ABSTRACT
Ultraviolet radiation (UV) is an important modulator of plant defenses against biotic stresses. We have recently described that different supplemental UV exposure times and irradiance intensities enhanced tomato (Solanum lycopersicum) resistance to Western flower thrips (Frankliniella occidentalis). UV increased jasmonic acid-isoleucine (JA-Ile) and salicylic acid (SA) levels, as well as the expression of JA- and SA-responsive genes, before thrips herbivory. Here we report how UV affects tomato defense responses upon thrips infestation, and resistance to pathogens that are susceptible to the activation of SA-associated defenses. Our experiments reveal that, at 7 days after thrips infestation, UV did not enhance the levels of jasmonates, auxin or abscisic acid. UV also did not affect the expression of JA-responsive genes in the cultivar Moneymaker, the jasmonate deficient mutant def-1, the type-VI trichome deficient mutant od-2, or their wild-type Castlemart. However, UV strongly activated SA-associated defense responses in def-1 after thrips infestation. Further bioassays showed that UV increased def-1 resistance to the hemi-biotrophic bacterial pathogen Pseudomonas syringae pv. tomato DC3000, which is susceptible to SA-mediated defenses. Our results suggest that UV might enhance tomato resistance to this pathogen in the JA deficient genotype through the activation of SA defenses.
KEYWORDS: Abscisic acid, auxin, jasmonates, light, salicylic acid, ultraviolet radiation, western flower thrips
One of the most important environmental factors determining plant performance is light. Light quality and quantity not only affect photosynthesis, but also plant adaptive responses to abiotic and biotic stresses. In particular, UV-B radiation (λ 280–320 nm), a small component of the solar radiation reaching terrestrial ecosystems, has a great influence on the plant’s physiology by inducing a range of molecular, biochemical, morphological and developmental responses.1–3 Although early research on the biological activity of UV-B radiation suggested detrimental effects on plants, recent evidence has demonstrated that ecologically-relevant levels are not deleterious.3 On the contrary, UV-B radiation has emerged as a light signal with potential uses to enhance plant protection against pests in agriculture systems, thereby increasing yields.4 This paradigm shift has led to the conception of UV exposure as an ‘eustress’. Eustress is defined as a positive form of stress with beneficial effects on plant health and adaptation to more severe stress conditions.5 Accordingly, UV-B has shown to confer cross-tolerance to high light stress, drought and high temperatures in plants.6 Furthermore, UV-B has been shown to promote plant resistance to a wide array of aboveground arthropod herbivores.6
Exposure to UV-B can alter constitutive (i.e., before herbivory) and inducible (i.e., upon herbivore) plant defenses to arthropod pests. For instance, UV-B can induce the accumulation of phenolic compounds in the leaf epidermis. This reduces the oxidative damage and UV-B penetration to the inner photosynthetic layers. At the same time, it can also reduce the palatability of plant tissues to herbivorous arthropods.7 Herbivore-mediated induction of plant chemical defenses can also be modulated by UV-B radiation. Upon perception of herbivory-associated molecular patterns, plants display specific defense responses that are mainly regulated by the phytohormones jasmonic acid (JA), salicylic acid (SA), ethylene (ET), and abscisic acid (ABA). These responses are fine-tuned by the cross-talk among these and other phytohormones such as auxins, cytokinins and giberellins.8 Activation of these defense-related hormone signaling pathways leads to the production of secondary metabolites (e.g., alkaloids, glucosinolates, terpenes) and defensive proteins (e.g., proteinase inhibitors and polyphenol oxidases) that hamper herbivore feeding and performance.7 UV-B is reported to enhance the induction of these plant defenses. In particular, increases in the JA and ethylene burst has been described after combined UV-B exposure and aboveground herbivory, resulting in enhanced plant resistance.9–14
