Abstract
Starch is one of the digestible natural polymers found in vascular plants. This natural polymer is the primary source of polysaccharides to produce energy for humans. In this work, starch was extracted from the defatted and dephenolated Limnophila aromatica (DFPLA) by using the alkaline method. The DFPLA contains starch with a purity of 70.43 % where 55.1 % of it is the resistant starch. Physicochemical properties of the DFPLA starch such as solubility, morphology, swelling power, crystallinity, gelatinization, retrogradation, decomposition temperature, pasting profile, and surface functional groups were evaluated. The DFPLA starch possesses a medium-amylose content of 23.78 %, and the particle diameters of the starch were varied from 3 to 6 μm. The swelling power and solubility of the DFPLA starch are increasing as the temperature increased, where at 90 °C the swelling power and solubility of the starch is 13.73 g/g and 7.26%, respectively. Starch from DFPLA has a high total dietary fiber (76.28%) which is comparable to that of starch extracted from staple foods. The results indicate that starch from DFPLA possesses good physicochemical properties; this alternative starch may have potential application as a new feedstock for food industries.
Keyword: Natural product chemistry
1. Introduction
Limnophila aromatica (Lam.) Merr. also known as rice paddy herb is a flowering weed plant that belongs to Scrophulariaceae (figwort, snapdragon) family. The plant is originated from Southeast Asia; it is easily cultivated in high moisture soil or watery area. L. aromatica can grow up to 4 mm long, and it can be harvested in 40 days after planting. Folks have usually cultivated the plants for the use of ornamental, food, and herb. The plant has a distinctive refreshing scent which resembles of basil and lemon, the plant is commonly extracted for essential oil due to its unique scent (Bui et al., 2004; Do et al., 2014; Gorai et al., 2014). Extraction of phenolics and flavonoids from L. aromatica has been reported (Bui et al., 2004; Do et al., 2014). The ethanolic extract of L. aromatica has a phenolic loading of 40.5 mg/g and flavonoid 31.11 mg/g (Do et al., 2014). The plant is favored as a complementary foodstuff. In this study, L. aromatica is proven to be a potential source of carbohydrate specifically starch.
Starch is known as a biocompatible, biodegradable, non-toxic, eco-friendly and inexpensive natural polysaccharide (Atwell et al., 1988; Rodrigues and Emeje, 2012). Starch composes of linear and branch polysaccharide namely amylose and amylopectin, respectively. Starch plays an essential role in providing energy for human activity; it also has a broad application in food and nonfood industries. Starch has been used as raw material in industries such as adhesives, cosmetic, pharmaceutical, paper, textile, and construction. Other advanced industries such as biodegradable plastic coating, printed circuit boards (PCB), and dry cell batteries also started to use starch as raw material due to its harmless property and environmental friendly (Rodrigues and Emeje, 2012; Colussi et al., 2014). Another consideration for some advanced industries for using starch as a raw material is to reduce the use of petrochemical resources by blending it with starch. The resulting polymer blends are known to have increased in biodegradability (Gupta et al., 2009; Oladunmoye et al., 2014).
Staple foods (e.g. corn, wheat, rice, cassava, and potato) are generally used as bio-source for starch isolation. This can lead to competition between humans and industry in the consumption of staple foods. Therefore, it is necessary to search for new and inexpensive sources of starch to balance the use of staple foods as starch producing sources. The use of L. aromatica to isolate starch can be a potential resolution for the problem.
Different biomass cultivars give different starch properties. Understanding of starch physical and chemical properties is vital for the application of starch since they affect the quality of the products produces; for example, in food applications, coating batter prepared using high-amylose starch produce more foods that are firmer and crispier after being fried while low-amylose starch is not (Richardson et al., 2000). In non-food application, a high viscosity starch can produce stronger gel adhesive (Orlando, 1998). Each application of starch in food or non-food industry requires particular functional characteristics. The chemical or biochemical modification is sometimes conducted if the physical or chemical characteristics of native starch do not fulfill certain applications. So far there have been no studies investigating the content and nature of starch from L. aromatica, which will be covered in this work. The extraction residue of L. aromatica was used for isolating starch by wet milling method. Physicochemical, structural, functional group, crystallinity, pasting and thermal properties of the isolated starch were investigated.
2. Experimental
2.1. Materials
Ethanol (95%) and n-Hexane (95%) were obtained from Echo Chemical (Miao Li, Taiwan) and Tedia (Fairfield, OH), respectively. The chemicals as follows were purchased from Sigma–Aldrich (St. Louis, MO): glucose standard, amylose standard (from potato), protease (EC 3.4.23.18), amyloglucosidase (EC 3.2.1.3), α-amylase (EC 3.2.1.1), and sodium bisulfate. Fresh L. aromatica was obtained from a Vietnamese store in Taipei, Taiwan. After removal of the roots, the plants are washed. Subsequently, the leaves and stems were freeze-dried for 2 days. The dried sample was grounded and sieved using the 60-mesh sieve. The fat in the powder was then extracted using n-hexane as the solvent. The solid residue was collected and dried. The dried residue (defatted L. aromatica - DFLA) was extracted using ethanol (6 h) to remove phenolic compounds. After phenolic extraction, the residue obtained (hereafter referred to as DFPLA) was air-dried and stored at 4 °C for further analyses.
