ABSTRACT
Development of bio-therapeutics has exhibited exponential growth in China over the past decade. However, no biosimilar drug has been approved in China (CN) due to the lack of a national biosimilar regulatory guidance. HLX01, a rituximab biosimilar developed in China under European Medicines Agency biosimilar guidelines and requirements, was the first such drug submitted for regulatory review in China, and it is expected to receive approval there as a biosimilar product. To demonstrate the analytical similarities of HLX01, CN-rituximab (sourced in China but manufactured in Europe) and EU-rituximab (sourced and manufactured in Europe), an extensive 3-way physicochemical and functional similarity assessment using a series of orthogonal and state-of-the-art techniques was conducted, following the similarity requirement guidelines recently published by China’s Center for Drug Evaluation. The results of the similarity study showed an identical protein amino acid sequence and highly similar primary structures between HLX01 and the reference product (RP) MabThera®, along with high similarities in higher order structures, potency, integrity, purity and impurity profiles, biological and immunological binding functions, as well as degradation behaviors under stress conditions. In addition, HLX01 presented slightly lower aggregates and better photostability compared with the RP. Despite slight changes in relative abundance of glycan moieties and heavy chain C-terminal lysine modification, no differences in biological activities and immunological properties were observed between the RP and HLX01. In conclusion, HLX01 is highly similar to CN- and EU-sourced RP in terms of physicochemical properties and biological activities, suggesting similar product quality, efficacy, and safety. The regulatory requirements interpreted and applied towards the HLX01 marketing application sets a precedent for analytical similarity assessment of biosimilar products in China.
KEYWORDS: Analytical similarity, biosimilar mAb, LC-MS characterization, physicochemical assessment, functional assessment, China biosimilar regulatory
Introduction
Therapeutic monoclonal antibodies (mAbs), developed for their predictable properties, controlled functions and long circulating half-life, now represent one of the fastest growing applicable biological medicines.1 However, the increasing use of therapeutic mAbs constitutes a major and increasing cost burden for health care systems.2,3 Due to high costs, therapeutic mAbs account for less than 5% of the biopharmaceutical market in China, far below the average global market share of 30%.4 Biosimilars are defined by China’s National Medical Product Administration (NMPA; formerly the China Food and Drug Administration) as biologically similar alternatives that are similar in quality, safety and efficacy to the approved reference products (RPs).5 The availability of biosimilars would improve affordability and bring increased access to essential therapeutics to a broader patient population, especially for patients in developing countries such as China.3
All of the world’s top five best-selling therapeutic mAbs (adalimumab, rituximab, bevacizumab, trastuzumab and infliximab) will lose patent protection by 2020; as a consequence, development of corresponding biosimilars has substantially expanded both in China and abroad in recent years.6,7 During the 12th five-year plan (2011–2015) of China, the Chinese government allocated about 40 billion RMB to research and development (R&D) of new drugs in the biomedical industry. Moreover, the R&D of therapeutic mAbs has been listed as a national Key Technology R&D Program by the Chinese government. Benefitting from these favorable policies, great progress in R&D and manufacturing technologies of therapeutic mAbs has been observed.4 More than 200 companies are now engaged in the development of therapeutic mAbs in China, and the NMPA has so far approved a total of 21 therapeutic antibodies. However, a substantially larger number of innovative mAb products are available on the US and European markets. For the market entry of biosimilar therapeutics, as of May 2018, the US Food and Drug Administration (US-FDA) has approved 11 biosimilars (including 7 mAbs) and the European Medicines Agency (EMA) has approved 39 biosimilars (including 12 mAbs). However, no true biosimilar drug has been approved in China so far due to the lack of a local biosimilar regulatory guidance before February 2015.7,8
In February 2015, the NMPA issued a tentative technical guideline for research, development and evaluation of biosimilars.5 The guideline is expected to improve accessibility and quality of biological drugs in China, while simultaneously excluding substandard “follow-on-biologics”, which often have a different protein amino acid sequence from their reference products, as biosimilars. It sets forth the basic principles for the technical review, the criteria for comparability, and the conditions under which extrapolations of indications would be permissible. The principles and key requirements for establishing biosimilarity are consistent with those of the US-FDA and EMA. 9–11 They call for comparisons of biosimilar and innovator drugs with an extensive array of comparative studies, including biochemical and physical characterizations on the structure of the molecules, and biological functions and properties. In addition, NMPA requires the use of RPs sourced locally, with an approval in China. A stepwise approach to examine similarity through comparative Chemistry, Manufacturing and Control (CMC)-quality data, non-clinical studies, and clinical studies is required. Furthermore, the methods used for similarity studies should be consistent with those used for the RP. Multiple and orthogonal methods should be used to demonstrate similarity of critical quality attributes (CQAs). Although it shares the same principles for technical evaluation and review, the NMPA biosimilar guideline did not provide detailed instructions for statistical evaluation of collected data compared to its US and European Union (EU) counterparts.12
Rituximab was the first mAb approved for treatment of B cell lymphoma.13 The chimeric mAb is composed of human-derived IgG1 heavy chains, kappa constant regions, and mouse-derived variable regions targeting CD20. The primary mechanism of action (MOA) of rituximab comprises targeting CD20 on the surface of B-lymphocytes to induce cell death by antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and apoptosis.14,15 As listed in Chart 1, EMA has approved 7 rituximab applications (including 6 biosimilar products), while US-FDA has approved 2 rituximab applications for the innovator drug. HLX01 was developed by Shanghai Henlius Biotech as a biosimilar to MabThera®, the Roche’s innovator rituximab marketed in China. Submitted to NMPA in October 2017, the new drug application (NDA) for HLX01 was the first NDA for a biosimilar mAb ever submitted to the agency.
Chart 1.
US-FDA and EMA approved rituximab applications.
To assess the similarity of HLX01 to the CN-rituximab and EU-rituximab RPs, a battery of state-of-the-art and orthogonal technologies for comparison of physicochemical and biological properties were applied, following the NMPA regulatory guideline on biosimilarity evaluation. Relevant guidelines of EMA, US-FDA and ICH Q5E were used as an infrastructure. The extensive 3-way similarity assessment of 12 batches of HLX01, 15 batches of CN-rituximab and 7 batches of EU-rituximab demonstrated that HLX01 was highly similar to the RPs.
Results
The function of a therapeutic mAb depends on its structure, which is fundamentally determined by its primary amino acid sequence, disulfide linkages and post-translational modifications (PTMs), which alter its size, mass, folding and stability. Accordingly, a set of highly sensitive, orthogonal and state-of-the-art analytical techniques were first employed to compare the structures, followed by evaluation of the critical physicochemical and biophysical attributes of HLX01 with the RPs (Table 1). CN-rituximab is manufactured in Germany and then shipped to China for packaging and labeling. Accordingly, the EU-rituximab was selected as a RP for similarity comparison as well. The attributes investigated included: the primary structure, higher order structures, charged variants, glycan structures, purity/impurity profiles, as well as various aspects of biological and immunological functions. CQAs were identified using a risk ranking approach,16 considering any attributes that may have an impact on the efficacy and safety of rituximab. The similarity of these quality attributes between the biosimilar HLX01, CN-rituximab and EU-rituximab was statistically assessed by a tiered approach.17 The evaluation of the analytical similarity was conducted as described below.
Table 1.
Summarized quality attributes and key findings in physiochemical and biological similarity assessment between HLX01 and the reference products.
| Category | Product Quality Attributes | Tier | Analytical Methods | Assessment |
|---|---|---|---|---|
| Physiochemical characteristics | ||||
| Primary structure | Amino acid sequence | I | LC-MS/MS | Identical to the RP |
| Intact and reduced molecular weights | II | LC-MS | Similar to the RP | |
| Disulfide linkage pattern | II | LC-MS/MS | Identical to the RP | |
| Free thiol groups | II | Ellman’s assay | Similar to the RP | |
| PTMs:Deamidation, Oxidation, Glycosylation, C-terminal and N-terminal Variants | II | LC-MS/MS | Same modification sites and similar level of modifications to the RP | |
| Higher order structure | Protein secondary and tertiary structure | II/III | DSC, CD, FTIR | Similar to the RP |
| Charge heterogeneity | Charge heterogeneity | II | CEX icIEF |
Similar to the RP, with higher basic variants due to unprocessed HC C-terminal lysine, not clinical meaningful |
| Isoelectric point | II | icIEF | Same to the RP | |
| Glycosylation | N-linked glycans | II | RapiFluor labeling and HILIC UPLC-FLD | Same glycan types with slightly difference in %G0F + %Man + %Gal + % Afuc, not clinical meaningful |
| Sialylation | II | DMB labeling and RPLC-FLD | Similar to the RP | |
| N-linked glycosylation site, and site occupancy | II | LC-MS/MS | Same to the RP | |
| Molecular variants | Soluble aggregates | I | SEC-HPLC | Slightly lower than the RP |
| Monomer | II | SEC-HPLC, CE-SDS | Similar to the RP with high level of purity | |
| Low molecular weight fragment | II | SEC-HPLC, CE-SDS | Slightly lower than the RP | |
| Biological characteristics | ||||
| Immunological functions | FcRn binding activity | I | SPR | Similar to the RP |
| FcγRIa binding activity | II | SPR | Similar to the RP | |
| FcγRIIa binding activity | ||||
| FcγRIIb/c binding activity | ||||
| FcγRIIIa (F) binding activity | ||||
| FcγRIIIa (V) binding activity | ||||
| FcγRIIIb binding activity | ||||
| C1q binding activity | II | ELISA | Similar to the RP | |
| Antigen binding activity | I | FACS | Similar to the RP | |
| Biological activities | CDC activity | I | Cell based assay | Similar to the RP |
| ADCC activity | II | Cell based assay | Similar to the RP | |
| Apoptosis | II | Cell based assay | Similar to the RP | |
| Safety of residues | Residual DNA | I | PCR | Similar to the RP |
| Residual HCP | I | ELISA | Similar to the RP | |
| Residual Protein A | I | ELISA | Similar to the RP | |
Primary structure of HLX01 is highly similar to that of the RPs, with identical amino acid sequence
The primary structure of HLX01, CN-rituximab and EU-rituximab was investigated using multiplex methods, including intact, reduced and deglycosylated, papain cleaved protein mass analyses, reduced peptide mapping, measurement of free thiol groups and disulfide linkage mapping.