Recently, we have demonstrated that supplemental UV (A + B) (λ 280–400 nm) can enhance tomato (Solanum lycopersicum) resistance to thrips (Frankliniella occidentalis), and this was dependent on the UV exposure time and irradiance intensity.15 We did metabolomics, hormone and gene expression analyses, and used the cultivar ‘Moneymaker’, the tomato mutants defenceless-1 (def-1), impaired in jasmonic acid (JA) biosynthesis, odorless-2 (od-2), defective in the production of functional type-VI trichomes, and their wild-type ‘Castlemart’, to demonstrate that UV-mediated induction of tomato resistance to thrips was not explained by changes in the leaf metabolome or trichome-mediated defenses, but probably by the activation of JA signalling.15 UV enhanced constitutive levels of JA-isoleucine (JA-Ile) and expression of JA-responsive genes, but it also induced SA signaling, before thrips herbivory.15 In the present study, we have further investigated whether UV promotes a stronger tomato defense response after thrips infestation. Furthermore, we have determined whether UV-mediated positive effects on SA signaling15 alters tomato resistance to a semi-biotrophic pathogen that is susceptible to the activation of this signaling pathway.16
Following the methodology described in,15 we present data on the effect of high UV irradiance intensity on the levels of jasmonates, SA, auxin and ABA, as well as the expression of JA- and SA-associated defense marker genes, in Moneymaker, Castlemart, def-1 and od-2 leaves after herbivory (Figures 1–2). In short, plants were subjected to supplemental high UV for 30 min d−1 (0.422 kJ m−2 d−1) or control (no UV) conditions for 28 days and subsequently infested with thrips as described in.15 At 7 days after thrips infestation, plants were sampled for hormone and gene expression analyses. Our results showed that the levels of the JA precursor 12-oxo-phytodienoic acid (OPDA) and ABA were significantly affected by UV, and generally lower in UV-treated plants after thrips infestation (Figure 1(a,f)). Levels of JA, JA-Ile and SA were not significantly affected by the light treatment (Figure 1(b,c,e)). Yet, the concentrations of JA and JA-Ile were slightly reduced, although not statistically significant, in UV-treated Moneymaker and od-2 plants after thrips infestation. Accordingly, UV significantly downregulated the expression of the JA-responsive genes TD-2 and JIP-21 in Moneymaker and, although not statistically significant, also in od-2 after thrips infestation (Figure 2(a,b)). We have previously described that high UV reduced thrips feeding damage in Moneymaker and od-2, but not in Castlemart and def-1.15 Thrips are susceptible to the induction of JA-associated defences,17–19 and our previous findings indicated that UV enhanced the constitutive (i.e. before herbivory) levels of jasmonates and expression of JA-responsive genes in UV-treated Moneymaker and od-2 plants.15 We speculated that this might have resulted in a stronger activation of these plant defenses upon thrips infestation as well. However, we observed the opposite at 7 days after thrips herbivory, and jasmonate levels were overall lower in UV-treated plants that were infested with thrips. This might be explained by the reduced thrips-associated feeding damage symptoms (‘silver damage’) observed in UV-treated Moneymaker and od-2 plants in comparison with their controls.15 Thrips feeding activates JA and ET signaling in tomato19,20 and the magnitude of this induction might be correlated with the intensity of the leaf damage caused by thrips. For instance, Schmelz et al.21 showed that infestation levels of maize plants with Spodoptera exigua caterpillars were positively correlated with JA production. Similarly, Thaler et al.22 described that JA-mediated induction of defensive proteins in tomato can respond in a positive dose-dependent manner. In addition, we do not rule out the possibility of a stronger activation of JA signaling in UV-treated plants at earlier times after thrips infestation, prior to our sampling time. For instance, Ðhin et al.12 detected a higher accumulation of JA and JA-Ile in UV-B-exposed Nicotiana attenuata plants at 3 days after herbivory. Likewise, Izaguirre et al.23 showed oscillations in the induced levels of JA-responsive defense proteins along time in N. attenuatta plants subjected to UV-B and simulated herbivory. Taken together, our data also suggest that UV-mediated effects on hormone-signaling events before infestation, and perhaps at early post-infestation times, were sufficient to reduce thrips feeding damage in tomato.
Figure 1.
