Abstract
Background:
It is unknown whether early cardiology involvement shortly after atrial fibrillation (AF) diagnosis is associated with favorable outcomes in AF patients who have cancer.
Objectives:
To examine the relationship between early cardiology involvement after AF diagnosis in patients with history of cancer.
Methods:
We examined associations of early cardiology involvement with oral anticoagulation use, stroke, and bleeding among non-valvular AF patients (N=388,045; mean age=68±15 years; 59% male) with history of cancer (past or active) from the MarketScan database (2009–2014). ICD-9 codes in any position were used to identify cancer diagnosis prior to AF diagnosis. Provider specialty and filled anticoagulant prescriptions 3 months prior to and 6 months after AF diagnosis were obtained. Poisson regression models were used to compute the probability of an oral anticoagulant prescription fill and Cox regression was used to estimate the risk of stroke and major bleeding.
Results:
64,016 (17%) AF patients had a history of cancer. Cardiology involvement was less likely to occur among patients with history of cancer than those without (relative risk=0.92, 95% confidence interval (0.91–0.93)). Patients with history of cancer were less likely to fill prescriptions for anticoagulants (0.89 (0.88–0.90)) than those without cancer, and similar results were observed across cancer types. Patients with cancer were more likely to fill prescriptions for anticoagulants (1.48 (1.45–1.52)) if seen by a cardiologist. A reduced risk of stroke (hazard ratio=0.89 (0.81–0.99)) was observed among all cancer patients who were seen by a cardiology provider, without an increased risk of bleeding (1.04 (0.95–1.13)). Similar results were observed when the analysis was stratified by active versus remote history of cancer.
Conclusion:
Although AF patients with cancer were less likely to see a cardiologist, or fill anticoagulant prescriptions, cardiology involvement was associated with increased anticoagulant prescription fills and favorable AF-related outcomes in AF patients with cancer.
Keywords: atrial fibrillation, anticoagulation, provider
CONDENSED ABSTRACT
In this analysis, we have shown that atrial fibrillation patients with a history of cancer were less likely to see a cardiologist, and less likely to fill an anticoagulant prescription than atrial fibrillation. Despite these differences, cardiology involvement was associated with increased anticoagulant prescription fills and reduced risk of stroke in AF patients with cancer, suggesting a beneficial role for cardiology providers to improve outcomes in AF patients with a history of cancer.
Cancer is an important comorbid condition associated with atrial fibrillation (AF) development (1–3). A higher prevalence of cardiovascular risk factors has been reported in AF patients who have cancer than AF patients without cancer (4), and concomitant malignancy may be associated with a higher risk of stroke in patients with AF (5). Accordingly, AF patients who have had cancer represent a high-risk group for adverse events, and careful attention is needed to improve outcomes in this subset of AF patients. Due to improvements in the detection and treatment of cancer, the number of Americans with a history of cancer will increase to nearly 19 million by 2024 (6). Due to demographic changes resulting in a greater number of older individuals who are at elevated risk of AF and cancer, increases in the number of AF patients with a history of cancer are expected. Consequently, providers will be faced with challenging decisions of how to care for these patients, particularly the initiation of oral anticoagulants in patients who possibly have an increased risk for bleeding events (7).
In the United States, it is unknown whether patients with AF and cancer receive antithrombotic therapies or have early cardiology involvement after their AF diagnosis similar to those without cancer. Early cardiology involvement within 90 days of AF diagnosis has been shown to be associated with greater oral anticoagulant use (8–10), and reduced risk of future stroke events (9,10). It is possible that patients with AF and history of cancer are less likely to receive antithrombotic therapies or see cardiology providers after their diagnosis due to negative perception of survival or a lack of perceived benefit with anticoagulant agents. This is supported by recent data demonstrating that patients with a history of cancer are less likely to receive guideline-recommended treatment for coronary heart disease events (11). Therefore, the purpose of this analysis was to examine the influence of cancer history on the association between provider specialty, anticoagulant prescription fills, and stroke risk in patients with AF.
METHODS
Study Design and Cohort
This study used data from the Truven Health MarketScan Commercial Claims and Encounter Database and the Medicare Supplemental and Coordination of Benefits Database (Truven Health Analytics, Ann Arbor, Michigan, USA) between January 1, 2009 and December 31, 2014. The MarketScan Commercial Claims and Encounter Database consists of health insurance claims from all levels of care from several large employers and health plans across the United States. The database is comprised of information from companies which provide private healthcare coverage for employees, their spouses, and other dependents. The MarketScan Medicare Supplemental Database includes claims from individuals and their dependents with employer-sponsored Medicare Supplemental plans. Both databases link medical and outpatient prescription drug claims and encounter data with patient enrollment data to provide individual clinical use, expenditure and outcomes information across inpatient and outpatient services and outpatient pharmacy services.
