Abstract
The stress-reducing effect of matcha, a high-quality fine-powdered green tea, has recently been clarified by animal experiments and clinical trials. However, the effect of matcha added to confectioneries is not clear. One aim of this study was to evaluate the relationship between matcha components and their stress-reducing effect in mice that were loaded with territorially-based stress. Adrenal hypertrophy, a marker of stress, was significantly suppressed in stress-loaded mice that had ingested matcha components, displaying a caffeine and epigallocatechin gallate to theanine and arginine (CE/TA) ratio of 2 or less. Another aim was to evaluate, in humans, the stress-reducing effect of matcha in cookies using test-matcha (CE/TA = 1.79) or placebo-matcha (CE/TA = 10.64). Participants, who were fifth year pharmacy college students, consumed 4.5 g of matcha in three pieces of cookie daily for 15 days. Salivary α-amylase activity, a stress marker, was significantly lower in the test-matcha group than in the placebo group. These results indicate that the CE/TA ratio of tea components is a key indicator for the suppression of stress. Moreover, matcha with a CE/TA ratio of 2 or less displays a stress-reducing effect, even if it is included in confectionery products. Such products may also benefit individuals who have no habit of drinking matcha as a beverage.
Keywords: Physiology, Food science
1. Introduction
Matcha is a high quality green tea that is a fine powder of tea leaves protected from sunlight. Since matcha has a unique taste, flavor and color, it is widely used not only as a beverage but also as a component of various confectioneries and dishes. Theanine, an amino acid unique to tea plant (Camellia sinensis (L.) Kuntze) [1], shows an excellent stress-reducing effect [2, 3, 4, 5] and exists at much higher levels in matcha than in other general green teas [6, 7]. Theanine content varies depending on the quality of green tea, but on average matcha contains about two-fold more theanine than sencha, a general green tea [8]. Modern life can cause stress in people, and the accumulation of stress is strongly linked to various diseases [9, 10, 11]. Reducing stress with matcha may become a new health-giving strategy for achieving good daily life. If it is possible to reduce stress by consuming matcha in confectionery products or meals, then individuals unaccustomed to drinking matcha as a beverage might benefit from the consumption of such products.
Previous studies suggested that differences in the quantities and ratios of matcha components affect the efficiency of its stress-reducing action. While caffeine and epigallocatechin gallate (EGCG) antagonize the stress-reducing effect of theanine [12, 13, 14], arginine (Arg), the second most abundant amino acid in Japanese green tea, enhances the effect of theanine [13]. When tea leaves are protected from direct sunlight, theanine levels remains high because it is broken down to glutamate and ethylamine when exposed to sunlight, and the former is used for the biosynthesis of EGCG [15, 16]. In addition, the amount of theanine depends on nitrogen supply absorbed from the roots [17]. Therefore, when leaves are insufficient shielded from sunlight and when nitrogen is limited, matcha with low levels of theanine and high levels of EGCG is produced. On the other hand, as caffeine is higher in the buds and young leaves of C. sinensis plants than in mature leaves, matcha has essentially a high content of caffeine [18, 19]. However, if theanine levels are sufficiently high, the anti-theanine action of caffeine is counteracted [13]. When matcha is consumed as a beverage to reduce stress, the concentration of theanine that is needed to achieve this effect is 50 mg or more, and the molar ratio of caffeine and EGCG to theanine and arginine (CE/TA) must be 2 or less [20].
Based on this background, we examined the relationship between different matcha components and their stress-reducing effect was examined in animal (mice) experiments. For human clinical trials, test matcha (effective for stress reduction) and placebo matcha were selected. Participants were assigned to a pharmacy practice outside the university, such as a hospital or a pharmacy. As their commitment to a new environment may lead to stressful conditions for young students, they consumed matcha daily in cookies and measured the activity of salivary α-amylase (sAA) as a stress marker of sympathetic excitement to assess their psychosocial and physiological stress response [4, 21].
2. Materials and methods
2.1. Selection of test- and placebo-matcha, and measurement of tea components by high performance liquid chromatography
The components in matcha were measured by high performance liquid chromatography (HPLC), as described previously [13]. In brief, according to the method of Horie, Ema, & Sumikawa [6], free amino acids (Arg, alanine, aspartic acid (Asp), asparagine (Asn), glutamic acid (Glu), glutamine (Gln), γ-amino butyric acid (GABA), serine (Ser), and theanine) were measured by HPLC (SCL-10Avp, Shimadzu, Kyoto, Japan; Develosil packed column ODS-HG-5, 150 × 4.6 mm, Nomura Chemical Co. Ltd., Seto, Japan) using glycylglycine as the internal standard [22]. These amino acids were detected at an excitation wavelength of 340 nm and at an emission wavelength of 450 nm (RF-535 UV detector, Shimadzu, Kyoto, Japan). Catechins and caffeine were measured by HPLC as described above at 280 nm. Based on these data, the molar ratio of CE/TA was calculated. In the clinical trial, two kinds of matcha were selected as test-matcha with a molar ratio of CE/TA 2 or les, and placebo-matcha with a molar ratio greater than 10.
