ABSTRACT
In Arabidopsis thaliana AtPEPR1 and AtPEPR2 act as the receptors for the endogenous AtPROPEP-derived Pep peptides and subsequently initiate defense-signaling cascades. In the previous work,9 the expression pattern of the genes encoding the PEPR receptors and the AtPROPEP peptide precursor proteins was studied using promoter-GUS reporter constructs. Here, using the same constructs to study their expression pattern under biotic and abiotic stress, including AtPep1, flg22, methyl jasmonate (MeJA), and NaCl treatments, we observed that in response to AtPep1 and flg22, the activation of AtPEPR1 promoter was different from AtPEPR2. We also found that these promoters were differentially activated in response to NaCl. Remarkably, we showed that it is possible to classify the genes of the AtPROPEP family, based on the response of their promoters to the various stimuli employed: thus, we classify AtPROPEP1 in one group; AtPROPEP2 and AtPROPEP3 in a second group; AtPROPEP4, AtPROPEP7 and AtPROPEP8 in a third group and AtPROPEP5 in a fourth group. Our finding, confirm non-redundant roles among the members of the AtPROPEP family and their corresponding receptors.
Keywords: Arabidopsis thaliana, AtPep, AtPEPR, biotic and abiotic stress, DAMP, endogenous elicitor, immune system, promoter activation, receptor
Introduction
In plants, pattern recognition receptors (PRRs) recognize microbe-derived signature components known as microbe-associated molecular patterns (MAMPs) and also damage-associated molecular patterns (DAMPs) which are host-derived danger signals that subsequently trigger plant immunity.1,2 Some of the well-studied PRRs are flagellin sensing 2 (known as FLS2), elongation factor Tu (known as EF-Tu) and chitin elicitor receptor kinase 1 (known as CERK1) from Arabidopsis thaliana, perceive the conserved N-terminal portion of flg22, EF-Tu, and fungal cell wall component chitin, respectively.3–6
The family of AtPeps, peptides which were initially identified by Huffaker et al. (2006)7 as endogenous peptide elicitors in A. thaliana, are considered to be DAMPs because they play a critical role in defense signaling, but they may also be involved in development.8 AtPep1 and its homologs (AtPep2-8) originate from the conserved C-terminal portion of their respective precursors AtPROPEP1–8, respectively.7,9 They are perceived through two plant cell surface PRRs, namely AtPEPR1 and AtPEPR2.10–12
Perception of Peps by the AtPEPRs, like perception of MAMPS by the corresponding PRRs, leads to the activation of downstream defense cascades including ion fluxes across the plasma membrane (e.g. increase in Ca2+ influx) within 30 s to 2 min.13 The Ca2+ elevation leads to the activation of the RbohD protein, which has crucial role in the production of reactive oxygen species which occurs in 2–3 min.3,14 Concomitantly, phosphorylation of mitogen-activated protein kinases (MAPKs; particularly of MPK3, MPK4 and MPK6) via MAP kinase cascades which occurs a few minutes after elicitor perception.15–17 In addition, stomatal closure is accrued after a few minutes upon elicitor perception.18 As well, ethylene (ET) biosynthesis is enhanced through activation of 1-amincyclopropane-1-carboxylate (ACS) synthase within 30 min.19 Furthermore, jasmonic acid-responsive genes involved in plant defense get activated in a few hours.20,21 Also, the accumulation of the plant defense hormone salicylic acid, which occurs in a few hours, is a subsequent event after elicitor perception.20–22 DAMP-triggered signaling cascades also lead to massive transcriptional reprogramming of the host cell, which may subsequently fine-tune effective and adequate defense responses.21
It has been observed that treatments of Arabidopsis plants with AtPeps induce the transcription of their own precursor genes and also other defense genes.21 Studies showed that the expression of the AtPROPEP1 gene is induced upon flg22 treatment, AtPep1 itself, wounding, ethylene (ET), and also methyl jasmonate (MeJA).7 It was also observed that WRKY transcription factors (TFs), which are the major regulators of MAMP-induced genes, have a role in expression of AtPROPEP2 and AtPROPEP3 expression.23 It seems that there is a positive feedback between Peps perception and activation of the immune system.22 It has also been observed that perception of Peps can increase resistance against bacterial or fungal infections in Arabidopsis and also in maize24-26 and it has recently been shown that AtPEPR1 and AtPEPR2 may play a crucial role in inducing local and systemic immunity.21
The PRRs AtPEPR1 and AtPEPR2 have been identified as the receptors for AtPeps. Both belong to the Receptor-like kinases with extracellular leucine-rich repeat motifs.11,12 AtPEPR1 and AtPEPR2 have different preferences for AtPeps perception: AtPep1 to AtPep8 are perceived by AtPEPR1, and they have similar activity in inducing AtPEPR1-mediated plant immune responses, while AtPEPR2 is only responsive to AtPep1 and AtPep2.10
Despite numerous attempts to determine the role of each AtPROPEP and also AtPEPR1 and AtPEPR2, the exact role of these elicitors and of the corresponding receptors is still unknown.8 Using promoter-β-glucuronidase fusion constructs including: pAtPEPR1::GUS, pAtPEPR2::GUS, pAtPROPEP1::GUS, pAtPROPEP2::GUS, pAtPROPEP3::GUS, pAtPROPEP4::GUS, pAtPROPEP5::GUS, pAtPROPEP7::GUS and pAtPROPEP8::GUS, we attempted to further evaluate and dissect the promoter activities of the genes encoding these endogenous peptide defense signals and also their corresponding receptors under different biotic and abiotic stresses and subsequently, to characterize and classify them into different groups.
Results
Differential, and tissue-specific activation of the promoters pAtPEPR1 and pAtPEPR2 studied in promoter-gus reporter lines in seedlings of A. thaliana in response to AtPep1 and flg22 treatment
We first studied the activities of the pAtPEPR1 and pAtPEPR2 promoters in seedlings harboring promoter-GUS fusions after elicitor treatment and compared them with mock-treated controls. We fixed the seedlings after 0, 1, 6 and 24 h and then performed a GUS staining.
We noticed that pAtPEPR1 and pAtPEPR2 promoters exhibited different expression patterns in response to these elicitor treatments. The pAtPEPR1 promoter was activated already 1 h after the AtPep1 treatment in cotyledons, in shoots and in roots, but neither in the hypocotyls nor in the root tips. Compared to the mock-treated control seedlings, 6 h after the AtPep1 treatment, the pAtPEPR1 promoter became activated even more higher level in cotyledon leaves and root tip. We did not observe the activation of pAtPEPR1 promoter in hypocotyls. Within 24-h posttreatment, compared to the mock-treated control seedlings, we noticed that the pAtPEPR1 promoter was activated in the shoot, but its activation was weaker than 6-h posttreatment (Figure 1; Table 1).