This analysis included health plan enrollees with ≥6 months of enrollment prior to the first nonvalvular AF diagnosis. A minimum of 6 months between enrollment and first AF diagnosis was selected to identify incident cases of AF. AF was defined by the presence of International Classification of Disease Ninth Revision, Clinical Modification (ICD-9-CM) codes 427.31 or 427.32 in any position on an inpatient claim or on two outpatient claims at least 7 days but less than 1 year apart, and without any inpatient diagnosis of mitral stenosis (ICD-9-CM 394.0) or mitral valve disorder (ICD-9-CM 424.0). A systematic review of studies using ICD-9-CM codes for AF identification reported a positive predicted value of ~90% and a sensitivity of ~80% (12). The AF diagnosis date was defined as the earlier of the following: 1) the discharge date for the qualifying inpatient claim; or 2) the service date of the second qualifying outpatient claim (13). The analytic sample was further restricted to patients who had at least one outpatient claim with a medical provider in a window 3 months prior to 6 months after AF diagnosis. We considered the period prior to AF diagnosis to account for imprecisions in the actual date of diagnosis for patients with outpatient AF, which required two outpatient claims. Participants with oral anticoagulant prescriptions more than 3 months prior to AF diagnosis were presumed to use these agents for other conditions (e.g., venous thromboembolism), and were excluded from the analysis. This analysis was approved by the Institutional Review Board at Emory University.
Cancer History
The MarketScan databases were used to identify prior history of the following cancers (past or active): colon, lung, breast, prostate, pancreas, hematologic, and others. These cancers were considered if the claims were present prior to AF diagnosis. ICD-9-CM codes to define each cancer are shown in Supplemental Table 1. Cancers of the skin (ICD-9-CM codes 172.x-173.x) were excluded from this analysis. Active cancer cases were identified by the use of chemotherapy, radiation therapy, or cancer surgery within 6 months prior to AF diagnosis, using a technique that has been previously described (14). Briefly, at least one of the following was used to identify active cases: 1) at least 1 claim for a chemotherapy J code or chemotherapy drug code; 2) at least 1 radiation therapy code; 3) active surgical treatment; 4) inpatient Medicare Severity Diagnosis Related Group with a cancer ICD-9-CM code in the primary position of the claim; or 5) outpatient cancer surgery coded with a cancer procedure code and coded with a cancer ICD-9-CM code in the primary diagnosis of the claim (Cancer Research Network [U24 CA171524]) (Supplemental Table 2). All analyses were stratified by active versus past history of cancer.
Provider Type
Cardiology and primary care outpatient claims were used to identify outpatient provider visits. Providers seen by patients in the hospital were not included, as the main purpose of the analysis was to determine the influence of outpatient cardiology involvement on oral anticoagulant use. Patients who saw a cardiology provider within the predetermined period (3 months prior to AF diagnosis to 6 months after AF diagnosis) were classified as the cardiology group, while patients seen exclusively by internal medicine, family practice, medical doctor, or unspecified multispecialty group were classified as primary care. Patients seen at least once by a cardiologist were included in the cardiology provider group, regardless of a primary care visit in the same period.
Oral Anticoagulant Use
Filled outpatient pharmaceutical claims are included in the MarketScan databases. Each claim includes the National Drug Code, prescription fill date, and the number of days supplied. All claims for oral anticoagulants in use during the study period were identified and included warfarin and direct oral anticoagulants (DOAC: dabigatran, rivaroxaban and apixaban). Oral anticoagulant prescriptions were limited to outpatient claims between 3 months prior to 6 months after AF diagnosis. A single claim during this period was used to define patients as recipients of oral anticoagulants. Although there is no information on the validity of DOAC claims, warfarin prescription in claims databases has been found to have positive predictive value of >99% (15). DOAC prescriptions were included independently of the dosage prescribed. We considered filled prescription claims as a proxy for actual anticoagulant prescription.
Covariates
Similar to the ascertainment of cancer history, we used the MarketScan databases to evaluate the presence of the following comorbid conditions: heart failure, hypertension, diabetes, stroke, myocardial infarction, peripheral artery disease, kidney disease, liver disease, alcohol use, and bleeding history. At least one ICD-9-CM diagnosis code, in any position, was considered evidence of the condition. Conditions were considered present if the claims were present prior to AF diagnosis. Additionally, the CHA2DS2-VASc and HAS-BLED scores were computed for each patient at the time of AF diagnosis (16,17). ICD-9-CM codes to define each condition are shown in Supplemental Table 3. Filled outpatient pharmaceutical claims also were assessed to ascertain the use of the following medications: angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, beta blockers, calcium channel blockers, diuretics, amiodarone, and digoxin. These medications were ascertained if filled prior to or at the time of AF diagnosis.
Outcomes
The main outcome variable was hospitalization for an ischemic stroke event that occurred after the diagnosis of AF. ICD-9-CM codes in the primary position of an inpatient claim were used to identify events. The codes used to detect ischemic stroke events are shown in Supplemental Table 4. Other outcomes examined included hospitalization for heart failure, myocardial infarction, major bleeding events (intracranial, gastrointestinal, or other), and hospitalization for AF using ICD-9-CM codes in the primary position, and the codes are shown in Supplemental Table 4. Additionally, we examined incident rhythm control strategies, defined as antiarrhythmic medications, catheter ablation, or direct current cardioversion. Filled outpatient pharmaceutical claims antiarrhythmic medications, and CPT and ICD-9-CM codes were used to identify if patients received these therapies during follow-up (Supplemental Table 5).
