ABSTRACT
Plant roots respond positively to gravity force and orientate it growth providing anchorage to the soil and gathering water and nutrient sources. The gravitropic response is a complex process wherein nitric oxide (NO) participates as a key signaling molecule. Here, we used genetically impaired genotypes to demonstrate the role of the nitrate reductase (NR) enzyme as a possible source of endogenous NO during gravitropic response in Arabidopsis thaliana (A. thaliana) roots. A. thaliana has two NR genes, NIA1 and NIA2. The single mutants nia1 and nia2, and the double mutant nia1/nia2 showed perturbed gravitropism. Complementation with the exogenous NO donor, S-nitroso-L-cysteine, partially rescued the wild-type phenotype in nia2 and nia1/nia2 but not in the nia1 mutant. Our findings showed that each NR gene differentially contributes to reaching the optimum level of NO during the gravitropic response, suggesting that NIA1 and NIA2 isoforms are not equivalent and have potential regulatory feedback to each other during the gravitropic response in A. thaliana roots.
KEYWORDS: Arabidopsis thaliana, gravitropism, nitrate reductase, nitric oxide, root
Text
Plant gravitropism involves the bending of roots in response to gravity. Positive gravitropism enables roots to grow downwards providing anchorage and facilitates soil exploration to take up water and mineral ions required for plant growth and development. Both signaling molecules, auxin and NO, are involved in gravitropism control.1,2 Gravitropism requires coordinated and asymmetric cell elongation of the lower and the upper sides of roots.3 A recent study in A. thaliana has showed that the asymmetric accumulation of NO between the upper and the lower sides of the root tip is critical for gravitropism.4 Diminished endogenous NO levels reduced and even reversed gravitropism, resulting in root upward bending.4 NO is a multifunctional lipophilic signaling molecule able to diffuse through membranes and possess a wide range of physiological functions.5 The best characterized NO production pathway in plants involves the reduction of NO2− to NO via different non-enzymatic or enzymatic mechanisms. The NR enzyme (EC 1.6.6.1) is one of the most important enzyme-based pathways for NO biosynthesis in plants.5–8 In addition to its reductase activity on nitrate (NO3−) to form nitrite (NO2−), NR is also able to catalyze the reduction of NO2− to NO.9 Mitochondrial electron transport chain and plasma membrane-bound nitrite-NO reductase (NiNOR) are other enzymatic systems that perform reductive NO production in plants. Oxidative mechanisms include animal NO synthase-like enzyme (NOS like), polyamines and hydroxylamines.10 The A. thaliana NR-impaired lines nia1, nia2, and nia1/nia2 have been used to establish the involvement of NR in NO production.9–12 Double mutant nia1/nia2 have only 0.5% of wild-type shoot NR activity and display very poor growth on media with NO3− as the unique form of nitrogen.13 Particularly, in seedlings, NIA2 isoform is responsible for 90% of the total NR activity, whereas NIA1 accounts for the remaining 10%. Both genes, NIA1 and NIA2, are expressed in leaves and roots; however, in roots, NIA1 is expressed at higher levels than NIA2.14 Primary root growth9 and root hair development15 is reduced in nia1/nia2 mutant and can be restored by exogenous NO application.
NR is necessary during early gravitropism in A. thaliana roots
To determine the contribution of NR to the gravitropic response, we adopted a genetic approach using single and double NR mutants. Firstly, we confirmed the endogenous NO level in roots using the cell-permeable NO-sensitive fluorophore 3-amino, 4-aminomethyl-2,7-difluorofluorescein diacetate (DAF-FM DA, Molecular Probes, USA) described by París et al. (2018).4 Roots of nia2 and nia1/nia2 displayed an endogenous NO level reduction of 19% and 37.5% in comparison to wt roots, respectively (Figure 1). However, nia1 roots did not show a statistically significant change in NO-associated fluorescence (Figure 1). These data are in agreement with previous results described in roots of nia1/nia2 seedlings, and with the decrease of endogenous NO reported in leaves of nia2 and nia1/nia2.9.11,16 Next, we took advantage of the decreased NO production mediated by NR in nia1, nia2, and nia1/nia2 to investigate how endogenous NO levels affect root gravitropism over time. Four-day-old A. thaliana seedlings were grown vertically and then rotated 90 degrees to induce gravistimulation. Images were taken sequentially every 30 min for 480 min to calculate the root tip angle relative to the horizontal line. Compared with wt plants, at 120 min after the onset of gravistimulation a statistically significant decrease in the average value of the root tip angle was detected in the three selected NR mutants (Figure 2). At 480 min after gravistimulation, the average values of tip angles were 80°, 60°, 43°, and 45° in wt, nia1, nia2, and nia1/nia2 roots, respectively.