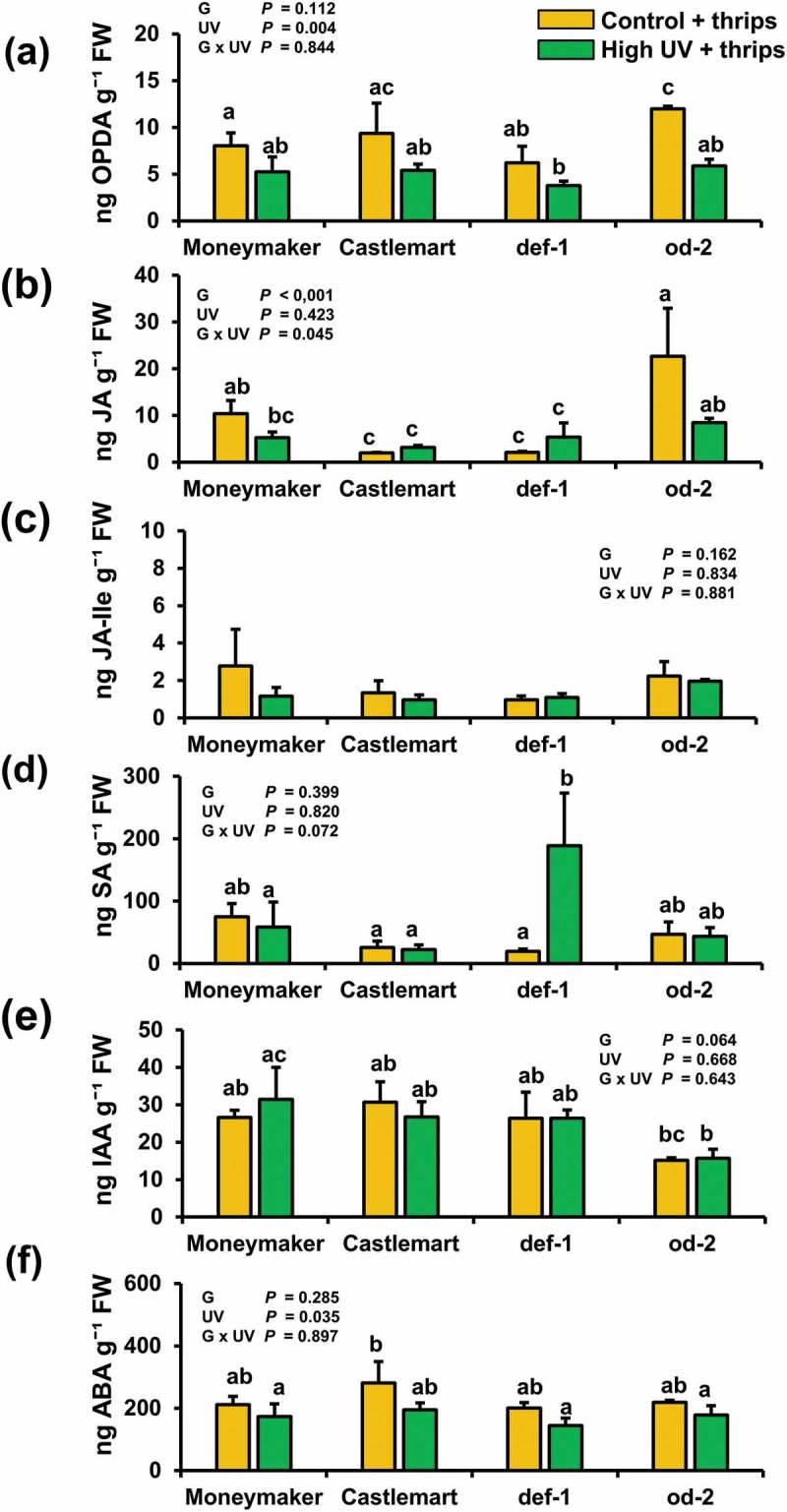
Concentrations of (a) 12-oxo-phytodienoic acid (OPDA), (b) jasmonic acid (JA), (c) jasmonic acid-isoleucine (JA-Ile), (d) salicylic acid (SA), (e) indole-3-acetic acid (IAA), and (f) abscisic acid (ABA) were determined in control and high UV-treated Moneymaker, Castlemart, defenceless-1 (def-1), and odorless-2 (od-2) plants at 7 days after thrips infestation. Plants were treated with supplemental control (no UV) or high UV for 30 min d−1 (0.422 kJ m−2 d−1) for 28 days, and subsequently used for non-choice whole plant thrips bioassays. Individual plants were infested with 20 adult thrips (18 females and 2 males). The analysis was performed on leaflets collected from the third and fourth youngest leaf. Values represent the mean + SEM (n = 5 individual plants). Data on OPDA levels were log (x + 1) transformed, while data on JA, JA-Ile and SA were log transformed prior to statistical analysis. Different letters above bars denote significant differences among groups tested by GLM followed by Fisher´s LSD test (P ≤ 0.05). The overall effects of genotype (g), UV, and their interaction tested by two-way ANOVA are indicated.
