Abstract
Objective:
The pore size of the scaffold is a critical factor in repairing large bone defect. Here, we investigated the potential of bone regeneration using novel nanocomposite polydopamine-laced hydroxyapatite collagen calcium silicate (HCCS-PDA) scaffolds with two different pore sizes, 250 and 500 μm.
Samples/Setting:
12 male Sprague-Dawley rats were implanted with HCCS-PDA scaffold with pore size of either 250 or 500 um into surgically created critical sized defect (CSD).
Methods:
HCCS-PDA scaffolds were fabricated using mold printing technique. The effect of pore size on mechanical strength of the scaffolds was assessed by compression testing. After seeding with rat mesenchymal stem cells (rMSCs), the scaffolds were implanted, and new bone formation was evaluated using microCT and histomorphometric analysis after 8 weeks.
Results:
MicroCT and histology analysis demonstrated restricted peripheral new bone formation in either dural or periosteal side and limited new bone formation in the 250 μm pore scaffold. Conversely, the 500 μm pore scaffold showed more penetration of new bone into the scaffold and greater bone regeneration in the rat CSD.
Conclusion:
Based on our results, which demonstrated improved new bone formation in 500 μm pores scaffold, we can conclude that effective scaffold pore size that induce osteointegration and bone regeneration is around 500 μm for HCCS-PDA nanocomposite scaffold.
Keywords: pore size, bone regeneration, CSD, HCCS-PDA
Introduction
Critical-sized large bone defects caused by nonunion fractures, tumor resections, trauma, infection and congenital etiology require extensive surgical intervention. Although autografting bone is the clinical gold standard, the limitation in supply and potential for donor site morbidity make it difficult to utilize autografting as a treatment option for patients. As a potential replacement that can overcome the drawbacks of the existing bone graft options, tissue engineered bone scaffold, a porous scaffold with osteoblasts seeded within the pores, has been explored for many years.
Scaffolds should consist of similar biochemical and biomechanical properties as natural bone to emulate the effects. In bone tissue engineering, the porous scaffold provides 3D structural environment that mimics the innate in vivo environment, where seeded mesenchymal stem cells (MSCs) can attach, secrete extracellular matrices (ECM), and facilitate vascularization and its replacement with new bone. The nanocomposite structure, which consists of collagen reinforced by hydroxyapatite provides high compressive strength in natural bone.
Recent technological advancement in biomaterials and printing technology has enabled the development of biomimetic nanocomposite biomaterial, polydopamine-laced hydroxyapatite collagen calcium silicate (HCCS-PDA), as an alternative scaffold material to repair a critical-sized defect (CSD). In a previous study, HCCS-PDA in particle form was tested for bone regeneration capacity both in vitro and in vivo to observe sole effect of HCCS-PDA material. The results showed that the HCCS-PDA enhanced local bone formation in calvarial CSD model, forming tight interface between HCCS-PDA particle and newly formed bone.2 With well-designed scaffold, the amount of bone regeneration is expected to increase. Computer-aided design (CAD) modeling has enabled precise control of the scaffold architecture, including the pore size, connectivity, dimensions, and shape.3 Due to the fast reacting property of HCCS-PDA material, the 3D mold printing technique is unsurpassed choice to fabricate customized HCCS-PDA, with interconnected pores. Effective optimization of scaffold material and structure is crucial in bone regeneration, especially, for the nanocomposite materials like HCCS-PDA. There is currently no information on the optimal pore size for effective bone regeneration in the CSD using porous HCCS-PDA scaffold.
