Abstract
Objectives:
To assess the potential of predicting adult facial types at different stages of mandibular development.
Setting and Sample Population:
941 participants from the Bolton-Brush, Denver, Fels, Iowa, Michigan, and Oregon growth studies with longitudinal lateral cephalograms (total of 7,166) between ages 6–21 years.
Material & Methods:
Each participant was placed into one of three facial types based on mandibular plane angle (MPA) from cephalograms taken closest to 18 years of age (range of 15–21 years): hypo-divergent (MPA<28°), normo-divergent (28°≤ MPA ≤39°), and hyper-divergent (MPA>39°). Cephalograms were categorized into 13 age groups 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18–21. Twenty-three two-dimensional anatomical landmarks were digitized on the mandible and superimposed using generalized Procrustes analysis, which projects landmarks into a common shape space. Data were analyzed within age categories using stepwise discriminant analysis to identify landmarks that distinguish adult facial types and by jackknife cross-validation to test how well young individuals can be re-classified into their adult facial types.
Results:
Although each category has multiple best discriminating landmarks among adult types, three landmarks were common across nearly all age categories: menton, gonion, and articulare. Individuals were correctly classified better than chance, even among the youngest age category. Cross-validation rates improved with age, and hyper- and hypo-divergent groups have better reclassification rates than the normo-divergent group.
Conclusions:
The discovery of important indicators of adult facial type in the developing mandible helps improve our capacity to predict adult facial types at a younger age.
Keywords: Mandibular shape, Geometric morphometrics, Facial type, Longitudinal growth
INTRODUCTION
Characterizing craniofacial growth has long been an important topic in biomedical research for both the clinician and the basic researcher. Various longitudinal growth studies have captured invaluable information on craniofacial growth in the form of sequential lateral cephalometric radiographs. The static and dynamic morphology of the face has been investigated extensively, and many approaches have been proposed to establish a relationship between the developing morphology and the final facial form (1–3).
Conventional cephalometric methods have been used for decades to assess craniofacial form and development (4–6), yet they produce only partial and localized descriptions of shape that may be biased by the reference structures used to align subjects (7).
In recent years, there has been an effort to supplement conventional cephalometric analysis with a variety of sophisticated morphometric methods incorporating mathematical and statistical analyses (7–10). Although there is no universal agreement between mathematicians, statisticians, researchers, and clinicians as to the most appropriate method for analyzing cephalograms, it is recognized that the erudite geometric morphometric techniques are a robust set of methods for deriving detailed and nuanced shape information from cephalograms (11).
Geometric morphometrics (GM) was developed and traditionally applied in the field of biology to analyze the geometric shape of biological structures between species (12). Since visualizing shape differences is important in understanding morphological variation, several researchers introduced GM to orthodontics (10, 12–15). Knowledge of mandibular growth is particularly critical to understanding facial growth, and therefore, to develop a treatment plan across all facial types (16–18). However, relating variation in mandibular shape to facial types throughout development requires a large longitudinal dataset with adequate representation of all facial types that was not previously available.
This study uses GM methods to identify mandibular landmarks that distinguish among adult facial types at different ages and to determine how adult facial type is reflected in the morphology of the developing mandible.
MATERIAL AND METHODS
This study was approved by the Institutional Review Board of the University of the Pacific (#15–83).
Data for the current study came from Craniofacial Growth Consortium Study (CGCS), which consists of a large longitudinal sample of normal healthy individuals participating in one of several longitudinal growth studies that include: the Fels Longitudinal Study, the Michigan Growth Study and others available from the American Association of Orthodontists Foundation Craniofacial Growth Legacy Collection (www.aaoflegacycollection.org) (Table 1). Landmark coordinates were collected for a large set of standard cephalometric points from each lateral cephalogram using the program eDigit, which was developed by Craniofacial Research Instrumentation Laboratory. Cephalometric landmarks were digitized independently by three calibrated judges. Outliers were excluded based on the established landmark-specific envelopes of error (19). The average values were recorded in a numerical database and used for further analysis. Radiographic enlargement was corrected using the specific correction factors established for each growth collection. For the present study, 23 two-dimensional landmarks on the mandible were collected to provide a comprehensive description of the morphology of the mandible (Table 2).
Table 1.
