Abstract
Objectives: This study aimed to identify potential prognostic factors for patients with complex atypical hyperplasia (CAH) or early-stage endometrial cancer (EC) who received progestin therapy to spare fertility and, thus, improve the management of this patient group.
Materials and methods: The PubMed, PMC, EMBASE, Web of Science, and Cochrane databases were searched for correlational studies published in English. Studies that evaluated the prognosis of patients with CAH or early-stage EC were pooled for a systematic review and meta-analysis.
Results: In total, 31 eligible studies, including 8 prospective and 23 retrospective studies involving 1099 patients, were included in this analysis. The most commonly used progestin agents were medroxyprogesterone acetate (MPA, 47.0%) and megestrol acetate (MA, 25.5%). The total complete response (CR) rate was 75.8% (833/1099), and the median time to CR with first-line progestin therapy was 6 months. In total, 294 (26.8%) patients who achieved CR became pregnant spontaneously (28 cases) or through assisted reproductive technology (127 cases). During the median follow-up of 39 months, 245 (22.3%) women developed recurrence. Only one patient (0.09%) died of the disease. The meta-analysis showed that compared to a BMI<25 kg/m2 and CAH, a body mass index (BMI) ≥25 kg/m2 (P=0.0004, odds ratios (OR), 0.4; 95% confidence interval, 0.3–0.6) and EC (P=0.0000, OR, 0.3; 95% confidence interval, 0.2–0.6) were significantly associated with a higher likelihood of a CR. Patients with a BMI≥25 kg/m2 (P=0.0007, OR, 2.5; 95% confidence interval, 1.4–4.3), PCOS (P=0.0006, OR, 3.4; 95% confidence interval, 1.5–7.9), and EC (P=0.0344, OR, 2.8; 95% confidence interval, 1.4–5.3) had a significantly higher risk of recurrence.
Conclusion: In general, patients with CAH or early-stage EC who were treated with progesterone therapy had a favorable prognosis. However, the recurrence risk was not insignificant. Weight control is crucial for improving the clinical management of this patient group.
Keywords: endometrial cancer, complex atypical hyperplasia, fertility-sparing treatment, progestogens, systematic review
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries.1 Although EC is typically thought to be a cancer affecting postmenopausal women, approximately 14% of cases occur in premenopausal women, and 5% of patients are aged 40 years or younger.2–4 Its precursor, ie, complex atypical hyperplasia (CAH), affects an even larger proportion of premenopausal women.5 The standard treatment for EC includes extrafascial hysterectomy, bilateral salpingo-oophorectomy, and pelvic and para-aortic lymphadenectomy, if indicated. This treatment is usually unacceptable for young patients, who are usually diagnosed at an early stage with well-differentiated disease 4,6 and usually desire a fertility-sparing procedure.
Figure 4.
the Egger’s test of each outcome of factor in the meta-analysis. (A) Egger’s test of the risk of obesity in disease complete response. (B) Egger’s test of the risk of obesity in disease recurrence. (C) Egger’s test of the risk of histology type in disease complete response. (D) Egger’s test of the risk of histology type in disease recurrence. (E) Egger's test of the risk of polycystic ovarian syndrome in disease complete response. (F) Egger's test of the risk of polycystic ovarian syndrome in disease recurrence. (G) Egger’s test of the risk of hormone type in disease complete response. (H) Egger’s test of the risk of hormone type in disease recurrence. (I) Egger’s test of the risk of age in disease complete response. (J) Egger’s test of the risk of age in disease recurrence.
Several previous studies have demonstrated that most “young” patients (premenopausal or aged 45 years or younger), who commonly have low-grade, minimally invasive tumors, have excellent clinical outcomes.7,8 In addition, the risk of myometrial invasion or lymph node metastasis in young patients is quite low.9,10 Thus, the cure rate among this patient group is very high. Fertility-sparing treatment to improve patients’ quality of life is an important consideration. The feasibility and safety of fertility-sparing treatment, mainly hormone therapy, in selected patients with early-stage EC or CAH have been demonstrated in multiple studies.11–13 However, most studies were limited by a small sample size and/or a single-center design, and definitive conclusions could not be drawn. In contrast, several other researchers have demonstrated that fertility preservation may have a nonnegligible negative impact on patients’ survival or risk of relapse.14–16 Consequently, the present systematic review was conducted to explore the potential prognostic factors of patients with early-stage EC and CAH who receive fertility preservation treatment. Reasonable suggestions and measures are proposed to improve the management of this patient group.
Materials and methods
Identification of literature
The PubMed, PMC, EMBASE, Web of Science, and Cochrane databases, where we considered those published before April 2018, were searched for correlational studies published in English. The search terms included “endometrial cancer”, “endometrial carcinoma”, “uterine cancer”, “uterine carcinoma”, “fertility-sparing”, “fertility preservation”, “fertility”, “preservation”, “conservative”, or “progestin”. The search strategy was based on medical subject headings and free text words in titles/abstracts, with connectives comprising “AND” or “OR.” We used this search strategy in PubMed, EMBASE, Web of science and the Cochrane databases, The full electronic search strategy for Pubmed is “(((((endometrial cancer [Title/Abstract]) OR endometrial carcinoma [Title/Abstract]) OR uterine cancer [Title/Abstract]) OR uterine carcinoma [Title/Abstract])) AND (((fertility sparing [Title/Abstract]) OR conservative [Title/Abstract]) OR progestin [Title/Abstract]).
Study selection and data extraction
The criteria for this systematic review were as follows: 1) patients staged based on the International Federation of Gynecology and Obstetrics (FIGO) staging system; 2) patients with early-stage EC (Stage IA, G1-G2) or CAH; 3) patients treated with progestin therapy to spare fertility; 4) available data regarding disease response and recurrence; and 5) full text and complete data available. The exclusion criteria were as follows: 1) review articles, case reports and meta-analyses; 2) patients with tumors invading the myometrium; 3) progestin use combined with surgical therapy; 4) studies that did not stratify the results to distinguish between hyperplasia with or without complex atypia; 5) non-English language; and 6) incomplete data.
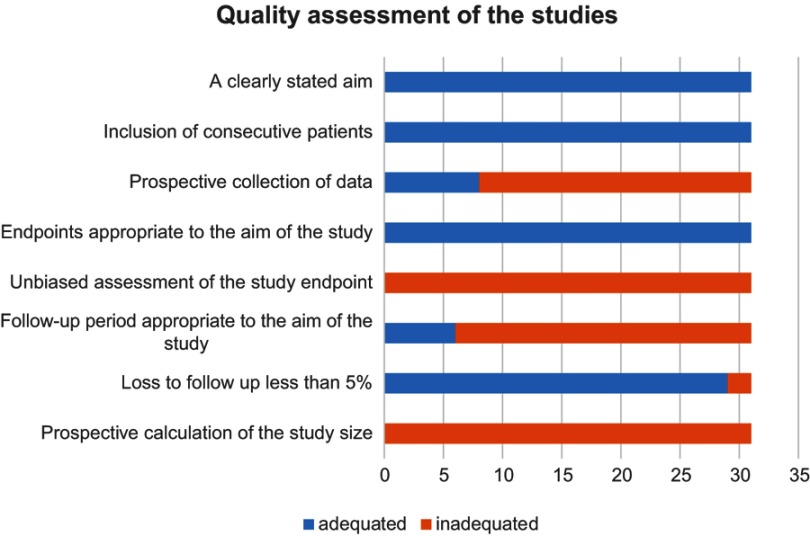
The literature was reviewed by two different readers independently (Miaomiao Li and Tao Guo). Disagreements were resolved by the arbitration of a third reviewer (Ran Cui). The Methodological Index for Non-Randomized Studies (MINORS) was implemented to assess the quality of the nonrandomized studies (Figure 2).17 The demographic data, including age, body mass index (BMI), diagnosis, medical comorbidities, type of hormonal agent used, patient response, and side effects of drug therapy, were collected. Information about the oncological and reproductive outcomes, including recurrence, survival, pregnancy rate and live birth rates, was also recorded. BMI was calculated as weight in kilograms divided by the square of height in meters. Complete response (CR) was defined as no microscopic evidence of either hyperplasia or cancer cells in endometrial histopathology. Partial response (PR) was defined as regression of CAH or EC to simple or complex hyperplasia without atypia. Stable disease (SD) was defined as the persistence of pretreatment lesions. The overall survival (OS) times were calculated in months from the date of the medical treatment to the death of the patients; survivors at the final follow-up visit were censored.
