Abstract
Patient: Female, 37
Final Diagnosis: Cardiac arrest due to Benzonatate overdose
Symptoms: Cardiac arrest • respiratory deterioration • seizure
Medication: Benzonatate
Clinical Procedure: Intubation • hypothermia protocol
Specialty: Critical Care Medicine
Objective:
Unknown ethiology
Background:
Benzonatate is one of the most widely prescribed nonnarcotic antitussives to relieve cough symptoms. As a structurally similar agent to other local anesthetics, including tetracaine and procaine, the risk to the public is not fully appreciated.
Case Report:
A 37-year-old female presented to the Emergency Department (ED) status post cardiac arrest. Advanced cardiac life support (ACLS) protocol was performed, and return of spontaneous circulation (ROSC) was achieved. Total downtime was 30 minutes. The patient was intubated, sedated, and hypothermia protocol was initiated. The patient developed bradyarrhythmia and mild coagulopathy suspicious for disseminated intravascular coagulation (DIC), thus hypothermia protocol was terminated later. A review of laboratory data showed acidosis with pH of 6.87, mixed acidosis secondary to high anion gap metabolic and respiratory acidosis with elevated liver enzymes. It was reported that approximately 2 hours prior to her presentation; the patient had ingested less than 30 pills of benzonatate 200 mg capsules with alcohol.
Conclusions:
Ingestion of benzonatate, a widely prescribed antitussive, may pose a risk to patients due to the potential for rapid development of life-threatening adverse events and limited treatment options in the overdose setting, not only in children but also in adults. Rational prescribing and patient education are needed.
MeSH Keywords: Acidosis; Antitussive Agents; Death, Sudden, Cardiac
Background
Benzonatate (Tessalon® or generic) has been a very widely prescribed non-narcotic antitussive to relieve cough symptoms since 1958. From 2004 to 2009, prescriptions of benzonatate increased by about 52% [1]. Benzonatate is a sodium channel-blocker and has anesthetic effects on the pulmonary stretch receptors. It is structurally related to tetracaine and other ester-type local anesthetics (Figure 1), thus the risk to the public may not be fully appreciated in clinical practice [2]. Here, we present an adult case with toxic ingestion of an overdose of benzonatate which results in severe mixed acidosis, seizure, and finally cardiac arrest; and we review the diagnostic approaches and treatment available.
Figure 1.
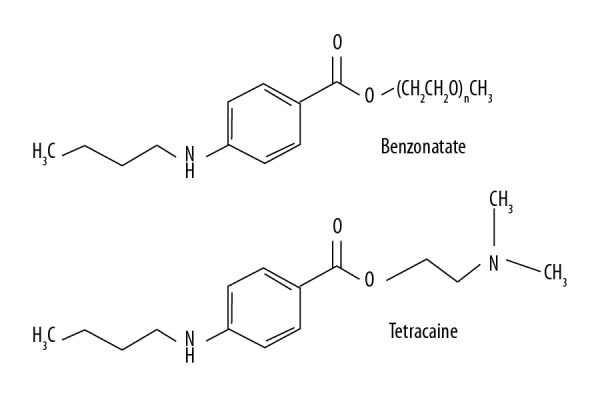
Structure comparison between benzonatate and tetracaine.
Case Report
A previously healthy 37-year-old female was found unresponsive on the couch with an unmarked pill bottle nearby, which was picked up by the responding emergency medical services (EMS) personnel and brought to the emergency department (ED), along with the patient. En route to the ED, the patient experienced episodes of seizures and later had a cardiac arrest. The patient was found to have bradycardia with asystole and became hypotensive. Advanced cardiac life support (ACLS) protocol was performed, and the return of spontaneous circulation (ROSC) was achieved. Total downtime was 30 minutes. The patient was intubated, sedated, and hypothermia protocol was initiated. The patient developed bradyarrhythmia and mild coagulopathy suspicious for disseminated intravascular coagulation (DIC), thus hypothermia protocol was later terminated. A review of laboratory data showed acidosis with pH of 6.87, mixed acidosis secondary to high anion gap metabolic and respiratory acidosis with elevated liver enzymes. It was reported that approximately 2 hours prior to her presentation; the patient had ingested less than 30 pills of benzonatate 200 mg capsules with alcohol. Unfortunately, due to severe anoxic brain injury, the patient did not survive her illness and was compassionately extubated as per the family wishes.