Sample size by growth collection
| Growth study | Males | Females | Total |
|---|---|---|---|
| Bolton Brush | 102 | 93 | 195 |
| Denver | 47 | 44 | 91 |
| Fels | 202 | 177 | 379 |
| Iowa | 16 | 29 | 45 |
| Michigan | 85 | 72 | 157 |
| Oregon | 32 | 42 | 74 |
| Total | 484 | 457 | 941 |
Table 2.
Definition of landmarks
| Number | Landmark | Description |
|---|---|---|
| 1 | LI edge | Tip of the incisal edge of the more anteriorly placed lower central incisor |
| 2 | Point B | Deepest point on the curvature of the anterior border of the mandible between pogonion and the alveolar crest of the lower central incisor |
| 3 | L1 apex | Point of intersection between the long axis of the most anteriorly positioned lower incisor and the contour of that tooth's root-end curvature |
| 4 | Pogonion | Anterior-most point of the bony chin at the midline |
| 5 | Menton | Inferior-most point on the mandible at the symphysis |
| 6 | Condyle | Point on the posterior-superior contour of the condyle that is the longest distance from pogonion |
| 7 | L6 MCP | Mesial contact point of the more anterior lower first molar |
| 8 | Post. ramus | Posterior ramus point where the inflection starts |
| 9 | Ant. articulare | Intersection between the basisphenoid synchondrosis and the anterior border of the neck of the condyle |
| 10 | Coronoid | Tip of coronoid process |
| 11 | Ant. ramus | Deepest point in the anterior ramus |
| 12 | Infradentale | Most superior and anterior point of the mandibular alveolar process |
| 13 | Lingual L1 | Most superior and anterior point of alveolar bone on the lingual surface |
| 14 | L Point B | Intersecting point of a posterior extension of point B parallel to the mandibular plane connecting gonion and menton on the lingual cortical plate of the symphysis |
| 15 | Pro-menti | Protuberance menti (suprapogonion): Point where the shape of the symphysis mentalis changes from convex to concave |
| 16 | L symph | Intersecting point of a posterior extension of pogonion parallel to the mandibular plane connecting gonion and menton on the lingual cortical plate of the symphysis |
| 17 | Gnathion | Point located by taking the midpoint between pogonion and menton of the bony chin |
| 18 | M Border | The most convex point of the mandibular lower border between menton and antegonial notch |
| 19 | Antegonion | Deepest point of the antergonial notch |
| 20 | Funct. Occlusal Post. | Posterior point of functional occlusal plane of the last molar |
| 21 | Funct. Occlusal Ant. | Anterior point of functional occlusal plane of the first premolar region |
| 22 | Articulare | Intersection between the basisphenoid synchondrosis and the posterior border of the neck of the condyle |
| 23 | Gonion | Lowest point of the curvature of the angle of the mandible where the inferior surface of the body of the mandible meets the ramus (averaged from both sides) |
Adult facial type was determined by the mandibular plane angle (MPA) for each individual based on the film closest to 18 years and within the range of 15–21 years of age. This age range allowed us to determine facial types that best represent the adult morphology while maximizing the available sample. MPA was calculated as the angle at the intersection of the mandibular plane (GO-Me) and Sella-Nasion (SN). Three different facial types were classified: hypo-divergent (MPA < 28°), norm-divergent (28° ≤ MPA ≤ 39°), and hyper-divergent (MPA > 39°) (Fig. 1).
Figure 1:
Lateral cephalograms demonstrating examples of hypo-divergent (a), normo-divergent (b), and hyper-divergent (c) facial types
Cephalograms with complete mandibular landmark sets were categorized into 13 age groups: 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18–21 yrs. If more than one cephalogram was present for an individual in a particular age group, the film recorded at a younger age was chosen for the analysis. Individuals were not required to have a film in each category to be included in the analysis. After removing films with missing data and duplicate individuals within each age group, the final sample consisted of 7,166 lateral cephalograms from 941 subjects (Table 3). Twelve subjects had cephalograms for all 13 age groups (7 males and 5 females) and 729 subjects had at least 6 radiographs (367 males and 362 females).
Table 3.
