Abstract
Commiphora, myrrha, is a pantropical genus and perform well in arid and semi-arid environments. This genus has economic importance. Distribution of Commiphora species and their associated species in Saudi Arabia has not been studied to date. The current study report on (a) characterization and distribution of plant communities including Commiphora species and (b) assessment of factors influencing ecological preferences of these species. Five species of Commiphora are recorded inhabiting mountain slopes, steep escarpments or hills consisting of igneous rocks, either granites or basalt with drought prone shallow soil. One hundred and twenty-six plant species belonging to 95 genera and 35 families were found associated with different Commiphora species. Therophytes showed the most frequent life form class and Sudanian region elements recorded the highest phytogeographical units (28%) followed by Tropical elements. Field study showed that Commiphora gileadensis and C. quadricincta preferred granite and basalt rocks exposed to erosion, while C. myrrah, C. kataf and C. habessinica grow on resistant coarse pink granite. The analysis of 240 sampling stands with TWINSPAN revealed the vegetation of Commiphora habitats into eight vegetation groups; each group represented a distinct microhabitat. Dendrogram obtained from a hierarchical classification showed that habitats of C. gileadinsis and C. quadricincta are more similar than those of other species. This similarity was confirmed by Jaccard and Sorenson similarity indices and by Pearson correlation coefficient. This investigation compiled the information/data to facilitate future range management of Commiphora species.
Keywords: Environmental science, Ecology
1. Introduction
Commiphora genus, Myrrha, is small trees or shrubs with short thistly branches. It is one of the most diverse genera of Burseraceae family. Myrrha trees have gained great economic importance since ancient times [31]. Their resinous exudates used as perfume, incense, or humankind [2, 19, 31, 46]. Commiphora is a pantropical in distribution performing well in arid and semi-arid environments occupying an ecological range; between 1 and 2100 m above sea level; striving best on aridisols [23, 49]. It is codominant over huge areas of the Horn of Africa and supports the large livestock populations of pastoral and agro pastoral communities. Some species of this genus are well adapted to a narrow ecological range [47]. Saudi Arabia is a huge arid land area covering major part of the Arabian Peninsula with a geologic structure contemporary of Alps [42]. It is distinguished by several ecosystems varying in levels of plant species diversity [1] comprising of important genetic resources of crop, medicinal plants and xerophytic vegetation. These make up the prominent features of the plant life in the kingdom [5, 6, 7, 53]. The flora of Saudi Arabia has about 2250 species [17] and include six Commiphora species inhabiting rocky stone hills. The trees and shrubs are scarce in arid environment of Saudi Arabia due to infrequent availability of water. However, due to rough topography, there is some order of grooving where runoff water gathers in specific habitats. These favored sites support lives of shrubs and trees like Commiphora species. Despite the great economic importance of Commiphora and other endangered species their phytosociology has not been studied in Saudi Arabia. Limited work is research is done in the world [21, 27, 28, 29, 30]. Our knowledge of Commiphora status and distribution in Saudi Arabia is inadequate. There is need for descriptive and ecological investigations. We know that vegetation classification is a widely used tool for the interpretation and explanation of natural ecosystems and habitats [14]. This also facilitates decision making for the management of protected areas [13]. We studied characteristics and distribution of plant communities including Commiphora species. Assessment of factors influencing ecological preferences in the area as a step for their conservation and propagation is presented.
2. Materials & methods
2.1. Study area
About 104 km2 were selected, including three governorates, Khulais, Osfan and Makkah, in Makkah region, western Saudi Arabia, (Fig. 1). The study area is located between 21°21′ to 22°11' (latitude) and from 39°34′ to 40°11' (longitude). The area belongs geologically to the Arab Shield unit, which is complex in its geological composition, consists generally of pre-Cambrian rocks, where underground rocky rocks are found in sedimentary rocks. In addition, Arabian lava fields (locally known as harrats) which were contemporary with the opening of the Red Sea predominate large part of the studied area.
Fig. 1.
Location map of the studied area, numbers indicates sites where Commiphora species are dominant.
The rainfall in the area is scarce and highly inconstant depends on elevation. The maximum annual precipitation is 18 mm through November to January. The average minimum and maximum annual temperature are 21 °C and 36.5 °C. The maximum temperature often reaches 41.9 °C during June, while the lowest minimum temperature touches below 16.4 °C during winter (Table 1).
Table 1.
Rainfall, temperature and relative humidity of the study species habitat during the study (from January 2016 to December 2018).