2.2. Animals and stress experiment
Male ddY mice (Slc: ddY, 4 weeks old) were purchased from Japan SLC Co. Ltd. (Shizuoka, Japan) and kept under conventional conditions in a temperature- and humidity-controlled environment with a 12/12 h light/dark cycle (light period, 8:00 a.m.–8:00 p.m.; temperature, 23 ± 1 °C; relative humidity, 55 ± 5%). Four-week-old mice were housed in a cage for five days in groups of four to allow them to adapt to co-habitation. Mice were fed a normal diet (CE-2; Clea Co. Ltd., Tokyo, Japan) and water ad libitum. All experimental protocols were approved by the University of Shizuoka Laboratory Animal Care Advisory Committee (approval No. 166197) and were in accordance with the guidelines of the U.S. National Institute of Health for the Care and Use of Laboratory Animals. To apply psychosocial stress to mice, confrontational housing was supplied in the form of a standard polycarbonate cage that was divided into two identical subunits by a stainless-steel partition as previously described [4]. In brief, two mice were housed in a partitioned cage for six days (single housing) to establish territorial consciousness. Then, the partition was removed to expose the mice to confrontational stress for 24 h (confrontational housing). To avoid visual social contact between cages, each cage was placed in a styrofoam box. At the end of 24 h of confrontational housing, mice were sacrificed and adrenal glands were weighed.
2.3. Ingestion of tea components by mice
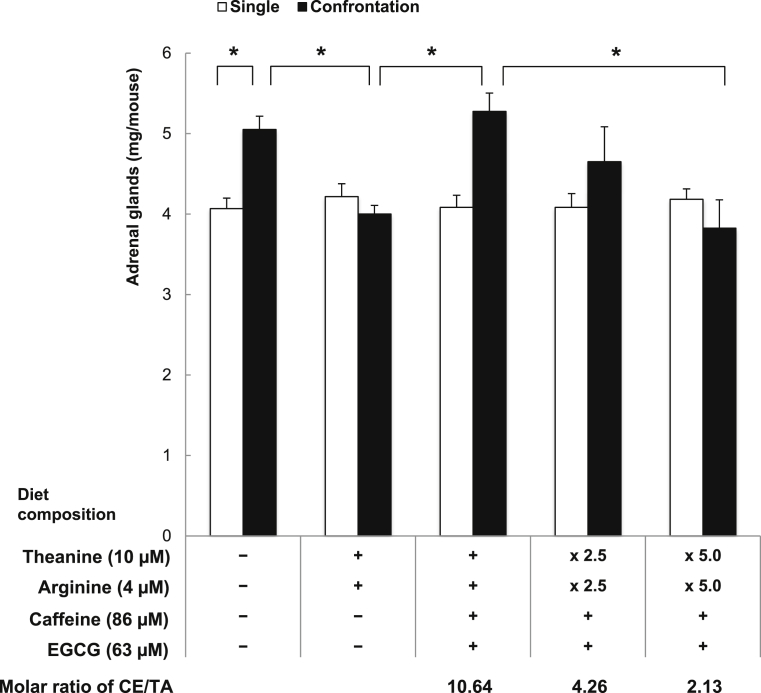
The effect of matcha was examined in mice (four mice/group, n = 40). Mice consumed tea components in a powder diet of CE-2 ad libitum for seven days (single housing for six days and confrontational housing for one day). The mice of the pre-treated group that did not experience confrontational housing were used as a standard and mice that underwent confrontational housing without ingesting any tea component were used as controls. Ingested food weight was measured using a special bait box to measure the exact ingestion volume (Roden CAFE®, Oriental Yeast Co., Ltd., Tokyo, Japan). Mouse body weight was measured on the last day of the experiment. Tea components used were as follows: L-theanine (Suntheanine; Taiyo Kagaku Co. Ltd., Yokkaichi, Japan), EGCG (Sunphenon EGCg, Taiyo Kagaku Co. Ltd.), caffeine, and Arg (Wako Pure Chemical Co. Ltd., Osaka, Japan). The interaction of these tea components on adrenal hypertrophy was examined in stressed mice. Mice ingested feed that contained each component as molar ratios of placebo-matcha sample with the following concentrations: theanine 10 μM, Arg 4 μM, caffeine 86 μM, and EGCG 63 μM (CE/TA = 10.64). The stress-reducing effect of tea components was compared among five groups, as follows: group 1 included mice fed a diet without any tea component; group 2 included mice fed a diet containing theanine and Arg; group 3 included mice fed a diet containing theanine, Arg, caffeine and EGCG, and with a CE/TA molar ratio = 10.64; group 4 included mice fed a diet containing a 2.5-fold higher amount of theanine and Arg, but the same amount of caffeine and EGCG as group 3, and a CE/TA molar ratio = 4.26; group 5 included mice fed a diet containing a 5-fold higher amount of theanine and Arg, but the same amount of caffeine and EGCG as group 3, and a CE/TA molar ratio = 2.13.