2.2. Isolation of starch
A slight modification of Wang and Wang's method was employed to isolate the starch from DFPLA (Wang and Wang, 2001). Briefly, DFPLA was soaked in NaOH solution (0.1%, 1:2 (w/v)) for 18 h under constant stirring. After the process completed, the solid was separated from the solution using filtration. The solid was rinsed several times with 0.1% sodium hydroxide solution and distilled water until the pH of rinse water is constant. The starch then suspended in distilled water (pH 6.5). The starch was separated from the suspension using the centrifugation method. The excess chemical was removed from the starch by rinsing with distilled water several times and dried in a freeze dryer for 2 days. The dried starch was kept in a desiccator before analysis.
2.3. Characterization of starch
Total starch, resistant starch content, amylose content, total dietary fiber, swelling, solubility, crystallinity degree, stability, Gelatinization, and retrogradation analysis of DFPLA starch were determined according to the defined procedures, the detail of the procedures can be found elsewhere (Yuliana et al., 2012). Pasting profile of the starch was determined by using a rapid visco analyzer (Newport Scientific, Australia). Detailed pasting profiling procedure can be found elsewhere (Yadav et al., 2016).
2.4. Surface morphology of starch
The starch sample was re-suspended in water and filtered under vacuum using a cellulose acetate membrane filter (0.2 μm). The starch sample was then freeze-dried for 2 days. The surface morphology of the starch was characterized using a scanning electron microscopy (SEM), JSM-6390LV, JEOL – USA. The SEM was operated at an accelerating voltage of 15 kV. Before the scanning analysis, the starch particles were coated with gold.
2.5. Fourier transforms infrared spectroscopy
The surface functional groups of the starch were investigated by using a Shimadzu FTIR-8400S. An accumulation scan of 128 and wavenumber resolution of 4 cm−1 was applied. The surface functional groups were examined at wavenumber range 4000 to 450 cm−1.
3. Results and discussion
3.1. The composition of isolated starch
The composition of the starch isolated from DFPLA is summarized in Table 1. The isolated starch product has starch content of 70.43 wt.% (55.10% of starch was resistant starch), total dietary fiber (TDF) content of 76.28 wt.%, protein 1.02%, ash 4.87%, water 2.05%, and others 0.45%. The TDF consists of polysaccharides, lignin, remnants of plant cells, and substances resistant to hydrolysis (digestion) by the alimentary enzymes of humans (Trowell et al., 1976). The resistant starch is one of insoluble dietary fiber. The starch of DFPLA consists of 23.78% amylose (insoluble starch) and 76.22% amylopectin (soluble starch). The yield of starch isolated from DFPLA using the alkaline method is 44.6%, thus 1 kg of dried DFPLA can produce approximately 446 g of starch. This value is still far below the yield of starch from various staple foods; specifically the starch yield for rice is 75.8%, wheat 71.9%, and corn 79.5% (Vegh, 2009). Although the yield is low, starch from DFPLA can be a potential mixture for starch from staple foods. In addition, L. aromatica is a secondary food; hence the use of L. aromatica can reduce competition from industry to the human in consuming of staple foods.
Table 1.
The composition of isolated starch.
| Component | wt.% |
|---|---|
| Starch | 70.43 ± 1.59 |
| TDF (without resistant starch) | 21.18 ± 2.51 |
| Protein | 1.02 ± 0.08 |
| Ash | 4.87 ± 0.34 |
| Water | 2.05 ± 0.33 |
| Others | 0.45 ± 0.30 |
3.2. Morphology
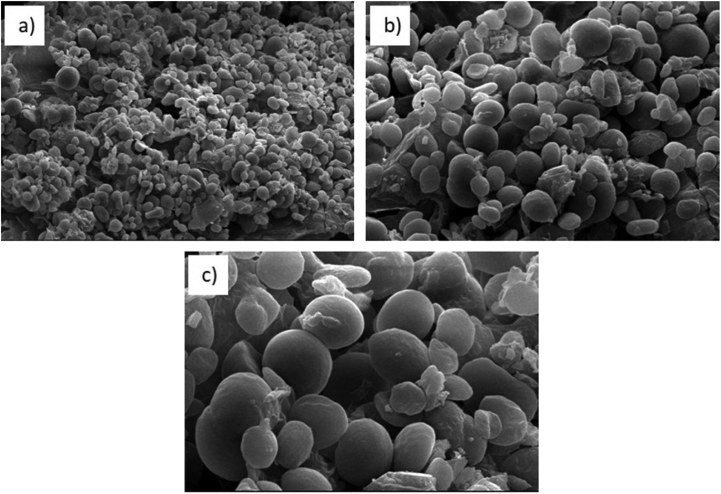
The surface morphological of the isolated starch from the leaves and stems DFPLA was shown in Fig. 1. Most of the starch granules have oval and round shapes which are 3–6 μm in size. Granule size plays an important role in determining the physical properties of starch, such as gelatinization, crystallinity, and solubility. The shape of starch granules is a mixture of granular, oval, and discoid which is a characteristic of starch isolated from photosynthetic tissues (Zeeman et al., 2002).
