Abstract
The implementation of nanotechnology in medicine has opened new research horizons particularly in the field of therapeutic delivery. Mesoporous silica particles have emerged as biocompatible drug delivery systems with an enormous potential in the treatment of cancer among many other pathologies. In this review, we focus on the unique properties of these particles as chemotherapy delivery carriers. Here, we summarize the general characteristics of these nanomaterials – including their physicochemical properties and customizable surfaces – different stimuli that can be used to trigger targeted drug release, biocompatibility and finally, the drawbacks of these types of nanomaterials, highlighting some of the most important features of mesoporous silica nanoparticles in drug delivery.
Keywords: nanocarrier, drug release, targeted drug delivery, biocompatibility, biodegradability, tumor
Introduction
Chemotherapy, tog ether with surgery, are the most used cancer treatments in oncology. Unfortunately, chemotherapeutic agents are applied systemically destroying both tumor and healthy cells and resulting in many of undesirable side effects.1 Encapsulated drug delivery systems offer the possibility to target therapies locally at adequate concentrations, maximizing the effect against cancer cells while reducing the side effects and cytotoxicity in healthy cells.2 In this sense, nanotechnology can help with the design of target-specific and controlled delivery systems, capable of transporting enough therapies to specific cells, releasing the drug in a controlled manner.2
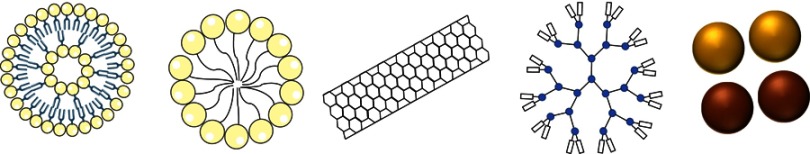
Different types of nanomaterials have been used as targeted carriers. Among others, the most employed are liposomes,3,4 polymeric micelles,5,6 carbon nanotubes,7 dendrimers,8–10 inorganic particles11 and silica-based materials12,13 (Figure 1). Recently, mesoporous silica particles (MSPs) have attracted much attention due to their singular properties.14 Here we discuss some of their characteristics and advantages in cancer drug delivery.15
Figure 1.
Schematic representation of different delivery systems. From left to right; liposomes, micelles, carbon nanotubes, dendrimer and gold (yellow) and iron (brown) nanoparticles.
Physicochemical properties of MSPs
MSPs have a well-defined internal mesopore structure (from 2 to 10 nm of diameter) with a large pore volume (0.6−1 cm3/g) and a high surface area (700−1,000 m2/g). Their size, nano- (50 nm) to submicron-scale (500 nm),16 as well as their shape17 and surface18 can be custom-designed offering many different possibilities for the loading of anticancer drugs such as docetaxel,19 paclitaxel20 or doxorubicin,21 among many others. Moreover, the cytotoxicity of these particles and cellular uptake have been demonstrated to be dependent on nanoparticle size and surface charge. Indeed, 15 nm diameter particles have been reported to trigger more cytotoxicity than 100 nm diameter particles in endothelial cells.22 Lu and collaborators showed that 50 nm diameter particles are the optimal for cellular uptake.23 When considering the particle surface charge, cationic silica particles appear to be more cytotoxic and have a faster cellular uptake than anionic or neutral silica particles.24,25 Davila-Ibáñez and co-workers used magnetic silica nanoparticles with DNA attached to the silica network to show how charges at the surface of the nanoparticles is a key issue to guarantee the cellular uptake.26,27 On the other hand, particles with a neutral charge do not appear to internalize the cell membrane of Caco-2 cells.
Targeting the cell/tissue of interest
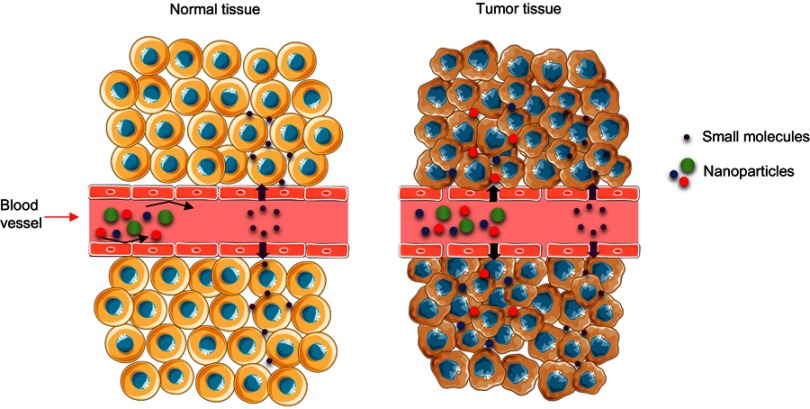
One of the most important goals to achieve in drug delivery is the possibility of targeting nanoparticles to a specific cell or tissue. In this regard, most nanomaterials including MSPs, have been reported to passively target solid tumors. Typically, when a tumor reaches a certain size, the normal vasculature present in the tumoral organ cannot irrigate all the cellular mass. This effect generates intra-tumoral hypoxia triggering the segregation of growth factors that activate the rapid sprouting of new blood vessels from the surrounding capillaries.28 This process known as angiogenesis generates irregular blood vessels displaying a discontinuous epithelium with an absent basal membrane.29 When blood components reach these abnormal and discontinuous vascular networks, the fenestrations between the endothelial cells offer little resistance to the extravasation of nanomaterials inside of the tumor.30 Particles/molecules smaller than 4 nm diffuse through the capillary endothelium back to the blood circulation and are reabsorbed,31 but macromolecules and nanomaterials do not naturally return to the blood vessels, accumulating in the perivascular tumoral space. In the nanomedicine field, this phenomenon is known as the Enhanced Permeability and Retention effect or “EPR” effect (Figure 2). The study carried out by Lee and co-workers, showed how MSPs decorated with multiple magnetite nanocrystals loaded with Doxorubicin (DOX), induced efficient cell death in a melanoma model, confirming in vivo passive targeting and accumulation of the nanoparticles in the tumor site.32 Huan and colleagues used MSPs functionalized with polyethyleneimine/polyethylene glycol (PEI/PEG) to carry doxorubicin together with P-glycoprotein siRNA. Their study demonstrated that these particles were effectively biodistributed, achieving an 8% of the enhanced permeability and retention effect at the tumor site in vivo.33 But there are many more examples in the literature.
Figure 2.
Image representing the blood transport mechanism of nanomaterials or molecules from normal tissue (left) and the enhanced permeability and retention effect in a tumor.