Figure 2.
The quality of the studies according to the MINORS checklist Figure 2. The appropriate follow-up period was defined as at least five years.
Statistical analyses
For calculations of median age and follow up times, individual data were used if the study reported these values. Otherwise, each subject in the study was assumed to be the reported mean or median value for the respective study. For the studies which did not provide any information, they were not included in the overall median estimates. Forest plots were created for each factor to show the pooled odds ratios (OR) with 95% confidence intervals (CI). The inconsistency index (I2) value across studies was used to evaluate heterogeneity. If the I2 statistic was >50%, a random-effects model was used. Otherwise, a fixed-effects model was used (I2<50%). The risk factors were compared with the Pearson chi-square test. The tests were two-sided. A P-value<0.05 was considered significant. All statistical analyses were performed using Review Manager 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK), SPSS software version 19 (Version X; IBM, Armonk, NY, USA) and Stata version 12.0 (StataCorp, College Station, TX, USA). Funnel plots were used to assess publication bias, which was quantified using Egger’s linear regression test.
Ethics statement
This article does not contain any studies with human participants performed by any of the authors.
Results
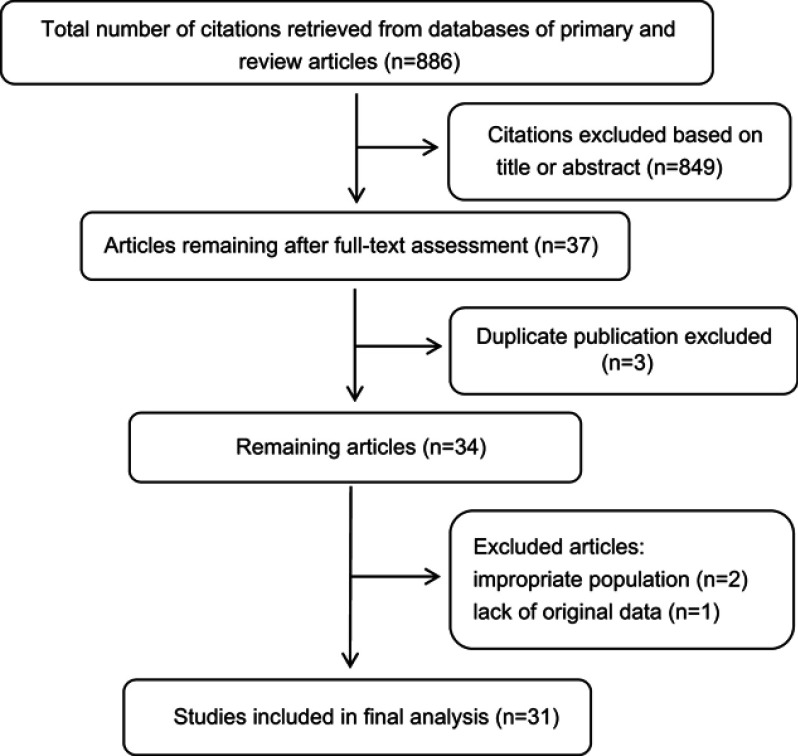
In total, 886 citations were retrieved from the databases by searching for the terms. Eight hundred forty-nine articles with irrelevant information based on reviews of the titles and abstracts were excluded. Three duplicated articles were also excluded. According to the inclusion criteria, three articles involving postmenopausal women (two articles) or lacking original data (one article) were further excluded. Thus, in total, thirty-one eligible studies, including eight prospective and twenty-three retrospective studies, involving 1099 patients were included in this analysis (Figure 1). The clinicopathological characteristics and oncologic and reproductive outcomes11–14,16–42 of the patients are shown in Tables 1 and 2, respectively.
Figure 1.
In total, thirty-one eligible studies, including eight prospective and twenty-three retrospective studies, involving 1099 patients were included in this analysis.
Table 1.
The characteristics of included studies
| Study | Study design | Age (years) | BMI (kg/m2) | Pathological type | Nulliparous | Medical co-morbidity | Intervention | |
|---|---|---|---|---|---|---|---|---|
| CAH | EC | |||||||
| Tamauchi 201819 | Retrospective | 34 (19–45) | 23.3 (18.1–45.0) | 30 | 9 | 37 | UK | MPA |
| Fukui 201720 | Retrospective | 33 (19–39) | 21.5 (17.7–34.9) | None | 35 | 34 | UK | MPA |
| Hwang 201721 | Retrospective | 30.4 (25–39) | 24.0 (18.5–30.5) | None | 5 | 5 | No | MPA+LNG-IUS |
| Park 201722 | Retrospective | 32 | 25.5 | None | 154 | 145 | Infertility: n=52, PCOS: n=31; Other medical disease: n=23 |
MA: n=51; MPA: n=103 |
| Kim 201723 | Retrospective | 36 (25–41) | 32.9 (21–70) | 29 | 21 | 44 | DM: n=3; previous malignancy tumor: n=4 |
MA: n=24; MPA: n=20; LNG-IUS: n=2 Micronized progesterone: n=2 |
| Chen 201624 | Retrospective | 32 (21–41) | UK | 16 | 37 | 48 | PCOS: n=18; DM: n=6; Family history of cancer: n=5 |
MA: n=21 (plus GNRHa: n=9, plus LNG-IUS: n=2); MPA: n=32 |
| Baek 201625 | Retrospective | 33 (20–41) | 23.1 (16.6–39.5) | 18 | 13 | 14 | PCOS: n=5 | MA: n=25; MPA: n=6 |
| Zhou 201526 | Retrospective | 30.4 (20–40) | 26.7 (17.6–36.0) | 13 | 19 | 23 | PCOS: n=6; Thyroid disease: n=3; High HbA1c: n=9 |
MA or MPA (metformin with high HbA1c) |
| Pronin 201514 | Prospective | 33 (28–42) | UK | 38 | 32 | UK | UK | Mirena: n=38 Mirena+GnRHa: n=32 |
| Mitsuhashi 201527 | Prospective | 33 (26–42) | 30.9 (18.8–52.7) | 17 | 19 | UK | PCOS: n=17; Abnormal glucose metabolism: n=16 |
MPA+Aspirin+Metformin |
| Simpson 201428 | Retrospective | 36.5 (26–44) | 25 (20–66) | 19 | 25 | 31 | DM: n=5; Family history Lynch syndrome: n=7 |
UK |
| Kudesia 201413 | Retrospective | 38.5 | CAH:28.6 EC:26.8 |
13 | 10 | 21 | UK | MA: n=9; LNG-IUS: n=6; LNG-IUS+Oral progestin: n=8 |
| Gonthier 201429 | Retrospective | 34.0 (23.0–40.0) | 26.9 (18–44) | 23 | 17 | 31 | PCOS: n=13; First degree with HNPCC associated cancer: n=2 |
oral progestin: n=28; GnRHa: n=5; LNG-IUS: n=5 |
| Park 201312 | Retrospective | 31.3 (21–40) | 24.98 (15.06–38.20) | None | 148 | 139 | PCOS: n=23; Other medical disease: n=20 |
MA: n=57; MPA: n=91 |
| Jafari 201330 | Prospective | 30 (24–35) | UK | None | 8 | 6 | PCOS: n=3 | MA |
