Abstract
Background/Aims:
Utilization of a psychosocial screener to identify families affected by a disorder/difference of sex development (DSD) and at risk for adjustment challenges may facilitate efficient use of team resources to optimize care. The Psychosocial Assessment Tool (PAT) has been used in other pediatric conditions. The current study explored the reliability and validity of the PAT (modified for use within the DSD population; PAT-DSD).
Methods:
Participants were197 families enrolled in the DSD-Translational Research Network (DSD-TRN) who completed a PAT-DSD during a DSD clinic visit. Psychosocial data were extracted from the DSD-TRN clinical registry. Internal reliability of the PAT-DSD was tested using the Kuder-Richardson-20 coefficient. Validity was examined by exploring the correlation of the PAT-DSD with other measures of caregiver distress and child emotional-behavioral functioning.
Results:
One-third of families demonstrated psychosocial risk (27.9% “Targeted” and 6.1% “Clinical” level of risk). Internal reliability of the PAT-DSD Total score was high (α = 0.86); 4 of 8 subscales met acceptable internal reliability. A priori predicted relationships between the PAT-DSD and other psychosocial measures were supported. The PAT-DSD Total score related to measures of caregiver distress (r = 0.40, P < 0.001) and to both caregiver-reported and patient self-reported behavioral problems (r = 0.61, P < 0.00; r = 0.37, P < 0.05).
Conclusions:
This study provides evidence for the reliability and validity of the PAT-DSD. Given variability in the internal reliability across subscales, this measure is best used to screen for overall family risk, rather than to assess specific psychosocial concerns.
Keywords: Disorders of Sex Development, DSD, Interdisciplinary Care, Intersex, Screening, Psychometric evaluation
Introduction
Disorders/differences of sex development (DSD)1 are a heterogeneous group of congenital conditions in which a person’s sex chromosomes, gonads or anatomy develop atypically or are discordant [1]. DSD are classified into 3 categories: sex chromosome DSD, 46, XY DSD and 46,XX DSD, with wide variability in the acuity of medical issues. Some DSD require little-to-no immediate medical or surgical management (e.g., proximal hypospadias, a urogenital sinus with clitoromegaly), whereas features of some DSD can be life-threatening without swift medical care (e.g., salt-wasting in congenital adrenal hyperplasia) [2]. DSD may present with internal or external reproductive anatomical differences, gonadal tumor risk requiring surveillance or gonadectomy, and reduced prospects for fertility. In addition, affected individuals frequently need lifelong hormone replacement. In the face of these challenges, the majority of families navigating DSD demonstrate improved coping after initial post-diagnosis distress [3], characteristic of resilience [4]. However, some affected individuals and their parents report significant distress related to aspects of the DSD experiences, such as coping with health care experiences, stigma concerns, and confusion related to diagnosis and decision-making [3, 5–10].
Families managing these DSD experiences are not doing so in a vacuum – there are a host of psychosocial (e.g., psychological, socioeconomic) contextual factors that may impact family resilience in the face of medical challenges (for review [11]). Family resources, social support networks, parent and child emotional-behavioral functioning, and previous interactions with health care providers have been implicated in adjustment to chronic conditions and may be more influential in shaping health and well-being outcomes than disease or biomedical variables [12]. Assessing and intervening along these factors may be critical for enhancing the overall outcomes for patients and families managing chronic conditions, including DSD.
Comprehensive services addressing psychological and social factors for optimizing outcomes in families with a DSD have been recommended in multiple consensus statements and care guidelines [1, 13–15]. For example, most recently, the 2018 European DSD Consensus Statement recommends “Holistic health care”, defined as “a system of comprehensive patient care that considers the physical, emotional, social, economic, and spiritual needs of the patient” and “the needs of family members of patients” [16]. To accomplish this care, DSD guidelines consistently endorse a multidisciplinary team that includes subspecialties with expertise in behavioral health, defined as the interdisciplinary field focused on “the application of behavioral and biomedical science knowledge and techniques to the maintenance of health and prevention of illness and dysfunction” [17]. Behavioral health providers typically include team members from psychology, psychiatry, and/or social work, each with their own areas of expertise.
Unfortunately, more than a decade after the 2006 Consensus statement which highlighted the importance of multidisciplinary care [1], many DSD teams still do not offer universal comprehensive services, and often lack, in particular, behavioral health providers [18, 19]. Barriers to inclusion of behavioral health in DSD comprehensive care have not been delineated but likely include lack of access to behavioral health providers (with DSD expertise, especially), inadequate funding models for multidisciplinary care [20], and/or the financial and stigma-related barriers found in rural or remote resource-poor communities [21, 22]. Even when DSD teams do adhere to a multidisciplinary model including behavioral health, busy clinic schedules involving multiple providers may preclude behavioral health providers from spending sufficient time with any individual family to review all psychosocial factors. For health care providers managing DSD care outside the context of a multidisciplinary team, involvement of behavioral health expertise may be even more challenging: for example, endocrinologists treating patients with DSD without a comprehensive team may be faced with recognizing psychosocial concerns, identifying community psychologists and helping families navigate barriers to access. Thus, families may not be receiving the behavioral health care that is needed. Indeed, in one study, 40% of the 317 parents of children with DSD noted that they had needed psychological support; of these parents identifying this need, 52% had not received any counseling [23].
Within the context of limited time or an incomplete multidisciplinary team, knowing which families are experiencing any of a range of psychosocial contextual challenges may be difficult for DSD care teams; while accurate identification of psychosocial challenges in DSD has not been investigated, research from general pediatric samples suggests that providers miss many patients/families experiencing difficulties and many patients/families do not readily discuss their psychosocial concerns [24, 25]. One strategy to better identify families experiencing challenges is to use some of the many validated measures available to assess numerous psychosocial factors that may impact family adjustment to a chronic condition. However, the use of multiple measures to assess the variety of potential risk factors poses an administrative burden within the health care system and can be limited by 1) the resources required to administer and score measures, 2) the behavioral health expertise required to interpret and communicate results, 3) financial costs to purchasing numerous measures, and 4) families’ willingness/ability to complete multiple measures. Preventive health models offer the framework to circumvent these barriers, particularly in terms of the screening approach, which involves the use of a brief validated measure to identify people currently or at risk of experiencing problems known to occur in a population [11]. Kazak and colleagues describe the Pediatric Psychosocial Preventative Health Model (PPPHM), which highlights 1) screening of all members of the population, 2) importance of universally delivering interventions that promote resiliency and prevent deterioration in family adaptation, and 3) providing specific interventions that match the level of need of families for whom risk factors are identified [11].
In line with this model [11], Kazak and colleagues developed the Psychosocial Assessment Tool (PAT) as a means of assessing risk and resiliency factors in families managing pediatric chronic conditions [26]. The PAT is a brief, broad screener of multiple psychosocial contextual factors that is used to triage families into 1 of 3 risk categories so that resources can be effectively and efficiently managed: Universal reflects transient distress but generally good adjustment; Targeted reflects families experiencing some acute distress and some psychosocial risk; and Clinical reflects on-going, escalating or high-intensity distress with multiple risk factors present. With few exceptions, studies employing the PAT find that the majority of families are coping well and fall into the Universal risk category (50%−76%), with fewer in the Targeted (19%−36%) and Clinical risk (3%−18%) levels [11]. The PAT was developed initially within the pediatric oncology population [26], and, with modest amendments in wording, has been used in multiple pediatric chronic illness populations such as kidney transplant [27], inflammatory bowel disease (IBD) [28] and headache [29]. In health care settings providing DSD care, utilization of a validated single screening measure to detect families at elevated psychosocial risk across multiple factors has the potential to facilitate the triaging and optimizing of resources and may overcome some of the barriers associated with providing integrated behavioral health care. However, the psychometric characteristics of the PAT when used within the context of DSD must first be determined.
