Abstract
Background:
To better characterize mortality among methamphetamine users, we estimated rates of all-cause mortality by HIV serostatus and smoking history in gay and bisexual men (GBM) treated for methamphetamine dependence, and explored associated clinical and sociobehavioral characteristics.
Methods:
We searched public records to identify deaths among men screened between 1998–2000 for a trial of outpatient therapy for GBM with methamphetamine dependence. Crude mortality rates (CMRs) were calculated and standardized mortality ratios (SMRs) estimated, comparing data with historical information from CDC WONDER. Associations of mortality with HIV infection, tobacco use, and other factors were explored using Kaplan-Meier survival analysis and Cox proportional hazards models.
Results:
Of 191 methamphetamine-dependent GBM (median age 35 years; majority Caucasian), 62.8% had HIV infection and 31.4% smoked tobacco at baseline. During the 20-year follow-up period, 12.6% died. Relative to controls, methamphetamine-dependent GBM had a three-fold higher 20-year SMR: 3.39, 95% CI: 2.69–4.09. Especially high mortality was observed among participants reporting tobacco use (adjusted HR 3.48, 95% CI: 1.54–7.89), club drug use prior to starting methamphetamine (2.63, 1.15–6.00), or other clinical diagnoses at baseline (3.89, 1.15–13.22). At 20 years, the CMR for HIV-infected participants (7.7 per 1,000 PY) was 1.5 times that for men without HIV (5.2 per 1,000 PY; p=0.22) and there was a 5-fold difference in CMRs for HIV-infected tobacco smokers (16.9 per 1,000 PY) compared to non-smokers (3.4 per 1,000 PY; p<0.01).
Conclusions:
In our sample of methamphetamine-dependent GBM, concomitant HIV infection and tobacco use were associated with dramatic increases in mortality.
Keywords: Gay and bisexual men (GBM), Methamphetamine, Human immunodeficiency virus (HIV), Tobacco, Mortality, Survival
1.0. Introduction:
The prevalence of recent methamphetamine use among gay and bisexual men (GBM) in the United States (U.S.) has been estimated as 25–45 times higher than in the U.S. general population (Forrest et al., 2010; Mimiaga et al., 2008). While some recent studies have reported an exceptionally high mortality rate among people who use methamphetamine (Callaghan et al., 2012; Darke et al., 2017; Herbeck et al., 2015; Kuo et al., 2011; Liang et al., 2010; Stenbacka et al., 2010), only one focused explicitly on GBM, and it was limited to men with HIV infection (Carrico et al., 2014). Some of these studies suggested an association between risk of death and male sex, social characteristics (Ronka et al., 2017) such as unemployment, lower education, and marital status (Kuo et al., 2011), and psychiatric comorbidity (Arendt et al., 2011). However, factors associated with mortality among GBM may be distinct due to the unique social vulnerabilities experienced by sexual minorities. While the HIV epidemic in the U.S. is concentrated among GBM, HIV incidence among GBM who use methamphetamine is more than twice as high than for non-users (Buchacz et al., 2005).
Recent research has proposed a synergistic interaction of HIV infection and methamphetamine use on adverse health outcomes, resulting in a worldwide call for new and better strategies to provide services to people with HIV infection who use methamphetamine (Degenhardt et al., 2010). Proposed biological mechanisms include increased multi-system inflammation and immune dysfunction (Samikkannu et al., 2013), and increased susceptibility to central nervous system (CNS) infection and inflammation (Najera et al., 2016). Studies examining methamphetamine use (Akhgari et al., 2017; Wijetunga et al., 2003) and HIV (Boccara et al., 2013; Hurwitz et al., 2004; Kelly et al., 2010) separately have highlighted their potential to produce adverse cardiovascular outcomes. However, these studies have not specifically addressed links between individual-scale pathology and morbidity with population-scale measures of mortality. We aimed to determine all-cause mortality rates and explore sociobehavioral and clinical characteristics associated with mortality among a cohort of HIV-infected and -uninfected GBM 20 years post-enrollment in an outpatient methamphetamine dependence treatment study.
2.0. Materials and Methods:
2.1. Participants and Recruitment
Participants were recruited from community venues serving GBM in the Hollywood area of Los Angeles County, California and through media outlets from 1998 to 2000. All participants were 18–65 years old and sought outpatient behavioral treatment for methamphetamine dependence. Exclusion criteria included medical or psychiatric conditions that precluded safe study involvement and methamphetamine dependence that necessitated inpatient substance abuse treatment.