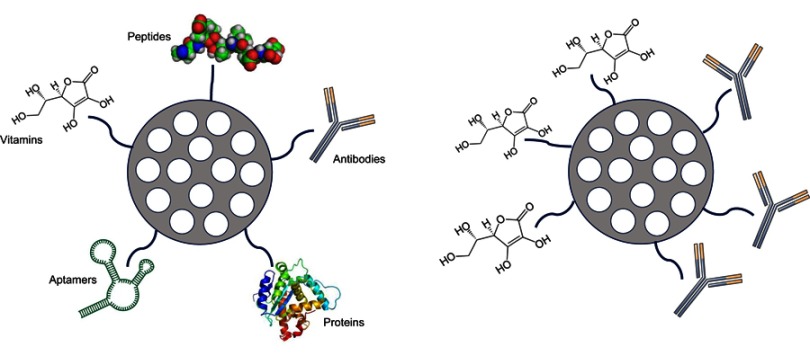
MSPs can also be functionalized to actively target tumors. One of the strategies used to reach this goal consists in the attachment of different ligand molecules – such as peptides, antibodies,34 aptamers,35 growth factors,36 vitamins, etc. – on their surface, so the particles interact with receptors on the cellular surface (Figure 3).37 This way, the entry mechanism of the nanodevice will be via receptor-mediated endocytosis, and the particle will be captured inside the endosomal membranes.37 In the study carried out by Kayuan and colleagues, HB5 aptamer-functionalized mesoporous silica-carbon-based DOX-loaded systems (MSCN-PEG-HB5/DOX) were used in vitro for chemo-photothermal combined therapy in Human Epithelial growth factor Receptor 2 (HER2)-positive breast cancer cells. This study demonstrated how HER2-positive breast cancer cells uptake these particles with more avidity than normal breast epithelial cells (MCF-10A). Additionally, cytotoxicity experiments demonstrated that combined therapy induces highest cell killing effect compared to chemotherapy and photothermal therapy by itself.38 Jianbin and colleagues showed how MSPs of 40 nm size, loaded with DOX and functionalized with selective αv-β3 integrin ligands on their surface displayed an enhanced targeting effect through the blood–brain barrier, penetrating glioblastoma cells. In summary, these targeted particles rapidly invaded cancer cells, delivering the drug intracellularly and improving the anticancer activity of the free drug. MSPs achieve satisfactory anti-glioblastoma efficacy avoiding toxic side effects in the healthy brain tissue thus, demonstrating active cell targeting.39
Figure 3.
Schematic description of active targeting possibilities on mesoporous silica particles (left). Dual targeting example (right).
Controlling drug release: gatekeepers
Another challenge in the design of nanotransporters is delivering the drug at the precise moment when the carrier reaches the tumor, or alternatively, when a signal is provided. MSPs are useful carrier systems due to their high surface and tunable porous structure. Drugs can be loaded inside their mesopores through simple diffusion mechanism. But, one of the main advantages of MSPs is the possibility to design “zero release” nanosystems by blocking the MSP pores using gatekeepers.13,40
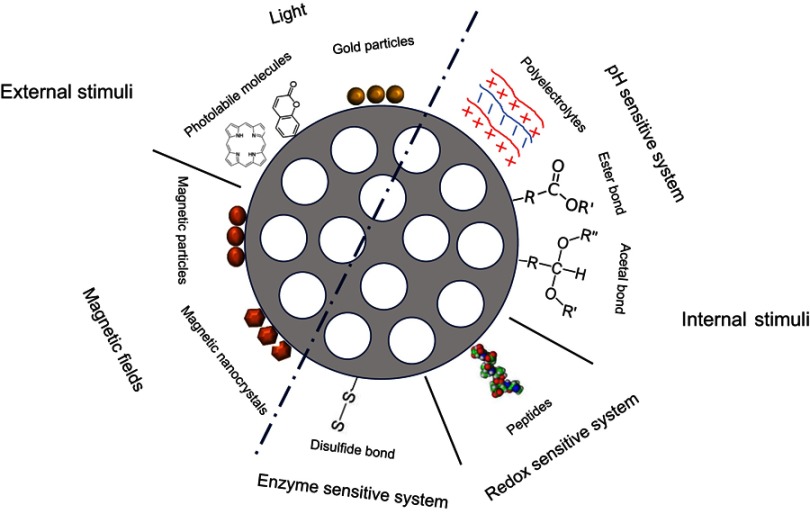
Once in the tumor, different internal or external stimuli can be employed to activate drug delivery. Some of the intra-tumoral stimuli used are the local pH conditions, the enzymes in the peritumoral tissue or the redox potential. In addition, external stimuli such as magnetic fields or light can also be applied to activate “on demand” drug release (Table 1, Figure 4).
Table 1.
Different gatekeepers that can be used to maintain the “zero release” of the drug and to trigger drug release
| External stimuli | Internal stimuli | |||||||
|---|---|---|---|---|---|---|---|---|
| Magnetic field | Light | pH Sensitive systems | Redox sensitive systems | Enzyme sensitive systems | ||||
| Magnetic particles | Magnetic nanocrystal | Gold nanoparticles | Photolabile molecules | Polyelectrolytes | Ester bond | Acetal bond | Peptides | Disulfude bond |
Figure 4.
Examples of different gatekeepers that can be used to maintain the “zero release” of the drug inside mesoporous silica particles and to trigger on demand the release.
Internal stimuli triggering drug delivery
The characteristics of the tumoral environment can help in the design of nanocarriers sensitive to internal or endogenous stimuli to ensure a controlled localized drug release.
pH-sensitive systems
One general feature of solid tumors is the presence of acidity in the tumor environment due to the “Warburg effect”. Healthy cells use the mitochondrial oxidative phosphorylation to produce energy. However, most cancer cells activate the glycolytic route.41,42 This process, also known as anaerobic glycolysis, is less efficient in terms of energy (adenosine triphosphate) production and increases the generation of additional metabolites – mostly lactic acid – generating local tumoral acidosis which can be beneficial for tumor proliferating cells.41,42
An effective strategy to control drug release is blocking the MSP pores with noncovalently bonded pH sensible polymers. Different polymers can be selected so they detach from the particle at low pH, releasing the drug at the tumoral site. Among these systems, one of the most commonly used methods is based on polyelectrolyte multilayers. These gatekeepers are based on the layer-by-layer technique (Figure 5).43–45 The composition, thickness or the molecular organization of the layers46 and the permeability/elasticity of the polymers can be modified so the system can be easily “tuned”.47 Feng et al synthesized MSPs coated with multilayers of Poly(Allylamine Hydrochloride) (PAH) and Poly(Styrene Sulfonate) (PSS) polyelectrolytes, loaded with DOX inside the pores.48 In this study, they demonstrated that the delivery of the drug was both pH and layer thickness dependent, and that the layer thickness has an exponential relationship with the number of polymer coats applied. This study also demonstrated that i) the biodistribution of the drug in vivo was smaller in major organs compared to that of free DOX, and that ii) these particles had lower systemic toxicity than free DOX, thus, concluding that these MSP-based nanoparticles were a good carrier system with high efficiency and low systemic toxicity. Also, Sun et al used multilayer-coated MSPs to load cisplatin and Rhodamine B (RhB).49 The outer polyelectrolyte multilayer was assembled from the cationic polyelectrolyte PAH, and a second negatively charged polyelectrolyte, P(DMA-co-TPAMA), consisting of N,N-Dimethylacrylamide (DMA) and 3,4,5,6-Tetrahydrophthalic Anhydride functionalized N-(3-Aminopropyl) Methacrylamide (TPAMA) monomer units, that exhibited pH-induced charge conversion characteristics. This way, cisplatin and rhodamine B were released in the tumor microenvironment upon a pH reduction from 7.4 to 5–6, typical in malignant tumors. Other interesting gatekeeper systems are based on pH-sensitive linkers. These linkers are cleaved in acidic conditions, triggering the release of the cargo from the carrier. Acetal bonds,50–52 hydrazine bonds,53–55 hydrazone bonds56,57 or ester bonds58,59 are some examples that have been used worldwide. In a study carried out by Ze-Yong Li et al, DOX was conjugated to MSPs using hydrazine bonds.60 They proved that when the particles were in vitro incubated at pH 6.5, a fast DOX release occurred due to the hydrolysis of the bonds. Lee et al were able to attach DOX to the inner wall of MSPs and release this drug in the endolysosomes of cancer cells in the liver.61 The conjugation of the drug was done by hydrazone bonds that released the drug upon endo-lysosomal maturation when the pH of the vesicles decreased.