| Avg. Temperature (°C) |
Min. Temperature (°C) |
Max. Temperature (°C) |
Precipitation/Rainfall (mm) |
|||||
|---|---|---|---|---|---|---|---|---|
| Khulais - Usfan | Makkah | Khulais - Usfan | Makkah | Khulais - Usfan | Makkah | Khulais - Usfan | Makkah | |
| January | 23.3 | 23.5 | 16.5 | 17.2 | 30.2 | 29.9 | 10 | 12 |
| February | 23.3 | 24.1 | 16.4 | 17.4 | 30.2 | 30.8 | 1 | 2 |
| March | 25.3 | 27.1 | 18.4 | 20.4 | 32.3 | 33.9 | 2 | 5 |
| April | 27.3 | 29.7 | 20 | 22.7 | 34.7 | 36.8 | 4 | 9 |
| May | 29.9 | 33.2 | 22.6 | 26.2 | 37.2 | 40.3 | 1 | 5 |
| June | 30.7 | 34.9 | 23.3 | 28 | 38.1 | 41.9 | 0 | 0 |
| July | 32 | 35.2 | 25.4 | 29.1 | 38.7 | 41.3 | 0 | 0 |
| August | 32.1 | 34.7 | 26 | 29 | 38.2 | 40.5 | 0 | 1 |
| September | 31.1 | 34.4 | 24.5 | 28 | 37.8 | 40.8 | 0 | 1 |
| October | 29.2 | 31.2 | 22.4 | 24.5 | 36.1 | 38 | 0 | 3 |
| November | 27.2 | 27.7 | 20.5 | 21.2 | 33.9 | 34.2 | 18 | 18 |
| December | 24.8 | 24.7 | 18.3 | 18.5 | 31.3 | 31 | 16 | 14 |
2.2. Sampled stands
A thorough survey of the study area was conducted to determine the Commiphora habitats and their distribution. 240 stands, each 50 m × 50 m in size, were distributed to cover the various habitats including Commiphora species. Due to the hot climate of the study area, samples were collected during January to April, prior to the disappearance of annuals. In each stand, listing of all species and their life forms and chorotypes were determined. The collected plant samples were identified and named according to [18, 35]; and [16]. Life forms of the identified species were determined depending upon the location of the regenerative buds and the parts that were shed through the unfavorable season [44]. The biogeographic affinities of the recorded species were determined according to [54] and [52]. In each stand, species present was recorded and plant cover was estimated visually. The density and frequency of different Commiphora and the associated species were determined by the data recorded from the sample stands following the methods described by [36].
2.3. Soil samples and analyses
Five soil samples were gathered from zero to 25 cm depth under different Commiphora species.
These five soil samples were pooled to form one composite sample, which was air-dried and thoroughly mixed. Soil texture was determined by the Bouyoucos hydrometer method, which provided quantitative data on the percentage of sand, silt, and clay. Soil water extracts of 1: 2.5 were prepared for soil pH determinations using a pH meter Model HI 8519, and electrical conductivity (Soil: water ratio, 1:1) was detected by a CMD 830 WPA conductivity meter. Soluble sulfates, chlorides, and bicarbonates (soil:water ratio, 1:5) were estimated according to [25]. Soil water extracts of 1:5 were prepared for detection of potassium and sodium cations by a flame photometer [4] and for determination of magnesium and calcium cations determination via EDTA (0.01 N) according to [25]. The least significant differences (One-WayANOVA) among the mean values for soil analysis were calculated as recommended by [11]; terms were considered significant at P = 0.05.
2.4. Data analysis
Vegetation classification technique was employed; the stand-species data matrix was classified into vegetation groups using the importance values of species by means of the Two Way Indicator Species Analysis (TWINSPAN) computer program [24]. Plant communities were named after their dominant species. Gamma species diversity (γ -diversity) was calculated as the total species number in each landscape or vegetation group. Species richness (α -diversity) of the vegetation cluster was calculated as the average number of species per stand. Species turnover (β -diversity) are calculated as (α γ −1). Shannon–Wiener index , for the relative evenness was calculated for each stand on the basis of the relative cover pi of the ith species [33, 39].
A hierarchical classification analysis based on presence/absence data with Wards' (minimum variance) method and Euclidean distances as a dissimilarity measure [50] were determined, the analysis was performed with the Statistica statistical software package ver. 8 (StatSoft, Inc., Tulsa, OK, USA). Depending on associated taxa of different Commiphora species, Pearson's simple linear correlation coefficient (r) were determined. In addition, two similarities indices, Jaccard and Sorenson, were applied based on presence/absence of species [15].
3. Results