2.2. Study Procedures
Potential participants completed a 2-week baseline period of three weekly visits where they provided urine samples, completed research measures (described below), attended up to four cognitive behavioral skills groups, and underwent a psychiatric interview (Structured Clinical Inventory for the DSM-IV, SCID). Following the baseline period, eligible participants were then randomized to receive either: 1) Cognitive behavioral therapy (CBT) only; 2) Contingency management (CM) only; 3) CBT + CM; or 4) gay-specific CBT (GCBT) (Shoptaw et al., 2005). Participants attended outpatient treatment three times per week for sixteen weeks, for a total of 48 sessions, and were required to provide a supervised urine sample at each study visit, which was analyzed using an enzyme multiplied immunoassay technique (EMIT) to test for methamphetamine metabolites. The Friends Research Institute West Coast IRB reviewed and approved all study procedures. Written informed consent was obtained from all participants prior to participation. The UCLA IRB reviewed and approved these retrospective analyses.
2.3. Measures
Participants completed the Addiction Severity Index (ASI) for drug use and related problematic behaviors and the Behavioral Questionnaire-Amphetamine (BQ-A) to assess HIV-related sexual risk behaviors. Felony history was assessed by self-report.
Online historical records (www.rootsweb.com, www.ancestry.com) were searched by name, date/place of birth, and Social Security numbers to identify deceased participants. These sites are open access databases that index publicly available records, including death certificates for U.S. citizens provided by the Social Security Death Index (SSDI), a public access database created and maintained by the U.S. Social Security Administration. Once a participant was identified as deceased, their death certificate was requested from the State of California.
2.4. Data Analysis
Bivariate analyses with chi-square and Fisher’s exact tests were used to estimate associations between participant characteristics and mortality among the whole sample and after stratifying by HIV infection status. Wilcoxon’s Rank Sum test measured differences in the distribution of non-parametric numeric variables between deceased and living participants. Cox proportional-hazard ratios (HRs) were used to measure associations between independent variables and mortality; the hazards proportionality assumption was verified.
Survival time for each participant was calculated from the beginning of the study period until the individual’s confirmed date of death or the end of the follow up period (January 01, 2018). Crude mortality rates (CMRs) were calculated after stratifying by HIV serostatus and/or self-reported tobacco use history. Standardized mortality ratios (SMRs) were calculated by dividing the observed number of deaths by the expected number of deaths, and compared with populations reported in the CDC WONDER database (matched according to geography, age range, and sex). Cumulative survival rates in our sample were estimated and compared with geographic-, age-, and sex-matched controls from the same time period using Kaplan-Meier (KM) methods. Log-rank test was used to compare the survival distribution between groups.
Sub-analyses of cumulative survival stratified by HIV infection and other variables significantly associated with mortality in this sub-group were conducted using KM methods. All analyses were conducted using Stata 12.0 (StataCorp, College Town, TX, USA). Pairwise deletion was performed for variables with missing data; < 5% of data were missing for any single variable.
3.0. Results:
The mean age of participants at baseline was 35 years, and mean age at first methamphetamine use was 28. Tobacco use was endorsed by 31.4% (60/191) of the participants, club drug use (e.g., cocaine, 3,4 methylenedioxymethamphetamine (ecstasy), lysergic acid diethylamide (LSD, acid), phencyclidine (PCP), or ketamine) prior to starting methamphetamine by 31.9% (61/191), and injection methamphetamine use by 44.0% (84/191). A felony history was reported by 8.4% (16/191) of participants. Other clinical diagnoses, including psychiatric and metabolic conditions were reported by 64.4% of participants (Table 1). Baseline HIV prevalence was 62.8% (120/191).
Table 1.