Figure 1.
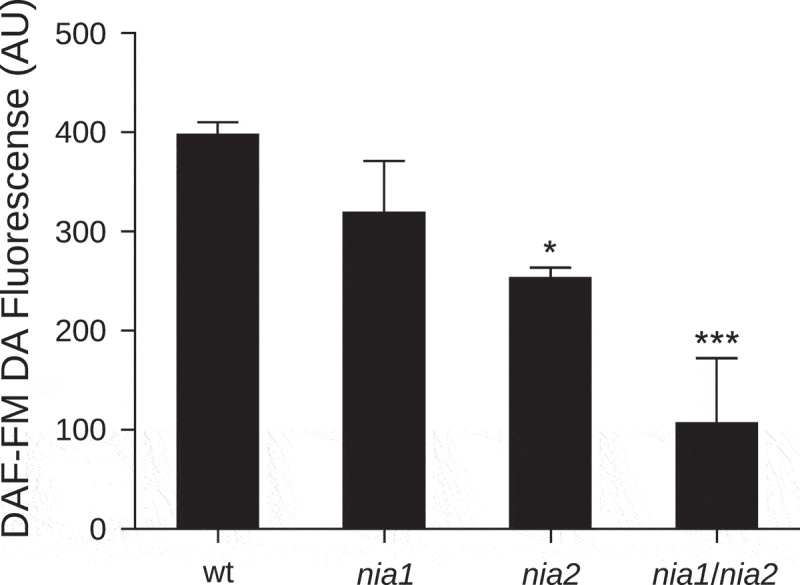
Nitric oxide quantification in roots of NR mutant lines.
Four-day-old wt, nia1, nia2 and nia1/nia2 A. thaliana seedlings were loaded with 10 µM DAF-FM DA probe as described by París et al. (2018)4. Roots were observed using a confocal microscope (Zeiss LSM333) 20 x objective, NA = 0.8, with an argon ion laser set at 488 nm; emission was collected between 490 and 561 nm. Associated fluorescence was quantified using FIJI.17 Means (±SE) are shown. P values in comparison to wt were calculated with two-tailed Mann–Whitney test, *p < 0.05 and ***p < 0.0001. The average values of 2 independent experiments were analyzed, n = 13.
Figure 2.
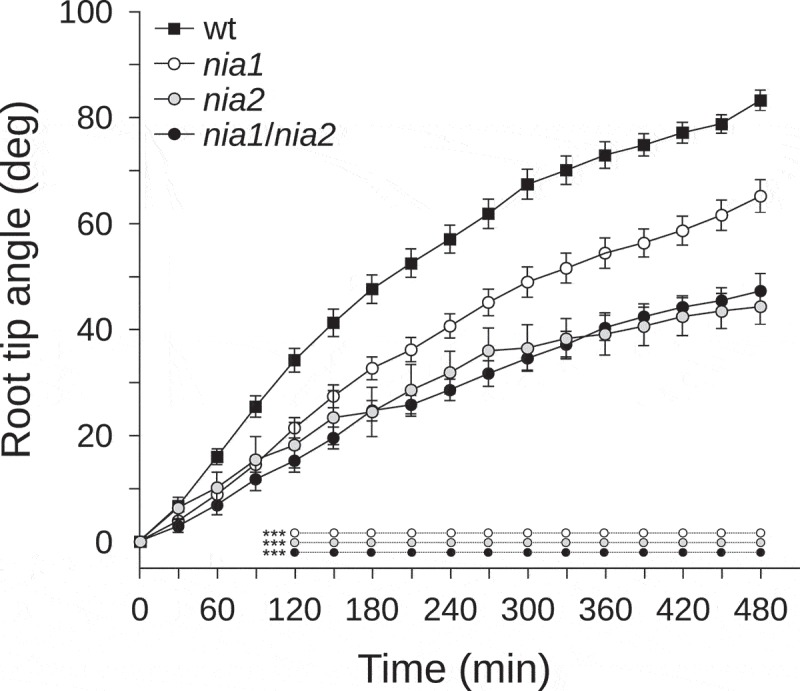
Root tip angle of NR mutants after gravistimulation.