Enzyme-responsive systems
Figure 5.
Scheme of the layer by layer technique in mesoporous silica particles.
Compared to healthy tissues or cells, many different enzymes, mostly proteases, are overexpressed by cancer cells.62 This peculiarity can also be an interesting stimulus to trigger enzyme-mediated drug release.63 The development of enzyme-released drug delivery systems based on MSPs has caught much attention. Liu et al used in their study a Matrix-Metalloproteinase (MMPs) responsive drug delivery system based on MSPs to reduce in vivo side effects of traditional chemotherapies.64 MSPs were loaded with DOX and coated with bovine serum albumin as an end-cap to seal the mesopores of the nanoparticles, using lactobionic acid as the targeting motif. The in vivo experiments showed that the DOX delivery system could be used to inhibit tumoral growth in mice with minimal side effects.
Redox-sensitive systems
Glutathione (GSH) is the most abundant non-protein thiol that acts as a reducing agent maintaining enzymes in an active state. In cancer cells, the intracellular concentration of GSH is three times higher than in normal cells.65 Hence, this is a good tool to prompt the release of drugs. Disulfide bonds66-70 (S-S) can be easily cleaved in the presence of GSH for being a redox-sensitive group, so they can be used to form capped systems with nanoparticles71,72 or polymers69,73,74 for instance. Gong et al were able to synthesize MSPs functionalized with polyethylene glycol using a disulfide bond linker.74 These authors demonstrate drug release upon GSH rise, while low GSH concentrations blocked the release. Apart from PEG,75,76 poly N-acryloxysuccinimide77 has also been used as an efficient method to deliver hydrophilic drugs to cancer cells improving the efficacy of the therapy.
External stimuli for drug delivery
Magnetic fields and light are external stimuli also used to control gatekeepers. Although these stimuli are less popular than endogenous stimuli, they are more reproducible and do not depend on the heterogeneous physiological conditions of the tumoral environment. Besides, these systems can be more precise in local drug release, minimizing toxicity and side effects.78 The two main strategies of these drug delivery systems are based on magnetic fields and light.
Magnetic fields
These drug delivery systems are based on the use of magnetic fields as external stimuli to guide the particles to the tumor environment and to locally increase the temperature, triggering cell death by controlled drug release and/or hyperthermia.79 Superparamagnetic iron oxide nanoparticles are the most used magnetic nanoparticles. They exhibit an extraordinary capacity to convert magnetic energy into heat.80,81 This ability allows the use of thermo-sensitive materials as gatekeepers capping the surface of MSPs, provoking the opening of the pores and the release of a drug using magnetic fields.82,83 Baeza and colleagues used a nanodevice based on MSPs with iron oxide nanocrystals inside the silica matrix.83 This device was coated with a copolymer of Poly(Ethyleneimine)-b-Poly (N Isopropylacrylamide) (PEI/NIPAM), which acts as a temperature-sensitive gatekeeper and retains proteins into the polymer shell linked by electrostatic forces or hydrogen bonds. Once these nanodevices are administrated into cancer cells, an alternative magnetic field is applied. The results demonstrate that the polymer can act as a gatekeeper, opening or closing the pores of the silica matrix, controlling the release of the macromolecules attached to the polymer branches. Moreover, Thomas et al used in their study DOX-loaded MSPs combined with magnetic nanocrystals that have been surface-modified with pseudorotaxanes.84 After the application of a magnetic field, the nanocrystals generate heat, causing the disassembly of the pseudorotaxanes, triggering the release of DOX and consequently, a cytotoxic effect in breast cancer cells.
Light
Among the external stimulus, light is a rapid, non-invasive, clean and efficient stimulus that can be used to control drug delivery with high spatial and temporal resolution.85,86 Although most photoreactions used in drug delivery are induced by UV light,85,87,88 the best wavelengths for good tissue penetration are those in the close IR, between 800 and 1,100 nm, which correspond to the so-called “water biological window”.89 The mechanism of these types of carriers to trigger drug release is based on the photo-sensitiveness of the gatekeeper that changes conformation upon light application. Guardado-Alvarez et al used MSPs with photolabile coumarin-based molecules capping the surface, noncovalently conjugated β-cyclodextrin to block the pores and rhodamine B inside the pores.90 This way, 800-nm two-photon excitation triggered the release of the bond holding the coumarin to the nanopore releasing both the β-cyclodextrin cap and the cargo. Martínez-Carmona and colleagues carried another study in vitro using an MSP-based device with porphyrin-caps attached with reactive oxygen species (ROS)-cleavable linkages.91 These bonds are sensitive to singlet-oxygen produced after exposure to visible light, releasing the cargo (Topotecan). The oxygen molecules produced by the porphyrin–nanocaps break the sensitive-linker uncapping the pores and releasing the entrapped drug. These particles have been used in osteosarcoma cancer cells demonstrating a controlled release of Topotecan inside the tumor cells. Another light-sensitive gatekeeper type is based on gold nanoparticles (AuNPs). These particles, combined with MSPs, are attractive devices for cancer cell imaging92-94 and can also heat when irradiated with a laser producing a photothermal effect.93 As for the magnetic nanoparticles, the heat generated upon light exposure can be used to release the anti-cancer therapy and/or trigger drug release. Wang and colleagues, for instance, designed a therapeutic delivery system, based on MSPs closed by AuNPs with RhB as the cargo.95 This carrier was studied in vitro showing a good release of the RhB when temperature increased. In the study done by Vivero-Escoto et al AuNP-capped-MSPs were useful to release a chemotherapeutic such as paclitaxel in human fibroblast and liver cells.96 This release could be easily controlled by low-power photoirradiation under physiological conditions.
Concerning the use of internal and external stimuli, it is important to mention the combination of both types of stimuli, light and alternating magnetic fields, to generate heat, to induce local hyperthermia increasing the pH97 and the enzymatic activity98 of the cells. Hence, this type of structured nanomaterials can be used as an interesting system for programmed site-specific drug delivery.
Triggering endolysosomal escape
Upon receptor-mediated endocytosis, MSPs are incorporated inside the endolysosomal membranes. Many nanoparticles after intracellular transit are eventually expelled from the cells by exocytosis.99–101 Thus, to avoid therapy degradation in the lysosomal due to the hostile chemical conditions and/or, exocytosis, nanocarriers need to escape into the cytoplasm. Thus far, different strategies have been developed to trigger lysosomal escape among these, the proton sponge effect102 and destabilization of the endosomal membrane are the most used.103 The first mechanism is based on the swelling of the vesicle, and the second, in the creation of pores that enable therapeutic release into the cytoplasm.