Number of images for each facial type per age category
| Age Category (years) | Hypo-divergent | Normo-divergent | Hyper- divergent | Total (n=941) | |||
|---|---|---|---|---|---|---|---|
| M (n=155) | F (n=101) | M (n=286) | F (n=298) | M (n=43) | F (n=58) | ||
| 6 | 88 | 62 | 143 | 165 | 21 | 28 | 507 |
| 7 | 90 | 56 | 153 | 160 | 22 | 31 | 512 |
| 8 | 97 | 61 | 153 | 167 | 20 | 34 | 532 |
| 9 | 99 | 66 | 163 | 177 | 27 | 39 | 571 |
| 10 | 97 | 70 | 161 | 169 | 21 | 38 | 556 |
| 11 | 97 | 61 | 166 | 173 | 24 | 35 | 556 |
| 12 | 99 | 63 | 162 | 199 | 29 | 43 | 595 |
| 13 | 99 | 65 | 202 | 201 | 29 | 37 | 633 |
| 14 | 101 | 63 | 172 | 197 | 33 | 35 | 601 |
| 15 | 103 | 57 | 200 | 190 | 26 | 38 | 614 |
| 16 | 103 | 59 | 169 | 195 | 28 | 31 | 585 |
| 17 | 78 | 58 | 119 | 157 | 21 | 25 | 458 |
| 18 | 76 | 52 | 134 | 140 | 20 | 24 | 446 |
| Total images | 1227 | 793 | 2097 | 2290 | 321 | 438 | 7166 |
The landmark configurations were superimposed separately for each age-sex group using a generalized Procrustes analysis, which removes variation attributed to scale, location, and orientation; any remaining variation among superimposed configurations was attributed to differences in shape (20, 21). The subsequent analyses had two main goals: (1) identify distinguishing landmarks among adult facial types within each age group and (2) using these landmarks to determine how morphological variation in the developing mandible reflects adult facial type. Analyses were performed separately for males and females. All statistical analyses were generated using SAS/STAT software, Version 9.4 of the SAS System for Windows (Copyright © 2018 SAS Institute Inc).
To address the first goal, a stepwise discriminant analysis was performed to identify those landmarks most useful for distinguishing adult facial types within each age group. At each step, the landmark coordinates were the dependent variables under consideration for the model with the adult facial types acting as the class levels. The significance level of an F-test from an analysis of covariance (ANCOVA) was used as the selection criterion for variables to enter or remain in the model. Variables already chosen from previous steps acted as covariates in subsequent ANCOVAs to determine if new variables provided a class distinction significant enough to be included in the model. When one of the two coordinates representing a single landmark was found to differentiate facial types, both coordinates for the landmark were included in the final set. Ultimately, a set of landmarks was identified that best differentiates adult facial types within each age group for males and females.
Second, canonical variates analysis (CVA) was performed within each age-sex group on the set of mandibular landmarks selected by the stepwise discriminant analysis, using adult facial type as the class or categorical variable. Jackknife cross-validation was employed to assess how robust adult facial type differences were by using a leave-one-out reclassification design. Each individual within an age-sex group was reclassified to an adult facial type based on mandibular shape using Mahalanobis distance as the metric for classification. A correct reclassification indicates that an individual was reclassified into the previously designated adult facial type.
RESULTS
Stepwise discriminant analysis (DA)
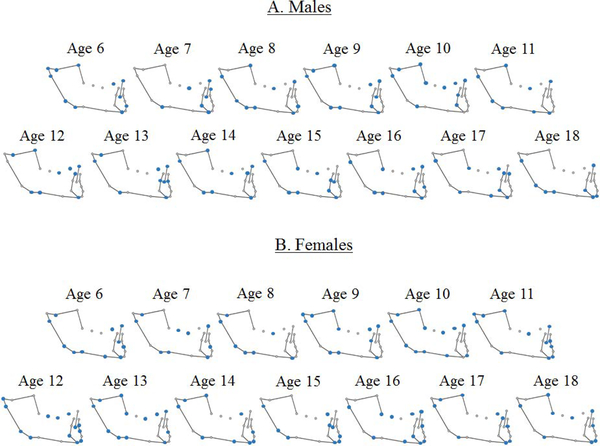
Results from stepwise discriminant analyses identified landmarks that best differentiate adult facial types within each age group for both males and females (Fig. 2). Some landmarks were consistently selected for the model across age groups and sexes. In particular, gonion, menton, and articulare commonly appeared as informative landmarks in both sexes: menton was not identified in only one female age group; articulare was not identified in two male age groups; gonion was selected for all age groups. Additionally, the tip of the incisor was identified in almost all male age groups. These informative landmarks represent anatomical regions that differ by adult facial type throughout development.
