Abstract
Objective: Our objective was to conduct a meta-analysis to investigate the clinicopathological features and prognostic value of programmed cell death ligand 1 (PD-L1) expression in patients with urothelial carcinoma (UC).
Materials and methods: Twenty-seven studies with 4,032 patients were included in the meta-analysis. Pooled ORs and 95% CIs were used to examine the associations between clinical factors and PD-L1 expression. HRs and 95% CIs were extracted from eligible studies. Heterogeneity was evaluated using the chi-squared-based Q test and I2 statistic.
Results: Expression of PD-L1 on tumor cells (TCs) was associated with muscle-invasive disease (OR=3.67, 95% CI: 2.53–5.33), and inversely associated with the history of intravesical bacilli Calmette-Guerin therapy (OR=0.39, 95% CI: 0.18–0.82) in bladder cancer patients. PD-L1 expression on TCs was associated with worse overall survival (HR=2.06, 95% CI: 1.38–3.06) in patients with organ-confined bladder cancer. PD-L1 expression in patients with UC was significantly related to better objective response rate after PD-1/PD-L1 antibody treatment.
Conclusions: Expression of PD-L1 on TCs was associated with muscle-invasive disease in patients with bladder cancer. Patients with PD-L1-positive UC had a significantly better response to PD-1/PD-L1 targeted treatment.
Keywords: urothelial carcinoma, programmed cell death ligand 1, immunotherapy, meta-analysis, prognosis
Introduction
Programmed cell death ligand 1 (PD-L1) is a cell surface glycoprotein that belongs to the B7/CD28 co-stimulatory factor superfamily.1 It functions as an inhibitor of the immune response through promoting T-cell apoptosis by either binding to programmed cell death-1 (PD-1) receptor, or a putative non-PD-1 receptor on the surface of T lymphocytes.1 Similar to self-antigen recognition, cancer cell can escape immune surveillance by upregulating PD-L1. Moreover, the PD-1/PD-L1 signaling axis may induce immune inhibitory/exhaustion signaling of activated T cells, and thus significantly impair the anti-tumor immune response.2 Therefore, it is hypothesized that blockade of the PD-1/PD-L1 pathway may restore the native anti-tumor function of T cells and facilitate tumor regression. In recent years, immune checkpoint inhibitors that can block PD-L1 expression and then enhance T cell function in cancers have been brought identified.
An association between high pretreatment tumor PD-L1 expression and poor survival has been reported in multiple cancers, including colorectal cancer and renal cell carcinoma.3,4 Several studies have indicated that PD-L1 expression on bladder cancer (BC) cells was related to multiple indicators of poor prognosis, such as high tumor grade, increased resistance to bacilli Calmette-Guerin therapy, and muscle-invasive disease.5,6 On the other hand, Xylinas et al suggested that PD-L1 expression was not associated with clinicopathological features in patients after radical cystectomy (RC).7 So far, data regarding the prognostic role of PD-L1 expression in BC are conflicting. Studies by Nakanishi et al revealed a higher risk of recurrence and shorter overall survival (OS) with high PD-L1 expression in patients with BC, though not all reports support this conclusion.8–10
Although blocking PD-L1 or PD-1 has emerged as a promising strategy for treating advanced urothelial carcinoma (UC), a consensus has not been reached regarding the prognostic value of PD-L1 expression. A previous meta-analysis suggested that patients with urothelial carcinoma with higher ratios of PD-L1-positive cells responded significantly better to anti-PD-1/PD-L1 therapy than those with lower ratios of PD-L1-positive cells.11 Because of the potential predictive value of PD-L1 expression on immune cells (ICs) in patients receiving checkpoint inhibitors for advanced urothelial carcinoma, more attention is being paid to the clinical significance of PD-L1 expression on ICs.12,13
In this meta-analysis, we aimed to assess PD-L1 expression and its association with clinical outcomes in urothelial carcinoma patients. Furthermore, this research attempts to show the potential of using PD-L1 as a biomarker to identify patients more likely to benefit from PD-1/PD-L1-targeted therapies.
Materials and methods
Literature search
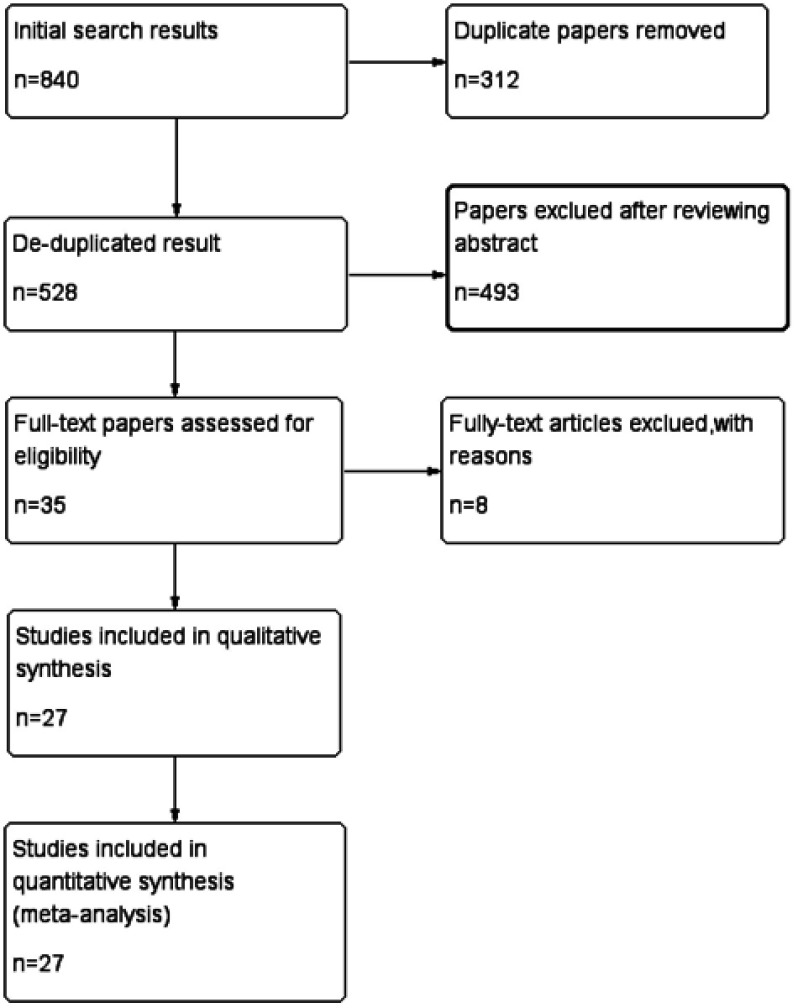
Two of the authors (QiaoChao Chen and Xiangli Ding) independently retrieved published literature up to December 22, 2017 from the PubMed, Cochrane Library, and the Web of Science online databases without region or time restrictions. The following medical subject and text words were used for the literature searches: (“Bladder cancer” OR “Bladder tumor” OR “Bladder carcinoma” OR “Urothelial cancer” OR “Urothelial tumor” OR “Urothelial carcinoma”) and (“PD-L1” OR “B7-H1” OR “CD274” OR “Programmed Cell Death 1 Ligand 1 Protein”) (Figure 1).
