Abstract
Non-pharmacological interventions designed to change cognitive function in older adults with Mild Cognitive Impairment have shown mixed results. Few studied interventions directly address preclinical disability. Slowing changes in disability are critical preserve independence and health related quality of life in older adults with Mild Cognitive Impairment. In this study, we discuss the design of the trial, challenges encountered, and solutions generated to guide future trials designed to prevent the onset of disability among at-risk older adults. We compared Strategy Training to enhanced-usual care in 30 older adults with Mild Cognitive Impairment. We recruited 79.7% (n = 188) of the potential participants through direct-to-consumer recruitment. We refined a three-step screening process, including a phone screen, initial in-person screening, and full in-person screening. This screening processes resulted in a high percentage of older adults completing the neuropsychological battery and adjudication of Mild Cognitive Impairment. Conducting a disability prevention among individuals without overt disability is a novel approach. Nevertheless, one of the greatest limitations to our project is the fact that follow-up is restricted to 1 year. Findings from this study can inform the design and conduct of future clinical trials that seek to slow progression of disability in older adults with Mild Cognitive Impairment.
Keywords: Prevention clinical trials, Mild cognitive impairment, Non-pharmacological interventions, Pre-clinical disability
1. Introduction
In this report, we describe the process for conducting an intervention study designed to prevent disability in older adults with Mild Cognitive Impairment (MCI) and depressive symptoms. MCI is the state between normal cognitive aging and dementia. Older adults with MCI have a high risk of developing dementia, as well as disability associated with dementia. Unfortunately, 50% of individuals with MCI experience elevated depressive symptoms, which confer accelerated cognitive decline [1] and increased risk of both disability and a diagnosis of dementia [2].
The extant literature on the progression to disability for individuals at-risk for dementia is inconclusive because disability was historically only considered once an individual was diagnosed with dementia. However, it is increasingly clear that individuals with MCI demonstrate preclinical disability [3]. Preclinical disability is defined as subtle differences in the effort required and adaptations noted in daily activities [4]. Individuals with MCI most often demonstrate preclinical disability in cognitively challenging activities (i.e., financial management and medication management) [3].
To date, non-pharmacological interventions designed to change cognitive function in older adults with MCI have shown mixed results [5]. Some cognitive training interventions, physical exercise, and psychotherapeutic interventions demonstrate greater improvements than care as usual in selected cognitive domains with small effect sizes [5]. Few directly address preclinical disability. Since preclinical disability emerges early in the disease trajectory, and is potentially more amenable to slowing, interventions that address preclinical disability are timely and critical with respect to the broader goal of preserving independence and health related quality of life.
We designed a pilot intervention study that aimed to delay the onset of overt disability by teaching individuals with MCI and depressive symptoms strategies to work through and around preclinical disability. Our intervention employed a process known as “Strategy Training”, which is based on the principles of behavioral activation, viz., avoidance behaviors are developed to limit exposure to negative stimuli or difficult activities [6,7]; however, such avoidant coping may lead to the worsening of disability and mood. By engaging in an intervention based on behavioral activation, the downward spiral of avoidant coping is disrupted by facilitating and reinforcing a positive problem orientation and active coping strategies, to maintain effective engagement in meaningful activities for as long as possible. In this report, we discuss the design of the trial, challenges encountered, and solutions generated to guide future trials designed to prevent the onset of disability among at-risk older adults.
2. Methods
2.1. Study design and specific aims
We conducted a randomized controlled trial to examine:
-
•
Whether differences existed in preclinical disability, as measured by the Performance Assessment of Self-Care Skills [8,9], at 12 months between Strategy Training and Enhanced-Usual Care groups.
-
•
relationships between clinical variables and preclinical disability. We sought to examine the relationships of (1) physical activity levels, (2) sleep, and (3) medical co-morbidity with disability.
Participants were randomized to Strategy Training and Enhanced-Usual Care using a 1:1 allocation with permuted blocks of varying size. We used a computer-generated random numbers table for randomization. Participants were stratified by whether a support person was involved because intervention uptake may be positively influenced by involvement of others in a participant's life.
2.2. Participants
Participants were adjudicated to have MCI (criteria provided below), a minimum of 60 years of age, living in the community, scored ≥1 on the Patient Health Questionnaire 2-item version (PHQ-2) assessing low mood and anhedonia [10], and had self-reported difficulty in at least 1 daily activity (see Table 1). Exclusion criteria included having complex medical history, such as a central nervous system disorder (other than MCI), presenting with current major depression, reporting alcohol or illicit substance use disorder in past 5 years, or having a lifetime history of bipolar disorder or schizophrenia. These criteria ensure that included participants had MCI and depressive symptoms but not conditions that may influence the diagnostic process or study outcomes for another reason. If potential participants presented with an exclusion criterion, they were referred for treatment outside of the study.