Figure 2:
Landmarks selected (in blue) by the stepwise discriminant analysis in each age group for males (A) and females (B).
The number of landmarks selected for the model in each age group varied from 7 to 13. A greater number of selected landmarks does not indicate that adult facial types are more or less morphologically distinct in a given age group, but instead reflects the anatomical differences that distinguish among adult facial types at that age, based on the criteria of the stepwise discriminant analysis. Shape variation within age groups that do not distinguish facial types would not be selected for the model.
Canonical variates analysis (CVA)
Fig 3 shows a percent correct reclassification plot by age group for males and females.
Figure 3:
Percent correct reclassification plot by age group for males (A) and females (B)
For males, the percentage of correct reclassifications ranged from approximately 47% to 88% (Table 4, Fig. 3A). All three facial types had higher reclassification rates in the oldest age group than the youngest age group. However, each facial type exhibited fluctuations from year to year, with the largest changes occurring at older ages in the hyper-divergent facial type.
Table 4:
Reclassification rates for jackknife cross-validation for each age group in males and females
| A. Male | ||||
|---|---|---|---|---|
| Age Category (years) | Reclassification | Hypo divergent | Normo- divergent | Hyper divergent |
| 6 years | # correct | 69 | 80 | 15 |
| Total | 88 | 143 | 21 | |
| % correct | 78.4% | 55.9% | 71.4% | |
| 7 years | # correct | 71 | 73 | 16 |
| Total | 90 | 153 | 22 | |
| % correct | 78.9% | 47.7% | 72.7% | |
| 8 years | # correct | 70 | 92 | 15 |
| Total | 97 | 153 | 20 | |
| % correct | 72.2% | 60.1% | 75.0% | |
| 9 years | # correct | 77 | 95 | 19 |
| Total | 99 | 163 | 27 | |
| % correct | 77.8% | 58.3% | 70.4% | |
| 10 years | # correct | 74 | 97 | 13 |
| Total | 97 | 161 | 21 | |
| % correct | 76.3% | 60.2% | 61.9% | |
| 11 years | # correct | 72 | 107 | 16 |
| Total | 97 | 166 | 24 | |
| % correct | 74.2% | 64.5% | 66.7% | |
| 12 years | # correct | 73 | 109 | 20 |
| Total | 99 | 162 | 29 | |
| % correct | 73.7% | 67.3% | 69.0% | |
| 13 years | # correct | 83 | 137 | 20 |
| Total | 99 | 202 | 29 | |
| % correct | 83.8% | 67.8% | 69.0% | |
| 14 years | # correct | 83 | 116 | 28 |
| Total | 101 | 172 | 33 | |
| % correct | 82.2% | 67.4% | 84.8% | |
| 15 years | # correct | 90 | 133 | 19 |
| Total | 103 | 200 | 26 | |
| % correct | 87.4% | 66.5% | 73.1% | |
| 16 years | # correct | 89 | 113 | 17 |
| Total | 103 | 169 | 28 | |
| % correct | 86.4% | 66.9% | 60.7% | |
| 17 years | # correct | 66 | 87 | 15 |
| Total | 78 | 119 | 21 | |
| % correct | 84.6% | 73.1% | 71.4% | |
| 18 years | # correct | 67 | 99 | 17 |
| Total | 76 | 134 | 20 | |
| % correct | 88.2% | 73.9% | 85.0% | |
| B. Female | ||||
| Age Category (years) | Reclassification | Hypo divergent | Normo- divergent | Hyper divergent |
| 6 years | # correct | 43 | 86 | 17 |
| Total | 62 | 165 | 28 | |
| % correct | 69.4% | 52.1% | 60.7% | |
| 7 years | # correct | 38 | 81 | 19 |
| Total | 56 | 160 | 31 | |
| % correct | 67.9% | 50.6% | 61.3% | |
| 8 years | # correct | 51 | 91 | 26 |
| Total | 61 | 167 | 34 | |
| % correct | 83.6% | 54.5% | 76.5% | |
| 9 years | # correct | 53 | 84 | 29 |
| Total | 66 | 177 | 39 | |
| % correct | 80.3% | 47.5% | 74.4% | |
| 10 years | # correct | 52 | 106 | 30 |
| Total | 70 | 169 | 38 | |
| % correct | 74.3% | 62.7% | 78.9% | |
| 11 years | # correct | 48 | 103 | 28 |
| Total | 61 | 173 | 35 | |
| % correct | 78.7% | 59.5% | 80.0% | |
| 12 years | # correct | 46 | 132 | 31 |
| Total | 63 | 199 | 43 | |
| % correct | 73.0% | 66.3% | 72.1% | |
| 13 years | # correct | 51 | 129 | 29 |
| Total | 65 | 201 | 37 | |
| % correct | 78.5% | 64.2% | 78.4% | |
| 14 years | # correct | 50 | 132 | 25 |
| Total | 63 | 197 | 35 | |
| % correct | 79.4% | 67.0% | 71.4% | |
| 15 years | # correct | 48 | 120 | 30 |
| Total | 57 | 190 | 38 | |
| % correct | 84.2% | 63.2% | 78.9% | |
| 16 years | # correct | 50 | 130 | 25 |
| Total | 59 | 195 | 31 | |
| % correct | 84.7% | 66.7% | 80.6% | |
| 17 years | # correct | 50 | 113 | 21 |