Fig. 1.
Scanning electron micrographs of starch from L. aromatic at different magnification, a) 1000×, b) 2,500×, and c) 5,000×.
3.3. Swelling power and solubility
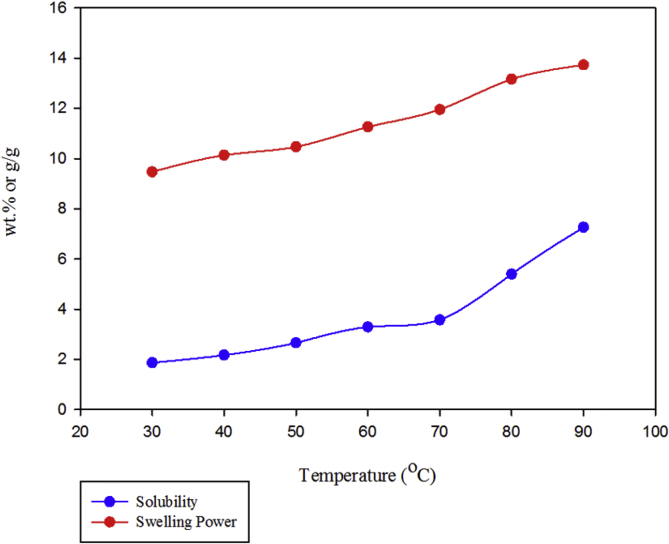
The starch swelling power indicates the uptake of water during the gelatinization of starch. In the gelatinization process, hot water is absorbed in the molecules of starch granules and caused them to swell. The hydrogen bonds between water and hydroxyl groups of amylopectin and amylose are disrupted during the process, and it will trigger the swelling and solubilization of the starch granules. Subsequently, some of the amylose content is released from the granules. Eventually, the starch granules absorbed more water and more swelled. Several factors influence the extent of this interaction is the amylose/amylopectin ratio, degree, and length of the branch of amylose and amylopectin, as well as the distribution of the molecular weight (Hoover, 2001). The swelling properties of starch isolated from DFPLA as a function of temperature were tested and the results are shown in Fig. 2. The swelling power of starch increases from 9.47 g/g at 30 °C to 13.73 g/g at 90 °C. The starch solubility increased from 1.87 wt.% at 30 °C to 7.26 wt.% at 90 °C.
Fig. 2.
Swelling power and solubility of starch from L. aromatica.
The properties of starch from different biomasses are given in Table 2. The swelling power of DFPLA starch is comparable to the wheat, potato, and cassava starch; but lower than that of banana, chestnut, corn, and rice. The difference in swelling power is due to the difference in amylose content, in which biomasses with lower amylose content tend to possess high swelling power. In the characteristic of solubility, the temperature is the most influential. The solubility of the starch is increased at a higher temperature and decreased at a lower temperature.
Table 2.
Comparison of amylase content, swelling power and solubility of starch from various biomasses.
| Biomass | Amylose (%) | Swelling power (g/g) | Solubility (%) | Reference |
|---|---|---|---|---|
| Banana | Not available | 16.0 (90 °C) | 8.1 (90 °C) | Kaur et al. (2011) |
| Chesnut from Yanquan | 35.10 | 13.6 (60 °C) 31.46 (95 °C) |
Not available | Yu et al. (2016) |
| Wheat (Triticum timopheevii) | 28.1–33.8 | 12.8–23.6 (92.5 °C) | Not available | Dennett et al. (2009) |
| Cassava | 19.5 | 9.0 (30 °C) | 2.2 (30 °C) | Oladunmoye et al. (2014) |
| Corn | 22.4–32.5 | 22 (95 °C) | 22 (95 °C) | Singh et al. (2003) |
| Rice | 7.83–18.86 | 26.06–33.20 (95 °C) | 0.29–0.36 (95 °C) | Singh et al. (2003) |
| Potato | 25.17 | 0.85 (60 °C) 8.64 (95 °C) |
1.87 (30 °C) | Reddy et al. (2015) |
| L. aromatica | 23.78 | 9.47 (30 °C) 13.73 (90 °C) |
1.87 (30 °C) 7.26 (90 °C) |
This work |
3.4. Pasting profile
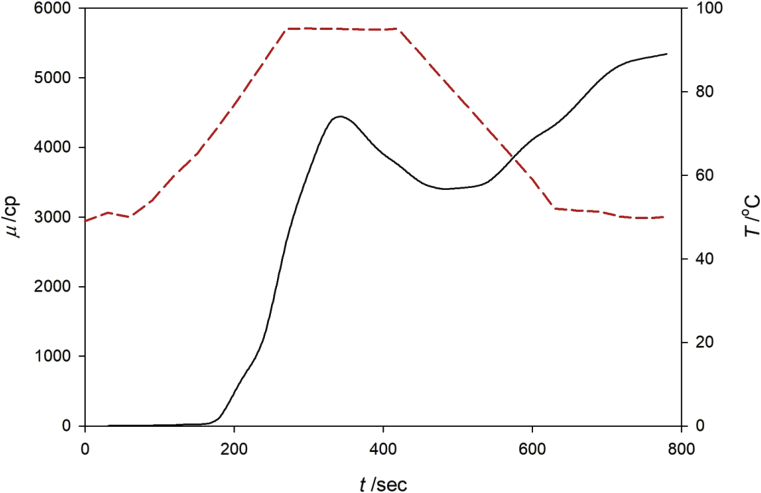