According to the studies performed by Karageorgiou and Kaplan et al., scaffolds with pore sizes greater than 300 μm are recommended for repairing large bone defect as they resulted in enhanced new bone and capillary formations.4 Among various types of scaffolds, excellent osteoinductions have been shown in the ones with pore sizes within the range of 500 to 1200 μm.5,6 It was also demonstrated that ceramic scaffold with 300 to 500 μm pores is optimal for osteogenesis; the ones with 300 μm pores, which exhibited inferior bone ingrowth.7 Regarding vascularization for the optimal pore size, Feng et al. reported that large pores in β-tricalcium phosphate scaffold was beneficial for the blood vessel ingrowth, and the pores smaller than 400 μm limit the growth of blood vessels.8
Varying outcomes in the differential materials make the conclusion elusive. In this regard, the optimal pore size of scaffold for bone regeneration should be investigated for each material prior to its use as a bone scaffold. The objectives of this study are to assess the feasibility of manufacturing porous HCCS-PDA scaffolds using 3D mold printing technique, optimize the suitable pore size beneficial for MSC survival and bone regeneration, and deduce the potential relevance of pore size and the resulting clinical biological performance. To determine the optimal pore size for bone regeneration, HCCS-PDA nanocomposite scaffolds with different pore sizes (250 and 300 μm) were fabricated through a mold printing technique that provide consistent porosity and pore size in orderly manner. Then the the effect of pore size on the bone regeneration was examined using rat calvarial CSD model.
Material and Methods
HCCS-PDA fabrication using 3D printed mold technique
Porous 3D scaffold was designed (8.5 mm in diameter, 1.2 mm in thickness with square pores, and 50% porosity) to fit in rat calvarial CSD using CAD. Wax molds of two different sizes of pores (250 and 500 um) were printed using Solidscape printer (Solidscape, Merrimack, NH). Hydroxyapatite and collagen (HC) slurry was biomimetically synthesized by co-precipitation method using in situ hybridization of calcium silicates (CS) with HC powder.9 The powders of HC (150 mg), dopamine (5 mg), and calcium hydroxide (100 mg) were mixed and cross-linked with 383 µL of enTMOS (bis [3-(trimethoxysilyl)-propyl] ethylenediamine) and APS (80 µL) on cold stage. The mixture was quickly poured into wax molds. After 20 minutes, the wax mold was immersed into acetone for 30 minutes to remove the mold, resulting an inverse HCCS-PDA scaffold. Residual wax around the scaffolds were completely dissolved in 95% ethanol overnight. The porous HCCS-PDA scaffolds were treated with 5% glutaraldehyde for 6 hours, then neutralized using 3% glycine solution with frequent changes. Then, the scaffolds were air-dried for 3 days, oven-dried for 3 days at 52°C, and sterilized with cold ethylene oxide gas.
Scanning Electron Microscopy (SEM)
Cross-sectioned HCCS-PDA scaffolds were fixed on a holder, sputter-coated with platinum (4 nm) in a vacuum and imaged using a Hitachi S-4700 Cold Cathode Field Emission SEM (Hitachi High Technologies America, Inc., Schaumburg, IL USA). The scaffolds were fixed in 10% neutral formalin for 24 hours, washed 3 times with deionized water, and followed by critical point drying process using EMS 850 Critical Point Dryer (Electron Microscopy Sciences, Hatfield, PA USA) before acquiring image using the SEM.
Mechanical test
The compressive strength of HCCS-PDA scaffolds with 250 and 500 µm were measured using Instron (model 4204, Canton, MA, USA). Scaffolds were compressed by 0.5 mm/min uniaxial force, with five scaffolds tested per each group. The maximum failure strength was measured from the stress-strain curve.
Isolation of MSCs from bone marrow
After Sprague-Dawley rats (8 weeks old) were sacrificed, their femurs were removed and cut at both ends to flush the bone marrow directly out into a 35-mm culture dish with growth media (Dulbecco’s Modified Eagle Medium (DMEM: Thermo Fisher Scientific Inc., Rockford, IL, USA) supplemented with 10% Fetal Bovine Serum (FBS: Sigma), 1% Penicillin & Straptomycin (P/S: Thermo Fisher Scientific Inc., Rockford, IL, USA), and 250 µL of GlutaMax® (Thermo Fisher Scientific Inc., Rockford, IL, USA)). The isolated bone marrow was cultured inside the incubator at 37°C and 5% CO2. After 24 hours, adhered cells were further expanded and passaged under growth media. In vitro experiments were performed with MSCs at passage 5 for in vivo study. Prior to use of MSCs for in vivo bone formation, the cells were characterized for their expression of specific surface markers and multilineage differentiation such as adipogenic, chondrogenic and osteogenic linages.
