ABSTRACT
Innate immune checkpoint CD47 has emerged as a prominent target for cancer immunotherapy and defining biomarkers predictive of response will be a crucial step towards clinical implementation. Hereto, we investigated the importance of a previously reported requisite for SLAM family member 7(SLAMF7) expression on cancer cell phagocytosis for effective CD47 antibody therapy.
KEYWORDS: SLAMF7, CD47, phagocytosis, DLBCL
CD47 is a cell surface receptor overexpressed on most, if not all, cancer cells. CD47 expression inhibits phagocytic removal of cancer cells by binding to phagocyte-expressed (signal regulatory protein alpha) SIRP-α. Specifically, SIRP-α is an immunoreceptor tyrosine-based inhibitory motiv (ITIM)-containing ligand that upon triggering inhibits phagocyte activity and reduces the immunogenic processing of cancer cells, leading to inhibition of not only innate but also adaptive immune responses.1 Antibodies that block the interaction of CD47 with SIRP-α can therapeutically augment phagocytosis of cancer cells, if and when the balance of pro and anti-phagocytic signals is sufficiently shifted towards phagocytic uptake.2 More importantly, co-treatment of anticancer monoclonal antibodies with CD47 blocking antibodies augments therapeutic anticancer activity in vivo and in vitro.3 In a recently reported phase 1B clinical trial the treatment of patients with relapsed or refractory Diffuse Large B-cell Lymphoma (DLBCL) or follicular lymphoma with CD47 monoclonal antibody (mAb) Hu5F9-G4 in combination with rituximab induced 50% objective response with up to 36% complete responses.4 Thus, it is clear that CD47 antibody therapy can have clinical benefit. To facilitate clinical implementation, it is imperative to search for appropriate biomarkers that can be used for patient stratification.
Several reports have highlighted potential immunoregulatory proteins that may impact on the efficacy of CD47-targeted therapy. For instance, expression of leukocyte immunoglobulin-like receptor B1 (LILRB1) on macrophages inhibited induction of cancer cell phagocytosis by a CD47-blocking antibody. Direct binding of LILRB1 to MHC class I resulted in inhibition of macrophage activity, which was reversed by antibody-mediated blocking of LILRB1 (Figure 1).5 Another immunoregulatory protein, calreticulin, has been reported as a pro-phagocytic signal that is counterbalanced by CD47 expression, with increase of surface calreticulin correlating with more phagocytosis upon CD47 mAb treatment (Figure 1).6 Next, it was recently reported that the expression of the pro-phagocytic receptor SLAM family member 7 (SLAMF7) on macrophages and cancer cells was required for phagocytosis induction upon treatment with a CD47 blocking therapeutic antibody. Specifically, macrophages obtained from SLAMF7 knock-out mice proved to be defective in phagocytosis of cancer cells. Further, SLAMF7 expression on hematopoietic cancer cells was reported as a requisite for phagocytosis upon treatment with a CD47 blocking antibody.7 The premise arising from this finding is that only hematopoietic cancers that express high levels of SLAMF7 are suitable targets for CD47 blocking therapy. DLBCL, the most common subtype of non-Hodgkin‘s lymphoma (NHL), was identified as a suitable target for CD47 blocking therapy based on its high SLAMF7 mRNA levels.
Figure 1.
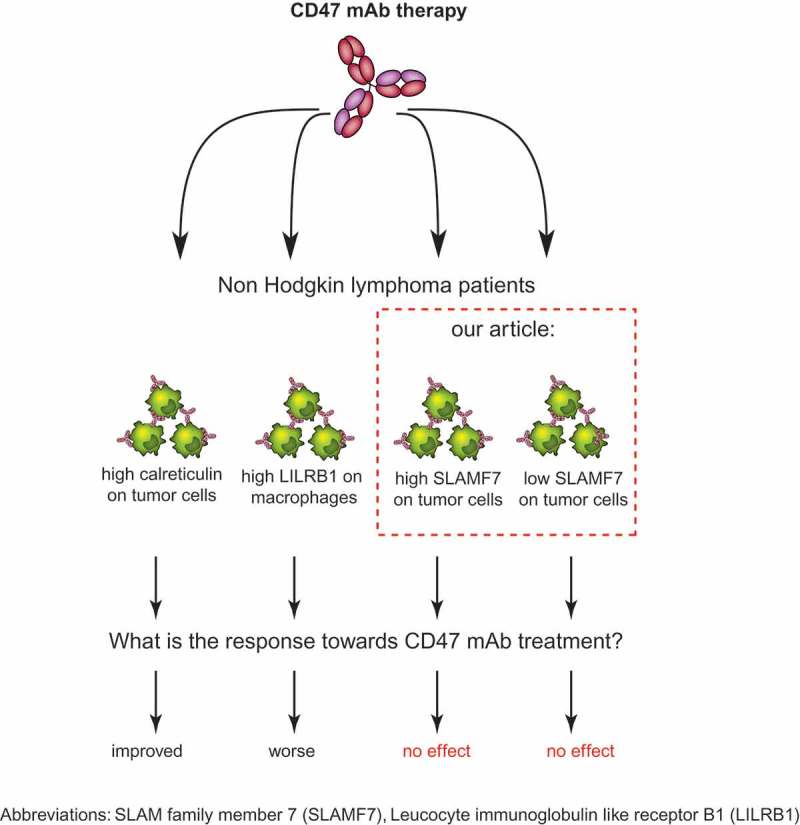
Overview of predictive biomarkers for response towards CD47 mAb treatmentAbbreviations: SLAM family member 7 (SLAMF7), Leucocyte immunoglobulin like receptor B1 (LILRB1)
Since SLAMF7 was reported to be critical for CD47 antibody-mediated phagocytosis and DLBCL was postulated to be a prime target for CD47 antibody therapy, we determined the expression of SLAMF7 in DLBCL. Surprisingly, using the same antibody clone as Chen et al., 7 only 1 out of 7 DLBCL cell lines were found to detectably express cell surface SLAMF7.