Sociobehavioral and Clinical Characteristics of GBM Treated for Methamphetamine Dependence in Los Angeles, CA between 1998–2000, stratified by HIV Serostatus and Mortality; N=191
| All participants | Participants with HIV infection | Participants without HIV infection | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total | Deceased (n=24) | Living (n=167) | Deceased (n=17) | Living (n=103) | Deceased (n=7) | Living (n=64) |
| Age at first use of meth | N=191 | 26 (22, 32) | 29 (23, 34) | 25 (20, 29) | 28 (23, 34) | 28 (26, 36) | 29 (21.5, 32.5) |
| Concurrent tobacco use | |||||||
| Yes | n=60 | 14 (58.3) | 46 (27.5) | 12 (70.6) | 31 (30.1) | 2 (28.6) | 15 (23.4) |
| No | n=131 | 10 (41.7) | 121 (72.5) | 5 (29.4) | 72 (69.9) | 5 (71.4) | 49 (76.6) |
| Other drug use prior to starting to use methamphetamine (participants could select more than one drug)*§ | |||||||
| Other psychostimulants | n=49 | 11 (45.8) | 38 (22.8) | 9 (52.9) | 26 (25.2) | 2 (28.6) | 12 (18.8) |
| Hypnosedatives | n=7 | 4 (16.7) | 3 (1.8) | 1 (5.9) | 1 (1.0) | 3 (42.9) | 2 (3.1) |
| Opioids | n=7 | 3 (12.5) | 4 (2.4) | 3 (17.7) | 4 (3.9) | 0 (0.0) | 0 (0.0) |
| Psychedelic drugs | n=27 | 12 (50.0) | 15 (9.0) | 8 (47.1) | 8 (7.8) | 4 (57.1) | 7 (10.9) |
| Cannabis | n=101 | 12 (50.0) | 89 (53.3) | 7 (41.2) | 54 (52.4) | 5 (71.4) | 35 (54.7) |
| Alcohol | n=91 | 11 (45.8) | 80 (47.9) | 10 (58.8) | 45 (43.7) | 1 (14.3) | 35 (54.7) |
| Amyl nitrates | n=13 | 4 (16.7) | 9 (5.4) | 4 (23.5) | 5 (4.9) | 0 (0.0) | 4 (6.3) |
| Injection methamphetamine use | |||||||
| Yes | n=74 | 10 (41.7) | 64 (38.3) | 8 (47.1) | 46 (44.7) | 2 (28.6) | 18 (28.1) |
| No | n-117 | 14 (58.3) | 103 (61.7) | 9 (52.9) | 57 (55.3) | 5 (71.4) | 46 (71.9) |
| Felony history | |||||||
| Yes | n=16 | 5 (20.8) | 11 (6.6) | 3 (17.7) | 4 (3.9) | 2 (28.6) | 7 (10.9) |
| No | n=175 | 19 (79.2) | 156 (93.4) | 14 (82.3) | 99 (96.1) | 5 (71.4) | 57 (89.1) |
| HIV infection | |||||||
| Yes | n=120 | 17 (70.8) | 103 (61.7) | ||||
| No | n=71 | 7 (29.2) | 64 (38.3) | ||||
| Other clinical diagnoses♯ | |||||||
| Any other diagnosis | n=123 | 21 (87.5) | 102 (61.1) | 15 (88.2) | 65 (63.1) | 6 (85.7) | 37 (57.8) |
| No other diagnosis | n=68 | 3 (12.5) | 65 (38.9) | 2 (11.8) | 38 (36.9) | 1 (14.3) | 27 (42.2) |
| Psychiatric diagnosis | n=118 | 19 (79.2) | 99 (59.3) | 14 (82.4) | 64 (62.1) | 5 (71.4) | 35 (54.7) |
| No psychiatric diagnosis | n=73 | 5 (20.8) | 68 (40.7) | 3 (17.7) | 29 (37.9) | 2 (28.6) | 29 (45.3) |
| Other physical diagnosis | n=6 | 1 (4.2) | 5 (3.0) | 0 (0.0) | 2 (1.9) | 1 (14.3) | 3 (4.7) |
| No other physical diagnosis | n=185 | 23 (95.8) | 162 (97.0) | 17 (100.0) | 101 (98.1) | 6 (85.7) | 61 (95.3) |
Bold text = p<0.05
Psychostimulants: cocaine, ecstasy; Hypnosedatives: benzodiazepines, hypnotics; Psychedelic drugs: LSD, acid, mushrooms, PCP, ketamine
All p-values for drug use prior to starting meth are bivariate comparisons between participants reporting use of the specified drug and those reporting no use of that drug.
Any other diagnoses: bipolar disorder, depression, anxiety, suicidality, diabetes, cancer, attention deficit hyperactivity disorder (ADHD), obstructive sleep apnea (OSA), schizotypal disorder, post-traumatic stress disorder (PTSD), cardiovascular (CV) disease; Psychiatric diagnoses: bipolar disorder, depression, anxiety, suicidality, ADHD, PTSD, schizotypal disorder; Other physical diagnoses: OSA, diabetes, CV disease
3.1. Causes of Death Recorded on Death Certificates
Causes of death recorded on death certificates for cases, stratified by HIV serostatus and reported tobacco use, are presented in Table 2. The most frequent circumstances of death recorded were AIDS-related (n=8) and cardiovascular (n=7). The mean age at death for cases with HIV infection was 44 years versus 48 years for cases without HIV infection (p=0.616).
Table 2.