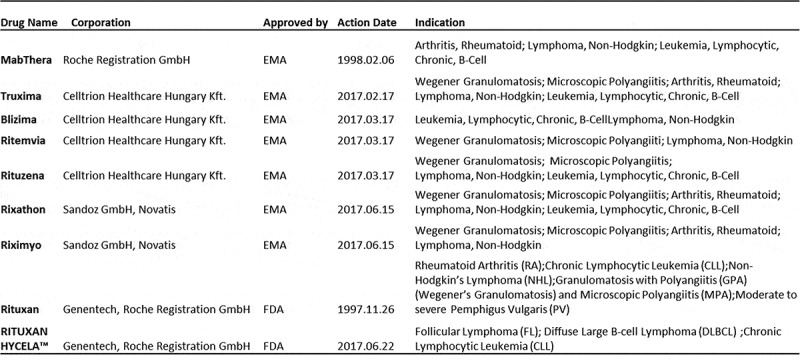
The predominant peaks in the intact protein mass spectrometry (MS) analysis of HLX01 and RPs closely matched the theoretical molecular weights of rituximab with different glycan forms (mass error < 5Da). As shown in Figure 1(a), the four highest MS peaks represent the 2 heavy chain (HC) glycoforms/modifications G0F/G0F/-2K/PyroQ(4), G0F/G1F/-2K/PyroQ(4), G1F/G1F/-2K/PyroQ(4), and G1F/G2F/-2K/PyroQ(4) of rituximab, respectively, in which “-2K” means C-terminal lysine truncation on both HCs and “PyroQ(4)” indicates N-terminal glutamate to pyroglutamate conversion on both HCs and both light chains (LCs). The molecular masses of reduced HLX01 and RPs provided further evidence that the LC and HC polypeptide compositions were similar between HLX01 and RPs (Figure 1(b,c)). In the MS spectra of reduced HC, three predominant MS peaks representing G0F/-K/PyroQ, G1F/-K/PyroQ and G2F/-K/PyroQ were observed, while only a 49060.3 Da peak (mass error < 3Da) belonging to -K/PyroQ HC was detected after deglycosylation (Figure 1(d)). At the same time, no glycosylation was observed on the LC, since only a single peak of 23035.7 Da (mass error < 1 Da) representing the PyroQ LC was observed both before and after the deglycosylation treatment. The above observations suggest that the different molecular masses between the biosimilar and RP antibodies are mainly due to the presence of various HC glycoforms. The mass spectra of papain-digested fragments further demonstrated that glycosylation occurred only on the Fc region of the HC (Supplemental Figure 1). In conclusion, the intact and reduced masses of HLX01 and the RPs are largely identical, with minor differences in the relative abundance of glycoforms, indicating that they have highly similar primary structures with similar levels of PTMs.
Figure 1.
Comparison of protein masses of HLX01 and RPs. The MS spectra for intact mAb (a), reduced LC (b), reduced HC (c) and reduced and deglycosylated HC (d) of HLX01, CN-rituximab and EU-rituximab.
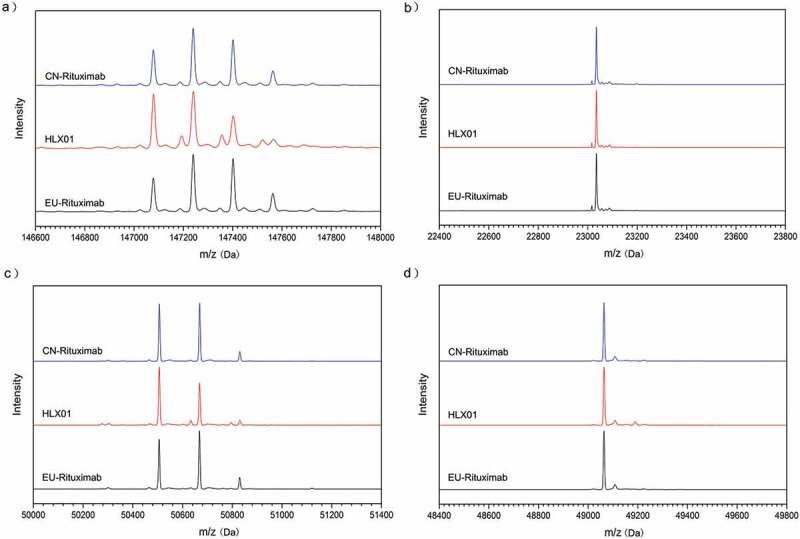
The reduced liquid chromatography (LC)-MS/MS peptide maps of HLX01, CN-rituximab and EU-rituximab exhibited the same peaks with similar intensities and retention times (Figure 2), and no additional peaks were observed in the HLX01 chromatograms compared with those of the RPs. This further confirmed that HLX01 possesses highly similar primary structure compared to the RPs. The sequences of the peptides identified from alternative trypsin and chymotrypsin digests covered 100% of the amino acid sequence of rituximab, demonstrating that the primary sequences of HLX01, CN-rituximab and EU-rituximab were identical, and matched the theoretical amino acid sequences of rituximab. The same PTM patterns, including N301-glycosylation, C-terminal K-truncation, N-terminal pyroQ, M-oxidation, N-deamidation and succinimide formation, were all detected in HLX01, EU-rituximab and CN-rituximab. The modified peptides, along with their PTM sites and PTM levels, are presented in Supplemental Table 1. Except for a slight difference in the amount of C-terminal lysine variants of HC, the identified PTM levels were similar between HLX01 and the RPs.
Figure 2.
Peptide maps of trypsin digested HLX01, CN-rituximab and EU-rituximab.
Non-reducing LC-MS/MS peptide maps showed that the same IgG1 disulfide linkage pattern in HLX01 as in the RPs. A total of 16 pairs of disulfide linkages were identified, including 12 intrachain and 4 interchain disulfide bonds (Supplemental Table 2). In addition, the total number of free thiols in all three mAb products was below the limit of quantification (0.688 μmol/L) of the Measure-iT thiol assay. These findings demonstrated that HLX01 and the RPs have the same disulfide linkages, and no mismatched disulfide bond was detected.
Higher order structures of HLX01 are highly similar to those of the RPs
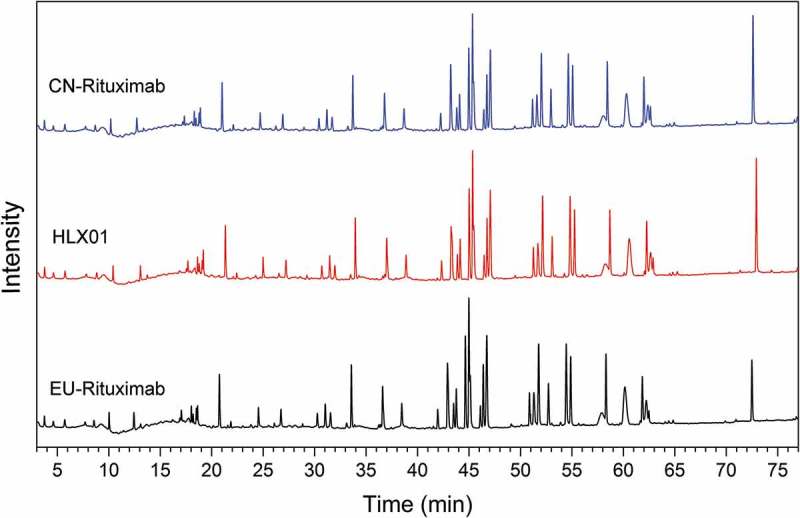
The higher order structures of HLX01, CN-rituximab and EU-rituximab were characterized using multiple biophysical techniques. The compactness, thermodynamic and kinetic stability of mAbs were examined by differential scanning calorimetry (DSC). As shown in Figure 3(a), both HLX01 and the RPs showed highly similar thermal unfolding profiles and identical transition temperatures (Tm) of 71.8°C, 75.2 ºC, and 83.4°C, with the confidence interval (CI) at 95% and standard deviation <0.02°C for all Tms. The three thermal transitions correspond to the denaturation of the constant CH2, the antigen-binding fragment (Fab) and constant CH3 domains, respectively.18 In the circular dichroism (CD) spectroscopy analysis, no differences in secondary structure (α-helix, β-sheet, β-turn and random coil) or tertiary structure were observed between HLX01 and the RPs in the far and near UV spectra (Figure 3(b,c)). The Fourier transform infrared spectroscopy (FTIR) profiles were visually similar between HLX01 and the RPs (Figure 3(d)). All spectra exhibit strong β-sheet bands at around 1639 cm−1 and 1689 cm−1 as well as a β-turn band at around 1670 cm−1, indicating the presence of antiparallel β-sheet structure typically observed in antibodies.19 When compared with the reference spectrum of RS201505020304-RM04, the similarity of all samples were greater than 0.99. Collectively, DSC, CD and FTIR results demonstrated that the higher order structures of HLX01 were highly similar to those of the RPs.