Statistical Analysis
Baseline characteristics were compared by cancer history. Categorical data were compared using the chi-square test and continuous data using the Student’s t-test. Poisson regression models with robust variance estimates were used to compute relative risk (RR) and 95% confidence intervals (CI) for cardiology involvement in patients with compared to without cancer (18). Models were adjusted for age, sex, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, amiodarone, digoxin, CHA2DS2-VASc, and HAS-BLED. As a secondary analysis we also performed a greedy propensity score-matched analysis using multivariable logistic regression to predict the probability of having a cancer history using the same covariates in the primary analysis. In instances when the absolute difference between propensity scores was ± 0.01 (N=189,220), 1:2 matching was performed (19). The original Poisson regression analysis was repeated in the propensity score-matched cohort.
We also examined the RR of oral anticoagulant prescription fills (any anticoagulant, DOACs, and warfarin) in patients with and without cancer in a multivariable-adjusted model with the same covariates as the primary analysis plus cardiology involvement. This analysis was repeated in the propensity-score matched cohort. To determine if cardiology specialists were positively associated with oral anticoagulant fills in patients with cancer, we restricted the sample to AF patients with history of cancer (N=64,016) and compared anticoagulant prescription fill patterns between those who had cardiology involvement versus those who did not. Models were adjusted for age, sex, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, amiodarone, digoxin, CHA2DS2-VASc, and HAS-BLED.
Cox regression was used to estimate the association of cardiology involvement (versus not) with the future risk of ischemic stroke, hospitalization for heart failure, myocardial infarction, major bleeding events, and hospitalization for AF among patients with cancer and AF (N=64,016), adjusting for the same covariates as the oral anticoagulant analysis.
All analyses were stratified by cancer status (active versus remote) and cancer type (colon, lung, breast, prostate, pancreas, hematologic system). Analyses for breast cancer were limited to women, and analyses for prostate cancer were limited to men. A sensitivity analyses was performed in which the relationship between cardiology involvement and oral anticoagulant prescription fills among AF patients who had cancer was stratified by high (HAS-BLED ≥3) versus low (HAS-BLED <3) bleeding risk. Due to the fact that cancers are more likely to develop in older patients (6), and advanced age may influence the association between cancer and the outcomes examined, we stratified the aforementioned analyses by age <75 versus ≥75 years of age. We also examined if cancer patients who saw a cardiology provider were more likely to receive rhythm control therapies (antiarrhythmic medications, catheter ablation, and direct current cardioversion) compared with those managed by primary care. The analyses for outcomes were stratified by cancer aggressiveness (breast/prostate versus colon/lung/pancreas/hematologic) due to the small number of events when stratified by individual cancer type. SAS Version 9.4 (Cary, NC) was used for all analyses.
RESULTS
There were 388,045 (mean age=68±15 years; 59% male) patients included in the final sample. Of these, 64,016 (17%) had a prior history of cancer. Prostate (26%) and breast (19%) were the most common sites of cancer. Patients with cancer were older, and more likely to have heart failure, hypertension, diabetes, stroke, myocardial infarction, and peripheral artery disease than those without cancer. Patients with a history of cancer also were more likely to fill prescriptions for cardiovascular medications. Baseline characteristics by cancer status are shown for the entire cohort in Table 1. Characteristics stratified by cardiology versus primary care involvement are shown in Supplemental Table 6, and the characteristics for the propensity score-matched cohort are shown in Supplemental Table 7.
Table 1.
Patient Characteristics at time of Nonvalvular Atrial Fibrillation Diagnosis by Cancer Status: MarketScan, 2009–2014 (N=388,045)
| Characteristic | Cancer (N= 64,016) |
No Cancer (N=324,029) |
P-value* |
|---|---|---|---|
| Age, mean ± SD, years | 74±12 | 67±15 | <0.001 |
| Female (%) | 40 | 41 | <0.001 |
| Heart failure (%) | 30 | 25 | <0.001 |
| Hypertension (%) | 79 | 71 | <0.001 |
| Diabetes (%) | 32 | 27 | <0.001 |
| Stroke (%) | 27 | 20 | <0.001 |
| Myocardial infarction (%) | 12 | 10 | <0.001 |
| Peripheral artery disease (%) | 2.7 | 2.3 | <0.001 |
| CHA2DS2-VASc, mean ± SD | 3.7±1.9 | 3.0±2.0 | <0.001 |
| Kidney disease (%) | 17 | 10 | <0.001 |
| Liver disease (%) | 11 | 5.1 | <0.001 |
| Alcohol abuse (%) | 2.3 | 2.5 | 0.037 |
| Bleeding history (%) | 31 | 16 | <0.001 |
| Antiplatelet agents (%) | 2.5 | 2.1 | <0.001 |
| HAS-BLED, mean ± SD | 2.4±1.2 | 1.8±1.2 | <0.001 |
| ACE inhibitors (%) | 33 | 31 | <0.001 |
| ARB (%) | 21 | 19 | <0.001 |
| Beta blockers (%) | 58 | 58 | <0.001 |
| Calcium channel blockers (%) | 34 | 30 | <0.001 |
| Diuretics (%) | 40 | 33 | <0.001 |
| Amiodarone (%) | 8.9 | 7.2 | <0.001 |
| Digoxin (%) | 10 | 9.1 | <0.001 |
| Cancer (%) | |||
| Colon | 12 | - | |
| Lung | 14 | - | |
| Breast | 19 | - | |
| Prostate | 26 | - | |
| Pancreas | 2 | - | |
| Hematologic | 14 | - | |
| Active cancer (%) | 41 | - | |
| Remote cancer (%) | 59 | - |
Statistical significance for continuous data was tested using the Student’s t-test and categorical data was tested using the Chi-square test.