The proton sponge effect
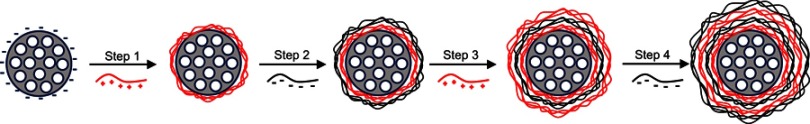
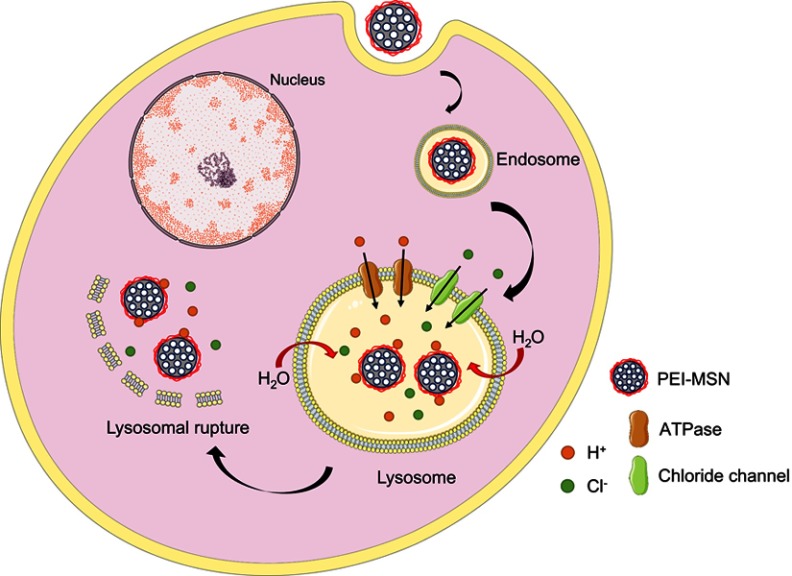
This effect relies on the rise of the proton concentration during hydrolysis that, in turn, causes an increase in the membrane potential, osmotic swelling and finally endo-lysosome bursting.104 This phenomenon occurs when polyplexes such as PEI or PAMAM are endocytosed. The amine groups of these molecules capture protons that accumulate in endosomes, gradually increasing the membrane potential and breaking the lysosomal membrane equilibrium. The diffusion of Cl− molecules into endosomes cause the increase of the osmotic pressure, swelling, expanding and finally tearing the lipid bilayer of the endolysosome, releasing the contents into the cytoplasm (Figure 6).102 The MSPs used by Wu et al could release siRNA and DOX into the cytoplasm of breast cancer cells in vitro and in vivo using a Poly-β-amino ester coating to provoke endolysosome bursting.105 The work carried out by Shen et al demonstrated that MSPs coated with PEI cannot only carry a siRNA but also deliver it to xenografted tumors, reducing the size of the tumoral mass.106
Figure 6.
Diagram of the proton sponge effect: particles coated with polyethyleneimine (PEI) are captured in the endolysosomal route. Lysosomal membranes tear apart, releasing the particles in the cytosol.
Abbreviation: PEI-MSN, mesoporous silica particles coated with PEI.
Destabilization of the endosomal membrane
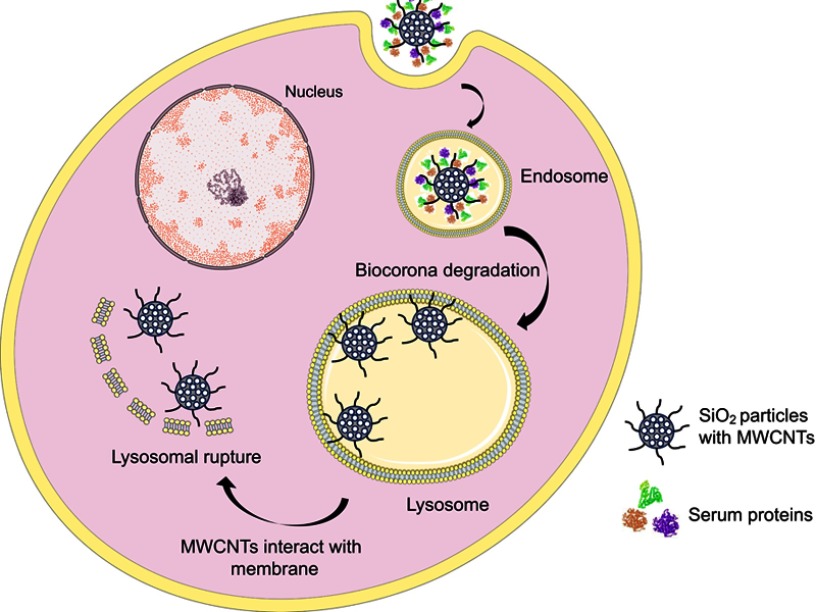
Other mechanisms to trigger particle endo-lysosomal escape are fusion lipids, cationic polymers, peptides107 or carbon nanotubes.103 In the study performed by Zhang et al, they synthesized polymer-lipid supported mesoporous silica nanoparticles (PLS-MSPs).108 These nanocarriers were able to release the anticancer drug (CPT -11) and maximize the effect of the treatment in MDR breast cancer cells. Apart from fusion lipids, many cell-penetrating peptides (fusogenic peptides) are being used based on bacterial or viral proteins. These peptides trigger vacuole-based endocytosis and/or to create discontinuities or pores on the cell membrane.109,110 For instance, Li and colleagues used MSPs coated with PEI and a fusogenic peptide to deliver siRNA to a tumor model showing an inhibition of the tumoral cell proliferation.111 Likewise, in some of our studies, we show how silica nanoparticles, when coated with multi-walled carbon nanotubes (MWCNT), can escape the endo-lysosomal route mimicking the viral spike fusion in lysosomes. The hypothesis is that proteins functionalizing the MWCNT surface are degraded in endolysosomes, exposing the surface of the nanotubes that are highly reactive and apolar. These stripped filaments now interact with the membrane of the endo-lysosomal vesicles, piercing and tearing it apart, triggering particle release into the cytoplasm (Figure 7).103
Figure 7.
Diagram of how mesoporous SiO2 particles with a multi-walled carbon nanotubes (MWCNT) coating, scape the endolysosomal route. When proteins of the biocorona are degraded, apolar MWCNTs interact with the membrane and help particles escape these vesicles.
Biocompatibility
Last, but not least, one of the most important features of MSPs is their biocompatibility. Different studies have demonstrated that silica nanoparticles are not toxic when administrated to different cell types at different dosages.99,112–114 Furthermore, there are several reports demonstrating that MSPs are degradable in water and in phosphate buffer saline.13,14 There are different parameters that can trigger MSPs in vitro degradation including i) particle morphology,115 ii) surface area116 and iii) surface functionalization117,118 among others. For instance, spherical particles are more degradable than with rod-shaped particles.115 Similarly, particles that have a high surface area are more degradable.116 Interestingly, the MSPs size is apparently not all that important in degradation in water or simulated body fluids.119,120 Moreover, MSP and their fragments have also been reported to be eliminated by renal clearance, in urine, and/or feces.113,121,122 Interestingly, positively charged MSPs are cleared faster than particles with a negative ζ potential. Also, PEGylated MSPs show a higher in vivo circulation time, since PEG avoids macrophage recognition and phagocytosis in the liver and spleen. Other studies are now developing to improve the interactions of nanomaterials with blood components. For instance, Roggers et al have demonstrated that the functionalization of MSPs with different lipids can be used to imitate red blood cell lipid membranes, improving their hemo-biocompatibility.121
Drawbacks of MSPs
Ideally, nanoparticles need i) to be stable, ii) to have a high loading capacity, iii) to be reproducible and iv) scalable in production. Reproducible MSPs synthesis is reasonably feasible when working at small scale, but the scaling up is not trivial therefore, reproducibility at industrial scale must be critically considered. Regarding the loading capacity of MSPs, not all drugs can be incorporated at an adequate concentration, and this critically influences the total concentration of nanoparticles that should be administrated to obtain an effective therapeutic effect. For instance, the tolerated dose of uncoated MSPs in murine models is ca. 50 mg/kg, but the human tolerance is so far unknown and needs to be evaluated.122 Also, most biodistribution and excretion studies have been performed in mice123 and must be reproduced in humans to understand the immune response and possible side-effects of these nanomaterials.