Wild-type, nia1, nia2, and nia1/nia2 A. thaliana seedlings were grown vertically on 0.5x Murashige and Skoog salt (MS), 0.8% agar and 1% sucrose for 4 days. Seedlings were transferred to fresh MS medium and plates were mounted in front of a photo scanner (Epson Perfection V600) and let set for 60 min before 90-degree rotation. For each time series of individual seedlings, root tip angle was measured on digital images using FIJI.17 Means (±SE) are shown. P values in comparison to wt were calculated with two-tailed Mann–Whitney test, ***p < 0.0001. The average values of at least four independent experiments were analyzed, n ≥ 20.
NO partially rescued the phenotype of NR mutants under gravitropism
Before gravistimiluation, we treated nia1, nia2, and nia1/nia2 roots with the physiological NO donor, S-nitrosylated version of L-cysteine (CysNO). Four-day-old seedlings were placed on supplemented plates with 1 or 100 µM CysNO and water as a control, and let to set vertically for 60 min before 90-degree rotation. Later, the root tip angle was recorded every 30 min for each root as described above. We found that both nia2 and nia1/nia2 altered gravitropic response was partially rescued by the addition of 1 µM CysNO. At 480 min after gravistimulation, root tip angle increased 14° and 12° for nia2 and nia1/nia2 respectively compared with control (Figure 3(b,c)). However, the application of CysNO did not rescue the nia1 gravitropic deficient phenotype (Figure 3(a)). Finally, 100 µM CysNO did not recover the gravitropic defect of any mutant. Additionally, the nia1/nia2 mutant has a reduced shoot and root growth rate, suggesting that NO is able to participate in critical physiological processes.11 To assess whether the NO production by NR is affecting the growth rate during the gravitropism, we measured the position of the root tip every 60 min. Both nia1 and nia1/nia2 mutants displayed a significantly lower growth rate during the gravitropic response in comparison to wt plants (Figure 4(a,c)). However, nia2 mutant did not show a significant decrease in the growth rate (Figure 4(b)). Interestingly, nia1/nia2 growth was rescued with the addition of 1 µM CysNO (Figure 4(c)); meanwhile, the nia1 phenotype was not rescued under our experimental conditions (Figure 4(a)). High CysNO concentrations of 100 µM were in detriment of the growth rate for the wt and the three mutant lines tested (Figure 4). Our study demonstrates that NR activity by NIA1 and NIA2 is likely to underlie the endogenous NO level required in A. thaliana gravitropism. In addition, this work shows that the functional contributions of each NR gene affect the gravitropic response. Thus, we speculate that NR isoforms have potential regulatory feed to each other during gravitropism. In leaves, a comparative study between the single mutants, nia1 and nia2 have shown that NIA1 despite being expressed at significantly lower levels than NIA2, is the most prominent NR enzyme in guard cells.19 Here, we demonstrated that despite nia1 mutant has a subtle reduction on the endogenous NO level, nia1 gravitropic response was diminished significantly. Additionally, nia2 mutant showed a similar reduction than nia1/nia2 double mutant on the gravitropic response. Both mutant phenotypes were partially rescued by exogenous NO treatment (Figure 3(b,c)), suggesting that mutations on nia1 and nia2 did not build an additive effect on this response. In consequence, our results reveal an uncharacterized signaling role for NR in A. thaliana during gravitropic response and support the idea that probably a tightly adjusted timing, concentration, and localization is necessary for NO action during gravitropism.