Table 1.
Inclusion and exclusion criteria and rationale for these decisions.
| Inclusion Criteria | Rationale |
|---|---|
| Age ≥60 years | Incidence and prevalence of MCI increases after age 60 Interventions designed to prevent disability in older adults are different than those for younger adults |
| Adjudicated to have MCI | Adjudication of MCI ensured commonality of cognitive changes experienced by participants |
| PHQ-2>0 (low mood or anhedonia endorsed) | Endorsement of cardinal symptoms of depression places individuals in the subthreshold range for depression, making them at greater risk for decline |
| Self-reported difficulty in an activity of daily living | Self-reported difficulty resulted in the inclusion of participants who may be more likely to identify areas for improvement |
| Community-dwelling |
Interventions designed to support individuals living in the community are different than interventions for individuals not living in the community |
|
Exclusion Criteria |
Rationale |
| Current episode of major depression | Comorbidity may influence the outcome. Treatment should be sought outside of this study |
| Central nervous system disorder (other than MCI) | Other central nervous system disorder may interfere with the ability to fully participate in the intervention |
| Alcohol or illicit substance use in the past 5 years | Comorbidity may influence the outcome. Treatment should be sought outside of this study |
| Lifetime history of bipolar of schizophrenia | Comorbidity may influence the outcome. Treatment should be sought outside of this study |
MCI diagnoses were conferred using a comprehensive assessment program suggested by National Institute on Aging-Alzheimer's Association criteria [11]. We used a three-step process comprised of a phone screen, initial in-person screening, and full in-person screening. The phone screen assessed for endorsement of depressive symptoms [10] and self-report of difficulty in a daily activity. The initial in-person screen was comprised of the Modified Mini-mental State Examination (3MS) [12], Trail Making Test A and B (Trails) [13], and the Quick Mild Cognitive Impairment Screen (Qmci) [14]. Individuals who scored <84 on the 3MS were excluded from the study due to potential dementia. Those who scored in the dementia range on Trails and Qmci (>2 SD below normed means) were also excluded from the study. Those who scored ≥1 SD below normed means on either or both Trails or Qmci proceeded to the full in-person screen.
The full in-person screen consisted of the Wide Range Achievement Test-IV Reading Subtest [15], Repeatable Battery for the Assessment of Neuropsychological Status [16], Delis-Kaplan Executive Function System Trail Making and Color-Word Tests [17], Measurement of Everyday Cognition [18], Older Americans Resources and Services [19], Clinical Dementia Rating Scale [20], Unified Parkinson's Disease Rating Scale [21], and Cumulative Illness Rating Scale- Geriatric [22]. Data gleaned during the assessments were reviewed by a neuropsychologist (MAB), neurologist (OL), and occupational therapist (JR), to diagnose MCI.
2.3. Recruitment
Participants were recruited from the Alzheimer's Disease Research Center (ADRC) at the University of Pittsburgh, as well as community-based outreach as appropriate. We engaged with partners across the University, including the Clinical and Translational Science Institute (CTSI).
2.4. Schedule of assessments
An independent assessment team, who was blinded from the condition to which participants were randomized, completed the assessments of study outcomes under the supervision of a neuropsychologist (MAB). Assessors were trained in neuropsychological and all other assessments before conducting assessments for the study.
The primary outcome was preclinical disability as measured with the Performance Assessment of Self-Care Skills (PASS) through standardized, criterion-referenced observations of cognitively challenging instrumental daily activities [3,8,9]. The activities assessed were shopping, bill paying, checkbook balancing, bill mailing, telephone use, medication management, critical information retrieval, and small device repair. We summed the number of cues the independent assessor provided the participant during the completion of the activities. The types of cues could have been verbal, gestural, or physical. The overall score has yielded good internal consistency rating, based on a Cronbach's alpha, of 0.71. The PASS has been found to have good reliability and validity. In particular, the tool has excellent measurement stability and is sensitive to change over time [8]. The PASS was administered pre-intervention, 6 weeks, 3, 6, 9- and 12-months follow-up.
We used additional assessments to measure the following domains: medical co-morbidity, depressive symptom severity, anxiety symptoms severity, perception of sleep quality, degree of daily physical activity, perceived level of social support, self-appraisal coping, and presence of worry (Table 2).
Table 2.