Figure 1.

Patterns of GUS staining in seedlings of Arabidopsis carrying pAtPEPR1:: GUS and pAtPEPR2::GUS reporter constructs, treated with 1 μM flg22 and 1 μM AtPep1. 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
Table 1.
pAtPEPR1::GUS and pAtPEPR2::GUS activity in response to AtPep1 and flg22 treatments.a.
| Shoot |
Root |
|||||||
|---|---|---|---|---|---|---|---|---|
| AtPEPRs | Treatment | Time point | Shoot meristem | Hypocotyl | Cotyledon | Tip | Lateral root | Ground Tissues |
| pAtPEPR1 | 1 h | + | - | + | - | - | + | |
| AtPep1 | 6 h | + | - | + | + | - | + | |
| 24 h | + | - | + | + | - | + | ||
| 1 h | + | - | + | - | - | - | ||
| flg22 | 6 h | + | - | + | - | - | - | |
| 24 h | + | - | + | - | - | + | ||
| pAtPEPR2 | 1 h | - | - | - | - | - | + | |
| AtPep1 | 6 h | + | - | - | - | - | + | |
| 24 h | + | - | - | - | - | + | ||
| 1 h | - | - | - | - | - | + | ||
| flg22 | 6 h | - | - | - | - | - | + | |
| 24 h | - | - | - | - | - | + | ||
a The signs in the table signify the following:
+: promoter is activated.
-: promoter is not activated.
On the other hand, 1-h posttreatment with flg22, the pAtPEPR1 promoter became activated in cotyledons but its activation remained more and less the same after 6 and 24-h posttreatment.
For the pAtPEPR2 promoter, 1-h posttreatment with AtPep1, compared to the mock-treated control seedlings, we noticed that the promoter became activated in roots 1 h after treatment. The response at 6 and 24 h was the same in roots but the promoter also became activated in the shoot meristem in response to AtPep1. We observed that 6-h posttreatment with AtPep1, the promoter activation of pAtPEPR2 was more robust than 24-h posttreatment. Compared to the mock-treated control seedlings in response to flg22, the promoter pAtPEPR2 became activated in the root 1-h posttreatment.
We observed the same response at 6 and 24-h posttreatment but the response was a bit weaker compared to the 1-h treatment. We also did not observe any activation of the promoter for pAtPEPR2 in shoots in response to flg22 and they were similar to mock-treated control seedlings. In a nutshell, it seems that the pAtPEPR1 promoter can be activated in different parts of the plant while pAtPEPR2 promoter expression is more restricted to the root.
Activation of the promoters of the AtPROPEPs and AtPEPR genes in response to MeJA in leaves
We used the transgenic Arabidopsis lines described earlier, carrying promoter-GUS-reporter constructs for each of the AtPROPEPs and AtPEPR genes available.9 All promoters were activated by a treatment with the phytohormone MeJA, as visualized by an increase of GUS expression, compared to a mock-treated control leaves. Interestingly, we noticed that all promoter reporter lines which we used in this study, became activated in response to MeJA treatment but overall, upon MeJA treatment, the levels of their activation were different from each other; therefore, it was also possible to differentiate the Pep family according to the response of their promoters upon MeJA treatment.
As it can be seen in Figure 2, in response to MeJA compared to the mock-treated control leaves, pAtPEPR1 promoter was strongly get activated and the level of activation was almost the same at different concentrations (1μM, 10 μM, 100 μM; Figure 2, Table 2). This indicates that even a low concentration of MeJA (1 μM) is adequate to activate the pAtPEPR1 promoter. This promoter was also slightly activated in mock-treated control leaves, especially 24-h posttreatment. On the other hand, as can be seen in Figure 2, the promoter for the pAtPEPR2 gene is just activated in veins and vascular tissues, and the level of the expression of these genes is similar at different time points (1, 6 and 24 h), and also at different concentrations of MeJA (1 μM, 10 μM, 100 μM). Remarkably, we observed that just 1 μM of MeJA activated the pAtPEPR1 promoter, 1-h posttreatment and the level of activation of this promoter increased over time as 24-h posttreatment with 1 μM of MeJA the stained leaves were almost completely blue (Figure 2). Treatment of the pAtPEPR1 promoter reporter line with 10 μM MeJA also led to the robust activation of this promoter; the level of activation of this promoter, comparing the 1 μM and 10 μM of MeJA treatments, was almost the same. We also observed that 100 μM of MeJA induced a very strong activation of this promoter, as the leaves were almost blue, especially 6 h after treatment. On the other hand, we observed that the pAtPEPR2 promoter was not so active compared to the pAtPEPR1 promoter. In other words, as can be seen in Figure 2, the level of activation of these two promoters (pAtPEPR1 and pAtPEPR2) was completely different from each other. Intriguingly, the pAtPEPR2 promoter, in response to MeJA, was only activated in the main vascular tissue. As shown in Figure 2, the pAtPEPR2 promoter was also a little active without any stimuli, but MeJA get activate this promoter slightly more than the control treatment. As also shown in Figure 2, 100 μM of MeJA treatment activated this promoter in the main vascular tissue clearly more than 1 μM and 10 μM of MeJA treatment. Cumulatively, these results indicate that there is a specific and also differential activation pattern between pAtPEPR1 and pAtPEPR2 promoters in response to MeJA compared to the control treatment.
Figure 2.

Patterns of GUS staining in seedlings of Arabidopsis carrying pAtPEPR1::GUS and pAtPEPR2::GUS reporter constructs, treated with Methyl Jasmonate (MeJA). 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
Table 2.
Schematic representation of the promoter activation of pAtPEPR1, pAtPEPR2, pAtPROPEP1, pAtPROPEP2, pAtPROPEP3, pAtPROPEP4, pAtPROPEP5, pAtPROPEP7 and pAtPROPEP8: GUS in leaves of Arabidopsis thaliana in response to elicitor treatmentsa.
 |
Concerning the promoters of the AtPROPEP genes, we observed that the pAtPROPEP1 to pAtPROPEP8 promoter activities were different from each other, in response to MeJA and also compared to the control. Table 2 schematically represents the activation of these promoters in response to the MeJA. Specifically, as can be seen in Figure 3, the pAtPROPEP1 promoter was activated by MeJA mainly in the main vascular tissue of the leaf. This hormone also activated the promoter in additional parts of the leaves beside the vascular tissue, leading to “blue patch” GUS staining. On the other hand, MeJA treatment activated the pAtPROPEP2 promoter in different parts of the leaves and also in the vascular tissue.
