.7% | Reference | - | ||
| No | HbA1c >8.0% | 0.94 (0.78–1.15) | 0.5662 | |
| HbA1c 6.5–8.0% | 1.12 (1.00–1.25) | 0.0521 | ||
| HbA1c 5.7–6.5% | 0.99 (0.93–1.07) | 0.8759 | ||
| HbA1c <5.7% | Reference | - | ||
| Modified Rankin scale at discharge (0–2 vs 3–6) | Interaction p-value (3df) | 0.6540 | ||
| Yes | HbA1c >8.0% | 1.28 (0.94–1.74) | 0.1150 | |
| HbA1c 6.5–8.0% | 1.33 (0.98–1.81) | 0.0707 | ||
| HbA1c 5.7–6.5% | 1.27 (0.91–1.78) | 0.1545 | ||
| HbA1c <5.7% | Reference | - | ||
| No | HbA1c >8.0% | 1.05 (0.70–1.57) | 0.8258 | |
| HbA1c 6.5–8.0% | 1.04 (0.85–1.27) | 0.7177 | ||
| HbA1c 5.7–6.5% | 1.10 (0.98–1.24) | 0.1099 | ||
| HbA1c <5.7% | Reference | - | ||
3.4. Clinical outcomes in non-diabetics (no prior history of DM nor prior use of anti-diabetic medications on admission)
In patients with no prior history of DM, patients with HbA1c >8% had 15.0% mortality as compared to patients with HbA1c 6.5–8.0% (13.5%), HbA1c 5.7–6.5% (13.7%), and HbA1c<5.7% (15%). Rates of discharge to home, good ambulatory status and discharge mRS were comparable across all groups (Table 3).
In multivariate model, patients with HbA1c 5.7–6.5% and HbA1c 6.5–8.0% had significantly lower mortality than patients with HbA1c <5.7%. However, mortality rates were similar in patients with HbA1c > 8.0 and HbA1c <5.7% (Table 4 and Fig. 1).
However, when the subset of patients with documented NIHSS were adjusted for admission injury severity; rates of mortality, discharge to home, independent ambulatory status and discharge mRS were similar across all groups (Table 5).
4. Discussion
Our study showed that in patients with known history of DM or exposure to any diabetic treatment prior to admission, higher HbA1c groups (5.7–6.5%, 6.5–8.0% and >8.0%) had lower mortality and better discharge outcomes when compared to lower HbA1c (<5.7%). The most remarkable characteristic of the study population was the direct correlation of the increasing HbA1c values with higher BMI. This could be a potential confounder as patients with higher HbA1c had better outcomes and may be suggestive of the obesity paradox seen in other literature especially in Percutaneous Coronary Intervention (PCI), where obesity has been found to be protective of bleeding [11, 12, 13].
The relationship of prior HbA1c and ICH prognosis has not been studied in large cohorts. Kazui et al. retrospectively reviewed the relationship of HbA1c and hematoma growth in 186 patients and found it to be directly associated with an interaction of fasting blood glucose (FBG) ≥141 mg/dL and systolic blood pressure (SBP) ≥200 mm Hg, and interaction of HbA1c ≥ 5.1% and SBP ≥200 mm Hg [14]. Zhang et al. retrospectively reviewed 332 patients with ICH from a prospectively collected dataset and found a direct correlation between higher HbA1c and greater hematoma expansion and poor outcome specifically in diabetic patients [7]. There are several studies showing poor outcome in ICH patients with poor admission glycemic state, though these studies were modest in size [2, 3, 15, 16, 17]. This relationship holds true in animal models with possible mechanisms including hematoma expansion and worsening of peri-hematoma edema [18, 19, 20]. However, this association becomes more complicated when groups are stratified according to prior clinical history of diabetes, which in most cases is underdiagnosed and may bias the results. Our study is unique as we tried to stratify groups based on their pre-stroke glycemic status (HbA1c), which is the true predictor of whether patient has diabetes, or not. The results of our study are contradictory to popular belief that higher HbA1c should always be associated with poor clinical outcome. One way to explain the discrepancy between our data and prior studies measuring admission hyperglycemia and ICH outcomes is that admission hyperglycemia may indicate a stress related response that is mostly unrelated to pre-ICH glycemic status. There has been suggestion in numerous studies that admission hyperglycemia and worsening outcome is more related to the initial injury severity and not the pre-morbid glycemic status of the patient [21]. Acute stress hyperglycemia may in fact be beneficial for critically ill patients [22, 23]. Prior use of anti-diabetic medications may reduce this beneficial effect and contribute to mortality and morbidity.