ACE=angiotensin-converting-enzyme; ARB=angiotensin II receptor blocker; CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; SD=standard deviation.
Cardiology involvement was less likely to occur among patients with a history of cancer than those without a history of cancer (54% vs. 62%; RR=0.92, 95%CI=0.91, 0.93) (Central Illustration). Similar differences were observed for cancers of the colon, lung, pancreas, and hematologic system. No difference in early cardiology involvement was observed for breast or prostate cancers. Similar results were observed in the propensity-score matched cohort, and when the analysis was stratified by active versus remote history of cancer (Supplemental Table 8).
Central Illustration. Association of Cancer History with Prevalence of Cardiology Involvement (A) and Anticoagulant Prescription Fills (B) in Patients with Nonvalvular Atrial Fibrillation: MarketScan 2009–2014 (N=388,045).
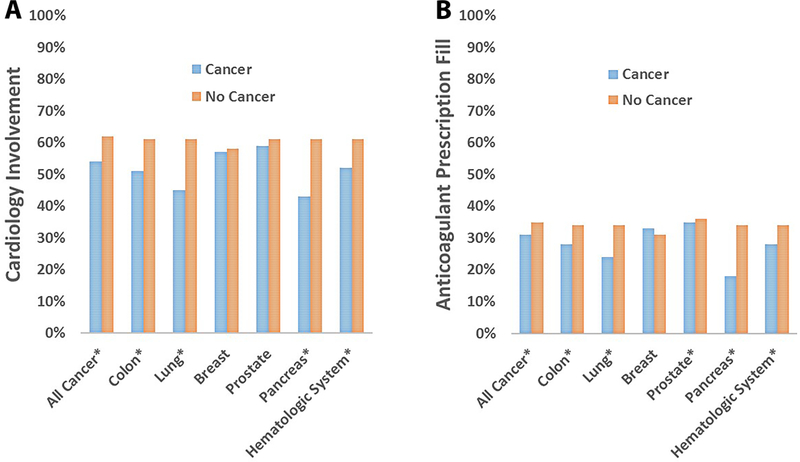
Cardiology involvement (A) was less likely to occur among patients with history of cancer than those without (RR=0.92, 95% CI=0.91–0.93), and similar differences were observed for cancers of the colon, lung, pancreas, and hematologic system. Anticoagulant prescription fills (B) were less likely among patients with history of cancer than those without cancer (RR=0.89, 95%CI=0.88–0.90), and similar results were observed for cancers of the colon, lung, pancreas, prostate, and hematologic system. *P<0.05.
Anticoagulant prescription fills by cancer status are shown in Table 2. Patients with a history of cancer were less likely to fill prescriptions for anticoagulants (RR=0.89, 95%CI=0.88, 0.90) than those without a history of cancer, and similar results were observed for cancers of the colon, lung, prostate, pancreas, and hematologic system (Central Illustration). No differences for anticoagulant prescription fills were observed for patients with a history of breast cancer. Similar results were observed in the propensity-score matched cohort, and when the analysis was stratified by active versus remote history of cancer (Table 2).
Table 2.
