Abstract
The dinoflagellate Prorocentrum donghaiense is a dominant harmful algal bloom (HAB) species on the East China Sea (ECS) coast. The co-occurrence of Karlodinium veneficum with P. donghaiense is often observed and can later develop into dense blooms. However, the role of K. veneficum in P. donghaiense population dynamics is unknown. In the current study, three K. veneficum (GM1, GM2, and GM3) strains were isolated from the ECS with one (GM1) from a mixed, dense bloom of P. donghaiense and other HAB species. All three isolates had identical ITS sequences that were concordant with the species designation. Unique karlotoxin congeners were isolated from one strain (GM2). The sterol compositions of P. donghaiense and K. veneficum were consistent with sensitivity to karlotoxin in the former and insensitivity in the latter. Additional experimentation showed that: (1)in monocultures, higher growth rate of P. donghaiense than K. veneficum is observed in nutrient-enriched and nutrient-depleted media. In co-cultures, the growth of P. donghaiense is inhibited; (2) feeding on P. donghaiense by K. veneficum is clearly demonstrated by fluorescent dye tracking; and (3) the isolated karlotoxin is lethal to P. donghaiense in a concentration-dependent manner. From these studies we propose that K. veneficum may play a negative role in P. donghaiense bloom maintenance and that P. donghaiense may in turn be a bloom initiator as a prey item for K. veneficum.
Keywords: Karlodinium veneficum, Prorocentrum donghaiense, Co-culture, Phagotrophy, Karlotoxin, Sterol
1. Introduction
Since the first description of Karlodinium veneficum from the East China Sea (ECS; Zhou et al., 2008a), both field (Xu et al., 2012; Dai et al., 2014) and laboratory studies (Wang et al., 2011; Zhou et al., 2011) of this species in China have increased. However, whether this species is endemic or was imported by ballast water is unknown. It is noted that K. veneficum is consistently detected in the phytoplankton community assemblages and in adjacent aquaculture ponds. In fact, some of the ECS K. veneficum isolates are genetically and phenotypically identical to strains of American origin as revealed by ITS sequencing and toxin profiling (unpublished data).
The ECS is well known for its frequent harmful algal blooms (HAB; Zhou et al., 2008b). The particular properties of the ECS contribute oceanographic and hydrographical characteristics that promote bloom forming in regional HAB species (Zhou, 2010). The dominant harmful bloom species in the coastal area of the ECS is Prorocentrum donghaiense (Lu and Goebel, 2001; Lu et al., 2002; Zhou et al., 2006; Li et al., 2010). Even though no toxin has been detected in P. donghaiense, the dense blooms can span thousands of kilometers and last as long as a month. The blooms can cause large economic losses to aquaculture and have adverse ecological impacts in the ECS (Zhou et al., 2006; Li et al., 2010).
The dinoflagellate Karlodinium veneficum has a global distribution with many well-documented harmful effects (Place et al.,2012). In addition to developing into dense single-species blooms (Braarud, 1957; Hall et al., 2008; Glibert and Terlizzi, 1999), it accompanies or co-occurs in the phytoplankton community with other HAB species (Marshall, 1980; Larsen and Moestrup, 1989), as well as showing mixotrophic and phagotrophic abilities (Li et al., 2000). It exhibits species specific toxins, karlotoxins (Van Wagoner et al., 2008; Kempton et al., 2002; Deeds et al., 2002), which play a role in feeding on the prey and deterring the predators (Adolf et al., 2006, 2007; Sheng et al., 2010). Sterol composition of the ambient organisms is critical to whether they will be karlotoxin sensitive (Deeds and Place, 2006).
In the ECS phytoplankton communities, Karlodinium veneficum is often detected with Prorocentrum donghaiense and its blooms. It has been noticed that K. veneficum can even develop into a dense bloom after the P. donghaiense bloom declines (Dai et al., 2014). The aim of this study was to assess whether P. donghaiense blooms are terminated or impacted by K. veneficum. The study focused on the role of K. veneficum, as a co-occurring species, in P. donghaiense population growth: whether either species influenced population growth in the other; whether K. veneficum feeds on P. donghaiense; whether the ECS strains produce karlotoxin; and if the toxin affects P. donghaiense.
2. Materials and methods
2.1. Algal cultures
The dinoflagellate Prorocentrum donghaiense (strain NMBjah045) was isolated from a dense bloom collected in Dongtou, ECS, in June 2005, in which Karenia mikimotoi dominated while both P. donghaiense and Karlodinium veneficum co-occurred (Zhou et al., 2011).
Three Karlodinium veneficum strains were isolated from different locations in the ECS at three different times. Strain NMBjah047 (GM1, GenBank code DQ459434, ITS sequence) was isolated from the above-mentioned bloom water in 2005. Strain NMB jah047–1 (GM2) was isolated from Yushan, Zhejiang province, in 2006. Strain NMB jah047–2 (GM3) was isolated most recently from an aquaculture pond in Zhaoan, Fujian province, in 2011. The ITS sequences of all three strains were identical (unpublished data). All of the monoalgal cultures were maintained in sterilized natural seawater enriched with f/2-Si at the Microalgae Collection in Ningbo University, China.