| Koskas 201231 | Retrospective | 28–40 | UK | 14 | 8 | 19 | No family history of HNPCC | MA: n=5; MPA: n=4; NA: n=7; CA: n=3; Lynestrenol: n=3 |
| Fujiwara201215 | Retrospective | 31 (21–42) | 23.3 (15–38) | None | 45 | UK | UK | MPA |
| Ricciardi 201232 | Retrospective | 32 (25–40) | UK | 13 | 1 | 11 | PCOS or infertility: n=13 | MA or MPA |
| Perri 201133 | Retrospective | 33.4 (24–43) | UK | None | 27 | UK | UK | MA: n=24; NA: n=1; Hydroxyprogesterone caproate: n=2 |
| Park 201134 | Retrospective | 30.0 (21–38) | 22.3 (17.0–33.0 | None | 14 | 12 | PCOS: n=6 | MA: n=12; MPA: n=2 |
| Kim 201118 | Retrospective | 38.4 (33–41) | 20.3 (11.4–36.7) | None | 5 | 5 | DM: n=1 | MPA+Mirena |
| Minig 201035 | Prospective | 34 (22–40) | 21 (17–41) | 20 | 14 | 29 | DM: n=1; HP: n=2; PCOS: n=4 |
LNG-IUS+GnRHa |
| Yu 200936 | Retrospective | CAH:29.9 EC:25.1 |
UK | 17 | 8 | UK | UK | MPA |
| Han 200937 | Retrospective | 32 (26–37) | UK | 3 | 7 | 9 | PCOS: n=8 Infertility: n=6 |
MA: n=7; MPA: n=2; Provera: n=1 |
| Hahn 200938 | Retrospective | 31 (21–43) | UK | None | 35 | 15 | Infertility:15 | MA: n=8; MPA: n=20; MPA+MA: n=7 |
| Signorelli 200839 | Prospective | 32 (21–40) | 27.7 (19–41) | 10 | 11 | UK | PCOS: n=5; Infertility: n=8; Hyperprolactinaemia: n=2. |
Natural progestin |
| Yamazawa 200740 | Prospective | 36 (28–40) | UK | None | 9 | 9 | Infertility: n=3 | MPA |
| Ushijima 200717 | Prospective | 31.7 (22–39) | 22.8 (16–32.7) | 17 | 28 | 45 | PCOS: n=7 | MPA+Aspirin |
| Yang 200541 | Prospective | 33 (27–39) | 21.9 (14.3–26.0) | None | 6 | 6 | Infertility: n=4 | MA |
| Yahata 200542 | Retrospective | 31.9 (26–37) | 25.4 (18–35) | None | 8 | 8 | MPA | |
| Gotlieb 200343 | Retrospective | 31 (23–40) | UK | None | 11 | 13 | Infertility: n=6 | MA: n=7; MPA: n=1; Others: n=3 |
Abbreviations: BMI, body mass index; CA, chlormadinone acetate; CAH, complex atypical hyperplasia; DM, diabetes mellitus; EC, endometrial cancer; GnRHa, gonadotropin-releasing hormone agonist; HNPCC, hereditary non-polyposis colorectal cancer; LNG-IUS, levonorgestrel intrauterine system; MA, megestrol acetate; MPA, medroxyprogesterone acetate; NA, nomegestrol acetate; PCOS, polycystic ovarian syndrome; UK, unknow.
Table 2.
Reproductive and oncological outcomes of CAH/early-stage EC patients who treated with fertility-preservation procedure
| Study | CR | Time of achieving CR(m) | PR or SD | PD | Recurrence | Time to recurrence(m) | Pregnancy | Gestational mode | Live birth | Hysterectomy | Follow-up time(m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tamauchi 201819 | CAH: n=28 | CAH:26 (10–63) | 3 | UK | CAH: n=14 | CAH: 72 (10–283) | 14 | IVF: n=10; | 7 | 3 | 52 (16–128) |
| EC: n=8 | EC:40 (26–53) | EC: n=7 | EC: 50 (24–272) | Spontaneous: n=4 | 3 | ||||||
| Fukui 201720 | 25 | UK | 10 | 0 | 8 | UK | 12 | UK | 11 | 15 | 89 (12–193) |
| Hwang 201721 | 3 | 11.0 (6–18) | 2 | 0 | 1 | 23 | 1 | IVF | 0 | 2 | 44.4 (12–71) |
| Park 201722 | 111 | 4.5 (0.8–55.5) | UK | UK | 43 | 57 (6–194) | 45 | UK | 35 | UK | 57 (6–194) |
| Kim 201723 | 22 | UK | 28 | 0 | 3 | UK | 10 | IVF: n=7; Others: n=3 |
5 | 27 | 23 (3–118) |
| Chen 201624 | CAH: n=12 | 6 (3–24) | 10 | 4 | CAH: n=3 | 28.5 (4–56) | 17 | IVF | 11 | 20 | 54 (4–148) |
| EC: n=27 | EC: n=7 | ||||||||||
| Baek 201625 | CAH: n=16 | CAH:3 (1–22) | 8 | 0 | CAH: n=2 | 8 (7–11) | 2 | ART | 2 | 8 | 11.5 (3–29) |
| EC: n=7 | EC 3 (2–9) | EC: n=4 | 2 (4–18) | ||||||||
| Zhou 201526 | 27 | 6.2 (0.8–41.5) | 5 | 0 | 9 | 8.5 (3–17.3) | 9 | ART | 5 | 2 | 32.2 (10–92) |
| Pronin 201514 | CAH: n=35 | UK | 9 | 0 | CAH: n=1 | 6–12 | 8 | Spontaneous | 8 | 9 | 17 (1–45) |
| EC: n=23 | EC: n=2 | ||||||||||
| Mitsuhashi 201527 | 29 | 6–9 | 5 | 2 | 3 | 38 (1–66) | 8 | IVF | 6 | 4 | 38 (9–66) |
| Simpson 201428 | 24 | 5.7 (2–24) | 20 | 0 | 13 | 42 | 5 | IVF: n=4; Spontaneous: n=1 |
2 | 21 | 39 (5–128) |
| Kudesia 201413 | CAH: n=5 | 13 (3–74) | 9 | 0 | UK | UK | 4 | IVF | 4 | 7 | 13 (3–74) |
| EC: n=7 | |||||||||||
| Gonthier 201429 | 29 | 2–6 | UK | UK | 6 | 3–37 | 14 | ART: n=9; Spontaneous: n=5 |
10 | UK | 23.4 (6–130) |
| Park 201312 | 115 | 4.5 (2–13.8) | 33 | 0 | 35 | 15 (4–61) | 44 | UK | 44 | 13 | 66 (14–194) |
| Jafari 201330 | 7 | 6 (3–9) | 1 | 0 | 3 | 14 (3–21) | 3 | IVF: n=2 | 2 | 3 | 34.5 (11–72) |
| Koskas 201231 | CAH: n=12 | 4.4 1(3–6) | 4 | 1 | 3 | 15.3 (6–31) | 8 | UK | 8 | 6 | 39 (14–86) |
| EC: n=5 | |||||||||||
| Fujiwara 201215 | 36 | 6.2 (3.3–17.5) | UK | UK | 17 | 12 (7–84) | UK | UK | UK | UK | 66 (11–251) |
| Ricciardi 201232 | 11 | UK | 3 | 0 | UK | UK | 4 | IVF | 4 | 3 | UK |
| Perri 201133 | 24 | 5 (1–17) | 3 | 0 | 9 | 39.9 (1.8–84) | 14 | IVF: n=9; Spontaneous: n=5 |
10 | 10 | 57.4(7.8–412) |
| Park 201134 | 13 | 6 (3–15) | 1 | 0 | 2 | 7–36 | 4 | ART: n=4 | 4 | 1 | 47.3(18–135) |