| Total | 58 | 157 | 25 | |
| % correct | 86.2% | 72.0% | 84.0% | |
| 18 years | # correct | 42 | 96 | 19 |
| Total | 52 | 140 | 24 | |
| % correct | 80.8% | 68.6% | 79.2% | |
#, number of images
For females, the percentage of correct reclassifications of each individual to the adult facial type ranged from approximately 47% to 86% (Table 4, Fig. 3B). Similar to males, all three facial types in females had a higher reclassification rate in the oldest age group (18–21 years) compared to the youngest age group (6 years). In addition, all female facial types exhibited a decrease in reclassification rate between the 17 and 18–21year old age groups. In females, correct reclassification rates into the adult normo-divergent type were the lowest of the three facial types. Hyper-divergent reclassification rates were, overall, slightly below those of the hypo-divergent facial type except between the ages of 10–13.
Generally, correct reclassification rates into adult hyper- and hypo-divergent types were higher compared to the normo-divergent facial type, with hyper-divergent reclassification rates often between hypo- and normo-divergent rates.
DISCUSSION
Craniofacial biologists, including orthodontists, have often sought to elucidate variations in size and shape of the face through univariate analyses, but they have also used multivariate techniques to understand the relationships between anatomical components or measurements of the face (11). Conventional cephalometric measurement methods continue to be used to investigate craniofacial growth and serve as the primary clinical method of assessing craniofacial form. This study extends current cephalometric approaches by exploring the link between mandibular shapes at different ages, quantified using geometric morphometric methods, with adult facial type characterized by mandibular plane angle.
Mandibular plane angle is a common, clinically-informative cephalometric measure of craniofacial shape (22) but it is susceptible to other sources of variation not reflective of morphology (e.g., steepness of cranial base (SN), open mouth, or head rotation etc.). In addition, individuals who have a MPA close to a boundary of categorization (28° or 39°) may appear to be shifting from one facial type to another when MPA is solely used for classifying facial type at each age. Therefore, a multivariate approach to quantifying mandibular shape was used in the present study to reduce the effects of other sources of variation and to evaluate which aspects of mandibular morphology contribute most to differentiating adult facial types in the developing mandible.
Geometric morphometrics were used to characterize mandibular shape which allowed for multivariate statistical analyses to be performed directly on landmark coordinates while retaining the spatial relationships of landmarks within each configuration. Through Procrustes superimposition, differences in location, rotation, and scale of the landmark configurations were removed so that only variation in shape remained. This was necessary in the context of facial type, as characterized by mandibular plane angle, since types are inherently descriptions of anatomical shape. This morphometric approach is well equipped for characterizing mandibular shape variation among adult facial types and identifying anatomical regions that contribute most to their separation.
Results of these analyses indicate that articulare, gonion, and menton are consistently identified as distinguishing adult facial types at every age in both males and females. Additionally, the tip of the incisor is an important discriminating landmark in almost all male age groups, but in just over half of the female age groups. This may indicate that gonial angle and dentoalveolar height at the symphysis region (menton-lower incisor tip) are important predictors for identifying different facial types as early as 6years of age. The anatomical regions represented by these landmarks suggest that variation in the overall geometry of the mandible reflects differences in adult facial types across all ages while other, more nuanced, aspects of mandibular shape variation are variably useful, depending on age and sex.