2.2. Co-culture of K. veneficum and P. donghaiense
Based on field and mesocosm observations, dinoflagellates frequently bloom after diatoms when the water is stratified (Smayda and Trainer, 2010). The two dinoflagellate species, Karlodinium veneficum and Prorocentrum donghaiense, bloom in the ECS in similar nutrient poor stratified waters (Dai et al., 2014). To mimic the oligotrophic conditions after a diatom bloom media, filtrates of a Thalassiosira rotula culture at the early stationary phase were used as culture media. The diatom T. rotula was isolated and purified from Nanji Islands, Zhejiang province, in 2006, and maintained in the Microalgae Collection in Ningbo University. The concentrations of nitrogen and phosphorus in the filtrates were 10.3 ± 2.7 μmol/l nitrate and 3.9 ± 0.9 μmol/l phosphate. Before inoculation, both media were filter sterilized. For nutrient-replete media, filtered natural seawater was enriched to a final nutrient solution that was two-fold that in the f/2-Si formula. The experimental cultures were inoculated by adding 100 ml of the cultures to 100 ml of the media in a 250-ml glass flask. A monoculture of the same initial cell density in each media was set as the control. The initial K. veneficum cell density was 0.5 ×104 cells/ml. For P. donghaiense, the initial cell density was 5 × 104 cells/ml and 0.5 × 104 cells/ml. The cultures were maintained in an air-conditioned culture room at 20 ± 2 °C, illuminated by cool white fluorescent bulbs 40 μmol m−2 s−1 illumination on a 12:12 dark:light cycle. Natural seawater from the coastal ECS 25 PSU (practical salinity units) was used. The cell number was determined using a hemocytometer (Sigma-Aldrich, USA) at the same time each day. The cells of these two dinoflagellates in co-culture can be easily distinguished by their shapes.
2.3. Feeding on P. donghaiense cells by K. veneficum ECS strain
To determine if Karlodinium veneficum ECS strain (GM3) feeds on Prorocentrum donghaiense in culture, a stationary-phase P. donghaiense culture was stained with fluorochrome carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich, USA) according to the methods of Rioboo et al. (2009) and Buhmann et al. (2013) with modifications. A CFSE stock solution was made by dissolving CFSE powder in DMSO (Sigma-Aldrich) to a final concentration of 5 mM. For the feeding experiments, 994 μl of a stationary-phase P. donghaiense culture, cell density 6.8 × 105 cells/ml, was incubated with 6 μl CFSE (5 mM) at a final concentration of 20 μM per 6.8 × 105 cells suspended in f/2-Si culture medium for 2 h at 20 °C in the dark. The CFSE-labeled cells were washed with f/2-Si medium three times by centrifugation at 3500 rpm for 15 min and resuspended in f/ 2-Si culture medium to 2 ml. The CFSE-labeled culture was put in a culture room for 1 day for the cells to recover from the treatments. CFSE-labeled P. donghaiense (200 μl) was added to a 48-well plate containing 200 μl stationary-phase K. veneficum (GM3) at a cell density of 5.9 × 104 cells/ml. The cell ratio of K. veneficum to P. donghaiense was ca. 1:5. The treatment was carried out eight times. The plate was returned to the culture room set at 20 °C with illumination of 40 μmol m−2 s−1 on a 12:12 (D:L) cycle.
After 1, 2, 3, and 24 h, the mixed culture from two wells of the 48-well plate were pipetted to two wells of another 48-well plate to be fixed with formaldehyde at a final concentration of 1.5% (v/v). The plate with the fixed cells was stored at 4 °C in darkness. After all of the sampling was done, the fixed samples were observed under an inverted microscope (Nikon Eclipse Ti-U equipped with Nikon Digital Sight DS-Ri1, Japan).
2.4. Karlotoxin isolation
The dinoflagellate Karlodinium veneficum (strain GM2) was grown in ESAW medium (Harrison et al., 1980) in 250-ml polycarbonate flasks in a culture room with natural light illumination (no direct sunlight) at 18–20 °C. When the culture had reached the stationary phase at a cell density of 1.63 × 105 cells/ml, 220 ml of the culture was vacuum filtered through a GF/F filter membrane (Whatman, USA). Karlotoxin in the filtrate was applied to a 3-ml packed volume tC-18 solid phase extraction cartridge column (Waters, USA) that had been activated by 100% methanol. The column was then eluted with 20 ml deionized water and 20%, 40%, 60%, and 80% methanol sequentially. The 60% and 80% methanol/water elute was collected and dried by rotary evaporation. The dried fraction was resuspended in 1 ml 20% methanol and stored at 4 °C until analyzed by LC-MS (Bachvaroff et al., 2008).
2.5. The effects of karlotoxin on P. donghaiense
The purified karlotoxin (GM2) was stored in 20% methanol in an amber glass bottle at 4 °C before use and was determined gravimetrically to be 240 μg/ml. Exponential phase Prorocentrum donghaiense (3.5 × 104 cells/ml) cultured in ESAW (25 PSU) was exposed to 0.048 μg/ml, 0.48 μg/ml, and 4.8 μg/ml purified karlotoxin. The total volume was adjusted to 100 μl with 20% methanol when necessary. Two controls were set up in this experiment. One control was set by adding 100 μl 20% methanol to a P. donghaiense culture (Control M). The other control was the original P. donghaiense culture (Control) with no added karlotoxin or methanol.
All of the treatments were duplicated over a 24-h period at 20 °C, 50 μmol photon/m2 s(L:D = 12:12). After 24 h exposure, cell number and cell size were analyzed in a Coulter™ Multisizer (Beckman, USA). Active chlorophyll a fluorescence was detected with a fluorometer (Turner Designs, USA). One hundred microliters of the cultures were sampled onto a 96-well culture plate, fixed by adding 50 μl 2% glutaraldehyde solution, and stored at 4 °C overnight to let the cells sink to the bottom. The fixed samples were used to analyze the proportion of empty cells by counting 100 cells under a light microscope.