Study assessments.
| Domain | Measure |
|---|---|
| Disability | Performance Assessment of Self-Care Skills [8] |
| Cognitive Status | Delis-Kaplan Executive Function System [17] Repeatable Battery for the Assessment of Neuropsychological Status [16] Modified Mini-Mental Status Examination [12] |
| Medical Co-Morbidity | Cumulative Illness Rating Scale- Geriatric [22] and current medication list |
| Depression symptom Severity | Patient Health Questionnaire- 9 [38] |
| Anxiety symptom severity | Generalized Anxiety Disorder- 7 [39] |
| Occurrence of Insomnia | Pittsburgh Sleep Quality Index- 1 [40] |
| Physical Activity | Physical Activity Scale for Elderly [41] |
| Social Support | Interpersonal Support Evaluation-12 [42] |
| Self-Appraisal of ability to take care of self | Appraisal of Self-Care Agency Scale [43] |
| Coping | Ways of Coping [44] |
| Worry | Penn State Worry Questionnaire [45] |
2.5. Intervention
2.5.1. Strategy Training
Strategy Training is based on the theoretical and empirical evidence associated with behavioral activation. It is a behavioral approach to help older adults who are beginning to experience preclinical disability to develop solutions and skills to preserve their abilities in daily activities (i.e., independence). Strategy Training optimizes engagement in meaningful daily activities through four integrated components: (i) generating self-selected goals that address activities [23], (ii) monitoring daily activities to provide information on baseline level of activities and to demonstrate the value of participating in activities, (iii) scheduling activities to increase contact with other individuals and participation in pleasurable activities, and (iv) problem-solving solutions to remove or circumvent barriers experienced when engaging in activities [5,[24], [25], [26], [27]]. The hallmark of Strategy Training is its focus on daily activities. Therapists facilitate participants' engagement in daily activities through prompts, questions, and problem-solving. Participants develop goals and develop their own strategies to improve their ability to perform daily activities. Behavioral activation suggests that behavior changes derived through this method are sustainable because participants focus on what they want to do and how they want to do it [28]. Strategy Training is promising in its potential for slowing decline to disability for older adults with MCI and depressive symptoms [5,23,24,29] because older adults with mild cognitive impairments become willing and able to maintain the ability to develop strategies for engaging in daily activities [30,31].
2.5.2. Enhanced-usual care
Participants assigned to Enhanced-Usual Care received the same assessments as those assigned to Strategy Training, but they did not receive the Strategy Training intervention or another intervention. All participants received feedback on their thinking and memory status, and, as appropriate, participants were referred to outside services. For example, if a participant presented with more than mild depressive or anxiety symptoms, they were referred to services; the same practice was followed in the intervention arm. Participants' experience was considered enhanced because they received close monitoring of their thinking, memory, and mood.
2.6. Interventionist training
Registered and licensed occupational therapists with expertise in the care of older adults served as interventionists for Strategy Training. Experts in Strategy Training (JR, ERS, KWG) trained the interventionists in the protocol using multiple modalities. First, an in-person session described the background and the intervention protocol. Therapists were then provided with video-recorded case examples to review. A subsequent in-person session provided a platform for questions and answers. An expert in Strategy Training (KWG) reviewed video and provided feedback to intervention therapists on protocol adherence and competence. Consistent protocol adherence and competence was generally reached after completion of 2 intervention participants. After training, all sessions are videotaped to assess treatment fidelity. A trained external rater assessed treatment fidelity in a random sample of 20% of the Strategy Training intervention sessions. Our assessment of treatment fidelity was multifaceted, and it included adherence and competence for the Strategy Training procedures and implementation of Strategy Training's active components. Adherence was rated as yes or no. Competence was rated as inadequate, adequate or exceptional.
2.7. Planned analysis
We plan to use descriptive statistics to assess the efficacy of Strategy Training on the primary and secondary outcomes. We will examine differences in preclinical disability, as measured by the PASS, at 12 months between Strategy Training and Enhanced-Usual Care groups. We will use an independent t-test to explore differences between Strategy Training and Enhanced-Usual Care at 12-months. The results of the t-test will provide parameter estimates, standard errors, and 95% confidence intervals, which will be examined to assess the magnitude of the between-group difference. Statistical significance will be determined based on an alpha level of ≤0.05. If t-test assumptions are not met, we will use the non-parametric equivalent. We will explore factors that may influence intervention response by examining associations between (1) physical activity, (2) sleep, and (3) medical co-morbidity with disability using correlational techniques. We will use Pearson correlations with continuous factors, and we will use Spearman correlations with ordinal factors.
2.8. Sample size justification
A formal sample size calculation was not performed due to the pilot feasibility nature of the study. We estimated that approximately 80% of participants will complete the intervention program with the 1-year follow-up (24/30 participants). Findings from this study will provide preliminary information relevant to conduct power analyses for future studies.
3. Results
Table 3 includes a list of the challenges encountered and the effective solutions. Our research team, including the staff and principal investigator, met on a weekly basis to discuss recruitment, assessments, intervention, and follow-up to develop effective solutions. Themes for (1) recruitment, (2) assessment procedures, and (3) intervention are below.
Table 3.