Association of Cancer History with Prevalence of Anticoagulant Prescription Fills in Patients with Nonvalvular Atrial Fibrillation: MarketScan 2009–2014 (N=388,045)
| Subgroup | Prescription Fill (%) | Primary Analysis RR (95%CI)*† |
Propensity-Score Matched RR (95%CI)*‡ | |
|---|---|---|---|---|
| Cancer | No Cancer | |||
| All Cancer | (N = 64,016) | (N = 324,029) | ||
| Any | 31 | 35 | 0.89 (0.88, 0.90) | 0.89 (0.88, 0.90) |
| Colon | 28 | 34 | 0.86 (0.83, 0.89) | 0.87 (0.84, 0.90) |
| Lung | 24 | 34 | 0.73 (0.71, 0.76) | 0.75 (0.72, 0.77) |
| Breast§ | 33 | 31 | 1.02 (0.99, 1.05) | 1.03 (1.01, 1.06) |
| Prostate** | 35 | 36 | 0.95 (0.93, 0.97) | 0.96 (0.94, 0.98) |
| Pancreas | 18 | 34 | 0.60 (0.52, 0.68) | 0.61 (0.53, 0.69) |
| Hematologic | 28 | 34 | 0.85 (0.83, 0.88) | 0.87 (0.84, 0.90) |
| Active Cancer | (N = 26,450) | (N = 324,029) | ||
| Any | 29 | 35 | 0.85 (0.83, 0.86) | 0.85 (0.83, 0.87) |
| Colon | 26 | 34 | 0.80 (0.76, 0.84) | 0.81 (0.77, 0.85) |
| Lung | 23 | 34 | 0.72 (0.69, 0.75) | 0.73 (0.69, 0.76) |
| Breast§ | 32 | 31 | 1.02 (0.98, 1.06) | 1.03 (0.99, 1.07) |
| Prostate** | 32 | 36 | 0.89 (0.86, 0.92) | 0.91 (0.87, 0.94) |
| Pancreas | 19 | 34 | 0.59 (0.50, 0.70) | 0.60 (0.51, 0.72) |
| Hematologic | 26 | 34 | 0.78 (0.75, 0.82) | 0.80 (0.76, 0.84) |
| Remote Cancer | (N = 37,556) | (N = 324,029) | ||
| Any | 32 | 35 | 0.91 (0.90, 0.92) | 0.91 (0.90, 0.93) |
| Colon | 31 | 35 | 0.89 (0.85, 0.93) | 0.90 (0.86, 0.95) |
| Lung | 25 | 35 | 0.74 (0.70, 0.78) | 0.75 (0.71, 0.79) |
| Breast§ | 33 | 31 | 1.00 (0.97, 1.04) | 1.02 (0.98, 1.05) |
| Prostate** | 36 | 37 | 0.96 (0.94, 0.99) | 0.97 (0.94, 0.99) |
| Pancreas | 18 | 34 | 0.58 (0.47, 0.70) | 0.58 (0.48, 0.71) |
| Hematologic | 31 | 35 | 0.91 (0.87, 0.94) | 0.92 (0.88, 0.96) |
Comparison between cancer and no cancer.
Relative risk of anticoagulant prescription fills for patients with vs. without cancer. Adjusted for cardiology involvement, age, sex, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, amiodarone, digoxin, CHA2DS2-VASc, and HAS-BLED.
Results of 1:2 propensity-matched analysis for patients with and without cancer. Propensity score was computed using multivariable logistic regression with the following variables: age, sex, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, amiodarone, digoxin, CHA2DS2-VASc, and HAS-BLED (N=189,220).
Analysis limited to women.
Analysis limited to men.
CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; CI=confidence interval; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; RR=relative risk.
Among patients with cancer (N=64,016), those seen by a cardiology provider around the time of their diagnosis, versus not seen by a cardiology provider, were more likely to fill prescriptions for anticoagulants (RR=1.48, 95%CI=1.45, 1.52) (Table 3). Similar results were observed for cancers of the colon, lung, breast, prostate, pancreas, and hematologic system. Additionally, the results were similar when stratified by active and remote history of cancer (Table 3). The positive association between cardiology involvement and anticoagulant prescription fill did not vary by HAS-BLED ≥3 (RR=1.51, 95%CI=1.45, 1.56) versus HAS-BLED <3 (RR=1.46, 95%CI=1.41, 1.51).
Table 3.
Association of Provider Specialty with Prevalence of Anticoagulant Prescription Fills in Patients with Nonvalvular Atrial Fibrillation and Cancer History: MarketScan 2009–2014 (N=64,016)
| Subgroup | Prescription Fill (%) | RR (95%CI)*† | |
|---|---|---|---|
| Cardiology | Primary Care | ||
| All Cancer (N=64,016) | (N=34,811) | (N=29,205) | |
| Any | 37 | 23 | 1.48 (1.45, 1.52) |
| Colon | 35 | 22 | 1.50 (1.39, 1.61) |
| Lung | 31 | 19 | 1.54 (1.43, 1.66) |
| Breast‡ | 38 | 26 | 1.39 (1.31, 1.46) |
| Prostate§ | 40 | 27 | 1.45 (1.39, 1.52) |
| Pancreas | 24 | 14 | 1.59 (1.23, 2.07) |
| Hematologic | 36 | 20 | 1.62 (1.51, 1.74) |
| Active Cancer (N=26,450) | (N=13,550) | (N=12,900) | |
| Any | 35 | 22 | 1.47 (1.41, 1.53) |
| Colon | 32 | 20 | 1.49 (1.33, 1.67) |
| Lung | 29 | 19 | 1.41 (1.28, 1.56) |
| Breast‡ | 37 | 26 | 1.34 (1.23, 1.45) |
| Prostate§ | 39 | 24 | 1.50 (1.38, 1.63) |
| Pancreas | 24 | 15 | 1.53 (1.09, 2.15) |
| Hematologic | 34 | 18 | 1.71 (1.54, 1.90) |
| Remote Cancer (N=37,566) | (N=21,261) | (N=16,305) | |
| Any | 38 | 24 | 1.48 (1.44, 1.53) |
| Colon | 37 | 23 | 1.48 (1.35, 1.64) |
| Lung | 33 | 18 | 1.74 (1.55, 1.96) |
| Breast‡ | 39 | 26 | 1.43 (1.33, 1.54) |
| Prostate§ | 41 | 28 | 1.42 (1.35, 1.50) |
| Pancreas** | 25 | 13 | - |
| Hematologic | 38 | 23 | 1.52 (1.38, 1.68) |
Comparison between cardiology and primary care.