2.6. Sterol analysis on K. veneficum and P. donghaiense
Dinoflagellates Karlodinium veneficum (GM1) and Prorocentrum donghaiense were cultured in f/2-Si media, respectively. The cultures were gradually scaled up to a final volume of 4000 ml in 5000 ml glass flasks. All of the cultures were maintained in a culture room at 20 ± 2 °C, 40 μmol m−2 s−1 illumination on a 12:12 light:dark cycle. The cultures were shaken twice daily. When the cultures had reached the stationary phase with a sufficient number of cells for the sterol analysis, the microalgae were harvested by centrifugation at 4000 rpm, freeze dried, and stored at −20 °C until analysis.
Total lipids were extracted using a modified Bligh-Dyer method (Bligh and Dyer, 1959). Sterol extraction and analysis were performed according to Xu et al. (2008). The lipids containing the sterol TMS ethers were dissolved in hexane and analyzed in a QP2010 GC-MS with an A0C-20 auto-sampler (Shimadzu Co., Japan) fitted with a 30 m × 0.25 mm × 0.25 mm SPB-50 column (Supelco Co., USA). The sterol structures were mainly identified by comparing the mass spectra according to the regular mass spectra patterns of sterol TMS derivatives (Miao et al., 2009) and relative retention times, NIST and WILEY library entries, and previously published GC/MS data on sterols (Barrett et al., 1995; Leblond and Chapman, 2002; Volkman et al., 1999; Rampen et al., 2009). Peak areas were used to determine the relative sterol proportions.
2.7. Data analysis
Culture growth rates (rt) were determined by the following equation: rt = (ln Nt - ln N0)/t, where N0 is the initial cell density (cells/ml), and Nt is the cell density at time t. Statistical analyses were conducted in SPSS (v19.0). Significant differences were determined by one-way ANOVA test.
3. Results
3.1. Population growth of K. veneficum and P. donghaiense in mono-and co-cultures
In enriched natural seawater (Fig. 1A and B), Karlodinium veneficum (GM1) monocultures grew to more than 10 times the initial cell density within 12 days. Growth rate in the first 4 days was 0.58 d-1. In the Prorocentrum donghaiense monocultures, the growth rate varied depending on the initial cell density. At higher initial cell densities (5 × 104 cells/ml), the growth rate was 0.30 d−1 for the first 4 days, while at the lower initial cell density (0.5 × 104 cells/ml) the growth rate was 0.73 d-1. When the two species were co-cultured, growth of P. donghaiense decreased significantly in the presence of K. veneficum. After a short period of initial growth for 3 days, P. donghaiense biomass declined in both co-cultures at the two initial densities. In contrast, K. veneficum population growth in co-culture was not affected. In the K/P = 1/10 co-culture (Fig. 1A), the growth rate in the first 4 days was slightly lower than that in the monoculture control (0.41 d−1 compared with 0.58 d−1). In the K/P= 1/1 co-culture (Fig. 1B), growth was also slightly inhibited in the first 3 days; however, it increased to the same level as that in the monoculture after the 4th day (0.5–0.6 d−1). The growth rate in the monoculture declined from the 8th day, while that in the co-culture was >0.3 d−1 until the end of the experiment. The final maximum K. veneficum biomass in the co-culture was significantly higher than that in the monoculture (P < 0.05).
Fig. 1.

The population growth of Karlodinium veneficum (K) and Prorocentrum donghaiense (P) in different media cultured alone and co-cultured at different initial cell densities (K/P). The data are the means of triplicates. Error bars (SD) have been omitted for the sake of clarity. (A) Enriched clean natural seawater (f/2-Si), inoculating cell number proportion K/P = 1:10.(B) Enriched clean natural seawater (f/2-Si), inoculating cell number proportion K/P = 1:1.(C) Oligotrophic media of diatom filtrates, inoculating cell number proportion K/P =1:10. (D) Oligotrophic media ofdiatom filtrates, inoculating cell number proportion K/P =1:1. Solid triangles (▲): K. veneficum growth in co-culture. Solid circles (⚫): P. donghaiense growth in co-culture. Hollow triangles (△): monoculture of K. veneficum as control. Hollow circles (⚪): monoculture of P. donghaiense as control.
It is showed that Karlodinium veneficum did not grow in the nutrient-depleted media from the diatom filtrates (Fig. 1C and D). Cell density remained at the initial inoculum density and then plummeted at day 5. However, in the co-cultures, K. veneficum survived and grew (Fig. 1C). In the K/P= 1/1 co-culture (Fig. 1D), the growth rate of K. veneficum (0.3 d−1 in 12 days) was significantly higher and reached a higher maximum cell number (1.7 × 105 cells/ml) than that in the K/P = 1/10 group (P < 0.05). In the Prorocentrum donghaiense monoculture, the scenario was basically the same as that in enriched clean natural seawater, except that the growth period was shorter and the population declined quickly after reaching the maximum cell number (Fig. 1C and D). The P. donghaiense growth rates in the diatom filtrates were lower than that in the enriched culture medium. Interestingly, in the diatom filtrates, monoculture P. donghaiense with higher initial cell numbers (5 × 104 cells/ml)also had a lower growth rate than that of the culture with lower initial cell numbers (0.5 × 104 cells/ml), 0.23 d−1 compared with 0.65 d−1, respectively. The growth of P. donghaiense in the co-cultures was also obviously affected by K. veneficum. In the K/P =1/1 co-culture, P. donghaiense declined sharply while K. veneficum grew quickly from day 4 to 5 (Fig. 1D). In the K/ P=1/10 co-culture (Fig. 1C), where there were relatively lower numbers of K. veneficum, the P. donghaiense population growth decline phase was delayed compared with that in K/P= 1/1.