| Kim 201118 | 4 | 5 (3–12) | 1 | 0 | 0 | —— | 1 | IVF | 1 | 0 | 10.2(6–16) |
| Minig 201035 | CAH: n=19 | 6–12 | 2 | 5 | CAH: n=4 | 36 (16–62) | 9 | UK | 7 | 13 | 29 (4–102) |
| EC: n=8 | EC: n=2 | ||||||||||
| Yu 200936 | 19 | CAH: 7.3(3–11) | 6 | 0 | 4 | CAH: 30 | 4 | IVF: n=3; Spontaneous: n=1 |
4 | 6 | CAH: 34.6 (7–114) |
| EC: 6.4 (3–10) | EC:11 (6–16) | EC: 31.8(5–90) | |||||||||
| Han 200937 | 10 | 5.2 (3–18) | 0 | 0 | 1 | UK | 9 | ART | 6 | 1 | 46.8 (13–75) |
| Hahn 200938 | 22 | 9 (2–12) | 13 | 0 | 9 | 12 (8–48) | 10 | ART: n=7; Spontaneous: n=3 |
8 | 16 | 39 (5–108) |
| Signorelli 200839 | 3 | 4 (3–9) | 18 | 0 | UK | UK | 9 | UK | UK | 9 | 98 (35–176) |
| Yamazawa 200740 | 8 | 5.3 (3–9) | 0 | 0 | 2 | 16 (10–22) | 4 | ART: n=4 | 3 | 2 | 39 (24–69) |
| Ushijima 200717 | CAH: n=16 | 12.5 (8–26) | 13 | 0 | 14 | CAH: 44.2 | 11 | ART: n=10; Spontaneous: n=1 |
7 | 18 | 39 (5–128) |
| G1EC: n=14 | EC: 34.6 | ||||||||||
| Yang 200541 | 4 | 3.5 (2–5) | 2 | 0 | 2 | 4.5 | 2 | UK | 2 | 4 | 48.8(14–132) |
| Yahata 200542 | 7 | 8.7 (4–14) | 1 | 0 | 7 | 11.6 (4–33) | 3 | ART: n=3 | 3 | 5 | 76.5(21–118) |
| Gotlieb 200342 | 11 | 3.5 (0–9) | 0 | 0 | 5 | 40 (19–358) | 6 | UK | 3 | 4 | 82 (6–358) |
Abbreviations: ART, assisted reproductive technology; IVF, in-vitro fertilization; IK unknown.
The average age of the patients at diagnosis was 32.8 (range: 19–45) years. Nulliparous women accounted for 87.4% of the sample. The average BMI was 24.9 (range: 11.4–70) kg/m2. Diabetes mellitus and abnormal glucose metabolism were identified in sixteen (1.5%) and twenty-five (2.3%) patients, respectively. Three patients had hypertension; in one case, hypertension was related to renal disease. Polycystic ovarian syndrome (PCOS) was identified in 148 (13.5%) patients. Nine (0.8%) patients had a family history of Lynch syndrome. CAH was identified in 316 (28.8%) patients. The remaining 783 (71.2%) patients had stage IA EC.
The most commonly used progestin agents were medroxyprogesterone acetate (MPA, 47.0%) and megestrol acetate (MA, 25.5%). The most common doses were 400–600 mg/d for MPA and 160–240 mg/d for MA. Other agents, including levonorgestrel intrauterine system, natural progesterone, hydroxyprogesterone caproate, norethisterone acetate, and gonadotropin-releasing hormone agonist, were also used either as a single agent or in combination. The most common adverse effects included weight gain (3.6%) and liver dysfunction (1.1%), followed by nausea (0.5%), diarrhea (0.4%), breast pain (0.5%), premature ovarian failure (0.09%), and antithrombin III and fibrinogen irregularities (0.09%). Grade 3–4 adverse effects associated with progestin treatment were identified in four patients (0.4%) and included body weight gain (two cases), liver dysfunction (one case), and premature ovarian failure (one case) No patient developed thromboembolism. The scheduled progesterone treatment was not delayed due to these side effects. No treatment-related deaths were identified.
All patients were closely followed during and after progesterone therapy. The median follow-up time was 39 months (range: 1–412 months). Endometrial resampling through endometrial curettage or endometrial biopsy was usually performed every 3 months (31.4%), every 3 to 6 months (19.4%) or every 6 months (6.4%). In three studies,14,16,19 the endometrial evaluations were performed more frequently (every 2 months after the initiation of hormonal therapy). CR was achieved in 806 (73.3%) patients with first-line progesterone therapy. Twenty-seven patients (2.5%) achieved CR with continued progestin treatment. Thus, the total CR rate was 75.8%, and the median time to CR with first-line progestin therapy was 6 months (range: 1–74 months). PR or SD was achieved in 210 (19.1%) patients. Twelve (1.1%) patients had progressive disease (PD) during hormonal therapy.
After achieving CR. There were 197 patients accepted fertility treatment, 65 patients accepted estrogen-progestin therapy, such as taking oral contraceptives, the 192 patients did not receive any treatment, just follow up regularly. There were 71 patients continued to receive progestins with treatment dose until pregnancy. The other 61 patients received low-dose cyclic progestin, such as dydrogesterone and progestin -releasing intrauterine device.
In total, 294 (26.8%) patients who achieved CR became pregnant spontaneously (28 cases) or with assisted reproductive technology (127 cases). The median time to achieve pregnancy was 12.5 months (range: 1–69.7 months). Forty-nine (4.5%) pregnant patients developed spontaneous abortion. In total, 225 (20.5%) pregnant patients had live births. Most (73.8%) pregnant women gave birth at full term.