Pasting profile of the starch from DFPLA is shown in Fig. 3, with peak viscosity (PV) of 4380 cp and final viscosity (FV) of 5340 cp. Pasting starts approximately after heating 3.5 min with a pasting temperature of 79.3 °C; starch can withstand heat up to 6.5 min before breaking down. The PV value of the DFPLA starch is found to be lower than that of banana and potato starch, but higher than that of rice and cassava starch (Kaur et al., 2011; Oladunmoye et al., 2014; Yadav et al., 2016); specifically, the PV order is potato (6179 cp) > banana (4577 cp) > DFPLA (4380 cp) > cassava (4372 cp) > rice (3380 cp). Some previous studies indicated that the PV value is affected by the total amylopectin content, where the higher amylopectin (lower amylose) content, the higher the PV (Sasaki et al., 2000; Gupta et al., 2009). The amylopectin content of the above mentioned starches is as follow: potato (83.12) > banana (81.67) > DFPLA (76.22) > cassava (71.80) < rice (79.55). Based on the above statement, the PV value of rice starch should be higher than that of DFPLA and cassava. The deviation and characteristic in pasting properties are affected by many factors such as amylopectin chain structure, lipid content, granule size, and protein content (Singh and Ali, 2008; Tsakama et al., 2010; Zhang et al., 2017). A study conducted by Kowittaya and Lumdubwong (2014) show that the PV value is affected by the structure of amylopectin, in which the higher the long-branched amylopectin content causes higher PV value (Kowittaya and Lumdubwong, 2014). Another study indicated that the molecular size of amylose related to that of starch viscosity (Obani and BeMiller, 1997; Elgadir et al., 2012).
Fig. 3.
Pasting profile of L. Aromatica starch, where μ is viscosity of starch in centipoises, T is temperature in °C and t is time in second.
3.5. Thermal analysis
The gelatinization temperature (To, Tp and Tc), enthalpy of gelatinization (ΔHgel) and Tc-To are mainly affected by the origin of starch, composition, moisture, solute, the presence of other biomaterials, processing and pretreatment conditions (Shyam, 2009). According to Krueger et al. (1987), high gelatinization temperatures (To, Tp, Tc) possibly due to the compact structure of starch granules and a higher degree of molecular order (Krueger et al., 1987). In general, starch is mainly composed of amorphous and crystalline phases of amylose and amylopectin. Starch gelatinization occurs in amorphous regions where hydrogen bonds weaken rapidly so that amylose is released, the amylose caused a decrease in the melting point of crystalline regions and energy of gelatinization (Kim et al., 1995).
High amylopectin starch is more stable in structure but more difficult to gelatinize due to the requirement of higher gelatinization starting energy. These phenomena will cause an increase in enthalpy and temperature of gelatinization (Barrichello et al., 1990). ΔH of gelatinization also affected by granular size; it will decrease with decreasing of granular size (Tang et al., 2000). The initial temperature of starch gelatinization was reported to correlate with the average branch chain length of starch (Jane et al., 1999). According to Tester and Morrison, DSC endotherm gives a measure of crystallinity quality (effectively double helix length) from Tp and overall crystallinity (quality and quantity) from ΔH (Tester and Morrision, 1992). Cooke and Gidley suggested that DSC endothermic enthalpy values primarily reflect the loss of double-helical order rather than loss of crystalline register (Cooke and Gidley, 1992). The degree of branching of amylopectin influences the Tc-To range, this temperature difference increases with the increase of the degree of branching (Biliaderis et al., 1980).