Figure 3.
The higher order structure analysis by DSC, CD and FT-IR for HLX01, CN-rituximab and EU-rituximab. (a) DSC thermograms, (b) Far-UV CD spectra, (c) Near-UV CD spectra, (d) FTIR spectra.
HLX01 has similar charge variant profiles to the RPs, except for slightly higher level of basic variants due to unprocessed HC c-terminal lysine
Charge variants of mAb typically resulting from PTMs might influence stability, biological activity, and pharmacokinetics of antibodies. Assessment of charge variants showed that HLX01 has similar charge variant profiles compared with CN-rituximab and EU-rituximab, except for a slightly higher level of basic variants representing more unprocessed HC C-terminal lysine. It has been established that the presence or truncation of C-terminal lysine does not affect efficacy and safety of antibody products.20
In cation exchange chromatography (CEX) analysis, charge variants were successively eluted in acid peaks, main peak, and basic peaks. As shown in Figure 4(a), the dominant peak in all three samples is the “-2K/PyroQ(4)” variant. LC-MS analysis of acidic or basic CEX fractions reveals that the acidic variants were mainly due to a sialic acid modification, while basic peak BP1 resulted from unprocessed C-terminal lysine on the HC, and BP2 resulted from N-terminal Q (not pyroglutamate) on the LC. Except for BP1, CEX profiles were visually similar between HLX01 and the RPs. The percentage of each charge variant was listed in Supplemental Table 3, which portrays higher basic variants and lower acidic variants in HLX01 compared with the RPs. After the removal of lysine by carboxypeptidase B (CpB), HLX01 exhibits similar levels of basic and acidic variants compared with the RPs (Figure 4(b), Supplemental Table 3), which confirmed that the surplus of basic charge variant was mainly due to the surplus of the unprocessed HC C-terminal lysine.
Figure 4.
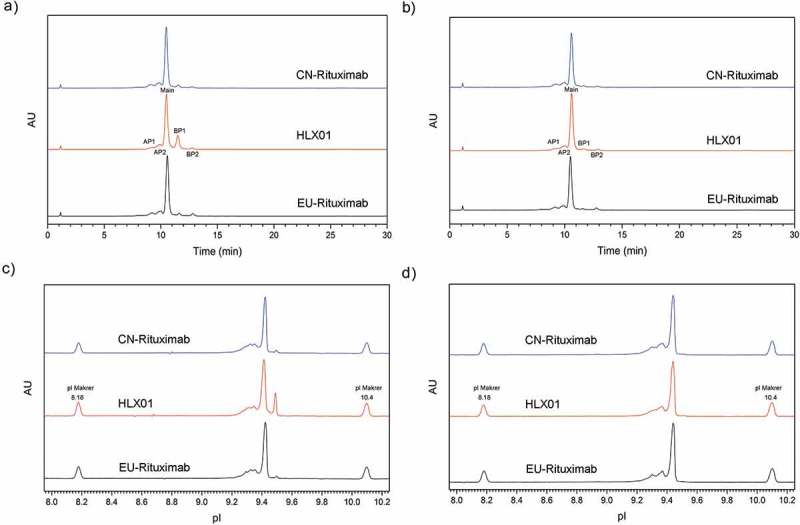
Comparison of the charge variant profiles among HLX01, CN-rituximab and EU-rituximab. (a) CEX chromatogram before CpB digestion, (b) CEX chromatogram after CpB digestion, (c) icIEF profiles before CpB digestion, (d) icIEF profiles after CpB digestion. “AP” indicates acidic peak, “Main” indicates main peak, “BP” indicates basic peak.
Similarly, the same charge species and similar profiles were observed by imaged capillary isoelectric focusing (icIEF) (Figure 4(c,d)). Again, the icIEF data before and after CpB digestion support the conclusion that HLX01 has a higher level of basic peaks due to unprocessed HC C-terminal lysine.
HLX01 has similarly high purity level and slightly lower aggregate/fragment compared with the RPs
Product and process-related impurities in medicinal products, especially high molecular weight aggregates and host cell proteins (HCP), which have been implicated in enhancing immunogenicity, may have effects on safety and efficacy.21 The high purity and similar product- and process-related impurity profiles in HLX01 and the RPs are demonstrated below.
The molecular size variants of HLX01, CN-rituximab and EU-rituximab were examined by size-exclusion chromatography (SEC) and capillary electrophoresis-sodium dodecyl sulfate (CE-SDS). SEC was performed to detect the levels of aggregates, monomers and fragments in HLX01, CN-rituximab and EU-rituximab under native conditions. A predominant peak (>98.9%) belonging to the monomeric mAb was observed with identical retention time in all samples, confirming low levels of impurities (Figure 5(a), Supplemental Table 4). Slightly lower levels of aggregates were observed in HLX01 as compared to the RPs. Since SEC analysis of the low molecular weight variants is limited due to poor resolution,22 CE-SDS serves as an orthogonal method to provide more accurate quantification. Both the reduced and non-reduced CE-SDS profiles of HLX01 and the RPs showed similar peak shapes and patterns (Figure 5(b), Supplemental Table 5).
Figure 5.
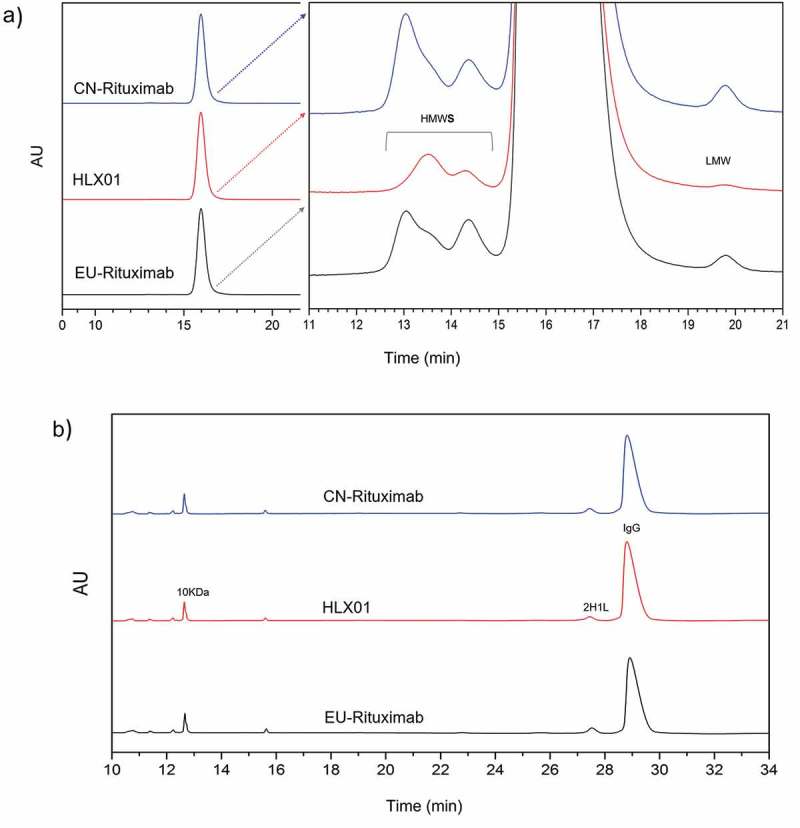
Comparison of the purity of HLX01, CN-rituximab and EU-rituximab. (a) SEC-HPLC chromatograms. The enlarged chromatograms are plotted on the right panel. The high molecular weight variants (HMW), monomer and low molecular weight variants (LMW) are separated. (b) Non-reduced CE-SDS chromatograms.