Causes of Death Recorded on Death Certificates for GBM Treated for Methamphetamine Dependence in Los Angeles, CA between 1998–2000, stratified by HIV Serostatus; N=24
| All Cases; N=24, (46y)* | Cases with HIV Infection; n=17, (44y) | Cases without HIV Infection; n=7, (48y) | |||
|---|---|---|---|---|---|
| Circumstances of death | S#; n=13, (44y) | NS; n=4, (47y) | S; n=2, (40y) | NS; n=5, (52y) | |
| Drug-related | 5 (45 years) | 3 (47 years) | 1 (39 years) | 1 (45 years) | 0 (N/A) |
| Likely drug overdose | 1 (44) | 1 (44) | 0 (N/A) | 0 (N/A) | 0 (N/A) |
| Multiple drug intoxication | 4 (45) | 2 (48) | 1 (39) | 1 (45) | 0 (N/A) |
| AIDS-related | 8 (46 years) | 6 (44 years) | 2 (50 years) | ||
| Unspecified | 1 (43) | 0 (N/A) | 1 (43) | ||
| Respiratory infection | 6 (47) | 5 (45) | 1 (56) | ||
| Cerebral infection | 1 (40) | 1 (40) | 0 (N/A) | ||
| Cardiovascular | 7 (51 years) | 2 (44 years) | 1 (49 years) | 0 (N/A) | 4 (55 years) |
| Atherosclerotic heart disease | 2 (48) | 0 (N/A) | 0 (N/A) | 0 (N/A) | 2 (48) |
| Ischemic cardiomyopathy | 2 (53) | 1 (50) | 0 (N/A) | 0 (N/A) | 1 (55) |
| Cardiopulmonary arrest | 2 (44) | 1 (38) | 1 (49) | 0 (N/A) | 0 (N/A) |
| Cerebral vascular accident | 1 (69) | 0 (N/A) | 0 (N/A) | 0 (N/A) | 1 (69) |
| Neoplastic | 2 (38 years) | 2 (38 years) | 0 (N/A) | 0 (N/A) | 0 (N/A) |
| Cholangiocarcinoma | 1 (37) | 1 (37) | 0 (N/A) | 0 (N/A) | 0 (N/A) |
| Squamous cell – tongue | 1 (38) | 1 (38) | 0 (N/A) | 0 (N/A) | 0 (N/A) |
| Other | 2 (37 years) | 0 (N/A) | 0 (N/A) | 1 (35 years) | 1 (38 years) |
| Diabetes, out of control | 1 (35) | 0 (N/A) | 0 (N/A) | 1 (35) | 0 (N/A) |
| Unknown | 1 (38) | 0 (N/A) | 0 (N/A) | 0 (N/A) | 1 (38) |
n (median age at death).
NS=non-smokers; S=smokers
3.2. Bivariate Analysis
Deceased participants were more likely to report a history of tobacco use (58.3% vs. 27.5%; p=0.002), club drug use prior to starting methamphetamine (58.3% vs. 28.1%; p=0.003), a felony conviction (20.8% vs. 6.6%; p=0.018), and to have other clinical diagnoses (87.5% vs. 61.1%; p=0.011; see Table 1 for the list of diagnoses).
3.3. Survival Analysis
Mortality during the 20-year follow-up period was 12.6% (24/191), or 6.8 per 1,000 person-years (PY). Mean follow-up time was 19.1 years. After adjusting for tobacco use, other drug use, and clinical diagnoses, especially high mortality was observed among men reporting tobacco use (adjusted HR [aHR], 95% CI: 3.48, 1.54–7.89), club drug use prior to starting methamphetamine (2.63, 1.15–6.00), and any other clinical diagnoses (3.89, 1.15–13.22; Table 2).
Estimates of progression to death among study participants, stratified by HIV serostatus, and compared to matched controls are highlighted in Figure 1. Mortality among GBM with methamphetamine dependence was significantly greater than for controls (Log-rank test: p<0.001). Relative to controls, GBM with methamphetamine dependence had a higher rate of mortality at 10 years (SMR 3.95, 95% CI: 2.89–5.01) and 20 years (3.39, 2.69–4.09). The 10-year crude mortality rate (CMR) for methamphetamine-dependent GBM with HIV infection (5.2 per 1,000 PY) was more than twice that for methamphetamine-dependent GBM without HIV infection (2.3 per 1,000 PY; p=0.216).
Figure 1.