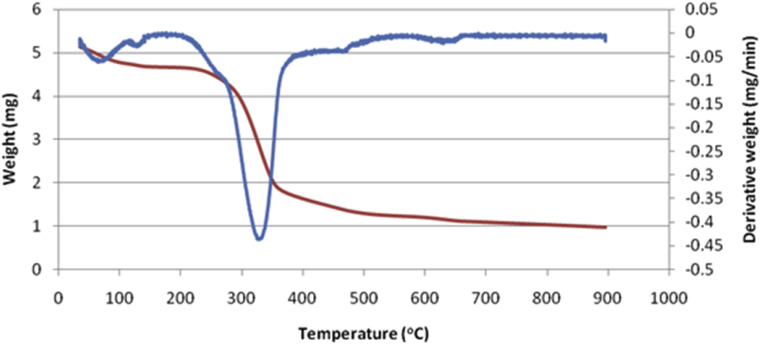
In this study, DSC was used to analyze the thermal properties of DFPLA starch. Gelatinization and retrogradation profile of the DFPLA starch were determined using starch with an original moisture content of 2.05 wt.% as well as starch mixed with DI water (1:4, w/w) and the results are shown in Table 3. The heat flow profile of gelatinization (Fig. 4) of the DFPLA starch has endothermic peaks at 59.3–81.2 °C with a peak temperature at 68.3 °C and corresponding gelatinization enthalpy of 3.7 J/g. At the onset temperature (To = 59.3 °C), the structural stability of the starch starts to be disrupted. The starch granules are swelled very rapidly in water environment at high temperature. As the temperature increases to the peak temperature (Tp = 68.3 °C), some of the crystallinity parts of the starch become amorphous due to the swelling of amylopectin region that causes dissociation and uncoils of its double helices (Stevens and Elton, 1971; Atwell et al., 1988). At this rate, the original appearance of the starch should not encounter a significant change. The apparent changes should occur at the completion temperature (Tc = 81.2 °C), in which the starch granules will interconnect to each other thus cause an increase in viscosity (Rincón-Londono et al., 2016). The Tp obtained for DFPLA is higher than that of rice starch (67.26 °C), but lower than that of corn starch (74.81 °C). The Tp is influenced by the amylose content in which higher amylose content will produce higher Tp and vice versa. The low amylose content signifies high amylopectin content; amylopectin has lower thermal stability due to its branched structure, in this case, starch which has low amylose content tend to have low Tp.
Table 3.
Thermal properties of L. Aromatica starch.
| Category | To (°C) | Tp (°C) | Tc (°C) | To-Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| Gelatinization | 59.3 | 68.3 | 81.2 | 21.8 | 3.7 |
| Retrogradation | 58.7 | 65.2 | 76.8 | 18.1 | 1.1 |
Fig. 4.
The TG/DTA curve of starch from L. Aromatica.
Retrogradation of starch was determined by keeping the gelatinized starch at refrigerator (4 °C) for 7 days, the results are shown in Table 2. The endothermic peaks of the DFPLA starch appeared between 58.7 and 76.8 °C with a retrogradation enthalpy of 1.1 J/g. Generally, the retrogradation temperatures and enthalpies are lower than those of the gelatinization (main peak). At a temperature range of 4–6 °C, the recrystallization of amylopectin will overcome the maximum rate of propagation and nucleation (Biliaderis et al., 1980). During that time, recombination of molecules will occur to create recrystallization in which hydrogen bonding between starch chains is weaker than before (Hoover, 2001). This is due to the change of the rheological properties (firmness), crystallinity, and water-holding ability (syneresis) of starch (Slade and Levine, 1987). The recrystallization of amylopectin branch chains in native starches had more ordered manner than stored starch gels (Ward et al., 1994).
Thermogravimetric (TG) curves obtained for DFPLA starch are presented in Fig. 4. The TG analysis began at a temperature of 34 °C. The first peak at a temperature range of 80–115 °C is indicated the evaporation of free moisture content of starch. Further increase in temperature causes the loss of water bond from the starch and some decomposition of some carbohydrate structure. The decomposition of cellulose, hemicellulose, and carbohydrate continued until the temperature reached 387 °C. At this stage, the loss of the sample was 26.81 %. As the heating continued, the decomposition of lignin and other dietary fiber continued, and almost 80% of the sample was lost at 800 °C. As seen in Fig. 3, a steep drop of the derivative curve occurred at 327.1 °C, this indicates that principal decomposition of starch occurred at this temperature.
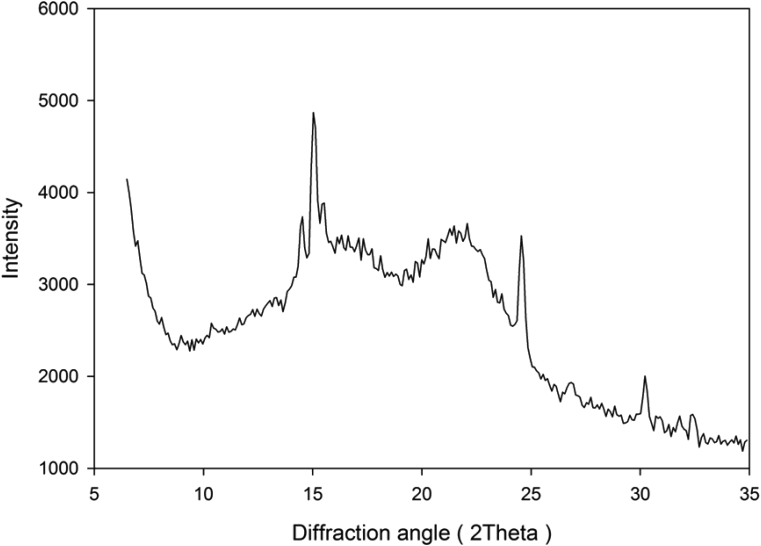
3.6. X-ray diffraction pattern
Fig. 5 depicts the X-ray diffractogram of DFPLA starch. In order to identify the crystalline type of starch, the diffraction pattern of DFPLA starch was compared with the diffraction pattern of other starches (Zobel, 1964, 1988; Cheetham and Tao, 1998). Different X-ray diffraction patterns represent a different type of starches. The starches of cereal usually have the A-type diffraction pattern, while for tuber and starches with a high amylose content usually possess B-type diffraction pattern. The starches of some fruit, roots, legume have C-type diffraction pattern. It is also believed, that C-type diffraction pattern is the combination of A-type and B-type starches (French, 1972; Banks and Greenwood, 1975; Sarko and Wu, 1978; Zobel, 1988).