Figure 3.

Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP1::GUS and pAtPROPEP2::GUS reporter constructs, treated with Methyl Jasmonate (MeJA). 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
Remarkably, the pattern of the pAtPROPEP2 promoter activation was exacerbated at higher concentrations of MeJA (Figure 3). Interestingly, this promoter was almost more active after 1 h after treatment than after 24 h after treatment. One of the most interesting findings in this study is the high activation of pAtPROPEP3 in response to MeJA treatment. As can be seen in Figure 4 and also Table 2, the pAtPROPEP3 promoter is activated in the vascular tissues and also additional parts of the leaves. We have observed that the pAtPROPEP3 promoter is highly active 1 h after treatment in response to 10 μM and also 100 μM MeJA treatment but we have not seen such a strong activation in treatment with 1 μM MeJA treatment. It seems that the concentration of MeJA is important for the activation of the pAtPROPEP3 promoter. Furthermore, as it can be seen in Figure 4, the pAtPROPEP3 promoter is also slightly active without any treatments and its activation is increased over the time of treatment. It seems that pAtPROPEP3 promoter activity is highly dynamic upon MeJA treatment. This observation is in line with the former investigations indicating that the AtPROPEP3 promoter region is highly active and dynamic.23 Note that we saw a slight activation of pAtPROPEP3 in some of the mock-treated control leaves.
Figure 4.
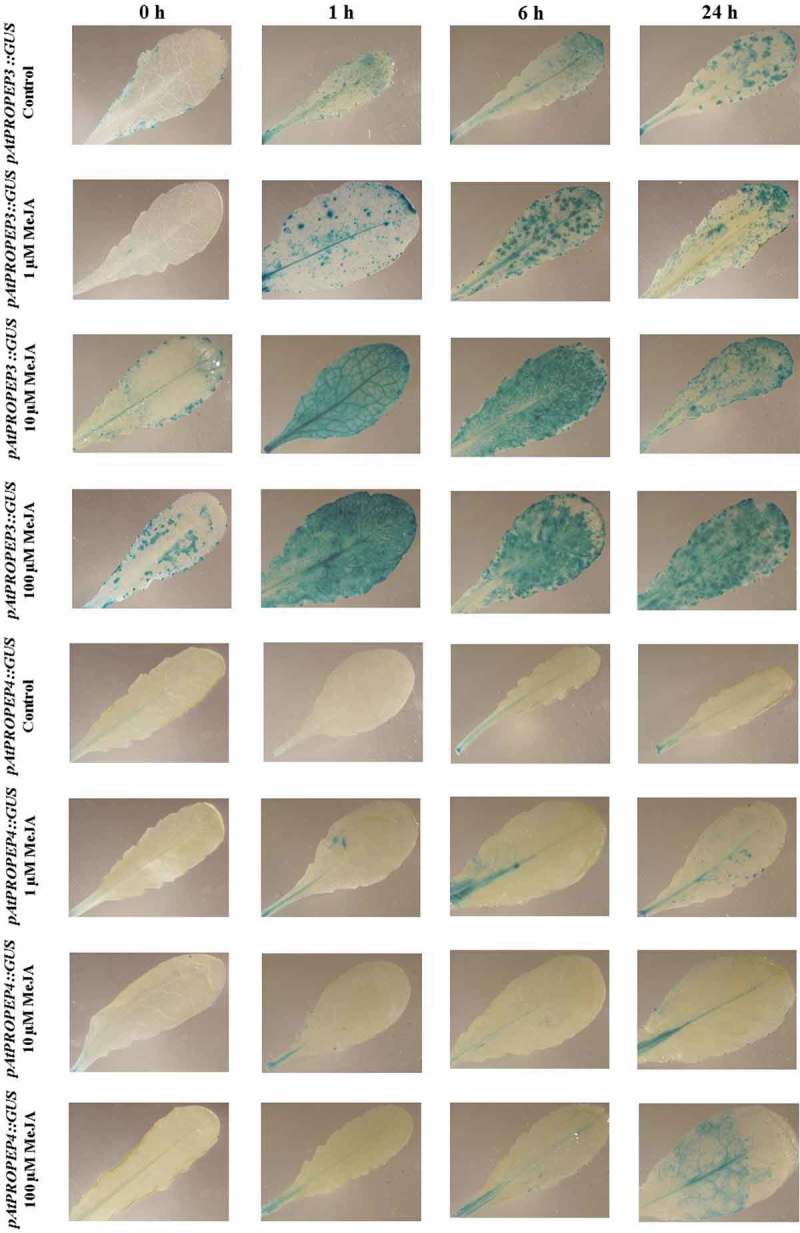
Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP3::GUS and pAtPROPEP4::GUS reporter constructs, treated with Methyl Jasmonate (MeJA). 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
On the other hand, as is represented in Figure 4, compared to the control leaves the pAtPROPEP4 promoter was just activated in the main vascular tissue, and the pattern of the activation of this promoter line was very different compared to pAtPROPEP3 (Figure 4; Table 2).
As illustrated in Figure 5, the pAtPROPEP5 promoter is highly inducible in all vascular tissues of the leaves compared to the mock-treated control leaves, and activation of this promoter increased at higher concentrations of MeJA. But as can be seen in Figure 5, the pattern of induction of the pAtPROPEP5 promoter is different compared with other promoters; it becomes activated in all vascular tissues in leaves but not in additional parts of the leaves. In addition, comparing the different concentrations, we observed that the higher the concentration of MeJA, the more this promoter was activated.
Figure 5.