Correct reclassification rates to each adult facial type generally increased by age group for both males and females. In females, individuals with hyper- and hypo-divergent facial types as adults were consistently classified into their adult facial types with greater than 70% accuracy from age 8 and onward. A similar rate of correct reclassification to the adult hypo-divergent type was demonstrated in males across all ages. Males exhibited greater fluctuation in correct reclassification rate to the adult hyper-divergent type, potentially due to smaller sample sizes, but never dropped below 60%. While correct reclassification rates to the adult normo-divergent type were lower in both sexes compared to other facial types, correct reclassification rates to this type were maintained above 60% by age 10 and older in both males and females.
These reclassification rates based on mandibular morphology are higher for the hypo-divergent facial type than would be expected for reclassification using only MPA at these ages. MPA tends to decrease with age and hypo-divergent individuals tend to have the greatest reduction in MPA among the three facial types, which leads to much lower accuracy in reclassifying adult facial type at younger ages using MPA (23).
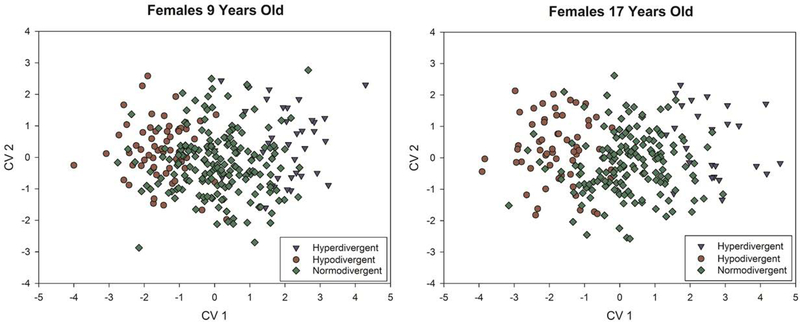
In the present study, normo-divergent reclassification rates were lower than that of the two other facial types. This is likely a result of the intermediate morphology of normo-divergent individuals that falls between the hyper- and hypo-divergent groups, allowing them to be misclassified into multiple groups (Fig. 4).
Figure 4:
Plots of the first two canonical variates axes for females at 9 years and 17 years to demonstrate the degree of overlap among facial types. These plots demonstrate examples of ages with lower (9 years) and higher (17 years) reclassification success.
The present study investigated the potential of predicting adult facial type at different stages of mandibular morphology by using GM to evaluate mandibular shape changes during growth and whether these changes are related to the initial mandibular shape pattern. A priori adult facial type classification was based solely on the measure of mandibular plane angle. However, reclassification of each individual through CVA, with jackknife crossvalidation, is based on the set of mandibular landmarks selected by the stepwise discriminant analysis to determine how well overall mandibular shape distinguishes facial types. Overall, the results from this analysis suggests that adult facial type can be determined reasonably well from mandibular shape at younger ages with increasing accuracy as individuals get older.
The integration of traditional cephalometric approaches used in clinical settings with more sophisticated morphometric techniques (e.g., geometric morphometrics) provides a way to maximize the anatomical information that can be extracted from a set of standard cephalometric points and can be used in a clinically-relevant context. Future studies include exploring ontogenetic trajectories of mandibular shape within each facial type for making growth predictions and evaluating changes in facial type within subjects over time. The results from this analysis provide a foundation for future work with the ultimate goal of providing accurate predictive assessments of craniofacial growth that are useful to clinicians in treatment planning.