Figure 2.
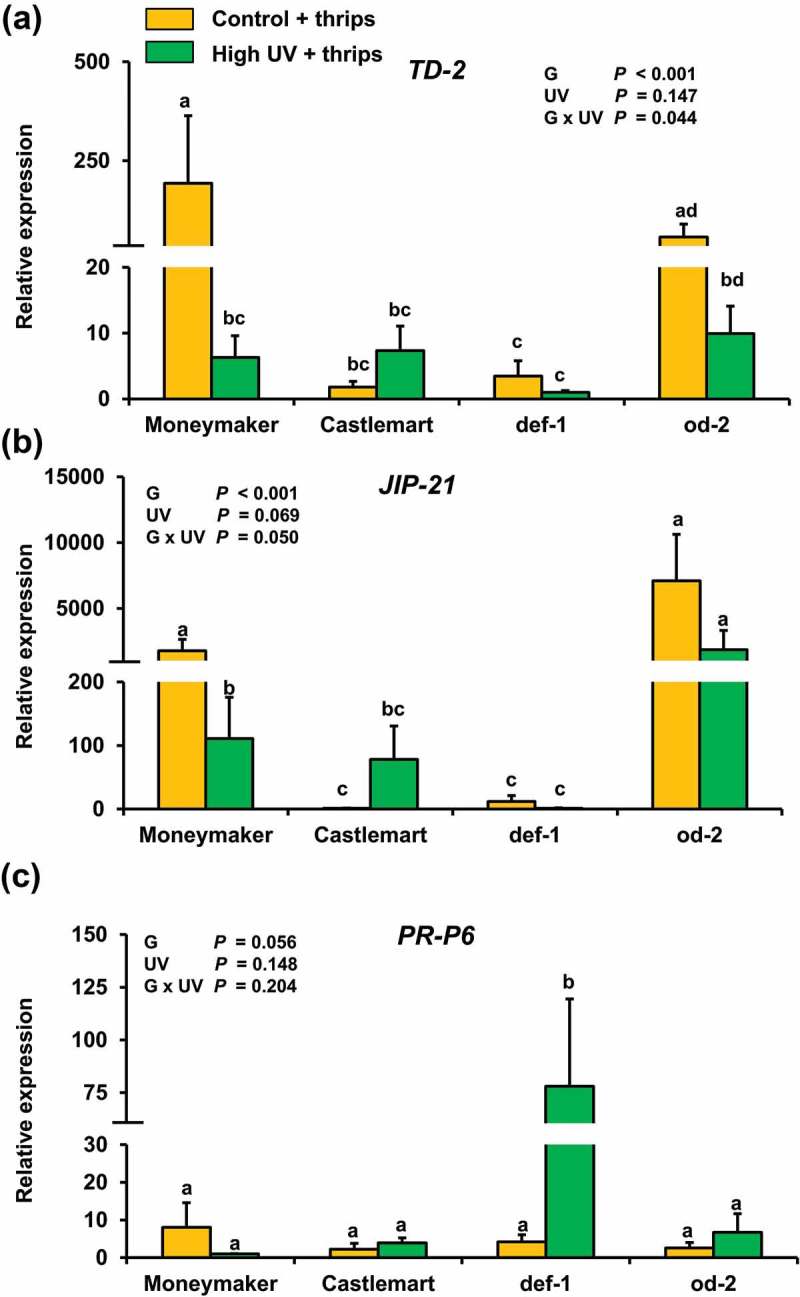
Relative transcript levels of the JA-responsive genes (a) threonine deaminase-2 (TD-2), and (b) jasmonate inducible protein-21 (JIP-21), and (c) the SA-responsive gene the pathogenesis related-protein 6 (PR-P6) measured in control (no UV) and high UV-treated Moneymaker, Castlemart, defenceless-1 (def-1), and odorless (od-2) plants at 7 days after thrips infestation. Plants were treated with supplemental control (no UV) or high UV for 30 min d−1 (0.422 kJ m−2 d−1) for 28 days, and subsequently used for non-choice whole plant thrips bioassays. Individual plants were infested with 20 adult thrips (18 females and 2 males). The analysis was performed on leaflets collected from the third and fourth youngest leaf. Values represent the mean (+ SEM) of relative expression of each treatment group (n = 5 individual plants, two technical replicates). Data were log transformed prior to statistical analysis. Different letters above bars denote significant differences among groups tested by GLM followed by Fisher´s LSD test (P ≤ 0.05). The overall effects of genotype (g), UV, and their interaction tested by two-way ANOVA are indicated.
Interestingly, SA levels were strongly induced in UV-treated def-1 plants after thrips infestation (Figure 1(d)), which was paralleled by a positive induction of the SA-responsive gene PR-P6 (Figure 2(c)). Notably, our previous data showed that PR-P6 was not significantly up-regulated in UV-treated def-1 plants prior to thrips infestation.15 These results suggest that thrips feeding might induce SA signaling in tomato, yet this induction might be generally repressed due to crosstalk mediated by JA.19 We previously showed that UV enhanced the concentration of SA in Moneymaker, Castlemart and, at lesser extent, in def-1 leaves before herbivory.15 This slight increase in SA levels might have stimulated or ‘primed’ the induction of these defenses in def-1 after thrips infestation due to the deficiency in JA signaling in this tomato mutant.24,25 Possibly, the UV-mediated reinforcement of JA signaling15 together with the subsequent induction of this signaling pathway by thrips infestation might have overridden SA-associated defense responses in Moneymaker and od-2.