Discussion
Benzonatate has been on the market for over a half century and is widely prescribed. In 2010, there was a review published by the National Poison Center Database System (2000–2006) that included 2173 patients who had ingested benzonatate alone, of which the mean age was 20 years and 30% of the patients were younger than 6 years of age [3].
Common adverse events of benzonatate overdose included convulsion, coma, and cardiac arrest [4,5]. A search of the US Food and Drug Administration (FDA) Adverse Event Reporting
System (AERS) database (1969–2010) identified 31 cases of benzonatate overdose (1 or 2 to 30 benzonatate capsules), of which 20 cases unfortunately had a fatal outcome. Seven children younger than 10 years of age had benzonatate overdose due to accidental ingestions [1]. Therefore, the FDA added Warnings/Precautions for a special population at risk for potentially fatal overdose of benzonatate as had been reported in children younger than 10 years of age [6]. In our patient’s case, overdose ingestion of less than 30 capsules of benzonatate caused rapid development of life-threatening adverse events, including seizure, severe mixed acidosis, and cardiac arrest.
High-performance liquid chromatography (HPLC) is a diagnostic technique available for quantitative analysis of benzonatate, but routine analysis for benzonatate is not common inside the hospital.
Animal studies and human case reports suggest intravenous lipid emulsion therapy to be a life-saving approach in the setting of severe local anesthetic overdose [7]. The benzonatate molecules are fat-soluble. Therefore, the lipid phase created by lipid emulsion can help to extract overdosed benzonatate from the plasma (aqueous phase), and finally decrease the rate of its metabolism and provide more time for the body to compensate for the acidosis.
Management of benzonatate toxicity begins with supportive care and continuous monitoring of neurologic and cardiovascular status. Seizures and cardiac arrhythmias should be anticipated and treated in a standard fashion with airway management.
Conclusions
An overdose of benzonatate can be life-threatening due to the rapid development of mixed acidosis, seizure, and cardiac arrest. We recommend rational prescribing with limited doses and ensuring benzonatate is kept out of the reach of children.
References:
- 1.McLawhorn MW, Goulding MR, Gill RK, Michele TM. Analysis of benzonatate overdoses among adults and children from 1969–2010 by the United States Food and Drug Administration. Pharmacotherapy. 2013;33(1):38–43. doi: 10.1002/phar.1153. [DOI] [PubMed] [Google Scholar]
- 2.Thimann DA, Huang CJ, Goto CS, Feng SY. Benzonatate toxicity in a teenager resulting in coma, seizures, and severe metabolic acidosis. J Pediatr Pharmacol Ther. 2012;17(3):270–73. doi: 10.5863/1551-6776-17.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter ML, Spiller HA, Griffith JR. Benzonatate ingestion reported to the National Poison Center Database System (NPDS) J Med Toxicol. 2010;6(4):398–402. doi: 10.1007/s13181-010-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen V, Jellinek SP, Stansfield L, et al. Cardiac arrest with residual blindness after overdose of Tessalon® (benzonatate) perles. J Emerg Med. 2011;41(2):166–71. doi: 10.1016/j.jemermed.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Thimann DA, Huang CJ, Goto CS, Feng SY. Benzonatate toxicity in a teenager resulting in coma, seizures, and severe metabolic acidosis. J Pediatr Pharmacol Ther. 2012;17(3):270–73. doi: 10.5863/1551-6776-17.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA Drug Safety Communication. Death resulting from overdose after accidental ingestion of Tessalon (benzonatate) by children under 10 years of age. https://www.fda.gov/drugs/drugsafety/ucm236651.htm.
- 7.Rosenblatt MA, Abel M, Fischer GW, et al. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105(1):217–18. doi: 10.1097/00000542-200607000-00033. [DOI] [PubMed] [Google Scholar]