Another important point regarding the use of MSPs in clinical trials is the fact that the Food and Drug Administration and the European Medicines Agency must evaluate drug delivery nanocarriers before bench-to-bed translation, even if loaded with drugs already approved for clinical use. This is a slow procedure that significantly delays all new developments in nanodelivery. Hopefully, soon new requirements will be developed to accelerate the translation from research to the clinic.
Conclusion
The field of nanotechnology is gaining a high interest in cancer medicine. MSPs can be customized on demand in order to engineer nanocarriers that can i) target cells specifically, ii) release drugs inside the desired tissues/cells reducing the side effects of the treatment, iii) invade the cytoplasm by scaping the endo-lysosomal membrane, so that the cargo is preserved and finally iv) be biodegraded or cleared from the organism to minimize toxicity. Although MSPs are being widely studied as nanocarrier systems in animal models to ensure they are safe, more research is needed in the field of nanodelivery in cancer.
Acknowledgments
This work has been supported by the Spanish MINECO and European Union FEDER Funds under Project Ref. PI16/00496 (AES 2016), CTM 2017-84050-R, MAT 2015-69508-P, NanoBioApp Network (MINECO-17-MAT2016-81955-REDT), Xunta de Galicia (Centro Singular de Investigación de Galicia - Accreditation 2016-2019 and EM2014/035), European Union (European Regional Development Fund-ERDF) and IDIVAL INNVAL15/15 and INNVAL17/11.
Images have been produced using the free software available at: https://smart.servier.com/.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441 [DOI] [PubMed] [Google Scholar]
- 2.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nano. 2007;2:751–760. doi: 10.1038/nnano.2007.387 [DOI] [PubMed] [Google Scholar]
- 3.Mo R, Jiang T, Gu Z. Enhanced anticancer efficacy by ATP-mediated liposomal drug delivery. Angew Int Ed Chemie. 2014;53:1–7. doi: 10.1002/anie.201400268 [DOI] [PubMed] [Google Scholar]
- 4.Dicheva BM, Ten Hagen TLM, Seynhaeve ALB, Amin M, Eggermont AMM, Koning GA. Enhanced specificity and drug delivery in tumors by cRGD - anchoring thermosensitive liposomes. Pharm Res. 2015;32(12):3862. doi: 10.1007/s11095-014-1538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talelli M, Barz M, Rijcken CJF, Kiessling F, Hennink WE, Lammers T. Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today. 2015;10(1):93–117. doi: 10.1016/j.nantod.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jhaveri AM, Vladimir P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5:1–26. doi: 10.3389/fphar.2014.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, Shi H, Zhang H, et al. Biomaterials Prostate stem cell antigen antibody-conjugated multiwalled carbon nanotubes for targeted ultrasound imaging and drug delivery. Biomaterials. 2014;35:5369–5380. doi: 10.1016/j.biomaterials.2014.03.038 [DOI] [PubMed] [Google Scholar]
- 8.Tang MX, Redemann CT, Szoka FC. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7(6):703–714. doi: 10.1021/bc9600630 [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Pan D, Luo K, She W, Guo C, Yang Y. Peptide dendrimer – doxorubicin conjugate-based nanoparticle as an enzyme-responsive drug delivery system for cancer therapy. Adv Healthc Mater. 2014;3(8):1299–1308. [DOI] [PubMed] [Google Scholar]
- 10.Yavuz B, Pehlivan SB, Imran Vural NÜ. In vitro/In vivo evaluation of dexamethasone-PAMAM dendrimer complexes for retinal drug delivery. Pharm Drug Deliv Pharm Technol. 2015;104:3814–3823. [DOI] [PubMed] [Google Scholar]
- 11.Maleki Dizaj S, Barzegar-Jalali M, Hossein Zarrintan M, Adibkia K, Lotfipour F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin Drug Deliv. 2015;12(10):1649–1660. doi: 10.1517/17425247.2015.1049530 [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Zhang W, Hong C, Pan C. Silica nanotubes decorated by pH-responsive diblock copolymers for controlled drug release. ACS Appl Mater Interfaces. 2015;7(6):3618–3625. doi: 10.1021/am507832n [DOI] [PubMed] [Google Scholar]
- 13.Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules. 2018;23(1):47. doi: 10.3390/molecules23010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poonia N, Lather V, Pandita D. Mesoporous silica nanoparticles: a smart nanosystem for management of breast cancer. Drug Discov Today. 2017;23(2):315–332. doi: 10.1016/j.drudis.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 15.Petrak K. Essential properties of drug-targeting delivery systems. Drug Discov Today. 2005;10(23):1667–1673. doi: 10.1016/S1359-6446(05)03698-6 [DOI] [PubMed] [Google Scholar]
- 16.Vogt C, Toprak MS, Muhammed M, Laurent S, Jean-Luc Bridot RNM. High quality and tuneable silica shell-magnetic core nanoparticles. J Nanopart Res. 2010;12(4):1137–1147. doi: 10.1007/s11051-009-9661-7 [DOI] [Google Scholar]
- 17.Slowing II, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. Jacs. 2007;129:8845–8849. doi: 10.1021/ja0719780 [DOI] [PubMed] [Google Scholar]
- 18.Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124–133. doi: 10.4103/2230-973X.160844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosravian P, Ardestani MS, Khoobi M, et al. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. Onco Targets Ther. 2016;9:7315–7330. doi: 10.2147/OTT.S113815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng H, Wang M, Liu H, et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano. 2015;9(4):3540–3557. doi: 10.1021/acsnano.5b00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenholm JM, Sahlgren C, Lindén M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment. Curr Drugs Targets. 2011;12:1166–1186. doi: 10.2174/138945011795906624 [DOI] [PubMed] [Google Scholar]
- 22.Napierska D, Thomassen LCJ, Rabolli V, et al. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5(7):846–853. doi: 10.1002/smll.200800461 [DOI] [PubMed] [Google Scholar]
- 23.Lu F, Wu S, Hung Y, Mou C. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small. 2009;5(12):1408–1413. doi: 10.1002/smll.v5:12 [DOI] [PubMed] [Google Scholar]
- 24.Oh W, Kim S, Choi M, et al. Cellular uptake, cytotoxicity, and innate immune response of silica-titania hollow nanoparticles based on size and surface functionality. ACS Nano. 2010;4(9):5301–5313. doi: 10.1021/nn100561e [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharjee S, Lhj DH, Evers NM, et al. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol. 2010;7(25):1–12. doi: 10.1186/1743-8977-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dávila-Ibáñez AB, Salgueirino V, Martinez-Zorzano V, et al. Magnetic silica nanoparticle cellular uptake and cytotoxicity regulated by electrostatic polyelectrolytes-DNA loading at their surface. ACS Nano. 2012;1:747–759. doi: 10.1021/nn204231g [DOI] [PubMed] [Google Scholar]