At the final contact, in total, 245 (22.3%) women had developed recurrence. The median time to recurrence was 20 (range: 1–358) months. Salvage progestin treatment was administered in 101 (41.2%) patients with recurrence, and nearly half (49.5%) of these patients achieved CR again. Hysterectomy with or without bilateral salpingo-oophorectomy was performed in patients with recurrent disease (eighty-three cases), PR or SD (137 cases), and PD (twelve cases). Extrauterine lesions were identified in eleven patients (1.0%) in the ovary (eight cases), fallopian tube (one case), uterine serosa (one case), bone and lymph gland (one case). In the entire series, in total, two deaths were described.16,26 One patient died of simultaneous peritoneal carcinoma and EC. Only one patient (0.09%) died of the disease; she developed bone metastasis and lymphadenopathy and died of the disease 14 months after the initial therapy.
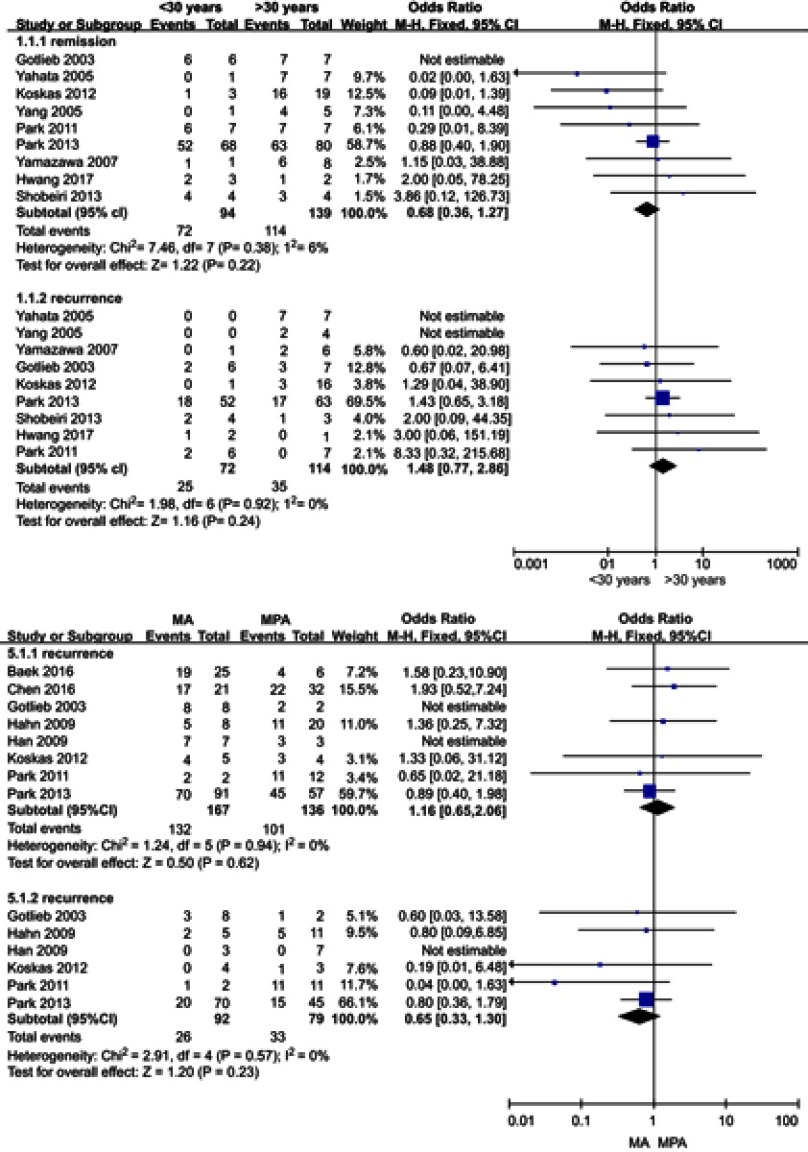
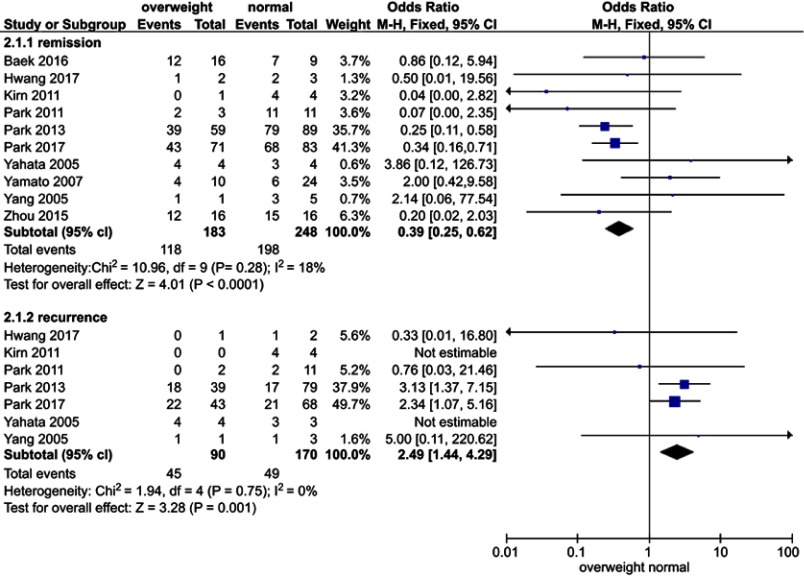
The potential predictors affecting the patients’ response to progestin therapy, including age, BMI, PCOS, type of hormone agent, and histology type (CAH or EC), were pooled for a meta-analysis (Figure 3). No substantial heterogeneity was observed in each analysis. The I2 values of each analysis were all less than 50% (6%, 18%, 0%, 0%, and 31%). Thus, a fixed-effects model was applied. According to the analysis, compared with a BMI<25 kg/m2 and CAH (P=0.0000, OR, 0.3; 95% confidence interval, 0.2–0.6), a BMI≥25 kg/m2 (P=0.0004, OR, 0.4; 95% confidence interval, 0.3–0.6) and EC were significantly associated with a higher likelihood of achieving CR. In contrast, age (P=0.3119), PCOS (P=0.2259), and hormonal agents (P=0.3265) had no impact on CR (Table 3).
Figure 3.

(Continued).
Figure 3.
(Continued).
Figure 3.
The potential predictors of patients’ responses to progestin therapy and recurrence Figure 3, including age, BMI, PCOS, type of hormonal agent used, and histology type (CAH or EC), were pooled for a meta-analysis. No substantial heterogeneity was found in any analysis of the patients’ response to progestin therapy. The I2 values in each analysis were all less than 50% (6%, 18%, 0%, 0%, and 31%). There was no substantial heterogeneity in any analysis of recurrence. The I2 values in all analyses were equal to zero.Abbreviation: PCOS, polycystic ovarian syndrome.
Table 3.
Risk factors and risk of bias for complete response and recurrence of CAH/ EC patients
| Risk factor | Complete Response | P valuea | P (Egger’s test) | Recurrence | Recurrence rate | P valuea | P (Egger’s test) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||||||
| Age | ≤30 years | 72 | 22 | 0.3119 | 0.301 | 25 | 47 | 34.7% | 0.5678 | 0.715 |
| >30 years | 114 | 25 | 35 | 79 | 30.7% | |||||
| BMI | Normal | 198 | 50 | 0.0004 | 0.533 | 49 | 121 | 28.8% | 0.0007 | 0.311 |
| Overweight | 118 | 65 | 45 | 45 | 50.0% | |||||
| PCOS | Yes | 173 | 60 | 0.2259 | 0.526 | 21 | 15 | 58.3% | 0.0006 | 0.282 |
| No | 51 | 25 | 30 | 98 | 23.4% | |||||
| Hormonal agents | MA | 132 | 35 | 0.3265 | 0.531 | 26 | 66 | 28.3% | 0.0639 | 0.152 |
| MPA | 101 | 35 | 33 | 46 | 41.8% | |||||
| Histology type | CAH | 192 | 37 | 0.0000 | 0.443 | 34 | 137 | 19.9% | 0.0344 | 0.133 |
| EC | 145 | 77 | 36 | 81 | 30.8% | |||||
Note: aPearson chi-square test.
