Abstract
Background
Cold-inducible RNA-binding protein (CIRP) has been identified as an inflammatory mediator that exerts its function in inflammatory diseases. However, the roles of CIRP in patients who received cardiovascular surgery necessitating cardiopulmonary bypass (CPB) are still unknown. The aim of this study was to examine CIRP levels and attempt to evaluate whether CIRP could serve as a predictor for lung dysfunction after cardiovascular surgery.
Material/Methods
Plasma CIRP levels were detected by ELISA in 31 patients who received cardiovascular surgery at different time points. Selective inflammatory cytokines (TNF-α, IL-6, IL-10, and TLR4) and mediators (Ang II, PAI-1, and soluble E-selectin) were also detected. Selective laboratory and clinical parameters were recorded at scheduled time points.
Results
Compared with pre-operation levels, CIRP levels significantly increased 6 h after cardiovascular surgery with CPB. Multiple linear regression analysis showed that the length of CPB time contributed to CIRP production (P=0.013). Furthermore, CIRP was associated with Ang II (r=0.438, P=0.016), PAI-1 (r=0.485, P=0.006), and soluble E-selectin (r=0.470, P=0.008), which partly reflected lung injuries. Multiple linear regression analysis showed that CIRP levels were independently associated with PaO2/FiO2 ratios (P=0.021).
Conclusions
The length of CPB time contributed to the upregulation of CIRP in patients who received cardiovascular surgery with CPB. CIRP levels could serve as a biomarker to predict the onset of lung injury induced by cardiovascular surgery.
MeSH Keywords: Acute Lung Injury, Cardiopulmonary Bypass, Cardiovascular Diseases, RNA-Binding Proteins
Background
During cardiovascular surgery, cardiopulmonary bypass (CPB) time is associated with organ dysfunction such as acute renal failure, myocardial dysfunction, and postoperative lung injury [1–3]. Prolonged CPB time induced by complicated cardiac diseases suggested exaggerated inflammatory responses that resulted in harmful mediators which could cause multiple organs dysfunction [4]. The lung are among the most vulnerable organs during the perioperative period of cardiovascular surgery with CPB [5]. Previous studies showed that 20% of patients who received cardiovascular surgery had acute lung injury during the perioperative period, with a mortality as high as 80% [6]. Those patients would receive much more successful treatment if the lung injury were detected earlier [7,8]. Therefore, a validated biomarker for lung injury in patients who underwent cardiovascular surgery would be helpful for diagnosis and therapy.
Cold-inducible RNA-binding protein (CIRP) is a 172-amino acid protein that belongs to the cold-shock protein family [9]. It is expressed constitutively at low levels in various tissues, and is upregulated when tissues are subjected to various stimulations, such as hypothermia, hypoxia, and several types of cancer [10]. Furthermore, CIRP plays important roles in tumor suppression or promotion [11,12] and has deleterious effects during hemorrhagic and septic shock [13]. Increased plasma CIRP is associated with poor prognosis of inflammatory-related diseases [14,15], and blockade of endogenous CIRP attenuates the multiple organ dysfunction [16,17]. It is well known that CIRP initiates a systemic inflammatory responses that leads to undesirable outcomes. However, in contrast to previous studies, a rat model study of deep hypothermic circulatory arrest showed that CIRP exerts its robust renoprotection roles through inhibiting apoptosis activities [18].
To date, the effects of plasma CIRP on patients who underwent cardiovascular surgery are unclear. In the present study we investigated the variation of plasma CIRP and explored the potential causes. We also assessed the correlation between CIRP and parameters that represent the outcomes of organs and explored whether CIRP could be a predictor for lung injury induced by cardiovascular surgery.
Material and Methods
Trial design and participants
This prospective observational study was conducted at Shanghai General Hospital, Jiaotong University, China. The study protocol and the consent form were approved by the Institutional Ethics Committee of Shanghai General Hospital (Approved number: [2017]30). The study was registered at chictr.org.cn (ChiCTR-IOR-17012381). Informed consent for participation in the study was obtained from patients who received selective cardiac surgery from 19/6/2017 to 28/3/2018 in Shanghai General Hospital. Patients over 18 years of age who underwent cardiovascular surgery with CPB were included. We excluded patients if they met 1 or more of the following criteria: received immunosuppressive therapy (e.g., corticosteroids), had preoperative history of organ dysfunction, had endocrine disease, had an infection, and patient refusal.
Standard anesthesia and cardiopulmonary bypass protocol
Anesthesia was managed according to a standard protocol, including induction with fentanyl (5–10 μg/kg), midazolam (0.1 mg/kg), and pipecuronium (0.08 mg/kg). Fentanyl, sevoflurane, and pipecuronium were used during anesthesia maintenance.
