The ductus arteriosus (DA) is a simple, yet enigmatic vessel that has confounded clinicians and investigators for centuries. Historical evidence suggests that Galen and other anatomists recognized the ductus as a unique anatomic structure as early as the second century A.D.1,2 As translated by Kühn, Galen’s writings recorded that
“This is the explanation of the communication of the vena cava with the vein-like artery [i.e., our pulmonary veins and left atrium] during foetal life. In as much as this latter vessel was then acting as the venous supply of the lung, its companion [i.e., the artery-like vein, or our pulmonary trunk and its branches] had to act as the arterial supply of that organ, and Nature therefore made a communication between it and the great artery [i.e., the aorta]. As, however, the two vessels were separated by a short distance, a small third vessel [i.e.; the ductus] was created by Nature to unite them…”1.
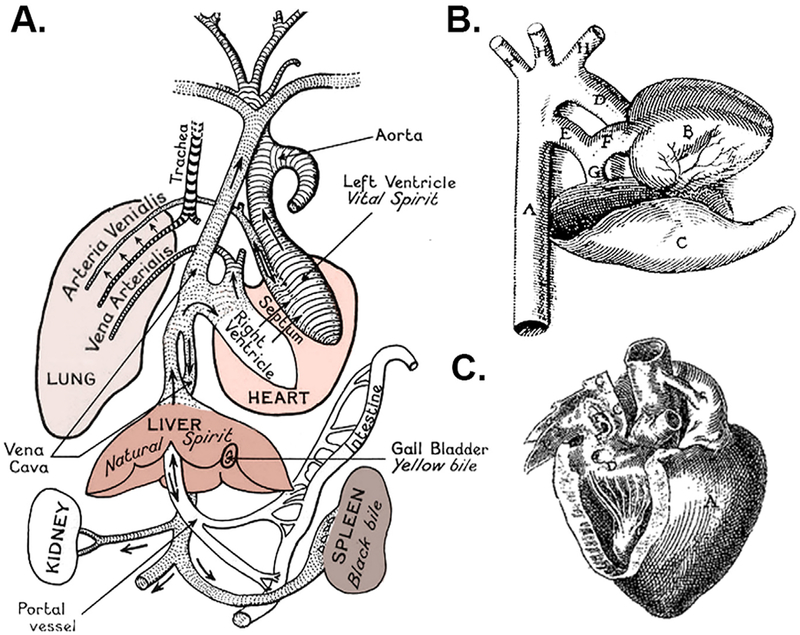
Galen’s paradigm for the mingling of blood and humors across invisible inter-ventricular pores of the heart (Fig. 1A) was accepted as dogma for centuries, while the DA was largely ignored or remained a curiosity. Ancient Persian documents from 200–600 A.D. suggested more accurate descriptions of blood flow from the heart to the lungs.3 Medical historians acknowledge Avicenna, Ibn-al-Nafis, and other influential scientific leaders from the Islamic Golden Age (9th–12th century) for their early theories on pulmonary circulation and cardiac function,4–6 but there are no known reports on the presence or significance of the DA from that era.
Fig. 1 –
Galen’s circulatory paradigm and early illustrations of the DA. (A) Galen’s scheme described transmission of blood across inter-ventricular pores to spread vital spirit in the body.19 (Adapted with permission from Dr. Barbara J. Becker, UC Irvine, and the Wellcome Collection (licensed under Creative Commons 4.0, CC BY).) (B) Hieronymus Fabricius of Aquapendente’s drawing shows the DA (“E”) as a prominent extension of the main pulmonary artery (“F”), similar in caliber to the ascending aorta (“D”), which was described as “the offshoot of the great artery to the artery-like vein, … a wide vessel in the fetus but becomes cord-like after birth.” (De formato fetu, 1600 AD). (Adapted with permission from Refs.1,20) (C) Johan van Horne’s addendum to illustrations in Leonardo Bottalo’s Opera Omnia (1660 AD) depicted the DA (canalis a pulmonali arteria tendens in aortam) in an unusual orientation. (Adapted with permission from Ref7).