Thus, the current study explored the reliability and construct validity of the PAT in the context of DSD care, utilizing clinical registry data from the DSD-Translational Research Network (DSD-TRN). The DSD-TRN is a clinical research network created in 2011, with funding from the National Institutes of Health, to advance discovery of genetic causes of DSD, standardize the diagnostic process, and systematically evaluate relationships between treatment strategies and health and quality of life outcomes of patients and families. Inclusion criteria for the DSD-TRN registry specifies that patients meet the DSD definition provided by the International Consensus Conference on Intersex in that they have “any congenital condition in which development of chromosomal, gonadal or anatomical sex is atypical” [1] and that they (or their caregivers) have provided consent for data to be entered into the registry. The governance structure of the NIH-supported DSD-TRN entails a Network Leadership Group headed by the principal investigators (D. Sandberg and E. Vilain) and identified leaders from each member site, workgroups involving DSD team members from specialty areas, and affected individuals and advocates (http://dsdtrn.org/ for more details). Standardized assessment protocols (based on evidence and care guidelines) are provided to all sites, and diagnoses and interventions are prospectively and longitudinally captured in the network’s patient registry [30]. The DSD-TRN currently comprises 12 US sites that provide multidisciplinary DSD care; at the time of this study, there were 10 sites, 9 of which provided data included in this study (see Appendix I).
As has been done in other studies using the PAT, and with the agreement of the questionnaire designers, the PAT was modified slightly for the DSD population to create the PAT-DSD (changes noted in methods section). We anticipated the breakdown into PAT-DSD risk categories of families affected by DSD would approximate the proportions predicted by the PPPHM model and be similar to the risk categories found in other pediatric conditions using the PAT [11]. We also predicted that the PAT-DSD would demonstrate adequate internal reliability, as shown in previous studies of the PAT [e.g.,26, 27–29]. Finally, we predicted that the PAT-DSD would demonstrate evidence of construct validity in its relationships to scores on independent caregiver- and child-reported psychosocial measures.
Participants and Methods
Participant group
The participants were caregivers and their children enrolled in the DSD-TRN clinical registry. Across the 6 (of 9) sites that provided adequate participation data for calculation, an average of 67% (53%−90%) of eligible families were enrolled in the DSD-TRN. Reasons for families declining to participate are unknown; reasons noted by sites for not approaching families include time constraints, high patient or family distress such that DSD-TRN introduction was delayed and ultimately not completed, guardianship issues, and concern for over-burdening families due to involvement in other research studies. At time of data extraction 325 families were enrolled in the DSD-TRN.
All caregiver-completed PAT-DSDs that were in the DSD-TRN clinical registry were extracted. PAT-DSDs had been completed by parents/caregivers in conjunction with a clinic visit at a DSD-TRN participating site. Families with multiple children with a DSD could consent for each child to be included in the DSD-TRN registry; however, to prevent individual families from being overrepresented within PAT-DSD risk categories, data from only the first child enrolled were included in the current analysis. The DSD-TRN psychosocial screening protocol calls for the PAT-DSD to be administered at the initial visit and annually thereafter [31]. The current analyses are restricted to the initial administration. Because the PAT-DSD was validated against other psychosocial measures completed by the same caregiver, three forms that listed the informant as “both parents” were excluded. The final data set comprised 197 PAT-DSD completed by caregivers (80% mothers; 17% fathers; 3% other caregivers) of 197 unique patients with a DSD; this corresponded with 61% of the enrolled families. Of note, there was considerable variability among sites in terms of PAT-DSD data availability (sites ranged from 6% - 89% of families with PAT-DSD data).
Measures
As part of the DSD-TRN model of care, caregivers and patients (8 years or older) completed a standardized assessment battery of commonly used and validated psychosocial measures [31]. Of note, patients and their caregivers were asked to complete the forms as part of standard clinical care, regardless of whether they had consented to have their clinical data entered into the DSD-TRN registry. Caregiver measures in the battery include the PAT-DSD, Patient Health Questionnaire-4 (PHQ-4) [32], Child Behavior Checklist (CBCL) [33] and 2 measures designed for the DSD-TRN (Support and Resource Assessment, Knowledge of Condition). Patient measures include the Youth Self-Report (YSR) [33], Self-Perception Profile (SPP) [34, 35], Body Image Scale [36], Multidimensional Gender Identity Scale [37], and a measure designed for the DSD-TRN (Knowledge of Condition). Instruments were selected by a working group including pediatric behavioral health specialists and members of DSD advocacy and support groups, were developmentally sensitive, and were included only if there was an expectation that they could deliver immediately actionable information to DSD team providers at the clinical sites about the patient and family (e.g., identification of caregiver stress leading to consultation with a behavioral health provider) [31]. Sites adopted different approaches for the administration of the psychosocial battery; for example, at one site families were given the measures to complete during their clinic visit, while at another site families completed measures at home and returned them by mail. Estimated maximum time to complete the entire battery, based on information provided by test developers and clinical experience, is 45 minutes for caregivers and 55 minutes for patients.
The Psychosocial Assessment Tool-DSD version (PAT-DSD).
The PAT is a brief measure that takes a parent/guardian approximately 10 minutes to complete (paper/pencil, web-based and REDcap administration are available), and takes approximately 5 minutes to score and interpret, with a variety of scoring templates available [11]. It assesses seven psychosocial domains thought to be related to overall family risk; subscales include Family Structure/Resources, Social Support, Child Problems, Sibling Problems, Family Problems, Caregiver Stress Reaction and Family Beliefs. Subscale scores are an average of item responses within the subscale, and range from 0–1, with higher scores indicating greater risk; the Total PAT score is a sum of subscale scores, and can range from 0–7. The total PAT score is used to determine risk category: < 1 = “Universal” risk; ≥ 1 and < 2 = “Targeted” risk; and ≥ 2 = “Clinical” risk. The PAT has been translated into multiple languages and the version on which the PAT-DSD was based has a fourth grade reading level [11]. Evidence for the reliability and validity of the PAT was first established in pediatric oncology [26], and has been found across a range of pediatric conditions with samples of caregivers of children from infancy through adolescence [27–29]. Of note, as has been done in other studies of the PAT, the PAT-DSD was modified slightly from the original PAT by substituting DSD-specific language in place of references to “cancer,” and the demographic section was expanded slightly. In addition, because stigma is a notable concern for affected individuals with DSD and their families [38, 39], 3 items about stigma were added to create a Stigma subscale. These items were chosen from previous published research on a DSD stigma measure that incorporated stigma items from other questionnaires as well as the input of caregivers of children with DSD on their stigma concerns [38], and were as follows: “My child will be treated differently because of his/her condition”, “Having a urogenital condition attaches a stigma or label to my child”, “My child’s condition will put limits on his/her having a good life”. These items were not included in the PAT Total score computation, and were added at the end of the PAT, thereby minimizing influence on caregiver responding to previous items.