The CPB circuit, which was identical for all patients, included a microporous hollow fiber membrane oxygenator (Dideco 901, Dideco Liliput, Italy; Medtronic, Inc, Minneapolis, USA) and a Stockert III roll pump (Stockert Instrument; Munich, Germany). Before aortic cannulation, 400 U/kg heparin was administered with the target kaolin-ACT value more than 480 s. The bypass circuit was primed with lactated Ringer’s solution 500 ml, hydroxyethyl starch 500 ml, and heparin 2000 IU. Pump flow rates ranged from 3.0 to 4.0 L·min−1·m−2. Core temperature was controlled at 30–32°C using a heat exchanger in the bypass circuit. At the end of CPB, to maintain the fluid balance, modified ultrafiltration was used to remove the excess fluid in the body according to the hematocrit (maintenance of hematocrit >24%) and the monitored blood pressure (aortic blood pressure 85–110/50–80 mmHg, left atrial pressure 5–12 mmHg, and right atrial pressure 5–10 mmHg according to the patient’ age and weight). During CPB, myocardial protection strategy was carried out as follows: after aortic cross-clamping, oxygenated blood and cardioplegia solution containing potassium (20 mmol/L) mixed at a 1: 4 ratio at 20°C were infused anterogradely through a cannula at the aortic root to achieve myocardial arrest, and this infusion was repeated every 30–40 min to prevent ischemic damage during CPB.
Blood sample and data collection
Blood samples were collected 1 day before surgery (T1), 6 h after surgery (T2), 1 day after surgery (T3), 3 days after surgery (T4), and 5 days after surgery (T5). All samples were centrifugated at 10 000 rpm for 10 min at 4°C. The plasma supernatants were collected and kept at −80°C until use. Inflammatory mediators – TNF-α, IL-6, IL-10, TLR4, Angiotensin (Ang II), plasminogen activator inhibitor-1(PAI-1), and soluble E-selectin – were measured by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s (CUSABIO) protocol.
Parameters of patients’ demographics information, including gender, age, BMI and ASA physical status; the parameters of clinical data during the surgery, such as CPB time and mechanical ventilation time; the scores of sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation II (APACHE II) were recorded or calculated according to patients’ clinical data at scheduled time points.
Statistical analysis
Quantitative data are expressed as mean ±SD or median [interquartile range (IQR)], as appropriate. The normality of data distribution was assessed by the Kolmogorov-Smirnov test. The Kruskal-Wallis test or one-way ANOVA was used in statistical analysis to compare differences between groups. Pearson’s correlation analysis was conducted to examine the relationship between CIRP and other variables. Multivariate regression analysis was then performed for the independent variables by ‘stepwise’ method. All the analyses were performed with SPSS software (version 17.0; SPSS Inc., Chicago, USA) and a P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics and clinical data
According to the inclusion/exclusion protocol, 31 patients (17 male and 14 female) patients who underwent cardiovascular surgery during the study period were enrolled. The median patient age was 60 years old. The types of surgeries are summarized in Supplementary Table 1. The mean CPB time was 88.8±43.3 min during surgeries. After the patients were admitted to the ICU, the mean mechanical ventilation time was 22.0±23.6 h. The nasopharyngeal temperature, hemorrhage volume, and some clinical parameters were recorded and are shown in the Table 1.
Table 1.
Patients characteristics and clinical data.
| Characteristics | |
|---|---|
| Ages (years) | 56.5±13.3 |
| Sex (Male/Female) | 17/14 |
| BMI (Kg/m2) | 23.1±10.1 |
| ASA physical status II, III | 15, 16 |
| Ejection fraction (%)a | 58.68±7.97 |
| Operation time (min) | 225±84.9 |
| CPB time (min) | 88.8±43.3 |
| Mechanical ventilation time (hours) | 22.0±23.6 |
| Hemorrhage volume (ml) | 541.9±535.3 |
| Blood transfusion (ml) | 680.6±304.9 |
| Fluid replacement (ml) | 1874.2±1191.4 |
| Temperature (°C)b | 36.6±0.1 |
| Temperature (°C)c | 31.6±1.4 |
| ICU stay (hours) | 42.3 (16–424) |
| In-hospital stay (days) | 10 (6–32) |
Data are presented as the mean ±SD or median (interquartile range) or in parameter counts (n).
Preoperative;
Before surgery;
During surgery.
ASA – American Society of Anesthesiologists; BMI – body mass index; CPB – cardiopulmonary bypass.
Plasma CIRP levels and laboratory parameters
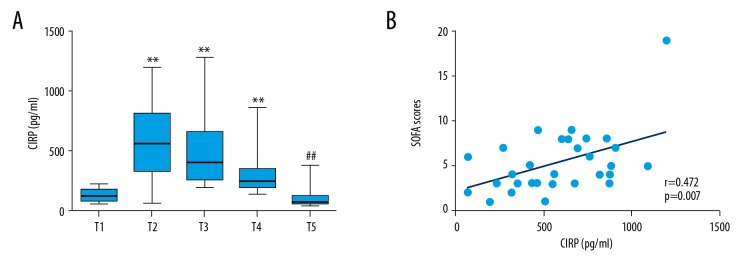
We measured the variation of CIRP level during cardiovascular surgery and investigated the correlation between laboratory parameters and CIRP levels. As shown in Table 2, we collected the laboratory parameters (including blood lactate, lymphocyte counts and serum creatinine) and calculated SOFA and APACHE II scores at different time points (Table 2). According to the values, even though cardiovascular surgery induced increased lactate, the blood lactate level alone was too low to have effects on outcomes (Table 2). However, the reduction of lymphocyte counts may represent the immunosuppression state induced by cardiovascular surgery with CPB (Table 2). As shown in Figure 1A, CIRP production was upregulated dramatically and peaked 6 h after cardiovascular surgery (T2 time point) and the level of CIRP returned to baseline 5 days after cardiovascular surgery (T5 time point); therefore, we analyzed the correlation between CIRP and other parameters at T2 time point. Univariate correlation analysis indicated that only SOFA score at T2 time point was positively correlated with CIRP production (r=0.472, P=0.007) (Figure 1B, Supplementary Table 2). Controversially, the SOFA scores at T2 timepoint show patients may in a good condition with high CIRP production, perhaps due to the balance between proinflammatory states and organ dysfunction.