Abbreviation: PCOS, polycystic ovarian syndrome.
The potential risk factors associated with recurrence, including age, BMI, PCOS, type of hormone agent, and histology type (CAH or EC), were also pooled for a meta-analysis Figure 3. There was no substantial heterogeneity in any analysis. The I2 values in each analysis were all equal to zero. Thus, a fixed-effects model was applied. Patients with a BMI≥25 kg/m2 (P=0.0007, OR, 2.5; 95% confidence interval, 1.4–4.3), PCOS (P=0.0006, OR, 3.4; 95% confidence interval, 1.5–7.9), and EC (P=0.0344, OR, 2.8; 95% confidence interval, 1.4–5.3) had a significantly higher risk of developing recurrence. Age (P=0.5678) and type of hormonal agent (P=0.0639) were not identified as risk factors of recurrence (Table 3).
Publication bias
According to assessments based on Egger’s test, there was no significant publication bias in the articles included in meta-analysis. The funnel diagrams with insignificant asymmetry are shown in Figure 4.
Discussion
As women increasingly choose to delay childbearing, young women diagnosed with CAH or well-differentiated early-stage EC often wish to maintain fertility. In general, patients who undergo fertility-sparing treatment with progestins have a good prognosis. In this analysis, the CR rate was 75.8%, and the median time to CR with first-line progestin therapy was 6 (range: 1–74) months. The OS rate was as high as 99.8%. Studies 43–46 in the literature have also demonstrated that progestin treatment is associated with a high response rate and a favorable clinical outcome. However, the recurrence risk associated with progestin treatment is not insignificant. Based on our data, the recurrence rate is 30.4%, which is within the range of rates reported in the literature (30.7%–50%).47–49 Therefore, exploring the prognostic predictors in CAH/EC patients who received fertility preservation is important for improving the clinical management of this patient group.
Obesity has been noted to have a linear relationship with all cancer types.50,51 An increased BMI and obesity are strongly associated with the incidence and mortality of EC.50 Young patients with CAH or EC frequently have a history of obesity, which is usually associated with prolonged, unopposed estrogen exposure, accounting for the increased risk factor of EC in obese women.52 This analysis showed that overweight or obese patients had a higher likelihood of recurrence and a lower likelihood of complete remission with progestin treatment, which is consistent with Koskas’s study.15 In the normal premenstrual endometrium, progesterone counters estrogen-driven proliferation and induces glandular differentiation and decidualization in the endometrial stroma.52 In the absence of progestin, the endometrium continuously proliferates, and the risk of EC increases. Therefore, obese patients may have prolonged estrogen exposure after progestin treatment, likely increasing the probability of disease recurrence. Courneya et.al53 found a general negative linear association between BMI and quality of life (QoL) in EC survivors. The QoL became progressively worse as the BMI increased from a normal weight to very severe obesity. Arem et.al54 evaluated the relationship between obesity and EC survival based on twelve studies, four of which suggested that obesity is associated with worse survival among women with EC (risk range from 1.86–2.76) with a BMI≥40 kg/m2 compared with nonobese weight women. We demonstrated that weight control had a positive effect on the prognosis of obese patients. Weight control is also vital for patients who receive progestin treatment.
Obesity is present in approximately 30–70% of women with PCOS.55 Women with PCOS have a three- to five-fold increased risk of EC.56 Patients with PCOS exhibit hyperandrogenism, chronic anovulation, and infertility.57 Chronic anovulation is a major risk factor for premenopausal EC and/or CAH.58 The results of this study indicate that patients with PCOS are more likely to develop relapse. Weight control is beneficial for PCOS patients to increase ovulation and decrease the risk of recurrence.
No progesterone treatment regimen has been established. MPA and MA are the most commonly used progestins in fertility-sparing treatment as described in this analysis. The potency of these two drugs (in terms of endometrial response) has been reported to be similar.59,60 The relative bioavailability of MA via the oral pathway is significantly higher than that of MPA.61 A meta-analysis also showed15 that the use of MA was associated with a higher response probability. The adverse effects associated with progestins were moderated, and no treatment-related deaths were identified in our review. Progestin treatment was well tolerated. However, its optimal regimen and duration require further evaluation.
The pregnancy and live birth rates in patients with EC after fertility-preserving treatment are also clinical concerns. Gallos et.al43 described live birth rates of 28% in EC patients and 26.3% in CAH patients, which are slightly higher than the rates (20.5%) found in this analysis. Assisted reproduction treatment had a higher success rate than spontaneous conception (39.4% VS 14.9%). A significant proportion of EC and CAH patients are obese and have anovulatory cycles with a history of infertility.62 The implementation of in vitro fertilization techniques not only increases the chance of conception but may also decrease the time to conception.63
After completing pregnancy, patients should be followed closely. The tissue biopsy methods used to diagnose endometrial lesions include endometrial aspiration, dilatation and curettage (D & C), and hysteroscopic biopsy. Endometrial aspiration biopsy is an easy, safe and cost-effective method that has been reported to be comparable to D&C in the diagnosis of endometrial hyperplasia and EC.64,65 However, Kim and colleagues 66 found that endometrial aspiration biopsy had a lower diagnostic accuracy (diagnostic concordance, 39.3%) than D&C. Gunderson et.al43 suggested recommending hysterectomy tor patients who had given birth or had persistent infertility to reduce the recurrence risk. EC patients who desire fertility preservation should be fully informed of the risk of recurrence and followed closely.
There are certain limitations in our review, First, most included studies were retrospective. No randomized control trials (RCT) focusing on fertility-sparing treatment for CAH and EC patients are available in the literature. The results of this analysis necessitate further evaluation. Second, the incomplete retrieval of identified research may result in the bias of our results. Although the possibility of publication and selection bias could not be excluded, no obvious bias was detected by the funnel plots.
In conclusion, patients with CAH or early-stage EC who were treated with progesterone therapy had a favorable prognosis. However, the recurrence risk was not insignificant. Weight control is crucial for improving the clinical management of this patient group. The optimal regimen and duration of progestin treatment require further evaluation.