Relative risk of anticoagulant prescription fills for patients seen by cardiology vs. primary care providers. Adjusted for age, sex, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, amiodarone, digoxin, CHA2DS2-VASc, and HAS-BLED.
Analysis limited to women.
Analysis limited to men.
Effect estimates for relative risk not generated due to small sample size.
CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; CI=confidence interval; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; RR=relative risk.
During a mean follow-up of 1.1 years after AF diagnosis, a reduced risk of ischemic stroke (HR=0.89, 95%CI=0.81, 0.99) was observed among cancer patients who were seen by a cardiology provider compared to those who were not, without an increased risk of bleeding (HR=1.04, 95%CI=0.95, 1.13) (Table 4). Additionally, AF patients with cancer and seen by a cardiologist were more likely to be hospitalized for heart failure and atrial fibrillation than those seen by primary care providers. No increased risk of myocardial infarction was observed. The risk of outcomes examined were similar when stratified by active and remote history of cancer (Supplemental Table 9).
Table 4.
Association of Provider Specialty with Ischemic Stroke and Major Bleeding Events in Patients with Nonvalvular Atrial Fibrillation and Cancer History: MarketScan 2009–2014 (N=64,016)
| Subgroup | No. Events | Person-years | Incidence Rate Per 1000 person-years |
HR (95%CI)* |
|---|---|---|---|---|
| Any Cancer (N=64,016) | ||||
| Ischemic Stroke | ||||
| Primary care | 680 | 39,286 | 17.3 | 1 (Ref) |
| Cardiology | 891 | 59,354 | 15.0 | 0.89 (0.81, 0.99) |
| Major Bleeding Events | ||||
| Primary care | 804 | 39,227 | 20.5 | 1 (Ref) |
| Cardiology | 1,220 | 59,020 | 20.7 | 1.04 (0.95, 1.13) |
| Myocardial Infarction | ||||
| Primary care | 310 | 39,696 | 7.8 | 1 (Ref) |
| Cardiology | 449 | 59,876 | 7.5 | 0.95 (0.82, 1.09) |
| Heart Failure | ||||
| Primary care | 961 | 39,145 | 24.5 | 1 (Ref) |
| Cardiology | 1,880 | 58,624 | 32.1 | 1.30 (1.20, 1.41) |
| AF Hospitalization | ||||
| Primary care | 1,121 | 38,607 | 29.0 | 1 (Ref) |
| Cardiology | 2,319 | 57,048 | 40.7 | 1.44 (1.34, 1.55) |
Results of multivariable Cox regression analysis adjusted for age, sex, heart failure, hypertension, diabetes, stroke, myocardial infarction, kidney disease, liver disease, bleeding history, alcohol use, antiplatelet agents, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics, amiodarone, digoxin, CHA2DS2-VASc, and HAS-BLED.
CHA2DS2-VASc=congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category; CI=confidence interval; HAS-BLED=hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly; HR=hazard ratio.
Patients with a history of cancer were less likely to see a cardiology provider if they were <75 years (RR=0.87, 95%CI=0.86, 0.88) compared with ≥75 years (RR=0.97, 95%CI=0.96, 0.98), and a similar association was observed for anticoagulant prescription fills by age (<75 years: RR=0.87, 95%CI=0.85, 0.88; ≥75 years: RR=0.94, 95%CI=0.92, 0.95) (Supplemental Table 10). However, similar beneficial associations were observed for cardiology involvement in patients with history of cancer for anticoagulant prescription fills, ischemic stroke, and major bleeding events by age <75 years versus ≥75 years (Supplemental Table 10). AF patients who had a history of cancer were more likely to receive rhythm control therapies if seen by a cardiology compared with primary care provider (RR=1.65, 95%CI=1.60, 1.70), and similar results were observed for cancers of the colon, lung, breast, prostate, pancreas, and hematologic system (Supplemental Table 11). The use of rhythm control therapies did not vary by active versus remote cancer history (Supplemental Table 11). The risks of each outcome associated with cardiology involvement among those with cancer were similar when the analyses were stratified by cancer aggressiveness (breast/prostate versus colon/lung/pancreas/hematologic), and these data are shown in Supplemental Table 12.
DISCUSSION
In this analysis from a large commercial claims database reflecting AF care in the United States, early cardiology involvement and oral anticoagulant prescription fills were less likely to occur in AF patients who had a history of cancer (active or remote) than those without. A beneficial association of early cardiology involvement was observed for AF patients with history of cancer, as these patients were more likely to fill oral anticoagulant prescriptions, and this coincided with a reduced risk of future stroke events without increased risk of bleeding. Overall, our data suggest that suboptimal antithrombotic care exists in AF patients who have a history of cancer, and early cardiology involvement appears to favorably influence oral anticoagulant prescription fills and AF-related outcomes in this subset of AF patients.