Figure 1.
Flowchart of study selection.
Eligibility criteria
Study inclusion criteria were: 1) sll patients had histologically confirmed urological/bladder carcinoma; 2) studies provided data regarding the correlation between PD-L1 expression and clinicopathological features; 3) studies reported Kaplan–Meier curves, HRs, and 95% CIs describing associations between OS and cancer-specific survival (CSS); 4) reported comparisons of PD-L1-positive versus PD-L1-negative patients receiving anti-PD-1/PD-L1 treatment; and 5) English-language publication. Studies that failed to meet the inclusion criteria were excluded. When duplicate publications were identified, only the newest or most recent article was used in the analysis.
Data extraction and quality assessment
The data were extracted independently by two reviewers (QiaoChao Chen and Hui Zhan), and any disagreements were resolved by achieving consensus with the assistance of a third reviewer (Xiangli Ding). The following information from each study enrolled was extracted: the first author’s name, year of publication, country of origin, number of patients, keywords used for indexing, technique used to assay PD-L1 values, cutoff points, PD-L1-positive expression (defined by tumor cells, tumor-infiltrating ICs, or combined positive score [CPS]), survival analysis method, HRs and 95% CIs for OS and CSS, and objective response rate (ORR) (defined as the percentage of patients with complete or partial response as per RECIST 1.1). For studies that reported survival data indirectly with a Kaplan–Meier curve, we used the method described by Tierney et al14 to estimate the log-transformed HR. Quality assessments were conducted independently for each study by two reviewers (Xiangli Ding and QiaoChao Chen) using a modified Newcastle-Ottawa Scale (NOS). The scale consisted of three parameters of quality: selection, comparability, and outcome assessment. The maximum possible score is 9 points, and NOS scores of more than 6 points were considered to indicate high-quality studies.15
Statistical analysis
Pooled ORs and its 95% CIs were used to present the associations between clinical factors, ORR of anti-PD1/PD-L1 treatment, and PD-L1 expression, and HRs and 95% CIs were extracted to evaluate the association between PD-L1 expression and prognosis (OS and CSS). Heterogeneity among studies was evaluated using the chi-squared-based Q test and I2 statistic. An I2>50% or p<0.1 represents significant heterogeneity between studies. When significant heterogeneity was present, a random effects model of analysis was used. Otherwise, a fixed-effects model was used. Subgroup analysis was performed to examine OS and CSS. Potential publication bias was examined by Egger’s and Begg’s tests. Data were analyzed by using STATA version 12.0 (Stata Corporation; College Station, TX, USA). All p-values and 95% CIs were two-sided, and p-values <0.05 were considered statistically significant.
Results
Search results and study characteristics
A total of 840 studies were identified in the literature searches. After removing duplicates, 528 titles and abstracts were screened, and 493 articles were excluded. After carefully reading the remaining 35 potentially eligible articles, 8 were excluded because key information was absent. Ultimately, 27 studies5,7–9,16–38 published from 2007 to 2017 that met the inclusion criteria were included in the analysis. The selection process is shown in Figure 1.
The characteristics of the included studies are shown in Table 1. The 27 studies included 4,032 patients; 716,18,20,21,23,24,26studies originated from Asian countries/regions (Japan, Taiwan, China), 925,27,29-35studies were multicentre and global studies, and the remaining studies originated from the United States, Germany, and France. The sample size ranged from 37 to 423. Seventeen studies were of retrospective design, 26 studies detected PD-L1 expression using immunohistochemistry , and 116 study examined the gene expression of PD-L1. OS was reported in 97,9,16–18,20,22,37,38 studies; 57,9,16–18 of them conducted multivariate analysis of OS. Six7,16,17,24,26,35 studies reported CSS and 216,26 studies conducted multivariate analysis of CSS. Study quality ranged from 5 to 8; thus, the studies were of relatively high quality.
Table 1.
Basic characteristics of 27 studies included in meta-analysis
| First author(y) | Conutry | Index | Patients | Material | Assay | Staining pattern | Cut-off | Number | PD-L1(+/-) TC, NO. | Positive(%) | survival analysis method | HR estimation | Quality assessment(score) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Bellmunt, J. | USA | Protein | Bladder cancer. | TMA | IHC | Membrane | Percentage (≥5%) | 160 | 32/128 | 20 | MA | Reported | 8 |
| 2Huang, Y. | China | Gene | Bladder cancer. | datasets | Datasets IHC | NA | Median PD-L1 expression levels | 165 | 40/125 | 24.2 | MA | Reported | 7 |
| 3Boorjian, S. A. | USA | Protein | Bladder cancer. | FFPE | IHC | Membrane | Percentage (≥5%) | 314 | 39/275 | 12.4 | MA | Reported | 8 |
| 4 Wang, Y. | China | Protein | Bladder cancer. | TMA | IHC | Membrane/ cytoplasm | Percentage (≥10%) | 50 | 36/14 | 72 | MA | Reported | 8 |
| 5Le Goux, C. | France | Protein | Bladder cancer. | FFPE | IHC | Membrane | Percentage (≥1%) | 108 | 55/53 | 50.9 | NA | NA | 7 |