Sensitive methods were selected to trace process-related impurities in these drug products; the corresponding results are listed in Supplemental Table 6. The RT-qPCR data indicate that the levels of residual DNA, which might cause oncogenesis, were lower than 0.1 pg/mg in all drug products (the release test acceptance criterion is below 100 pg/dose or 1 pg/mg). The results of HCP quantitation by ELISA showed that the residual HCP concentrations of HLX01 and the RPs were all below 2 ppm (the release test acceptance criterion for HLX01 is below 10 ppm). The concentrations of residual protein A in all samples were below the detection limit of the ELISA assay.
Overall, it can be concluded that HLX01 has the same level of high purity and low impurity profiles, well within the product specifications of CN-rituximab and EU-rituximab. Furthermore, HLX01 possesses slightly lower aggregates and fragments when compared to the RPs, demonstrating the quality of the manufacturing process of HLX01.
HLX01 has the same glycosylation site and occupancy, and same glycans with minor differences in their relative abundances compared with the RPs
Glycosylation plays a significant role in the function, efficacy, in vivo half-life, and immunogenicity of an antibody. LC-MS/MS peptide mapping confirmed that there are no O-linked glycosylation modifications in both HLX01 and the RPs, but, as expected, one N-linked glycosylation site exists at Asn 301 of the HC. The reduced CE-SDS results indicated that >99.6% HC N301 was glycosylated (Supplemental Table 5) for all tested samples. Ultra-high performance liquid chromatography with fluorescence detector (UHPLC-FLD) profiles of the PNGase F-released N-glycans indicated that the same N-glycan species and similar abundance distribution were detected in these samples (Figure 6). G0F and G1F were the major N-glycan species. The relative G0F content is higher in HLX01, resulting in lower relative content of galactose-contained glycans (Gal, see Supplemental Table 7 for classification of glycan types) compared with that of the RPs. The average Gal % of HLX01, CN-rituximab and EU-rituximab are 43.6%, 53.5% and 53.7% (Supplemental Table 7), respectively. Galactose content has a weak effect on CDC activity,23 but a difference of 10% in galactose between HLX01 and the RPs would not cause noticeable changes in their CDC activities, which has been confirmed by the cell-based bioassay for CDC (Figure 7(c)). The content differences of other types of N-glycans, including high mannosylated (Man), sialylated (Sialy) and afucosylated (Afuc), among HLX01, and CN-rituximab and EU-rituximab were all less than 2%.
Figure 6.
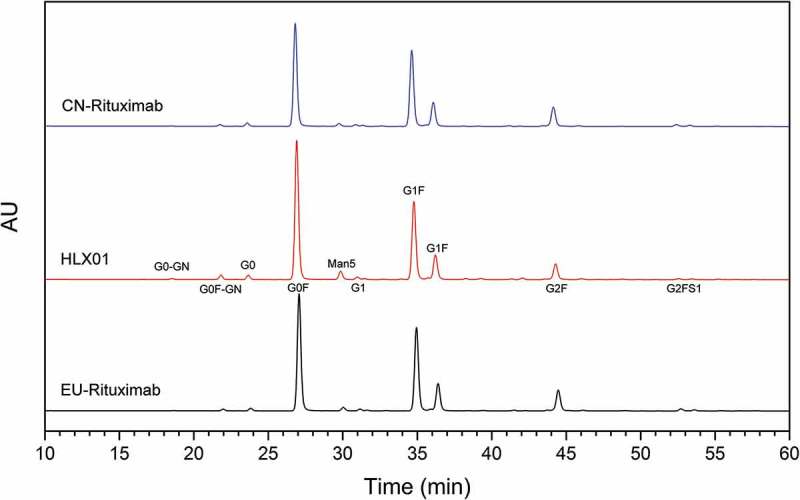
Comparison of N-glycan species of HLX01, CN-rituximab and EU-rituximab. The major types of glycans are labelled beside corresponding peaks in the middle panel (HLX01).
Figure 7.
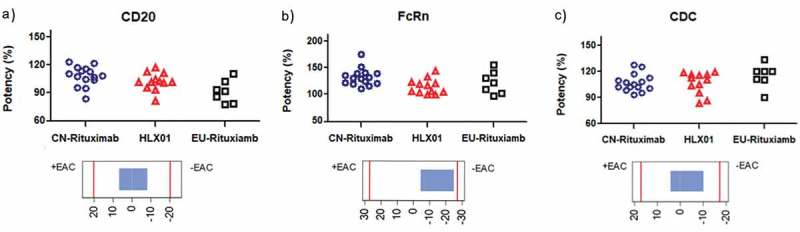
Comparison of Tier 1 biological quality attributes of HLX01, CN-rituximab and EU-rituximab. The dot plots of (a) CD20 binding affinity, (b) FcRn binding affinity and (c) CDC activity were plotted above corresponding equivalence test results showing 90% CI. Each marker shows the activity value of a specific batch: the blue dots show the values of CN-rituximab, red triangles show the values of HLX01, and black squares show the values of EU-rituximab.
Sialylation can affect the bioactivity and safety of antibody drugs, especially the half-life of protein drugs in the human body. The absolute amount of sialic acid was quantified after acid hydrolysis and 1, 2 – diamino −4, 5 -methyleneoxybenzene (DMB) derivation followed by HPLC-FLD. In line with the results of N-glycan analysis, HLX01 exhibited a slightly lower content of N-acetylneuraminic acid (NANA) (0.038–0.075 mol/mol) than the RPs (0.115–0.215 mol/mol). N-Glycolylneuraminic acid (NGNA), a cause of potential immunogenicity in humans, was not detectable in most batches of HLX01, except for a batch in which 0.001 mol NGNA per mol of antibody was detected. The content of NGNA was detected at trace amounts of 0.003–0.006 mol/mol antibody in CN-rituximab and EU-rituximab (Supplemental Table 7). In summary, these small differences in glycosylation are not expected to affect biological activities and immunological properties between HLX01 and MabThera® mAbs as demonstrated below.
Biological and immunological activities of HLX01 are similar to those of the RPs
Rituximab can induce death of CD20 + B cells by CDC, ADCC, and apoptosis. According to the theoretical MOA of rituximab, 12 biological and immunological tests were conducted and evaluated in tiers following US-FDA guidance,24 including CD20 binding, Fc-receptor bindings, ADCC, CDC and apoptosis assays (Table 2).
Table 2.
Summary of functional assay results for HLX01, CN-rituximab and EU-rituximab.
| Sample | CD20-binding mean |
FcRn-binding mean |
FcγRIa-binding mean |
FcγRIIa-binding mean |
FcγRIIb/c-binding mean |
FcγRIIIa(V) -binding mean |
FcγRIIIa(F) -binding mean |
FcγRIIIb-binding mean |
C1q-binding mean |
CDC mean |
ADCC mean |
Apoptosis mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (range) | (range) | (range) | (range) | (range) | (range) | (range) | (range) | (range) | (range) | (range) | (range) | |
| HLX01 | 107% | 113% | 103% | 102% | 90% | 95% | 98% | 102% | 102% | 107% | 95% | 99% |
| (83–123%) | (98%–143%) | (93%–124%) | (97%–109%) | (81%–99%) | (84%–101%) | (89%–118%) | (93%–116%) | (90%–115%) | (83%–119%) | (77%–110%) | (73%–114%) | |
| CN-rituximab | 91% | 131% | 99% | 105% | 99% | 85% | 80% | 85% | 102% | 107% | 77% | 98% |
| (77–110%) | (110%–174%) | (80%–110%) | (98%–111%) | (83%–114%) | (74%–102%) | (67%–100%) | (72%–100%) | (91%–122%) | (93–127%) | (63–97%) | (61–121%) | |
| EU-rituximab | 102% | 121% | 118% | 109% | 100% | 84% | 76% | 90% | 106% | 115% | 83% | 97% |
| (81%–117%) | (96%–155%) | (111%–124%) | (104%–113%) | (69%–123%) | (76%–93%) | (64%–89%) | (83%–99%) | (101%–114%) | (90–133%) | (62–107%) | (68–112%) |
CD20 binding, FcRn binding and CDC activity were evaluated as Tier 1 for the following reasons. The binding capacity to the CD20 molecule on the surface of B cell is the basis of rituximab-inducing cell death. In the fluorescence-activated cell sorting assay, the binding affinity of HLX01 to Raji cells was highly similar to the RPs (Figure 7(a)). FcRn participating in the recycling of IgG can potentially affect the half-life of mAb drugs in serum. As shown in Figure 7(b), in vitro FcRn-binding assay results suggested similar binding capabilities for each antibody. To assess CDC activity, WIL2-S cells were incubated with HLX01 and the RPs in the presence of serum complement source. The dose-dependence analysis revealed that similar cytotoxic effects were detected for all antibodies. Figure 7(c) shows that HLX01 induced similar CDC effects compared to those observed from the RPs.