Table 2.
The laboratory parameters during the cardiovascular surgery.
| T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|
| Blood lactate | 1.4 (1.1–1.7) | 1.9 (1.3–2.6)a | 2. 1 (1.5–2.8)b | 1.3 (1.0–1.7)c | 1.8 (1.12–2.20) |
| Lymphocyte counts (×106) | 1.78 (1.41–2.15) | 0.49 (0.36–0.69)b | 0.47 (0.34–0.77)b | 1.19 (0.81–1.34)b | 1.37 (1.13–1.6)c |
| SOFA scores | 1.0 (0–1) | 5 (3–7)b | 2 (1–5)b | 2 (1–3)a,c | 1 (0–3)c |
| APACHE II scores | 3 (2–5) | 10 (7–14)b | 6 (5–8)b | 7 (5–9)b | 7 (4–8)b,c |
| Serum creatinine | 78 (64–88) | 71 (63.4–91.8) | 75.9 (64–101) | 63.7 (54–80.2) | 69.4 (55.2–94.3) |
Data are presented as median [interquartile range (IQR)]. T1 – 1 day before surgery; T2 – 6 hours after surgery; T3 – 1 day after surgery; T4 – 3 days after surgery; T5 – 5 days after surgery.
P<0.05 vs. T1 time point;
P<0.01 vs. T1 time point;
P<0.01 vs. T2 time point.
Figure 1.
Plasma CIRP levels at scheduled time points and the relationship between CIRP and SOFA scores. (A). Plasma CIRP levels at scheduled time points. Data are presented as median [interquartile range (IQR)] and analyzed by Kruskal-Wallis test. ** P<0.01 vs. T1 time point, ## P<0.01 vs. T2 time point. (B) CIRP levels association with SOFA scores 6 h after CPB (r=0.472, P=0.007).
Correlations between plasma CIRP level and cytokines production
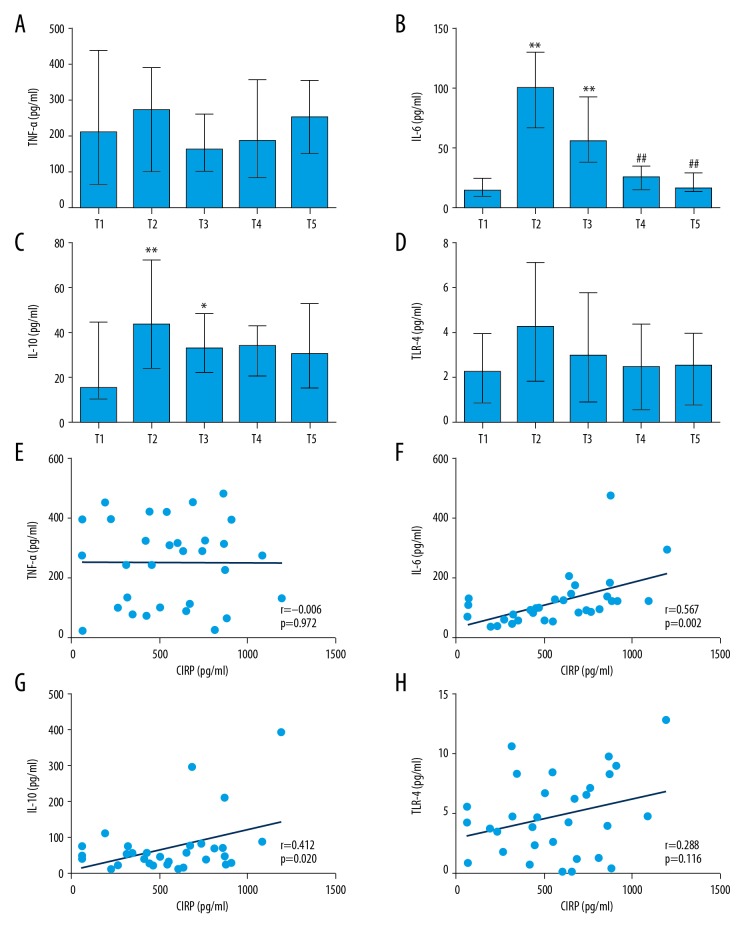
We found that the levels of IL-6 and IL-10 increased significantly 6 h after cardiovascular surgery. The level of IL-6 returned to baseline at 5 days after surgery, while the IL-10 level remained in high until 5 days after surgery (Figure 2A–2D). However, inconsistent with expectation, there was no significant difference among those time points for TNF-α and TLR4, although there was an increasing trend. Univariate correlation analysis (Supplementary Table 3) showed that plasma CIRP level was positively correlated with IL-6 (r=0.567, P=0.002) and IL-10 (r=0.412, P=0.02) (Figure 2E–2H) at T2 time point.