Our study also suggested a trend towards poor outcome in patients with HbA1c>8% without a prior diagnosis of diabetes. This finding suggests that absence of glycemic control does have some role to play in mortality and morbidity in ICH patients with undiagnosed diabetes. There is also a trend of higher mortality in patients with HbA1c <5.7% in non-diabetic population who were not exposed to anti-diabetic medications. Patients with low HbA1c especially <5.0% have been shown to have higher all-cause mortality in previously published literature and considered a generalized marker of mortality risk in general population [24]. Additionally, a recent population-based study showed that low blood glucose was a risk factor for incident ICH [25]. There is also a possible lack of stress response to insult in the group with low HbA1c [26]. It is now clear why low HbA1c could be related to poor ICH outcome. Potential explanations include that low HbA1c has been associated with liver disease, low fibrinogen, and anemia, which could predispose to increased bleeding risk and larger hematoma volume.
4.1. Limitations
There are several limitations in our study. A large proportion of patients in our cohort had missing HbA1c values. The initial NIHSS Score in those patients was significantly higher than patients with documented HbA1c values. These patients also had higher in-hospital mortality and mRS at discharge. Thus, patients with missing HBA1c likely had larger, more severe ICH and there was less chance or perceived need to measure HBA1c in the hospital. These patients with missing HBA1c were more likely to have lower, not higher, random blood glucose levels which might suggest that they also had lower HBA1c levels, consistent with the findings from our analysis of those with HbA1c measured. The data are drawn from hospitals participating in GWTG-Stroke and may not apply to patients and hospitals that have different characteristics. The accuracy of the data is dependent on accurate reporting by participating sites, though good reliability of data collection has been shown in a prior paper by Xian et al [27]. Even though severity of presentation could be detected from NIHSS, neither GCS nor ICH score were available for analysis. HbA1c might not accurately reflect glycemic status in the minority of patients with chronic renal insufficiency. We also did not have information regarding the degree of anemia in patients, which may be associated with lower HbA1c values and has been an independent predictor of worse ICH outcomes in other studies [28, 29], or the different etiologies of ICH or the cause of mortality.
5. Conclusions
Lower HbA1c, either intentional in diabetic patients or unintentional in non-diabetic patients, in the weeks leading up to intracerebral hemorrhage is associated with poor outcomes. Further prospective studies are needed to study this effect in detail to establish any sort of cause-effect relationship.
Declarations
Author contribution statement
Sudeepta Dandapat, Fazeel M Siddiqui, Gregg C. Fonarow, Deepak L Bhatt, Haolin Xu, Roland Matsouaka, Paul A. Heidenreich, Ying Xian, Lee H Schwamm, Eric E. Smith: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by an American Heart Association (AHA) seed grant and data was acquired from the Get With The Guidelines (GWTG) registry.
Competing interest statement
The authors declare the following conflict of interests:
Sudeepta Dandapat: No conflicts.
Fazeel M. Siddiqui: No conflicts.
Gregg C. Fonarow: Research, Patient Centered Outcome Research Institute.
Deepak L. Bhatt: Advisory Board - Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Cleveland Clinic, Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda.
Haolin Xu: No conflicts.
Roland Matsouaka: No conflicts.
Paul A. Heidenreich: No conflicts.
Ying Xian: No conflicts.
Lee H Schwamm: GWTG-Stroke Clinical workgroup (Chair).
Eric Smith: No conflicts.
Additional information
No additional information is available for this paper.