3.2. Feeding on P. donghaiense by K. veneficum
For the first time, the feeding activity of Karlodinium veneficum (GM3) in the presence of CFSE-labeled Prorocentrum donghaiense cells was clearly observed (Fig. 2). Frequently, K. veneficum cell(s) actively attached to P. donghaiense, as shown in Fig. 2A. After 1.5 h, K. veneficum cells with small CFSE fluorescence particle(s) were observed. At 2 h, obvious feeding activity was observed. The interesting feeding behavior is described as follows: K. veneficum appeared to drag P. donghaiense around rapidly. However, K. veneficum did not completely engulf P. donghaiense cells but swam away after feeding for some period, occasionally leaving behind an empty thecal shell of the prey. In the fixed samples, K. veneficum cells with different quantities of CFSE-labeled material were found at different time points (Fig. 2E and F). Not all K. veneficum cells fed because some were observed with no CFSE fluorescence in the fixed samples (Fig. 2C and D).
Fig. 2.

Feeding on P. donghaiense cells by K. veneficum. (A)K. veneficum (solid arrows➨) actively attached P. donghaiense (hollow arrow ➬). Arrow head indicates an empty P. donghaiense theca. (B) CFSE-labeled P. donghaiense cell. (C) K. veneficum and CFSE-labeled P. donghaiense. (D) Same view of (C) observed under fluorescence microscope, showing CFSE-labeled P. donghaiense (hollow arrows) and K. veneficum (solid arrows) without CFSE fluorescence. (E) K veneficum cell with CFSE-labeled P. donghaiense components (arrows). (F) K. veneficum cell with CFSE-labeled P. donghaiense components. E or F was assembled images taken by a differential interference contrast (DIC) microscope and a fluorescence microscope.
3.3. The effect of karlotoxin on P. donghaiense
The karlotoxin isolated from Karlodinium veneficum (GM2) is a KmTx-2-like molecule. As shown in Fig. 3, both sulfated and unsulfated congeners were present. The UV spectra of the congeners are consistent with the terminal chlorine found in KmTx 2. However, the mass spectra (Fig. 4) indicated that these are unique congeners not previously reported. The toxin cell quota was estimated at 6.6 pg/cell and 2.5 pg/cell for the nonsulfate and sulfate congeners, respectively.
Fig. 3.

Reverse phase chromatograms (LiChrosphere 125 mm × 4 mm × 5 μm bead size RP-8) of the 60% (A) and 80% (B) methanol fractions from strain NMB jah047–1 culture filtrates fractionated with a Sep-Pak tC-18 cartridge prior to chromatography. Injected toxin was equivalent to 3.5 × 107 cells. The UV spectra of the peaks (1,2, and 3) for each fraction were identical and are shown as an inset in Fig. 2B. The 235-nm UV maximum is characteristic of a terminal chlorine (Bachvaroff et al., 2008).
Fig. 4.

Mass spectra of the UV peaks (1,2, and 3) shown in Fig. 3. Putative identifications of the different ions (sodiated ions assumed) are shown. The karlotoxins in peak 1 appeared to be the sulfated forms of those in peaks 2 and 3.
When Prorocentrum donghaiense was exposed to the nonsulfate congeners at a concentration of 0.048–4.8 μg/ml, significant effects were recorded. After exposure to 4.8 μg/ml for 24 h, the cell number decreased to 58% that of the controls (Fig. 5A), the cell size significantly decreased compared with the other groups (Fig. 5B), the chlorophyll a fluorescence declined to 39% that of the control (Fig. 5C), and 75% of the cells were empty thecal shells (Fig. 5D). No negative effects on P. donghaiense cells exposed to methanol were observed in this experiment. The chlorophyll fluorescence was sensitive enough to reflect the effect of karlotoxin according to the Turner Designs reads, and it declined to 85% of the control when the cells were exposed to 0.48 μg/ml of the toxin (Fig. 5C), while there was no significant variation from other parameters, such as cell number or cell size. Empty P. donghaiense thecae were obvious when the fixed cells were observed under the microscope. The epitheca and hypotheca were either fully attached or slightly separated with no cellular contents. Empty cells were proportional to the amount of karlotoxin in the culture.
Fig. 5.