- 27.Dávila-Ibáñez AB, Buurma NJ, Salgueriño V. Assessment of DNA complexation onto polyelectrolyte-coated magnetic silica nanoparticles. Nanoscale. 2013;5:4797–4807. doi: 10.1039/c3nr34358h [DOI] [PubMed] [Google Scholar]
- 28.Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM, Le L. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat. 2002;200:581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noguchi Y, Wu J, Duncan R, Ulbrich K, Akaike T. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J Cancer Res. 1998;89:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JE, Lee N, Kim H, et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. Jacs. 2010;132:552–557. doi: 10.1021/ja905793q [DOI] [PubMed] [Google Scholar]
- 33.Meng H, Mai WX, Zhang H, et al. Codelivery of an optimal Drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7(2):994–1005. doi: 10.1021/nn3044066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durfee PN, Lin Y, Dunphy DR, et al. Mesoporous silica nanoparticle-supported lipid bilayers (Protocells) for active targeting and delivery to individual leukemia cells. ACS Nano. 2016;10(9):8325–8345. doi: 10.1021/acsnano.6b02819 [DOI] [PubMed] [Google Scholar]
- 35.Babaei M, Abnous K, Taghdisi SM, et al. Synthesis of theranostic epithelial cell adhesion molecule targeted mesoporous silica nanoparticle with gold gatekeeper for hepatocellular carcinoma. Nanomedicine. 2017;12(11):1261–1279. doi: 10.2217/nnm-2017-0028 [DOI] [PubMed] [Google Scholar]
- 36.Zhou S, Wu D, Yin X, et al. Intracellular pH-responsive and rituximab- conjugated mesoporous silica nanoparticles for targeted drug delivery to lymphoma B cells. J Exp Clin Cancer Res. 2017;36(24):1–14. doi: 10.1186/s13046-016-0473-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Zhang W, Wang B, Gao Y, Song Z, Zheng QC. Ligand-based targeted therapy: a novel strategy for hepatocellular carcinoma. Int J Nanomedicine. 2016;1:5645–5669. doi: 10.2147/IJN.S115727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Yao H, Meng Y, Wang Y, Yan X, Huang R. Specific aptamer-conjugated mesoporous silica-carbon nanoparticles for HER2-targeted chemo-photothermal combined therapy. Acta Biomater. 2015;16:196–205. doi: 10.1016/j.actbio.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 39.Mo J, He L, Ma B, Chen T. Tailoring particle size of mesoporous silica nanosystem to antagonize glioblastoma and overcome blood-brain barrier. ACS Appl Mater Interfaces. 2016;8(11):6811–6825. doi: 10.1021/acsami.5b11730 [DOI] [PubMed] [Google Scholar]
- 40.Slowing II, Vivero-Escoto JL, Wu C, Lin VS. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 41.Warburg O, Franz Wind AEN. The metabolism of tumors in the body. J Gen Physiol. 1926;8:519–530. doi: 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. [DOI] [PubMed] [Google Scholar]
- 43.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science (80-). 1997;277:1232–1237. doi: 10.1126/science.277.5330.1232 [DOI] [Google Scholar]
- 44.Sukhorukov GB, Donath E, Lichtenfeld H, et al. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surf A. 1998;137:253–266. doi: 10.1016/S0927-7757(98)00213-1 [DOI] [Google Scholar]
- 45.Donath E, Sukhorukov GB, Caruso F, Davis SA, Möhwald H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew Int Ed Chemie. 1998;37(16):2201–2205. doi: [DOI] [PubMed] [Google Scholar]
- 46.Feng W, Nie W, He C, et al. Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake and biodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl Mater Interfaces. 2014;6(11):8447–8460. doi: 10.1021/am501337s [DOI] [PubMed] [Google Scholar]
- 47.Xu R, Sun G, Li Q, Wang E, Gu J. A dual-responsive superparamagnetic Fe3O4/Silica/PAH/PSS material used for controlled release of chemotherapeutic agent, keggin polyoxotungstate, PM −19. Solid State Sci. 2010;12(10):1720–1725. doi: 10.1016/j.solidstatesciences.2010.06.026 [DOI] [Google Scholar]
- 48.Feng W, Zhou X, He C, et al. Polyelectrolyte multilayer functionalized mesoporous silica nanoparticles for pH-responsive drug delivery: layer thickness-dependent release profiles and biocompatibility. J Mater Chem B. 2013;1:5886–5898. doi: 10.1039/c3tb21193b [DOI] [PubMed] [Google Scholar]
- 49.Wan X, Zhang G, Liu S. pH-disintegrable polyelectrolyte multilayer- coated mesoporous silica nanoparticles exhibiting triggered co-release of cisplatin and model drug molecules. Macromol Rapid Commun. 2011;32(14):1082–1089. doi: 10.1002/marc.201100198 [DOI] [PubMed] [Google Scholar]
- 50.Chen T, Hao Y, Yang N, Wang M, Dinga C, Fu J. Graphene quantum dot-capped mesoporous silica nanoparticles through an acid-cleavable acetal bond for intracellular drug delivery and imaging. Mater Chem B. 2014;2:4979–4982. doi: 10.1039/C4TB00849A [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Ai K, Liu J, Sun G, Yin Q, Lu L. Multifunctional envelope-type mesoporous silica nanoparticles for pH-responsive drug delivery and magnetic resonance imaging. Biomaterials. 2015;60:111–120. doi: 10.1016/j.biomaterials.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 52.Martínez-Carmona M, Lozano D, Vallet-Regí M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018;65:393–404. doi: 10.1016/j.actbio.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 53.Chem JM, Cheng S, Liao W, Chen L, Lee C. pH-controllable release using functionalized mesoporous silica nanoparticles as an oral drug delivery system. J Mater Chem. 2011;21:7130–7137. doi: 10.1039/c0jm04490c [DOI] [Google Scholar]
- 54.Huang I, Sun S, Cheng S, Lee C, Wu C. Enhanced chemotherapy of cancer using pH-sensitive mesoporous silica nanoparticles to antagonize P-glycoprotein-mediated drug resistance. Ther Discov. 2011;10(5):761–769. [DOI] [PubMed] [Google Scholar]
- 55.Lin C, Cheng S, Liao W, et al. Mesoporous silica nanoparticles for the improved anticancer efficacy of cis-platin. Int J Pharm. 2012;429:138–147. doi: 10.1016/j.ijpharm.2012.03.026 [DOI] [PubMed] [Google Scholar]
- 56.Lin J, Du J, Yang Y, Li L, Zhang D. pH and redox dual stimulate-responsive nanocarriers based on hyaluronic acid coated mesoporous silica for targeted drug delivery. Mater Sci Eng C Mater Biol Appl. 2017;81:478–484. doi: 10.1016/j.msec.2017.08.036 [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, Jia L, Ying K, et al. Ingenious pH-sensitive dextran/mesoporous silica nanoparticles based drug delivery systems for controlled intracellular drug release. Int J Biol Macromol. 2017;98:691–700. doi: 10.1016/j.ijbiomac.2017.01.136 [DOI] [PubMed] [Google Scholar]
- 58.Sun L, Zhang X, An J, Su C, Guo Q, Li C. Boronate ester bond-based core–shell nanocarriers with pH response for anticancer drug delivery. RSC Adv. 2014;4:20208–20215. doi: 10.1039/C4RA01812E [DOI] [Google Scholar]