Validation measures.
To demonstrate evidence of construct validity of the PAT-DSD in this new population, only measures from the psychosocial battery which, a priori, were predicted to be related to domains represented in the PAT-DSD were included in the data analyses. These measures are listed in Table 1. Caregivers complete the PHQ-4 [32], a self-reported screener for anxiety and depression and the parent-proxy (CBCL) [33] for children 1.5 years of age and older to screen for child social competencies and emotional/behavioral problems; both of these measures are completed at the first visit and annually thereafter. Patients with a DSD who are 8 years of age or older complete the SPP for Children/Adolescents [34, 35] to assess self-perceptions of domain-specific competencies or adequacies, and patients ≥ 11 years old complete the YSR [33], a self-report measure that parallels the CBCL. These measures are completed at the first visit that children meet the age eligibility requirement, with the CBCL and YSR completed annually and the SPP biannually thereafter. Only data from measures completed within 90 days of the PAT-DSD were used in analyses, and, for caregiver-report measures, only reports completed by the same caregiver completing the PAT-DSD were used. Sites were variable in how closely they followed the recommended administration schedule for measures. Thus, while the total n for the PAT-DSD is 197 families, the n is smaller for the validity analyses.
Table 1.
Validation measures for the Psychosocial Assessment Tool-DSDa with a priori predicted relationships noted
| Measure | N | Domains assessed |
Reporter | Score used |
PAT-DSD scale score associations to establish construct validity |
|
|---|---|---|---|---|---|---|
| Criterion Validity | Convergent Validity | |||||
| Patient Health Questionnaire-4 [32] | 113 | Caregiver anxiety and depression | Caregiver | Total score | PAT-DSD Total score | PAT-DSD Family Problems PAT-DSD Caregiver Stress Reaction |
| Child Behavior Checklist [33] | 84 | Child emotional-behavioral functioning | Caregiver | Total T score | PAT-DSD Total score | PAT-DSD Child Problems |
| Youth Self-Report [33] | 45 | Child emotional-behavioral functioning | Patient | Total T score | PAT-DSD Total score | PAT-DSD Child Problems |
| Self-Perception Profile [34,35] | 41 | Perceptions of domain-specific competencies or adequacies | Patient | Global Self-Worth score | NA | PAT-DSD Child Problems |
Psychosocial Assessment Tool-DSD based on the Psychosocial Assessment Tool [26]
Statistical Analyses
Demographic information was extracted from the PAT-DSD form and diagnostic information was extracted from the DSD-TRN patient registry. Descriptive analyses were conducted on the demographic and diagnostic information as well as on the PAT-DSD Total and subscale scores. Risk category frequencies were also calculated. Internal consistency reliability (that is, the degree to which items are interrelated) for the PAT-DSD Total and each subscale was tested using a modified Cronbach’s alpha appropriate for binary responses (i.e., Kuder-Richardson-20 coefficient), with α ≥ 0.70 considered “acceptable” [40]. Criterion and convergent validity of the PAT-DSD were explored to document evidence of construct validity [41, 42]. Criterion validity measures the degree to which a test predicts a specific outcome. In this case, we examined the ability of the PAT-DSD Total score to predict functioning as ascertained by the validation measures (i.e., PHQ-4, CBCL, YSR and SPP) by first estimating bivariate correlations of the Total score with those measures and, second, by conducting analyses of variance (Welch’s robust ANOVAs with post hoc Tukey HSD tests) to determine if classification according to PAT-DSD risk category was associated with mean differences on those measures. Convergent validity of the PAT-DSD subscales was evaluated by calculating bivariate correlation coefficients of specific subscales with validation measures assessing similar constructs (Table 1). Alpha was set to 0.05 for all analyses, with P values < 0.1 considered to be a statistical trend. Statistical analyses were completed using SPSS Version 23.
Results
Participant group characteristics
The group was primarily white and non-Hispanic (Table 2). The median patient age at time of PAT-DSD completion was 6.7 years; 22% of the patients were less than 1 years old. The majority of patients were being raised as girls (59%); 1 child (1 month old at time of PAT-DSD completion) was being raised gender-neutral and 1 youth (17 years old) had initially been assigned female gender at birth but had self-initiated gender reassignment to male as an adolescent. 46,XY and 46,XX DSD were equally represented in the group, and were more prevalent than sex chromosome DSD (41.6%, 42.1%, 10.2%, respectively). While 49.2% of patients had received a confirmed genetic diagnosis, many patients carried a confirmed or provisional diagnosis based on a combination of endocrinologic and phenotypic findings. Diagnoses in the 46,XY category included partial and complete androgen insensitivity, 17-beta hydroxysteroid dehydrogenase deficiency, and penoscrotal hypospadias (etiology unknown); in the 46,XX category included congenital adrenal hyperplasia, ovotesticular DSD, and Mayer-Rokitansky-Küster-Hauser syndrome; and in the Sex Chromosome DSD category included mixed gonadal dysgenesis, mosaic Turner syndrome, and case-specific deletions (e.g., Xp22.31 deletion). PAT-DSDs were typically completed by mothers, and the majority of caregiver informants had some college education.
Table 2.
Participant group demographic and diagnostic information (n = 197)
| Children | ||||
| Age (yrs), median (range) | 6.7 (1 day – 22.5 years) | |||
| White, n (%) | 137 (69.5) | |||
| Hispanic, n (%) | 57 (28.9) | |||
| Gender at time of study, n (%) | ||||
| Girl | 117 (59.4) | |||
| Boya | 79 (40.1) | |||
| Gender-neutral | 1 (0.5) | |||
| Gender n (% of category) |
||||
| DSD category, n (%) | Girl | Boy | Gender-neutral | |
| Sex chromosome DSD | 20 (10.2) | 7 (35.0) | 13 (65.0) | 0 (0.0) |
| 46,XY DSD | 82 (41.6) | 30 (36.6) | 52 (63.4)a | 0 (0.0) |
| 46,XX DSD | 83 (42.1) | 73 (88.0) | 9 (10.8) | 1 (1.2) |
| Karyotype unavailable | 12 (6.1) | 7 (58.3) | 5 (41.7) | 0 (0.0) |
| Caregiver Informants | ||||
| Mother, n (%) | 150 (76.1) | |||
| White, n (%) | 138 (70.1) | |||
| Educational background n (%)b | ||||
| ≤ High school education | 43 (21.8) | |||
| College courses/degrees | 113 (57.4) | |||
| Postgraduate education | 39 (19.8) | |||
Includes one youth who was assigned female gender at birth but in adolescence self-initiated reassignment to male gender.
Educational background missing for 2 caregivers
PAT-DSD Total and subscale scores were comparable to the values found in other studies of pediatric conditions using the PAT [29, 43] (Table 3). The mean (SD) Total score for the group was 0.86 (0.65), which fell in the Universal level of risk. However, nearly a third of the group demonstrated some level of psychosocial risk (66.0% Universal risk, 27.9% Targeted risk and 6.1% Clinical level of risk), mirroring the general distribution of risk categories previously identified by the PAT (see Figure 1 for comparison with a sampling of conditions [11, 28, 43, 44]; for more complete comparison see review [11]).