Activation of SA-associated defenses can confer plant resistance to biotrophic pathogens.16 Hence, we further determined the effect of UV on tomato resistance to the hemi-biotrophic bacterial pathogen Pseudomonas syringae (Pst) pv. tomato DC3000. Control and UV-treated Moneymaker, Castlemart, def-1 and od-2 plants were inoculated with Pst by dipping all the plant leaves into a bacterial suspension [108 colony-forming units (cfu) ml−1] for 2–3 seconds. Determination of bacterial growth in the plants was conducted at 1 h and 4 d after inoculation. For this, three leaf discs (Ø 1.4 cm) per plant, taken from the second, third and fourth youngest leaves from the apex, were pooled and homogenized in 1 ml of 10 mM MgCl2. Bacterial growth was determined by plating appropriate dilutions in King’s B medium with 100 μg ml−1 rifampicin. CFU were counted after incubation for 72 h at room temperature. Our results showed that there were no significant differences in the Pst population among the plant genotypes at 1 h after inoculation (Figure 3(a)). However, a slightly greater Pst population was detected in leaves of UV-treated Moneymaker plants when compared to their controls. At 4 days after bacteria inoculation, there were significant differences in Pst population growth among the different tomato genotypes. Castlemart and def-1 were more susceptible to Pst than Moneymaker and od-2 (Figure 3(b)). UV did not generally affect tomato susceptibility to Pst, but there was a significant interactive effect between the plant genotype and the light treatment on plant susceptibility to Pst. UV significantly reduced the bacterial population in def-1 leaves, while no effect was observed for the other genotypes. Pst pv. tomato DC3000 produces the phytotoxin coronatine, whose structure mimics the bioactive JA conjugate JA-Ile and activates JA-associated defenses while suppressing SA signalling.26 Activation of JA signaling promotes Pst parasitism in the host plant. In turn, induction of SA defenses in Pst-infected tomato mutants deficient in the perception of coronatine has been reported to increase resistance.27 Theoretically, if UV supplementation reinforced JA defenses in Moneymaker and od-2, an increased susceptibility to this pathogen was expected in these genotypes. We hypothesized that the reinforcement of both JA and SA by the UV treatment in Moneymaker might have resulted in a negative cross-talk.8 In def-1, however, UV has been previously reported to enhance SA defenses only,15 which might have promoted a stronger activation of these defenses by Pst infection, as SA is also induced by this pathogen albeit to a lesser extent than JA.28 It would be interesting to test whether UV increases resistance to other pathogens that activate and are susceptible to SA defenses in future investigations.