| 6Wu, C. T. | Taiwan | Protein | Bladder cancer. | TMA | IHC | NA | IRS scoring grade ≥2 | 120 | 58/62 | 48.3 | UA | Calculated | 8 |
| 7Inman, B. A. | USA | Protein | Bladder cancer. | FFPE | IHC | Membrane | Percentage (≥1%) | 280 | 77/203 | 27.5 | NA | NA | 6 |
| 8Wang, Y. H. | China | Protein | Bladder cancer. | TMA | IHC | Membrane | Percentage (≥10%) | 60 | 43/17 | 71.7 | NA | NA | 6 |
| 9Erlmeier.F | Germany | Protein | Bladder cancer. | FFPE | IHC | Membrane | Percentage (≥10%) | 64 | 19/45 | 29.7 | UA | Calculated | 6 |
| 10Faraj, S. F. | USA | Protein | Bladder cancer. | TMA | IHC | Membrane | Percentage (≥5%) | 56 | 10/46 | 17.9 | NA | NA | 8 |
| 11Liu, Z. H. | China | Protein | Bladder cancer. | FFPE | IHC | Membrane/ cytoplasm | Percentage (≥10%) | 55 | 31/24 | 56.4 | NA | NA | 6 |
| 12Noro, D. | Japan | Protein | Bladder cancer. | FFPE | IHC | Membrane | Percentage (≥5%) | 102 | 38/64 | 37.3 | UA | Calculated | 8 |
| 13Xylinas, E. | USA | Protein | Bladder cancer. | TMA | IHC | Membrane | Percentage (≥5%) | 302 | 76/226 | 25 | MA | Reported | 8 |
| 14Powles, T. | NA | Protein | Bladder cancer. | FFPE | IHC | NA | Percentage (≥5%) | 205 | 22/183 | 11 | NA | NA | 8 |
| 15Zhang, B. | China | Protein | UTUC | FFPE | IHC | Membrane | Percentage (≥5%) | 162 | 20/142 | 12.3 | MA | Reported | 8 |
| 16Rosenberg, J. E. | NA | Protein | Bladder, Renal pelvis, Ureter Urethra. | FFPE | IHC | Membrane | Percentage (≥5%) | 310 | 100/210 | 32.3 | UA | Calculated | 8 |
| 17Plimack, E. R. | USA and Israel | Protein | Bladder, Renal pelvis, Ureter, Urethra. | FFPE | IHC | Membrane | Percentage (≥1%) | 115 | 61/54 | 53 | NA | NA | 8 |
| 18Balar, A. V. | NA | Protein | Bladder, Renal pelvis, Ureter, Urethra. | FFPE | IHC | Membrane | Percentage (≥5%) | 119 | 32/87 | 26.9 | NA | Calculated | 8 |
| 19Apolo, A. B. | NA | Protein | Bladder, Renal pelvis, Ureter, Urethra. | FFPE | IHC | Membrane | Percentage (≥5%) Percentage | 37 | 13/24 | 35.1 | NA | Calculated | 8 |
| 20Sharma, P. | NA | Protein | Bladder, Renal pelvis, Ureter, Urethra. | FFPE | IHC | Membrane | (≥1%) | 265 | 122/143 | 46 | NA | Calculated | 8 |
| 21Sharma, P. | NA | Protein | Bladder, Renal pelvis. | FFPE | IHC | Membrane | Percentage (≥1%) | 67 | 25/42 | 37.3 | NA | NA | 7 |
| 22Massard, C. | NA | Protein | Bladder cancer. | FFPE | IHC | Membrane | Percentage (≥25%) | 61 | 40/21 | 65.6 | NA | NA | 8 |
| 23Powles, T. | NA | Protein | Urothelial carcinoma | FFPE | IHC | Membrane | Percentage (≥25%) | 177 | 98/79 | 55.3 | NA | Calculated | 8 |
| 24Krabbe, L.M. | NA | Protein | UTUC. | TMA | Membrane/ cytoplasm | Percentage (≥1%) | 423 | 111/312 | 26.2 | MA | Reported | 8 | |
| 25Skala, S.L. | USA | Protein | UTUC. | TMA | IHC | Membrane | Percentage (≥5%) | 149 | 35/114 | 23.5 | MA | Reported | 8 |
| 26Tretiakova, M. | USA | Protein | Urothelial carcinoma | TMA | IHC | Membrane | Composite score | 101 | 21/80 | 20.8 | UA | Reported | 7 |
| 27Pichler, R. | Austria | Protein | Urothelial carcinoma | FFPE | IHC | Membrane | Percentage (≥5%) | 61 | 16/61 | 26.2 | UA | Reported | 7 |
Abbreviations: IHC, immunohistochemistry; FFPE, formalin-fixed paraffin-embedded; TMA, tissue microarray; UA, univariate analysis; MA, multivariate analysis; NA, not available; TC, tumor cell; UTUC, urothelial carcinoma of the upper urinary tract.
Correlation between clinicopathological parameters and PD-L1 expression in urothelial carcinoma
We explored the correlation between PD-L1 expression and clinicopathological characteristics of 2,200 patients from 135,7–9,17–22,26,35,37 studies, of which 226,35 studies were about upper urinary tract urothelial carcinoma (UTUC). Data including tumor stage, tumor grade, sex, smoking history, preoperative use of chemotherapy, intravesical instillation of bacilli Calmette-Guérin (BCG), lymph node metastasis, distant metastasis, recurrence of UTUC, and death due to UTUC were extracted and then pooled ORs and 95% CIs were computed. As shown in Figure 2, the meta-analysis of 115,7–9,17–22,37 relevant studies on tumor stage demonstrated a significantly higher incidence of PD-L1 expression in the muscle-invasive-bladder cancer (MIBC) group relative to the non-muscle-invasive-bladder cancer (NMIBC) group (OR=3.67, 95% CI: 2.53–5.33; p=0.668; fixed-effects) (Figure 2A1), while tumor stage was not significantly associated with PD-L1 expression on tumor-infiltrating ICs in patients with BC (n=4, OR=1.43, 95% CI: 0.93–2.21; p=0.211; fixed-effects) (Figure 2B1). In addition, we noted a significantly decreased expression of PD-L1 in specimens from patients who received intravesical therapy of BCG before cystectomy (n=3, OR=0.39, 95% CI: 0.18–0.82; p=0.309; fixed-effects) (Figure 2C).
Figure 2.

Association between PD-L1 expression and (A1) tumor stage; (B1) tumor stage; (C) intravesical therapy of BCG; (D1) tumor grade; (E1) sex; (F1) smoking history; (G1) prior use of chemotherapy; (H1) lymph node metastases; and (I1) distant metastases in bladder cancer. Association between PD-L1 expression and (A2) tumor stage; (B2) tumor stage; (D2) tumor grade; (E2) sex; (F2) smoking history; (G2) prior use of chemotherapy; (H2) lymph node metastases; (I2) distant metastases; (J) recurrence; (K) death due to UTUC for patients with UTUC. Figure 2(A, C–K) expression of PD-L1 was defined by tumor cells. Figure 2(B) expression of PD-L1 was defined by tumor-infiltrating immune cells.