Figure 3.
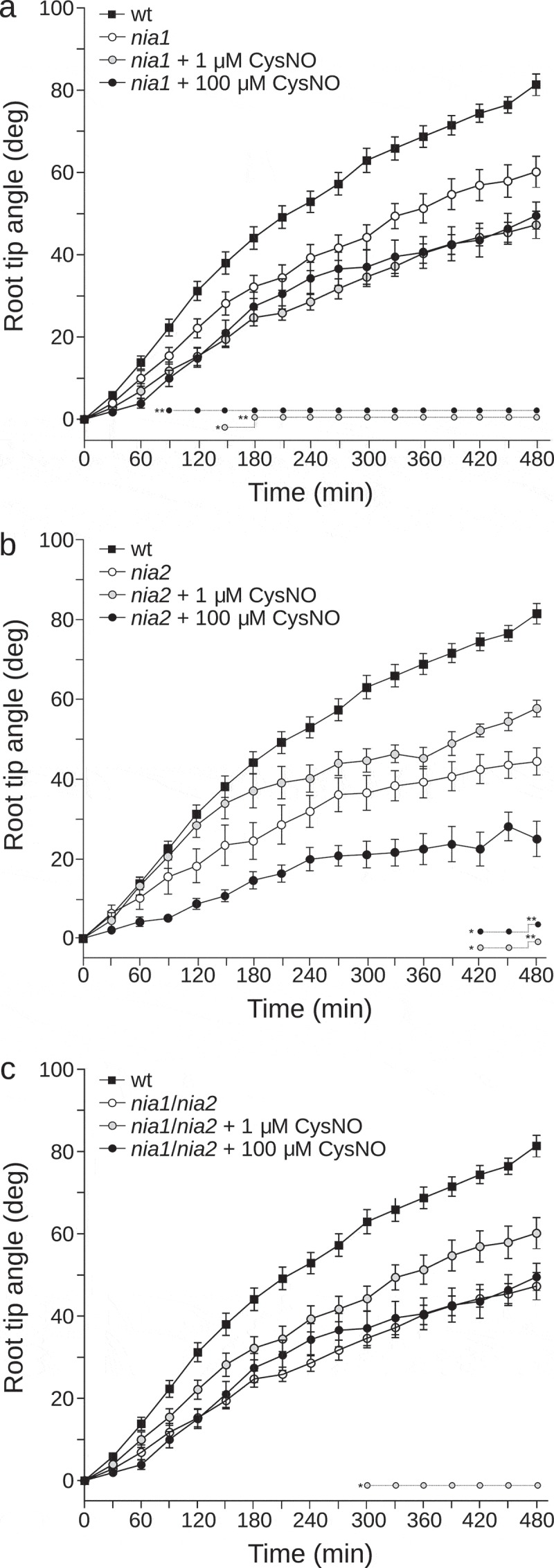
Tip angle of NO-treated roots mutants after gravistimulation.
Four-day-old seedlings (a.) nia1, (b.) nia2 and (c.) nia1/nia2, were transferred to fresh MS medium, containing 1 µM or 100 µM of CysNO, or water as a control. Solutions were also poured at the surface of each root to ensure homogeneous absorption and action. Stock solutions of CysNO were prepared as previously described.18 A gravitropic assay was performed as described in Figure 1. P values in comparison to untreated mutant were calculated with two-tailed Mann–Whitney test, *p < 0.05 and **p < 0.005. The average values of at least four independent experiments were analyzed, n ≥ 20.
Figure 4.