- 59.Tan L, Yang M, Wu H, et al. Glucose- and pH-responsive nanogated ensemble based on polymeric network capped mesoporous silica. ACS Appl Mater Interfaces. 2015;7:6310−6316. doi: 10.1021/acsami.5b00631 [DOI] [PubMed] [Google Scholar]
- 60.Li Z, Liu Y, Wang X, et al. One-pot construction of functional mesoporous silica nanoparticles for the tumor-acidity-activated synergistic chemotherapy of glioblastoma. ACS Appl Mater Interfaces. 2013;5:7995−8001. doi: 10.1021/am401486h [DOI] [PubMed] [Google Scholar]
- 61.Lee C, Cheng S, Huang I, et al. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew Int Ed Chemie. 2010;49:8214–8219. doi: 10.1002/anie.201002639 [DOI] [PubMed] [Google Scholar]
- 62.Cheng Y, Luo G, Zhu J, et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl Mater Interfaces. 2015;7:9078−9087. doi: 10.1021/acsami.5b00752 [DOI] [PubMed] [Google Scholar]
- 63.De la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012;64(11):967–978. doi: 10.1016/j.addr.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Ding X, Li J, et al. Enzyme responsive drug delivery system based on mesoporous silica nanoparticles for tumor therapy in vivo. Nanotechnology. 2015;26(14):145102. doi: 10.1088/0957-4484/26/14/145102 [DOI] [PubMed] [Google Scholar]
- 65.Saito G, Swanson JA, Lee K. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: rola and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Sun H, Hu J, Han X, Liu H, Hu Y. Biointerfaces Transferrin gated mesoporous silica nanoparticles for redox-responsive and targeted drug delivery. Colloids Surf B Biointerfaces. 2017;152:77–84. doi: 10.1016/j.colsurfb.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 67.Mortera R, Vivero-Escoto J, Slowing II, Garrone E, Onida B, Lin VS. Cell-induced intracellular controlled release of membrane impermeable cysteine from a mesoporous silica nanoparticle-based drug delivery system. Chem Commun. 2009;(22):3219–3221. doi: 10.1039/b900559e [DOI] [PubMed] [Google Scholar]
- 68.Ma X, Nguyen KT, Borah P, Ang CY, Zhao Y. Functional silica nanoparticles for redox-triggered Drug/ssDNA co-delivery. Adv Healthc Mater. 2012;1(6):690–697. doi: 10.1002/adhm.201200123 [DOI] [PubMed] [Google Scholar]
- 69.Nadrah P, Porta F, Planinsek O, Krosb A, Gabersek M. Poly(propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release: smaller is better. Phys Chem Chem Phys. 2013;15:10740–10748. doi: 10.1039/c3cp44614j [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Niemelä M, Westermarck J, Rosenholm JM. Mesoporous silica nanoparticles with redoxresponsive surface linkers for charge-reversible loading and release of short oligonucleotide. Dalt Trans. 2014;43:4115–4126. doi: 10.1039/c3dt53071j [DOI] [PubMed] [Google Scholar]
- 71.Torney F, Trewyn BG, Lin VS-Y, Wang K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol. 2007;2:295–300. doi: 10.1038/nnano.2007.108 [DOI] [PubMed] [Google Scholar]
- 72.Giri S, Trewyn BG, Stellmaker MP, Lin VS-Y. Stimuli-Responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew Chem. 2005;117:5166–5172. doi: 10.1002/ange.200501819 [DOI] [PubMed] [Google Scholar]
- 73.Yi Z, Hussain HI, Feng C, et al. Functionalized mesoporous silica nanoparticles with redox- responsive short-chain gatekeepers for agrochemical delivery. ACS Appl Mater Interfaces. 2015;7(18):9937–9946. doi: 10.1021/acsami.5b02131 [DOI] [PubMed] [Google Scholar]
- 74.Gong H, Xie Z, Liu M, Zhu H, Sun H. Redox-sensitive mesoporous silica nanoparticles functionalized with PEG through a disulfide bond linker for potential anticancer drug delivery. RSC Adv. 2015;5:59576–59582. doi: 10.1039/C5RA09774F [DOI] [Google Scholar]
- 75.Palanikumar L, Choi ES, Cheon JY, Joo SH, Ryu J-H. Noncovalent polymer-gatekeeper in mesoporous silica nanoparticles as a targeted drug delivery platform. Adv Funct Mater. 2014;25(6):957–965. doi: 10.1002/adfm.201402755 [DOI] [Google Scholar]
- 76.Gimenez C, De La Torre C, Gorbe M, et al. Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir. 2015;31(12):3753–3762. doi: 10.1021/acs.langmuir.5b00139 [DOI] [PubMed] [Google Scholar]
- 77.Liu R, Zhao X, Wu T, Feng P. Tunable redox-responsive hybrid nanogated ensembles. Jacs. 2008;130:14418–14419. doi: 10.1021/ja8060886 [DOI] [PubMed] [Google Scholar]
- 78.Watermann A, Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials. 2017;7(7):189. doi: 10.3390/nano7120458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Publ Gr. 2013;12(11):991–1003. [DOI] [PubMed] [Google Scholar]
- 80.Laurent S, Dutz S, Häfeli UO, Mahmoudi M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166(1–2):8–23. doi: 10.1016/j.cis.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 81.Torres-Lugo M, Rinaldi C. Thermal potentiation of chemotherapy by magnetic nanoparticles. Nanomedicine. 2013;8(10):1689–1707. doi: 10.2217/nnm.13.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guisasola E, Baeza A, Talelli M, et al. Magnetic-responsive release controlled by hot spot effect. Langmuir. 2015;31(46):12777–12782. doi: 10.1021/acs.langmuir.5b03470 [DOI] [PubMed] [Google Scholar]
- 83.Baeza A, Guisasola E, Ruiz-Herna E, Vallet-Regí M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem Mater. 2012;24:517−524. doi: 10.1021/cm203000u [DOI] [Google Scholar]
- 84.Thomas CR, Ferris DP, Lee J, et al. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. Jacs. 2010;132:10623–10625. doi: 10.1021/ja1022267 [DOI] [PubMed] [Google Scholar]
- 85.Ferris DP, Zhao Y, Khashab NM, Khatib HA, Stoddart JF, Zink JI. Light-operated mechanized nanoparticles. Jacs. 2009;131:1686–1688. [DOI] [PubMed] [Google Scholar]
- 86.Wu S, Butt H-J. Near-infrared-sensitive materials based on upconverting nanoparticles. Adv Mater. 2016;28:1208–1226. [DOI] [PubMed] [Google Scholar]
- 87.Inoue Y, Kuad P, Okumura Y, Takashima Y, Yamaguchi H, Harada A. Thermal and photochemical switching of conformation of Poly(ethylene glycol)-substituted cyclodextrin with an azobenzene group at the chain end. Jacs. 2007;129:6396–6397. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z, Johns VK, Liao Y. Controlled release of fragrant molecules with visible light. Chem Eur J. 2014;20:1–5. [DOI] [PubMed] [Google Scholar]
- 89.Mekaru JLH, Tamanoi F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv Drug Deliv Rev. 2015;95:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guardado-Álvarez TM, Devi LS, Russell MM, Schwartz BJ, Zink JI. Activation of snap-top capped mesoporous silica nanocontainers using two near-infrared photons. Jacs. 2013;135(38):14000–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martínez-Carmona M, Lozano D, Baeza A, Colillaa M, Vallet-Regí M. A novel visible light responsive nanosystem for cancer treatment. Nanoscale. 2017;9(41):15967–15973. [DOI] [PubMed] [Google Scholar]