Figure 2.
Inflammatory cytokines levels at scheduled time points and the correlation between CIRP and cytokines. (A–D). Inflammatory cytokines levels at scheduled time points. Data are presented as median [interquartile range (IQR)] and analyzed by Kruskal-Wallis test. ** P<0.01 vs. T1 time point, * P<0.05 vs. T1 time point, ## P<0.01 vs. T2 time point. (E–H). CIRP positively associated with inflammatory cytokines levels 6 h after CPB. (F). CIRP and IL-6: r=0.567, P=0.002; (G). CIRP and IL-10: r=0.412, P=0.02.
CPB time contributes to the production of plasma CIRP
We analyzed the correlation between CIRP levels and some clinical factors that can affect CIRP production. Univariate analysis indicated that plasma CIRP level was positively correlated with CPB time, as well as inflammatory cytokines (IL-6 and IL-10), at T2 time point (Supplementary Tables 3, 4). To investigate the causes of CIRP upregulation, we used a stepwise multiple linear regression model to adjust age, BMI, operation time, CPB time, mechanical time, temperature (during CPB), and inflammatory cytokines (including TNF-α, IL-6, TLR4, and IL-10). Interestingly, CPB time and IL-6 level were associated with CIRP production (CPB time: P=0.013; IL-6: P=0.008) (Table 3). Because IL-6 may secreted by macrophages when stimulated with recombinant CIRP, we proposed that the length of CPB time contributed to the increasement of CIRP production (Table 3).
Table 3.
Multiple linear regression model analysis of independent risk factors associated with CIRP production 6 h after cardiac surgery.
| Variables | Standard regression coefficient | 95% Confidence interval | P Value |
|---|---|---|---|
| CPB time | 0.394 | [0.628, 4.918] | 0.013 |
| IL-6 | 0.423 | [−0.407, 2.524] | 0.008 |
Plasma CIRP predicts lung injury induced by cardiopulmonary bypass
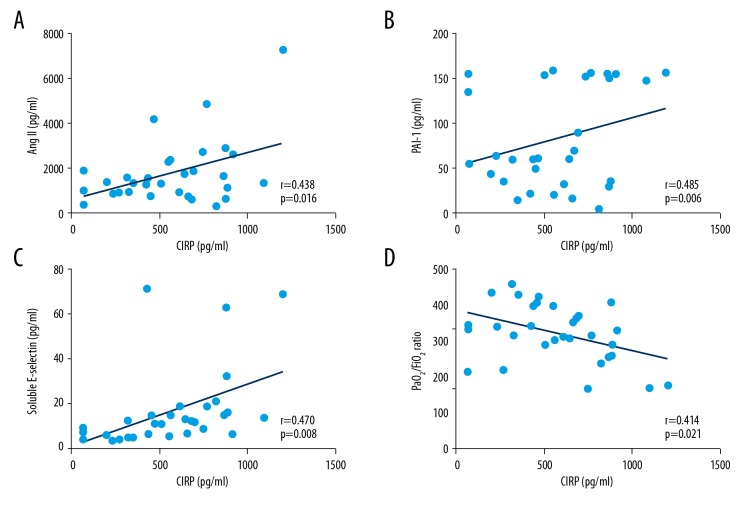
As shown in Figure 3A–3C, CIRP was associated with Ang II (r=0.438, P=0.016), PAI-1(r=0.485, P=0.006), and soluble E-selectin (r=0.470, P=0.008), which partly reflect lung injuries (Supplementary Table 5). We speculated that CIRP is involved in lung injury during cardiovascular surgery at T2 time point. Furthermore, univariate analysis indicated that CIRP production is correlated with severity of lung injury, as reflected by PaO2/FiO2 ratio at T2 time point (r=−0.414, P=0.02) (Figure 3D). Therefore, we used CIRP value at this time point for further subsequent multivariable analysis. As expected, in a stepwise multiple linear regression model, plasma CIRP level was independently associated with PaO2/FiO2 ratio (P=0.021, 95%CI: [−0.203, −0.018]) after adjusting for age, BMI, operation time, CPB time, mechanical time, hemorrhage volume, blood transfusion, temperature (during CPB), and inflammatory mediators such as TNF-α and IL-6 (Supplementary Table 6).
Figure 3.
The correlation between CIRP and biomarker that represented lung dysfunction. Data were enrolled at 6 h after cardiovascular surgery and analyzed by Pearson’s correlation analysis. (A). CIRP and Ang II: r=0.438, P=0.016. (B). CIRP and PAI-1: r=0.485, P=0.006. (C). CIRP and soluble E-selectin: r=0.470, P=0.008. (D). CIRP and PaO2/FiO2 ratio: r=−0.414, P=0.021.