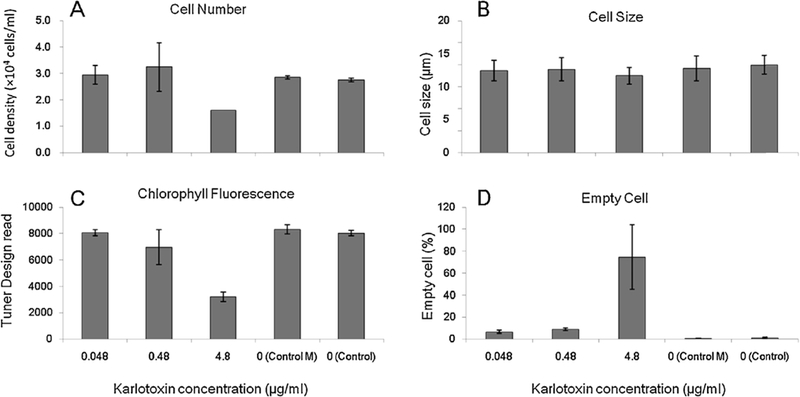
Variations in Prorocentrum donghaiense cells after exposure to different amounts ofkarlotoxin in 24 h. Error bars are standard deviations. Control M: P. donghaiense culture exposed to 20% methanol. Control: Original P. donghaiense culture.
3.4. Sterol profiles of K. veneficum and P. donghaiense
The sterol analysis results for the two species are shown in Table 1. The dinoflagellate Karlodinium veneficum contained (24S)- 4α-methyl-5α-ergosta-8(14), 22 diene-3β-ol (gymnodinosterol), 27-nor(24S)-4α-methyl-5α-ergosta-8(14), and 22 diene-3β-ol (brevesterol) as the major sterols, representing more than 70% of the total sterol, together with two unknown C29:2 sterols (12.84% and 3.24%); the minor sterols were 24-ethylcholesta-5-en-3β-ol (sitosterol), 4α,23,24-trimethylcholest-5,22-dien-3β-ol (dehydrodinosterol) and its isomer (3.73% and 2.82%, respectively), and Choles-5-en-3β-ol (cholesterol; 3.6%). The results are similar to that described by Place et al. (2004) for other K. veneficum strains, in which, gymnodinosterol and brevesterol represented 85% of the sterols. These two sterols are considered to be important players for host immunity against karlotoxin because of the 4α- methyl and 8(14) ethylenic bond in their sterol backbone.
Table 1.
The sterol contents of Karlodinium veneficum and Prorocentrum donghaiense (the data shown inparentheses are the percentages of sterols).
| Sterols (%) | Prorocentrum donghaiense | Karlodinium veneficum | |
|---|---|---|---|
| C23 sterol | C23:1(Δ/4Me) (trace) | ||
| C27 sterol | C27:1(Δ5) (1.34) | C27:1(Δ5) (3.6) | |
| C 28 sterol | C28:2(Δ5,22/24Me) (diatomsterol) (4.37) | C28:2(Δ8(14),22/24,4-dime,27-nor)NED (24.94) | |
| C28:2(Δ5,24(28)/24Me) (24.57) | |||
| C28:2 (8.9) | |||
| C29 sterol | C29:1(Δ7/4,24diMe) (1.35) | C29:2(Δ8(14),22/4,24dime)ED (47.68) | |
| C29:2 | Isomer 1 (12.84%) | ||
| Isomer 2 (3.24%) | |||
| C29:1(Δ5/24ethyl) (1.15) | |||
| C30 sterol | C30:2(7.12) | C30:2 | Isomer 1 (3.73%) |
| C30:1(Δ22/4,23,24triMe) (dinosterol) (45.5) | Isomer 2 (2.82%) | ||
| Unknown | (5.86) | ||
| (0.99) | |||
The dinoflagellate Prorocentrum donghaiense contained 4α,23,24-trimethyl-5α-cholest-22-en-3β-ol (dinosterol) and 24- methyl-5α-cholest-24(28)-en-3β-ol as the major sterols, 45.5% and 24.5%, respectively. The minor sterols were 4α,23,24- trimethylcholest-5,22-dien-3β-ol (dehydrodinosterol isomer; 7.12%), 4α,24-dimethylcholest-7-en-3β-ol (1.35%), 24-methyl- cholest-5,22-dien-3β-ol (diatomsterol; 4.37%), C28:2 (8.9%), and Choles-5-en-3β-ol (cholesterol; 1.3%), as well as other unknown sterols.
4. Discussion
The dinoflagellate Prorocentrum donghaiense is the most frequently reported blooming alga in the ECS, with a wide distribution throughout the region. The ability to adapt and grow in a wide range of temperatures from 10 °C to 27 °C and salinities from 20 PSU to 40 PSU enables this species to dominate and bloom in the ECS (Xu et al., 2010). After concentrating in the mid-water column, it can then migrate to the surface to develop into blooms with cell densities as high as 108 cells/ml (data from State Oceanic Administration, China). It is usually found that Karlodinium veneficum accompanies other blooms, including P. donghaiense. The present study shows that laboratory co-culture of K. veneficum and P. donghaiense results in population decline of the latter under various nutrient and initial ratio conditions. In contrast, K. veneficum exhibited enhanced growth in the co-cultures compared with the monocultures under the same nutrient and ratio conditions.
It has been previously observed that Prorocentrum donghaiense thrives in eutrophic waters (Zhou et al., 2003). The growth rate of P. donghaiense monocultured in f/2-Si medium with an initial cell density of 5 × 104 cells/ml reached 0.3 d−1, which is in the same range reported by Li et al. (2012). The P. donghaiense monoculture grew in the oligotrophic diatom filtrate medium. It has been demonstrated that P. donghaiense is a mixotrophic dinoflagellate, which can graze on cyanobacteria and cryptophytes (Jeong et al., 2005a,b). Such abilities allow P. donghaiense to outgrow other blooming species when nutrients are limited (Li et al., 2009). The Karlodinium veneficum monocultures crashed at low nutrient conditions, but survived and steadily increased in the presence of P. donghaiense to a biomass of 1.6 × 104 cells/ml by the end of the experiment. This observation agrees with that of Dai et al. (2014) on the bloom dynamics. It is found that K. veneficum initially at low cell densities in P. donghaiense blooms can later develop into denser blooms, becoming the dominant species.