To our knowledge, only one prior study has examined if variation exists in the antithrombotic care of AF patients by history of cancer. Data from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) reported that AF patients with a history of cancer were equally as likely to use antithrombotic agents compared with patients without a history of cancer (4). The findings differed from our data, as we have shown that AF patients were less likely to fill an oral anticoagulant prescription if a history of cancer was present. Antithrombotic care in ORBIT-AF was tabulated by providers in a relatively small sample (N=9,749), that consisted of >80% cardiology specialists (20). Also, ORBIT-AF included both incident and prevalent AF cases, and the ability to study AF care shortly after diagnosis in patients with history of cancer was not possible. In comparison, our study likely better reflects what occurs among newly-diagnosed AF in persons with a history of cancer, as our data came from filled pharmaceutical claims rather than relying on provider-driven data. We also were able to further delineate patients as having either active or remote history of cancer through the use of a large commercial claims database. Additionally, the population of ORBIT-AF was older than the current cohort, and we observed that the magnitude of the association for oral anticoagulant prescription fills was greater for patients <75 years compared with ≥75 years (RR=0.87 versus RR=0.94). This suggests that greater differences in oral anticoagulant fills will be observed in AF patients with a history of cancer who are younger, and this possibly is related to provider perception of poor survival in younger patients with a history of cancer. Alternatively, cancer history may have limited additive influence in older adults, as they are less likely to see a cardiology provider or fill an anticoagulant prescription regardless of cancer history (10). However, the explanations provided for variation by age are speculative and further research is needed to understand this difference.
We also examined whether there was a beneficial association of early cardiology involvement around the time of AF diagnosis among patients who had a history of cancer. In addition to confirming this beneficial association (9,10), we have demonstrated that early cardiology involvement shortly after AF diagnosis is less likely in those who have a history of cancer. Due to the favorable association of cardiology provider involvement on oral anticoagulant prescription fills in AF patients with a history of cancer, early referral to cardiology specialists shortly after diagnosis may be beneficial to increase the use of antithrombotic agents and reduce the risk of future stroke events in this subset of AF patients.
Prescription fills for oral anticoagulants and cardiology involvement varied by cancer type, but differences were similar when stratified by active versus remote history. Differences were not observed for cancers that are more likely to be detected at an earlier stage (breast and prostate) (6). Therefore, providers may be less likely to prescribe antithrombotic agents or to refer to cardiology specialists if a lack of perceived benefit exists, especially with cancers commonly detected at more advanced stages (e.g., lung and pancreas). Although the decision to initiate antithrombotic therapy or refer to a cardiology provider in AF patients with cancer history should be individualized based on patient characteristics, such as life expectancy and bleeding risk, the data in this report suggest that cardiology providers positively influence outcomes among AF patients with a history of cancer, and this is not limited to patients with a remote history of cancer.
We observed an increased risk for hospitalizations for heart failure and AF in our cohort of cancer patients. Similar findings were reported from the Retrospective Evaluation and Assessment of Therapies in AF study from the Veterans Health Administration, where AF patients who were seen by cardiology providers had increased risks for decompensated heart failure and admission for AF (9). Explanations for these findings possibly were related to more aggressive cardiovascular care for patients who were seen by cardiology providers. This was evident in our analysis, as cancer patients in our cohort who were seen by cardiology providers were more likely to receive rhythm control therapies. Therefore, medication side effects could explain the higher risk of hospital admission for these conditions. Additionally, this observation possibly was due to variation in disease severity between patients who were seen by both specialties, as more patients with heart failure at baseline were observed among those who saw a cardiology provider. The increased risk would then be expected due to the underlying referral bias between both groups.
AF represents an important comorbid condition in patients with cancer. Several common predisposing factors exist for both conditions that may explain the increased prevalence of AF in those with cancer, including advanced age and inflammation (7). Additionally, the development of AF in patients with cancer has been linked to certain chemotherapeutic agents (21). As the number of cancer survivors grows in coming decades due to improvements in the detection and treatment of certain cancers (6), a simultaneous increase in patients with AF and a history of cancer is expected. Accordingly, the care for AF patients with a history of cancer will pose a challenge for clinicians, especially as AF patients with a history of cancer possibly have a negative perception regarding the benefit of certain AF therapies (e.g., anticoagulation). This possibly explains the suboptimal rates of oral anticoagulant prescription fills observed for patients with a history of cancer. Similarly, the efficacy of certain anticoagulant therapies in AF patients with a history of cancer is uncertain, due to their exclusion in recent clinical trials (22,23). Therefore, further research is needed to determine optimal treatment strategies for AF patients with a history of cancer, including initiatives to enhance early cardiology involvement to reduce morbidity and mortality.