2.4. Participants
Thirty-six healthy students (23 ± 0.9 years old, 18 men and 18 women) who participated in the experiment received verbal and written information about the study. Participants were fifth year college students of the University of Shizuoka, School of Pharmaceutical Sciences, Japan. They were selected for a double-blind randomized controlled trial, and signed an informed consent form before participating in the study. None of the participants indicated any acute or chronic diseases, regular intake of medication, or habitual smoking. Participants were instructed to not consume theanine- and caffeine-rich beverages, such as coffee, green tea, black tea, and soda, throughout the experiment. They were also instructed not to consume caffeine-rich chocolate and candy. Participants were allowed to drink water and barley tea freely, but were not permitted to consume alcohol at night. They were randomly divided into two groups: test-matcha (n = 19) and placebo-matcha (n = 17).
The participants were assigned to a practice outside the university for 11 weeks either in a hospital or at a pharmacy. However, the participants in this study already experienced another pharmacy practice, either in a hospital or a pharmacy, for three months up to one month ago. Seven days of routine university life and the first 8 days of the pharmacy practice program were analyzed. The study was conducted in accordance with the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects (Public Notice of the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare, 2008). This study was approved in Japan. The study protocol, which was approved by the Ethics Committee of the University of Shizuoka (No. 29-15), was registered at the University Hospital Medical Information Network (UMIN) (registration No. UMIN28529). The study period was from August to September 2017.
2.5. Procedure
The participants were randomly assigned to test- or placebo-matcha groups. This study was based on a group comparison design. Matcha (4.5 g) was mixed with flour 31.5 g, butter 26.1 g, sugar 13.5 g and egg yolk 1.8 g, molded into three cookies, then baked (at Patisserie Rond Point, Shizuoka, Japan). The participants did not know whether they were consuming test- or placebo-matcha. The consumption of cookies containing test- or placebo-matcha started from seven days prior to pharmacy practice and continued for eight days into the practice period, for a total of 15 days. The participants had three pieces of cookie per day. The state of anxiety of participants was examined before pharmacy practice and on the eighth day of pharmacy practice using a state-trait anxiety inventory (STAI) test (Japanese STAI Form X-1, Sankyobo, Kyoto, Japan).
Participants were requested to complete a questionnaire after each day's practice, and for a total of 15 days, that included feedback on their physical condition, subjective stress, and achievement emotion. The physical condition of participants was assigned an ordinal scale (5, very good; 4, good; 3, normal; 2, slightly bad; 1, bad). Subjective stress was evaluated using visual analogue scales (VAS: 0–10) from very relaxed to highly stressed. Achievement emotion was assigned an ordinal scale compared to the standard level (5, completely; 4, better; 3, a little better; 2, a little worse; 1, much worse). Sleeping hours were also recorded. Each participant's average data at the university and pharmacy practice were used for statistical analysis.
2.6. Measurement of sAA
For assessing the physiological stress response, sAA was measured using a colorimetric system (Nipro Co., Osaka, Japan) [23]. One unit of activity (U) per mass of enzyme is defined as the production of 1 μmol of the reduction sugar in 1 min (NC-IUBMB, EC 3.2.1.1). Participants washed their mouths with water before sampling, and measured their own sAA immediately after saliva was collected for 30 s using a sampling tip. Saliva was measured in the morning after waking up (sAAm). Next, the participants measured sAA every evening (sAAe) for seven days during routine daily life at the university, and successively for eight days during pharmacy practice. Each participant's average sAA at the university and pharmacy practice was used for statistical analysis.
2.7. Statistical analyses
Data are expressed as the mean ± standard error of the mean (SEM). Statistical analyses in the animal experiment were performed using a student's t-test and one-way analysis of variance followed by Bonferroni's post-hoc test for multiple comparisons. All statistical analyses in the clinical study were performed in a statistical analysis program, JMP ver.13 (SAS Institute Inc., Cary, NC, US). Differences were considered to be significant at p < 0.05.