Discussion
In this study, we investigated the roles of perioperative plasma CIRP in patients who underwent cardiovascular surgery with CPB. We reported for the first time that plasma CIRP was significantly upregulated immediately after CPB. The elevated levels were correlated with inflammatory cytokines IL-6 and IL-10. Furthermore, the length of CPB time was associated with CIRP production, while CIRP level was correlated with severity of lung dysfunction. Therefore, plasma CIRP could serve as a predictor for lung injury after cardiovascular surgery with CPB.
CIRP is a highly conserved RNA-binding nuclear protein. It migrates from nucleus to cytoplasm and then releases into the circulation [13]. It exerts its function as a translational regulator for numerous genes or a harmful mediator that induces organ dysfunction [13,19]. In this study, we found that, compared with preoperative level, plasma CIRP increased immediately after cardiovascular surgery with CPB, and univariate analysis indicated that CIRP is correlated with SOFA scores. These findings suggest CIRP plays detrimental roles during cardiovascular surgery with CPB, in agreement with previous studies [20,21].
Besides CIRP production, inflammatory cytokines (e.g., IL-6 and IL-10) exhibit a similar variation tendency after CPB, as described previously [22,23]. Multiple linear regression analysis indicated that the length of CPB time and IL-6 level contribute to CIRP production. However, Wang et al. found that CIRP derived from macrophages induced IL-6 secretion, which exacerbated organ damage [13,16]. Based on these findings, we speculated that the length of CPB time is an important contributor to upregulation of CIRP. Interestingly, our multiple linear regression analysis found that the temperature value we kept during CPB has little effect on CIRP production, but this finding is inconsistent with previous evidence that CIRP is induced by hypothermia [18,24]. Considering the hypothermia level during CPB, we speculate that the degree of temperature reduction partly explains this strange phenomenon.
As previous studies described, plasma Ang II, PAI-1, and soluble E-selectin partly indicate lung injury [25–27]. In the present study we found that these proteins increased dramatically and were positively correlated with CIRP production after cardiovascular surgery. Considering that CIRP participates in the dysfunction of endothelial cells via activation of the NLRP3 inflammasome [21], we speculated that CIRP is correlated with lung injury induced by cardiovascular surgery with CPB. As expected, after adjusting other factors, multiple linear regression analysis indicated that CIRP levels were independently associated with PaO2/FiO2 and reflect the severity of lung injury [28]. These findings strengthen the conclusion that CIRP is a predictor for lung injury after cardiovascular surgery with CPB.
Our study has several limitations. Firstly, inflammatory and stress responses induced by surgeries were different in this study, which may have affected CIRP production. Furthermore, the sample size was too small to make a precise analysis. We did not perform power analysis calculation, although we achieved the expected results. This study is a preliminary investigation of the relationship between CIRP and organ dysfunction during cardiovascular surgery following CPB, and further research is warranted.
Conclusions
CIRP level can act as a biomarker for onset of lung injury after cardiovascular surgery with CPB. Insight into the roles of CIRP in post-cardiovascular surgery provides a potential therapeutic target for pulmonary dysfunction induced by cardiovascular surgery necessitating CPB.
Supplementary Tables
Supplementary Table 1.
Surgery type of patients.
| Surgery type | Counts (n, %) |
|---|---|
| CABG | 6 (19.4) |
| CABG+MVR | 2 (6.5) |
| MVR | 4 (12.9) |
| AVR | 6 (19.4) |
| MVR+AVR | 4 (12.9) |
| VSDR | 4 (12.9) |
| ASD+TVP | 1 (3.2) |
| Myxomas excision | 1 (3.2) |
| TAAR | 1 (3.2) |
| AAR+AVR | 2 (6.5) |
CABG – coronary artery bypass grafting; MVR – mitral valve replacement; AVR – aortic valve replacement; TVP – tricuspid valve replacement; VSD – ventricular septal defect repair; ASD – atrial septal defect repair; TAAR – total aortic arch replacement; AAR – ascending aortic replacement.
Supplementary Table 2.
The correlation between CIRP and laboratory parameters.
| Clinical parameters | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| Blood lactate | T2 | 0.214 | [−0.152, 0.529] | 0.247 |
| T3 | 0.331 | [−0.027, 0.614] | 0.069 | |
| Lymphocyte count | T2 | 0.03 | [−0.326, 0.382] | 0.865 |
| T3 | −0.228 | [−0.539, 0.138] | 0.218 | |
| SOFA scores | T2 | 0.472 | [0.1411, 0.708] | 0.007 |
| T3 | 0.022 | [−0.335, 0.373] | 0.908 | |
| APACHE II scores | T2 | 0.160 | [−0.207, 0.487] | 0.391 |
| T3 | 0.212 | [−0.154, 0.527] | 0.252 | |
| Serum creatinine | T2 | 0.139 | [−0.227, 0.470] | 0.457 |
| T3 | −0.346 | [−0.624, 0.009] | 0.056 | |
Supplementary Table 3.
Correlation between cytokines and CIRP level.
| Cytokines | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| TNF-α | T2 | −0.006 | [−0.360, 0.369] | 0.972 |
| T3 | 0.192 | [−0.175, 0.512] | 0.301 | |
| IL-6 | T2 | 0.527 | [0.213, 0.743] | 0.002 |
| T3 | 0.154 | [−0.212, 0.482] | 0.408 | |
| TLR4 | T2 | 0.288 | [−0.074, 0.583] | 0.116 |
| T3 | 0.463 | [0.130, 0.702] | 0.009 | |
| IL-10 | T2 | 0.412 | [0.07, 0.669] | 0.02 |
| T3 | 0.100 | [−0.264, 0.439] | 0.592 | |
Supplementary Table 4.