Acknowledgment
This study was funded by the Clinical Medicine Foundation of Beijing Hospital Administration (grant number: ZYLX201713).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Evans-Metcalf ER, Brooks SE, Reale FR, Baker SP. Profile of women 45 years of age and younger with endometrial cancer. Obstet Gynecol. 1998;91(3):349–354. [DOI] [PubMed] [Google Scholar]
- 3.Lee TS, Jung JY, Kim JW, et al. Feasibility of ovarian preservation in patients with early stage endometrial carcinoma. Gynecol Oncol. 2007;104(1):52–57. doi: 10.1016/j.ygyno.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 4.Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001;83(2):388–393. doi: 10.1006/gyno.2001.6434 [DOI] [PubMed] [Google Scholar]
- 5.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64(3):417–420. [PubMed] [Google Scholar]
- 6.Soliman PT, Oh JC, Schmeler KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105(3):575–580. doi: 10.1097/01.AOG.0000154151.14516.f7 [DOI] [PubMed] [Google Scholar]
- 7.Gressel G, Parkash V, Pal L. Management options and fertility-preserving therapy for premenopausal endometrial hyperplasia and early-stage endometrial cancer. Int J Gynaecol Obstet. 2015;131(3):234–239. doi: 10.1016/j.ijgo.2015.06.031 [DOI] [PubMed] [Google Scholar]
- 8.Kalogiannidis I, Agorastos T. Conservative management of young patients with endometrial highly-differentiated adenocarcinoma. J Obstet Gynaecol. 2011;31(1):13–17. doi: 10.3109/01443615.2010.532249 [DOI] [PubMed] [Google Scholar]
- 9.Creasman W, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035–2041. [DOI] [PubMed] [Google Scholar]
- 10.Boronow R, Morrow CP, Creasman WT, et al. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63(6):825–832. [PubMed] [Google Scholar]
- 11.Park J-Y, Kim D-Y, Kim J-H, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur J Cancer. 2013;49(4):868–874. doi: 10.1016/j.ejca.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 12.Kudesia R, Singer T, Caputo TA, et al. Reproductive and oncologic outcomes after progestin therapy for endometrial complex atypical hyperplasia or carcinoma. Am J Obstet Gynecol. 2014;210(3):255.e1–4. doi: 10.1016/j.ajog.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Pronin SM, Novikova OV, Andreeva JY, Novikova EG. Fertility-Sparing Treatment of Early Endometrial Cancer and Complex Atypical Hyperplasia in Young Women of Childbearing Potential. Int J Gynecol Cancer. 2015;25(6):1010–1014. doi: 10.1097/IGC.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara H, Jobo T, Takei Y, et al. Fertility-sparing treatment using medroxyprogesterone acetate for endometrial carcinoma. Oncol Lett. 2012;3(5):1002–1006. doi: 10.3892/ol.2012.602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koskas M, Uzan J, Luton D, Rouzier R, Daraï E. Prognostic factors of oncologic and reproductive outcomes in fertility-sparing management of endometrial atypical hyperplasia and adenocarcinoma: systematic review and meta-analysis. Fertil Steril. 2014;101(3):785–794. doi: 10.1016/j.fertnstert.2013.11.028 [DOI] [PubMed] [Google Scholar]
- 16.Ushijima K, Yahata H, Yoshikawa H, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25(19):2798–2803. doi: 10.1200/JCO.2006.08.8344 [DOI] [PubMed] [Google Scholar]
- 17.Kim MK, Yoon BS, Park H, et al. Conservative treatment with medroxyprogesterone acetate plus levonorgestrel intrauterine system for early-stage endometrial cancer in young women: pilot study. Int J Gynecol Cancer. 2011;21(4):673–677. doi: 10.1111/IGC.0b013e3181fd9a06 [DOI] [PubMed] [Google Scholar]
- 18.Tamauchi S, Kajiyama H, Utsumi F, et al. Efficacy of medroxyprogesterone acetate treatment and retreatment for atypical endometrial hyperplasia and endometrial cancer. J Obstet Gynaecol Res. 2018;44(1):151–156. doi: 10.1111/jog.13473 [DOI] [PubMed] [Google Scholar]
- 19.Fukui Y, Taguchi A, Adachi K, et al. Polycystic ovarian morphology may be a positive prognostic factor in patients with endometrial cancer who achieved complete remission after fertility-sparing therapy with progestin. Asian Pac J Cancer Prev. 2017;18(11):3111–3116. doi: 10.22034/APJCP.2017.18.11.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang JY, Kim DH, Bae HS, et al. Combined oral medroxyprogesterone/levonorgestrel-intrauterine system treatment for women with grade 2 stage IA endometrial cancer. Int J Gynecol Cancer. 2017;27(4):738–742. doi: 10.1097/IGC.0000000000000927 [DOI] [PubMed] [Google Scholar]
- 21.Park J-Y, Seong SJ, Kim T-J, Kim JW, Bae D-S, Nam J-H. Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol Oncol. 2017;146(1):39–43. doi: 10.1016/j.ygyno.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Kim SR, van der Zanden C, Ikiz H, Kuzelijevic B, Havelock J, Kwon JS. Fertility-sparing management using progestin for young women with endometrial cancer from a population-based study. J Obstet Gynaecol Can. 2018;40(3):328–333. doi: 10.1016/j.jogc.2017.06.037 [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Jin Y, Li Y, Bi Y, Shan Y, Pan L. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynaecol Obstet. 2016;132(1):34–38. doi: 10.1016/j.ijgo.2015.06.046 [DOI] [PubMed] [Google Scholar]
- 24.Baek JS, Lee WH, Kang WD, Kim SM. Fertility-preserving treatment in complex atypical hyperplasia and early endometrial cancer in young women with oral progestin: is it effective? Obstet Gynecol Sci. 2016;59(1):24–31. doi: 10.5468/ogs.2016.59.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou R, Yang Y, Lu Q, et al. Prognostic factors of oncological and reproductive outcomes in fertility-sparing treatment of complex atypical hyperplasia and low-grade endometrial cancer using oral progestin in Chinese patients. Gynecol Oncol. 2015;139(3):424–428. doi: 10.1016/j.ygyno.2015.09.078 [DOI] [PubMed] [Google Scholar]
- 26.Mitsuhashi A, Sato Y, Kiyokawa T, Koshizaka M, Hanaoka H, Shozu M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann Oncol. 2016;27(2):262–266. doi: 10.1093/annonc/mdv539 [DOI] [PubMed] [Google Scholar]
- 27.Simpson AN, Feigenberg T, Clarke BA, et al. Fertility sparing treatment of complex atypical hyperplasia and low grade endometrial cancer using oral progestin. Gynecol Oncol. 2014;133(2):229–233. doi: 10.1016/j.ygyno.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 28.Gonthier C, Walker F, Luton D, Yazbeck C, Madelenat P, Koskas M. Impact of obesity on the results of fertility-sparing management for atypical hyperplasia and grade 1 endometrial cancer. Gynecol Oncol. 2014;133(1):33–37. doi: 10.1016/j.ygyno.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 29.Jafari Shobeiri, M, Gharabaghi, PM, Esmaeili, H, et al. Fertility sparing treatment in young patients with early endometrial adenocarcinoma. Pak J Med Sci. 2013;29(2):651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koskas M, Azria E, Walker F, Luton D, Madelenat P, Yazbeck C. Progestin treatment of atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium to preserve fertility. Anticancer Res. 2012;32(3):1037–1043. [PubMed] [Google Scholar]
- 31.Ricciardi E, Maniglio P, Frega A, Marci R, Caserta D, Moscarini M. Fertility-sparing treatment of endometrial cancer precursors among young women: a reproductive point of view. Eur Rev Med Pharmacol Sci. 2012;16(14):1934–1937. [PubMed] [Google Scholar]