Table 3.
Descriptive statistics and internal consistency for the Psychosocial Assessment Tool-DSD Total score and subscales
| PAT-DSD Scales | n | # items | Range | Mean | SD | Cronbach α (n) |
|---|---|---|---|---|---|---|
| T otal Score | 197 | 62 | 0 – 3.58 | 0.86 | 0.65 | .86 (86) |
| Family Structure/Resources | 197 | 8 | 0 – 0.75 | 0.14 | 0.17 | .56 (197) |
| Social Support | 193 | 4 | 0 – 1.00 | 0.06 | 0.18 | .73 (184) |
| Child Problems | 197 | 15 | 0 – 0.87 | 0.23 | 0.24 | .86 (197) |
| Sibling Problems | 100 | 17 | 0 – 0.59 | 0.10 | 0.15 | .80 (94) |
| Family Problems | 194 | 8 | 0 – 1 | 0.19 | 0.21 | .71 (188) |
| Caregiver Stress Reaction | 197 | 3 | 0 – 1 | 0.04 | 0.15 | .65 (197) |
| Caregiver Beliefs | 197 | 4 | 0 – 1 | 0.13 | 0.18 | .41 (197) |
| Caregiver Stigma Concerns | 197 | 3 | 0 – 1 | 0.13 | 0.25 | .58 (197) |
Note: Sample sizes for Cronbach a calculations differ from the samp e sizes for descriptive statistics because listwise deletion was used for reliability estimates, whereas descriptive statistics were based on subscale scores, which were calculated as long as 50% of items needed to compute a subscale score were non-missing.
Figure 1.
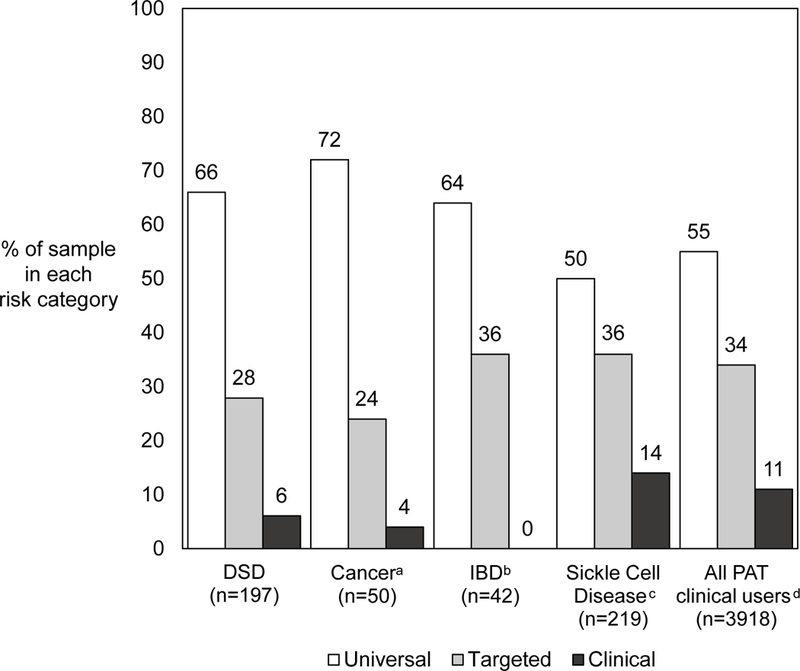
Comparison of the Psychosocial Assessment Tool-DSD (PAT-DSD) risk category distribution (in current study, n = 197) versus Psychosocial Assessment Tool (PAT) risk category distribution in comparable pediatric populations.
Internal Consistency Reliability
The PAT-DSD Total score demonstrated good internal consistency reliability (α = 0.86; Table 3), comparable to previous studies of the PAT that reported internal consistency (e.g., oncology α = 0.81 [26]; kidney transplant α = 0.82 [27]; IBD α = 0.82 [28]; headache α = 0.80 [29]). PAT-DSD Social Support, Child Problems, Sibling Problems and Family Problems all showed acceptable reliability (all α > 0.70), with Caregiver Stress Reaction slightly below the acceptable range (α = 0.65). The subscales of Family Resources/Structure and Caregiver Beliefs demonstrated inadequate reliability; previous studies have found these same subscales to show poorer reliability [26–29]. Our newly added Stigma Concerns subscale did not meet acceptable criteria for reliability.
Construct Validity
As evidence of criterion validity, the PAT-DSD Total score correlated positively with the predicted caregiver-reported (PHQ-4: r = 0.43, P < 0.001; CBCL: r = 0.61, P < 0.001) and patient self-reported (YSR: r = 0.37, P = 0.011) measures of parent and child emotional distress/behavioral disturbance (Table 4). In addition, caregiver distress varied significantly according to PAT-DSD risk category [F(2, 15.12) = 5.91, P = 0.013], with mean (SD) PHQ-4 scores of 1.03 (1.88), 1.78 (2.26) and 5.43 (3.60) for the Universal, Targeted and Clinical risk levels, respectively; Universal and Targeted groups differed significantly from the Clinical risk group, but not from each other. Similarly, scores on measures of child emotional/behavioral functioning on both the caregiver-reported CBCL [F(2, 14.18) = 13.07, P < 0.001] and the patient self-reported YSR [F(2, 9.82) = 5.86, P = 0.021] differed significantly according to PAT-DSD risk category. For the CBCL, scores for the Targeted and Clinical risk groups were significantly higher than the Universal risk group, but not different from each other; the mean (SD) CBCL Total score was 44.19 (11.09), 55.89 (12.21) and 62.67 (11.38) for the Universal, Targeted and Clinical risk levels, respectively. For the YSR, the Universal and Targeted risk groups significantly differed; the mean (SD) YSR Total T score was 47.41 (6.52), 56.28 (9.00) and 54.00 (15.91) for the Universal, Targeted and Clinical risk levels, respectively.
Table 4.
Correlations between the Psychosocial Assessment Tool-DSD (PAT-DSD) scales and construct validation measures (n)
| Criterion Validity |
Convergent Validity |
|||||
|---|---|---|---|---|---|---|
| PAT-DSD Total score |
PAT-DSD subscalesa | |||||
| Social Support | Child Problems | Sibling Problems |
Family Problems |
Caregiver Stress Reaction | ||
| Caregiver-report, r (n) | ||||||
| PHQ-4b | 0.43*** (113) | 0.29** (113) | 0.13 (113) | 0.06 (63) | 0.47*** (112) | 0.46*** (113) |
| CBCLc | 0.61*** (84) | 0.05 (84) | 0.80*** (84) | 0.29* (55) | 0.40*** (84) | −0.05 (84) |
| Child self-report, r (n) | ||||||
| YSRd | 0.37* (45) | 0.09 (45) | 0.43** (45) | 0.31 (31) | 0.47** (45) | −0.13 (45) |
| SPPe | −0.11 (41) | 0.17 (40) | −0.35* (41) | −0.14 (26) | −0.26 (41) | 0.04 (41) |
Evidence for convergent validity was also demonstrated (Table 4). As predicted, the PAT-DSD Child Problems subscale positively correlated with the CBCL (r = 0.80, P < 0.001) and YSR (r = 0.43, P = 0.003) and negatively correlated with the SPP Global Self-Worth scale (r = −0.35, P = 0.024). Also as predicted, the PAT-DSD Family Problems and Caregiver Stress Reaction subscales were both positively correlated with the PHQ-4 (r = 0.47, P < 0.001; r = 0.46, P < 0.001, respectively). Although not predicted a priori, the PAT-DSD Family Problems subscale also correlated with both caregiver- and self-reported patient emotional/behavioral problems (CBCL: r = 0.40, P < 0.001; YSR: r = 0.47, P = 0.001). Smaller unpredicted associations were found between the PAT-DSD Social Support subscale and PHQ-4 (r = 0.29, P = 0.002) and the PAT-DSD Sibling Problems subscale and CBCL (r = 0.29, P = 0.032), such that 1) higher caregiver distress was related to lower social support and 2) higher caregiver-reported patient emotional/behavioral concerns were associated with higher caregiver-reported sibling problems.