Correlations between plasma CIRP level and clinical variables.
| Clinical variables | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| Ages | T2 | 0.143 | [−0.223, 0.473] | 0.444 |
| T3 | 0.119 | [−0.246, 0.454] | 0.524 | |
| BMI | T2 | 0.152 | [−0.214, 0.480] | 0.418 |
| T3 | 0.08 | [−0.284, 0.421] | 0.676 | |
| Operation time | T2 | 0.243 | [−0.122, 0.555] | 0.188 |
| T3 | −0.001 | [−0.355, 0.354] | 0.997 | |
| CPB time | T2 | 0.507 | [0.186, 0.730] | 0.04 |
| T3 | 0.245 | [−0.12, 0.551] | 0.184 | |
| Mechanical time | T2 | 0.249 | [−0.116, 0.554] | 0.178 |
| T3 | 0.005 | [−0.350, 0.359] | 0.978 | |
| Hemorrhage volume | T2 | 0.008 | [−0.279, 0.426] | 0.653 |
| T3 | −0.224 | [−0.536, 0.14] | 0.226 | |
| Blood transfusion | T2 | 0.150 | [−0.216, 0.479] | 0.420 |
| T3 | −0.10 | [−0.438, 0.264] | 0.595 | |
| Fluid replacement | T2 | 0.05 | [−0.313, 0.395] | 0.803 |
| T3 | −0.166 | [−0.492, 0.20] | 0.371 | |
| Temperature (during CPB) | T2 | −0.171 | [−0.495, 0.195] | 0.358 |
| T3 | −0.110 | [−0.447, 0.254] | 0.556 | |
| ICU stay | T2 | 0.04 | [−0.323, 0.397] | 0.824 |
| T3 | 0.104 | [−0.266, 0.448] | 0.583 | |
| In-hospital stay | T2 | 0.036 | [−0.329, 0.391] | 0.851 |
| T3 | −0.023 | [−0.380, 0.341] | 0.906 | |
Supplementary Table 5.
Correlations between plasma CIRP level and lung injury-related protein.
| Parameter | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| Ang II | T2 | 0.427 | −0.086–0.629 | 0.017 |
| T3 | 0.258 | −0.107–0.561 | 0.162 | |
| PAI-1 | T2 | 0.278 | −0.085–0.576 | 0.130 |
| T3 | 0.470 | 0.139–0.707 | 0.008 | |
| Soluble E-selectin | T2 | 0.450 | 0.114–0.692 | 0.01 |
| T3 | 0.214 | −0.152–0.528 | 0.248 | |
Supplementary Table 6.
Multiple linear regression model analysis of independent risk factors associated with PaO2/FiO2 ratio 6 h after cardiac surgery.
| Variables | Standard regression coefficient | 95% CI | P value |
|---|---|---|---|
| CIRP | 0.414 | [−0.203, −0.018] | 0.021 |
Acknowledgements
We thank Junyan Yao for providing many helpful suggestions. We also thank Yun Zou for technical assistance.
Footnotes
Source of support: This work was supported by grant 81571877 from the Natural Science Foundation of China
Conflict of interest
None.
References
- 1.Sirvinskas E, Andrejaitiene J, Raliene L, et al. Cardiopulmonary bypass management and acute renal failure: Risk factors and prognosis. Perfusion. 2008;23(6):323–27. doi: 10.1177/0267659109105251. [DOI] [PubMed] [Google Scholar]
- 2.Aass T, Stangeland L, Moen CA, et al. Myocardial function after polarizing versus depolarizing cardiac arrest with blood cardioplegia in a porcine model of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2016;50(1):130–39. doi: 10.1093/ejcts/ezv488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosour C, Dragosavac D, Antunes N, et al. Effect of ultrafiltration on pulmonary function and interleukins in patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2016;30(4):884–90. doi: 10.1053/j.jvca.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Esper SA, Subramaniam K, Tanaka KA. Pathophysiology of cardiopulmonary bypass: Current strategies for the prevention and treatment of anemia, coagulopathy, and organ dysfunction. Semin Cardiothorac Vasc Anesth. 2014;18(2):161–76. doi: 10.1177/1089253214532375. [DOI] [PubMed] [Google Scholar]
- 5.Fujii M, Miyagi Y, Bessho R, et al. Effect of a neutrophil elastase inhibitor on acute lung injury after cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2010;10(6):859–62. doi: 10.1510/icvts.2009.225243. [DOI] [PubMed] [Google Scholar]
- 6.Stephens RS, Shah AS, Whitman GJ. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann Thorac Surg. 2013;95(3):1122–29. doi: 10.1016/j.athoracsur.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Turnbull AE, Ruhl AP, Lau BM, et al. Timing of limitations in life support in acute lung injury patients: A multisite study. Crit Care Med. 2014;42(2):296–302. doi: 10.1097/CCM.0b013e3182a272db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohata K, Chen-Yoshikawa TF, Menju T, et al. Protective effect of Inhaled Rho-kinase inhibitor on lung ischemia-reperfusion injury. Ann Thorac Surg. 2017;103(2):476–83. doi: 10.1016/j.athoracsur.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 9.Lujan DA, Ochoa JL, Hartley RS. Cold-inducible RNA binding protein in cancer and inflammation. Wiley Interdiscip Rev RNA. 2018;9(2) doi: 10.1002/wrna.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao Y, Tong L, Tang L, Wu S. The role of cold-inducible RNA binding protein in cell stress response. Int J Cancer. 2017;141(11):2164–73. doi: 10.1002/ijc.30833. [DOI] [PubMed] [Google Scholar]