Acknowledgements
The data was acquired from the Get With The Guidelines (GWTG) registry.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Flaherty M.L., Haverbusch M., Sekar P., Kissela B., Kleindorfer D., Moomaw C.J. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66(8):1182–1186. doi: 10.1212/01.wnl.0000208400.08722.7c. [DOI] [PubMed] [Google Scholar]
- 2.Saxena A., Anderson C.S., Wang X., Sato S., Arima H., Chan E. Prognostic significance of hyperglycemia in acute intracerebral hemorrhage: the INTERACT2 study. Stroke; J. Cereb. Circ. 2016;47(3):682–688. doi: 10.1161/STROKEAHA.115.011627. [DOI] [PubMed] [Google Scholar]
- 3.Bejot Y., Aboa-Eboule C., Hervieu M., Jacquin A., Osseby G.V., Rouaud O. The deleterious effect of admission hyperglycemia on survival and functional outcome in patients with intracerebral hemorrhage. Stroke; J. Cereb. Circ. 2012;43(1):243–245. doi: 10.1161/STROKEAHA.111.632950. [DOI] [PubMed] [Google Scholar]
- 4.Gray C.S., Scott J.F., French J.M., Alberti K.G., O'Connell J.E. Prevalence and prediction of unrecognised diabetes mellitus and impaired glucose tolerance following acute stroke. Age Ageing. 2004;33(1):71–77. doi: 10.1093/ageing/afh026. [DOI] [PubMed] [Google Scholar]
- 5.Roquer J., Rodriguez-Campello A., Cuadrado-Godia E., Giralt-Steinhauer E., Jimenez-Conde J., Soriano C. The role of HbA1c determination in detecting unknown glucose disturbances in ischemic stroke. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koga M., Yamagami H., Okuda S., Okada Y., Kimura K., Shiokawa Y. Blood glucose levels during the initial 72 h and 3-month functional outcomes in acute intracerebral hemorrhage: the SAMURAI-ICH study. J. Neurol. Sci. 2015;350(1-2):75–78. doi: 10.1016/j.jns.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Zhang G., Wu F., Xu Y., Feng J., Cai Z., Xu B. Prestroke glycemic status is associated with the functional outcome in spontaneous intracerebral hemorrhage. Neurol. Sci. 2015;36(6):927–934. doi: 10.1007/s10072-014-2057-1. [DOI] [PubMed] [Google Scholar]
- 8.Schwamm L.H., Fonarow G.C., Reeves M.J., Pan W., Frankel M.R., Smith E.E. Get with the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119(1):107–115. doi: 10.1161/CIRCULATIONAHA.108.783688. [DOI] [PubMed] [Google Scholar]
- 9.Hong Y., LaBresh K.A. Overview of the American Heart Association "Get with the Guidelines" programs: coronary heart disease, stroke, and heart failure. Crit. Pathw. Cardiol. 2006;5(4):179–186. doi: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 10.Smaha L.A., American Heart A. The American heart association Get with the Guidelines program. Am. Heart J. 2004;148(5 Suppl):S46–S48. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Payvar S., Kim S., Rao S.V., Krone R., Neely M., Paladugu N. In-hospital outcomes of percutaneous coronary interventions in extremely obese and normal-weight patients: findings from the NCDR (National Cardiovascular Data Registry) J. Am. Coll. Cardiol. 2013;62(8):692–696. doi: 10.1016/j.jacc.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Byrne J., Spence M.S., Fretz E., Mildenberger R., Chase A., Berry B. Body mass index, periprocedural bleeding, and outcome following percutaneous coronary intervention (from the British Columbia Cardiac Registry) Am. J. Cardiol. 2009;103(4):507–511. doi: 10.1016/j.amjcard.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Numasawa Y., Kohsaka S., Miyata H., Kawamura A., Noma S., Suzuki M. Impact of body mass index on in-hospital complications in patients undergoing percutaneous coronary intervention in a Japanese real-world multicenter registry. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazui S., Naritomi H., Yamamoto H., Sawada T., Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke; J. Cereb. Circ. 1996;27(10):1783–1787. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- 15.Guo X., Li H., Zhang Z., Li S., Zhang L., Zhang J. Hyperglycemia and mortality risk in patients with primary intracerebral hemorrhage: a meta-analysis. Mol. Neurobiol. 2016;53(4):2269–2275. doi: 10.1007/s12035-015-9184-4. [DOI] [PubMed] [Google Scholar]