3. Results
3.1. Anti-stress effects of matcha in a mouse model of psychosocial stress
The relationship between the intake of matcha components and suppression of stress was examined in a mouse model. Psychosocial stress alters the hypothalamus-pituitary-adrenal (HPA) axis function in humans and other animals. We have found that significant adrenal hypertrophy was observed in mice during confrontational housing [3]. In contrast, no change in adrenal weight was observed in mice housed singly [3]. The weight of adrenal glands increased to about 5 mg in mice after confrontational housing from about 4 mg before confrontation [20]. When mice ingested theanine and Arg without caffeine and EGCG, adrenal hypertrophy was significantly suppressed (Fig. 1). In this case, the ingested volume of theanine was 0.3 mg/kg. This dose was enough to suppress adrenal hypertrophy under stress [13]. However, the suppressive effect was counteracted in mice that also ingested caffeine and EGCG with a CE/TA molar ratio = 10.64. In mice fed a 2.5-fold higher amount of theanine and Arg than the former, adrenal hypertrophy was not suppressed when caffeine and EGCG coexisted and where the CE/TA molar ratio was 4.26. In mice fed a five-fold higher amount of theanine and Arg than at a CE/TA molar ratio of 10.64, adrenal hypertrophy was significantly suppressed, even though caffeine and EGCG coexistend with a CE/TA molar ratio of 2.13. These results indicate that matcha with a CE/TA molar ratio exceeding 10 could not suppress physiological and psychological stress. However, when the ratio was about 2, a significant stress-reducing effect was observed.
Fig. 1.
Suppression of adrenal hypertrophy in stressed mice. Mice consumed a CE-2 powder diet containing theanine, Arg, caffeine, and EGCG for seven days (single rearing for six days and confrontational rearing for one day). Mice ingested feed that contained the following components: theanine 10 μM, Arg 4 μM, caffeine 86 μM, and EGCG 63 μM. The mice of the pre-treated group that did not experience confrontational housing were used as a standard. The stress-reducing effect of tea components was compared among five groups, as follows: group 1 included mice fed a diet without any tea component; group 2 included mice fed a diet containing theanine and Arg; group 3 included mice fed a diet containing theanine, Arg, caffeine and EGCG; group 4 included mice fed a diet containing 2.5-fold more theanine and Arg, and the same amount of caffeine and EGCG as group 3; group 5 included mice fed a diet containing 5-fold more theanine and Arg, and the same amount of caffeine and EGCG as group 3. Bars indicate the mean ± SEM (n = 4; * p < 0.05).
3.2. Effect of ingestion of matcha cookies on stress in students assigned pharmacy practice outside the university
Participants consumed 4.5 g of test- or placebo-matcha daily that were present in three pieces of cookie. The components of matcha ingested by participants are shown in Table 1. The CE/TA molar ratio was 1.79 in test-matcha and 10.64 in placebo matcha (Table 1).
Table 1.
Matcha components ingested daily by each participant.
| Matcha | Amino acids (mg/day) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Theanine | Arg | Glu | Asp | Asn | Ser | Gln | GABA | Total | |
| Test | 78.4 ± 0.8 | 55.5 ± 1.4 | 24.2 ± 0.2 | 34.2 ± 0.6 | 14.3 ± 0.1 | 6.0 ± 0.0 | 3.8 ± 0.0 | 0.6 ± 0.0 | 226.4 ± 3.2 |
| Placebo | 17.6 ± 0.4 | 6.4 ± 0.5 | 12.7 ± 0.2 | 13.2 ± 0.3 | 3.7 ± 0.1 | 2.6 ± 0.0 | 1.7 ± 0.0 | 1.2 ± 0.0 | 66.1 ± 1.7 |
| Matcha | Caffeine |