Phagotrophy is a prominent feature of Karlodinium veneficum and involves diatoms, cryptophytes, and even zooplankton (Li et al., 2000; Place et al., 2012). In this laboratory study, K. veneficum (GM3) isolated from the ECS actively fed on Prorocentrum donghaiense. K. veneficum (GM3) did not engulf P. donghaiense as observed when feeding on either Rhinomonas reticulata or Hemiselmis brunnescens (Place et al., 2012). Instead, CFSE-labeled P. donghaiense cell contents were transferred (i.e., myzocytosis) to the K. veneficum food vacuole. K. veneficum may sip (via its peduncle) the cell contents of the larger P. donghaiense rather than engulf the whole cell and leave the empty theca in the medium. Hence, phagotrophic activity in K. veneficum may have been responsible for the decline in P. donghaiense cell density seen in the co-cultures (Fig. 1). This P. donghaiense growth inhibition appears to be specific to K. veneficum. P. donghaiense is not significantly affected by co-culture with Karenia brevis (Li et al., 2012). Further studies on the feeding activity of K. veneficum on P. donghaiense in situ are warranted, especially as it relates to bloom dynamics.
The cells of Prorocentrum donghaiense are lysed in a dose-dependent manner upon addition of purified karlotoxin from the Karlodinium veneficum ECS strain. The prominent karlotoxin toxicity mechanism is through membrane pore formation (Deeds et al., 2014), which disrupts membrane potential. This membrane disruption is consistent with the chlorophyll a fluorescence decrease measured in P. donghaiense in the presence of karlotoxin. Cell diameter shrank significantly after a 24-h exposure at the highest karlotoxin concentration. Concomitant with exposure intracellular cell content was released, resulting in empty thecae. The mechanism ofkarlotoxin pore formation is sterol-dependent (Deeds and Place, 2006). In the current study, the absence of gymnodinosterol and brevesterol is clearly evident in P. donghaiense, which may explain the toxicity of karlotoxin to this species. Members of the genus Prorocentrum basically have broadly similar sterol compositions (dinosterol being dominant), but none have an 8(14) ethylenic bond in their sterol backbone (Volkman et al., 1999).
In the Li et al. (2012) study, Karlodinium veneficum inhibited cocultured P. minimum population growth rate to about 40% of the monoculture. Ozbay et al. (2014) found that when P. minimum was treated with 0.256 μg/mlkarlotoxin(KmTx2)for24 h, it experienced mortality and cell diameter increases. The P. minimum is sensitive to karlotoxin but only at high concentrations (Ozbay et al., 2014).
Allelopathic effects between Prorocentrum donghaiense and other algae are quite common. Wang et al. (2007) found that P. donghaiense growth is inhibited by fresh macroalgae (Ulva linza, Corallina pilulifera, and Sargassum thunbergii) and their culture medium filtrates. The growth of P. donghaiense was also negatively affected by Alexandrium tamarense. However, the negative potency was not correlated with the content of paralytic shellfish poison (PSP) toxin of A. tamarense but with its hemolytic properties (Yang et al., 2010). The P. donghaiense cell membrane may be particularly sensitive to the hemolytic properties of the allelopathic substances.
5. Conclusions
After more than 15 years of intensive study, Karlodinium veneficum is now recognized as a globally distributed and toxic harmful alga (Place et al., 2012). Our current work extends this distribution to the East China Sea (ECS). We describe new karlotoxin congeners that are highly toxic to sympatric Prorocentrum donghaiense and may allow the ECS K. veneficum strain to feed on its sympatric cousin. We also propose that P. donghaiense blooms may initiate subsequent K. veneficum blooms in the ECS and that K. veneficum may in turn terminate P. donghaiense blooms.