Abbreviations: PD-L1, programmed cell death-1; BCG, bacilli Calmette-Guerin; UTUC, upper urinary tract urothelial carcinoma; OR, odds ratio.
No significant relation was observed between PD-L1 expression on BC tumor cells and higher tumor grade (grade III) (n=5, OR=1.25, 95% CI: 0.85–1.84; p=0.003; random-effects) (Figure 2D1), sex (n=9, OR=1.01, 95% CI: 0.73–1.40; p=0.138; fixed-effects) (Figure 2E1), smoking history (n=3, OR=1.15, 95% CI: 0.65–2.04; p=0.902; fixed-effects) (Figure 2F1), prior use of chemotherapy (n=4, OR=0.72, 95% CI: 0.31–1.69; p=0.066; random-effects) (Figure 2G1), lymph node metastases (n=7, OR =1.04, 95% CI: 0.74–1.45, p=0.230; fixed-effects) (Figure 2H1), and distant metastases (n=1, OR=2.18, 95% CI: 0.75–6.35) (Figure 2I1). The analysis suggested that positive PD-L1 expression on TCs in patients with BC could be considered a significant biomarker for diagnosis of advanced stage disease.
For UTUC, no statistically significant significance was observed between PD-L1 expression on TCs and advanced (≥T3) stage disease (n=2, OR=1.05, 95% CI: 0.70–1.58; p=0.230; fixed-effects) (Figure 2A2), sex (n=3, OR=0.76, 95% CI: 0.54–1.09; p=0.593; fixed-effects) (Figure 2E2), smoking history (n=1, OR=1.48, 95% CI: 0.62–3.51) (Figure 2F2), prior use of chemotherapy (n=1, OR =0.92, 95% CI: 0.28–3.01) (Figure2G2), lymph node metastases (n=2, OR=1.49, 95% CI: 0.80–2.80) (Fig. 2H2), distant metastases (n=1, OR =1.11, 95% CI: 0.45–2.76) (Figure 2I2), recurrence (n=2, OR=0.71, 95% CI: 0.46–1.09; p=0.886; fixed-effects) (Figure 2J), and death due to UTUC (n=2, OR=1.08, 95% CI: 0.49–2.40; p=0.130; random-effects) (Figure 2K).
Prognostic value of PD-L1 expression for OS and CSS
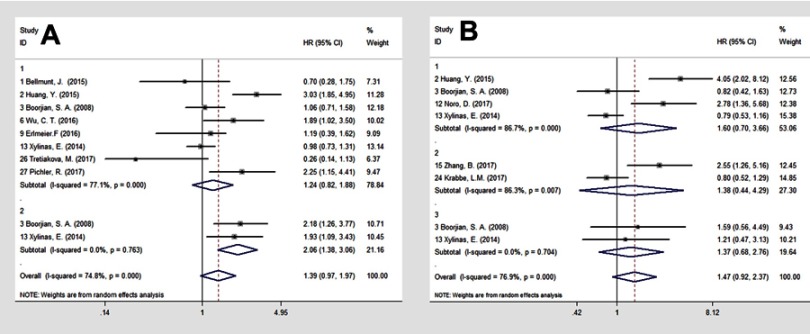
We evaluated the association between PD-L1 expression on TCs and OS and CSS in BC patients without anti-PD-1/PD-L1 treatment. A total of eight7,9,16,17,20,22,37,38 studies reported OS data of BC by univariate analysis. Significant heterogeneity existed among the studies (I2= 77.1%, p=0.000). Three16,20,38 studies indicated that PD-L1 expression was associated with poor prognosis of BC. The pooled results revealed PD-L1 expression was not significantly related to OS (HR=1.24, 95% CI: 0.82–1.88; p=0.000; random-effects) (Figure 3A1). For patients with organ-confined disease,7,17 PD-L1 expression on TCs was a significant predictor of all-cause mortality following RC (HR=2.06, 95% CI: 1.38–3.06; p=0.763; fixed-effects) (Figure 3A2).
Figure 3.
Forest plot to assess effect of PD-L1 tumor cell expression on (A1) OS for BC; (A2) OS for organ-confined BC, and (B1) CSS for BC; (B2) CSS for UTUC; (B3) CSS for organ-confined BC.
Abbreviations: PD-L1, programmed cell death ligand 1; OS, overall survival; BC, bladder cancer; CSS, cancer specific survival; UTUC, upper urinary tact urothelial carcinoma.
Four7,16,17,24 studies provided CSS data for BC, and two7,17 studies for organ-confined BC. Pooled results revealed PD-L1 expression had no significant effect on CSS for either BC (HR=1.60, 95% CI: 0.70–3.66; p=0.000; random-effects) (Figure 3B1) or organ-confined BC (HR=1.37, 95% CI: 0.68–2.76; p=0.704; fixed-effects) (Figure 3B3). Two26,35 studies provided data of UTUC, and analysis showed that PD-L1 expression had no impact on CSS (HR 1.38, 95% CI: 0.44–4.29; p=0.007; random-effects) (Figure 3B2).
Subgroup analyses
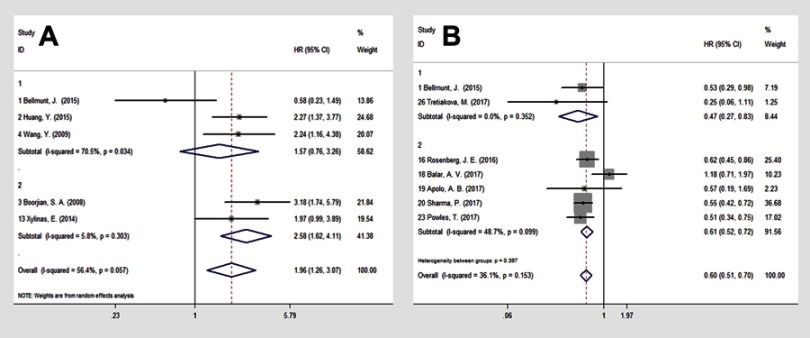
To obtain further insight, we performed subgroup analysis stratified by the survival analysis method and receiving anti-PD-1/PD-L1 treatment to evaluate the prognostic significance of PD-L1 expression on TC. For OS, the subgroup of the survival analysis revealed a merged HR for multivariate analysis of 1.57 (n=3, 95% CI: 0.76–3.26; p=0.034; random-effect) for BC (Figure 4A1), 2.58 (n=2, 95% CI: 1.62–4.11; p=0.303; fixed-effect) for patients with organ-confined BC following RC (Figure 4A2).