Catechin (mg/day) |
|||
|---|---|---|---|---|---|
| (mg/day) | EGCG | ECG | EGC | EC | |
| Test | 175.3 ± 2.5 | 221.2 ± 3.1 | 114.3 ± 3.1 | 44.6 ± 0.4 | 14.8 ± 0.1 |
| Placebo | 166.8 ± 1.0 | 290.7 ± 19.4 | 137.4 ± 6.8 | 140.4 ± 1.8 | 30.4 ± 0.5 |
| Matcha | Ingestion (mmol) |
Molar ratio of CE/TA | |||
|---|---|---|---|---|---|
| Theanine | Arg | Caffeine | EGCG | ||
| Test | 0.45 ± 0.01 | 0.32 ± 0.01 | 0.90 ± 0.01 | 0.48 ± 0.01 | 1.79 |
| Placebo | 0.10 ± 0.00 | 0.04 ± 0.00 | 0.86 ± 0.01 | 0.63 ± 0.04 | 10.64 |
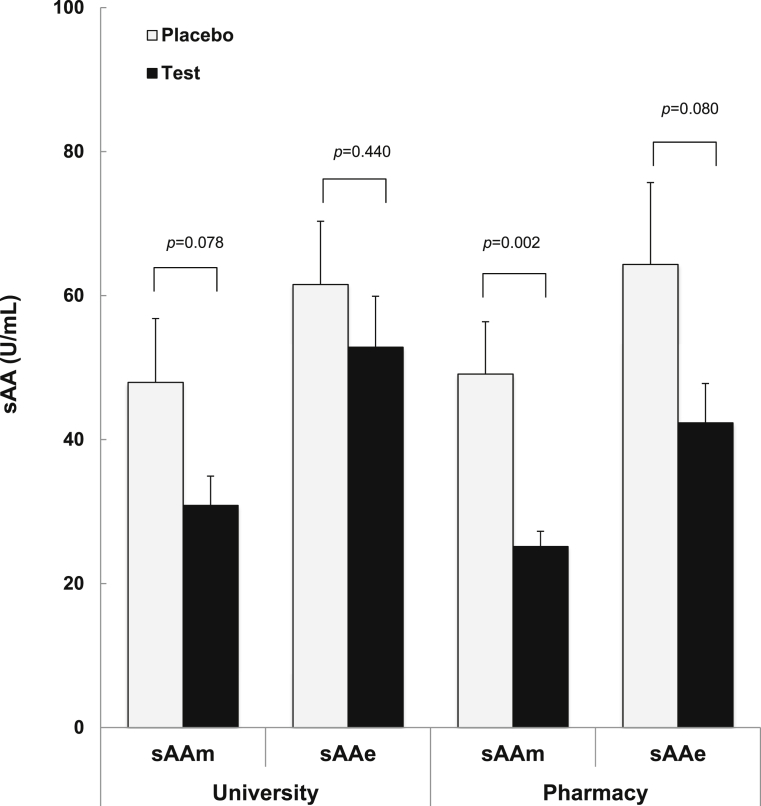
The basal level of sAAm before the ingestion of matcha was not different between the participants of the test- and placebo-matcha groups (43.2 ± 7.7 and 45.9 ± 7.0, respectively). However, the level of sAAm lowered to 27.9 in the first seven days at university and to 23.2 in the next eight days at the pharmacy after the ingestion of test-matcha. These results indicate that daily ingestion of test-matcha effectively lowered sAAm. In contrast, the level of sAAm did not change over the 15 days after the ingestion of placebo-matcha (Fig. 2). The level of sAAm at university tended to be lower than placebo-matcha group following the intake of test-matcha (p = 0.078, Fig. 2). Furthermore, during pharmacy practice, the level of sAAm was significantly lower in the test-matcha group than in the placebo-matcha group (p = 0.002). A lower level of sAA indicates lower physiological and psychological stress [21], so these results indicate that the ingestion of test-matcha reduced stress, whereas placebo-matcha did not.
Fig. 2.
Effect of matcha ingestion on students during university and pharmacy practice. The level of sAAm was measured in participants every morning. The median sAAm of each participant at the university and pharmacy practice was used for statistical analysis. Bars indicate the mean ± SEM (test matcha, n = 19; placebo matcha, n = 17).
The levels of sAAe were higher than sAAm in both groups. They were lower in the test-matcha group than in the placebo-matcha group during pharmacy practice but not significant (p = 0.080). Other parameters such as STAI, physical condition, sleeping time, and achievement emotion were not significantly different between the two groups (Table 2).
Table 2.
Effect of consumption of test- and placebo-matcha by students at university and pharmacy practice on psychosocial responses, as assessed from a questionnaire.
| Questionnaire Item | University |
Pharmacy |
||||
|---|---|---|---|---|---|---|
| Matcha |
Matcha |
|||||
| Test | Placebo | p value | Test | Placebo | p value | |
| STAI (20–80) | 45.8 ± 2.0 | 46.8 ± 2.8 | 0.76 | 42.3 ± 2.2 | 44.6 ± 2.7 | 0.51 |
| Subjective stress (0–10) | 4.01 ± 0.33 | 4.07 ± 0.37 | 0.89 | 4.66 ± 0.41 | 4.90 ± 0.45 | 0.69 |
| Physical condition (1–5) | 3.46 ± 0.13 | 3.58 ± 0.15 | 0.56 | 3.24 ± 0.14 | 3.40 ± 0.20 | 0.93 |
| Achievement emotion (1–5) | (−) | (−) | 3.29 ± 0.20 | 3.76 ± 0.13 | 0.07 | |
| Sleep time (h) | 6.64 ± 0.18 | 6.33 ± 0.12 | 0.17 | 6.07 ± 0.19 | 6.38 ± 0.17 | 0.24 |
The STAI test consisted of 20 questions. The degree of anxiety per item was evaluated by 1–4 points. Subjective stress was evaluated using visual analogue scales (VAS: 0–10) from very relaxed to highly stressed. The physical condition of participants was assigned an ordinal scale (5, very good; 4, good; 3, normal; 2, slightly bad; 1, bad). Achievement emotion was assigned an ordinal scale compared to the standard level (5, completely; 4, better; 3, a little better; 2, a little worse; 1, much worse).