Fc-related binding, including FcγRIa, FcγRIIa, FcγRIIb/c, FcγRIIIa-F, FcγRIIIa-V and FcγRIIIb were performed by surface plasmon resonance (SPR). The binding capability to C1q was assessed by ELISA. Fcγ expressed on white blood cells are related to the ADCC activities of therapeutic antibodies. Similarity evaluation by the quality range approach of Tier 2 suggested that HLX01, CN-rituximab and EU-rituximab have similar binding affinities to FcγRIa, FcγRIIa, FcγRIIb/c, FcγRIIIa-F, FcγRIIIa-V and FcγRIIIb (Figure 8(a–f)). The binding of the Fc to C1q could initiate the classical pathway of complement activation, then lead to dissolution of target cells. The CDC-related C1q-binding affinity was confirmed to be similar between HLX01 and the RPs by ELISA (Figure 8(g)).
Figure 8.
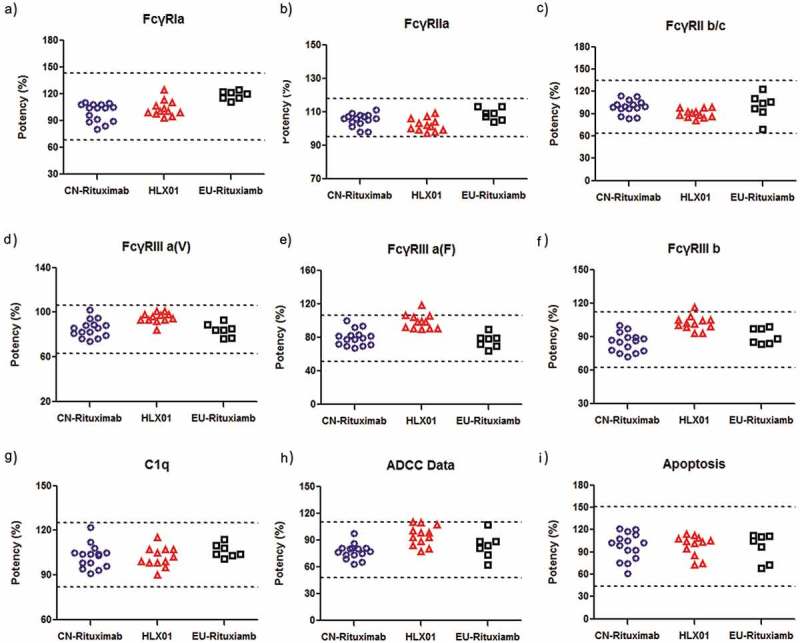
Comparison of Tier 2 Fab and Fc related binding functions of HLX01, CN-rituximab and EU-rituximab. The dot plots of (a) FcγRIa binding affinity, (b) FcγRIIa binding affinity, (c) FcγRIIb/c binding affinity, (d) FcγRIIIa-F binding affinity, (e) FcγRIIIa-V biding affinity, (f) FcγRIIIb binding affinity, (g) C1q binding affinity, (h) ADCC activity, (i) Apoptosis activity. Each marker shows the activity value of a specific batch: the blue dots show the values of CN-rituximab, red triangles show the values of HLX01, and black squares show the values of EU-rituximab. Dashed lines represent quality range according to the suggestion of FDA (mean ± 3 SD).
To evaluate the Fc-effector function of rituximab, the relative activities of ADCC and apoptosis were analyzed for HLX01, CN-rituximab and EU-rituximab. Luciferase, a product of natural killer cell pathway activation in the effector cell, was quantified to evaluate ADCC activity. In the co-culture of WIL2-S and Jurkat cells, all antibodies developed a dose-dependent ADCC response with no significant differences among them. As shown in Figure 8(h), HLX01, CN-rituximab and EU-rituximab induced highly similar ADCC responses. Concurrently, the apoptosis assay using Raji cells suggested similar results and conclusion (Figure 8(i)) as well.
In summary, the biological functions, including CD20 binding, Fc receptor binding, ADCC, CDC and apoptosis of HLX01 are similar to those of the RPs from 7 EU-rituximab and 15 CN-rituximab batches.
HLX01, EU-rituximab and CN-rituximab showed good stability and consistent degradation behaviors under accelerated and stress conditions
Three batches of HLX01, CN-rituximab and EU-rituximab were randomly picked for forced degradation comparative study. Changes in the stability-indicating attributes were assessed using SEC, CEX, non-reduced CE-SDS, LC-MS and cell-based assays.
Except for continuous shaking, which had little effect on the physiochemical and biological properties of rituximab, most forced degradation conditions (high temperature, light exposure, strong acid, strong base, and strong oxidizer) accelerated the degradation of all samples. The results of CEX, SEC, CE-SDS and LC-MS indicate that HLX01 and the RPs have a consistent degradation behavior and result in the same corresponding PTMs. However, compared with HLX01, CN-rituximab and EU-rituximab showed a faster degradation rate and higher proportion of oxidation under light exposure (Supplemental Figure 2). Under the treatment of high temperature, light exposure, strong acid or strong base for longer than a week, the CDC activity of both HLX01 and the RPs decreased slightly (less than 30%), which indicates good stability of all the tested mAbs. In summary, HLX01, CN-rituximab, and EU-rituximab exhibited similar stability upon stress treatments by high temperature, strong acid, strong base, strong oxidizer and shaking conditions.
Discussion
Biosimilar mAbs, which can provide affordable access to biologic therapies, can ameliorate a substantially unmet medical need in populous nations such as China. HLX01, developed by Henlius, is the first product to enter regulatory review in China following the biosimilar guidance, which was published by China’s Center for Drug Evaluation in February 2015. In the study presented here, we applied NMPA biosimilar regulatory requirements for the first time to evaluate the analytical similarities between a biosimilar and innovator mAb. The systematic physiochemical and functional comparison of the HLX01, CN-rituximab and EU-rituximab demonstrated an example for analytical similarity assessment of biosimilar mAb in China.
Despite sharing the same principles for establishing similarity, NMPA guidelines present notable differences in the detailed operational requirements from the US and EU regulatory authorities, including the choice of the RPs, the classification and analytical requirement for quality attributes, as well as the similarity evaluation criteria.5,25–28 In this study, we take NMPA guidelines as the fundamental criteria, and also refer to the guidance of EMA, US-FDA and ICH Q5E. In the choice of the RPs, NMPA requires multiple batches obtained in China;5 US-FDA requires a minimum of 10 reference batches;24 and EMA requires multiple batches covering the life cycle of the RPs.27 The shelf life of MabThera® is 3 years; accordingly, we selected 15 batches of CN-rituximab with expiring dates from March 2015 to February 2018. Moreover, 7 batches of EU-rituximab, which were manufactured in the same regional factories as CN-rituximab, were selected for comparison as well, which may extend the similarity study for potential future EMA applications. For the quality attribute evaluation, NMPA requires analysis of multiple product quality attributes (PQAs), including primary and higher order structures, biological activities, purity/impurities, immunology properties and forced degradation behaviors.5 In terms of analytical methods, NMPA encourages the use of analytical methods consistent with those for innovator analysis originally applied by the innovator company, while EU and US agencies encourage the use of state-of-the-art technology taking into account relevant and up-to-date information.25,27 As a result, state-of-the-art orthogonal analytical technologies were applied in comparative analyses of certain PQAs of HLX01 and the RPs, if the methods used for the PQA analyses of the innovator were not readily available from the public or official sources. NMPA guidelines also require setting similarity acceptance criteria according to the potential clinical impact of each PQA. However, NMPA does not provide methods for classification and statistical analysis for the collected data of the PQAs. Following US-FDA guidelines24 and ICH Q8, we classified the identified PQAs into three tiers according to their criticality to clinical outcomes, and then performed corresponding statistical analyses to demonstrate similarity between HLX01 and the RPs for Tier 1 and 2 attributes of biological and immunological functions. For Tier 3 attributes, they are simply required to be visually similar.
All the comparison results demonstrated that HLX01 is analytically similar to CN-rituximab and EU-rituximab (Table 1). Firstly, a series of orthogonal and state-of-the-art LC-MS analyses suggested identical amino acid sequence and highly similar primary structures, including the same glycosylation species, disulfide linkages, and PTM sites and percentages among HLX01, CN-rituximab and EU-rituximab. This meets the fundamental requirement of NMPA’s recently released similarity evaluation guidance, also demanded by EMA and US-FDA, that the amino acid sequence of biosimilars must be consistent with that of the innovator drug.5 Secondly, CD, DSC and FTIR attested to the highly similar secondary and tertiary structures of HLX01 compared to MabThera®, further proving the same disulfide linkage pattern and indicating similar biological activities and functions could be expected. Thirdly, the purity and heterogeneity profiles (by SEC, CE-SDS, CEX, icIEF, glycan profiling) and process-related residuals (residual HCP, DNA, and protein A) analyses indicated that HLX01 has similarly high purity and even slightly less aggregates and fragments than the RPs (demonstrated by SEC and CE-SDS). Fourthly, HLX01 showed similar degradation behavior under stress conditions and better photostability compared with RPs. Finally, antigen binding activity, Fc receptor binding affinity, and Fc-effector function (CDC, ADCC and apoptosis) are all statistically similar among HLX01, CN-rituximab and EU-rituximab. The totality of these results demonstrated the analytical similarity of HLX01 to MabThera®, indicating its similar quality, efficacy, and safety.