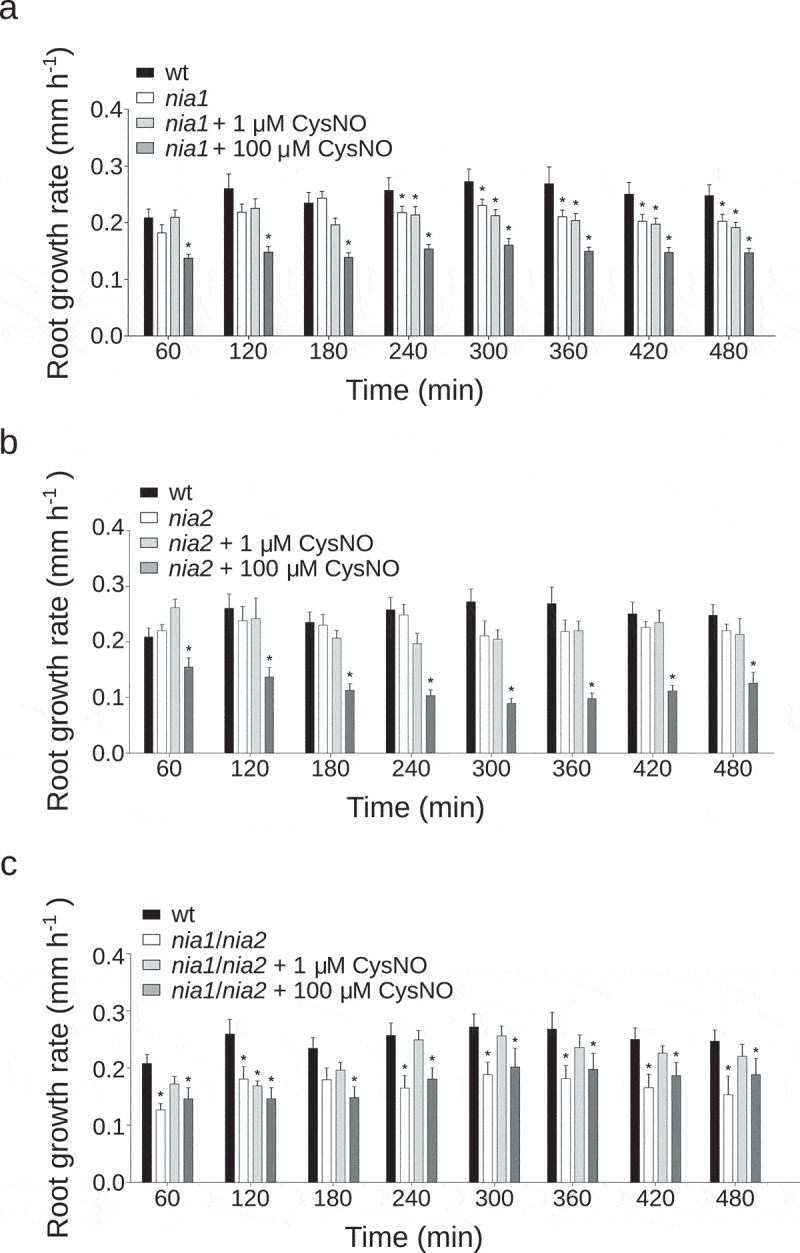
Growth rate of graviestimulated NO-treated root mutants.
Four-day-old seedlings (a.) nia1, (b.) nia2 and (c.) nia1/nia2, were grown and treated as described in Figure 3. Roots were monitored every 60 min for 480 min. For each time series of individual roots, root tip position and distance were measured on digital images using FIJI17 to calculate average values (±SE) of growth rate expressed as mm h−1. P values in comparison to wt were calculated with two-tailed Mann–Whitney test, *p ≤ 0.05. The average values of at least four independent experiments were analyzed, n ≥ 20.
In this work, we showed that simple and double NR mutants are not completely impaired in root bending (Figure 2). In soybean primary roots both NOS-like enzyme and NR have been proposed to regulate the gravitropic root response.2 If complementarily to NR, NO generation by NOS-like, NiNOR, and mitochondrial nitrite reduction could be participating during the signaling of gravitropic response in A. thaliana roots has to be demonstrated. Moreover, we could not rule out the possible participation of other nitrate metabolism-dependent signals during gravitropism. Further biochemical, cellular and genetic investigations on NO metabolism and signaling during the gravitropic response would contribute to elucidate this complex tropic response.
Funding Statement
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas [0601]; Fondo para la Investigación Científica y Tecnológica [0373]; Fondo para la Investigación Científica y Tecnológica [2421].