Seeding MSCs in the scaffold and implantation in rat critical sized defect (CSD)
On the day of implantation, 5 × 106 rat mesenchymal stem cells (rMSCs) were seeded into each porous HCCS-PDA scaffold (8.5 mm in diameter, 1.2 mm in thickness) mixing in matrigel (Corning: Corning, NY USA) containing 10 µg/mL of BMP-2 (Shenandoah biotechnology Inc., Warwick, PA USA) for cell delivery into pores. MSC-matrigel solution (30 µL/scaffold) at 4 °C was dropped throughout the pores on the scaffold surface and solidified at 37 °C. After adding osteogenic media, the scaffolds were kept under 37 °C chamber until implantation in CSD. The detailed surgical procedure has been well-described in a previous study.10 Male rats were chosen to minimize factors, especially osteoporotic hormonal effects, that can influence bone regeneration. Briefly, twelve young male Sprague-Dawley rats (260 to 320 g) were randomly assigned to two different experimental groups to implant HCCS-PDA scaffolds with 250 µm pores (n=6) and that with 500 µm pores (n=6). Animals were anesthetized by injecting 95 mg/kg Xylazine/ketamine intraperitoneally. Their heads were shaven, and the surgical site was sterilized with Betadine and 70 % ethanol. A midline incision was taken through the periosteum to expose the underlying bone. After a CSD was created using 8 mm trephine burr, MSC seeded HCCS-PDA scaffold was inserted into the defect. The periosteum was then closed with 5–0 cat-gut suture. After placing titanium mesh above the defect site, the skin was closed with 4–0 polypropylene suture. 22.5 mg/mL Cefazolin, 5 mg/kg ketoprofen, and 0.01 mg/kg buprenorphine were administered for 1 week of post-surgery.
MicroCT Analysis
Calvaria were harvested 8 weeks post-implantation, immediately fixed in 10% neutral formalin solution for 3 days. Then, the calvaria were scanned with a Skyscan microCT (Skyscan 1076; Skyscan, Aartselaar, Belgium) to acquire images at 40 kV, 1000 mA, 20 µm in resolution with a 720 ms integration time. Three dimensional images were reconstructed using ITK-SNAP software. Following the reconstruction, newly formed bone in the CSDs of three animals from each group was measured using Geomagic Design X™ (3D Systems Inc., SC USA) software. The bone volume was represented in mm3.
Histology and new bone formation (NBF) measurement
After microCT scanning, the calvaria were dehydrated, embedded in Techovit® (Electron Microscopy Sciences, Hatfield, PA USA), and grinded 3 sections to expose a mid-sagittal view of the calvaria in 60 µm thickness using Exakt grinding instrument (Hamburg-Norderstedt, Germany). Stevenel’s blue and Van Gieson staining were performed to assess new bone formation. Color images of the histologic sections were acquired under 10x magnification using a Nikon Eclipse Ti-U camera with an automatic stage (Melville, NY USA) from three independent samples, and image analysis was performed by measuring in pixels using Image J software (U.S. National Institutes of Health, Bethesda, MD USA). The newly formed bone area was calculated as a percent of total defect area.
Statistics
The one tail Student t-test was used to compare the means between the groups.
Results
Stereoscopic Images of HCCS-PDA scaffolds
HCCS-PDA scaffolds with different pore sizes (250 and 500 μm) were successfully fabricated using the 3D mold printing technique (Fig. 2A). The dimensions of the fabricated disks were 1.2 mm in thickness and 8.5 mm in diameter (Fig. 2B). SEM images on cross-sectional views of both 250 and 500 μm pore HCCS-PDA scaffolds showed internal pore surface and structure in each scaffold (Fig. 2C and D).
Figure 2.

(A) Stereomicroscopic photograph showing 3D printed wax mold and (B) HCCS-PDA scaffolds after wax mold removed. Pores were represented as square channels and scaffolds oriented at 90° among three layers. Pores extend from top to bottom layer. SEM images of interconnected pore surface morphology of scaffolds with pore sizes of (C) 250 and (D) 500 um. (E) Compression tests performed on 3D porous HCCS-PDA scaffolds. (n = 5, *p < 0.05).