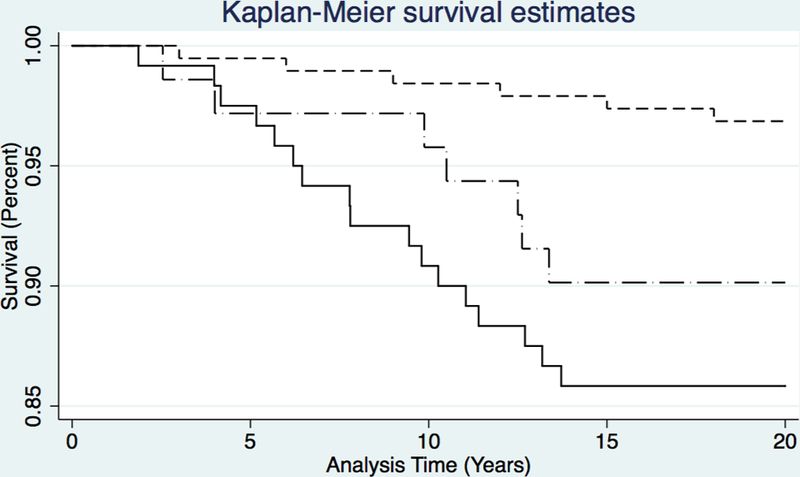
Kaplan-Meier (K-M) Survival Curves for GBM Treated for Methamphetamine Dependence in Los Angeles, CA between 1998–2000, stratified by HIV status, versus Age-Matched Control Population of HIV-Negative Men Who do Not Use Methamphetamine from Urban California; N = 382. Short-dashed line = Control group (No HIV infection, No methamphetamine use); Dash-dot line = GBM treated for meth use (No HIV infection); Solid line = GBM with HIV infection treated for meth use.
Figure 2 shows cumulative mortality stratified by HIV serostatus and tobacco use. Mortality for HIV-infected participants who used tobacco was significantly greater than for those who did not use tobacco (Log-rank test: p<0.001). This association between HIV infection, tobacco use, and mortality among methamphetamine-dependent GBM is also reflected in the dramatic difference in 20-year CMRs for HIV-infected tobacco smokers (16.9 per 1,000 PY) compared to non-smokers (3.4 per 1,000 PY; p=0.0016).
Figure 2.
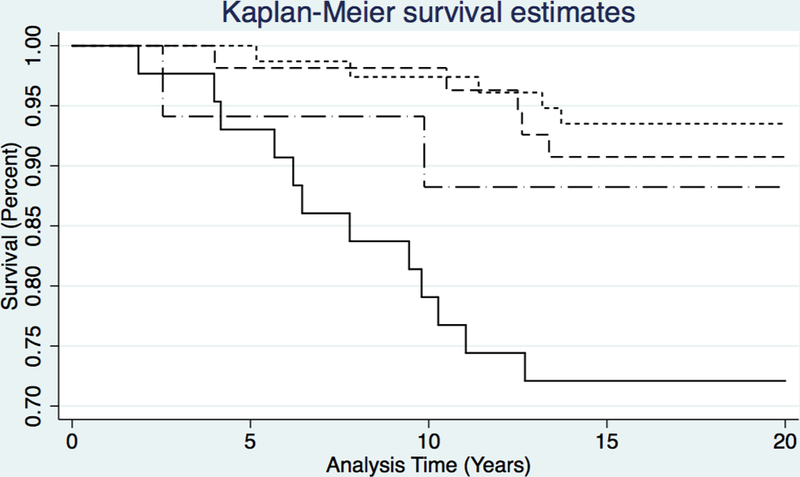
Kaplan-Meier (K-M) Survival Curves for GBM Treated for Methamphetamine Dependence in Los Angeles, CA between 1998–2000, stratified by HIV status and Tobacco Use; N = 191. Short-dashed line = HIV infection, but no tobacco use; Dashed line = No HIV infection of tobacco use; Dash-dot line = Tobacco use, but no HIV infection; Solid line = Concomitant HIV infection and tobacco use.
4.0. Discussion:
In this sample of GBM who enrolled in a methamphetamine dependence treatment study, the short-term risk of mortality was high compared with matched population controls, and significantly accelerated by both HIV infection and tobacco use. To our knowledge, this study is the first to compare rates of death in this vulnerable population by HIV serostatus, and the second to report data on the high risk of mortality among GBM who use methamphetamine (Carrico et al., 2014). While previous studies have emphasized the high likelihood of mortality associated with methamphetamine use, they have stopped short of identifying factors unique to distinct subgroups within this high-risk population (Callaghan et al., 2012; Herbeck et al., 2015; Kuo et al., 2011). Our focus on GBM allows us to highlight that methamphetamine-dependent GBM are at particularly high risk of mortality, and to identify potentially modifiable factors associated with mortality for future interventions (Carrico et al., 2016). Understanding how the interaction between methamphetamine dependence, HIV, and other factors may influence survival time in GBM is critical to improving health outcomes in communities that maintain high burdens of both HIV infection and methamphetamine use (Colfax and Shoptaw, 2005; Vu et al., 2015).