Challenges and solutions.
| Challenges | Solutions |
|---|---|
| Recruitment | |
|
|
|
|
|
|
| Assessment | |
|
|
|
|
|
|
|
|
| Intervention | |
|
|
|
|
|
|
3.1. Recruitment
Identifying and recruiting individuals with mild cognitive impairments and depressive symptoms required a multipronged approach. In addition to using developed infrastructure at the ADRC and the University of Pittsburgh (such as the CTSI), we invested resources to develop several community-based partnerships to reach older adults who may be interested in participating in research. We built relationships with independent living facilities, public libraries, senior high-rises, senior centers, community centers, and via participation in community services and events (e.g., health fairs). We employed a variety of methods for recruitment with the help of these relationships, including visual advertisements in print- and on-air media, radio advertisements, internet advertising, and community mailings. Based on these partnerships, we gave presentations on the influence of aging-related changes in thinking and memory on daily activities. Afterward, interested participants provided contact information for further screening.
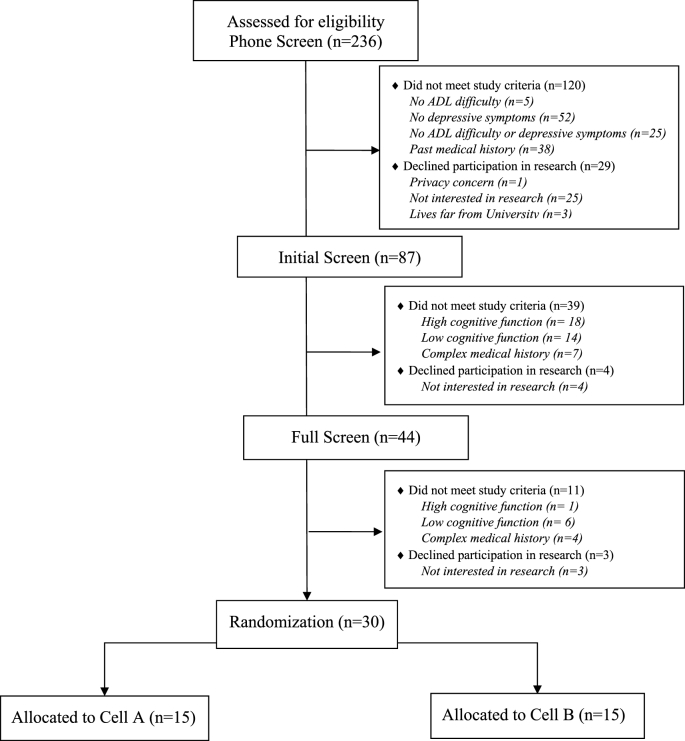
Fig. 1 shows our CONSORT diagram up until the point of randomization where we assessed 236 individuals for eligibility and randomized 30 individuals. Among those we assessed, we recruited 79.7% (n = 188) of the potential participants through direct-to-consumer recruitment (e.g., community-based partnership talks, radio-advertising, social media, Craigslist). The rest were recruited through university-related research registries (18.2%, n = 43), and primary care practices (2.1%, n = 5).
Fig. 1.
CONSORT diagram.
Table 4 provides the descriptive statistics for demographic characteristics for all 30 participants at baseline. In brief, participants were, on average, older than 75 years, majority female, majority white, with high school education or above, reported mild depressive symptoms, mild anxiety, fairly good sleep, and average levels of physical activity.
Table 4.
Baseline Characteristics (N = 30) mean (sd).