Fig. 5.
X-ray diffraction spectrum of starch from L. Aromatica.
The X-ray diffraction pattern of DFPLA starch indicates that the amorphous structure more dominant than the crystalline structure. Several characteristic peaks of A-type starch and B-type starch were observed in the XRD pattern of DFPLA starch (2θ around 15°, 17°, 22° and 24°). The characteristic of A-type starch indicated by strong reflection at 2θ = 15° and a lower intensity peak at 17°, while the B-type starch indicated by peaks around 2θ = 22° and 24°. The presence of both A-type and B-type of starches in DFPLA starch indicates that the crystalline structure of the starch is C-type starch.
The ratio between the crystalline area in the XRD diffraction pattern and total area in the diffraction pattern determines the relative crystallinity of the starch (Nara and Komiya, 1983). Based on the result of TOPAS Version 4.2 XRD data analysis program, the relative crystallinity of the starch isolated from DFPLA was found to be 32.18 %. This relative crystallinity is within the range of natural starches, specifically 15–45% (Zobel, 1988).
3.7. Surface functional groups analysis
FTIR spectroscopy was used to affirm the surface functional group of the starch extracted from DFPLA. The selected functional groups are presented in Table 4, functional groups that should be present in ordinary starch were also observed in FTIR of DFPLA starch (Colussi et al., 2014; Liu et al., 2016). The starch from DFPLA has a broad FTIR peak at 3383 cm−1 which represent the stretching of O–H group. The peak at 2895 cm−1 was assigned to C–H stretching. The presence of water molecules associated with the starch is indicated by the peak at 1635 cm−1. Other organic functional groups also observed in DFPLA starch are at 1421 cm−1 and 1079 cm−1 which assigned to C–H bending and C–O stretching, respectively.
Table 4.
Selected FTIR functional group of L. aromatica starch.
| Wavenumber/cm−1 | Assignment |
|---|---|
| 3383 | O–H stretching |
| 2895 | C–H stretching |
| 1635 | O–H bending (H2O) |
| 1421 | C–H bending |
| 1079 | C–O stretching |
4. Conclusion
Starch was successfully isolated from the defatted and de-phenolics L. aromatica (DFPLA) by using the alkaline method. DFPLA starch contains 70.43% starch, 1.02% protein, 21.18% total dietary fiber (without resistant starch), 4.87% ash, 2.05% water, and others. Resistant starch content and amylose content of the DFPLA starch is 55.1% and 23.78 %, respectively. The shape of starch granules is a mixture of granular, oval, and discoid with sizes of approximately 3–6 μm. Given the physicochemical properties, the medium-amylose starch from DFPLA can be used to partially substitute the potato or rice or corn starches which have similar amylose content and features. DFPLA starch has a quite high viscosity and potential to be an adhesive. L. aromatica can be potential biomass to reduce the use of staple foods as starch producing biomass.
Declarations
Author contribution statement
Suryadi Ismadji: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Cynthia Wijaya: Performed the experiments; Wrote the paper.
Quy Diem Do: Performed the experiments.
Yi-Hsu Ju: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Shella P. Santoso: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jindra Nyoo, Livy Laysandra: Analyzed and interpreted the data; Wrote the paper.
Felycia Edi-Soetaredjo: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express their sincere gratitude to the Department of Chemical Engineering at National Taiwan University of Science and Technology, Taiwan, for undergraduate internship grant and providing all the research facilities.
Contributor Information
Yi Hsu Ju, Email: yhju@mail.ntust.edu.tw.
Suryadi Ismadji, Email: suryadiismadji@yahoo.com.