4. Discussion
The animal (mouse) experiment in this study demonstrated that the CE/TA molar ratio of tea components is key for the suppression of stress. In addition, our clinical human study showed that a significant stress-reducing effect was observed in participants that ingested test-matcha cookies in which the CE/TA molar ratio was 1.79. However, a stress-reducing effect was not observed in participants that ingested placebo-matcha cookies with a CE/TA molar ratio of 10.64.
The reason why a CE/TA molar ratio of 2 or less is necessary to suppress stress has not been clarified yet. Theanine has been reported to inhibit the excitatory effects of caffeine [12]. In addition, Arg is considered to be an important regulator in the central nervous system through the synthesis of nitric oxide [24]. The stress-reducing effects of theanine and Arg in mice [13] may be related to these actions. Glu is the principal excitatory neurotransmitter and GABA is the main inhibitory neurotransmitter in the mammalian central nervous system [25]. Theanine, when incorporated into the brain, reduces the incorporation of extracellular Gln into neurons, which suppresses the conversion of Gln to Glu by glutaminase [26, 27]. On the other hand, Glu can be decarboxylated into GABA. In the hippocampus of mice that consumed theanine for two weeks, the level of GABA increased, and conversely the level of Glu was significantly reduced [28], suggesting that theanine may modulate the balance between GABA and Glu. In contrast, EGCG suppresses over-expression of the GABA pathway [29]. Suitable synaptic excitation is important but excessive excitation causes synaptic deficits and triggers neurodegenerative disorders [30, 31]. Therefore, theanine and Arg may be required at a certain ratio to suppress excessive excitation in the coexistence of caffeine and EGCG under stress loading.
Since matcha has a unique taste, aroma and color, it is widely used for confectionery products such as cookies, cakes, chocolates, and ice cream around the world. However, inexpensive matcha of which theanine content is low and catechins are high is often used for processing. Low-grade green teas with a low amino acid content are also sold as "matcha" in the US [32], indicating that a quality check of matcha is needed not only in the US but also in other countries. Especially, a reference value of matcha components is required to vouch for the stress-reducing effect of matcha. If the CE/TA molar ratio of matcha in confectionery products is 2 or less, then the stress-reducing effect of matcha is not disrupted by caffeine and EGCG. To prevent the accumulation of stress in everyday life, the consumption of confectioneries that include matcha may be a beneficial method for many people who do not have the habit of consuming tea.
This study, despite exploring the differences that matcha green tea components have on stress, has several limitations. First, in this study, there was no significant difference in the STAI value between the test- and placebo-matcha groups. Since participants in this study already experienced another pharmacy practice, either in a hospital or a pharmacy, for three months up to one month ago, it was considered that their state-anxiety did not become too high, even in the placebo group. It is better, in a future study, to examine the effect of matcha under a more stressful condition, such as in the first experience of pharmacy practice. Nevertheless, the levels of sAA were significantly lower in the test-matcha group than in the placebo-matcha group. Lower sAA implies lower physiological and psychological stress [21], indicating that the ingestion of test-matcha reduced stress. Measurement of sAA based on physiological response may be less affected by psychological bias than the STAI value based on psychological questions. Second, participants consumed 4.5 g of test-matcha daily, but it is necessary to examine if a lower amount of matcha can also reduce stress. Third, as this study is the result of young participants in their twenties, it is also necessary to examine middle-aged and elderly people to assess if the anti-stress effect is age-dependent. In addition, baking can decrease the content of total catechins by 19% compared to dough, although the content of epimerized catechin increases [33]. In the case of heated matcha products, studying the effect of epimerized catechin may be needed.
We have previously clarified that both the amount of theanine and the molar ratio of caffeine and EGCG, which counteracts the stress-reducing effect of theanine and Arg, are important for the function of matcha [20]. In this study, we furthermore clarified that it is possible to actually reduce stress when test-matcha is included in cookies.