Figure 3.
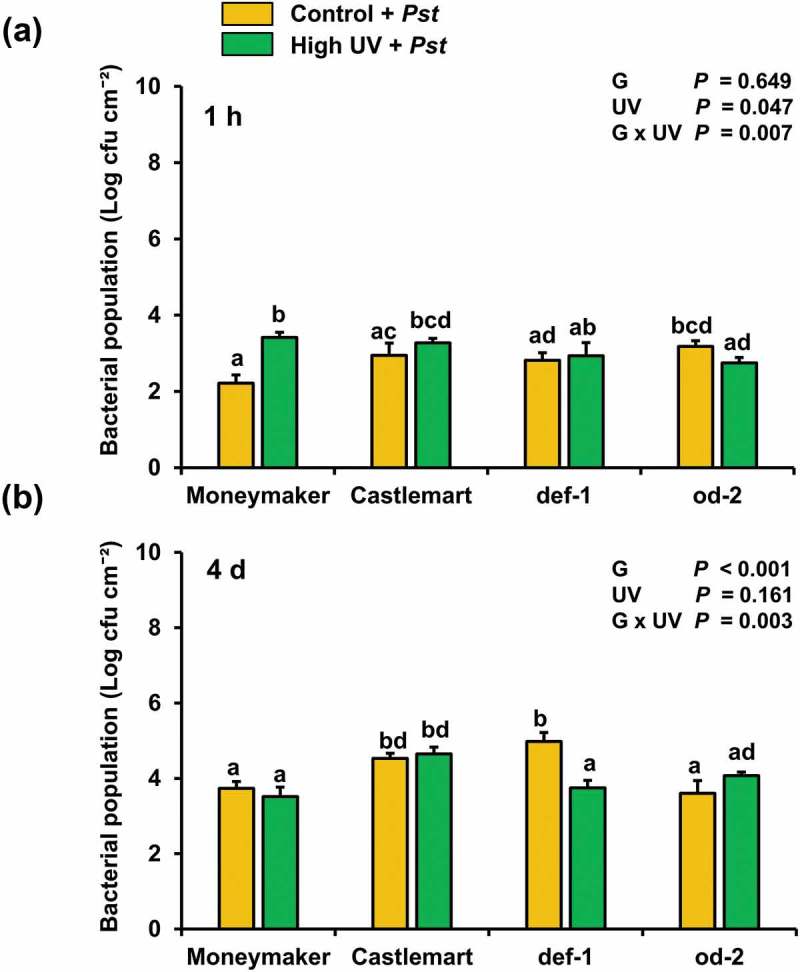
Effect of supplemental UV radiation on tomato resistance to Pseudomonas syringae (Pst) pv. tomato DC3000. Bacterial population was determined at (a) 1 h and (b) 5 days after inoculation in control (UV) and high UV-treated Moneymaker, Castlemart, defenceless-1 (def-1), and odorless (od-2) plants. Plants were treated with supplemental control (no UV) or high UV for 30 min d−1 (0.422 kJ m−2 d−1) for 28 days, and subsequently inoculated with P. syringae. Values represent the mean of Log transformed colony forming units (cfu) (+SEM) of five biological replicates, each replicate consisting of three leaf-discs taken from the second, third and fourth youngest leaves from the apex, respectively. Data on cfu determined at 5 days were log transformed prior statistical analysis. Different letters above bars denote significant differences among groups tested by GLM followed by Fisher´s LSD test (P ≤ 0.05). The overall effects of genotype (g), UV, and their interaction tested by two-way ANOVA are indicated.