References
- Atwell W.A., Hood L., Lineback D., Varriano-Morston E., Zobel H. The terminology and methodology associated with basic starch phenomena. Cereal Food World. 1988;33:306–311. [Google Scholar]
- Banks W., Greenwood C.T. Edinburgh University Press; Edinburg, England: 1975. Starch and its Components. [Google Scholar]
- Barrichello V., Yada R.Y., Coffin R.H., Stanley D.W. Low-temperature sweetening in susceptible and resistant potatoes: starch structure and composition. J. Food Sci. 1990;55:1054–1059. [Google Scholar]
- Biliaderis C.G., Maurice T.J., Vose J.R. Starch gelatinization phenomena studied by differential scanning calorimetry. J. Food Sci. 1980;45:1669–1674. [Google Scholar]
- Bui M.L., Grayer R.J., Veitch N.C., Kite G.C., Tran H., Nguyen Q.C.K. Uncommon 8-oxygenated flavonoids from Limnophila aromatica (Scrophulariaceae) Biochem. Syst. Ecol. 2004;32:943–947. [Google Scholar]
- Cheetham N.W.H., Tao L. Variation in crystalline type with amylose content in maize starch granules: X-ray powder diffraction study. Carbohydr. Polym. 1998;36:277–284. [Google Scholar]
- Colussi R., Pinto V.Z., Halal S.L.M.E., Vanier N.L., Villanova F.A., Silva RMe, Zavareze EdR., Dias A.R.G. Structural, morphological, and physicochemical properties of acetylated high-, medium-, and low-amylose rice starches. Carbohydr. Polym. 2014;103:405–413. doi: 10.1016/j.carbpol.2013.12.070. [DOI] [PubMed] [Google Scholar]
- Cooke D., Gidley M.J. Loss of crystalline and molecular order during starch gelatinization: the origin of the enthalpy transition. Carbohydr. Res. 1992;227:103–112. [Google Scholar]
- Dennett A.L., Schofield P.R., Roake J.E., Howes N.K., Chin J. Starch swelling power and amylose content of triticale and Triticum timopheevii germplasm. J. Cereal Sci. 2009;49:393–397. [Google Scholar]
- Do Q.D., Angkawijaya A.E., Tran-Nguyen P.L., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgadir M.A., Akanda M.J.H., Ferdosh S., Mehrnoush A., Karim A.A., Noda T., Sarker M.Z.I. Mixed biopolymer systems based on starch. Molecules. 2012;17:584–597. doi: 10.3390/molecules17010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French D. Fine structure of starch and its relationship to the organization of starch granules. J. Jpn. Soc. Starch Sci. 1972;19:8–25. [Google Scholar]
- Gorai D., Jash S.K., Singh R.K. Chemical and pharmacological aspects of Limnophila heterophylla (Scrophulariaceae): an overview. Int. J. Pharm. Sci. Rev. Res. 2014;25:100–102. [Google Scholar]
- Gupta M., Bawa A.S., Semwal A.D. Morphological, thermal, pasting, and rheological properties of barley starch and their blends. Int. J. Food Prop. 2009;12:587–604. [Google Scholar]
- Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr. Polym. 2001;45:253–267. [Google Scholar]
- Jane J., Chen Y.Y., Lee L.F., McPherson A.E., Wong K.S., Radosavljevic M., Kasemsuwan T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch 1. Cereal Chem. 1999;76:629–637. [Google Scholar]
- Kaur M., Oberoi D.P.S., Sogi D.S., Gill B.S. Physicochemical, morphological and pasting properties of acid treated starches from different botanical sources. J. Food Sci. Technol. 2011;48:460–465. doi: 10.1007/s13197-010-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.S., Wiesenborn D.P., Orr P.H., Grant L.A. Screening potato starch for novel properties using differential scanning calorimetry. J. Food Sci. 1995;60:1060–1065. [Google Scholar]
- Kowittaya C., Lumdubwong N. Molecular weight, chain profile of rice amylopectin and starch pasting properties. Carbohydr. Polym. 2014;108:216–223. doi: 10.1016/j.carbpol.2014.02.081. [DOI] [PubMed] [Google Scholar]
- Krueger B.R., Knutson C.A., Inglett G.E., Walker C.E. A differential scanning calorimetry study on the effect of annealing on gelatinization behavior of cornstarch. J. Food Sci. 1987;52:715–718. [Google Scholar]
- Liu Y., Xie H., Shi M. Effect of ethanol–water solution on the crystallization of short chain amylose from potato starch. Starch Stärke. 2016;68:1–8. [Google Scholar]
- Nara S., Komiya T. Studies on the relationship between water-saturated state and crystallinity by the diffraction method for moistened potato starch. Starch Stärke. 1983;35:407–410. [Google Scholar]
- Obani M., BeMiller J.N. Properties of some starch blends. Cereal Chem. 1997;74:431–436. [Google Scholar]
- Oladunmoye O.O., Aworh O.C., Maziya-Dixon B., Erukainure O.L., Elemo G.N. Chemical and functional properties of cassava starch, durum wheat semolina flour, and their blends. Food Sci. Nutr. 2014;2:132–138. doi: 10.1002/fsn3.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando T.E. Vol. 9. Rapra Technology Ltd.; 1998. (Solvent-Free Adhesives). Rapra Review Reports. [Google Scholar]