Figure 4.
Forest plot of the combined overall survival (OS) for (A1) bladder cancer patients; (A2) patients with organ-confined BC; analyzed with multivariate analysis, with PD-L1 status defined by TCs and OS (B1) without and (B2) with anti-PD-1/PD-L1 treatment in urothelial carcinoma patients with PD-L1 status stratified by ICs.
Abbreviations: OS, overall survival; BC, bladder cancer; TCs, tumor cells; PD-L1, programmed cell death ligand 1; ICs, tumor-infiltrating immune cells.
In subgroup analysis of receiving anti-PD-1/PD-L1 treatment, high PD-L1 expression on ICs was significantly associated with better OS for UC patients without (n=2, HR=0.47; 95% CI: 0.27–0.83; p=0.352; fixed-effects) (Figure 4B1) and with (n=5, HR =0.61; 95% CI: 0.52–0.72; p=0.099; fixed-effects) anti-PD-1/PD-L1 treatment (UC) (Figure 4B2).
Association between location of PD-L1 expression and ORR of PD-L1/PD-1 antibody treatment
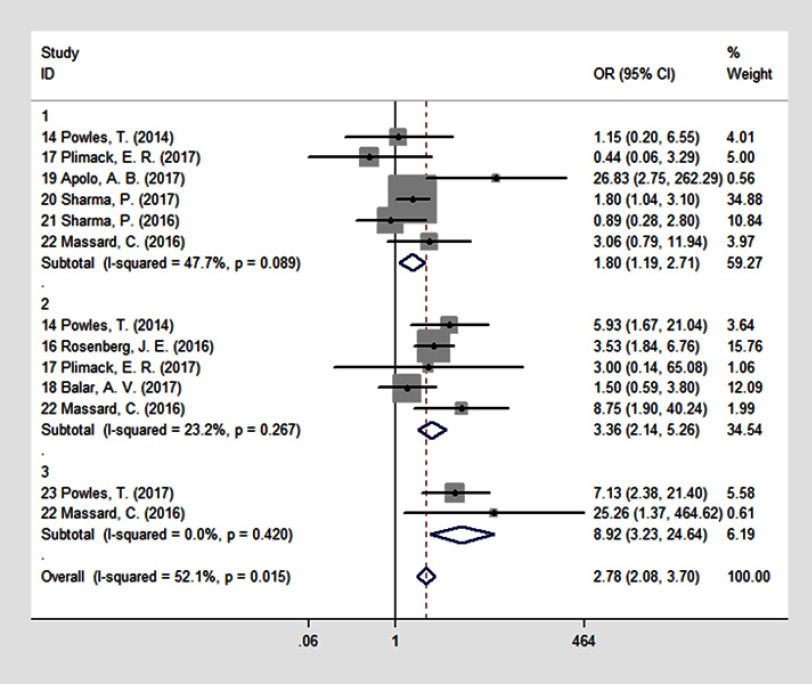
Nine25,27–34 studies reported ORR data, and their results are summarized in Figure 5. We explored defining PD-L1 status based on TCs, ICs, or CPS and the ORR of PD-1/PD-L1 antibody treatment. Patients with PD-L1 expression had a significantly better ORR after PD-1/PD-L1 antibody treatment (n=13, OR=2.78, 95% CI: 2.08–3.70; p=0.015). Subgroup analysis showed that when PD-L1 expression was defined by TC status, the ORRs were 27.4% in the PD-L1-positive group and 19.3% in the PD-L1-negative group (n=6, OR=1.80, 95% CI: 1.19–2.71; p=0.089) (Figure 5.1). When PD-L1 expression was defined by IC status, the ORRs were 30% in the PD-L1-positive group and 12.2% in the PD-L1-negative group (n=5, OR=3.36, 95% CI: 2.14–5.26; p=0.0267) (Figure 5.2). In addition to TC- or IC-independent definitions, a combined TC/IC algorithm (CPS, defined as the percentage of tumor and infiltrating ICs with PD-L1 expression of the total number of tumor cells) was developed and seemed to show a clear dichotomy between responding and nonresponding subgroups: the ORR was 31.74% in PD-L1-high patients and 4.3% in PD-L1-low or negative patients (n=2, OR=8.92, 95% CI: 3.23–24.64; p=0.015) (Figure 5.3).
Figure 5.
Forest plot of the combined overall response rate (ORR) of anti-PD-1/PD-L1 antibody treatment for UC with PD-L1 status defined by 1) TCs; 2) ICs; 3) CPS.
Abbreviations: ORR, overall response rate; PD-1/PD-L1, programmed cell death-1/programmed cell death ligand 1; UC, urothelial carcinoma; TCs, tumor cells; ICs, tumor-infiltrating immune cells; CPS, combined positive score.
Publication bias
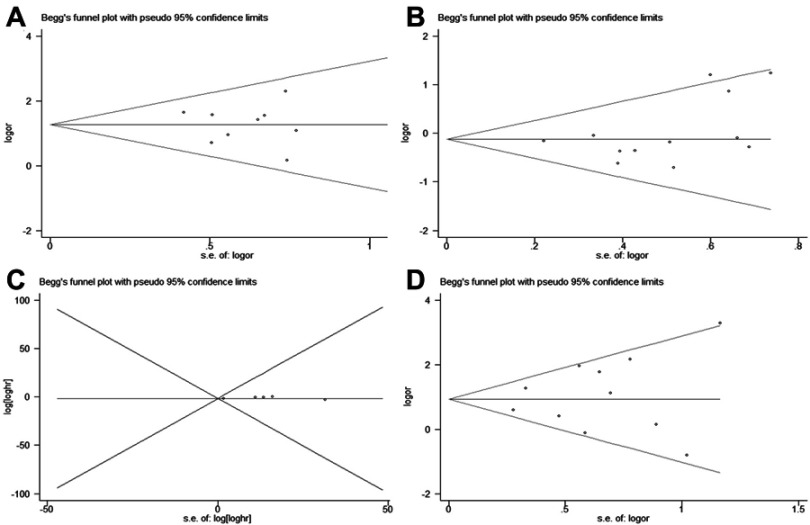
Funnel plots for the meta-analysis of PD-L1 expression and clinical features, as well as OS and CSS, were evaluated by Egger’s and Begg’s tests (Figure 6). The funnel plots were symmetric, indicating no obvious publication bias (Egger’s p: tumor stage (n=11), p=0.461; sex (n=12): p=0.209; OS (n=8), p=0.169; ORR: p=0.556 (n=13) (Figure 6).