Fast forward to the 16th century, when renaissance scholars including Vesalius, Servetus, and Columbo among others, are credited with modern descriptions of the pulmonary circulation. Complete descriptions and the earliest illustrations of the DA appeared around the turn of the 17th century by Falloppio, Aranzio, Carcano, Fabricius, van Horne (Fig. 1B and C), and others.1,7,8 In the mid-1600s, a pivotal shift in understanding cardiopulmonary circulation is attributed to Sir William Harvey, an English physician who studied under Fabricius and likely drew upon the observations of Middle Eastern and European scholars. Harvey noted the presence of the DA in the fetus as ‘two roots’ of the great artery arising from the heart, and described its ‘withering’ after birth, similar to the fate of the umbilical vessels.8 Over the past 200 years, theories on the mechanisms for spontaneous closure of the DA have included thrombosis, mechanical compression by the newly inflated lungs, kinking or angular constriction, twisting or torsional constriction, stretch by attachment to the pericardium, collapse from cessation of flow, closure of a purported valve-like fold at the junction of the ductus and aorta, or acute narrowing of the angle formed by the ductus’ insertion into the aorta. Unfortunately, we still lack consensus on the steps leading to final DA closure and share uncertainty regarding the need for treatment.
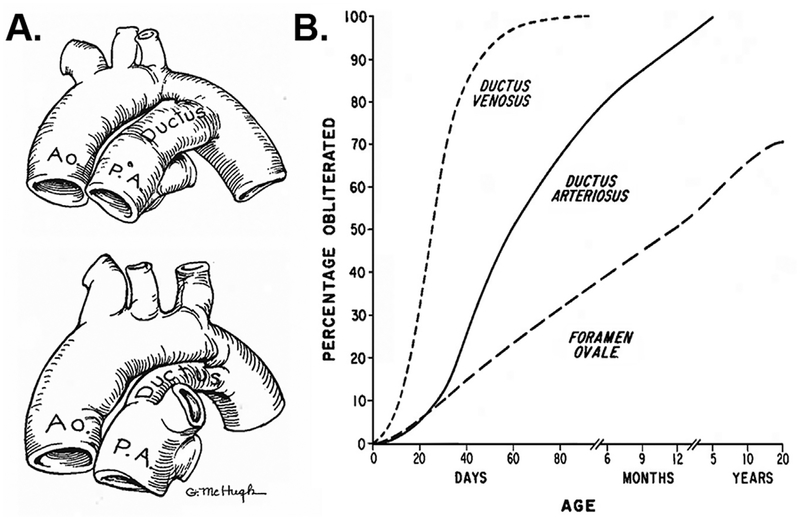
A greater understanding of the mechanisms and timing for spontaneous DA closure is critical for efforts to “leave the PDA alone”,9 or deciding when to intervene. Some argue that PDAs will close on their own and only a small proportion of preterm infants will benefit from treatment to close the ductus.10,11 On the other hand, studies suggest that only 18– 20% of infants will achieve spontaneous closure.12–14 Higher spontaneous closure rates have been reported, but increased PDA-related morbidity and mortality is concerning.15 Galen noted that postnatal closure typically occurred within a few days of birth. By the 16th century, however, Carcano and other anatomists reported postmortem cases where the DA remained patent at several months of age.1 Indeed, efforts to accurately document the normal timing of DA closure were dependent on autopsy (Fig. 2A),16,17 angiography,18 or clinical expertise until the advent of ultrasound and more recent imaging techniques. Although our current NICU practice of “waiting for spontaneous DA closure” is applied to much more immature infants than in 1918, the long time-course for eventual DA closure that was reported in infants 100 years ago (Fig. 2B) is remarkably similar to recent observations in some current-day preterm infants.12,13 Thus, nearly two millenia of observations have provided valuable information on DA morphology and expected closure rates. Yet there is insufficient information to resolve important questions on the mechanisms and management of PDA.