5. Conclusions
Matcha, which has a stress-reducing effect, contains less caffeine and EGCG than twice the molar amount of theanine and Arg. Even when matcha is included in confectionery products, a stress-reducing effect was observed. Daily intake of matcha with a low CE/TA molar ratio in confectioneries may benefit people who do not drink green tea, and serve as a simple and practical way to prevent the accumulation of stress.
Declarations
Author contribution statement
Keiko Unno: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Daisuke Furushima: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Shingo Hamamoto: Analyzed and interpreted the data.
Kazuaki Iguchi: Performed the experiments.
Hiroshi Yamada: Conceived and designed the experiments; Analyzed and interpreted the data.
Akio Morita: Contributed reagents, materials, analysis tools or data.
Monira Pervin: Performed the experiments.
Yoriyuki Nakamura: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI 15K00828), a Honjo International Scholarship Foundation and a grant for specifically promoted research of the University of Shizuoka.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the participants of this study.
References
- 1.Vuong Q.V., Bowyer M.C., Roach P.D. L-Theanine: properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011;91:1931–1939. doi: 10.1002/jsfa.4373. [DOI] [PubMed] [Google Scholar]
- 2.Kimura K., Ozeki M., Juneja L.R., Ohira H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007;74:39–45. doi: 10.1016/j.biopsycho.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Unno K., Fujitani K., Takamori N., Takabayashi F., Maeda K., Miyazaki H., Tanida N., Iguchi K., Shimoi K., Hoshino M. Theanine intake improves the shortened lifespan, cognitive dysfunction and behavioural depression that are induced by chronic psychosocial stress in mice. Free Radic. Res. 2011;45:966–974. doi: 10.3109/10715762.2011.566869. [DOI] [PubMed] [Google Scholar]
- 4.Unno K., Iguchi K., Tanida N., Fujitani K., Takamori N., Yamamoto H., Ishii N., Nagano H., Nagashima T., Hara A., Shimoi K., Hoshino M. Ingestion of theanine, an amino acid in tea, suppresses psychosocial stress in mice. Exp. Physiol. 2013;98:290–303. doi: 10.1113/expphysiol.2012.065532. [DOI] [PubMed] [Google Scholar]
- 5.Unno K., Tanida H., Ishii N., Yamamoto H., Iguchi K., Hoshino M., Takeda A., Ozawa H., Ohkubo T., Juneja L.R., Yamada H. Anti-stress effect of theanine on students during pharmacy practice: positive correlation among salivary α-amylase activity, trait anxiety and subjective stress. Pharmacol. Biochem. Behav. 2013;111:128–135. doi: 10.1016/j.pbb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Ikegaya K., Takayanagi H., Anan T. Chemical composition of matcha. Tea Res. J. (Chagyo Kenkyu Hokoku) 1984;60:79–81. [Google Scholar]
- 7.Horie H., Ema K., Sumikawa O. Chemical components of Matcha and powdered green tea. J. Cook. Sci. Jap. (Nippon Chourikagaku Kaishi) 2017;50:182–188. [Google Scholar]
- 8.Goto T., Horie H., Ozeki Y., Masuda H., Warashina J. Chemical composition of Japanese green teas on market. Tea Res. J. (Chagyo Kenkyu Hokoku) 1994;80:23–28. [Google Scholar]
- 9.Wirtz P.H., von Känel R. Psychological stress, inflammation, and coronary heart disease. Curr. Cardiol. Rep. 2017;19:111. doi: 10.1007/s11886-017-0919-x. [DOI] [PubMed] [Google Scholar]
- 10.Piirainen S., Youssef A., Song C., Kalueff A.V., Landreth G.E., Malm T., Tian L. Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer's disease: the emerging role for microglia? Neurosci. Biobehav. Rev. 2017;77:148–164. doi: 10.1016/j.neubiorev.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Munakata M. Clinical significance of stress-related increase in blood pressure: current evidence in office and out-of-office settings. Hypertens. Res. 2018;41(8):553–569. doi: 10.1038/s41440-018-0053-1. [DOI] [PubMed] [Google Scholar]
- 12.Kakuda T., Nozawa A., Unno T., Okamura N., Okai O. Inhibiting effects of theanine on caffeine stimulation evaluated by EEG in the rat. Biosci. Biotechnol. Biochem. 2000;64:287–293. doi: 10.1271/bbb.64.287. [DOI] [PubMed] [Google Scholar]