3.1. Commiphora habitats and their associated taxa
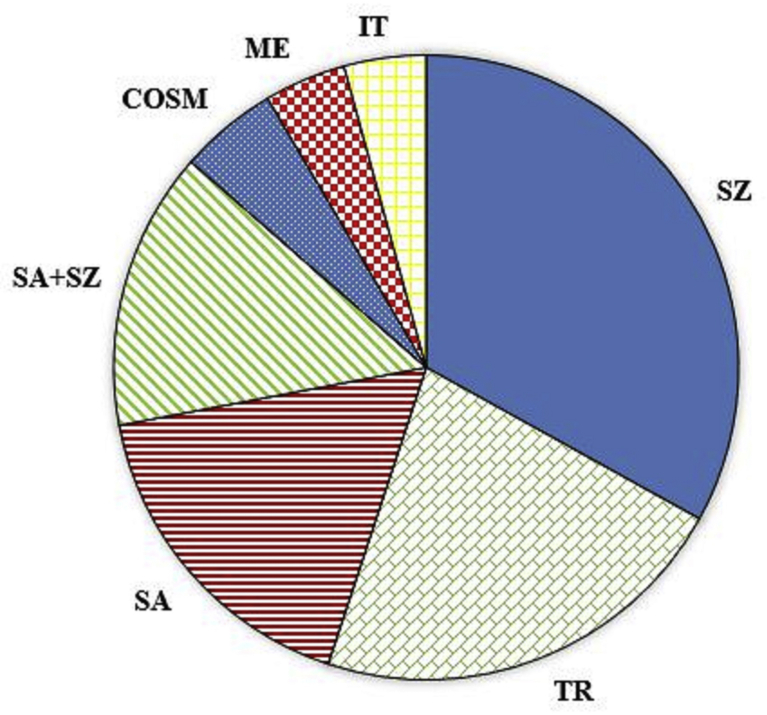
Five species were recorded; C. gileadensis (L.) C.Chr., C. habessinica (O.Berg) Engl., C. kataf (Forssk.) Engl., C. myrrha (Nees) Engl. and C. quadricincta Schweinf. In general, the all recorded Commiphora species were recorded in Rocky habitats, mountain slopes, steep escarpments or hills which consists of igneous rocks, either granites or basalt, with drought prone shallow soil at an elevation ranged between 80 and 1200 m a.s.l. Commiphora species were never recorded in either inland or coastal plains with deep soil. Table 2 Soil analysis shows that there are differences in Geological substratum. C. gileadensis and C. quadricincta were found in granite and basalt rocks that were exposed to erosion, while C. myrrha, C. kataf and C. habessinica were found in resistant, coarse pink granite, mixed with grey diorite and granodiorite. A total of 126 plant species belonging to 95 genera and 35 families were identified as associated with different Commiphora species (Appendix). The major associated families were Poaceae and Fabaceae (13 species for each) followed by Lamiaceae (eight species). While 13 families were represented by only one species. More than 64% of the recorded species (Appendix) belong to only nine species rich families. Blepharis attenuate Napper, Acacia ehrenbergiana (Forssk.) Hayne, Indigofera spinosa Frossk, Boerhavia diffusa L., Cenchrus ciliaris L. and Fagonia indica Burm. f. were recorded as an associated species with all Commiphora species. Table 3 shows eleven species that recorded more than 50% (frequency) with at least one Commiphora species, five of them were recorded with all Commiphora species, namely Acacia mellifera, Caralluma retrospiciens, Grewia tenax, Indigofera spinosa, and Lycium shawii. The highest frequent species was A. hamulosa with C. giladeansis while, Indigofera spinosa recorded the highest frequencies with the other four species. Life forms of the associated species were exhibited a great diversity and reflects a typical desert flora. The most frequent life form class was Therophytes with the maximum number of species (48.8%), followed by Chaemophytes (23.9%), Phanerophytes (18.9%) and Hemicryptophytes (7.8%), while the least frequent life form class was Geophytes (0.8 %) (Fig. 2). Biogeographical affinities (phytogeography) of the recorded associated flora showed that Sudano-Zambezian region elements exhibited the highest number (28%) followed by Tropical elements and Saharo-Arabian elements, 22% and 17%, respectively (Fig. 3). In addition to plant species that belong to the Sudano-Zambezian elements and the Saharo-Arabian associated with Commiphora species in the target region, it has numbers of plant species which dominate in the other uniregional region, such as Mediterranean and Irano-Turanian (5 species for each). The highest Bioregional elements were recorded by Saharao-Arabian- Sudano-Zambezian (15%), followed by Saharo-Arabian – Tropical while, the cosmopolitan were eight species.
Table 2.
Soil parameters registered along different sites where Commiphora species grow. The results are the means of 5 replicates.
| C. giladensis | C. quadricincta | C. myrrha | C. kataf | C. habessinica | L.S.D. (P < 0.05) | |
|---|---|---|---|---|---|---|
| Coarse sand (%) | 7.1 | 0.57 | 5.6 | 3.8 | 8.2 | 2.10 |
| Fine sand (%) | 79.4 | 87.13 | 79.3 | 83.6 | 80.1 | 11.30 |
| Silt (%) | 9.8 | 9.3 | 10.9 | 9.2 | 8.2 | 1.74 |
| Clay (%) | 3.7 | 3 | 4.2 | 3.4 | 3.5 | 1.30 |
| pH | 7.70 | 7.60 | 7.50 | 7.70 | 7.40 | 0.40 |
| E.C. (mS/cm) | 1.43 | 1.13 | 1.43 | 1.05 | 1.40 | 0.51 |
| SO4-- (mg/Kgm) | 90.1 | 86.0 | 135.0 | 89.0 | 42.0 | 4.2 |
| Cl− (mg//Kgm) | 22.5 | 82.0 | 110.1 | 89.0 | 39.1 | 1.3 |
| HCO3- (mg//Kgm) | 33.8 | 67.8 | 79.0 | 119.0 | 90.3 | 1.6 |
| K+ (mg//Kgm) | 4.0 | 11.8 | 3.40 | 2.0 | 2.1 | 0.1 |
| Na+/Kgm) | 2.0 | 11.4 | 4.30 | 45.1 | 9.4 | 3.0 |
| Mg++ (/Kgm) | 15.5 | 28.0 | 39.1 | 26.5 | 22.2 | 2.1 |
| Ca++ (/Kgm) | 15.0 | 42.4 | 41.0 | 42.8 | 23.1 | 1.4 |
| Topography | Mountain slops, Mountain cliffs | Lava hills - Mountain slops | Mountain slops | Escarpment slope | Escarpment slope | |
| Altitude range (m) | 100-750 | 80–300 | 250–1200 | 260–1200 | 500–1150 | |
| Geological substratum | Eroded granite and black basalt | Eroded granite and black basaltic | Coarse pink granite mixed with diorite | Coarse pink granite mixed with diorite | Coarse pink granite mixed with diorite |
Table 3.