Acknowledgments
We thank Marisa S. Otegui (University of Wisconsin-Madison, WI, United States) for assistance via intellectual discussions. We thank Dr. Ramiro E. Rodriguez, Lic. Camila Godoy and Rodrigo Vena (Universidad Nacional de Rosario, Argentina) for the assistance with the microscopy image acquisition. We are also grateful to Lorenzo Lamattina (Universidad Nacional de Mar del Plata, Argentina) for providing seed material. This work was supported by grants PICT N° 0373 and PICT N° 2421 from Agencia Nacional de Promoción Científica y Tecnológica, to CAC; PIP0601 from CONICET to MCT and RP. CAC and RP are members of the research staff from CONICET. MMV is a graduate fellow from the same institution.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Abbreviations
- CysNO
S-nitroso-L-cysteine
- DAF-FM DA
3-amino, 4-aminomethyl-2,7-difluorofluorescein diacetate
- NO
nitric oxide
- NR
nitrate reductase
References
- 1.Vanneste S, Friml J.. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Hu X, Neill SJ, Tang Z, Cai W.. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baster P, Robert S, Kleine-Vehn J, Vanneste S, Kania U, Grunewald W, De Rybel B, Beeckman T, Friml J. SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. Embo J. 2013;32:260–274. doi: 10.1038/emboj.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.París R, Vazquez MM, Graziano M, Terrile MC, Miller ND, Spalding EP, Otegui MS, Casalongué CA. Dynamical spatiotemporal distribution of endogenous NO modulates auxindependent PIN2 localization during early gravitropism. Front Plant Sci. 2018;9:495. doi: 10.3389/fpls.2018.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu M, Lamattina L, Spoel SH, Loake GJ. Nitric oxide function in plant biology: a redox cue in deconvolution. New Phytol. 2014;202:1142–1156. doi: 10.1111/nph.12739. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ID, Neill SJ, Hancock JT. Nitric oxide synthesis and signalling in plants. Plant Cell Environ. 2008;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki H, Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000;468:89–92. doi: 10.1016/S0014-5793(00)01203-5. [DOI] [PubMed] [Google Scholar]
- 8.Domingos P, Prado AM, Wong A, Gehring C, Feijo JA. Nitric oxide: a multitasked signaling gas in plants. Mol Plant. 2015;8:506–520. doi: 10.1016/j.molp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Meyer C, Lea US, Provan F, Kaiser WM, Lillo C. Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth Res. 2005;83:181–189. doi: 10.1007/s11120-004-3548-3. [DOI] [PubMed] [Google Scholar]
- 10.Astier J, Gross I, Durner J. Nitric oxide production in plants: an update. J Exp Bot. 2018;69:3401–3411. doi: 10.1093/jxb/erx420. [DOI] [PubMed] [Google Scholar]
- 11.Lozano-Juste J, Leon J. Enhanced abscisic acid-mediated responses in nia1nia2noa1-2 triple mutant impaired in NIA/NR- and AtNOA1-dependent nitric oxide biosynthesis in Arabidopsis. Plant Physiol. 2010;152:891–903. doi: 10.1104/pp.109.148023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desikan R, Griffiths R, Hancock J, Neill S. A new role for an old enzyme : nitrate reductase- mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson JQ, Crawford NM. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol Gen Genet. 1993;239:289–297. doi: 10.1007/BF00281630. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CL, Acedo GN, Dewdney J, Goodman HM, Conkling MA. Differential expression of the two Arabidopsis nitrate reductase genes. Plant Physiol. 1991;96:275–279. doi: 10.1104/pp.96.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M-G, Chen L, Zhang -L-L, Zhang W-H. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009;151:755–767. doi: 10.1104/pp.109.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Preibisch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2019;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Ruiz A, Lamas S. Chapter seventeen - proteomic identification of S-nitrosylated proteins in endothelial cells. Cardiovasc Proteomics Methods Protoc. 2007;215–223. doi: 10.1385/1597452149. [DOI] [PubMed] [Google Scholar]
- 19.Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]