- 13.Unno K., Hara A., Nakagawa A., Iguchi K., Ohshio M., Morita A., Nakamura Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine. 2016;23:1365–1374. doi: 10.1016/j.phymed.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Giles G.E., Mahoney C.R., Brunyé T.T., Taylor H.A., Kanarek R.B. Caffeine and theanine exert opposite effects on attention under emotional arousal. Can. J. Physiol. Pharmacol. 2017;95:93–100. doi: 10.1139/cjpp-2016-0498. [DOI] [PubMed] [Google Scholar]
- 15.Anan T., Nakagawa M. Effect of light on chemical constituents in the tea leaves. Nippon. Nogeikagaku Kaishi. 1974;48:91–96. [Google Scholar]
- 16.Ashihara H. Occurrence, biosynthesis and metabolism of theanine (γ-glutamyl-L-ethylamide) in plants: a comprehensive review. Nat. Prod. Commun. 2015;10:803–810. [PubMed] [Google Scholar]
- 17.Ruan J., Haerdter R., Gerendás J. Impact of nitrogen supply on carbon/nitrogen allocation: a case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol. (Stuttg) 2010;12:724–734. doi: 10.1111/j.1438-8677.2009.00288.x. [DOI] [PubMed] [Google Scholar]
- 18.Goto T., Nagashima H., Yoshida Y., Kiso M. Contents of individual tea catechins and caffeine in Japanese green tea. Tea Res. J. (Chagyo Kenkyu Hokoku) 1996;83:21–28. [Google Scholar]
- 19.Ashihara H., Suzuki T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004;9:1864–1876. doi: 10.2741/1367. [DOI] [PubMed] [Google Scholar]
- 20.Unno K., Furushima D., Hamamoto S., Iguchi K., Yamada H., Morita A., Horie H., Nakamura Y. Stress-reducing function of matcha green tea in animal experiments and clinical trials. Nutrients. 2018;10:1468. doi: 10.3390/nu10101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Stegeren A., Rohleder N., Everaerd W., Wolf O.T. Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Goto T., Horie H., Mukai T. Analysis of major amino acids in green tea by high-performance liquid chromatography coupled with OPA precolumn derivatization. Tea Res. J. (Chagyo Kenkyu Hokoku) 1993;77:29–33. [Google Scholar]
- 23.Yamaguchi M., Kanemori T., Kanemaru M., Takai N., Mizuno Y., Yoshida H. Performance evaluation of salivary amylase activity monitor. Biosens. Bioelectron. 2004;20:491–497. doi: 10.1016/j.bios.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Virarkar M., Alappat L., Bradford P.G., Awad A.B. L-Arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2013;53:1157–1167. doi: 10.1080/10408398.2011.573885. [DOI] [PubMed] [Google Scholar]
- 25.Schousboe A. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem. Res. 2003;28:347–352. doi: 10.1023/a:1022397704922. [DOI] [PubMed] [Google Scholar]
- 26.Kakuda T., Hinoi E., Abe A., Nozawa A., Ogura M., Yoneda Y. Theanine, an ingredient of green tea, inhibits [3H]glutamine transport in neurons and astroglia in rat brain. J. Neurosci. Res. 2008;86:1846–1856. doi: 10.1002/jnr.21637. [DOI] [PubMed] [Google Scholar]
- 27.Kakuda T. Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction. Pharmacol. Res. 2011;64:162–168. doi: 10.1016/j.phrs.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Inoue K., Miyazaki Y., Unno K., Min J.Z., Todoroki K., Toyo'oka T. Stable isotope dilution HILIC-MS/MS method for accurate quantification of glutamic acid, glutamine, pyroglutamic acid, GABA and theanine in mouse brain tissues. Biomed. Chromatogr. 2016;30:55–61. doi: 10.1002/bmc.3502. [DOI] [PubMed] [Google Scholar]
- 29.Souchet B., Guedj F., Penke-Verdier Z., Daubigney F., Duchon A., Herault Y., Bizot J.C., Janel N., Créau N., Delatour B., Delabar J.M. Pharmacological correction of excitation/inhibition imbalance in Down syndrome mouse models. Front. Behav. Neurosci. 2015;9:267. doi: 10.3389/fnbeh.2015.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta A., Prabhakar M., Kumar P., Deshmukh R., Sharma P.L. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Kvartan M.D., Bradbrook K.E., Dantrassy H.M., Bailey A.M., Thompson S.M. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J. Neurophysiol. 2015;114:1713–1724. doi: 10.1152/jn.00359.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horie H., Ema K., Nishikawa H., Nakamura Y. Comparison of the chemical components of powdered green tea sold in the US. JARQ. 2018;52:143–147. [Google Scholar]
- 33.Phongnarisorn B., Orfila C., Holmes M., Marshall L.J. Enrichment of biscuits with matcha green tea powder: its impact on consumer acceptability and acute metabolic response. Foods. 2018;7 doi: 10.3390/foods7020017. pii: E17. [DOI] [PMC free article] [PubMed] [Google Scholar]