Frequencies (%) of the major associated species with the different recorded Commiphora species.
| C. giladensis | C. quadricincta | C. myrrha | C. kataf | C. habessinica | |
|---|---|---|---|---|---|
| Acacia hamulosa | 95 | 80 | 60 | 50 | 50 |
| Acacia mellifera | 45 | 65 | 17 | 10 | 5 |
| Acacia etbaica | 0 | 0 | 30 | 20 | 20 |
| Cadaba farinosa | 50 | 55 | 25 | 0 | 0 |
| Cadaba glandulosa | 66 | 40 | 20 | 0 | 0 |
| Caralluma retrospiciens | 10 | 5 | 10 | 15 | 10 |
| Euphorbia cuneata | 75 | 70 | 0 | 0 | 0 |
| Grewia tenax | 30 | 25 | 80 | 50 | 50 |
| Indigofera spinosa | 85 | 90 | 90 | 95 | 95 |
| Lycium shawii | 50 | 50 | 30 | 50 | 50 |
| Tephrosia nubica | 30 | 10 | 40 | 0 | 0 |
Fig. 2.
Proportinal percentage of life forms for the associated species.
Fig. 3.
Chorological types of the the associated species. Abbreviations are in the appendix.
3.2. Vegetation groups
During the long dry period, there are many dry woody herbs and grasses, which are not clearly apparent among rocks. Next rains fall, an incredible flush of plant growing and many plants, previously dry, become unmistakable while others not obvious before appear. Classification of the recorded species in the 240 stands with TWINSPAN separated the vegetation of Commiphora habitats into two main divisions, which further divided at five level to eight vegetation groups (Fig. 4); each group represented a distinct microhabitat.
Fig. 4.
Vegetation clusters resulting from the TWINSPAN classification.
3.2.1. VG (I)
Acacia hamulosa- Cadaba farinosa community in a granite outcrop habitats, 103 m a.s.l., represented this group, which exhibited the lowest species number. The most frequent species was Indigofera spinosa, while Euphorbia cuneata was abundant and each of Blepharis attenuate, Tripulus macropterus, Senna italica and Commiphora quadricincta, with a density of 2/hectare, were prominent.
3.2.2. VG (II)
This group occurred in a mountain escarpment, 300–600 m a.s.l., in Makkah city. The community found in this group was Commiphora gileadinses- Acacia mellifera community. A. hamulosa, A. tortilis and Euphorbia arabica have been usually abundant; also a variety of annual grasses grow, including Aristida mutabilis, Tephrosia nubica, Stipa capensis and Cenchrus ciliaris among the most abundant species, C. gileadinses recorded a density of 30/hectare.
3.2.3. VG (III)
This group was recorded at a stony plateau, 140 m a.s.l., covered with lava flows, which are divided into a sheet of rocks known as 'harra'. In this vegetation group Commiphora quadricincta- A. hamulosa community was distinguished with A. mellifera, C. gileadensis and Euphorbia cuneate as codominant species. Among the woody herbs, which form a natural rockery of great beauty, after rainfall, are included Aerva javanica and Tribulus macropterus, in patches colonies. The most distinguishing perennial herbs of the hills were Cymbopogon schoenanthus, Anastatica hierochuntica, Aizoon canariense and Blepharis attenuate while Leguminosae was well represented with Indigofera spinosa. C. quadricincta and C. gileadensis were recorded in this community with a density of 50 and 3/hectare, respectively.
3.2.4. (IV)
Likewise the previous group, this group included two Commiphora species, C. quadricincta and C. gileadensis. The group was found on a mountain slopes, 105–140 ma.s.l., covered by lava rocks. Surprisingly, it is characterized by a larger number of plant species than the previous group, upper plateau, because of the accumulation of soil particles between the rocks in larger quantities than harra plateau. The community, which recorded in this group, was Cadaba glandulosa- Commiphora quadricincta community with A. hamulosa, A. melliferea as codominant. The most frequent species were Tephorosia apollineae, Ochradenus baccatus, and Forsskaolea tenacissima, while C. gileadensis was frequent in this vegetation group. C. quadricincta and C. gileadensis were recorded in this community with a density of 20 and 5/hectare, respectively.
3.2.5. VG (V)
This group was found in a hill covered with weathering basalt rocks, 130–270 m a.sl. Two different communities were recorded in this vegetation group. The first community was A. tortilis - A. ehrenbergiana community occurred on the stony slopes at 130 m a.s.l., with E. cuneata and A. hamulosa as common small trees on the stony slopes. In sheltered glens, Commiphora gileadensis, Lycium shawii and Cadaba glandulosa were abundant, the trees often spaced as close as 6 × 6 m, though they do not form a canopy. The second community, C. gileadensis - Lycium shawii was recorded at 235 m a.s.l. A. tortilis disappeared in this community and C. gileadensis increased with increasing elevation, furthermore Indigofera spinosa, Stipa capensis, Lindenbergia indica, and Grewia tenax were frequent. C. gileadensis was recorded in this group with a mean density of 25/hectare.
3.2.6. VG (VI)
This vegetation group showed the highest species number (Table 4), gamma diversity, and include three Commiphora species (C. Myrrha, C. kataf and C. habessinica) in three different plant communities:
Table 4.