| Variable | Descriptive Statistics |
||
|---|---|---|---|
| Range | |||
| Age, mean (SD) | 77.97 | (8.68) | 62–96 |
| Female, n (%) | 20 | (66.7) | |
| White, n (%) | 26 | (86.7) | |
| Education, n (%) | |||
| High School Educated | 13 | (43.4) | |
| Vocational or Associates | 4 | (13.3) | |
| Bachelors | 6 | (20.0) | |
| Master and above | 7 | (23.3) | |
| Performance Assessment of Self-Care Skills (range: 0–486), mean (SD)* | 17.97 | (9.69) | 2–37 |
| DKEFS (range: 0–19; mean:10; SD:3), mean (SD) | |||
| Trail Making Test: Set Shifting Scaled Score | 9.07 | (3.79) | 3–14 |
| Trail Making Test: Speed Scaled Score | 10.50 | (2.24) | 5–14 |
| Trail Making Test: Set Shifting vs. Motor | 8.57 | (3.45) | 2–15 |
| Color Word: Inhibition-Switching Scaled Score | 10.86 | (3.34) | 1–16 |
| RBANS (range: 40–160; mean:100; SD:15), mean (SD) | |||
| Immediate Memory Index | 96.77 | (12.35) | 76–120 |
| Language Memory Index | 99.10 | (12.57) | 71–127 |
| Delayed Memory Index | 95.03 | (14.44) | 52–126 |
| Attention Index | 101.60 | (18.36) | 68–142 |
| Visuospatial Index | 93.87 | (14.91) | 58–121 |
| Total Score Index | 95.70 | (11.04) | 82–133 |
| Modified Mini-Mental State Examination (range: 1–100), mean (SD) | 93.6 | (3.91) | 84–99 |
| Patient Health Questionnaire-9 (range: 0–27), mean (SD)* | 5.6 | (4.75) | 0–20 |
| Generalized Anxiety Disorder-7 (range: 0–21), mean (SD)* | 3.9 | (4.16) | 0–18 |
| Pittsburgh Sleep Quality Index-1 (range: 0–3), mean (SD)* | 0.97 | (0.93) | 0–3 |
| Physical Activity Scale for the Elderly Questionnaire (range: 0–400), mean (SD) | 109.25 | (72.70) | 20.8–278.1 |
| Interpersonal Support Evaluation List- 12 (range: 12–48), mean (SD)* | 38.5 | (6.49) | 27–47 |
| Appraisal of Self-Care Agency Scale (range: 24–120), mean (SD) | 83.33 | (4.80) | 73–91 |
| Ways of Coping (range: 0–198), mean (SD) | 82.63 | (25.20) | 31–123 |
| Penn State Worry Questionnaire (range: 16–80), mean (SD)* | 43.67 | (11.62) | 22–67 |
Notes: DKEFS: Delis-Kaplin Executive Function System; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; *lower is better.
3.2. Assessment procedures
We employed a multistep screening process such that only individuals most likely to have MCI completed the neuropsychological examinations. Among the individuals contacted for phone-screen, 63.1% (n = 149) were deemed ineligible. The primary reasons for exclusion were lack of depressive symptoms (34.9%, n = 52) and complexity of past medical history (25.5%, n = 38). Forty-three (49.4%) potential participants did not meet study criteria after the initial screen. Individuals were excluded due to high cognitive function 41.9% (n = 18), low cognitive function 32.6% (n = 14), and a complex medical history 16.3% (n = 7). Additionally, 4 individuals withdrew due to lack of interest in research participation. Fourteen (31.8%) potential participants did not meet study criteria after the full clinical and neuropsychological examinations. Individuals were excluded due to high cognitive function 7.1% (n = 1), low cognitive function 42.9% (n = 6), and a complex medical history 28.6% (n = 4). Per our recruitment and screening process, 30 out of 45 individuals who completed the neuropsychological examinations were adjudicated to have MCI. Three participants withdrew before randomization. In the end, 30 were adjudicated to have MCI and depressive symptoms and were randomly assigned to Strategy Training or Enhanced-Usual Care.
3.3. Intervention
We developed a standardized goal-setting process that was modified from the Activity Card Sort [32]. Described elsewhere, it is a theoretical hallmark of behavioral activation [28]. It ensures that the intervention is personally meaningful to the participants. Administration of the goal-setting process took between one to three sessions to complete. Guided by the occupational therapist, participants would identify activities and prioritize a minimum of 5 personally meaningful goals.
After the first two study participants, we recognized that participants quickly demonstrated the acquisition and use of the global strategy in the addressed activity; that is, participants were learning how to apply their self-identified strategies to their activity of interest. We noticed, however, that participants were not using their effective strategies in related daily activities; they were not generalizing their strategies to other activities. Generalization is considered the use of the same strategy with a different activity or in a new context. Thus, we increased our emphasis on the generalization of strategy use during the intervention sessions. This aligns with behavioral activation and encourages active problem solving and use of coping strategies to main engagement in daily activities. We enhanced generalization by implementing questions in each intervention session about effective and ineffective strategies and related activities or contexts where effective strategies could be applied.
Informal feedback from participants indicated satisfaction with the Strategy Training intervention. One participant said, “this was the first [study] so far that made an impact on me … This [study] is the one that gave something back to me.” Another participant indicated that s/he was able to develop effective strategies to work through and around barriers to daily activities, saying, “When I have a problem, I think about the fact that I have a plan".
3.4. Intervention fidelity
In our preliminary studies, Strategy Training therapists demonstrated excellent treatment fidelity, demonstrated that our training and supervision achieved good treatment fidelity. Therapists implemented and demonstrated competence in the Strategy Training procedures and active components more than 90% of the time. Table 5 depicts the percentages of adherence and competence to the procedures and active components.
Table 5.
Percentages of adherence and competence to treatment principles.
| Variable | Percent |
|---|---|
| Adherence to procedures | 93 |
| Competence with procedures | 95 |
| Adherence to active components | 97 |
| Competence with active components | 98 |
4. Discussion
This pilot intervention development study is based on the theoretical framework of behavioral activation and is designed to delay the onset of disability by teaching individuals with MCI and depressive symptoms strategies to work through and around preclinical disability. Since preclinical disability emerges early in the disease trajectory [3,33], interventions that address preclinical disability and daily life are timely and critical. This intervention approach is unique in its focus on preclinical disability; few current nonpharmacological interventions address or influence preclinical disability [5].