- 11.Nishiyama H, Itoh K, Kaneko Y, et al. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137(4):899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng Y, Kulkarni P, Inoue T, Getzenberg RH. Down-regulating cold shock protein genes impairs cancer cell survival and enhances chemosensitivity. J Cell Biochem. 2009;107(1):179–88. doi: 10.1002/jcb.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiang X, Yang WL, Wu R, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19(11):1489–95. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan MM, Yang WL, Brenner M, et al. Cold-inducible RNA-binding protein (CIRP) causes sepsis-associated acute lung injury via induction of endoplasmic reticulum stress. Sci Rep. 2017;7:41363. doi: 10.1038/srep41363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ode Y, Aziz M, Wang P. CIRP increases ICAM-1(+) phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J Leukoc Biol. 2018;103(4):693–707. doi: 10.1002/JLB.3A0817-327RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cen C, McGinn J, Aziz M, et al. Deficiency in cold-inducible RNA-binding protein attenuates acute respiratory distress syndrome induced by intestinal ischemia-reperfusion. Surgery. 2017;162(4):917–27. doi: 10.1016/j.surg.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 17.McGinn J, Zhang F, Aziz M, et al. The protective effect of a short peptide derived from cold-inducible RNA-binding protein in renal ischemia-reperfusion injury. Shock. 2018;49(3):269–76. doi: 10.1097/SHK.0000000000000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L, Gu T, Liu Y, et al. Cold-inducible ribonucleic acid-binding protein attenuates acute kidney injuries after deep hypothermic circulatory arrest in rats. Interact Cardiovasc Thorac Surg. 2018;26(1):124–30. doi: 10.1093/icvts/ivx262. [DOI] [PubMed] [Google Scholar]
- 19.Roilo M, Kullmann MK, Hengst L. Cold-inducible RNA-binding protein (CIRP) induces translation of the cell-cycle inhibitor p27Kip1. Nucleic Acids Res. 2018;46(6):3198–210. doi: 10.1093/nar/gkx1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Fan EK, Liu J, et al. Cold-inducible RNA-binding protein through TLR4 signaling induces mitochondrial DNA fragmentation and regulates macrophage cell death after trauma. Cell Death Dis. 2017;8(5):e2775. doi: 10.1038/cddis.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WL, Sharma A, Wang Z, et al. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci Rep. 2016;6:26571. doi: 10.1038/srep26571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justus G, Walker C, Rosenthal LM, et al. Immunodepression after CPB: Cytokine dynamics and clinics after pediatric cardiac surgery – A prospective trial. Cytokine. :2017. doi: 10.1016/j.cyto.2017.03.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Howell KW, Cleveland JC, Jr, Meng X, et al. Interleukin 6 production during cardiac surgery correlates with increasing age. J Surg Res. 2016;201(1):76–81. doi: 10.1016/j.jss.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Larrayoz IM, Rey-Funes M, Contartese DS, et al. Cold shock proteins are expressed in the retina following exposure to low temperatures. PLoS One. 2016;11(8):e0161458. doi: 10.1371/journal.pone.0161458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware LB, Zhao Z, Koyama T, et al. Derivation and validation of a two-biomarker panel for diagnosis of ARDS in patients with severe traumatic injuries. Trauma Surg Acute Care Open. 2017;2(1):e000121. doi: 10.1136/tsaco-2017-000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolthuis EK, Vlaar AP, Hofstra JJ, et al. Plasminogen activator inhibitor-type I gene deficient mice show reduced influx of neutrophils in ventilator-induced lung injury. Crit Care Res Pract. 2011;2011:217896. doi: 10.1155/2011/217896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okajima K, Harada N, Sakurai G, et al. Rapid assay for plasma soluble E-selectin predicts the development of acute respiratory distress syndrome in patients with systemic inflammatory response syndrome. Transl Res. 2006;148(6):295–300. doi: 10.1016/j.trsl.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Chen Q, Luo Y, et al. Plasma levels of alarmin HNPs 1–3 associate with lung dysfunction after cardiac surgery in children. BMC Pulm Med. 2017;17(1):218. doi: 10.1186/s12890-017-0558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Surgery type of patients.
| Surgery type | Counts (n, %) |
|---|---|
| CABG | 6 (19.4) |
| CABG+MVR | 2 (6.5) |
| MVR | 4 (12.9) |
| AVR | 6 (19.4) |
| MVR+AVR | 4 (12.9) |
| VSDR | 4 (12.9) |
| ASD+TVP | 1 (3.2) |
| Myxomas excision | 1 (3.2) |
| TAAR | 1 (3.2) |
| AAR+AVR | 2 (6.5) |
CABG – coronary artery bypass grafting; MVR – mitral valve replacement; AVR – aortic valve replacement; TVP – tricuspid valve replacement; VSD – ventricular septal defect repair; ASD – atrial septal defect repair; TAAR – total aortic arch replacement; AAR – ascending aortic replacement.