Mechanical Property Assessment
The mean values of the compressive strength were 32.76 ± 4.37 and 67.98 ± 5.64 kgf for HCCS-PDA with 250 and 500 μm pores, respectively. The compressive strength of the HCCS-PDA with 500 μm pores was twice higher than that with 250 μm pores. It is clear that pore size had an influence on structural strength of 3D porous HCCS-PDA scaffold (Fig. 3).
Figure 3.
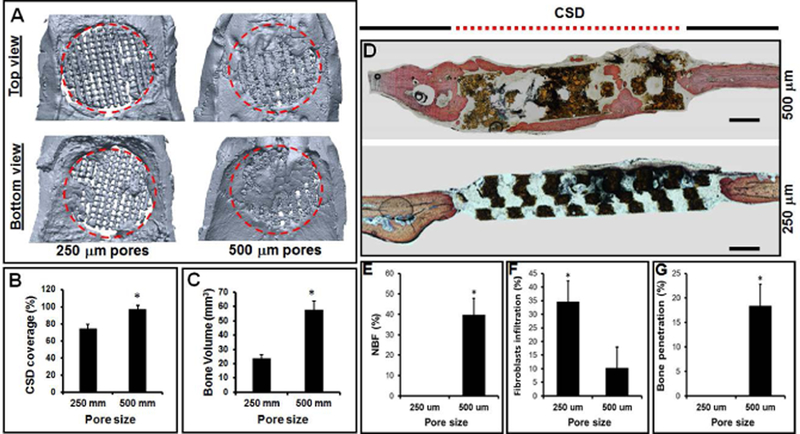
(A) MicroCT images and (D) histological sections processed after 8 weeks of implantation of HCCS-PDA scaffolds (250 and 500 um pores) seeded with MSCs into rat calvarial CSD (red dotted circle). (B) CSD coverage and (C) bone volume in the defect calculated in % and mm3, respectively. (E) The area of new bone formation (NFB), (F) fibroblasts infiltration, and (G) bone penetration in to the pores quantified in % (mean ± STD).
Isolation and Characterization of Bone Marrow rMSCs
The method of bone marrow MSCs isolation from Sprague Dawley rats has been well described in the previous study.10 rMSC specific surface markers confirmed positive expression for CD44 and CD90 and negative for CD45 and CD34 antibodies. Chondrogenic, osteogenic and adipogenic differentiation of rMSC was verified by positive on Safranin O staining, Alizarin Red S staining and Oil Red O staining, respectively. (Fig. 1D).
Figure 1.
Scheme of the study. (A) 3D porous wax mold and (B) wax molds designed and printed using Solidscape® Studio 3D printer. (C) Porous HCCS-PDA scaffolds casted using printed wax molds. (D) Image of MSC isolation method and characterization, (E) Surgical implantation of HCCS-PDA scaffold into the CSD of rat calvaria. (F) MicroCT and histomorphometric analysis after 8 weeks of implantation
MicroCT and Histomorphometric Analysis
After 8 weeks post-implantation, the entire calvaria was explanted and underwent fixation. MicroCT analysis revealed the % coverage on the CSD (Fig. 3B) implanting with HCCS-PDA scaffolds with 500 μm pore (96.96 ± 4.57%) was more evident than 250 μm pore size group (74 ± 5.31%). The % volume of the newly formed bone (Fig. 3C) in the HCCS-PDA with 500 μm pores (57.67 ± 6.14 %) was higher than that in the HCCS-PDA with 250 um pores (23.39 ± 2.75 %). Figure 3A indicates that the new bone ingrowth from both dural and periosteal sides merged together to make scaffold-new bone a stable structure to effectively heal the CSD. Overall, the HCCS-PDA with 500 μm pores with rMSCs showed superior bone regeneration capacity in the defect site by filling the pores with newly formed bony tissue compared to the HCCS-PDA scaffold group with 250 μm pores.