Our experience suggests that with a strong emphasis on recruitment, it is feasible to recruit community-dwelling older adults with MCI and depressive symptoms for a disability-prevention study. Multiple direct consumer recruitment methods were successful in reaching older adults who were interested in participating in research. Ultimately, the majority (80%) of our interested participants were recruited through direct consumer recruitment methods. These methods require delivering presentations to groups of older adults about aging and the progression of disability. While community-based sampling methods require investment from staff and consistent monitoring to ensure enrollment of interested individuals, we reached our recruitment targets.
Our stepped screening and assessment procedures were helpful in optimizing efficient use of staff resources and in reducing participant burden. Two-thirds of the potential participants who completed the neuropsychological examinations during the full assessment phase, were adjudicated to have MCI. With the prevalence of MCI being an estimated 5.9 (5.5–6.3)% of older adults [34], our protocol was efficient in identifying older adults for the study who were likely to have MCI.
Non-pharmacological interventions, if they are to be beneficial for older adults with cognitive decline, are likely to be complex interventions. The Medical Research Council guidelines for developing and evaluating complex interventions encourages careful conceptualization and operationalization of the intervention using a sound theoretical background to understand how change occurs [35]. With the Medical Research Council guidelines in mind, the combination of the Strategy Training components for older adults with MCI and depressive symptoms were carefully conceptualized and operationalized for further implementation and study. We trained three occupational therapists to implement Strategy Training for older adults with MCI and depressive symptoms. They subjectively reported satisfaction with the training and protocol clarity. They indicated that Strategy Training provides an explicit framework for what they think to be best practice. One therapist commented, “This intervention has made me a better therapist”, and “I learned skills that work with everyone that I treat, whether in research or my practice.” This subjective feedback suggests that we have the infrastructure required to conduct therapist training.
Several additional points from this study are worth highlighting. This project necessarily involves a multidisciplinary team. A research groups with diverse expertise was required to address the complex issues related to cognitive decline and the risk architecture associated with disablement. Our team included an occupational therapist with expertise in disability, a neurologist, a neuropsychologist, a geriatric psychiatrist, and a methodologist with expertise in prevention trials. Prevention of disability in a group of older adults with MCI and depressive symptoms requires this expertise to assess cognition and conduct adjudications, monitor depressive symptoms, and deliver the intervention protocol designed to slow decline to overt disability.
Conducting a disability prevention among individuals without overt disability is a novel approach. Nevertheless, one of the greatest limitations to our project is the fact that follow-up is restricted to 1 year. While this is similar to other prevention intervention protocols [36], an ideal protocol would follow participants longer, because (1) preclinical disability, while present, is subtle [33] and (2) the rate of conversion from MCI to dementia is around 10% per year [37]. An ideal protocol would follow older adult with MCI until the onset of overt disability which may not occur until the onset of dementia. Then, the time of onset of disability could be compared between groups.
Given the great personal and societal effects of disability associated with dementia and limited numbers of effective interventions that influence disease pathology, a promising non-pharmacological intervention designed to slow decline to disability is critical. We will report findings from this trial once available.
Funding sources
Funding was received from the National Institute of Health grants KL2 TR001856, P30 MH090333, P50 AG05133, R01 AG056351, UL1 TR000005, American Occupational Therapy Foundation, and the UPMC Endowed Chair in Geriatric Psychiatry.