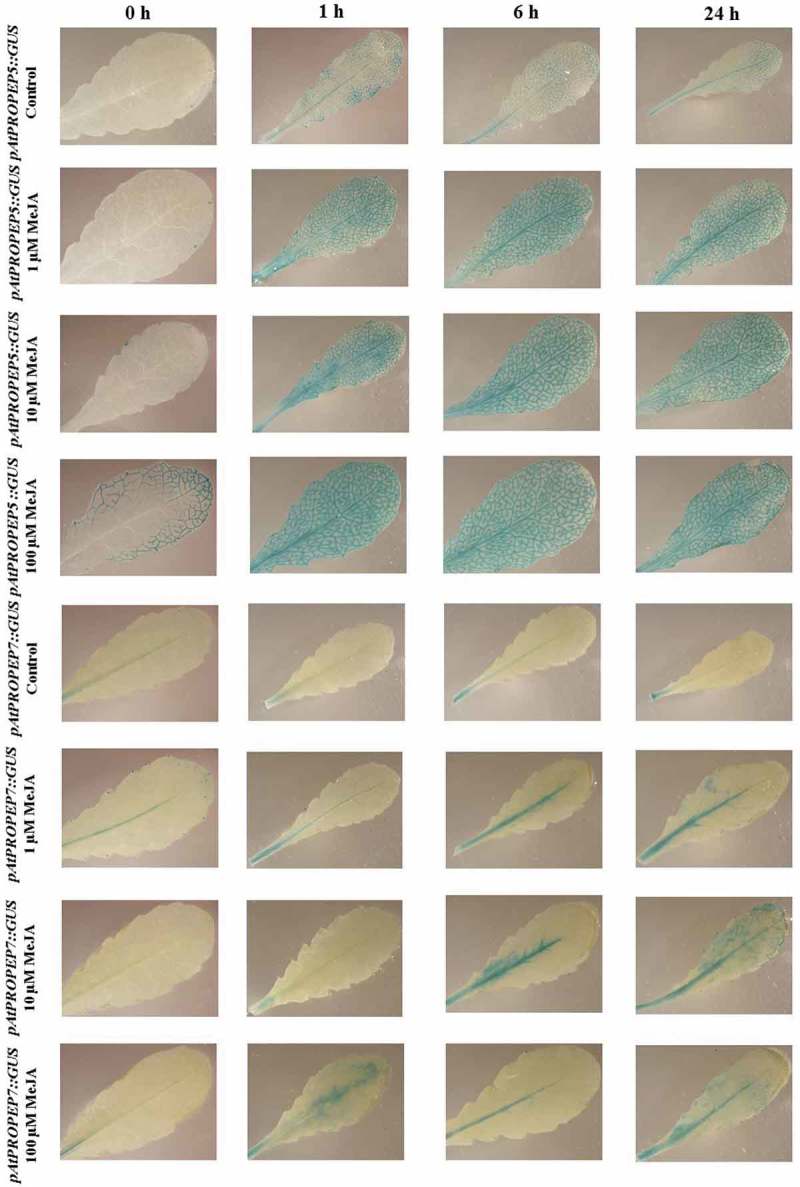
Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP5::GUS and pAtPROPEP7::GUS reporter constructs, treated with Methyl Jasmonate (MeJA). 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
We observed that in response to MeJA and compared to the mock-treated control leaves, the pAtPROPEP7 promoter was activated only in the main vascular tissue. We noticed that the pAtPROPEP7 promoter was more responsive to 10 μM MeJA. As it can be seen in Figure 5 and in Table 2, compared to the mock-treated control leaves, the pAtPROPEP7 promoter responded almost in the same way as pAtPROPEP4. Concerning the pAtPROPEP8 promoter, we did not observe high activity of this promoter in response to MeJA treatment when compared to the mock-treated control leaves; this promoter was just active in the main vascular tissues (Figure 6; Table 2) and responded almost the same way to MeJA as the pAtPROPEP4 and pAtPROPEP7 promoters.
Figure 6.
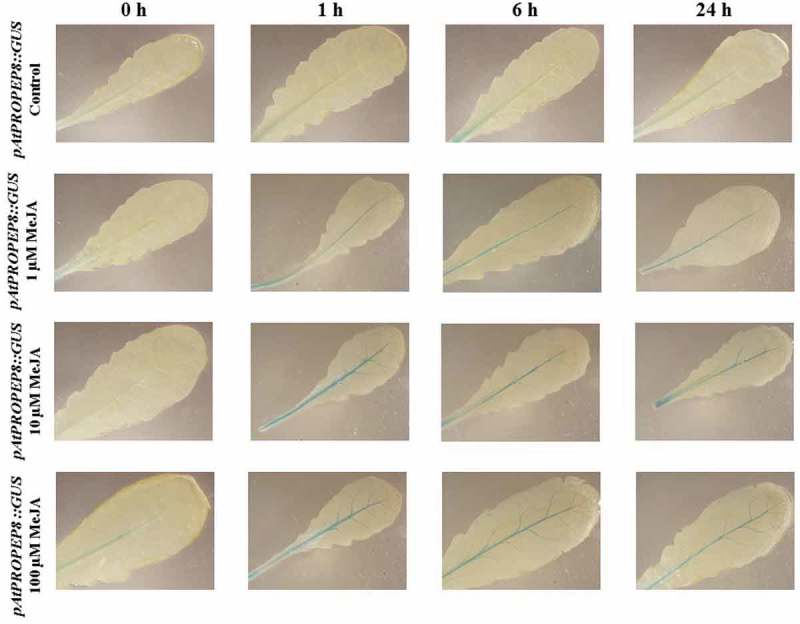
Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP8::GUS reporter construct, treated with Methyl Jasmonate (MeJA). 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
In conclusion, in response to MeJA and compared to the mock-treated control leaves, we have noticed that it is possible to classify the Pep family promoter activation in response to MeJA (Figure 2-6; Table 2). We can classify pAtPROPEP1 in one group as it expressed almost uniformly; pAtPROPEP2 and pAtPROPEP3 in the second group as they are highly activated in response to MeJA treatment; pAtPROPEP4, pAtPROPEP7 and pAtPROPEP8 in the third group; and as pAtPROPEP5 promoter did respond differentially comparing with others, we grouped it in the fourth group.
The AtPROPEPs and AtPEPR promoters are induced in response to AtPep1 and flg22
We observed that the promoters of AtPROPEP 1-8 and also of the genes encoding the corresponding receptors (AtPEPR1 and AtPEPR2) responded differently in treatment with AtPep1 and also flg22 compared to the mock treatments. Briefly, we observed that in response to AtPep1, the pAtPEPR1 promoter, is highly activated already after 1 h of treatment, while we did not observe a robust activation of the pAtPEPR2 promoter in response to AtPep1 treatment and this promoter was most active in the main vascular tissue and the highest activation of this promoter was 24-h posttreatment (Figure 7; Table 2). We also observed that the pAtPEPR2 promoter was almost inactive in control untreated leaves.
Figure 7.