Next, we generated macrophages (MØ) with M0-like phenotype (primed with Granulocyte-macrophage colony-stimulating factor(GM-CSF)/macrophage colony-stimulating factor(M-CSF)), M1-like phenotype (primed with lipopolysaccharide(LPS)/interferon gamma(IFN-γ)), and M2-like phenotype (primed with interleukin 10 (IL-10)). Importantly, despite the absence of cancer cell-expressed SLAMF7, CD47 mAb treatment induced significant phagocytosis in 7 out of 7 DLBCL cell-lines upon mixed culture experiments with these macrophages.8 Of note, in these experiments we used fragment antigen binding (F(ab’))2 of a hIgG4 CD47 mAb, which lacks a constant Fc domain. Consequently, the phagocytic effect demonstrated during our experiments is due to blocking of CD47/SIRP-α and not due to potential confounding Fc/Fc-receptor (Fc/FcR)-mediated effects. Indeed, such F(ab’)2-mediated blocking of CD47/SIRP-α was also reported by Chen et al.7
To further investigate the potential relevance of cancer-expressed SLAMF7, other B cell NHL cell lines displaying varying levels of surface SLAMF7 were evaluated for phagocytosis upon CD47-targeting. Specifically, the NHL cell line Raji, BJAB, and Z138 significantly expressed cell surface SLAMF7, whereas Daudi and Ramos had weak and non-significant expression of SLAMF7. Nevertheless, all of these cell lines were significantly phagocytosed upon treatment with CD47 F(ab)2 irrespective of the level expression of SLAMF7, further illustrated by the lack of correlation between phagocytosis and SLAMF7 surface expression.8
Interestingly, in primary patient-derived DLBCL and mantle cell lymphoma (MCL) samples also no SLAMF7 surface expression was detected. This in contrast with high expression of SLAMF7 on the surface of primary autologous macrophages, obtained from these DBLCL and MCL patients. To investigate whether the absence of SLAMF7 expression on primary material negatively affected CD47 mAb therapy, we used an IgG4 containing antibody called Inhibrix, currently being evaluated in clinical trials for B cell malignancies including DBLCL (NCT02367196). Inhibrix also effectively induced phagocytosis upon treatment of SLAMF7-negative primary patient-derived DLBCL and MCL cells by autologous patient-derived macrophages, yielding significant increases in phagocytosis of ~15% and 8%, respectively.8 Thus, in an autologous setting with primary patient-derived material, expression of SLAMF7 was not required for phagocytosis upon CD47 mAb treatment.
In line with the data obtained for CD47 mAb-based targeting, SLAMF7 expression also did not impact on macrophage-mediated phagocytosis of DLBCL cell lines treated with the CD20 antibody rituximab. Correspondingly, SLAMF7 mRNA expression also did not correlate with overall survival after R-CHOP treatment in a large transcriptomic dataset of gene expression profiles (GEP) of 680 DLBCL patients, whereas expression of CD47 did correlate with survival.8
In conclusion, mRNA and/or protein expression levels of SLAMF7 on hematopoietic cancer cells should not be used as selection/exclusion criterion for future clinical studies that evaluate the therapeutic potential of CD47-blockade or the combination with CD47 blocking therapy. Further, SLAMF7 is not a predictive marker for response to rituximab in vitro or R-CHOP therapy in DBLCL patients.
Funding Statement
Supported by grants RUG2012-5541, RUG2013-6209, RUG2014-6986, RUG2015-7887 (EB) and RUG 2014-6727 (TvM) from the Dutch Cancer Society.
References
- 1.Wiersma VR, van Bommel P, de Bruyn M, Helfrich W, Bremer E.. CD47, a multi-facetted target for cancer immunotherapy. Atlas Genet Cytogenet Oncol Haematol. 2015;19(6):417–431. doi: 10.4267/2042/62150. [DOI] [Google Scholar]
- 2.Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim JS, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Investig. 2016. July 1;126(7):2610–2620. PMID:27294525. doi: 10.1172/JCI81603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010. September 3;142(5):699–713. PMID: 20813259. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med. 2018. November 1;379(18):1711–1721. PMID: 30380386. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018. January;19(1):76–84. PMID: 29180808. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, et al. Calereticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010. December 22;2(63):63ra94 PMID: 21178137. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Zhong MC, Guo H, Davidson D, Mishel S, Lu Y, Rhee I, Pérez-Quintero LA, Zhang S, Cruz-Munoz ME, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017. April 27;544(7651):493–497. PMID: 28424516. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y, Bouwstra R, Wiersma VR, de Jong M, Jan LH, Fehrmann R, de Bruyn M, Ammatuna E, Huls G, van Meerten T, et al. Cancer cell-expressed SLAMF7 is not required for CD47-mediated phagocytosis. Nat Commun. 2019. February 1;10(1):533 PMID: 30710089. doi: 10.1038/s41467-018-08013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