Discussion
This study is the first to examine the psychometric properties of a broad psychosocial screener, the PAT-DSD, in the pediatric DSD population. Our study provided evidence for the reliability and validity of the PAT-DSD; in particular, the PAT-DSD Total score demonstrated good internal reliability and evidence of criterion validity in that it was correlated with other measures of emotional/behavioral functioning as predicted. The PAT-DSD Total score was positively correlated to measures completed by both the same informant (PHQ-4 and CBCL) and a different informant (YSR), strengthening confidence in this finding. In addition, the classification of the PAT-DSD Total score into household levels of risk resulted in a risk stratification similar to other pediatric populations, with the risk groups differing in both caregiver and child emotional-behavioral functioning. The PAT-DSD subscales were variable in terms of internal reliability, with subscales measuring the emotional/behavioral functioning of family members and family social support (Social Support, Child Problems, Sibling Problems, Family Problems and Caregiver Stress Reaction) demonstrating the highest internal consistency. Convergent validity of subscales was also supported as these subscales significantly correlated in the expected direction with independent measures of overlapping constructs. Thus, our study provides preliminary support for the use of the PAT-DSD as a screener for psychosocial risk in families with a patient with DSD.
Given that a third of these families affected by DSD reported risk factors that might be barriers to resilient adaptation, identification of a broad and user-friendly screener for the DSD population may have a beneficial clinical impact. In other populations, use of the PAT has correlated with increased mention of psychosocial risk factors in the medical record, and increased utilization of psychosocial services relative to standard of care [44, 45]. Level of psychosocial follow-up care aligned with risk category in some [27, 45], but not all [46] previous studies, suggesting that identification of high-risk families may lead to greater allocation of resources. Notably, in a randomized controlled pilot study with a sample of children with cancer, those families whose PAT information at Time 1 was systematically provided to their treatment team demonstrated significant reduction in risk on the PAT as measured 6 months later relative to families whose PAT information was not communicated [47]. While these findings using the PAT in other conditions are encouraging, future studes of utility of the PAT-DSD within the DSD population are needed.
Health prevention models emphasize that families in all risk categories benefit from interventions designed to promote resilience and mitigate risk factors. Potential DSD-specific interventions across risk level categories are listed in Table 5. Universal care for all families should include high-quality education, connection with peer support, and team emphasis on essential components of resiliency including developmentally-sensitive information-sharing with children. In addition, all families should be offered consultation with a behavioral health specialist familiar with DSD and their implications. Families identified as falling in the Targeted risk category can be prioritized to have access to behavioral health providers with interventions addressing specific concerns, such as a pediatric psychologist providing brief behavioral interventions to assist with medication adherence or involvement of social work to assist with resource identification. Families scoring in the Clinical risk range will likely require extended access to behavioral health during clinic visits, with identification of outpatient support, close follow-up by the multidisciplinary care team and, possibly, more extensive utilization of community agencies providing wrap-around services. Research in other pediatric populations suggests that parent- and family-focused interventions such as cognitive-behavioral therapy or problem-solving interventions can benefit parent adaptation, child coping and medical symptoms [48]. Of course, in resource-poor communities, bolstering family resilience may remain challenging, but the use of a screening tool may focus efforts on those with most need.
Table 5.
Example DSD-related interventions across the Psychosocial Assessment Tool-DSD risk levels
| Risk level | Potential interventions |
|---|---|
| Universal | Connect with peer support Provide high-quality written and verbal education Specific DSD etiology, course and treatment options Distinctions between biological sex (sex chromosomes, gonads, internal/external anatomy) and psychosocial differentiation (gender identity, gender role, sexual orientation) Natural variability in appearance Promote family-centered care and active involvement in shared decision-making Discuss importance of information sharing (particularly to affected child) Offer behavioral health consultation (unless universally involved) Address complex shared-decision making challenges (e.g., prior to child’s ability to participate themselves in irrevocable non-urgent decisions) Explore stigma-related concerns (e.g., distinguish “privacy” vs “shame/secrecy,” problem-solve social challenges) Provide routine clinic follow-up |
| Targeted | Offer Universal interventions (see above) Meet with in-clinic behavioral health specialist / psychologist for further assessment of identified concerns Practice communication skills (e.g., disclosure to others, developmentally appropriate education to child) Involve other hospital resources to address specific concerns (e.g., Social Work, Chaplaincy, Child Life) Implement behavioral interventions (e.g., medication adherence, healthy food choices/ exercise for weight management) Teach brief emotion regulation strategies (e.g., to assist with coping with medical exams/ procedures, mild/transient mood concerns) Teach problem-solving interventions (e.g., medication management during overnight party) Provide shorter-term follow-up to assess efficacy of interventions and need for more intensive services |
| Clinical | Offer appropriately timed Targeted and Universal interventions (Note: these may not be effective when families are in acute crisis) Conduct safety assessment & planning Provide immediate crisis intervention Refer for outpatient counseling Involve community services Provide close phone follow-up to support follow-through with additional referrals/services |
Any screening tool must be integrated into clinic flow for it to be effectively utilized with patients, and future research will need to determine the utility of the PAT for health care providers working with patients affected by a DSD. Providers will need to determine the most efficient system based on available resources: for example, in one setting a behavioral health provider may administer, score and review with the family and team, whereas in another setting a nurse may administer and score and then convey risk level to a behavioral health provider and/or the medical team. Previous research on stakeholder perspectives of the PAT in other populations find that caregivers describe the PAT as short and easy to complete, and likely to facilitate difficult conversations about risk factors [49]; studies of health providers find that some providers have concern about the length of time it takes to assist families completing the PAT; however, some providers in systems with a standardized work flow for the PAT report that PAT administration and scoring is simple with a minimal impact on workflow [50]. There are a number of administration and scoring options (e.g., paper and pencil, web-based; https://www.psychosocialassessmenttool.org/using-pat). Provider stakeholders have also noted that having a member of the team who will educate other members on the importance of screening and advance the screening process is essential for successful implementation [50]. The optimal screening schedule may differ according to the pediatric population, but certainly time of diagnosis or first contact with family offers the opportunity to provide preventive psychosocial care. Finally, health care systems must have a financial model that supports access to behavioral health providers and allied health professionals trained in evidence-based approaches to ameliorating psychosocial risk and identifying and supporting family strengths to optimize adjustment.