Acknowledgments
This work was funded by the Zhejiang Marine Biotechnology Innovation Team (ZMBIT) (2010R50029), Natural Science Foundation of Zhejiang Province (LY12D06001; LQ13B020004), PhD Programs Foundation of Ministry of Education of China (20133305120007), and sponsored by the K.C. Wong Magna Fund, Ningbo University. This is contribution #15–162 from the Institute of Marine and Environmental Technology and contribution #5065 from the University of Maryland Center for Environmental Sciences. This research was supported in part by grants from OHH NIH R01ES021949–01/NSF0CE1313888 to ARP.[SS]
References
- Adolf JE, Bachvaroff T, Krupatkina DN, Nonogaki H, Brown PJP, Lewitus AJ, 2006. Species specificity and potential roles of Karlodinium micrum toxin. Afr. J. Med. Sci. 28, 415–421. [Google Scholar]
- Adolf JE, Krupatkina D, Bachvaroff T, Place AR, 2007. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum. Harmful Algae 6,400–412. [Google Scholar]
- Bachvaroff TR, Adolf JE, Squier AH, Harvey HR, Place AR, 2008. Characterization and quantification of karlotoxins by liquid chromatography - mass spectrometry. Harmful Algae 7, 473–484. [Google Scholar]
- Bligh EG, Dyer WJ, 1959. A rapid method of total lipid extraction and purification. Can. Biochem. Physiol. 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Barrett SM, Volkman JK, Dunstan GA, LeRoi JM, 1995. Sterols of 14 species of marine diatoms. J. Phycol. 31, 360–369. [Google Scholar]
- Braarud T, 1957. A red water organism from Walvis Bay (Gymnodinium galathean- umn spp.). Galathea Rep. 1, 137–138. [Google Scholar]
- Buhmann MT, Day JG, Kroth PG, 2013. Post-cryopreservation viability of the benthic freshwater diatom Planothidium frequentissimum depends on light levels. Cryobiology 67, 23–29. [DOI] [PubMed] [Google Scholar]
- Dai XF, Lu DD, Guan WB, Wang HX, He PX, Xia P, Yang HJ, 2014. Newly recorded Karlodinium veneficum dinoflagellate blooms in stratified water of the East China Sea. Deep-Sea Res. II 101, 237–243. [Google Scholar]
- Deeds JR, Hoesch RE, Kao JPY, Place AR, 2014. The cytotoxic mechanism of karlotoxin 2 (KmTx 2) from Karlodinium veneficum (Dinophyceae). Aquat. Toxicol. 159, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeds JR, Place AR, 2006. Sterol specific membrane interactions with the toxins from Karlodinium micrum (Dinophyceae) - a strategy for self-protection. Afr. J. Med. Sci. 28, 421–427. [Google Scholar]
- Deeds JR, Terlizzi DE, Adolf JE, Stoecker DK, PLace AR, 2002. Toxic activity from cultures of Karlodinium micrum (=Gyrodinium galatheanum) (Dinophyceae) - a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae 1, 169–189. [Google Scholar]
- Glibert PM, Terlizzi DE, 1999. Cooccurence of elevated urea levels and dinoflagellate blooms in temperate estuarine aquaculture ponds. Appl. Environ. Microbiol. 65, 5594–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NS, Litaker RW, Fensin E, Adolf JE, Bowers HA, Place AR, Paerl HW, 2008. Environmental factors contributing to the development and demise of a toxic dinoflagellate (Karlodinium veneficum) bloom in a shallow, eutrophic, lagoonal estuary. Estuar. Coast. 31, 402–418. [Google Scholar]
- Harrison PJ, Waters RE, Taylor FJR, 1980. A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 16, 28–35. [Google Scholar]
- Jeong HJ, Du YY, Park JY, Song JY, Kim ST, Lee SH, Kim KY, Yih WH, 2005a. Feeding by phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. Aquat. Microb. Ecol. 40, 133–150. [Google Scholar]
- Jeong HJ, Park JY, Nho JH, Park MO, Ha JH, Seong KA, Jeng C, Seong CN, Lee KY, Yih WH, 2005b. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquat. Microb. Ecol. 41, 131–143. [Google Scholar]
- Kempton JW, Lewitus AJ, Deeds JR, Law JM, Place AR, 2002. Toxicity of Karlodinium micrum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae 1, 233–241. [Google Scholar]
- Larsen J, Moestrup O, 1989. Guide to Toxic and Potentially Toxic Marine Algae. Fish Inspection Service, Ministry of fisheries, Copenhagen, pp. 61. [Google Scholar]
- Leblond JD, Chapman PJ, 2002. A survey the composition ofthe marine dinoflagellates Karenia brevis, Karenia mikimotoi, and Karlodinium micrum: distribution of sterols within other members ofthe class dinophyceae. J. Phycol. 38 (4), 670–682. [Google Scholar]
- Li J, Glibert PM, Alexander JA, Molina ME, 2012. Growth and competition of several harmful dinoflagellates under different nutrient and light conditions. Harmful Algae 13, 112–125. [Google Scholar]
- Li J, Glibert PM, Zhou M, 2010. Temporal and spatial variability in nitrogen uptake kinetics during dinoflagellate blooms in the East China Sea. Harmful Algae 9 (6), 531–539. [Google Scholar]
- Li J, Glibert PM, Zhou MJ, Lu SH, Lu DD, 2009. Relationships between nitrogen and phosphorus forms and ratios and the development ofdinoflagellate blooms in the East China Sea. Mar. Ecol. Prog. Ser. 383, 11–26. [Google Scholar]
- Li A, Stoecker DK, Coats DW, 2000. Spatial and temporal aspects of Gyrodinium galatheanum in Chesapeake Bay: distribution and mixotrophy. J. Plankton Res. 22, 2105–2124. [Google Scholar]