The crude mortality rate (CMR) in this study was similar to data reported in previous studies of methamphetamine users in multiple international settings (Callaghan et al., 2012; Carrico et al., 2014; Darke et al., 2017; Herbeck et al., 2015; Kuo et al., 2011; Liang et al., 2010; Singleton et al., 2009). The only one of these that was both domestic and reported underlying causes of death also found “cardiovascular” and “HIV” to be the most frequently reported causes (Herbeck et al., 2015). While our SMR was lower than others have reported (Callaghan et al., 2012; Herbeck et al., 2015; Kuo et al., 2011), those studies were limited to individuals who were either hospitalized for methamphetamine use (Callaghan et al., 2012; Kuo et al., 2011) or who had never received treatment (Herbeck et al., 2015). As our sample was restricted to GBM who were enrolled in outpatient treatment, our findings may underestimate the mortality of the larger population of methamphetamine users who exist in the community and do not seek treatment for their methamphetamine use (Anglin et al., 2000). In this context, the high mortality rate we report is even more alarming.
Our survival analysis suggests that HIV infection contributes to short-term mortality in GBM who are dependent on methamphetamine. Though this finding is not surprising, it supports the hypothesis that methamphetamine use and HIV infection together increase systemic inflammatory processes that increase risk of morbidity and mortality (Fulcher, 2018; Samikkannu et al., 2013). Animal models suggest that methamphetamine may be critical to CNS inflammation and susceptibility to HIV infection (Najera et al., 2016; Passaro et al., 2015). Methamphetamine use has also been associated with poor antiretroviral medication adherence among gay and bisexual men (Marquez et al., 2009; Reback et al., 2003). Importantly, the stratification of our survival analysis by HIV serostatus and tobacco use suggests that the synergistic health consequences of tobacco use and HIV infection may be worse than those of HIV infection alone, particularly for GBM dependent upon methamphetamine.
Several studies have explored the potential for overlapping biologic mechanisms for cardiovascular pathologies caused by HIV infection, tobacco use, and stimulant use (Akhgari et al., 2017; Callaghan et al., 2016; Kelly et al., 2010; Shirley et al., 2013). One study identified a synergistic effect of HIV infection and tobacco use on atherosclerotic plaque development (Kelly et al., 2010), while another identified methamphetamine as a cause of clinically significant histologic changes in cardiac tissue (Akhgari et al., 2017). Other studies have documented poor performance indicators of HIV control, including medication adherence and viral load, among people who smoke tobacco (O’Cleirigh et al., 2015) and people who use stimulants (Rajasingham et al., 2012). The profound association of HIV and tobacco use with mortality in our sample of methamphetamine-dependent GBM underscores the importance of further research exploring the impact of these factors on vascular and central nervous system (CNS) inflammation and antiretroviral adherence, as well as the development of effective smoking prevention and cessation programs for this vulnerable population.
Our findings should be considered in the context of several limitations. First, we did not systematically collect follow-up data on participants between 2000 and 2018, and thus do not know whether participants continued to use methamphetamine, continued to smoke, or received additional treatment for substance use during this time period. Moreover, we do not have data on antiretroviral treatment and adherence, clinical progression of HIV, or other social support variables that may have mediated the relationship between HIV and mortality. We only know participants’ HIV serostatus at study baseline or as recorded on their death certificate, and expect that some men in this high-risk population acquired HIV during the 20 years post-methamphetamine abuse treatment and did not die of HIV-related causes. Additionally, our sample was geographically limited to GBM seeking treatment in the Hollywood area of Los Angeles County, California and, therefore, may not be generalizable to the larger GBM or methamphetamine-using communities. Finally, our sample size was small, limiting the number of variables we could adjust for during modeling.
5.0. Conclusions:
These results are the first to highlight the roles of HIV infection and tobacco use in the mortality of GBM who used methamphetamine. We identify potentially modifiable risk factors in this high-risk subgroup of methamphetamine-dependent GBM, particularly smoking cessation. Future studies should explore the synergy between HIV infection, tobacco use, and methamphetamine use on morbidity and mortality outcomes among GBM, their effect on cardiovascular and CNS inflammatory processes, and the impact of innovative support programs on smoking cessation and treatment adherence in this vulnerable population.
Table 3.