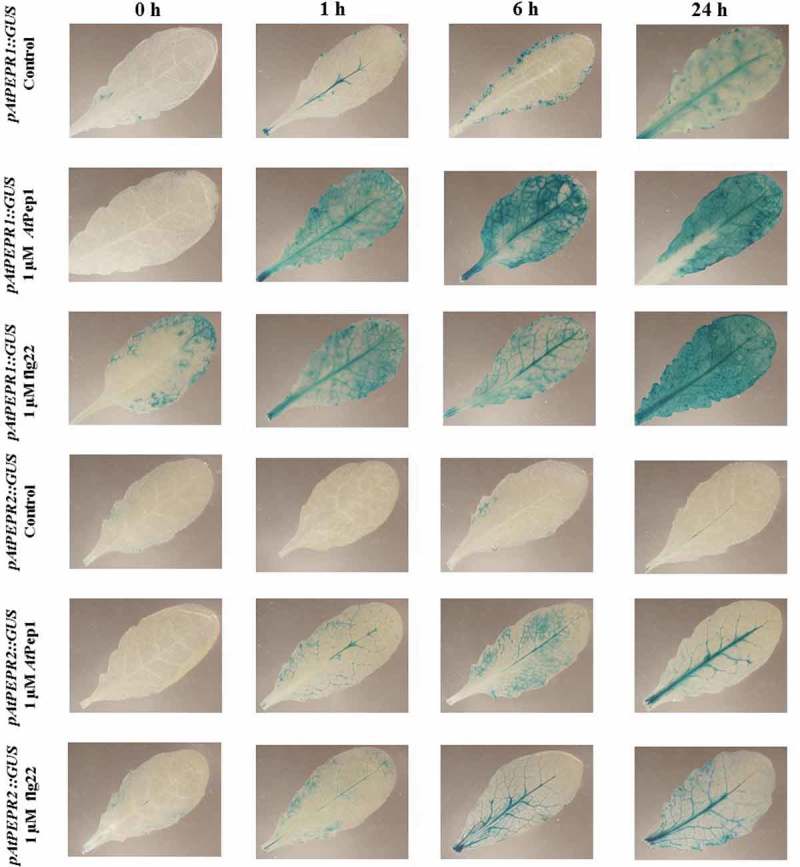
Patterns of GUS staining in leaves of Arabidopsis carrying pAtPEPR1::GUS and pAtPEPR2::GUS reporter constructs, treated with 1 μM AtPep1 and 1 μM flg22. 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
One of the interesting findings of this research was that flg22 differentially activated the pAtPEPR1 promoter and to a lower extent the pAtPEPR2 promoter. We observed that the pattern of activation of these two promoters was different. More specifically, the pAtPEPR1 promoter was active in both the vascular tissues and also additional parts of the leaves but the pAtPEPR2 promoter was only active in the main vascular tissues. We also observed that after 24 h of treatment, the highest activation of both promoters occurred, compared to the mock-treated control leaves (Figure 7). This observation is in line with former studies indicating that flg22 and Pep system work as a positive loop.
On the other hand, contrary to expectations, AtPep1 did not highly activate its own promoter (Figure 8; Table 2). However, AtPep1 activated the pAtPROPEP3 promoter and to a lower extent the AtPROPEP2 promoter (Figure 8 and Figure 9).
Figure 8.

Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP1::GUS and pAtPROPEP2::GUS reporter constructs, treated with 1 μM AtPep1 and 1 μM flg22. 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
Figure 9.

Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP3::GUS and pAtPROPEP4::GUS reporter constructs, treated with 1 μM AtPep1 and 1 μM flg22. 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
Interestingly, we observed that in response to flg22 and also AtPep1, the pAtPROPEP2 promoter was not activated in the main vascular tissue but it was activated in additional parts of the leaves. A similar observation was made for the pAtPROPEP3 promoter, with the exception that 24-h posttreatment with AtPep1, beside additional parts of the leaves, we observed the activation of this promoter in the main vascular tissue too (Figure 9).
The pAtPROPEP4 promoter activated at a very low level in response to AtPep1 and also flg22 (Figure 9). Concerning the pAtPROPEP7 promoter, we did not observe any activation of this promoter in response to AtPep1 and flg22.
Regarding the pAtPROPEP5 promoter, we observed a very low activation of this promoter in response to AtPep1 and flg22. As can be seen in Figure 10, the pAtPROPEP5 promoter was activated to similar degree at 1, 6 and 24-h posttreatment with AtPep1, while the activation of this promoter by flg22 showed a different time course; flg22 activated this promoter mainly 6 h after treatment. It seems that in response to AtPep1, the activation of the pAtPROPEP5 promoter is higher than in response to flg22. For the pAtPROPEP8 promoter, as shown in Figure 10, we did not observe an activation of this promoter in response to flg22 and AtPep1, except for the main vein. Briefly, AtPep1 activated this promoter in the main vascular tissue, and the highest activation of this promoter was 24-h posttreatment. Flg22 similarly activated this promoter in the main vascular tissue 6 and 24-h posttreatment.
Figure 10.
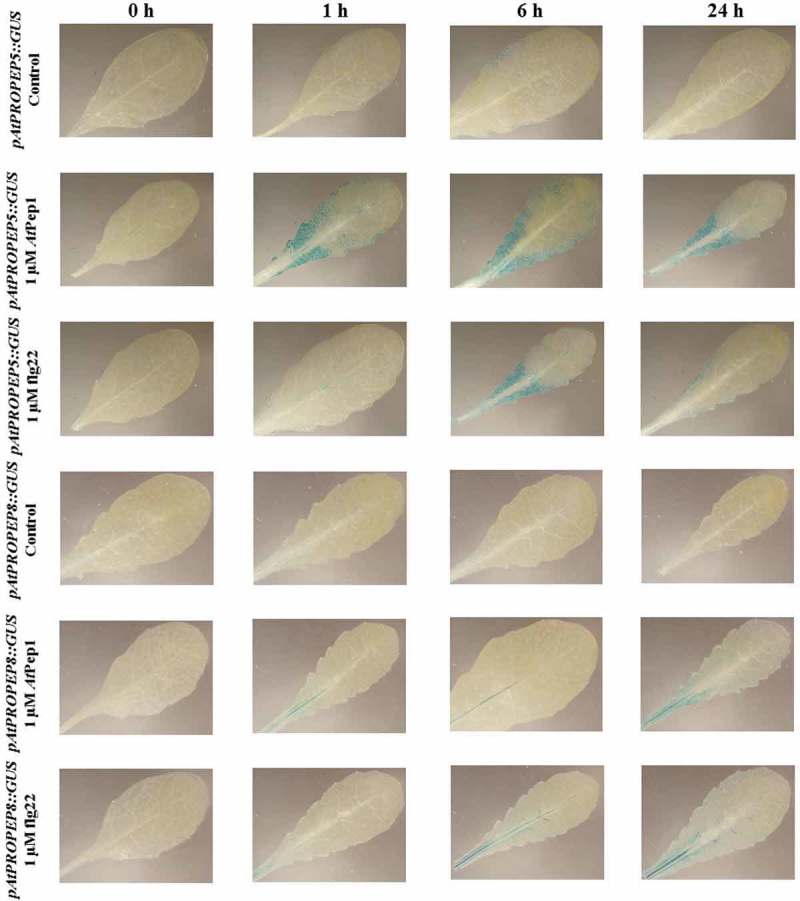
Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP5::GUS and pAtPROPEP8::GUS reporter constructs, treated with 1 μM AtPep1 and 1 μM flg22. 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
pAtPROPEP1, pAtPROPEP3 and pAtPEPR1 promoters are induced in response to NaCl treatment
We observed that compared to the mock-treated control leaves, treatment with NaCl (100 and 250 mM concentration) strongly activated the pAtPROPEP1, pAtPROPEP3 and also pAtPEPR1 promoters in leaves (Figures 11 and 12). Briefly, as it can be seen in Figure 11, 24-h posttreatment with NaCl, the pAtPROPEP1 promoter was activated in response to 100 mM NaCl. As is illustrated in Figure 11, the level of activation of this promoter increased once the leaves were treated with 250 mM NaCl. In addition, as can be seen in Figure 11, the level of the pAtPROPEP1 promoter activation in response to 250 mM NaCl treatment was increased over different time points.