The recorded vegetation groups with their biodiversity indices and densities (individual/hectare) of different Commiphora species.
| Vegetation groups |
||||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | |
| C. giladensis | 0 | 30 | 3 | 5 | 25 | 0 | 0 | 0 |
| C. quadricincta | 2 | 0 | 50 | 20 | 0 | 0 | 0 | 0 |
| C. myrrha | 0 | 0 | 0 | 0 | 0 | 30 | 35 | 5 |
| C. kataf | 0 | 0 | 0 | 0 | 0 | 20 | 0 | 0 |
| C. habessinica | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Number of stands | 10 | 20 | 40 | 10 | 40 | 40 | 40 | 40 |
| Total species number (Gamma species diversity | 21 | 17 | 25 | 31 | 28 | 71 | 59 | 36 |
| Species richness (sp stand-1 | 5.3 ± 0.03 | 5.1 ± 0.02 | 4.5 ± 0.02 | 2.57 ± 0.01 | 10.5 ± 0.95 | 11.5 ± 0.91 | 8.3 ± 0.90 | 7.4 ± 0.8 |
| Shanon index | 1.10 ± 0.08 | 1.06 ± 0.7 | 0.93 ± 0.07 | 0.53 ± 0.02 | 2.1 ± 0.09 | 2.4 ± 0.09 | 1.73 ± 0.07 | 1.54 ± 0.08 |
(A) Commiphora myrrha-Acacia etbaica community, between 12000 and 800 m trees occur on the hillsides at orchard spacing (5–20 m), among which, C. kataf, A. ehrenbergiana and Ficus cordata were frequent, together with Grewia tenax, and others, all of which become more closely spaced in small ravines. Cucumis prophetarum, Lycium shawii and Pupalia lappacea were almost abundant. In some small narrow valleys, the trees were almost thick enough to shape a light woodland. C. myrrha and C. kataf were recorded in this community with a density of 30 and 20/hectare.
(B) Acacia ehrenbergiana- A. hamulosa community.
This community was appeared at elevation (1000- 700 m) at a north facing mountain slope.
Few individuals of Commiphora habessinica were recorded in this community with a density of 2/hectare. Commelina benghalensis, Ocimum forsskaolii, Phlomis brachyodon and Scrophularia argute were abundant in higher elevation, where high water content present.
(C) Acacia hamulosa - Triumfetta flavescens community.
This community was appeared along the base of the foothills where the substratum is coarser (600–700 m), small-scattered trees of Acalypha fruticosa and Commiphora myrrha occur, furthermore Blepharis attenuate, Indigofera spinosa, Abutilon pannosum and Tephrosia purpurea were frequent.
3.2.7. VG (VII)
A. hamulosa- Commiphora myrrha community was recorded in this group on more gentle granite slopes, 450 m a.s.l, with Lycium persicum, Grewia tenax and Acacia etbaica were abundant, also Heliotropium strigosum, Euphorbia Arabica, Anticharis glandulosa, all become more frequent. C. myrrha recorded its highest density (35/hectare) in this community.
3.2.8. VG (VIII)
A. mellifera- C. myrrha community was recorded in this group and found at an escarpment consisted of granite and marble at the upstream of a tributary of al-Nu'man valley, 600 m a.s.l,. The most common species that always occurred were Grewia tenax, Rhazya stricta, Caralluma retrospiciens, Ephedra foliata and Ochradenus baccatus. Lindenbergia indica, Cocculus pendulus, Corbichonia decumbens and Tephrosia nubica were frequent, whilst Premna resinosa and Cleome chrysantha, which recorded just in this group.
3.3. Similarity
The hierarchical classification of Commiphora species, according to their associated species, (Fig. 5) resulted a dendrogram of two main groups, one included C. gileadinsis and C. quadricincta and the second included the other three species. Jaccard and Sorenson similarities (Table 5) were highest between C. gileadensis and C. quadricincta, followed by that obtained between C. kataf and C. habessinica. The previous similarities results were confirmed by both Pearson correlation coefficient (Table 6).
Fig. 5.
Hierarchical classification of the Commiphora's habitats based on their floristic composition (incidence data), obtained using Ward's method and Euclidean distances as measures of Linkage Distance.
Table 5.
Sorenson (bold) and Jaccard similarities between different Commiphora species.
| C. gileadensis | C. myrrha | C. quadricincta | C. habessinica | C. kataf | |
|---|---|---|---|---|---|
| C. gileadensis | 1 | 0.56 | 0.91 | 0.49 | 0.48 |
| C. myrrha | 0.39 | 1 | 0.55 | 0.69 | 0.47 |
| C. quadricincta | 0.84 | 0.38 | 1 | 0.47 | 0.47 |
| C. habessinica | 0.32 | 0.53 | 0.30 | 1 | 0.82 |
| C. kataf | 0.31 | 0.53 | 0.31 | 0.70 | 1 |
Table 6.
Pearson correlation coefficient between different Commiphora species.