Hazard Ratios for Mortality for GBM Treated for Methamphetamine Dependence in Los Angeles, CA between 1998–2000, stratified by HIV Serostatus; N=191
| Characteristic or behavior |
All Participants (N=191) | Participants with HIV Infection (n=120) | Participants without HIV Infection (n=71) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | aHR | (95% CI) | HR | (95% CI) | aHR | (95% CI) | HR | (95% CI) | |
| Concurrent tobacco use | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 3.47 | (1.54, 7.82) | 3.48 | (1.54, 7.89) | 5.01 | (1.76, 14.22) | 5.12 | (1.80, 14.58) | 1.35 | (0.26, 6.95) |
| Club drug use prior to starting meth* | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 3.35 | (1.49, 7.54) | 2.63 | (1.15, 6.00) | 3.34 | (1.27, 8.78) | 2.64 | (0.98, 7.07) | 3.27 | (0.73, 14.63) |
| Any other clinical diagnoses♯ | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | |||||
| Yes | 4.21 | (1.25, 14.10) | 3.89 | (1.15, 13.22) | 4.11 | (0.94, 17.98) | 3.62 | (0.81, 16.26) | 4.13 | (0.50, 34.33) |
Bold text = p<0.05
HR, hazard ratio; aHR, adjusted hazard ratio
Club drugs: cocaine, ecstasy, LSD, acid, PCP, ketamine
Any other clinical diagnoses: bipolar disorder, depression, anxiety, suicidality, diabetes, cancer, attention deficit hyperactivity disorder (ADHD), obstructive sleep apnea (OSA), schizotypal disorder, post-traumatic stress disorder (PTSD), cardiovascular (CV) disease
Footnotes
We have no conflicts of interest to disclose.
REFERENCES
- Center for Disease Control and Prevention, National Center for Health Statistics. Compressed Mortality File 1999–2015 on CDC WONDER Online Database, released December 2016 Data are from the Compressed Mortality File 1999–2015 Series 20 No. 2U, 2016, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Accessed at http://wonder.cdc.gov/cmf-icd10.html on Dec 7, 2017. 1:27:13 PM. [Google Scholar]
- Substance Abuse and Mental Health Administration. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52) Rockville, MD, Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/. [Google Scholar]
- Akhgari M, Mobaraki H, Etemadi-Aleagha A, 2017. Histopathological study of cardiac lesions in methamphetamine poisoning-related deaths. Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences 25(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S, 2000. History of the Methamphetamine Problem. Journal of psychoactive drugs 32(2), 137–141. [DOI] [PubMed] [Google Scholar]
- Arendt M, Munk-Jorgensen P, Sher L, Jensen SO, 2011. Mortality among individuals with cannabis, cocaine, amphetamine, MDMA, and opioid use disorders: a nationwide follow-up study of Danish substance users in treatment. Drug Alcohol Depend 114(2–3), 134–139. [DOI] [PubMed] [Google Scholar]
- Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, Capeau J, Cohen A, 2013. HIV and coronary heart disease: time for a better understanding. Journal of the American College of Cardiology 61(5), 511–523. [DOI] [PubMed] [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, Klausner JD, 2005. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS (London, England) 19(13), 1423–1424. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Verdichevski M, Sykes J, Jaffer SR, Kish SJ, 2012. All-cause mortality among individuals with disorders related to the use of methamphetamine: a comparative cohort study. Drug Alcohol Depend 125(3), 290–294. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Gatley JM, Sykes J, Taylor L, 2016. The prominence of smoking-related mortality among individuals with alcohol- or drug-use disorders. Drug and alcohol review. [DOI] [PubMed] [Google Scholar]
- Carrico AW, Jain J, Discepola MV, Olem D, Andrews R, Woods WJ, Neilands TB, Shoptaw S, Gomez W, Dilworth SE, Moskowitz JT, 2016. A community-engaged randomized controlled trial of an integrative intervention with HIV-positive, methamphetamine-using men who have sex with men. BMC Public Health 16, 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Shoptaw S, Cox C, Stall R, Li X, Ostrow DG, Vlahov D, Plankey MW, 2014. Stimulant use and progression to AIDS or mortality after the initiation of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 67(5), 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colfax G, Shoptaw S, 2005. The methamphetamine epidemic: implications for HIV prevention and treatment. Current HIV/AIDS reports 2(4), 194–199. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, Duflou J, 2017. Rates, characteristics and circumstances of methamphetamine-related death in Australia: a national 7-year study. Addiction (Abingdon, England) 112(12), 2191–2201. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers B, Guarinieri M, Panda S, Phillips B, Strathdee SA, Tyndall M, Wiessing L, Wodak A, Howard J, 2010. Meth/amphetamine use and associated HIV: Implications for global policy and public health. The International journal on drug policy 21(5), 347–358. [DOI] [PubMed] [Google Scholar]