Figure 11.
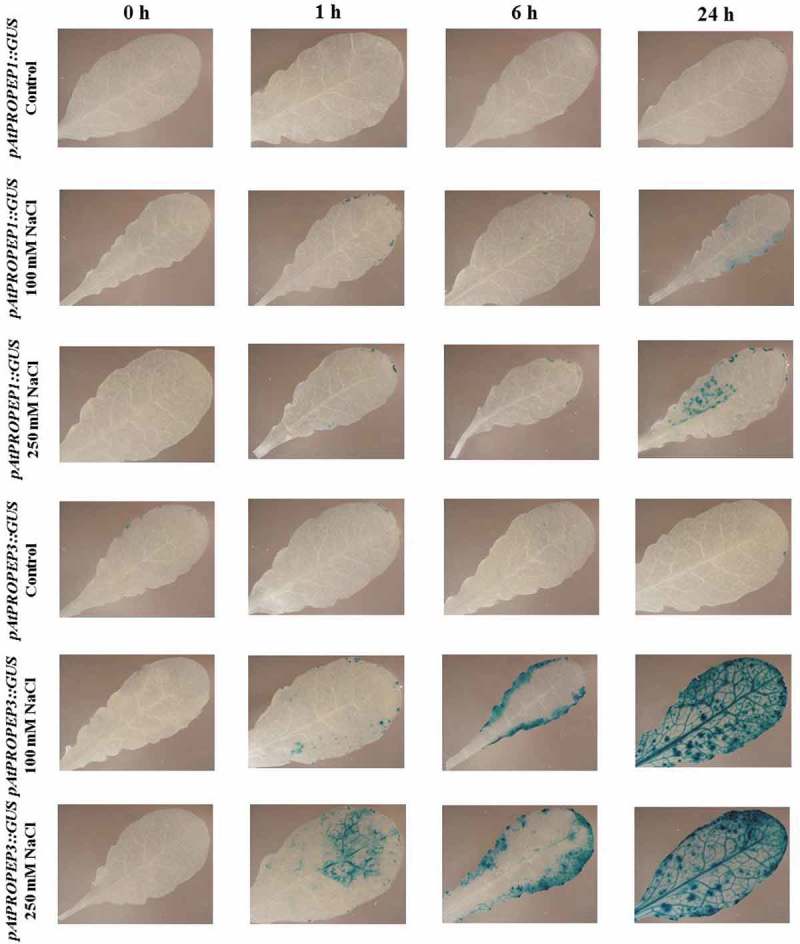
Patterns of GUS staining in leaves of Arabidopsis carrying pAtPROPEP1::GUS and pAtPROPEP3::GUS reporter constructs, treated with 100 mM and 250 mM NaCl. 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
Figure 12.
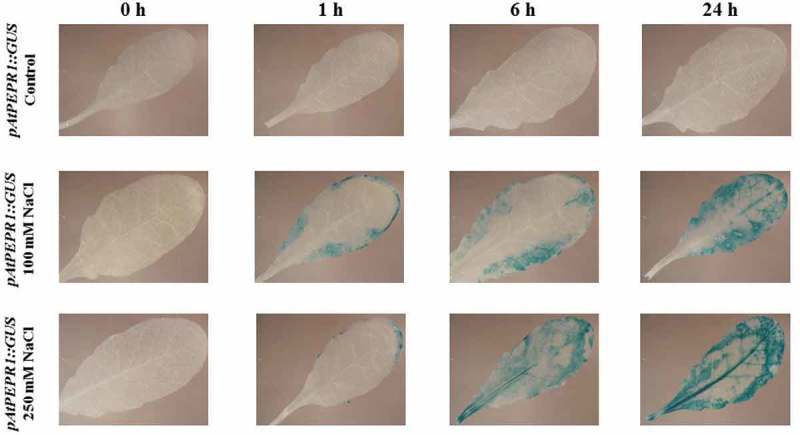
Patterns of GUS staining in leaves of Arabidopsis carrying pAtPEPR1::GUS reporter construct, treated with 100 mM and 250 mM NaCl. 0 h: 0 time point; 1 h: one hour after treatment; 6 h: six hours after treatment; 24 h: 24 hours after treatment.
Remarkably, we noticed that the pAtPROPEP3 promoter produced a stronger GUS staining compared to the pAtPROPEP1 promoter. As presented in Figure 11, compared to the mock-treated control leaves, 1 h after treatment with 100 mM NaCl, the pAtPROPEP3 promoter was activated, and activation of this promoter increased with time. Furthermore, treatment with 250 mM NaCl led to stronger activation of pAtPROPEP3 promoter. At 24-h posttreatment with 250 mM NaCl, the pAtPROPEP3 promoter was activated in the main vascular tissue and also in additional parts of the leaf.