Three sagittal sections of calvaria were stained with Stevenel’s Blue and counter-stained with Van Gieson. Histological analysis revealed bone regeneration in the HCCS-PDA scaffolds with both 250 and 500 μm pores at 8 weeks post-implantation (Fig. 3D). Newly formed bone was stained brighter red color by Van Gieson staining than the host bone located near the defect site. The NBF in the 500 μm pores was well integrated at the interface between the scaffold and newly formed bone in the defect without any sign of fibrous tissue infiltration from the surrounding, whereas 34.55 ± 7.80% of fibrous tissues were detected in the HCCS-PDA scaffold with 250 μm pores (Fig. 3F). In the HCCS-PDA with 250 μm pore group, there was an absence of NBF, with intervening fibrous tissue evident, whereas new bone formation was regenerated through he pores and along the surface of the HCCS-PDA scaffold with 500 μm pore group (39.66 ± 8.24 %) for the HCCS-PDA with 500 μm pores. Histology picture confirmed that the HCCS-PDA with 500 μm pores seeded with rMSC led to a significant amount of penetration of bony tissue into the pores after 8 weeks (18.38 ± 4.4). In contrast, the HCCS-PDA group with 250 μm pores indicated no penetration of bone into the pores. (Fig. 3G).
Discussion
Together with stem cells and growth factors, scaffold is one of the critical factors in bone regeneration using tissue engineering. Over the past decade, the aspiration to regenerate bone has driven significant progress in the synthesis of innovative scaffold materials and fabrication. In particular, porous structure has become a fundamental scaffold characteristic to induce osteoconduction, osteoinduction and osteointegration.
Originally, four different groups of scaffolds with pore sizes less than 100, 200–250, 450–500, and larger than 700 μm were considered. However, the scaffold with pore size less than 100 μm resulted in hollow space in the middle of the scaffold once the wax mold was dissolved. The scaffold with pores larger than 700 μm, called megaporous scaffold, also showed problem in delivering cells into the pores and caused incomplete bone formation in the pore structures. Due to such prior founding, we focused on comparing bone regeneration only between the two scaffolds with intermediate pore sizes (200–250 and 450–500 μm).
The mechanical property is important in maintaining the structural stability of the scaffold, but it is largely influenced by the pores.11 Most of the HCCS-PDA scaffolds with 250 μm pores resulted either no bone formation or only minor bone formation. The scaffold might have disintegrated during implantation surgery or post-surgical period biomechemically or biochemically in vivo condition due to its weaker mechanical stability. Either case could have caused micromovement of the disintegrated pieces in the defect site, prohibiting new bone formation in the scaffold.
3D porous HCCS-PDA scaffold improved technical deficiency of previous study using the particulate form of grafts in delivering cells to the CSD site. Seeding cells in the evenly distributed pores can result in enhanced bone formation through the interconnected pores. Though many studies reported a higher in vivo bone regeneration as an effect of seeded MSCs, the detail interaction between material and seeded cells after implantation remains unclear presently. Further research should also be conducted by tracing the seeded cells during bone formation to define their fate as of whether the seeded MSCs directly affect osteogenesis by depositing minerals or indirectly stimulate bone regeneration by secreting biological factors. For the new bone formation in the pore structure of HCCS-PDA scaffold, effective strategy for cell delivery into the pores has been developed. Using matrigel to deliver and retain the cells in 3D distribution improved bone regeneration in the pores significantly compare to ordinary cell seeding using culture media because the matrigel entraps cells and allows even cell distribution within each pore.
After 8 weeks of post-implantation, the entire calvaria was harvested for microCT and histomorphometric analysis. Both analysis showed that the group with 500 μm pores had more bone regeneration than the 250 μm pores group, indicated by greater bone volume, coverage of defect area, and NBF (Fig. 3). MicroCT could not differentiate between the newly formed bone in the pores and the HCCS-PDA material. Thus, the result showed only 2D image of bone regeneration status in the pores from the top view, not cross-sectional view of the pores. As it is challenging to measure the volumes of scaffold and new bone separately, both were included in the percentage calculation and histological analysis using the cross-sectional view of the HCCS-PDA scaffold has been included to provide further support for new bone formation in the pores. It revealed that the new bone penetration into 500 μm pores was significantly higher than into the scaffold with 250 μm pores (Fig. 3G). Previously, it was reported that the amount of fibrous tissue ingrowth increased with the decrease in pore size.12 The result was based on the β-TCP scaffold materials with no seeding of MSCs in the scaffold, which increased risk of fibrous tissue infiltration into the empty pores. Likewise, our previous data indicated that a high amount of fibrous tissue was observed in the pores of HCCS-PDA scaffold without any seeded cells.