Limitations of the study
A limitation of our study is that several PAT-DSD subscales did not meet criteria for adequate internal reliability. Of note, these subscales typically had fewer items in the scale (e.g., Caregiver Stress and Caregiver Beliefs); additional items might strengthen internal reliability [51]. In particular, the 3-item Caregiver Stigma Concern subscale that we added to modify the PAT specifically for the DSD population did not demonstrate adequate internal reliability. Caregivers highlight significant concerns at the time of diagnosis related to stigma, and indicate that they make treatment decisions related to these concerns [38, 52]. Accordingly, further work on identifying different or additional items that, as a group, more reliably screen for stigma is indicated. These items could be identified by looking at other measures of stigma in this population [38]. However, one strength of the PAT is its brevity, so any additional items should be carefully selected and tested to avoid unnecessarily lengthening the measure. In addition, because these data were collected as part of a clinical registry protocol conducted at multiple sites with varying availability of administrative resources, patient enrollment and measure administration rates differed across sites, and we lack detailed information related to family nonparticipation in the DSD-TRN and our incomplete measure data.
Conclusion
Our study provides evidence supporting the psychometric merit of the PAT-DSD, particularly the Total score and risk categories. The variability in internal consistency of the PAT-DSD subscales underscores that the PAT-DSD is best understood as a screener of overall psychosocial risk, and individual items within the subscales should guide clinical conversations rather than using subscale scores to diagnose any specific psychological disorder or to capture change in response to an intervention. Our study also suggests that families with DSD may have psychosocial risk factors that could complicate DSD care. A family’s ability to manage treatment burden will likely be enhanced when these risk factors are identified and resources are provided to mitigate psychosocial risk. Use of a psychosocial screener, such as the PAT-DSD, at initial point of entry may be a step toward providing comprehensive care as envisioned in multiple care guidelines for individuals affected by DSD and their families [1, 13–16], particularly in less resource-rich communities. Given that DSDs pose long-term health, psychological and socioeconomic challenges for some individuals [53–55], early prevention or intervention efforts may set the stage for more resilient adaptation over a lifetime. Future studies should investigate the utility of the PAT-DSD for triaging available resources to families and testing the impact of these resource on the quality of life of affected persons and their families.
Acknowledgements
The authors express sincere gratitude to the patients with DSD and their families who have agreed to participate in the DSD-TRN patient registry in order to contribute to shared knowledge and improve DSD care. We thank the following members of the DSD-TRN Leadership group for overseeing site management and participant recruitment: Michael DiSandro, Courtney Finlayson, Veronica Gomez-Lobo, Janet Green, Stephen Rosenthal and Kathleen van Leeuwen. We also thank the members of the DSD-TRN Psychosocial Work Group for designing and/or implementing the battery of psychosocial measures at participating DSD-TRN sites: Diane Chen, Carmel Foley, Sue Kearns, Kim Kennedy, Tom Mazur, Linda Ramsdell, Vernon Rosario, Melissa Sharp, and Beverly Yashar.
Further information about the Psychosocial Assessment Tool can be found at https://www.psychosocialassessmenttool.org/using-pat.
Funding Sources
The DSD-TRN was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant numbers: R01 HD068138, DSD-Translational Research Network.
Appendix
The DSD-TRN is currently comprised of the following member and affiliate sites: Children’s National Health System, Cincinnati Children’s Hospital Medical Center, Cohen Children’s Medical Center of New York/Northwell Health, Doernbecher Children’s Hospital – Oregon Health & Science University, Le Bonheur Children’s Hospital, Lurie Children’s Hospital, Michigan Medicine, Phoenix Children’s Hospital, Seattle Children’s Hospital, University of California-San Francisco, and St. Louis Children’s Hospital – Washington University in St. Louis.
The following sites provided data utilized in this study: Cincinnati Children’s Hospital Medical Center, Children’s National Health System, Cohen Children’s Medical Center of New York/Northwell Health, Lurie Children’s Hospital, Michigan Medicine, Phoenix Children’s Hospital, Seattle Children’s Hospital, University of California-Los Angeles, and University of California-San Francisco.
The DSD-TRN Leadership Group consisted of the following members at the time of this study: Emmanuéle Délot, Michael DiSandro, Patricia Y. Fechner, Courtney Finlayson, Veronica Gomez-Lobo, Janet Green, Stephen Rosenthal, Meilan M. Rutter, David E. Sandberg, Margarett Shnorhavorian, Phyllis Speiser, Kathy van Leeuwen, and Eric Vilain.
The DSD-TRN Psychosocial Workgroup consisted of the following members at the time of this study: Erica M. Weidler, Diane Chen, Tiger Devore, Michelle M. Ernst, Peter Ferren, Carmel Foley, Michelle Fox, Elaine Goldberg, Sue Kearns, Kim Kennedy, Tom Mazur, Elizabeth McCauley, Miriam Muscarella, Linda Ramsdell, Vernon Rosario, Melissa Sharp, and Beverly Yashar.
Footnotes
Statement of Ethics
Caregivers gave their written informed consent for participation in the clinical registry; patients gave their written assent beginning at age 8 years old. The study protocol was approved by each DSD-TRN member site’s committee on human research and conformed to the ethical standards of the World Medical Association Declaration of Helsinki.
Disclosure Statement
The authors have no conflicts of interest to declare.
There is a lack of agreement on the terminology used to describe these conditions; while “disorders of sex development” was proposed in the 2006 International DSD Consensus Statement (with the input of affected individuals and advocates) as a means of decreasing stigmatizing language and standardizing terminology to promote clinical and research rigor, many affected individuals prefer other terms such as “intersex” or diagnosis-specific terms.