Cumulatively, as can be seen in Figure 11 and also in Figure 12, the activation of these promoters (pAtPROPEP1, pAtPROPEP3 and pAtPEPR1) was increased 24 h after treatment. We observed an increased activity of the pAtPROPEP3 promoter in all parts of the leaves but for the pAtPROPEP1 promoter, it was activated only in some parts of the leaves.
Interestingly, the AtPEPR1 promoter was also activated in response to NaCl treatment. As can be seen in Figure 12, treatment with 100 mM NaCl led to the activation of the AtPEPR1 promoter 1 h after treatment and the activation of this promoter increased over time. Furthermore, 250 mM NaCl treatment strongly activated the AtPEPR1 promoter, especially 24-h posttreatment. In summary, treatment with NaCl activated the pAtPROPEP1, pAtPROPEP3 and pAtPEPR1 promoters in a time and concentration-dependent manner. The promoters of the other AtPROPEP genes were not activated in response to NaCl treatment.
Comparative analysis of PEP-family promoters in response to different treatments
In Table 2, we compared the activation of the pAtPEPR1, pAtPEPR2, pAtPROPEP1, pAtPROPEP2, pAtPROPEP3, pAtPROPEP4, pAtPROPEP5, pAtPROPEP7 and pAtPROPEP8 promoters in leaves upon different treatments including AtPep1, flg22, MeJA and NaCl. This table highlights several features of Pep-family promoter activation. As can be seen in this Table, the pAtPEPR1 and pAtPROPEP3 promoters were highly active upon AtPep1, flg22, MeJA and NaCl treatments. Interestingly, in response to different stimuli, the activation of these two promoters increased over time. The pAtPROPEP2 promoter also followed the same pattern of activation; the difference is that the response of this promoter was weaker compared to the pAtPROPEP3 promoter. On the other hand, pAtPEPR2 is not highly active compared to pAtPEPR1. It was almost only active in main vascular tissue on one hand and on the other hand, the activation of this promoter did not increase over time.
As shown in Table 2, pAtPROPEP1 was activated only slightly in response to AtPep1 and also flg22, and it is worth mentioning that the activation of this promoter upon different stimuli did not follow the same pattern as it was highly active 6 h after flg22 treatment but for NaCl treatment, it was more active 24-h posttreatment. As illustrated in Table 2, pAtPROPEP1 responded differentially upon different treatments, and it was highly active in response to MeJA but differentially respond to AtPep1 and also flg22 treatment. It is worth mentioning that the pAtPROPEP4, pAtPROPEP7 and pAtPROPEP8 promoters responded very weakly and similarly to these stimuli. Nevertheless, their response over time after treatment was somehow different. The highest activation of the pAtPROPEP4 promoter in response to AtPep1 occurred 6 h after treatment while for the pAtPROPEP8 promoter, it was 24 h after treatment.
It is interesting to note that, the pAtPROPEP7 promoter showed the weakest response to different stimuli compared to the other promoters studied here. In addition, as it is represented in Table 2, almost all promoters had some activity without any stimuli but the level of their activity was very low.
Discussion
Endogenous host molecules, termed as DAMPs, trigger a plant defense immune system through PRRs, which are released as a result of tissue damage.1 DAMP signaling cascades intensify or prolong the stereotypical defense responses that are also triggered by MAMPs.12 Using promoter reporter lines, we were able to confirm a strong induction of the pAtPROPEP promoters upon biotic and abiotic stresses. We observed a great similarity in the activation of pAtPROPEP1 and pAtPROPEP3 in response to these stimuli (AtPep1, flg22, MeJA, and NaCl). In other words, whatever stimuli can activate the pAtPROPEP1 promoter, can also activate the pAtPROPEP3 promoter. It seems that there is a positive feedback in the activation of these two promoters. Interestingly, in all treatments, the pAtPROPEP3 promoter activation was much more robust, and the pattern of induction was also different. Furthermore, we have not observed a robust induction of pAtPROPEP4, pAtPROPEP7 and also pAtPROPEP8. This observation is in line with the finding of Bartels et al. (2013).9 They also did not observe an induction of the pAtPROPEP4 and pAtPROPEP7 promoters in response to any induction or wounding.
Although studies on Pep research focused on their role in the plant immune system,12,28,29 there is emerging evidence indicating that Pep family also has a role in the development of the plants.30,31 Ma et al. (2014)31 have reported that the pAtPEPR2 promoter has strong activity in the vascular tissues of the roots. In transcriptional profiling analysis, they have shown that expression of 75% of AtPep1‐modulated genes in roots is fully dependent on pAtPEPR2. They have shown that Pep affects the regulation of GLUTAMINE DUMPER (GDU) genes. They also have shown that atpepr2 mutants exhibit shorter root phenotype. Ma et al. (2014)31 have also observed that AtPEPR2 mediates most of the AtPep1-induced transcriptional responses in the roots. Remarkably, we have noticed that pAtPEPR2 promoter is in strongly activated in root in response to AtPep1 perception. Based on what we have found in this research, coupled with Ma et al. (2014),31 it seems that the pAtPEPR2 has an important role in the AtPep1‐mediated signaling in the roots and taken together, we postulate that pAtPEPR2 may have a role in root development of Arabidopsis. Additionally, recently, Poncini et al. (2017)32 showed that AtPep1 is a stronger elicitor of immune signaling that flg22 or the chitin heptamer. They showed that AtPep1 can trigger a higher number and intensity of immune responses in root when it is compared to flg22. Our finding is in line with their findings as we also observed that compared to flg22, AtPep1 is more active in the root.
Gully et al. (2015)30 also have found a new possible function of Peps apart from their presumed major role in the plant immune system. They showed that dark-induced leaf senescence was affected by the presence of Peps. They also observed that this response was dependent on ET signaling and inhibited by the addition of growth hormone cytokinins. Interestingly, flg22 or elf18, both of which are potent inducers of PTI, did not induce an early start of leaf senescence. Based on their finding, it seems that Pep-perception affects and accelerates dark/starvation-induced senescence via an early induction of chlorophyll degradation and autophagy which is unrelated to PTI.
Concerning the activity of these promoters without any stimuli, Bartels et al. (2013)9 also showed that these promoters were not active even after 24 h of staining and they barely observed a visible blue precipitate in the leaves. Klauser et al. (2015)33 also showed that promoters of the AtPROPEP family were inactive in the absence of biotic and abiotic stresses. It seems that without any stress or stimuli, the activity of these genes is very low. This observation is in line with our finding indicating that the promoters of the AtPROPEP and AtPEPR gene families (pAtPEPR1, pAtPEPR2, pAtPROPEP1, pAtPROPEP2, pAtPROPEP3, pAtPROPEP4, pAtPROPEP5, pAtPROPEP7 and pAtPROPEP8) are almost inactive in the absence of any treatments. Furthermore, they also observed that MeJA has positive affect on the activation of the Pep family. In addition, it was shown previously that the Pep family is positively affected with the phytohormone jasmonic acid.31 We have also observed MeJA has positive affect in the activation Pep family promoters.