Fig. 2 –
Assessment of DA closure. (A) Before sonography, DA closure rates were determined by the degree of ductus patency found at autopsy. (Adapted with permission from Refs.21,22) (B) Although patient populations have evolved over the past century, spontaneous closure rates recorded by postmortem examinations in 1918 (Adapted with permission from Refs.17,23) show a similar trend to current studies demonstrating successful DA closure in preterm infants as late as 18–24 months of age.12,13
In this issue of the journal, a series of articles are presented that set the stage for a greater understanding of the DA and its pathophysiological associations. Based on comparative studies from his laboratory and others, Edward Dzialowski provides an overview of DA development and function among diverse vertebrate species. Despite common features and shared embryonic origins, interesting differences in fetal circulatory patterns and DA regulation become apparent when comparing the DA of lungfish, egg-laying terrestrial and aquatic vertebrates, birds, and higher-order mammals. A second article on the unique genetic makeup of the DA is presented by Yarboro and colleagues, who performed a systematic analysis of microarray studies to examine the DA transcriptome of rodents. Data from a human RNA-seq study was evaluated to identify genes enriched in the DA and permit comparison of rodent and human studies in order to identify commonly detected genes. The importance of finding DA-specific genes for therapeutic advantage is addressed by Shelton and colleagues in the third article in this series. Here, DA regulatory molecules that are not related to nitric oxide, prostaglandin signaling, or other canonical DA pathways are considered. Specifically, receptors, enzymes, ion channels/transporters and other members of the “druggable genome” are discussed, with evidence for select potassium channel modulators as an example of emerging targets for therapeutic drug development.
A second series of articles presents different perspectives on the contribution of patent ductus arteriosus (PDA) to adverse pulmonary outcomes. Jensen and colleagues performed a systematic review and subsequent meta-analysis of studies that address the association of prophylactic indomethacin and death or bronchopulmonary dysplasia (BPD). Although a weak association between prophylaxis and decreased odds of mortality was identified in pooled data from two large observational studies, a randomized trial would be required to resolve this effect. A related article by Ron Clyman comprehensively explores the rationale for the long-held belief that PDA has a pathophysiologic basis for contributing to BPD. The implications of recent quality improvement studies and a randomized clinical trial (RCT) that was just completed were considered in light of older RCTs. A window of opportunity for pharmacologic treatment in the first week of life may offer the best chance to prevent the effects of prolonged PDA exposure on BPD, but this requires further study.
A final set of articles addresses evolving information on different PDA treatment strategies. McNamara and colleagues critically reviewed the rapidly expanding data on acetaminophen (paracetamol) for treatment of PDA. The promise of an effective treatment with lower toxicity may finally be realized by selective use of acetaminophen in small preterm infants. In this population, acetaminophen catabolic pathways are less mature and might be protective against accumulation of the toxic metabolites that affect older infants and children. Next, an article by Patrick and colleagues highlights the changing temporal and geographical patterns of PDA ligation in the United States over a 12-year period. Analysis of a large all-payer database of hospitalized children revealed an uptrend in ligation rates until 2006, with a notable decline thereafter. Mortality rates decreased while some morbidities increased over time, leaving smaller, more complex infants at risk for complications. Finally, Backes and colleagues reviewed current information on catheter-based closure of PDA with particular emphasis on smaller infants and earlier time points for intervention. The success of this approach may provide long-sought answers regarding the benefits of closing the PDA in small preterm infants without exposure to potentially harmful drugs. Future decisions regarding PDA treatment (or decisions to refrain from active treatment) will likely depend on the outcome of upcoming genetic, pharmacologic, and catheter-based studies outlined in these reviews.