To date, variety of bone scaffolds have been examined for new bone formation in vivo. Crucial challenges, such as developing consistent and reliable manufacturing techniques for delicate material like nanocomposite for direct printing method still remain. Although we here defined ideal pore size of HCCS-PDA scaffold for enhanced bone ingrowth through the pores, the current protocols and parameters are still insufficient to accomplish complete bone regeneration. Additionally, it will be important to investigate our regeneration strategy for gender-related osteoporotic bone regeneration to confirm its effectiveness. For our future studies, heavier focus on effective cell delivery and their differentiation efficiency in the pores would be necessary to achieve complete healing of the CSD. Moreover, controlling the degradation rate of any scaffold material to match the bone regeneration rate is of interest for future development.
Conclusion
The novel HCCS-PDA biomaterial with a hierarchical pore architecture could be fabricated with 3D printing technique using wax mold to generate inversely casted HCCS-PDA scaffolds with 500 and 250 μm pores. It was found that the scaffolds with 500 μm pores possessed about 2 times higher compressive strength than the scaffolds with 250 μm pores. In addition, implantation of these scaffolds into a rat calvarial CSD yielded an effective bone formation in the scaffold with 500 μm pores, contrary to minimal bone formation in the scaffold with 250 μm pores. Here, we confirmed that 500 μm pores in HCCS-PDA scaffold facilitated mineralization by bone ingrowth through the pores. Overall, our results indicate that the 3D HCCS-PDA biomaterial can serve as an osteoconductive scaffolding material, with optimal pore size around 500 μm with additional custom fit design for therapeutic bone tissue engineering applications.
Acknowledgements
This work was supported by NIH/NIDCR R01DE022816.
Footnotes
All animal studies followed the guidelines for Institutional Animal Care and Use Committee (ICAUC) at the University of North Carolina at Chapel Hill (Approved protocol number 15-273).
References
- 1.Ko CC, Guez C, Lee DJ, Wang Z, Tseng H. Advances in Bioceramics and Biotechnologies II: Ceramic Transactions California: Wiley-American Ceramic Society; 2014. 135–148 p. [Google Scholar]
- 2.Lee DJ, Lee YT, Zou R, Daniel R, Ko CC. Polydopamine-Laced Biomimetic Material Stimulation of Bone Marrow Derived Mesenchymal Stem Cells to Promote Osteogenic Effects. Sci Rep 2017;7(1):12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do A, Khorsand B, Geary SM, Salem AK. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv Health Mater 2015;4(12):1742–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005;26:5474–5491. [DOI] [PubMed] [Google Scholar]
- 5.Jinyu Li, Wei Zhi, Taotao Xu, Feng Shi, Ke Duan, Jianxin Wang, Yandong Mu, Jie Weng. Ectopic osteogenesis and angiogenesis regulated by porous architecture of hydroxyapatite scaffolds with similar interconnecting structure in vivo. Regen Biomater 2016:3(5): 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng Part A 2012;18(13–14): 1376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng B, Jinkang Z, Zhen W, Jianxi L, Jiang C, Jian L, Guolin M, Xin D. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed Mater 2011;6(1): 015007. [DOI] [PubMed] [Google Scholar]
- 8.Chang MC, Ko CC, Douglas WH. Preparation of hydroxyapatite-gelatin nanocomposite. Biomaterials 2003;24(17): 2853–2862. [DOI] [PubMed] [Google Scholar]
- 9.Lee DJ, Padilla R, Zhang H, Ko CC. Biological Assessment of a Calcium Silicate Incorporated Hydroxyapatite-Gelatin Nanocomposite: A Comparison to Decellularized Bone Matrix. BioMed Research International 2014;2014(9): 837524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AG. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc 2012:7(10): 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater. Res. B Appl Biomater 2005;74(2): 782–788. [DOI] [PubMed] [Google Scholar]
- 12.Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD, Stelling FH. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res 1970;4: 433–456. [DOI] [PubMed] [Google Scholar]