- 32.Perri T, Korach J, Gotlieb WH, et al. Prolonged conservative treatment of endometrial cancer patients: more than 1 pregnancy can be achieved. Int J Gynecol Cancer. 2011;21(1):72–78. doi: 10.1097/IGC.0b013e31820003de [DOI] [PubMed] [Google Scholar]
- 33.Park H, Seok JM, Yoon BS, et al. Effectiveness of high-dose progestin and long-term outcomes in young women with early-stage, well-differentiated endometrioid adenocarcinoma of uterine endometrium. Arch Gynecol Obstet. 2012;285(2):473–478. doi: 10.1007/s00404-011-1959-x [DOI] [PubMed] [Google Scholar]
- 34.Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011;22(3):643–649. doi: 10.1093/annonc/mdq463 [DOI] [PubMed] [Google Scholar]
- 35.Yu M, Yang J-X, Wu M, Lang J-H, Huo Z, Shen K. Fertility-preserving treatment in young women with well-differentiated endometrial carcinoma and severe atypical hyperplasia of endometrium. Fertil Steril. 2009;92(6):2122–2124. doi: 10.1016/j.fertnstert.2009.06.013 [DOI] [PubMed] [Google Scholar]
- 36.Han AR, Kwon Y-S, Kim DY, et al. Pregnancy outcomes using assisted reproductive technology after fertility-preserving therapy in patients with endometrial adenocarcinoma or atypical complex hyperplasia. Int J Gynecol Cancer. 2009;19(1):147–151. doi: 10.1111/IGC.0b013e31819960ba [DOI] [PubMed] [Google Scholar]
- 37.Hahn HS, Yoon SG, Hong JS, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. 2009;19(6):1068–1073. doi: 10.1111/IGC.0b013e3181aae1fb [DOI] [PubMed] [Google Scholar]
- 38.Signorelli M, Caspani G, Bonazzi C, Chiappa V, Perego P, Mangioni C. Fertility-sparing treatment in young women with endometrial cancer or atypical complex hyperplasia: a prospective single-institution experience of 21 cases. BJOG. 2009;116(1):114–118. doi: 10.1111/j.1471-0528.2008.02024.x [DOI] [PubMed] [Google Scholar]
- 39.Yamazawa K, Hirai M, Fujito A, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22(7):1953–1958. doi: 10.1093/humrep/dem088 [DOI] [PubMed] [Google Scholar]
- 40.Yang Y-C, Wu -C-C, Chen C-P, Chang C-L, Wang K-L. Reevaluating the safety of fertility-sparing hormonal therapy for early endometrial cancer. Gynecol Oncol. 2005;99(2):287–293. doi: 10.1016/j.ygyno.2005.06.018 [DOI] [PubMed] [Google Scholar]
- 41.Yahata T, Fujita K, Aoki Y, Tanaka K. Long-term conservative therapy for endometrial adenocarcinoma in young women. Hum Reprod. 2006;21(4):1070–1075. doi: 10.1093/humrep/dei434 [DOI] [PubMed] [Google Scholar]
- 42.Gotlieb W. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet Gynecol. 2003;102(4):718–725. [DOI] [PubMed] [Google Scholar]
- 43.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207(4):266 e1–12. doi: 10.1016/j.ajog.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 44.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125(2):477–482. doi: 10.1016/j.ygyno.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 45.Minig L, Franchi D, Valero de Bernabé J, Sideri M. Controversies of the hormonal conservative treatment of endometrial cancer. Gynecol Obstet Invest. 2013;75(3):145–151. doi: 10.1159/000349891 [DOI] [PubMed] [Google Scholar]
- 46.Park JY, Nam JH. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist. 2015;20(3):270–278. doi: 10.1634/theoncologist.2013-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erkanli S, Ayhan A. Fertility-Sparing Therapy in Young Women With Endometrial Cancer. Intl J Gynecol Cancer. 2010;20(7):1170–1187. [DOI] [PubMed] [Google Scholar]
- 48.Feichtinger M, Rodriguez-Wallberg KA. Fertility preservation in women with cervical, endometrial or ovarian cancers. Gynecol Oncol Res Pract. 2016;3:8. doi: 10.1186/s40661-016-0029-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan Z, Li H, Hu R, Liu Y, Liu X, Gu L. Fertility-Preserving treatment in young women with grade 1 presumed stage IA endometrial adenocarcinoma: a meta-analysis. Int J Gynecol Cancer. 2018;28(2):385–393. doi: 10.1097/IGC.0000000000001164 [DOI] [PubMed] [Google Scholar]
- 50.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bessonova L, Marshall SF, Ziogas A, et al. The association of body mass index with mortality in the California Teachers Study. Int J Cancer. 2011;129(10):2492–2501. doi: 10.1002/ijc.25905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011;205(6):518–525. doi: 10.1016/j.ajog.2011.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courneya K, Karvinen KH, Campbell KL, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97(2):422–430. doi: 10.1016/j.ygyno.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 54.Arem H, Irwin ML. Obesity and endometrial cancer survival: a systematic review. Int J Obes (Lond). 2013;37(5):634–639. doi: 10.1038/ijo.2012.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vrbikova J, Hainer V. Obesity and polycystic ovary syndrome. Obes Facts. 2009;2(1):26–35. doi: 10.1159/000194971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piltonen TT. Polycystic ovary syndrome: Endometrial markers. Best Pract Res Clin Obstet Gynaecol. 2016;37:66–79. doi: 10.1016/j.bpobgyn.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 57.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853–861. doi: 10.1056/NEJM199509283331307 [DOI] [PubMed] [Google Scholar]
- 58.Okamura Y, Saito F, Takaishi K, et al. Polycystic ovary syndrome: early diagnosis and intervention are necessary for fertility preservation in young women with endometrial cancer under 35 years of age. Reprod Med Biol. 2017;16(1):67–71. doi: 10.1002/rmb2.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000;65(10–11):629–636. [DOI] [PubMed] [Google Scholar]
- 60.Schindler A, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. [DOI] [PubMed] [Google Scholar]
- 61.Adlercreutz H, Eriksen P, Christensen M. Plasma concentrations of megestrol acetate and medroxyprogesterone acetate after single oral administration to healthy subjects. J Pharm Biomed Anal. 1983;1(2):153–162. [DOI] [PubMed] [Google Scholar]
- 62.Schmeler, KM, Soliman, PT, Sun, CC, et al. Endometrial cancer in young, normal-weight women. Gynecol Oncol. 2005;99(2):388–392. [DOI] [PubMed] [Google Scholar]
- 63.Gadducci A, Spirito N, Baroni E, Tana R, Genazzani AR. The fertility-sparing treatment in patients with endometrial atypical hyperplasia and early endometrial cancer: a debated therapeutic option. Gynecol Endocrinol. 2009;25(10):683–691. doi: 10.1080/09513590902733733 [DOI] [PubMed] [Google Scholar]
- 64.Dijkhuizen FP, Mol BW, Brölmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000;89(8):1765–1772. [PubMed] [Google Scholar]
- 65.Stovall TG, Photopulos GJ, Poston WM, Ling FW, Sandles LG. Pipelle endometrial sampling in patients with known endometrial carcinoma. Obstet Gynecol. 1991;77(6):954–956. [PubMed] [Google Scholar]
- 66.Kim DH, Seong SJ, Kim MK, et al. Dilatation and curettage is more accurate than endometrial aspiration biopsy in early-stage endometrial cancer patients treated with high dose oral progestin and levonorgestrel intrauterine system. J Gynecol Oncol. 2017;28(1):e1. doi: 10.3802/jgo.2017.28.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]