References
- 1.Palmer K., Di Iulio F., Varsi A.E., Gianni W., Sancesario G., Caltagirone C., Spalletta G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J. Alzheimer's Dis. 2010;20:175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulos G.S., Kiosses D.N., Klimstra S., Kalayam B., Bruce M.L. Clinical presentation of the "depression-executive dysfunction syndrome" of late lif. Am. J. Geriatr. Psychiatry. May 18, 2018:98–106. http://www.ncbi.nlm.nih.gov/pubmed/11790640 10 (n.d.) accessed. [PubMed] [Google Scholar]
- 3.Rodakowski J., Skidmore E.R.E.R., Reynolds C.F.C.F., Dew M.A.M.A., Butters M.A.M.A., Holm M.B.M.B., Lopez O.L.O.L., Rogers J.C.J.C. Can performance on daily activities discriminate between older adults with normal cognitive function and those with mild cognitive impairment? J. Am. Geriatr. Soc. 2014;62:1347–1352. doi: 10.1111/jgs.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazer D. Neurocognitive disorders in DSM-5. Am. J. Psychiatry. 2013;170:585–587. doi: 10.1176/appi.ajp.2013.13020179. [DOI] [PubMed] [Google Scholar]
- 5.Rodakowski J., Saghafi E., Butters M.A., Skidmore E.R. Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: an updated scoping review. Mol. Aspect. Med. 2015:43–44. doi: 10.1016/j.mam.2015.06.003. 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuijpers P., van Straten A., Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin. Psychol. Rev. 2007;27:318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Rovner B.W., Casten R.J., Hegel M.T., Leiby B.E. Preventing cognitive decline in older African Americans with mild cognitive impairment: design and methods of a randomized clinical trial. Contemp. Clin. Trials. 2012;33:712–720. doi: 10.1016/j.cct.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holm M.B., Rogers J.C. The performance assessment of self-care skills ( PASS ) In: Hemphill-Pearson B., editor. Assessments Occup. Ther. Ment. Heal. second ed. SLACK; ThoroFare, NJ: 2008. pp. 101–110. [Google Scholar]
- 9.Rogers J., Holm M. 1989. Performance Assessment of Self-Care Skills (PASS, Versions 3.1) Pittsburgh. [Google Scholar]
- 10.Kroenke K., Spitzer R.L., Williams J.B.W. The patient health questionnaire-2. Med. Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 11.Beekly D.L., Ramos E.M., Lee W.W., Deitrich W.D., Jacka M.E., Wu J., Hubbard J.L., Koepsell T.D., Morris J.C., Kukull W.A. The National Alzheimer's coordinating center (NACC) database: the uniform data set. Alzheimers Dis. Assoc. Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 12.Teng E.L., Chui H.C. The modified mini-mental state (3MS) examination. J. Clin. Psychiatry. 1987;48:314–318. http://www.ncbi.nlm.nih.gov/pubmed/3611032 accessed. [PubMed] [Google Scholar]
- 13.Tombaugh T. Trail Making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 14.O'Caoimh R., Gao Y., McGlade C., Healy L., Gallagher P., Timmons S., Molloy D.W. Comparison of the quick mild cognitive impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing. 2012;41:624–629. doi: 10.1093/ageing/afs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kareken D.A., Gur R.C., Saykin A.J. Reading on the Wide range achievement test-revised and parental education as predictors of IQ: comparison with the barona formula. Arch. Clin. Neuropsychol. 1995;10:147–157. [PubMed] [Google Scholar]
- 16.Randolph C., Tierney M.C., Mohr E., Chase T.N. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 17.Delis D. D-KEFS; 2001. Delis-Kaplan Executive Function System.http://www.worldcat.org/title/delis-kaplan-executive-function-system-d-kefs/oclc/812888503 accessed. [Google Scholar]
- 18.Tomaszewski Farias S., Mungas D., Harvey D.J., Simmons A., Reed B.R., Decarli C. The measurement of everyday cognition: development and validation of a short form of the Everyday Cognition scales. Alzheimers. Dement. 2011;7:593–601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.G.G. Fillenbaum, Multidimensional Functional Assessment of Older Adults: the Duke Older Americans Resources and Services Procedures., (n.d).
- 20.Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. http://www.ncbi.nlm.nih.gov/pubmed/7104545 accessed. [DOI] [PubMed] [Google Scholar]
- 21.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease The unified Parkinson's disease rating scale (UPDRS): status and recommendations. Mov. Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 22.Linn B.S., Linn M.W., Gurel L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. http://www.ncbi.nlm.nih.gov/pubmed/5646906 accessed. [DOI] [PubMed] [Google Scholar]
- 23.Rodakowski J., Becker A.M., Golias K.W. Activity-based goals generated by older adults with mild cognitive impairment. OTJR Occup. Participation Health. 2018 doi: 10.1177/1539449217751357. 153944921775135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodakowski J., Reynolds C.F., Lopez O.L., Butters M.A., Dew M.A., Skidmore E.R. Developing a non-pharmacological intervention for individuals with mild cognitive impairment. J. Appl. Gerontol. 2016 doi: 10.1177/0733464816645808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson D., Richardson J., Troyer A., Binns M., Clark A., Polatajko H., Winocur G., Hunt A., Bar Y. An occupation-based strategy training approach to managing age-related executive changes: a pilot randomized controlled trial. Clin. Rehabil. 2014;28:118–127. doi: 10.1177/0269215513492541. [DOI] [PubMed] [Google Scholar]