| C. gileadensis | C. myrrha | C. quadricincta | C. habessinica | C. kataf | |
|---|---|---|---|---|---|
| C. gileadensis | 1 | −0.2256 | 0.8259 | 0.02758 | −0.02437 |
| C. myrrha | −0.2256 | 1 | −0.2122 | 0.4243 | 0.3708 |
| C. quadricincta | 0.8259 | −0.2122 | 1 | 0.004584 | −0.01291 |
| C. habessinica | 0.02758 | 0.4243 | 0.004584 | 1 | 0.6951 |
| C. kataf | −0.02437 | 0.3708 | −0.01291 | 0.6951 | 1 |
4. Discussion
The field survey of this study and also earlier investigation revealed that Commiphora species inhabit rocky habitats with soil accumulated in pockets and crevices. Geologically, the study area characterized by Precambrian basement rocks that have been deformed, and then eroded over hundreds of millions of years. The study area is distinguished by late Precambrian outcrops of granitic gneisses and Arabian lava fields (locally called harrat), creating scattered rock as habitats suitable for Commiphora species. We recorded five species of Commiphora in the study area out of six species of Commiphora of Saudi's flora indicating the availability of appropriate habitats for Commiphora. In the habitats of Commiphora the soils receives water steadily almost independent of rainfall changes. The relatively large surface area of the rocks harvest water to saturate the nearby soil pockets with water even in moderately dry years. Worldwide, a large number of plant species are confined to relatively open, shallow-soil, rocky habitats [12, 26, 34, 38, 41]. There is growing evidence that during the dry season, water held within the underlying bedrock is essential for meeting the transpiration demands of shrubs and trees [43, 45]. [40] suggested that the strong habitat specificity of many shallow soil endemics related to the degree of edaphic specialization needed to establish and survive in these harsh habitats and the incompatibility of these adaptations with deeper soil environments explaining the absence of Commiphora species in the deep soil of sandy plains. Commiphora species were not recorded in earlier study by [7] of habitat with similar rock type, but with higher elevation, differing in temperature and rainfall. Rock type, rainfall and temperature have a strong effect on the distribution of plant species [9, 10]. The presence associated species exhibited that a few families are of floristically importance as in most tropical and subtropical regions, where majority of plant species belong to a restricted number of plant families, a distinguishing character of the floristic structure of Saudi's flora [6]. Our data revealed that of the 35 families, 13 families (37%) are represented by one species per family. This is a common character of desert flora indicating that only a few of species of individual families have adapted and survived the harsh environment. The other species that could not adapt and endure did not survive. Therophytes as expect showed more than 48% of the total associated species, they usually bloom and form luxurious growth, when moisture collect after rainfall. These results explain that the life form/spectra of desert habitats in many parts of Saudi Arabia (e.g. [3, 7, 8, 16, 18, 20, 22]. The presence of five species of Commiphora in the study area could be attributed to the fact that the present-day flora of the West Saudi Arabia are the result of the evolutions taken place in the palaeotropical flora of ancient times [48, 51]. The Southwestern Arabian Peninsula and the northeastern region of Africa belong to a single phytogeographic entity and more than half of Commiphora species are native to the Horn of Africa [47]. Sudano-Zambezian region elements recorded the highest number as the studied area belongs to The Nubo-Sindian Province, which is a part of the Sudano-Zambezian Region [54]. Saharo-Arabian elements showed a high species number because plant species in this region adapted to aridity and very high temperatures. The ecological optimum of species is well reflected in the density of its individuals and dominance, as it reflects optimal conditions of the habitats. It is a reliable marker of phytosociolgical units [32]. stated that species with narrow ecological ranges, such as Commiphora, in their habitat are highly competitive with a plant of wider ranges, which explain the dominance of C. gileadensis, C. quadricincta and C. myrrah, for some communities.
A number of narrow ranged species of rocky habitats in the desert provide description of the plant communities confining to these habitats. These could be classified as exclusives and some of them are even endemic to these habitats. TWINSPAN classification divided the vegetation of Commiphora habitats into two main divisions, one includes C. gileadensis and C. quadricincta and the second includes the other three species. The difference between the two divisions habitats is clear in rock type, the first division characterized by granite and basalt rocks that were exposed to erosion, while the second division characterized by resistant, coarse pink granite, mixed with grey diorite and granodiorite. This indicates that the type of rock has an effect on Commiphora species distribution. Vegetation group (VI) exhibited the highest species number because of its geomorphological heterogeneity due to the range of differing aspects and slopes (topography). Difference in aspect and soil drainage has proven to be an important predictor of plant diversity [37]. In addition, dense fog at high altitudes condenses on the surface of the rocks and turns into water absorbed by the soil collected between the cracks of the rocks increases soil water content at high places. On the other hand, vegetation group (II) recorded the lowest species number because of its small area. The distribution of some plant species overlaps many groups, in some cases, owing to a wider ecological role of these species or the similarities between some habitats. The similarity between C. gileadinsis and C. quadricincta may be due to the degree to which they tolerate drought more than other species.
5. Conclusion
The present study is an attempt to compile the current available knowledge of Commiphora specie probably to be broadly representative of many parts of western Saudi Arabia. Investigations underline the significance of local sites for Commiphora. It is suggested that the description and classification of the different vegetation groups will facilitate understanding of the Commiphora life in the study area in particular and of Saudi Arabia as a whole. Our results can be usefully applied in the conservation and management of the area.
