Figure 1.
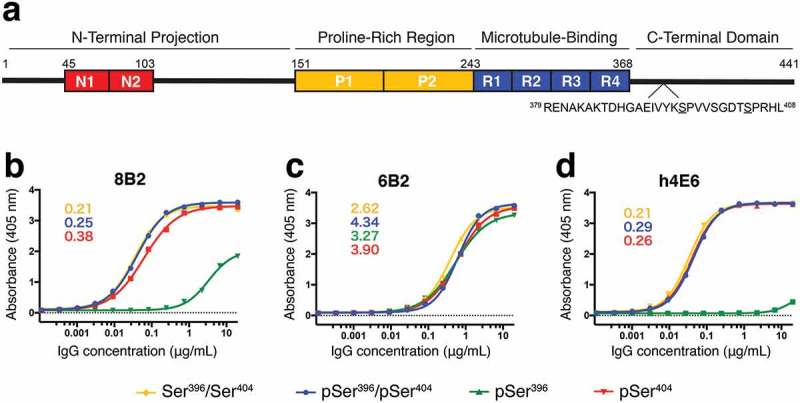
Antibody affinity to tau peptide measured by ELISA. (a) Schematic of tau 2N4R isoform labeled by known functional domains, including the two N-terminal acidic inserts (red, residues 45–103), two proline-rich regions (yellow, residues 151–243), microtubule-binding domains (blue, residues 244–368), and the C-terminal domain. The sequence of the region (residues 379–408) of interest is shown below with Ser396 and Ser404 underlined. (b) – (d) Binding curves from ELISA measurements for mAbs 8B2 (b), 6B2 (c), and h4E6 (d) comparing recognition of four differentially phosphorylated peptides (Table 1). Peptides pSer396/pSer404-tau (blue), pSer404-tau (red), and non-phosphorylated Ser396/Ser404-tau (yellow) were bound by all three mAbs while pSer396-tau (green) was only bound by 6B2 and, with a much lower affinity, by 8B2. Number insets are estimated Kd values (nM) from the best binding curves.