References
- 1.Lee PA, Houk CP, Ahmed SF, Hughes IA. Consensus statement on management of intersex disorders. Pediatrics. 2006;118(2):e488–500. doi: 10.1542/peds.2006-0738. [DOI] [PubMed] [Google Scholar]
- 2.Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HF, Miller WL, Murad MH, Oberfield SE, White, PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043–88. doi: 10.1210/jc.2018-01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellens RE, Bakula DM, Mullins AJ, Reyes KJS, Austin P, Baskin L, Bernabé K, Cheng EY, Fried A, Frimberger D. Psychological adjustment in parents of children born with atypical genitalia one year after their child undergoes genitoplasty. J Urol. 2017198;(4):914–20. doi: 10.1016/j.juro.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh F Family resilience: a framework for clinical practice. Family process. 2003;42(1):1–18. [DOI] [PubMed] [Google Scholar]
- 5.Alpern AN, Gardner M, Kogan B, Sandberg DE, Quittner AL. Development of health-related quality of life instruments for young children with disorders of sex development (DSD) and their parents. J Pediatr Psychol. 2016;42(5):544–58. doi: 10.1093/jpepsy/jsw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engberg H, Strandqvist A, Nordenström A, Butwicka A, Nordenskjöld A, Hirschberg AL, Frisén L. Increased psychiatric morbidity in women with complete androgen insensitivity syndrome or complete gonadal dysgenesis. J Psychosom Res. 2017;101:122–27. doi: 10.1016/j.jpsychores.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Meyer-Bahlburg HF, Khuri J, Reyes-Portillo J, New MI. Stigma in medical settings as reported retrospectively by women with congenital adrenal hyperplasia (CAH) for their childhood and adolescence. J Pediatr Psychol. 2016;42(5):496–503. doi: 10.1093/jpepsy/jsw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasterski V, Mastroyannopoulou K, Wright D, Zucker KJ, Hughes IA. Predictors of posttraumatic stress in parents of children diagnosed with a disorder of sex development. Arch Sex Behav. 2014;43(2):369–75. doi: 10.1007/s10508-013-0196-8. [DOI] [PubMed] [Google Scholar]
- 9.Schützmann K, Brinkmann L, Schacht M, Richter-Appelt H. Psychological distress, self-harming behavior, and suicidal tendencies in adults with disorders of sex development. Arch Sex Behav. 2009;38(1):16–33. doi: 10.1007/s10508-007-9241-9. [DOI] [PubMed] [Google Scholar]
- 10.Suorsa KI, Mullins AJ, Tackett AP, Reyes KJS, Austin P, Baskin L, Bernabé K, Cheng E, Fried A, Frimberger D, Galan D, Gonzalez L, Greenfield S, Kropp B, Meyer T, Meyer S, Nokoff N, Palmer B, Poppas B, Paradis A, Yerkes E, Wisniewski AB, Mullins LL. Characterizing early psychosocial functioning of parents of children with moderate to severe genital ambiguity due to disorders of sex development. J Urol. 2015;194(6):1737–42 doi: 10.1016/j.juro.2015.06.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazak AE, Schneider S, Didonato S, Pai AL. Family psychosocial risk screening guided by the pediatric psychosocial preventative health model (PPPHM) using the Psychosocial Assessment Tool (PAT). Acta Oncol. 2015;54(5):574–80. doi: 10.3109/0284186X.2014.995774. [DOI] [PubMed] [Google Scholar]
- 12.Ernst MM, Marino BS, Cassedy A, Piazza-Waggoner C, Franklin RC, Brown K, Wray J. Biopsychosocial predictors of quality of life outcomes in pediatric congenital heart disease. Pediatr Cardiol. 2018;39(1):79–88. doi: 10.1007/s00246-017-1730-6. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, Elford S, Hughes IA, Izatt L, Krone N, Miles H, O’Toole S, Perry L, Sanders C, Simmonds M, Watt A, Willis D. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015). Clin Endocrinol. 2016;84(5):771–88. doi: 10.1111/cen.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakula DM, Sharkey CM, Wolfe-Christensen C, Mullins AJ, Meyer J, Mullins LL, Wisniewski AB. Recommendations for the establishment of disorders/differences of sex development interdisciplinary care clinics for youth. J Pediatr Nurs. 2017;37:79–85. doi: 10.1016/j.pedn.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Hiort O, Birnbaum W, Marshall L, Wünsch L, Werner R, Schröder T, Döhnert U, Holterhus PM. Management of disorders of sex development. Nat Rev Endocrinol. 2014;10(9):520–29 doi: 10.1038/nrendo.2014.108. [DOI] [PubMed] [Google Scholar]
- 16.Cools M, Nordenström A, Robeva R, Hall J, Westerveld P, Flück C, Köhler B, Berra M, Springer A, Schweizer K. Caring for individuals with a difference of sex development (DSD): a Consensus Statement. Nature Reviews Endocrinology. 2018:1. doi: 10.1038/s41574-018-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matarazzo JD. Behavioral health and behavioral medicine: frontiers for a new health psychology. Am Psychol. 1980;35(9):807. [DOI] [PubMed] [Google Scholar]
- 18.Rolston AM, Gardner M, van Leeuwen K, Mohnach L, Keegan C, Délot E, Vilain E, Sandberg DE, members of the DSD-TRN Advocacy Advisory Network Accord Alliance. Disorders of sex development (DSD): clinical service delivery in the United States. Am J Med GenetC Semin Med Genet. 2017;175(2):268–78. doi: 10.1002/ajmg.c.31558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyriakou A, Dessens A, Bryce J, lotova V, Juul A, Krawczynski M, Nordenskjold A, Rozas M, Sanders C, Hiort O, Ahmed SF. Current models of care for disorders of sex development - results from an international survey of specialist centres. Orphanet J Rare Dis. 2016; 11(1): 155. doi: 10.1186/s13023-016-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernst MM, Liao L-M, Baratz AB, Sandberg DE. Disorders of sex development/intersex: gaps in psychosocial care for children. Pediatrics. 2018;142(2). doi: 10.1542/peds.2017-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saraceno B, van Ommeren M, Batniji R, Cohen A, Gureje O, Mahoney J, Sridhar D, Underhill C. Barriers to improvement of mental health services in low-income and middle-income countries. Lancet. 2007;370(9593):1164–74. doi: 10.1016/S0140-6736(07)61263-X. [DOI] [PubMed] [Google Scholar]
- 22.Warne GL, Raza J. Disorders of sex development (DSDs), their presentation and management in different cultures. Rev Endocr Metab Disord. 2008;9(3):227–36. doi: 10.1007/s11154-008-9084-2. [DOI] [PubMed] [Google Scholar]
- 23.Bennecke E, Werner-Rosen K, Thyen U, Kleinemeier E, Lux A, Jurgensen M, Gruters A, Kohler B. Subjective need for psychological support (PsySupp) in parents of children with dsd: results from the German clinical evaluation study. Eur J Pediatr. 2015;174(10):1287–97. doi: 10.1007/s00431-015-2530-8. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz SM, Leaf PJ, Leventhal JM. Identification of psychosocial problems in pediatric primary care: do family attitudes make a difference? Arch Pediatr Adolesc Med. 1998;152(4):367–71. [DOI] [PubMed] [Google Scholar]
- 25.Sayal K, Taylor E. Detection of child mental health disorders by general practitioners. Br J Gen Pract. 2004;54(502):348–52. [PMC free article] [PubMed] [Google Scholar]