Declarations
Author contribution statement
Emad A. Alsherif: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was funded by the Deanship of Scientific Research (DSR) University of Jeddah, Jeddah, Saudi Arabia under grant no (UJ-05-18-DR).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2019.e01615.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Abdel Khalik K., Al-Gohary I., Al-Sodany Y. Floristic composition and vegetation: environmental relationships of wadi fatimah, mecca, Saudi Arabia. Arid Land Res. Manag. 2017;31(3):1–19. [Google Scholar]
- 2.Abdellatif D.A., Abdellahi B.M., Deida M.F., HucherN, Malhiac C., Renou F. Chemical and physicochemical characterizations of the water-soluble fraction of the Commiphora Africana exudate. Food Hydrocolloids. 2019;86:2–10. [Google Scholar]
- 3.Alatar A., El-Sheikh M.A., Thomas J. Vegetation analysis of Wadi Al-Jufair, a hyper arid region in Najd, Saudi Arabia. Saudi J. Biol. Sci. 2012;19(3):357–368. doi: 10.1016/j.sjbs.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Allen S.E., Grimshaw H.M., Parkinson J.A., Quarmby C., Roberts J.D. In: Methods in Plant Ecology. 2end ed. Moore P.D., Chapman S.B., editors. Blackwell Scientific Publications; Oxford: 1986. pp. 411–466. [Google Scholar]
- 5.Alsherif E.A. Exploration of unconventional fodder for arid land rehabilitation. Arid Land Res. Manag. 2018;32(3):337–350. [Google Scholar]
- 6.Al-Sherif E.A., Ayesh A.M., Rawi S.M. Floristic composition, life form and chorology of plant life at Khulais region, Western Saudi Arabia. Pakistan J. Bot. 2013;45:29–38. [Google Scholar]
- 7.Alsherif E.A., Fadl M.A. Floristic study of the Al-shafa highlands in taif, western Saudi Arabia. Flora. 2016;225:20–29. [Google Scholar]
- 8.Al-Turki T.A., Al-Qlayan H.A. Contribution to the flora of Saudi Arabia: hail region. Saudi J. Biol. Sci. 2003;10:190–222. [Google Scholar]
- 9.Austin M.P. Vegetation. In: Austin M.P., Cocks K.D., editors. vol. 2. CSIRO; Melbourne: 1978. pp. 44–46. (Land Use on the South Coast of New South Wales). [Google Scholar]
- 10.Austin M.P., Cunningham R.B., Fleming P.M. New approaches to direct gradient analysis using environmental scalars and statistical curve-fitting procedures. Vegetatio. 1984;55:11–27. [Google Scholar]
- 11.Bailey N.T.J. third ed. Cambridge UniversityPress; London: 1994. Statistical Methods in Biology. [Google Scholar]
- 12.Baskin J.M., Baskin C.C. Endemism in rock outcrop plant communities of unglaciated eastern United States: an evaluation of the roles of the edaphic, genetic and light factors. J. Biogeogr. 1988;15:829–840. [Google Scholar]
- 13.Bredenkamp G.J., Theron G.K. A synecological account of the Suikerbosrand Nature Reserve, Part I: the phytosociology of the Witwatersrand geological system. Bothalia. 1978;12:513–529. [Google Scholar]
- 14.Brown L.R., Du Preez P.J., Bezuidenhout H., Bredenkamp G.J., Mostert T.H.C., Collins N.B. Guidelines for phytosociological classifications and descriptions of vegetation in southern Africa. Koedoe. 2013;55:1–10. [Google Scholar]
- 15.Castro S.A., Jaksic F.M. Patterns of turnover and floristic similarity show a non-random distribution of naturalized flora in Chile, South America. Rev. Hist.Nat. 2008;81:111–121. [Google Scholar]
- 16.Chaudhary S. Flora of the kingdom o/Saudi Arabia. Ministry of Agriculture and Water, Riyadh. 2001;2(3):1–432. [Google Scholar]
- 17.Collenette S. Corpion publishing Ltd.; London: 1985. An Illustrated Guide to the Flowers of Saudi Arabia. [Google Scholar]
- 18.Collentette S. National Commission for Wildlife Conservation and Development; Riyadh: 1999. Wild Flowers of Saudi Arabia. [Google Scholar]
- 19.Cunninghama A.B., Brinckmannc J.A., Kullolid R.N., Schippmanne U. Rising trade, declining stocks: the global gugul (Commiphora wightii) trade. J. Ethnopharmacol. 2018;223:22–32. doi: 10.1016/j.jep.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 20.Daur I. Plant flora in the rangeland of western Saudi Arabia. Pakistan J. Bot. 2012;44:23–26. [Google Scholar]
- 21.Dixit A.M., Rao S. Observation on distribution and habitat characteristics of Guggal (Commiphora wightii) in the arid region of Kachchh, Gujarat (India) Trop. Ecol. 2000;41(1):81–88. [Google Scholar]
- 22.El-Demerdash M.A., Hegazy A.K., Zilaym A.M. Distribution of plant communities in Tihamah coastal plains of Jazan region, Saudi Arabia. Vegetatio. 1994;112:141–151. [Google Scholar]
- 23.Gostel M.R., Phillipson P.B., Weeks A. Phylogenetic reconstruction of the myrrh genus, Commiphora (Burseraceae), reveals multiple radiations in Madagascar and clarifies infrageneric relationships. Syst. Bot. 2016;41(1):67–81. [Google Scholar]
- 24.Hill M.O. Cornell University; Ithaca, NY, US: 1979. TWINSPAN – A FORTRAN Program for Arranging Multivariate Data in an Ordered Two-Way Table by Classification of the Individuals and Attributes. [Google Scholar]
- 25.Jackson M.L. Constable and Co. Ltd; London: 1962. Soil Chemical Analysis. [Google Scholar]