Supplementary Table 2.
The correlation between CIRP and laboratory parameters.
| Clinical parameters | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| Blood lactate | T2 | 0.214 | [−0.152, 0.529] | 0.247 |
| T3 | 0.331 | [−0.027, 0.614] | 0.069 | |
| Lymphocyte count | T2 | 0.03 | [−0.326, 0.382] | 0.865 |
| T3 | −0.228 | [−0.539, 0.138] | 0.218 | |
| SOFA scores | T2 | 0.472 | [0.1411, 0.708] | 0.007 |
| T3 | 0.022 | [−0.335, 0.373] | 0.908 | |
| APACHE II scores | T2 | 0.160 | [−0.207, 0.487] | 0.391 |
| T3 | 0.212 | [−0.154, 0.527] | 0.252 | |
| Serum creatinine | T2 | 0.139 | [−0.227, 0.470] | 0.457 |
| T3 | −0.346 | [−0.624, 0.009] | 0.056 | |
Supplementary Table 3.
Correlation between cytokines and CIRP level.
| Cytokines | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| TNF-α | T2 | −0.006 | [−0.360, 0.369] | 0.972 |
| T3 | 0.192 | [−0.175, 0.512] | 0.301 | |
| IL-6 | T2 | 0.527 | [0.213, 0.743] | 0.002 |
| T3 | 0.154 | [−0.212, 0.482] | 0.408 | |
| TLR4 | T2 | 0.288 | [−0.074, 0.583] | 0.116 |
| T3 | 0.463 | [0.130, 0.702] | 0.009 | |
| IL-10 | T2 | 0.412 | [0.07, 0.669] | 0.02 |
| T3 | 0.100 | [−0.264, 0.439] | 0.592 | |
Supplementary Table 4.
Correlations between plasma CIRP level and clinical variables.
| Clinical variables | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| Ages | T2 | 0.143 | [−0.223, 0.473] | 0.444 |
| T3 | 0.119 | [−0.246, 0.454] | 0.524 | |
| BMI | T2 | 0.152 | [−0.214, 0.480] | 0.418 |
| T3 | 0.08 | [−0.284, 0.421] | 0.676 | |
| Operation time | T2 | 0.243 | [−0.122, 0.555] | 0.188 |
| T3 | −0.001 | [−0.355, 0.354] | 0.997 | |
| CPB time | T2 | 0.507 | [0.186, 0.730] | 0.04 |
| T3 | 0.245 | [−0.12, 0.551] | 0.184 | |
| Mechanical time | T2 | 0.249 | [−0.116, 0.554] | 0.178 |
| T3 | 0.005 | [−0.350, 0.359] | 0.978 | |
| Hemorrhage volume | T2 | 0.008 | [−0.279, 0.426] | 0.653 |
| T3 | −0.224 | [−0.536, 0.14] | 0.226 | |
| Blood transfusion | T2 | 0.150 | [−0.216, 0.479] | 0.420 |
| T3 | −0.10 | [−0.438, 0.264] | 0.595 | |
| Fluid replacement | T2 | 0.05 | [−0.313, 0.395] | 0.803 |
| T3 | −0.166 | [−0.492, 0.20] | 0.371 | |
| Temperature (during CPB) | T2 | −0.171 | [−0.495, 0.195] | 0.358 |
| T3 | −0.110 | [−0.447, 0.254] | 0.556 | |
| ICU stay | T2 | 0.04 | [−0.323, 0.397] | 0.824 |
| T3 | 0.104 | [−0.266, 0.448] | 0.583 | |
| In-hospital stay | T2 | 0.036 | [−0.329, 0.391] | 0.851 |
| T3 | −0.023 | [−0.380, 0.341] | 0.906 | |
Supplementary Table 5.
Correlations between plasma CIRP level and lung injury-related protein.
| Parameter | CIRP | |||
|---|---|---|---|---|
| Pearson r | 95% CI | P value | ||
| Ang II | T2 | 0.427 | −0.086–0.629 | 0.017 |
| T3 | 0.258 | −0.107–0.561 | 0.162 | |
| PAI-1 | T2 | 0.278 | −0.085–0.576 | 0.130 |
| T3 | 0.470 | 0.139–0.707 | 0.008 | |
| Soluble E-selectin | T2 | 0.450 | 0.114–0.692 | 0.01 |
| T3 | 0.214 | −0.152–0.528 | 0.248 | |
Supplementary Table 6.
Multiple linear regression model analysis of independent risk factors associated with PaO2/FiO2 ratio 6 h after cardiac surgery.
| Variables | Standard regression coefficient | 95% CI | P value |
|---|---|---|---|
| CIRP | 0.414 | [−0.203, −0.018] | 0.021 |