- 92.Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2011;62(11):1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tong L, Wei Q, Wei A, Cheng J. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol. 2009;85:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ke H, Wang J, Dai Z, et al. Gold-nanoshelled microcapsules: a theranostic agent for ultrasound contrast imaging and photothermal therapy. Angew Int Ed Chemie. 2011;50:3017–3021. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Tan -L-L, Li X, et al. Smart mesoporous silica nanoparticles gated by pillararene-modified gold nanoparticles for on-demand cargo release. Chem Commun. 2016;52:13775–13778. [DOI] [PubMed] [Google Scholar]
- 96.Vivero-Escoto JL, Slowing II, Wu C-W, Lin VS-Y. Photoinduced intracellular controlled release drug delivery in human cells by gold-capped mesoporous silica nanosphere. Jacs. 2009;131:3462–3463. [DOI] [PubMed] [Google Scholar]
- 97.Suit HD, Gerweck LE. Potential for hyperthermia and radiation therapy. Cancer Res. 1979;39:2290–2298. [PubMed] [Google Scholar]
- 98.Deswal K, Chohan IS. Effects of hyperthermia on enzymes and electrolytes in blood and cerebrospinal fluid in dogs. Int J Biometeor. 1981;25(3):227–233. [DOI] [PubMed] [Google Scholar]
- 99.González-Domíngez E, Iturrioz-Rodríguez N, Padín-González E, et al. Carbon nanotubes gathered onto silica particles lose their biomimetic properties with the cytoskeleton becoming biocompatible. Int J Nanomed. 2017;12:6317–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014;9:51–63. doi: 10.2147/IJN.S26592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu L, Mao Z, Zhang Y, Gao C. Influences of size of silica particles on the cellular endocytosis, exocytosis and cell activity of HepG2 cells. J Nanosci Lett. 2011;1(1):1–16. [Google Scholar]
- 102.Freeman EC, Weiland LM, Meng WS. Modeling the proton sponge hypothesis: examining proton sponge effectiveness for enhancing intracellular gene delivery through multiescale modeling. J Biomater Sci Poly Ed. 2014;24(4):398–416. doi: 10.1080/09205063.2012.690282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iturrioz-Rodriguez N, González-Lavado E, Marín- L, Araújo BV, Pérez-Lorenzo M, Fanarraga ML. A biomimetic escape strategy for cytoplasm invasion by synthetic particles. Angew Int Ed Chemie. 2017;56(44):13736–13740. doi: 10.1002/anie.201707769 [DOI] [PubMed] [Google Scholar]
- 104.Nelson N. Structure and pharmacology of the proton-ATPases. Trends Pharmacol Sci. 1991;12:71–75. [DOI] [PubMed] [Google Scholar]
- 105.Wu M, Meng Q, Chen Y, et al. Large pore-sized hollow mesoporous organosilica for redox-responsive gene delivery and synergistic cancer chemotherapy. Adv Mater. 2016;28:1963–1969. doi: 10.1002/adma.201505524 [DOI] [PubMed] [Google Scholar]
- 106.Shen J, Kim H, Su H, et al. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics. 2014;4:487–497. doi: 10.7150/thno.8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma D. Enhancing endosomal escape for nanoparticle mediated siRNA delivery. Nanoscale. 2014;6:6415–6425. doi: 10.1039/c4nr00018h [DOI] [PubMed] [Google Scholar]
- 108.Zhang X, Li F, Guo S, et al. Biofunctionalized polymer-lipid supported mesoporous silica nanoparticles for release of chemotherapeutics in multidrug resistant cancer cells. Biomaterials. 2014;35(11):3650–3665. doi: 10.1016/j.biomaterials.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 109.Crombez L, Charnet A, Morris MC, Aldrian-Herrada G, Heitz F, Divita G. A non-covalent peptide-based strategy for siRNA delivery. Biochem Soc Trans. 2007;35:44–46. doi: 10.1042/BST0350044 [DOI] [PubMed] [Google Scholar]
- 110.Nakase I, Akita H, Kogure K, et al. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc Chem Res. 2012;45(7):1132–1139. doi: 10.1021/ar200256e [DOI] [PubMed] [Google Scholar]
- 111.Li X, Chen Y, Wang M, Ma Y, Xia W, Gu H. A mesoporous silica nanoparticle e PEI e Fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013;34:1391–1401. doi: 10.1016/j.biomaterials.2012.10.072 [DOI] [PubMed] [Google Scholar]
- 112.Agostini A, Mondragón L, Bernardos A, et al. Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew Int Ed Chemie. 2012;51:1–6. doi: 10.1002/anie.201204663 [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, Chen H, Shi J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater. 2013;25:1–33. doi: 10.1002/adma.201205292 [DOI] [PubMed] [Google Scholar]
- 114.Shi Y, Hélary C, Haye B, Coradin T. Extracellular versus Intracellular Degradation of Nanostructured Silica Particles. Langmuir. 2018;34:406–415. doi: 10.1021/acs.langmuir.7b02231 [DOI] [PubMed] [Google Scholar]
- 115.Chen D, Hao N, Liu H, et al. In vitro degradation behavior of silica nanoparticles under physiological conditions in vitro degradation behavior of silica nanoparticles under physiological conditions. J Nanosci Nanotechnol. 2012;12:6346–6354. [DOI] [PubMed] [Google Scholar]
- 116.Huang X, Young NP, Townley HE. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater Nanotechnol. 2014;4(2):1–15. doi: 10.5772/58290 [DOI] [Google Scholar]
- 117.Paris JL, Cabañas MV, Manzano M, Vallet-Regí M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano. 2015;9(11):11023–11033. doi: 10.1021/acsnano.5b04378 [DOI] [PubMed] [Google Scholar]
- 118.Cauda V, Argyo C, Bein T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J Mater Chem. 2010;20:8693–8699. doi: 10.1039/c0jm01390k [DOI] [Google Scholar]
- 119.Yamada H, Urata C, Aoyama Y, Osada S, Yamauchi Y, Kuroda K. Preparation of colloidal mesoporous silica nanoparticles with different diameters and their unique degradation behavior in static aqueous systems. Chem Mater. 2012;24:1462−1471. doi: 10.1021/cm3001688 [DOI] [Google Scholar]
- 120.Braun K, Pochert A, Beck M, Fiedler R. Dissolution kinetics of mesoporous silica nanoparticles in different simulated body fluids. J Sol-Gel Sci Technol. 2016;79(2):319–327. doi: 10.1007/s10971-016-4053-9 [DOI] [Google Scholar]
- 121.Roggers RA, Joglekar M, Valenstein JS, Trewyn BG. Mimicking red blood cell lipid membrane to enhance the hemocompatibility of large-pore mesoporous silica. ACS Appl Mater Interfaces. 2014;6(3):1675–1681. doi: 10.1021/am4045713 [DOI] [PubMed] [Google Scholar]
- 122.Lu J, Li Z, Zink JI, Tamanoi F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: enhanced efficacy by folate modification. Nanomedicine NBM. 2012;8(2):212–220. doi: 10.1016/j.nano.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He Q, Zhang Z, Gao F, Li Y, Shi J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and pegylation. Small. 2011;7(2):271–280. doi: 10.1002/smll.201001459 [DOI] [PubMed] [Google Scholar]