Although minor differences such as charge heterogeneity and relative abundance of glycans were observed between rituximab and HLX01, the similarity of results in biological activity and immunogenicity function confirmed that these differences had no effect on MOA and the potential safety. Charge heterogeneity due to PTMs can potentially influence stability, biological activity, and pharmacokinetics of mAbs.29 The slightly higher level of basic variants in HLX01 compared with EU-rituximab and CN-rituximab resulted from unprocessed HC C-terminal lysine. The C-terminal lysine variants commonly observed in biopharmaceutical mAbs, which are the main contributor to basic peak variants, range from 10–30% in different batches of rituximab innovator drugs,30 which indicates that different lots of MabThera® exhibit broader C-terminal heterogeneity differences than that between HLX01 and MabThera®. Meanwhile, all tested lots of MabThera® remained on the market with unaltered labels in the tested time frame, indicating that the observed changes were predicted not to result in an altered clinical profile, and are therefore determined to be acceptable by the health authorities.30 Previous publications demonstrated that the C-terminal lysine residues can be rapidly cleaved by endogenous CpB in vivo after intravenous injection with a half-life time of about an hour.31 The C-terminal lysine of an antibody was therefore reported to have little effect on structure, biological activity and safety.20,32 After the removal of C-terminal lysine by CpB, HLX01 exhibited slightly lower acidic variants and higher main peak proportions compared to the RPs. The acidic variants of HLX01 and the RPs were identified due to the sialic acid of glycans, which may affect ADCC activity.33 Accordingly, the production process of HLX01 strictly controlled the acid species. As a result, both the NANA and NGNA levels were slightly lower than those in RPs. Due to potential safety issues associated with immunogenicity, NGNA should not be present in mAb products.
Glycosylation can influence in vivo half-life, immunogenicity, and biological activities such as CDC and ADCC of mAbs.34,35 Even a well-controlled product may contain different glycoforms.30 The glycosylation pattern is often inherently variable from one batch to another, and changes in the manufacturing process can add to the variabilities.36 In a quality study of multiple batches of Aranesp®, MabThera® and Enbrel®,30 substantial alterations of the glycosylation profile were observed for all the tested products. MabThera® with expiry dates from September 2007 to October 2011 exhibited abundance differences in afucosylated G0 and Man5, which induced slight variation in ADCC activity among batches.30 As cited before, the differences were predicted not to result in an altered clinical profile. Compared with the RPs, HLX01 has identical types of N-glycans, detected with small differences in relative abundances. The relative content of galactose-containing glycans (Gal), which may enhance FcγRIIIa binding and CDC activity,37 is 10% lower in HLX01 compared with the RPs. Gal is known to mediated CDC through C1q binding of the Fc region. The Gal content of Herceptin® decreased from about 40% in the batches produced before August 2015 to about 22% in those after August 2015, while little or even no C1q binding activity drift in breast cancer cell lines was observed.36 Studies have shown that tripling Gal content only increases CDC activity from 60% to 100%.38 Therefore, the content difference of 10% Gal between HLX01 and MabThera® should not cause a noticeable difference in CDC activity. The CDC and ADCC activities, as well as Fcγ binding affinities, of HLX01, CN-rituximab and EU-rituximab were confirmed to lack statistically significant differences. Except for Gal, only minor differences (<2%) of high Man, Sialylation and Afuc among HLX01, CN-rituximab and EU-rituximab were detected. According to the similarity results, these glycosylation relative abundance differences have no effect on biological activities and immunological properties.
Taken together, the 3-way similarity assessment demonstrates that HLX01 is a highly similar product to CN-rituximab and EU-rituximab in primary and higher order structures, PTMs, purity, impurity profiles, biological activities, immunological properties, forced degradation behavior and trends, indicating similar product quality, efficacy, and safety. These were confirmed by the comparable results of head-to-head non-clinical, and Phase 1 and 3 clinical trials.39–42
Materials and methods
Reference product EU-rituximab (MabThera®, 10 mg/mL) and CN-rituximab (MabThera®, 10 mg/mL) were purchased from Roche. HLX01 (10 mg/mL) was manufactured by Shanghai Henlius Biopharmaceuticals, China. The batches of CN-rituximab, EU-rituximab and HLX01 were chosen to cover a range of shelf life; their corresponding lot numbers and expiration dates are listed in Supplemental Table 8. To avoid repeated freeze/thaw cycles, each sample was stored in multiple aliquots according to the manufacturer’s instruction and at −80°C before expiration. Chemicals were purchased from Sigma without special mention.
Intact and reduced molecular weight analysis
The molecular weights of intact, reduced and papain cleaved HLX01, EU-rituximab and CN-rituximab were determined by Waters ACQUITY UPLC (C4 BEH column, 1.7 μm 300 Å, 2.1 × 50 mm) coupled online to Waters Xevo G2-XS Q-TOF mass spectrometer. The samples used for reduced analysis were treated with dithiothreitol (DTT) for reduction, and PNGase F (New England Biolabs) for the N- linked glycan removal. 0.5 μg of each sample was injected and data in the m/z range of 500–4000 Da were acquired. Waters BiopharmaLynx software was used to process and interpret the collected LC-MS data.
Peptide mapping and PTM identification
The amino acid sequence and PTMs, including deamidation, oxidation and glycosylation, were assessed by peptide mapping of trypsin digests of reduced mAbs using reversed-phase (RP) LC-MS/MS (Waters ACQUITY UPLC-Xevo G2-XS Q-TOF system). Samples were solubilized in 6 M guanidine hydrochloride and 50 mM Tris hydrochloride (Tris-HCl) at pH 8.0, reduced with 10 mM DTT and alkylated with 20 mM iodoacetamide (IAM). The reduced alkylated samples were then desalted and buffer exchanged to 50 mM Tris-HCl 10 mmol/L CaCl2 (pH 8.0) with a NAP-5 column (GE Healthcare), and then digested with trypsin or chymotrypsin (Promega). For N-glycosylation site profiling, the samples were further digested with PNGase F (New England Biolabs). The resulting peptides were subsequently injected onto a UPLC C18 column (Acquity UPLC BEH, 1.7 mm, 2.1 × 150 mm; Waters) coupled online to Waters Xevo G2-XS Q-TOF MS system for separation and detection. MS data were processed by Waters BiopharmaLynx software.
Disulfide bonds were analyzed by non-reduced peptide maps. The samples for native peptide mapping was solubilized in 50 mM Tris-HCl (pH 8.0), and alkylated with 10 mM n-ethylmaleimide. RapiGest (Waters) was added to facilitate trypsin digestion. The resulting disulfide bond linked peptides were identified by LC-MS/MS analysis as mentioned above.
Free thiol analysis
The free thiol (SH) groups in the samples were determined by Measure-iT thiol assay kit (Thermo Fisher). Briefly, the standard and samples were mixed with Ellman’s reagent according to the manufacturer’s instructions. The fluorescence signal was then detected using a microplate reader (excitation/emission light: 490 nm/517 nm, Molecular Devices SpectraMax i3X). The content of free thiol groups was estimated by comparison to a standard curve composed of known concentrations of cysteine.
Glycan profiling
HC N-glycans were released by PNGase F digestion and then labeled by RapiFluor-MS N-Glycan kit (Waters), followed by hydrophilic interaction liquid chromatography (HILIC) purification. Purified glycans were separated by a Waters BEH Glycan Amide column (1.7 µm, 1.7 × 150 mm) on a UHPLC system (Agilent 1290) equipped with a fluorescence detector (excitation/emission light of 265 nm/425 nm).
Sialic acid (NGNA and NANA) assay
The content of sialic acid in mAb samples was analyzed by RP-LC after labeling with DMB (Takara). Briefly, HCl was added to the sample to reach a final concentration of 50 mM. Reagent and coupling solution were then added, and the mixture was incubated at 50°C for 2.5 hours protected from light. The coupling reaction was submerged in an ice bath for 5 minutes. The sample was then separated by a C18 column (Phenomenex Jupiter, 250 × 2.0 mm, 5 µm) with Agilent 1260 HPLC equipped with a fluorescence detector (excitation/emission light: 373 nm/448 nm).
Higher order structure analysis
Thermal stability of the samples was evaluated by measuring their Tm values using a TA Nano DSC. Samples were diluted to 1 mg/ml with reference buffer and scanned from 20 to 95°C with a scan rate of 1°C/min. Nano Analyze software was used for data analysis.
CD experiments were carried out on a JASCO J-715 spectrometer equipped with a Peltier-type cell holder. Spectra were recorded at 4 s response time and a band width of 1 nm. Near-UV CD spectra were recorded from 250 to 340 nm using a path length of 0.1 nm at a scanning speed of 50 nm/min. Far-UV CD spectra were recorded for the range of 195–250 nm using path length of 0.2 nm at a scanning speed of 100 nm/min.