- Reddy C.K., Pramila S., Haripriya S. Pasting, textural and thermal properties of resistant starch prepared from potato (Solanum tuberosum) starch using pullulanase enzyme. J. Food Sci. Technol. 2015;52:1594–1601. doi: 10.1007/s13197-013-1151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P.H., Jeffcoat R., Shi Y.C. High-amylose starches: from biosynthesis to their use as food ingredients. MRS Bull. 2000 December 2000. [Google Scholar]
- Rincón-Londono N., Vega-Rojas L.J., Contreras-Padilla M., Acosta-Osorio A.A., Rodríguez-García M.E. Analysis of the pasting profile in corn starch: structural, morphological, and thermal transformations, Part I. Int. J. Biol. Macromol. 2016;91:106–114. doi: 10.1016/j.ijbiomac.2016.05.070. [DOI] [PubMed] [Google Scholar]
- Rodrigues A., Emeje M. Recent applications of starch derivatives in nanodrug delivery. Carbohydr. Polym. 2012;87:987–994. [Google Scholar]
- Sarko A., Wu H.C.H. The crystal structures of A-, B-, and C-polymorphs of amylose and starch. Starch Stärke. 1978;30:73–78. [Google Scholar]
- Sasaki T., Yasui T., Matsuki Effect of amylose content on gelatinization, retrogradation and pasting properties of starches from waxy and non-waxy wheat and their F1 seeds. Cereal Chem. 2000;77:58–63. [Google Scholar]
- Shyam S. second ed. Food Properties Handbook CRC Press; 2009. Gelatinization of Starch; pp. 8–27. [Google Scholar]
- Singh N., Singh J., Kaur L., Sodhi N.S., Gill B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81:219–231. [Google Scholar]
- Singh V., Ali S.Z. Properties of starches modified by different acids. Int. J. Food Prop. 2008;11:495–507. [Google Scholar]
- Slade H., Levine L. Gordon and Breach Science; New York: 1987. Recent Advances in Starch Retrogradation. Industrial Polysaccharides; pp. 387–430. [Google Scholar]
- Stevens D.J., Elton G.A.H. Thermal properties of the starch/water system. Starch Stärke. 1971;23:8–11. [Google Scholar]
- Tang H., Ando H., Watanabe K., Takeda Y., Mitsunaga T. Some physicochemical properties of small-, medium-, and large-granule starches in fractions of waxy barley grain. Cereal Chem. 2000;77:27–31. [Google Scholar]
- Tester R.F., Morrison W.R. Swelling and gelatinization of cereal starches. III. Some properties of waxy and normal nonwaxy barley starches. Cereal Chem. 1992;69:654–658. [Google Scholar]
- Trowell H.C., Southgate D.A.T., Wolever T.M.S., Leeds A.R., Gassull M.A., Jenkins D.J.A. Dietary fiber redefined. Lancet. 1976;1:967. doi: 10.1016/s0140-6736(76)92750-1. [DOI] [PubMed] [Google Scholar]
- Tsakama M., Mwangwela A.M., Manani T.A., Mahungu N.M. Physicochemical and pasting properties of starch extracted from eleven sweetpotato varieties. Afr. J. Food Sci. Technol. 2010;1:90–98. [Google Scholar]
- Vegh K.R. Cultivated Plants, Primarily as Food Sources. Vol. 1. 2009. Starch bearing crops as food sources. (Encyclopedia of Life Support Systems). [Google Scholar]
- Wang L., Wang Y.J. Comparison of protease digestion at neutral pH with alkaline steeping method for Rice starch isolation. Cereal Chem. 2001;78:690–692. [Google Scholar]
- Ward K.E.J., Hoseney R.C., Seib P.A. Retrogradation of amylopectin from maize and wheat starches. Cereal Chem. 1994;71:150–155. [Google Scholar]
- Yadav R.B., Kumar N., Yadav B.S. Characterization of banana, potato, and rice starch blends for their physicochemical and pasting properties. Food Sci. Technol. 2016;2 112787-112783. [Google Scholar]
- Yu S., Liu J., Yang Y., Ren J., Zheng X., Kopparapu N.K. Effects of amylose content on the physicochemical properties of Chinese chestnut starch. Starch Stärke. 2016;68:112–118. [Google Scholar]
- Yuliana M., Huynh L.H., Ho Q.P., Truong C.T., Ju Y.H. Defatted cashew nut shell starch as renewable polymeric material: isolation and characterization. Carbohydr. Polym. 2012;87:2576–2581. [Google Scholar]
- Zeeman S.C., Tiessen A., Pilling E., Kato K.L., Donald A.M., Smith A.M. Starch synthesis in Arabidopsis. Granule synthesis, composition, and structure. Plant Physiol. 2002;129:516–529. doi: 10.1104/pp.003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Xu F., Wu G., Tan L. Molecular structure of starch isolated from jackfruit and its relationship with physicochemical properties. Sci. Rep. 2017;7:13423. doi: 10.1038/s41598-017-13435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel H.F. Starch Academic Press; New York: 1964. Methods in Carbohydrate Chemistry; pp. 109–113. [Google Scholar]
- Zobel H.F. Molecules to granules: a comprehensive starch review. Starch Stärke. 1988;40:1–7. [Google Scholar]