Figure 6.
Funnel plot evaluating possible publication bias for (A) tumor stage; (B) sex; (C) OS; (D) ORR.
Abbreviations: OS, overall survival; ORR, objective response rate.
Discussion
PD-L1, a member of the B7 family, has the ability to regulate T cell functions through engagement with PD-1, and is expressed on dendritic cells (immature, mature, and follicular) and on many types of cancer cells.39 Agents blocking either PD-1 or PD-L1 are thought to increase the T cell-mediated immune response to tumor cells. In recent years, PD-L1 expression on tumor cells and tumor-infiltrating cells is thought to have distinct implications on tumor response to anti-PD-1/PD-L1.40 However, the prognostic value of PD-L1 in UC has varied between clinical trials. The conflicting data from different research studies urged us to perform this meta-analysis. Our meta-analysis included 27 studies with 4,032 patients, and demonstrated that PD-L1 expression on TCs was associated with MIBC and shortened OS for organ-confined BC patients. The pooled survival analysis results indicated that PD-L1 expression on ICs was a predictor of better OS for patients with and without anti-PD-1/PD-L1 antibody treatment. Moreover, the studies also supported the hypothesis that positive PD-L1 expression based on staining different cellular populations (tumor cells, tumor-infiltrating ICs, or both) might be associated with improved response to PD-1/PD-L1 inhibitors in UC patients. These results indicate that PD-L1 expression on TCs may be valuable for evaluation of tumor aggressiveness, and that PD-L1 status may be used to select patients more likely to respond to anti-PD-1/PD-L1 treatment.
An association between higher PD-L1 expression in TCs and tumor aggressiveness has been reported in a two human malignancies.41,42 Forced or constitutive PD-L1 expression by tumors in vivo and in vitro leads to immune tolerance, while blockade of PD-L1/PD-1 enhances anti-tumor immunity and inhibits tumor growth.43,44The presence of PD-L1 glycoprotein on the surface of UC cells may affect malignant stage progression via impairing host anti-tumor immunity, which may explain the positive correlation between PD-L1 expression and high-risk prognostic factors. Our results regarding PD-1 pathway activation in MIBC (OR =3.67) were in consistent with those of prior studies.5,7,17,19 In addition, Le Goux et al reported low overexpression of both PD-1 and PD-L1 genes in NMIBC tissue, with no significant difference in mRNA expression as compared with normal bladder tissue,19 suggesting that immune checkpoints may be involved in the pathogenesis of BC.
Emerging data suggest that PD-L1 is expressed by many tumor types, and is associated with poor prognosis in several, including UC, lung adenocarcinoma, and renal cell carcinoma.41,45,46 However, in contrast PD-L1 expression on ICs is an indicator of a favorable prognosis for vulvar squamous cell carcinoma,47 ovarian carcinoma,48 and breast cancer.49 In this meta-analysis, only two studies reported improved OS with PD-L1 expression on tumor cells.9,37 Thus, no conclusive conclusions with respect to clinical outcomes and PD-L1 expression on tumors could be reached. Based on the potential predictive role of PD-L1 expression on ICs in UC patients receiving checkpoint inhibitors, attention has recently switched toward analysis of PD-L1 expression in ICs instead of TCs. Two studies reported improved OS with PD-L1 expression in tumor-infiltrating ICs, with a merged HR=0.47.9,37 The role of PD-L1 expression in predicting outcomes remains controversial for BC, likely due to the variability of methodologies for evaluating PD-L1 expression and its scoring between trials.50
The success of BCG in treating NMIBC has emphasized BC as an immune sensitive disease, and the role of immune checkpoints inhibitors like pembrolizumab is also being researched in NMIBC patients, relapsing after the BCG treatment (KEYNOTE-057).51 Inman et al reported that PD-L1 tumor cell expression was associated with increased resistance to BCG therapy, which suggests that TCs might be protected from attack by ICs through immune checkpoints, since a fully functional immune system is prerequisite for BCG efficacy.52 Notably, in our analysis PD-L1 expression was inversely correlated with prior use of BCG.
In patients with organ-confined BC after RC, higher PD-L1 expression in TCs was associated with an increased risk of all-cause mortality in both univariate and multivariate analysis. These findings are in accordance with those of Boorjian et al who reported that PD-L1 expression on TCs was associated with all-cause mortality in 167 BC patients with organ-confined disease treated with RC.17 The accurate prediction of outcomes for patients with organ-confined tumors has recently raised interest.53,54 Approximately 80% of patients with organ-confined BC may be cured by RC.55 Our observation that PD-L1 positivity predicts postoperative mortality may help identify patients with organ-confined tumors who are at high risk for disease progression and who may benefit from treatment in addition to surgery.
An ongoing challenge to UC immunotherapy is how to identify patients who are most likely to benefit from these therapies. It seems reasonable that PD-L1, highly expressed in UC and associated with aggressive tumors,10,56 might be a predictive biomarker, but the data are inconclusive. An association between increased PD-L1 expression on tumor-infiltrating ICs and an increased response rate to atezolizumab27 and nivolumab31 has been reported. These results, however, were in direct contrast to the results of the Phase II study IMvigor 210, which showed increased PD-L1 expression in locally advanced and metastatic UC did not improve treatment responses.29 While a lower HR for death was confirmed for patients with PD-L1-positive UC (HR=0.57) relative to the total pembrolizumab population (HR=0.73) in one recent Phase Ⅲ experience, which suggested that positive PD-L1 status (defined as a CPS of ≥10%) was a negative predictive factor. At the same time, pembrolizumab improved the median OS in all patients compared to standard chemotherapy, regardless of PD-L1 status.57
In this study, the ORR HR was 1.89 when PD-L1 status defined by TCs, and when PD-L1 status was defined by tumor-infiltrating ICs, the pooled HR was 3.36. Evaluating both TCs and ICs in a combined definition of PD-L1 status (CPS) seemed to show that PD-L1 was a positive predictive factor for ORR (HR=0.92). It remains to be seen whether this difference in ORR would translate into long-term differences since a recent Phase Ⅲ trial (IMvigor211) that randomized 931 patients with metastatic UC to treatment with either atezolizumab or chemotherapy found a lower ORR and shorter OS in both intention-to-treat populations than in the PD-L1-positive-population, for both atezolizumab-treated and chemotherapy-treated patients, negating the potentially predictive value of PD-L1 expression.58
For PD-L1, three drug-specific tests have now been approved by the US Food and Drug Administration as either companion or complementary immunohistochemical assays.59 However, variations in staining platforms, antibodies, scoring guidelines, and definitions of PD-L1 positivity ranging from any expression to 50% complicate the use of PD-L1 as a biomarker in UC.60 Our findings supported the hypothesis that combined assessment of PD-L1 staining of TCs and ICs can predict the response to PD-1/PD-L1 antibody treatment in patients with UC. There are some biological rationale for evaluating IC PD-L1 expression as part of PD-L1 testing in future clinical trials.