FTIR measurements were performed using Thermo Scientific NicoletTM iSTM5 system with reflective mode and DTGS KBr detector at a resolution of 4 cm−1 and scan number of 64. Spectral similarity was calculated using Thermo Scientific OMNICTM 8.2.
Charge heterogeneity assays
The icIEF method was used to determine isoelectric point (pI) values of charge variants in the sample. MAb samples were treated with CpB (100:1 w/w) for 2 hours to remove the unprocessed Lys residue at the C terminus of the heavy chain. Both the CpB-treated sample and untreated sample were mixed with Pharmalyte 8–10.5, 1% methyl cellulose, pI marker (8.18/10.10) and distilled water. The mixture was loaded onto an iCE3 icIEF instrument (Protein Simple) and resolved by pre-focusing for 1 min at 1500 V, and focusing for 6 min at 3000 V. The pIs of the sample peaks were determined using a linear regression between two pI marker peaks.
The distribution of charge variant was evaluated by CEX analysis using ProPac WCX-10 (Thermo Scientific, 4.0 mm × 250 mm) on an Agilent 1260 HPLC system. UV detection was performed at a wavelength of 280 nm. Results of both CpB-treated and native samples were reported as relative percentages of charge variants.
Molecular size variant assay
SEC-HPLC was performed to quantify molecular size variants on an Agilent 1260 HPLC system with Tosoh TSKgel G3000WXL (300 × 7.8 mm, 5 µm) column. Analytes were monitored by UV absorbance at 280 nm. CE-SDS was performed on Beckman Coulter PA800 Plus to separate size variants under reduced or non-reduced conditions. MAb samples were denatured using sodium dodecyl sulfate and incubated at 70°C for 10 minutes before injection. For reduced conditions,β-mercaptoethanol was added to reduce the disulfide bonds. Analytes were monitored by UV absorbance at 220 nm. The content of corresponding variants was evaluated by determining the peak area of each species as a percentage of the total peak area.
CD20 binding assay
Binding of HLX01, EU-rituximab and CN-rituximab to CD20 was measured by a cell-based competitive binding assay. Test samples were used to displace a fluorescently labeled mAb on human Raji cells. The fluorescence is detected using flow cytometer (Accuri C5, BD). The intensity of the fluorescent signal is directly proportional to the amount of mAb bound to CD20 on cells. The CD20 binding activity was determined by concentration for 50% of maximal effect (EC50).
C1q binding assay
The binding activity to the human C1q was determined by ELISA. Different concentrations of mAb samples were added onto a 96-well plate. After blocking with milk, 25 µg/mL C1q (Abcam) was added and incubated for 1 hr at room temperature. Then anti-C1q-HRP antibody conjugate (1:300 Abcam) and TMB, which generates a colorimetric reaction, was added to complete the reaction. The C1q binding activity of mAbs was determined by EC50.
Fc receptor binding assays
The affinity of mAb samples to human Fcγ and FcRn receptors was determined by SPR using a Biacore T200 (GE Healthcare). Recombinant Fcγ receptors were immobilized covalently on CM5 sensor chips. The mAb sample was serially diluted and loaded on the chip surface. The results of Fc receptor binding activity were analyzed by the multi-cyclic kinetics strategy and reported by comparing the KD ratio of the test sample to that of a reference.
Fc-effector function bioassays
Biological activity was measured in human B cell lines by evaluating the cytotoxicity in the presence of HLX01, EU-rituximab or CN-rituximab. ADCC was evaluated by colorimetric luciferase release assay. Briefly, WIL2-S cells (1.25 × 104) and Jurkat cells (7.5 × 104) were seeded in a 96-well plate with 4-fold serially diluted test sample at a concentration from 3000 ~ 0.01 ng/ml. After incubation at 37°C, 5% CO2 for 6 hrs, the cell cytotoxicity was measured by quantification of released luciferase and reported as ADCC activity. To assess CDC activity, WIL2-S cells were incubated with 2-fold serially diluted mAbs (30 μg/ml ~ 117 ng/ml) in the presence of normal human serum as a complement source. Target cell depletion was monitored by determining cell viability at the end of the incubation with CellTiter-Glo® reagent (Promega). Relative potencies of both ADCC and CDC were determined using a parallel logistic assay (4-parameter fit) by comparing the EC50 of the sample to a reference.
The apoptosis bioassay measured the rituximab-induced externalization of phosphatidylserine on Raji B cells. After 24 hrs incubation with rituximab, the cells were harvested and stained with FITC Annexin-V Apoptosis Detection Kit II (BD Pharmingen TM). The apoptosis activity was evaluated by reading the fluorescence intensity of the dead cells using a flow cytometer (Acuri 5, BD).
Residual safety assays
Real-time fluorescence quantitative polymerase chain reaction (RT-qPCR) on Thermo Fast 7500 qPCR instrument was used to detect the residual DNA of host cells in the monoclonal antibody samples expressed by Chinese hamster ovary cells (CHO). The HCP impurities were quantitatively measured by a customized CHO HCP ELISA kit (Cygnus Technologies). A Cygnus Technologies Protein A ELISA kit was used to detect the residual Protein A in the samples. ELISA experiments were performed by MD microplate reader and SoftMax Pro was used for data analysis.
Forced degradation study
The degradation profiles were determined under the circumstances of high temperature (50°C), light (4500 ± 500 lux) exposure, strong acid (pH 4), strong alkali (pH 10), strong oxidizer (1.0% tBHP) or shaking at speed of 1000 rpm and 25°C. The time points for each treatment are listed in supplemental Table 9. Subsequent characterization of the degradation samples was conducted by using LC-MS, CEX, SEC, CE-SDS and CDC as listed in Supplemental Table 9.
Statistical analysis
The quality attributes of rituximab were ranked according to the potential impact on clinical efficacy and safety. Equivalence testing is applied for attributes ranked as Tier 1 with the highest potential clinical impact. An interval (−1.5SD, 1.5SD) that can support 90% confidence interval is recommended in two one-sided tests to determine the similarity of HLX01 and the RPs. A quality range approach is recommended for attributes categorized as Tier 2 with high risk ranking. Standard deviation of the RPs is used to determine similarity acceptance criteria. Similarity with RP can be determined, when 90% of HLX01 data falls in the quality range (mean – 3SD, mean + 3SD). Tier 3 attributes with lowest risk ranking were evaluated by visual comparison.
Abbreviations
- ADCC
Antibody-Dependent Cell-Mediated Cytotoxicity
- CD
Circular Dichroism
- CDC
Complement-Dependent Cytotoxicity
- CDE
Center for Drug Evaluation
- CE-SDS
Capillary Electrophoresis - Sodium Dodecyl Sulfate
- CEX
Cation Exchange Chromatography
- CFDA
China Food and Drug Administration (renamed to NMPA, China National Medical Product Administration, on August 29th 2018)
- CHO
Chinese Hamster Ovary Cells
- CI
Confidence Interval
- CMC
Chemistry and Manufacturing Control
- CN
China
- CpB
Carboxypeptidase B
- CQA
Critical Quality Attribute
- DSC
Differential Scanning Calorimetry
- DMB
1, 2 - Diamino −4, 5 –Methyleneoxybenzene
- DTT
Dithiothreitol
- EC50
50% of Maximal Effect
- EMA
European Medicines Agency
- FLD
Fluorescence Detector
- FTIR
Fourier Transform Infrared Spectroscopy
- HC
Heavy Chain
- HCP
Host Cell Proteins
- HILIC
Hydrophilic Interaction Liquid Chromatography
- HPLC
High Performance Liquid Chromatography
- icIEF
Imaged Capillary Isoelectric Focusing
- LC
Light Chain
- LC-MS
Liquid Chromatography-Mass Spectrometry
- mAb
Monoclonal Antibody
- MOA
Mechanism of Action
- MS
Mass Spectrometry
- NANA
N-acetyl-neuraminic Acid
- NMPA
National Medical Product Administration
- NDA
New Drug Application
- NGNA
N-glycolylneuraminic Acid
- PQA
Product Quality Attribute
- PTM
Post-Translational Modification
- R&D
Research and Development
- RP
Reference Product
- RP-LC
Reversed Phase Liquid Chromatography
- SEC
Size-Exclusion Chromatography
- SPR
Surface Plasmon Resonance
- US-FDA
US Food and Drug Administration
Acknowledgments
We gratefully thank Ms. Dorothy Huang for critical English review as well as the scientists of Department of Bioassay and Analytical Development, Shanghai Henlius Biotech, Inc. and Departments of Quality Control and Manufacturing, Shanghai Henlius Biopharmaceuticals, Inc. for assistance with experimental execution, figures preparation, and HLX01 manufacturing. Shanghai Henlius Biopharmaceuticals, Inc. is a subsidiary company of Shanghai Henlius Biotech, Inc.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–15. doi: 10.4161/mabs.19931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu P, Graff R, Zheng L, Khawli L, Epstein A. Preclinical characterization of CTL-1, A biosimilar anti-EGFR monoclonal antibody for cetuximab. SM J Bioequiv Availab. 2017;1:1003. [Google Scholar]
- 3.McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91:405–17. doi: 10.1038/clpt.2011.343. [DOI] [PubMed] [Google Scholar]
- 4.Shan-Shan M, Su-Yong M, Guang-Rong Z. The status and development prospect of Chinese antibody drug industry. China Biotechnol. 2015;35:103–08. http://html.rhhz.net/ZGSWGCZZ/html/20151216.htm. [Google Scholar]
- 5.Center for drug evaluation of China National Medical Product Administration Technical guideline for development and evaluation of biosimilars (Interim) [accessed 2015. February 28]. http://samr.cfda.gov.cn/WS01/CL1616/115104.html.