REFERENCES
- 1.Franklin KJ. A survey of the growth of knowledge about certain parts of the foetal cardio-vascular apparatus, and about the foetal circulation, in man and some other mammals. Part I: Galen to Harvey. Ann Sci. 1941;5:57–89. [Google Scholar]
- 2.Boyer NH. Patent ductus arteriosus: some historical highlights. Ann Thorac Surg. 1967;4:570–573. [DOI] [PubMed] [Google Scholar]
- 3.Zargaran A Ancient Persian medical views on the heart and blood in the Sassanid era (224–637 AD). Int J Cardiol. 2014;172: 307–312. [DOI] [PubMed] [Google Scholar]
- 4.Pagel W. Vesalius and the pulmonary transit of venous blood. J Hist Med Allied Sci. 1964;19:327–341. [DOI] [PubMed] [Google Scholar]
- 5.West JB. Ibn al-Nafis, the pulmonary circulation, and the Islamic Golden Age. J Appl Physiol. 2008;105:1877–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarshenas MM, Zargaran A. A review on the Avicenna’s contribution to the field of cardiology. Int J Cardiol. 2015;182:237–241. [DOI] [PubMed] [Google Scholar]
- 7.Alexi-Meskishvili VV, Bottcher W. The first closure of the persistent ductus arteriosus. Ann Thorac Surg. 2010;90:349–356. [DOI] [PubMed] [Google Scholar]
- 8.Obladen M History of the ductus arteriosus: 1. Anatomy and spontaneous closure. Neonatology. 2011;99:83–89. [DOI] [PubMed] [Google Scholar]
- 9.Benitz WE. Hey, doctor, leave the PDA alone. Pediatrics. 2017;140. [DOI] [PubMed] [Google Scholar]
- 10.Laughon MM, Simmons MA, Bose CL. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated? Curr Opin Pediatr. 2004;16:146–151. [DOI] [PubMed] [Google Scholar]
- 11.Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. 2010;30:241–252. [DOI] [PubMed] [Google Scholar]
- 12.Herrman K, Bose C, Lewis K, Laughon M. Spontaneous closure of the patent ductus arteriosus in very low birth weight infants following discharge from the neonatal unit. Arch Dis Childhood. 2009;94:F48–F50. [DOI] [PubMed] [Google Scholar]
- 13.Weber SC, Weiss K, Buhrer C, Hansmann G, Koehne P, Sallmon H. Natural history of patent ductus arteriosus in very low birth weight infants after discharge. J Pediatr. 2015;167: 1149–1151. [DOI] [PubMed] [Google Scholar]
- 14.Reese J, Laughon MM. The patent ductus arteriosus problem: infants who still need treatment. J Pediatr. 2015;167: 954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semberova J, Sirc J, Miletin J, et al. Spontaneous closure of patent ductus arteriosus in infants /=1500 g. Pediatrics. 2017:140. [DOI] [PubMed] [Google Scholar]
- 16.Christie A Normal closing time of the foramen ovale and the ductus arteriosus: an anatomic and statistical study. Am J Dis Child. 1930;40:323–326. [Google Scholar]
- 17.Adams FH, Moss AJ, Emmanouilides GC. Closure of the ductus arteriosus and foramen ovale In: Cassels DE, ed, The Heart and Circulation in the Newborn Infant. New York and London: Grune and Stratton; 1966. 80–87. [Google Scholar]
- 18.Lind J, Wegelius C. Human fetal circulation: changes in the cardiovascular system at birth and disturbances in the postnatal closure of the foramen ovale and ductus arteriosus. Cold Spring Harb Symp Quant Biol. 1954;19:109–125. [DOI] [PubMed] [Google Scholar]
- 19.Singer C The evolution of anatomy: a short history of anatomical and physiological discovery to Harvey, being the substance of the Fitzpatrick lectures delivered at The Royal College of Physicians of London in the years 1923 and 1924. London: Kegan Paul, Trench, Trubner; 1925;1925. [Google Scholar]
- 20.Dunn PM. Andreas Vesalius (1514–1564), Padua, and the fetal shunts. Arch Dis Childhood. 2003;88:F157–F159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RR. Post-mortem observations on contraction of the human ductus arteriosus. Br Med J. 1958;1:810–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassels DE. The Ductus Arteriosus. Springfield, IL: Charles C. Thomas; 1973;1973. [Google Scholar]
- 23.Scammon R, Norris E. On the time of the post-natal obliteration of the fetal blood-pasages (foramen ovale, ductus arteriosus, ductus venosus). Anat Rec. 1918;15:165–180. [Google Scholar]