CONCLUSIONS
Individuals were correctly reclassified in their adult facial types with greater than 60% success rate from age 10 and older
Landmarks important for distinguishing adult facial type at almost all age-sex groups were articulare, menton, and gonion
In general, correct reclassification rates to adult hyper- and hypo-divergent types were higher compared to the adult normo-divergent type
The discovery of important indicators of adult facial type in the developing mandibles can help improve our capacity to diagnose and treat individuals at a younger age
Geometric morphometric methods show promise in clinical evaluation of different facial types and quantification of the changes in size and shape that occur during mandibular growth
ACKNOWLEDGMENTS:
We are grateful to the AAOF Craniofacial Growth Legacy Collection. Research reported in this publication was supported by the National Institute of Dental & Craniofacial Research of the National Institutes of Health under Award Number R01DE024732. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Bahita SN, Wright GW, Leighton BC, A proposed multivariate model for prediction of facial growth. Am J Orthod 1979;75:264–281 [DOI] [PubMed] [Google Scholar]
- 2.Hixon EH: Prediction of facial growth. Trans. Eur. Orthod. Sot, pp 127–139, 1968. [PubMed] [Google Scholar]
- 3.Ricketts RM: Planning treatment on the basis of the facial pattern and estimate of its growth, Angle Orthod 1957;27: 14–37. [Google Scholar]
- 4.Downs WB. Analysis of the dentofacial profile. Angle Orthod 1956; 26: 191–212 [Google Scholar]
- 5.Steiner CC. The use of cephalometrics as an aid to planning and assessing orthodontic treatment. Am J Orthod 1960;46: 721–735. [Google Scholar]
- 6.McNamara JA. A method of cephalometric evaluation. Am J Orthod 1984;86: 449–469. [DOI] [PubMed] [Google Scholar]
- 7.Halazonetis DJ. Morphometrics for cephalometric diagnosis. Am J Orthod Dentofac Orthop 2004;125(5):571–81. [DOI] [PubMed] [Google Scholar]
- 8.Singh GD, McNamara JA, Lozanoff S. Finite-element morphometry of soft tissue morphology in subjects with untreated Class III malocclusions. Angle Orthod 1999;69: 215–224. [DOI] [PubMed] [Google Scholar]
- 9.Franchi L, Baccetti T, McNamara JAJ. Thin-plate spline analysis of mandibular growth. Angle Orthod 2001;71(2):83–9. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre GT, Mossey PA. Size and shape measurement in contemporary cephalometrics. Eur J Orthod 2003;25 231–242 [DOI] [PubMed] [Google Scholar]
- 11.Chvatal BA, Behrents RG, Ceen RF, Buschang PH. 2005. Development and testing of multilevel models for longitudinal craniofacial growth prediction. Am J Orthod Dentofacial Orthop 128(1):45–56. [DOI] [PubMed] [Google Scholar]
- 12.Ghislanzoni LH, Lione R, Cozza P, Franchi L. Measuring 3D shape in orthodontics through geometric morphometrics. Progress in Orthodontics 2017;18:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoloni V, Lione R, Farisco F, Halazonetis DJ, Franchi L, Cozza P. Morphometric covariation between palatal shape and skeletal pattern in Class II growing subjects. European Journal of Orthodontics 2017, 371–376 [DOI] [PubMed] [Google Scholar]
- 14.Singh GD. Morphospatial analysis of soft-tissue profile in patients with Class II Division 1 malocclusion treated using twin block appliances: geometric morphometrics. Orthod Craniofac Res. 2002;51(1):38–50. [DOI] [PubMed] [Google Scholar]
- 15.Katsadouris A and Halazonetis D. Geometric morphometric analysis of craniofacial growth between the ages of 12 and 14 in normal humans. European Journal of Orthodontics 2017, 386–394. [DOI] [PubMed] [Google Scholar]
- 16.Aki T, Nanda RS, Frans Currier B, Nanda SK. Assessment of symphysis morphology as a predictorof the direction of mandibular growth. Am J Orthod Dentofacial Orthop 1994;106:60–9. [DOI] [PubMed] [Google Scholar]
- 17.Bjork A Prediction of mandibular growth rotation. Am J Orthod.1969;55:585–599. [DOI] [PubMed] [Google Scholar]
- 18.Davidovitch M, Eleftheriadi I, Kostaki A, Shpack N. The use of Bjork’s indications of growth for evaluation of extremes of skeletal morphology. European Journal of Orthodontics 2016, 555–562. [DOI] [PubMed] [Google Scholar]
- 19.Baumrind S, Miller D. Computer-aided head film analysis: the University of California San Francisco method. Am J Orthod 1980;78:41–65. [DOI] [PubMed] [Google Scholar]
- 20.Gower JC. Generalized Procrustes Analysis. Psychometrika 1975;40:33–51. [Google Scholar]
- 21.Rohlf FJ, Slice D Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- 22.Bishara SE, Augspurger EF Jr. 1975. The role of mandibular plane inclination in orthodontic diagnosis. Angle Orthod 45(4):273–281. [DOI] [PubMed] [Google Scholar]
- 23.Hardin AM, Knigge RP, Duren DL, Oh HS, Valiathan M, Mcnulty KP, Leary EV, Sherwood RJ. Growth-related change in the mandibular plane angle with clinical implications. Submitted to Brit. Dent. J [DOI] [PMC free article] [PubMed] [Google Scholar]