- 6.Beck A. Biosimilar, biobetter and next generation therapeutic antibodies. MAbs. Taylor & Francis 2011;3:107–10. doi: 10.4161/mabs.3.2.14785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Min L, Ri-Wei W. The market overview of monoclonal antibodies in both domestic and abroad. China Biotechnol. 2017;37:106–14. http://html.rhhz.net/ZGSWGCZZ/html/20170315.htm. [Google Scholar]
- 8.Zhang J. Biosimilars in China. Pharmaceutical Executive; 2018. http://www.pharmexec.com/biosimilars-china
- 9.Committee for Medicinal Products for Human Use (CHMP) of European Medicines Agency (EMA) Guideline on similar biological medicinal products [accessed 2014. March 11]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf.
- 10.U.S. Food and Drug Administration (FDA) Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. U.S. Department of Health and Human Services [accessed 2014. March 1]. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf.
- 11.WHO Expert Committee on Biological Standardization Guidelines on evaluation of Similar Biotherapeutic Products (SBPs); 2014. [accessed 2014 March 1]. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.
- 12.Wang K. Biosimilars are regulated differently in China. pharma DJ; 2015. https://www.internationallawoffice.com/Newsletters/Healthcare-Life-Sciences/China/Ropes-Gray-LLP/Biosimilars-are-regulated-differently-in-China.
- 13.Leget GA, Czuczman MS. Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol. 1998;10:548–51. [DOI] [PubMed] [Google Scholar]
- 14.Dorvignit D, Palacios JL, Merino M, Hernández T, Sosa K, Casacó A, López-Requena A, Mateo de Acosta C. Expression and biological characterization of an anti-CD20 biosimilar candidate antibody: a case study. MAbs: Taylor & Francis. 2012;4:488–96. doi: 10.4161/mabs.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri G-M, Bernasconi S, Tedesco F, Rambaldi A, Introna M. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–08. [PubMed] [Google Scholar]
- 16.Alt N, Zhang TY, Motchnik P, Taticek R, Quarmby V, Schlothauer T, Beck H, Emrich T, Harris RJ. Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biologicals. 2016;44:291–305. doi: 10.1016/j.biologicals.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Chow S-C, Song F, Bai H. Analytical similarity assessment in biosimilar studies. Aaps J. 2016;18:670–77. doi: 10.1208/s12248-016-9882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ionescu RM, Vlasak J, Price C, Kirchmeier M. Contribution of variable domains to the stability of humanized IgG1 monoclonal antibodies. J Pharm Sci. 2008;97:1414–26. doi: 10.1002/jps.21104. [DOI] [PubMed] [Google Scholar]
- 19.Seo N, Polozova A, Zhang M, Yates Z, Cao S, Li H, Kuhns S, Maher G, McBridge HJ, Liu J. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. mAbs. Taylor & Francis 2018:10:678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Zhang J, Ren D, Tsai WL, Li F, Amanullah A, Hudson T. Probing of C‐terminal lysine variation in a recombinant monoclonal antibody production using Chinese hamster ovary cells with chemically defined media. Biotechnol Bioeng. 2012;109:2306–15. doi: 10.1002/bit.24510. [DOI] [PubMed] [Google Scholar]
- 21.Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;11:99–109. doi: 10.3109/1547691X.2013.821564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G. Potential inaccurate quantitation and sizing of protein aggregates by size exclusion chromatography: essential need to use orthogonal methods to assure the quality of therapeutic protein products. J Pharm Sci. 2010;99:2200–08. doi: 10.1002/jps.21989. [DOI] [PubMed] [Google Scholar]
- 23.Raju TS, Jordan RE. Galactosylation variations in marketed therapeutic antibodies. MAbs. Taylor & Francis 2012;4:385–91. doi: 10.4161/mabs.19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration (FDA) Guidance for industry: Statistical approaches to evaluate analytical similarity [accessed 2017. September 22].
- 25.U.S. Food and Drug Administration (FDA) Guidance for industry: quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. [assessed 2015. April]. https://www.fda.gov/downloads/drugs/guidances/ucm291134.pdf.
- 26.Committee for Medicinal Products for Human Use (CHMP) of European Medicines Agency (EMA) Guideline on development, production, characterisation and specification for monoclonal antibodies and related products [assessed 2016. July 21]. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/08/WC500211640.pdf.
- 27.Committee for Medicinal Products for Human Use (CHMP) of European Medicines Agency (EMA) Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1) [accessed 2014. May 22]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/06/WC500167838.pdf.
- 28.Schiestl M, Li J, Abas A, Vallin A, Millband J, Gao K, Joung J, Pluschkell S, Go T, Kang H-N. The role of the quality assessment in the determination of overall biosimilarity: a simulated case study exercise. Biologicals. 2014;42:128–32. doi: 10.1016/j.biologicals.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, Yao Z, Sreedhara A, Cano T, Tesar DB, et al. Charge variants in IgG1: isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs. Taylor & Francis 2010;2:613–24. doi: 10.4161/mabs.2.6.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiestl M, Stangler T, Torella C, Čepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 2011;29:310. doi: 10.1038/nbt.1867. [DOI] [PubMed] [Google Scholar]
- 31.Cai B, Pan H, Flynn GC. C‐terminal lysine processing of human immunoglobulin G2 heavy chain in vivo. Biotechnol Bioeng. 2011;108:404–12. doi: 10.1002/bit.22933. [DOI] [PubMed] [Google Scholar]
- 32.Antes B, Amon S, Rizzi A, Wiederkum S, Kainer M, Szolar O, Fido M, Kircheis R, Nechansky A. Analysis of lysine clipping of a humanized Lewis-Y specific IgG antibody and its relation to Fc-mediated effector function. J Chromatogr B. 2007;852:250–56. doi: 10.1016/j.jchromb.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Mimura Y, Kelly RM, Unwin L, Albrecht S, Jefferis R, Goodall M, Mizukami Y, Mimura-Kimura Y, Matsumoto T, Ueoka H, et al. Enhanced sialylation of a human chimeric IgG1 variant produced in human and rodent cell lines. J Immunol Methods. 2016;428:30–36. doi: 10.1016/j.jim.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Zheng K, Bantog C, Bayer R. The impact of glycosylation on monoclonal antibody conformation and stability. MAbs: Taylor & Francis. 2011;3:568–76. doi: 10.4161/mabs.3.6.17922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefferis R. Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol Sci. 2009;30:356–62. doi: 10.1016/j.tips.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Song J, Park S, Ham S, Paek K, Kang M, Chae Y, Seo H, Kim H-C, Flores M. Drifts in ADCC-related quality attributes of Herceptin®: impact on development of a trastuzumab biosimilar. MAbs: Taylor & Francis. 2017;9:704–14. doi: 10.1080/19420862.2017.1305530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abès R, Teillaud J-L. Impact of glycosylation on effector functions of therapeutic IgG. Pharmaceuticals. 2010;3:146–57. doi: 10.3390/ph3010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–78. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Biotech H. Important news! Henlius completed its first phase III study of HLX01 (Rituximab injection); 2018. http://www.henlius.com/en/NewsD-894.html.
- 40.Shi Y, Zhang Q, Han X, Song Y, Qin Y, Hong X, et al The pharmacokinetics, pharmacodynamics and safety profiles of HLX01 are equivalent to MabThera in B-cell lymphoma patients who have reached complete response (CR) or complete response uncertain (CRu). CSCO Annual Congress; 2018; China: CSCO-Xiamen. [Google Scholar]
- 41.Shi Y, Zhang Q, Han X, Song Y, Qin Y, Hong X, Ke X, Feng J, Wang D, Li W, et al. First china-manufactured proposed rituximab biosimilar met primary efficacy and safety endpoints in CD20-positive diffuse large B-cell lymphoma. Ann Oncol. Singapore 2018;29. doi: 10.1093/annonc/mdy437.005. [DOI] [Google Scholar]
- 42.Shi Y-K. The clinical journey of HLX01: from phase 1 clinical trial to potentially first MabThera® biosimilar in China. CSCO Annual Congress; 2018; CSCO; Xiamen, China. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