- Forrest DW, Metsch LR, LaLota M, Cardenas G, Beck DW, Jeanty Y, 2010. Crystal methamphetamine use and sexual risk behaviors among HIV-positive and HIV-negative men who have sex with men in South Florida. Journal of urban health : bulletin of the New York Academy of Medicine 87(3), 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher JA, Shoptaw S, Makgoeng SB, Elliott J, Ibarrondo FJ, Ragsdale A, Brookmeyer R, Anton PA, Gorbach PM, 2018. Brief report: Recent methamphetamine use is associated with rectal mucosal inflammatory cytokinds, regardless of HIV-1 status. Journal of Acquired Immune Deficiency Syndrome 78(1), 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck DM, Brecht ML, Lovinger K, 2015. Mortality, causes of death, and health status among methamphetamine users. Journal of addictive diseases 34(1), 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz BE, Klimas NG, Llabre MM, Maher KJ, Skyler JS, Bilsker MS, McPherson-Baker S, Lawrence PJ, Laperriere AR, Greeson JM, Klaus JR, Lawrence R, Schneiderman N, 2004. HIV, metabolic syndrome X, inflammation, oxidative stress, and coronary heart disease risk : role of protease inhibitor exposure. Cardiovascular toxicology 4(3), 303–316. [DOI] [PubMed] [Google Scholar]
- Kelly JA, Amirkhanian YA, Seal DW, Galletly CM, Difranceisco W, Glasman LR, Stevenson LY, Rosado N, 2010. Levels and Predictors of Sexual HIV Risk in Social Networks of Men who Have Sex with Men in the Midwest. AIDS education and prevention : official publication of the International Society for AIDS Education 22(6), 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CJ, Liao YT, Chen WJ, Tsai SY, Lin SK, Chen CC, 2011. Causes of death of patients with methamphetamine dependence: a record-linkage study. Drug and alcohol review 30(6), 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LJ, Huang D, Brecht ML, Hser YI, 2010. Differences in Mortality among Heroin, Cocaine, and Methamphetamine Users: A Hierarchical Bayesian Approach. Journal of drug issues 40(1), 121–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD, 2009. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care 21(5), 575–582. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Fair AD, Mayer KH, Koenen K, Gortmaker S, Tetu AM, Hobson J, Safren SA, 2008. Experiences and sexual behaviors of HIV-infected MSM who acquired HIV in the context of crystal methamphetamine use. AIDS education and prevention : official publication of the International Society for AIDS Education 20(1), 30–41. [DOI] [PubMed] [Google Scholar]
- Najera JA, Bustamante EA, Bortell N, Morsey B, Fox HS, Ravasi T, Marcondes MC, 2016. Methamphetamine abuse affects gene expression in brain-derived microglia of SIV-infected macaques to enhance inflammation and promote virus targets. BMC immunology 17(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Cleirigh C, Valentine SE, Pinkston M, Herman D, Bedoya CA, Gordon JR, Safren SA, 2015. The unique challenges facing HIV-positive patients who smoke cigarettes: HIV viremia, ART adherence, engagement in HIV care, and concurrent substance use. AIDS Behav 19(1), 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro RC, Pandhare J, Qian H-Z, Dash C, 2015. The Complex Interaction between Methamphetamine Abuse and HIV-1 pathogenesis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 10(3), 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasingham R, Mimiaga MJ, White JM, Pinkston MM, Baden RP, Mitty JA, 2012. A systematic review of behavioral and treatment outcome studies among HIV-infected men who have sex with men who abuse crystal methamphetamine. AIDS Patient Care STDS 26(1), 36–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reback CJ, Larkins S, Shoptaw S, 2003. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care 15(6), 775–785. [DOI] [PubMed] [Google Scholar]
- Ronka S, Karjalainen K, Martikainen P, Makela P, 2017. Social determinants of drug-related mortality in a general population. Drug Alcohol Depend 181, 37–43. [DOI] [PubMed] [Google Scholar]
- Samikkannu T, Rao KV, Arias AY, Kalaichezian A, Sagar V, Yoo C, Nair MP, 2013. HIV infection and drugs of abuse: role of acute phase proteins. Journal of neuroinflammation 10, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley DK, Kaner RJ, Glesby MJ, 2013. Effects of smoking on non-AIDS-related morbidity in HIV-infected patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 57(2), 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C, 2005. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend 78(2), 125–134. [DOI] [PubMed] [Google Scholar]
- Singleton J, Degenhardt L, Hall W, Zabransky T, 2009. Mortality among amphetamine users: a systematic review of cohort studies. Drug Alcohol Depend 105(1–2), 1–8. [DOI] [PubMed] [Google Scholar]
- Stenbacka M, Leifman A, Romelsjo A, 2010. Mortality and cause of death among 1705 illicit drug users: a 37 year follow up. Drug and alcohol review 29(1), 21–27. [DOI] [PubMed] [Google Scholar]
- Vu NT, Maher L, Zablotska I, 2015. Amphetamine-type stimulants and HIV infection among men who have sex with men: implications on HIV research and prevention from a systematic review and meta-analysis. Journal of the International AIDS Society 18, 19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijetunga M, Seto T, Lindsay J, Schatz I, 2003. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? Journal of toxicology. Clinical toxicology 41(7), 981–986. [DOI] [PubMed] [Google Scholar]