- 26.Kruckeberg A.R., Rabinowitz D. Biological aspects of endemism in higher plants. Annu. Rev. Ecol. Systemat. 1985;16:447–479. [Google Scholar]
- 27.Kulloli R.N., Purohit C.S., Kumar S., Jindal S.K., Rawat K., Acharya D. Rajasthan with Special Emphasis on its Conservation Planning in Arid Areas. Vegetos; 2013. Distribution of Commiphora wightii (arnt.) bhand. pp. 113–120. [Google Scholar]
- 28.Kumar R. Distribution and abundance of Commiphora wightii (Arn) Bhandari, in the forest of north Gujarat region (NGR) Gujarat. India. Ind J Pl Sci. 2013;2(1):43–51. http://www.cibtech.org/J-Plant-Sciences/PUBLICATIONS/2013/Vol-2-No-1/08004...Rajendra...Distribution%E2%80%A6India...43-51.pdf [Google Scholar]
- 29.Kumar S., Kulloli R.N. Effect of associated species on distribution of Commiphora wightii in indaian arid zone. Taiwania. 2017;26(1):43–49. [Google Scholar]
- 30.Lal H., Kasera P.K. Status and distribution range of guggal: a critically endangered medicinal plant from the Indian Thar desert. Sci. Cult. 2010;76:531–533. [Google Scholar]
- 31.Langenheim J.H. Timber Press; Portland, Cambridge: 2003. Plant Resins: Chemistry, Evolution, Ecology and Ethnobotany. [Google Scholar]
- 32.Lavergne S., Thompson J.D., Garnier E., Debussche M. The biology and ecology of narrow endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos. 2004;107:505–518. [Google Scholar]
- 33.Magurran A.E. PrincetonUniversity Press; New Jersey: 1988. Ecological Diversity and its Measurements. [Google Scholar]
- 34.Médail F., Verlaque R. Ecological characteristics and rarity of endemic plants from southeast France and Corsica: implications for biodiversity conservation. Biol. Conserv. 1997;80:269–281. [Google Scholar]
- 35.Migahid A.M. King Saud University Press; Riyadh: 1996. Flora of Saudi Arabia. [Google Scholar]
- 36.Misra R. Oxford and IBH Publishing Co.; New Delhi: 1968. Ecology Work Book. [Google Scholar]
- 37.Nichols W.F., Killingbeck K.T., Augustt P.V. The influence of geomorphological heterogeneity on biodiversity II. A landscape perspective. Conserv. Biol. 1998;12(2):371–379. [Google Scholar]
- 38.Pate J.S., Hopper S.D. Rare and common plants in ecosystems, with special reference to the South-west Australian flora. In: Schulze E.D., Mooney H.A., editors. Biodiversity and Ecosystem Function. Springer-Verlag; New York: 1993. pp. 293–325. [Google Scholar]
- 39.Pielou E.C. first ed. Wiely Interscience; New York: 1975. Ecological Diversity. [Google Scholar]
- 40.Poot P., Hopper S.D., van Diggelen J.M.H. Exploring rock fissures: does a specialized root morphology explain endemism on granite outcrops? Ann. Bot. 2012;110:291–300. doi: 10.1093/aob/mcr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porembski S., Barthlott W. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol. 2000;151:19–28. [Google Scholar]
- 42.Powers R.W., Ramirez L.F., Redmond C.D., Elberg E.L. U.S. Geological Survey Professional Paper 560-D; 1966. Geology of the Arabian Peninsula: Sedimentary Geology of Saudi Arabia; p. 147. [Google Scholar]
- 43.Querejeta J.I., Egerton-Warburton L.M., Allen M.F. Hydraulic lift may buffer rhizosphere hyphae against the negative effects of severe soil drying in a California oak savanna. Soil Biol. Biochem. 2007;39:409–417. [Google Scholar]
- 44.Raunkiaer C. 1934. Life Forms of Plants and Statistical Geography. Oxford, 632 P. [Google Scholar]
- 45.Rose K., Graham R., Parker D. Water source utilization by Pinus jeffreyi and Arctostaphylos patula on thin soils over bedrock. Oecol. 2003;134:46–54. doi: 10.1007/s00442-002-1084-4. [DOI] [PubMed] [Google Scholar]
- 46.Shen T., Li G., Wang X., Lou H. The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2012;142:319–330. doi: 10.1016/j.jep.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 47.Soromessa T. Ecological phytogeography: a case study of Commiphora species. Sci. Technol. Arts Res. J. 2013;2(3):93–104. https://www.ajol.info/index.php/star/article/viewFile/98910/88193 [Google Scholar]
- 48.Thomas J., Alfarhan A.H., Ali A., Miller A.G., Othman L. An account on the eastern limits of Afro-Arabian plants in South Asia. Bas App Dryd Res. 2008;2:12–22. [Google Scholar]
- 49.Vollesen K. Vol. 3. Addis Ababa University Press; 1989. pp. 442–478. (Burseraceae, Flora of Ethiopia). AddisAbaba. [Google Scholar]
- 50.Ward J.H. Hierarchical grouping to optimize an objective function. Am. Stat. Assoc. J. 1963;58:236–244. [Google Scholar]
- 51.White F., Leonard J. Phytogeographical links between Africa and southwest asia. Flora Veg. Mundi. 1991;9:229–246. [Google Scholar]
- 52.Wickens G.E. HMSO; London: 1976. The flora of Jebel Morra (Sudan Republic) and its Geographical Affinities. Kew Bulletin Additional Series V. [Google Scholar]
- 53.Zahran M. King Abdel Aziz University Press; Jeddah, Saudi Arabia: 1982. Vegetation Types of Saudi Arabia. [Google Scholar]
- 54.Zohary M. Fisher-Verlag; Stuttgart: 1973. Geobotanical Foundation of the Middle East 2 Vols. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.