Study limitations
Although extensive literature retrieval was conducted to provide a comprehensive analysis of PD-L1 and prognosis in patients with UC, there are limitations to this study. First, a lack of standardized assays and metrics for defining PD-L1 positivity implies that further studies are needed to establish a baseline for positive PD-L1expression. Second, some HRs and 95% CIs were calculated based on data extracted from Kaplan–Meier curves, and might be affected by the precision of the original data. Third, we were unable to evaluate the prognostic value of PD-L1 by stratifying patients according to their clinical features since most of the primary studies did not provide sufficient data for this analysis.
Conclusion
In conclusion, this meta-analysis suggests that PD-L1 expression on BC TCs is associated with more aggressive clinical features and reduced OS in patients with organ-confined disease. PD-1/PD-L1 targeted treatment in patients with UC was analyzed for response on the basis of expression of PD-L1 on both TCs and ICs. Although the benefit is greater in patients who are PD-L1-positive, patients with PD-L1-negative can also respond to anti-PD-1/PD-L1 therapy, so a more helpful marker is needed to determine the appropriate patient for anti-PD-1/PD-L1 therapy. Data on PD-L1 as a prognosis/predictor are currently being developed. Detection of these indicators before UC patients receive anti-PD-1/PD-L1 therapy should be included in future clinical trials.
Acknowledgments
This study was supported by Project of National Natural Science Foundation of China [No. 81860453 and No. 81602644], Basic Scientific Research Operating Expenses for Central Universities [No. buctrc201910], Major Project of Yunnan Provincial Department of Science and Technology Item [No. 2018ZF009] and National Key Research and Development Program [No. 2017YFA0105900].
Abbreviations
BC, bladder cancer; BCG, bacilli Calmette-Guerin; CPS, combined positive score; CSS, cancer-specific survival; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; FDA, Food and Drug Administration; IC, tumor-infiltrating immune cell; ICs, tumor-infiltrating immune cells; ORR, objective response rate; OS, overall survival; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand 1; RC, radical cystectomy; TC, tumor cell; UA, univariate analysis; UTUC, upper urinary tract urothelial carcinoma; UC, urothelial carcinoma.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704 Epub 2008/ 01/05. doi: 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Park HE, Cho NY, Lee HS, Kang GH. Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer. 2016;115(4):490–496. Epub 2016/ 07/13. doi: 10.1038/bjc.2016.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song M, Chen D, Lu B, et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS One. 2013;8(6):e65821 Epub 2013/ 06/21. doi: 10.1371/journal.pone.0065821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann oncol. 2014;25(11):2178–2184. Epub 2014/ 09/07. doi: 10.1093/annonc/mdu445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499–1505. Epub 2007/ 03/07. doi: 10.1002/cncr.22588 [DOI] [PubMed] [Google Scholar]
- 6.Boorjian SA, Frank I, Lohse CM, et al. T cell coregulatory molecule expression in urothelial cell carcinoma: A potential target for therapy. J Urol. 2008;179(4):265. doi: 10.1016/S0022-5347(08)60768-5 [DOI] [Google Scholar]
- 7.Xylinas E, Robinson BD, Kluth LA, et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur j surg oncol. 2014;40(1):121–127. Epub 2013/ 10/22. doi: 10.1016/j.ejso.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 8.Faraj SF, Munari E, Guner G, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology. 2015;85(3):703e1–703e6. Epub 2015/ 03/04. doi: 10.1016/j.urology.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann oncol. 2015;26(4):812–817. Epub 2015/ 01/21. doi: 10.1093/annonc/mdv009 [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immuno. 2007;56(8):1173–1182. doi: 10.1007/s00262-006-0266-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Zhang C, Hu J, et al. Effectiveness of anti-PD-1/PD-L1 antibodies in urothelial carcinoma patients with different PD-L1 expression levels: a meta-analysis. Oncotarget. 9(15):12400–12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. Epub 2014/ 11/28. doi: 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 Epub 2007/ 06/09. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. Epub 2010/ 07/24. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Zhang SD, McCrudden C, Chan KW, Lin Y, Kwok HF. The prognostic significance of PD-L1 in bladder cancer. Oncol Rep. 2015;33(6):3075–3084. Epub 2015/ 05/13. doi: 10.3892/or.2015.3933 [DOI] [PubMed] [Google Scholar]
- 17.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14(15):4800–4808. Epub 2008/ 08/05. doi: 10.1158/1078-0432.CCR-08-0731 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhuang Q, Zhou S, Hu Z, Lan R. Costimulatory molecule B7-H1 on the immune escape of bladder cancer and its clinical significance. J Huazhong Univ Sci Technol Medl sci. 2009;29(1):77–79. Epub 2009/ 02/19. doi: 10.1007/s11596-009-0116-2 [DOI] [PubMed] [Google Scholar]
- 19.Le Goux C, Damotte D, Vacher S, et al. Correlation between messenger RNA expression and protein expression of immune checkpoint-associated molecules in bladder urothelial carcinoma: A retrospective study. Urol Oncol. 2017;35(5):257–263. Epub 2017/ 03/16. doi: 10.1016/j.urolonc.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 20.Wu CT, Chen WC, Chang YH, Lin WY, Chen MF. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci Rep. 2016;6:19740 Epub 2016/ 01/26. doi: 10.1038/srep19740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YH, Cao YW, Yang XC, et al. Effect of TLR4 and B7-H1 on immune escape of urothelial bladder cancer and its clinical significance. Asian Pac J Cancer Prev. 2014;15(3):1321–1326. Epub 2014/ 03/13. [DOI] [PubMed] [Google Scholar]
- 22.Erlmeier F, Seitz AK, Hatzichristodoulou G, et al. The role of PD-L1 expression and intratumoral lymphocytes in response to perioperative chemotherapy for urothelial carcinoma. Bladder Cancer. 2016;2(4):425–432. Epub 2016/ 12/31. doi: 10.3233/BLC-160067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu ZH, Zheng FF, Mao YL, et al. Effects of programmed death-ligand 1 expression on OK-432 immunotherapy following transurethral resection in non-muscle invasive bladder cancer. Oncol Lett. 2017;13(6):4818–4824. Epub 2017/ 06/11. doi: 10.3892/ol.2017.6080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noro D, Hatakeyama S, Yoneyama T, et al. Post-chemotherapy PD-L1 expression correlates with clinical outcomes in Japanese bladder cancer patients treated with total cystectomy. Med Oncol. 2017;34:6. doi: 10.1007/s12032-017-0977-3 [DOI] [PubMed] [Google Scholar]