- 26.Pai AL, Patino-Fernandez AM, McSherry M, Beele D, Alderfer MA, Reilly AT, Hwang WT, Kazak AE. The Psychosocial Assessment Tool (PAT2.0): psychometric properties of a screener for psychosocial distress in families of children newly diagnosed with cancer. J Pediatr Psychol. 2008;33(1):50–62. doi: 10.1093/jpepsy/jsm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai AL, Tackett A, Ittenbach RF, Goebel J. Psychosocial Assessment Tool 2.0_general: validity of a psychosocial risk screener in a pediatric kidney transplant sample. Pediatr Transplant. 2012;16(1):92–98. doi: 10.1111/j.1399-3046.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 28.Pai AL, Tackett A, Hente EA, Ernst MM, Denson LA, Hommel KA. Assessing psychosocial risk in pediatric inflammatory bowel disease: validation of the Psychosocial Assessment Tool 2.0_general. J Pediatr Gastroenterol Nutr. 2014;58(1):51–56. doi: 10.1097/MPG.0b013e3182a938b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods K, Ostrowski-Delahanty S. Psychometric properties of the Psychosocial Assessment Tool-chronic pain version in families of children with headache. J Child Neurol. 2017;32(8):766–73. doi: 10.1177/0883073817707111. [DOI] [PubMed] [Google Scholar]
- 30.Delot EC, Papp JC , DSD-TRN Genetics Workgroup, Sandberg DE, Vilain E. Genetics of disorders of sex development: the DSD-TRN experience. Endocrinol Metab Clin North Am. 2017;46(2):519–37. doi: 10.1016/j.ecl.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandberg DE, Gardner M, Callens N, Mazur T, DSD-TRN Psychosocial Workgroup, the DSD-TRN Advocacy Advisory Network, Accord Alliance. Interdisciplinary care in disorders/differences of sex development (DSD): the psychosocial component of the DSD-Translational research network. Am J Med Genet C Semin Med Genet. 2017;175(2):279–92. doi: 10.1002/ajmg.c.31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–21. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 33.Achenbach TM. The Achenbach system of empirically based assessment (ASEBA): development, findings, theory and applications. Burlington, VT: University of Vermont Research Center for Children, Youth and Families; 2009. [Google Scholar]
- 34.Harter S Manual for the self-perception profile for children. Denver, CO: Univeristy of Denver; 1988. [Google Scholar]
- 35.Harter S Manual for the self-percepton profile for adolescents. Denver, CO: Unversity of Denver; 1988. [Google Scholar]
- 36.Lindgren TW, Pauly IB. A body image scale for evaluating transsexuals. Arch Sex Behav. 1975;4(6):639–56. [DOI] [PubMed] [Google Scholar]
- 37.Egan SK, Perry DG. Gender identity: a multidimensional analysis with implications for psychosocial adjustment. Dev Psychol. 2001;37(4):451–63. [DOI] [PubMed] [Google Scholar]
- 38.Rolston AM, Gardner M, Vilain E, Sandberg DE. Parental reports of stigma associated with child’s disorder of sex development. Int J Endocrinol. 2015;2015:980121. doi: 10.1155/2015/980121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Bahlburg HF, Reyes-Portillo JA, Khuri J, Ehrhardt AA, New MI. Syndrome-related stigma in the general social environment as reported by women with classical congenital adrenal hyperplasia. Arch Sex Behav. 2017;46(2):341–51. doi: 10.1007/s10508-016-0862-8. [DOI] [PubMed] [Google Scholar]
- 40.Nunnally JC. Psychometric theory (2nd ed). New York, NY: McGraw Hill; 1978. [Google Scholar]
- 41.Kazdin AE. Research design in clinical psychology. 4th ed Boston: Allyn and Bacon; 2003. [Google Scholar]
- 42.DeVon HA, Block ME, Moyle-Wright P, Ernst DM, Hayden SJ, Lazzara DJ, Savoy SM, Kostas-Polston E. A psychometric toolbox for testing validity and reliability. J Nurs Scholarsh. 2007;39(2):155–64. doi: 10.1111/j.1547-5069.2007.00161.x. [DOI] [PubMed] [Google Scholar]
- 43.Karlson CW, Leist-Haynes S, Smith M, Faith MA, Elkin TD, Megason G. Examination of risk and resiliency in a pediatric sickle cell disease population using the Psychosocial Assessment Tool 2.0. J Pediatr Psychol. 2012;37(9):1031–40. doi: 10.1093/jpepsy/jss087. [DOI] [PubMed] [Google Scholar]
- 44.Kazak AE, Barakat LP, Ditaranto S, Biros D, Hwang WT, Beele D, Kersun L, Alderfer MA, Mougianis I, Hocking MC, Reilly A. Screening for psychosocial risk at pediatric cancer diagnosis: the Psychosocial Assessment Tool. J Pediatr Hematol Oncol. 2011;33(4):289–94. doi: 10.1097/MPH.0b013e31820c3b52. [DOI] [PubMed] [Google Scholar]
- 45.Kazak AE, Barakat LP, Hwang WT, Ditaranto S, Biros D, Beele D, Kersun L, Hocking MC, Reilly A. Association of psychosocial risk screening in pediatric cancer with psychosocial services provided. Psychooncology. 2011;20(7):715–23. doi: 10.1002/pon.1972. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy MC, DeGraves S, Wakefield CE, Bowden MJ, Marks LV, Williams LK. The association of psychosocial screening and service provision in pediatric oncology: the Psychosocial Assessment Tool (PAT2.0) into clinical practice. Support Care Cancer. 2016;24(7):2945–52. doi: 10.1007/s00520-016-3107-4. [DOI] [PubMed] [Google Scholar]
- 47.Barrera M, Hancock K, Rokeach A, Atenafu E, Cataudella D, Punnett A, Johnston D, Cassidy M, Zelcer S, Silva M, Jansen P, Bartels U, Nathan P, Shama W, Greenberg C. Does the use of the revised Psychosocial Assessment Tool (PATrev) result in improved quality of life and reduced psychosocial risk in Canadian families with a child newly diagnosed with cancer? Psychooncology. 2014;23(2):165–72. doi: 10.1002/pon.3386. [DOI] [PubMed] [Google Scholar]
- 48.Eccleston C, Fisher E, Law E, Bartlett J, Palermo TM. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2015(4):CD009660. doi: 10.1002/14651858.CD009660.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reader SK, Ruppe NM, Deatrick JA, Rash-Ellis DL, Wadman JR, Miller RE, Kazak AE. Caregiver perspectives on family psychosocial risks and resiliencies in pediatric sickle cell disease: informing the adaptation of the Psychosocial Assessment Tool. Clin Pract Pediatr Psychol. 2017;5(4):330–41. doi: 10.1037/cpp0000208. [DOI] [Google Scholar]
- 50.Kazak AE, Barakat LP, Askins MA, McCafferty M, Lattomus A, Ruppe N, Deatrick J. Provider perspectives on the implementation of psychosocial risk screening in pediatric cancer. J Pediatr Psychol. 2017;42(6):700–10. doi: 10.1093/jpepsy/jsw110. [DOI] [PubMed] [Google Scholar]
- 51.Simms LJ, Watson D. The construct validation approach to personality scale construction; in Robins RW, Frayley RC, Kruger RF (eds): Handbook of research methods in personality psychology. New York, Guilford, 2007. 240–58p. [Google Scholar]
- 52.Crissman HP, Warner L, Gardner M, Carr M, Schast A, Quittner AL, Kogan B, Sandberg DE. Children with disorders of sex development: a qualitative study of early parental experience. Int J Pediatr Endocrinol. 2011;2011(1):10. doi: 10.1186/1687-9856-2011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beale JM, Creighton SM. Long-term health issues related to disorders or differences in sex development/intersex. Maturitas. 2016;94:143–48. doi: 10.1016/j.maturitas.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Jürgensen M, Kleinemeier E, Lux A, Steensma TD, Cohen-Kettenis PT, Hiort O, Thyen U, Köhler B, DSD Networking Group. Psychosexual development in adolescents and adults with disorders of sex development—results from the german clinical evaluation study. J Sex Med. 2013;10(11):2703–14. doi: 10.1111/j.1743-6109.2012.02751.x. [DOI] [PubMed] [Google Scholar]
- 55.Strandqvist A, Falhammar H, Lichtenstein P, Hirschberg A, Wedell A, Norrby C, Nordenskjöld A, Frisén L, Nordenström A. Suboptimal psychosocial outcomes in patients with congenital adrenal hyperplasia: epidemiological studies in a nonbiased national cohort in Sweden. J Clin Endocrinol Metab. 2014;99(4):1425–32. doi: 10.1210/jc.2013-3326. [DOI] [PubMed] [Google Scholar]