- 16.Tapia-Perez J.H., Gehring S., Zilke R., Schneider T. Effect of increased glucose levels on short-term outcome in hypertensive spontaneous intracerebral hemorrhage. Clin. Neurol. Neurosurg. 2014;118:37–43. doi: 10.1016/j.clineuro.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi A.I., Palesch Y.Y., Martin R., Novitzke J., Cruz-Flores S., Ehtisham A. Association of serum glucose concentrations during acute hospitalization with hematoma expansion, perihematomal edema, and three month outcome among patients with intracerebral hemorrhage. Neurocritical Care. 2011;15(3):428–435. doi: 10.1007/s12028-011-9541-8. [DOI] [PubMed] [Google Scholar]
- 18.Song E.C., Chu K., Jeong S.W., Jung K.H., Kim S.H., Kim M. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke; J. Cereb. Circ. 2003;34(9):2215–2220. doi: 10.1161/01.STR.0000088060.83709.2C. [DOI] [PubMed] [Google Scholar]
- 19.Aljada A., Ghanim H., Mohanty P., Syed T., Bandyopadhyay A., Dandona P. Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am. J. Clin. Nutr. 2004;80(1):51–57. doi: 10.1093/ajcn/80.1.51. [DOI] [PubMed] [Google Scholar]
- 20.Chiu C.D., Chen C.C., Shen C.C., Chin L.T., Ma H.I., Chuang H.Y. Hyperglycemia exacerbates intracerebral hemorrhage via the downregulation of aquaporin-4: temporal assessment with magnetic resonance imaging. Stroke; J. Cereb. Circ. 2013;44(6):1682–1689. doi: 10.1161/STROKEAHA.113.675983. [DOI] [PubMed] [Google Scholar]
- 21.Tetri S., Juvela S., Saloheimo P., Pyhtinen J., Hillbom M. Hypertension and diabetes as predictors of early death after spontaneous intracerebral hemorrhage. J. Neurosurg. 2009;110(3):411–417. doi: 10.3171/2008.8.JNS08445. [DOI] [PubMed] [Google Scholar]
- 22.Wernly B., Lichtenauer M., Hoppe U.C., Jung C. Hyperglycemia in septic patients: an essential stress survival response in all, a robust marker for risk stratification in some, to be messed with in none. J. Thorac. Dis. 2016;8(7):E621–E624. doi: 10.21037/jtd.2016.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marik P.E., Bellomo R. Stress hyperglycemia: an essential survival response! Crit. Care Med. 2013;41(6):e93–e94. doi: 10.1097/CCM.0b013e318283d124. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal V., Schneider A.L.C., Selvin E. Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care. 2012;35(10):2055–2060. doi: 10.2337/dc11-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin C., Li G., Rexrode K.M., Gurol M.E., Yuan X., Hui Y. Prospective study of fasting blood glucose and intracerebral hemorrhagic risk. Stroke; J. Cereb. Circ. 2018;49(1):27–33. doi: 10.1161/STROKEAHA.117.019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marik P.E., Bellomo R. Stress hyperglycemia: an essential survival response! Crit. Care. 2013;17(2):305. doi: 10.1186/cc12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xian Y., Fonarow G.C., Reeves M.J., Webb L.E., Blevins J., Demyanenko V.S. Data quality in the American Heart Association Get with the Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. Am. Heart J. 2012;163(3):392–398. doi: 10.1016/j.ahj.2011.12.012. 8 e1. [DOI] [PubMed] [Google Scholar]
- 28.Kumar M.A., Rost N.S., Snider R.W., Chanderraj R., Greenberg S.M., Smith E.E. Anemia and hematoma volume in acute intracerebral hemorrhage. Crit. Care Med. 2009;37(4):1442–1447. doi: 10.1097/CCM.0b013e31819ced3a. [DOI] [PubMed] [Google Scholar]
- 29.Kuramatsu J.B., Gerner S.T., Lucking H., Kloska S.P., Schellinger P.D., Kohrmann M. Anemia is an independent prognostic factor in intracerebral hemorrhage: an observational cohort study. Crit. Care. 2013;17(4):R148. doi: 10.1186/cc12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.