- 25.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558. doi: 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Yu W, Feng XR, et al. Prognostic significance of PD-L1 expression on tumor cells and tumor-infiltrating mononuclear cells in upper tract urothelial carcinoma. Med Oncol. 2017;34(5). doi: 10.1007/s12032-017-0941-2 [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. Epub 2016/ 03/10. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017. Available from:: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/885/CN-01299885/frame.html. doi: 10.1016/S1470-2045(17)30007-4 [DOI] [PubMed] [Google Scholar]
- 29.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J clin oncol. 2017;35(19):2117–2124. Epub 2017/ 04/05. doi: 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. Epub 2017/ 01/31. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 32.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590–1598. Epub 2016/ 10/14. doi: 10.1016/S1470-2045(16)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J clin oncol. 2016;34(26):3119–3125. Epub 2016/ 06/09. doi: 10.1200/JCO.2016.67.9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA oncol. 2017;3(9):e172411 Epub 2017/ 08/18. doi: 10.1001/jamaoncol.2017.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krabbe L-M, Heitplatz B, Preuss S, et al. Prognostic value of PD-1 and PD-L1 expression in patients with high grade upper tract urothelial carcinoma. J Urol. 2017;198(6):1254–1263. doi: 10.1016/j.juro.2017.06.086 [DOI] [PubMed] [Google Scholar]
- 36.Skala SL, Liu T-Y, Udager AM, et al. Programmed death-ligand 1 expression in upper tract urothelial carcinoma. Eur Urol Focus. 2017;3(4–5):502–509. doi: 10.1016/j.euf.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 37.Tretiakova M, Fulton R, Kocherginsky M, et al. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: comparison of four commonly used antibodies and RNA expression. Mod Pathol. 2017. Epub 2017/ 12/23. [DOI] [PubMed] [Google Scholar]
- 38.Pichler R, Heidegger I, Fritz J, et al. PD-L1 expression in bladder cancer and metastasis and its influence on oncologic outcome after cystectomy. Oncotarget. 2017;8(40):66849–66864. doi: 10.18632/oncotarget.19913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–1266. Epub 2003/ 01/23. [DOI] [PubMed] [Google Scholar]
- 40.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. Epub 2012/ 06/05. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101(49):17174–17179. Epub 2004/ 12/01. doi: 10.1073/pnas.0406351101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. Epub 2007/ 03/16. doi: 10.1073/pnas.0611533104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. Epub 2002/ 07/02. doi: 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 44.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. Epub 2005/ 02/12. [PubMed] [Google Scholar]
- 45.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. Epub 2010/ 04/08. doi: 10.1007/s12032-010-9515-2 [DOI] [PubMed] [Google Scholar]
- 46.Noro D, Hatakeyama S, Yoneyama T, et al. Post-chemotherapy PD-L1 expression correlates with clinical outcomes in Japanese bladder cancer patients treated with total cystectomy. Med Oncol. 2017;34(6):117 Epub 2017/ 05/14. doi: 10.1007/s12032-017-0977-3 [DOI] [PubMed] [Google Scholar]
- 47.Sznurkowski JJ, Zawrocki A, Sznurkowska K, Peksa R, Biernat W. PD-L1 expression on immune cells is a favorable prognostic factor for vulvar squamous cell carcinoma patients. Oncotarget. 2017;8(52):89903–89912. Epub 2017/ 11/23. doi: 10.18632/oncotarget.20911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan D, Sheng L, Yi Q. Correlation of PD-1/PD-L1 polymorphisms and expressions with clinicopathologic features and prognosis of ovarian cancer. Cancer Biomarkers. 2017. Epub 2017/ 11/25. [DOI] [PubMed] [Google Scholar]
- 49.AiErken N, Shi HJ, Zhou Y, et al. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in chinese triple negative breast cancer patients. Int J Biol Sci. 2017;13(9):1172–1179. Epub 2017/ 11/07. doi: 10.7150/ijbs.20868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67 Epub 2017/ 02/20. doi: 10.1016/j.ctrv.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 51.Popovic LS, Matovina-Brko G, Popovic M. Checkpoint inhibitors in the treatment of urological malignancies. ESMO Open. 2017;2(2):e000165 Epub 2017/ 08/02. doi: 10.1136/esmoopen-2017-000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499–1505. doi: 10.1002/cncr.22588 [DOI] [PubMed] [Google Scholar]
- 53.Karakiewicz PI, Shariat SF, Palapattu GS, et al. Precystectomy nomogram for prediction of advanced bladder cancer stage. Eur Urol. 2006;50(6):1254–60;discussion 61–2 Epub 2006/ 07/13. doi: 10.1016/j.eururo.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 54.Shariat SF, Chromecki TF, Cha EK, et al. Risk stratification of organ confined bladder cancer after radical cystectomy using cell cycle related biomarkers. J Urol. 2012;187(2):457–462. Epub 2011/ 12/20. doi: 10.1016/j.juro.2011.10.031 [DOI] [PubMed] [Google Scholar]
- 55.von Rundstedt FC, Mata DA, Groshen S, et al. Significance of lymphovascular invasion in organ-confined, node-negative urothelial cancer of the bladder: data from the prospective p53-MVAC trial. BJU Int. 2015;116(1):44–49. Epub 2014/ 11/22. doi: 10.1111/bju.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boorjian SA, Sheinin Y, Crispen PL, et al. T-Cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14(15):4800–4808. doi: 10.1158/1078-0432.CCR-08-0731 [DOI] [PubMed] [Google Scholar]
- 57.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2017. Epub 2017/ 12/23. [DOI] [PubMed] [Google Scholar]
- 59.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA oncol. 2017;3(8):1051–1058. Epub 2017/ 03/10. doi: 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Nunno V, De Luca E, Buttigliero C, et al. Immune-checkpoint inhibitors in previously treated patients with advanced or metastatic urothelial carcinoma: A systematic review and meta-analysis. 1879–0461. (Electronic). [DOI] [PubMed] [Google Scholar]