- Lu DD, Goebel J, 2001. Five red tide species in genus Prorocentrum including the description of Prorocentrum donghaiense Lu Sp. Nov. from the East China Sea. Chin. J. Oceanol. Limnol. 4, 337–344. [Google Scholar]
- Lu D, Goebel J, Qi Y, Zou J, Han X, Gao Y, Li Y, 2002. Prorocentrum donghaiense - a high biomass bloom-forming species in the East China Sea. IOC Newsl. Toxic Algae Algal Bloom. 23, 1–5. [Google Scholar]
- Marshall HG, 1980. Seasonal phytoplankton composition in the lower Chesapeake Bay and Old Plantation Creek, Cape Charles, Virginia. Estuaries 3, 207–216. [Google Scholar]
- Miao M, Yan XJ, Xu JL, Hou YD, 2009. Study of the regular pattern of mass spectrometry of TMS derivatives of sterols. Chin. Mass Spec. 30 (3), 65–74. [Google Scholar]
- Ozbay G, Chambliss SS, Wikfors GH, Adolf JE, Chintapenta LK, Place AR, 2014. The growth response of Prorocentrum minimum Pavill. (Dinophyta) to karlotoxin exposure. Int. J. Algae 16 (1), 95–105. [Google Scholar]
- Place AR, Bowers HA, Bachvaroff TR, Adolf JE, Deeds JR, Sheng J, 2012. Karlodinium veneficum - the little dinoflagellate with a big bite. Harmful Algae 14, 179–195. [Google Scholar]
- Place AR, Harvey HR, Bai X, Coats DW, 2004. Sneaking under the toxin surveillance radar: parasitism and sterol content. Afr. J. Mar. Sci. 28 (2), 347–351. [Google Scholar]
- Rampen SW, Schouten S, Hopmans EA, Noordeloos AAM, van Bleijswijk JDL, Geenevasen JAJ, Sinninghe Damste JS, 2009. Diatoms as a source for 4- desmethyl-23,24-dimethyl steroids in sediments and petroleum. Geochim. Cosmochim. Acta 73 (2), 377–387. [Google Scholar]
- Rioboo C, O’Connor JE, Prado R, Herrero C, Cid A, 2009. Cell proliferation alterations in Chlorella cells under stress conditions. Aquat. Toxicol. 94 (3), 229–237. [DOI] [PubMed] [Google Scholar]
- Smayda TJ, Trainer VL, 2010. Dinoflagellate blooms in upwelling systems: seeding, variability, and contrasts with diatom bloom behavior. Prog. Oceanogr. 85, 92–107. [Google Scholar]
- Sheng J, Malkiel E, Katz J, Adolf JE, Place AR, 2010. A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proc. Natl. Acad. Sci. U.S.A. 107 (5), 2083–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner RM, Deeds JR, Satake M, Ribeiro AA, Place AR, Wright JLC, 2008. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum. Tetrahedron Lett. 49, 6457–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman JK, Rijpstra WIC, de Leeuw JW, Mansour MP, Jackson AE, Blackburn SI, 1999. Sterols of four dinoflagellates from the genus Prorocentrum. Phytochemistry 52 (4) , 695–668. [Google Scholar]
- Wang HX, Lu DD, Huang HY, GobelB EL,J, Dai XF, Xia P, 2011. First observation of Karlodinium veneficum from the East China Sea and the coastal waters of Germany. Acta. Oceanol. Sin. 32 (6), 112–121. [Google Scholar]
- Wang RJ, Xiao H, Wang Y, Zhou WL, Tang XX, 2007. Effects of three macro-algae, Ulva linza (Chlorophyta), Corallina pilulifera (Rhodophyta) and Sargassum thunbergii (Phaeophyta) on the growth of the red tide microalga Prorocentrum donghaiense under laboratory conditions. J. Sea Res. 58, 189–197. [Google Scholar]
- Xu N, Duan SS, Li AF, Zhang CW, Cai ZP, Hu ZX, 2010. Effects oftemperature, salinity and irradiance on the growth of the harmful dinoflagellate Prorocentrum donghaiense Lu. Harmful Algae 9, 13–17. [Google Scholar]
- Xu N, Pang SJ, Liu F, 2012. Molecular identification of a bloom-forming species isolated from Sanggou Bay in Shandong Province. Mar. Sci. 36 (4), 13–18 (in Chinese). [Google Scholar]
- Xu ZB, Yan XJ, Pei LQ, Luo QJ, Xu JL, 2008. Changes in fatty acids and sterols during batch growth of Pavlova viridis in photobioreactor. Appl. Phycol. 20,237–243. [Google Scholar]
- Yang WD, Xie J, van Rijssel M, Li HY, Liu JS, 2010. Allelopathic effects of Alexandrium spp. on Prorocentrum donghaiense. Harmful Algae 10, 116–120. [Google Scholar]
- Zhou MJ, 2010. In: Ishimatsu A, Lie H-J (Eds.), Environmental Settings and Harmful Algal Blooms in the Sea Area Adjacent to the Changjiang River Estuary. Coastal Environmental and Ecosystem Issues of the East China Sea. TERRAPUB and Nagasaki University, pp. 133–149. [Google Scholar]
- Zhou CX, Fernández N, Chen HM, You YR, Yan XJ, 2011. Toxicological studies of Karlodinium micrum (Dinophyceae) isolated from East China Sea. Toxicon 57, 9–18. [DOI] [PubMed] [Google Scholar]
- Zhou CX, Sun X, Feng J, Yan XJ, 2008a. Microscopic observations and molecular identification of toxic unarmoured dinoflagellates Karlodinium micrum (Dinophyceae) from the East China Sea (ECS). Mar. Sci. Bull. 27 (3), 32–37 (in Chinese). [Google Scholar]
- Zhou MJ, Shen ZL, Yu RC, 2008b. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) river. Cont. Shelf Res. 28, 1483–1489. [Google Scholar]
- Zhou M, Yan T, Zou J, 2003. Preliminary analysis of the characteristics of red tide areas in Changjiang River estuary and its adjacent sea. Chin. J. Appl. Ecol. 14, 1031–1038. [PubMed] [Google Scholar]
- Zhou W, Yin K, Zhu D, 2006. Phytoplankton biomass and high frequency of Prorocentrum donghaiense harmful algal bloom in Zhoushan sea area in spring. Chin. J. Appl. Ecol. 17 (5), 887–893 (in Chinese). [PubMed] [Google Scholar]