- 26.Skidmore E.R., Whyte E.M., Butters M.A., Terhorst L., Reynolds C.F. Strategy training during inpatient rehabilitation may prevent apathy symptoms after acute stroke. Pharm. Manag. PM R. 2015;7:562–570. doi: 10.1016/j.pmrj.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skidmore E.R., Butters M., Whyte E., Grattan E., Shen J., Terhorst L. Guided training relative to direct skill training for individuals with cognitive impairments after stroke: a pilot randomized trial. Arch. Phys. Med. Rehabil. 2017;98:673–680. doi: 10.1016/j.apmr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanter J.W., Manos R.C., Bowe W.M., Baruch D.E., Busch A.M., Rusch L.C. What is behavioral activation?A review of the empirical literature. Clin. Psychol. Rev. 2010;30:608–620. doi: 10.1016/j.cpr.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Rodakowski J., Skidmore E.R. Non-pharmacological interventions for early cognitive decline. Am. J. Geriatr. Psychiatry. 2017;25 doi: 10.1016/j.jagp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Ballesteros R., Zamarrón M.D., Tárraga L., Moya R., Iñiguez J. Cognitive plasticity in healthy, mild cognitive impairment (MCI) subjects and Alzheimer's disease patients: a research project in Spain. Eur. Psychol. 2003;8:148–159. [Google Scholar]
- 31.Mufson E.J., Ikonomovic M.D., Styren S.D., Counts S.E., Wuu J., Leurgans S., Bennett D.A., Cochran E.J., DeKosky S.T. Preservation of brain nerve growth factor in mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2003;60:1143–1148. doi: 10.1001/archneur.60.8.1143. [DOI] [PubMed] [Google Scholar]
- 32.Baum D.F., M C., Edwards . second ed. AOTA Press; Bethesda, MD: 2008. Activity Card Sort Test Manual. [Google Scholar]
- 33.Jekel K., Damian M., Wattmo C., Hausner L., Bullock R., Connelly P.J., Dubois B., Eriksdotter M., Ewers M., Graessel E., Kramberger M.G., Law E., Mecocci P., Molinuevo J.L., Nygård L., Olde-Rikkert M.G., Orgogozo J.-M., Pasquier F., Peres K., Salmon E., Sikkes S.A., Sobow T., Spiegel R., Tsolaki M., Winblad B., Frölich L. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimer's Res. Ther. 2015;7:17. doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachdev P.S., Lipnicki D.M., Kochan N.A., Crawford J.D., Thalamuthu A., Andrews G., Brayne C., Matthews F.E., Stephan B.C.M., Lipton R.B., Katz M.J., Ritchie K., Carrière I., Ancelin M.-L., Lam L.C.W., Wong C.H.Y., Fung A.W.T., Guaita A., Vaccaro R., Davin A., Ganguli M., Dodge H., Hughes T., Anstey K.J., Cherbuin N., Butterworth P., Ng T.P., Gao Q., Reppermund S., Brodaty H., Schupf N., Manly J., Stern Y., Lobo A., Lopez-Anton R., Santabárbara J., C.S., M An I.C. Cohort studies of memory in an international consortium (COSMIC), the prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2769032&tool=pmcentrez&rendertype=abstract accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gildengers A.G., Butters M.A., Albert S.M., Anderson S.J., Dew M.A., Erickson K., Garand L., Karp J.F., Lockovich M.H., Morse J., Reynolds C.F. Design and implementation of an intervention development study: retaining cognition while avoiding late-life depression (ReCALL) Am. J. Geriatr. Psychiatry. 2016;24:444–454. doi: 10.1016/j.jagp.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell A.J., Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K., Spitzer R.L. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr. Ann. 2002;32:509–515. [Google Scholar]
- 39.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 40.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Washburn R.A., Smith K.W., Jette A.M., Janney C.A. The physical activity scale for the elderly (PASE): development and evaluation. J. Clin. Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 42.Brookings J.B., Bolton B. Confirmatory factor analysis of the interpersonal support evaluation list. Am. J. Community Psychol. 1988;16:137–147. doi: 10.1007/BF00906076. [DOI] [PubMed] [Google Scholar]
- 43.Sousa V.D., Zauszniewski J.A., Bergquist-Beringer S., Musil C.M., Neese J.B., Jaber A.F. Reliability, validity and factor structure of the appraisal of self-care agency scale-revised (ASAS-R) J. Eval. Clin. Pract. 2010;16:1031–1040. doi: 10.1111/j.1365-2753.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- 44.N.M. Wineman, E.J. Durand, B.J. McCulloch, Examination of the factor structure of the Ways of Coping Questionnaire with clinical populations., Nurs. Res. 43 (n.d.) 268-73. http://www.ncbi.nlm.nih.gov/pubmed/7937172 (accessed July 26, 2016). [PubMed]
- 45.Yao B., Sripada R.K., Klumpp H., Abelson J.L., Muzik M., Zhao Z., Rosenblum K., Briggs H., Kaston M., Warren R. Penn State Worry Questionnaire – 10: a new tool for measurement-based care. Psychiatr. Res. 2016;239:62–67. doi: 10.1016/j.psychres.2016.02.069. [DOI] [PubMed] [Google Scholar]