In summary, we showed that UV-mediated positive effects on JA signaling were not evident at a late stage of thrips infestation in tomato. Further studies using time-course analyses could determine whether UV might modulate the magnitude and timing of the induction of tomato defenses upon herbivory. In addition, we demonstrated that the positive effects of UV on constitutive jasmonate levels did not increase susceptibility to the hemi-biotrophic pathogen Pst DC3000, but that UV likely mediated the observed enhanced resistance in JA-deficient tomato mutants via hormonal crosstalk. Our study highlights the potential dual role of UV in protection against herbivores and pathogens. This, opening new venues for the use of UV as a ‘priming’ agent in agricultural systems.
Funding Statement
This work was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek [13553].
Acknowledgments
This work was supported by the STW Perspective program “Green Defense against Pests” (GAP) (Ref.13553). We thank the companies involved in the GAP project: Rijk Zwaan, Dümmen Orange, Deliflor, Dekker Chrysanten, and Incotec for financial support. Katharina Grosser and Nicole M. Van Dam gratefully acknowledge the support of the German Center for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig funded by the German Research Foundation (FZT 118).
Author contribution
R.E.B, P.G.L.K and G.C. designed the experiments. R.E.B. conducted the thrips bioassays. R.E.B. and G.C. conduced the bacterial assays. R.E.B. performed the gene expression analyses. R.E.B. and K.G. carried out the hormone analyses. All the authors critically discussed the results. R.E.B. wrote the manuscript with input from all the authors.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
References
- 1.Paul ND, Gwynn-Jones D.. Ecological roles of solar UV radiation: towards an integrated approach. Trends Ecol Evol. 2003;18:48–55. doi: 10.1016/S0169-5347(02)00014-9. [DOI] [Google Scholar]
- 2.Ballare CL, Caldwell MM, Flint SD, Robinson SA, Bornman JF.. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem Photobiol Sci. 2011;10:226–241. doi: 10.1039/c0pp90035d. [DOI] [PubMed] [Google Scholar]
- 3.Morales LO, Brosché M, Vainonen J, Jenkins GI, Wargent JJ, Sipari N, Strid Å, Lindfors AV, Tegelberg R, Aphalo PJ. Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultraviolet radiation. Plant Physiol. 2013;161:744–759. doi: 10.1104/pp.112.211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robson T, Klem K, Urban O, Jansen MA. Re‐interpreting plant morphological responses to UV‐B radiation. Plant Cell Environ. 2015;38:856–866. doi: 10.1111/pce.12374. [DOI] [PubMed] [Google Scholar]
- 5.Mazza CA, Giménez PI, Kantolic AG, Ballaré CL. Beneficial effects of solar UV‐B radiation on soybean yield mediated by reduced insect herbivory under field conditions. Physiol Plant. 2013;147:307–315. doi: 10.1111/j.1399-3054.2012.01661.x. [DOI] [PubMed] [Google Scholar]
- 6.Escobar-Bravo R, Klinkhamer PG, Leiss KA. Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Front Plant Sci. 2017;8:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zavala JA, Mazza CA, Dillon FM, Chludil HD, Ballare CL. Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV‐B radiation and correlates with isoflavonoid content in pods under field conditions. Plant. Cell Environ. 2015;38:920–928. doi: 10.1111/pce.12368. [DOI] [PubMed] [Google Scholar]
- 8.Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012;17:250–259. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazza CA, Zavala J, Scopel AL, Ballaré CL. Perception of solar UVB radiation by phytophagous insects: behavioral responses and ecosystem implications. Proc Natl Acad Sci. 1999;96:980–985. doi: 10.1073/pnas.96.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL. Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol. 2003;132:1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caputo C, Rutitzky M, Ballaré CL. Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia. 2006;149:81–90. doi: 10.1007/s00442-006-0422-3. [DOI] [PubMed] [Google Scholar]