The current analysis should be interpreted in the context of several limitations. The misclassification of AF cases, cancer history, comorbid conditions, and AF-related outcomes was possible as we relied on administrative claims. We did not separate cases of AF related to secondary precipitants (e.g., cardiac surgery), and these cases often are considered transient without a need for long-term anticoagulation. However, recent data have suggested that long-term AF-related stroke and mortality risks are similar between individuals with and without secondary AF precipitants (24). The methodology to define active versus remote history of cancer possibly was incomplete. Additionally, we considered cardiology involvement if a patient had a specialty outpatient claim within 3 months prior to 6 months after AF diagnosis, and did not consider cardiology involvement if it occurred outside this time period. We were unable to account for the competing risk of death in our analyses, as mortality data were not captured in the MarketScan databases. Nonetheless, modeling the effect of cardiology involvement on the cause-specific hazard of each outcome was the most appropriate analysis, as described in a recent review (25). Furthermore, the large sample size rendered the statistical analysis sensitive to the influence of residual confounding and the effect estimates should be interpreted with caution, especially in the context of nonrandomized comparisons with very large sample sizes.
In conclusion, patients with AF and a history of cancer (active or remote) are less likely to fill oral anticoagulant prescriptions and to have early cardiology involvement after their diagnosis compared with those without a history of cancer. Despite these differences, when early cardiology involvement occurs for cancer patients with AF, the patients were more likely to fill anticoagulant prescriptions, and there were lower rates of stroke without an increased risk of bleeding. Early evaluation by a cardiology provider shortly after AF diagnosis should be considered to improve outcomes in AF patients who have a history of cancer.
Supplementary Material
PERSPECTIVES COMPETENCY IN MEDICAL KNOWLEDGE
Although suboptimal antithrombotic care exists in patients who have atrial fibrillation and a history of cancer, early cardiology involvement appears to favorably influence oral anticoagulant prescription fills and outcomes in this subset of patients who have atrial fibrillation.
TRANSLATIONAL OUTLOOK
Further studies are needed to determine optimal treatment strategies for patients who have atrial fibrillation with a history of cancer, including initiatives to enhance early cardiology involvement to improve outcomes.
Acknowledgments
FUNDING
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health undeSr award numbers R01-HL122200 and F32-HL134290, by the National Institute on Aging of the National Institutes of Health under award number R21-AG058445, and by the American Heart Association under award number 16EIA26410001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
ABBREVIATIONS
- AF
atrial fibrillation
- CI
confidence interval
- CHA2DS2-VASc
congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–75 years, and sex category
- DOAC
direct oral anticoagulants
- HAS-BLED
hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age >65 years), drugs/alcohol concomitantly
- HR
hazard ratio
- ICD-9-CM
International Classification of Disease Ninth Revision, Clinical Modification
- RR
relative risk
Footnotes
Disclosures: None.
References
- 1.Erichsen R, Christiansen CF, Mehnert F, Weiss NS, Baron JA, Sorensen HT. Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case-control study. Intern Emerg Med 2012;7:431–8. [DOI] [PubMed] [Google Scholar]
- 2.Guzzetti S, Costantino G, Vernocchi A, Sada S, Fundaro C. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med 2008;3:227–31. [DOI] [PubMed] [Google Scholar]
- 3.O’Neal WT, Lakoski SG, Qureshi W et al. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am J Cardiol 2015;115:1090–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melloni C, Shrader P, Carver J et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes 2017;3:192–197. [DOI] [PubMed] [Google Scholar]
- 5.Hu YF, Liu CJ, Chang PM et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol 2013;165:355–7. [DOI] [PubMed] [Google Scholar]
- 6.DeSantis CE, Lin CC, Mariotto AB et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71. [DOI] [PubMed] [Google Scholar]
- 7.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol 2014;63:945–53. [DOI] [PubMed] [Google Scholar]
- 8.Turakhia MP, Hoang DD, Xu X et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J 2013;165:93–101 e1. [DOI] [PubMed] [Google Scholar]
- 9.Perino AC, Fan J, Schmitt SK et al. Treating Specialty and Outcomes in Newly Diagnosed Atrial Fibrillation: From the TREAT-AF Study. J Am Coll Cardiol 2017;70:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neal WT, Sandesara PB, Claxton JS et al. Provider Specialty, Anticoagulation Prescription Patterns, and Stroke Risk in Atrial Fibrillation. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrmann S, Witassek F, Erne P, Rickli H, Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care 2017:2048872617729636. [DOI] [PubMed] [Google Scholar]
- 12.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccini JP, Hammill BG, Sinner MF et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah S, Norby FL, Datta YH et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv 2018;2:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg RK, Glazer NL, Wiggins KL et al. Ascertainment of warfarin and aspirin use by medical record review compared with automated pharmacy data. Pharmacoepidemiol Drug Saf 2011;20:313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 17.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 18.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fosbol EL, Holmes DN, Piccini JP et al. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT-AF registry. J Am Heart Assoc 2013;2:e000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaakeh Y, Overholser BR, Lopshire JC, Tisdale JE. Drug-induced atrial fibrillation. Drugs 2012;72:1617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 23.Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 24.Lubitz SA, Yin X, Rienstra M et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation 2015;131:1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016;133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