- 12.Đinh ST, Galis I, Baldwin IT. UVB radiation and 17‐hydroxygeranyllinalool diterpene glycosides provide durable resistance against mirid (Tupiocoris notatus) attack in field‐grown Nicotiana attenuata plants. Plant Cell Environ. 2013;36:590–606. doi: 10.1111/j.1365-3040.2012.02598.x. [DOI] [PubMed] [Google Scholar]
- 13.Dillon FM, Tejedor MD, Ilina N, Chludil HD, Mithöfer A, Pagano EA, Zavala JA. Solar UV‐B radiation and ethylene play a key role in modulating effective defenses against Anticarsia gemmatalis larvae in field‐grown soybean. Plant Cell Environ. 2018;41:383–394. doi: 10.1111/pce.13104. [DOI] [PubMed] [Google Scholar]
- 14.Qi J, Zhang M, Lu C, Hettenhausen C, Tan Q, Cao G, Zhu X, Wu G, Wu J. Ultraviolet-B enhances the resistance of multiple plant species to lepidopteran insect herbivory through the jasmonic acid pathway. Sci Rep. 2018;8:277. doi: 10.1038/s41598-017-18600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escobar-Bravo R, Cheng G, Kyong Kim H, Grosser K, van Dam NM, Leiss KA, Klinkhamer PG. Ultraviolet radiation exposure time and intensity modulate tomato resistance against herbivory through activation of the jasmonic acid signaling. J Exp Bot. 2018. doi: 10.1093/jxb/ery347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 17.Abe H, Ohnishi J, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M. Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol. 2008;49:68–80. doi: 10.1093/pcp/pcm168. [DOI] [PubMed] [Google Scholar]
- 18.Abe H, Shimoda T, Ohnishi J, Kugimiya S, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M. Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol. 2009;9:97. doi: 10.1186/1471-2229-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escobar-Bravo R, Klinkhamer P, Leiss KA. Induction of jasmonic acid-associated defenses by thrips alters host suitability for conspecifics and correlates with increased trichome densities in tomato. Plant Cell Physiol. 2017;58:622–634. doi: 10.1093/pcp/pcx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawazu K, Mochizuki A, Sato Y, Sugeno W, Murata M, Seo S, Mitsuhara I. Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod Plant Interact. 2012;6:221–230. doi: 10.1007/s11829-011-9174-z. [DOI] [Google Scholar]
- 21.Schmelz EA, Alborn HT, Banchio E, Tumlinson JH. Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta. 2003;216:665–673. doi: 10.1007/s00425-002-0898-y. [DOI] [PubMed] [Google Scholar]
- 22.Thaler JS, Stout MJ, Karban R, Duffey SS. Jasmonate‐mediated induced plant resistance affects a community of herbivores. Ecol Entomol. 2001;26:312–324. doi: 10.1046/j.1365-2311.2001.00324.x. [DOI] [Google Scholar]
- 23.Izaguirre MM, Mazza CA, Svatoš A, Baldwin IT, Ballaré CL. Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora. Ann Bot. 2007;99:103–109. doi: 10.1093/aob/mcl226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost CJ, Mescher MC, Carlson JE, De Moraes CM. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol. 2008;146:818–824. doi: 10.1104/pp.107.113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Medina A, Flors V, Heil M, Mauch-Mani B, Pieterse CM, Pozo MJ, Ton J, van Dam NM, Conrath U. Recognizing plant defense priming. Trends Plant Sci. 2016;21:818–822. doi: 10.1016/j.tplants.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Geng X, Jin L, Shimada M, Kim MG, Mackey D. The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae. Planta. 2014;240:1149–1165. doi: 10.1007/s00425-014-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 2003;36:485–499. [DOI] [PubMed] [Google Scholar]
- 28.Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, Bender CL. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol Plant Micro Inter. 2007;20:955–965. doi: 10.1094/MPMI-20-8-0955. [DOI] [PubMed] [Google Scholar]