Host transcriptional responses are considered as a vital and crucial component of the plant defense system, and so far, several TFs have been identified as important regulators of plant immunity (reviewed by Eulgem, 2005; Moore et al. 2011).34,35 Recently, Logemann et al. (2013)23 described cis-regulatory modules (CRMs) of the AtPROPEP2 and AtPROPEP3 promoters, using Petroselinum crispum protoplasts and transgenic A. thaliana plants harboring promoter-reporter constructs that report MAMP responsiveness. They also detected a specific TF inducing transcription of the AtPROPEP2 and also AtPROPEP3 by chromatin immunoprecipitation and also DNA interaction studies. They identified conserved regions of the AtPROPEP3 locus in different Brassicaceae species. They confirmed that WRKY-type TFs played the most important role in regulating transcriptional outputs of the AtPROPEP2 and AtPROPEP3. They observed that in the AtPROPEP3 promoter, the CRM contained six W boxes. This observation may explain why the pAtPROPEP3 promoter is so active upon biotic and also abiotic stresses. However, although Logemann et al. (2013),23 have observed the same regulatory element for AtPROPEP2 and AtPROPEP3 promoter, we have not observed a similarly strong activation for the AtPROPEP2 promoter: Activation of the AtPROPEP2 promoter was much weaker than that of the AtPROPEP3 promoter. It seems that there are other factors affecting the activation of these promoters that must be studied in more detail.
Although the members of the AtPep family show similarity in amino acid sequence (Supplementary Fig. S1), the pattern of expression of their precursors is quite different. As an example, it has been observed that the promoter of AtPROPEP1 responds to infection with pathogens while the promoter of AtPROPEP3 is activated upon herbivory attack.25,33 Therefore, as AtPROPEPs, AtPEPR1 and AtPEPR2 promoter activation is very different, it seems that the AtPROPEPs, and also AtPEPR1 and AtPEPR2 have different roles. For instance, we have observed that the AtPROPEP3 promoter is strongly activated by various stress treatments, while we have not seen such an activation for others and especially the promoters of AtPROPEP4 and AtPROPEP7, which were almost inactive.
Recently, Bartels and Boller (2015),8 summarized the transcriptional landscape of the Arabidopsis AtPEPRs and AtPROPEPs genes in a review based on previous publications. They compared the activation of the various AtPROPEP promoters in different tissues of A. thaliana in response to various treatment including MAMPs, AtPeps, hormones, oral secretion of herbivores, pathogens, wounding and also darkness. Our findings have some similarities and differences with their report. Briefly, they summarized that, based on former publications, the AtPEPR1 and AtPEPR2 promoters were activated in response to flg22, AtPeps (AtPep1 to 6 for AtPEPR1 and AtPep1, 2 and 4 for AtPEPR2), elf18, MeJA, ET, and wounding. Our observation is in line with their review for the AtPEPR receptors with respect to the statement that the AtPEPR1 promoter is more active than the AtPEPR2 promoter. In their review, they also state that the promoters of AtPROPEP1, AtPROPEP2 and AtPROPEP3 are activated by flg22, AtPeps, elf18, MeJA, ET, and wounding. We also have confirmed that these three promoters are indeed strongly activated by the treatments mentioned. However, it is worth mentioning that there are some differences in the robustness of activation of the AtPROPEP1, AtPROPEP2 and AtPROPEP3 promoters. In comparison between the AtPROPEP1, AtPROPEP2 and AtPROPEP3 in response to MeJA, AtPep1, flg22 and NaCl, we found that the AtPROPEP1 promoter was little active in response to MeJA, while AtPROPEP2 promoter responded strongly to MeJA stimulus but the activity of AtPROPEP2 promoter was less than the AtPROPEP3 promoter in response to MeJA. In response to the AtPep1 treatment, the AtPROPEP1 promoter was more restricted to the vascular tissue while we observed that in response to AtPep1 treatment, the AtPROPEP2 promoter was activated in additional parts of the leaves but not in main vascular tissue. On the other hand, in response to AtPep1 treatment, the AtPROPEP3 promoter was strongly activated in the main vascular tissues and also additional parts of the leaves (especially 24-h posttreatment). They have summarized that the AtPROPEP4, AtPROPEP5 promoters are not activated in response to flg22, AtPeps and MeJA while we have shown that they respond to these stimuli differentially. In addition, they have not shown any data about the AtPROPEP7 and AtPROPEP8 promoters, while we present evidence that both the AtPROPEP7 and AtPROPEP8 promoters are active upon MeJA treatment but their activation is not strong and they were mainly active in the main vascular tissues. In comparison, in response to flg22 treatment and also AtPep1 treatment the AtPROPEP8 promoter responds slightly while AtPROPEP7 promoter did not respond to these stimuli (flg22 and also AtPep1 treatment). In addition, none of them responded to NaCl treatment.
Our observations confirm the former findings about the activation of the AtPEPR1 and AtPEPR2 genes. However, there are some differences among the robustness of their activation, as the AtPEPR1 promoter has proven to be highly active in response to different stimuli (Table 2), while the activation of the AtPEPR2 promoter in response to these stimuli was less robust, and restricted essentially to the main vascular tissue.
In addition, comparing the FLS2 receptor and AtPEPRs, FLS2 receptor can only perceive flg22 and closely related peptides,1 while AtPEPR1 and AtPEPR2 can perceive elicitors with more divergent amino acid sequences (AtPEPR1 can perceive AtPep1 to AtPep8; AtPEPR2 can perceive AtPep1 and AtPep2). It seems that AtPEPR1 and AtPEPR2 can perceive the broad spectrum of ligand in amino acid sequence in contrast with the FLS2 receptor. Therefore, it may be speculated that AtPEPR1 and AtPEPR2 might have more functions in addition to their role in immunity.8
From the discovery of the Pep family in Arabidopsis in 2006, and up to now that these endogenous peptide elicitors have been discovered in other plant species, little is known about the specific activation and function of Pep family. This study was an initial attempt to determine the expression of Pep family in response to biotic and abiotic stresses in A. thaliana. Based on what we have found, and comparing our results with former investigations, we can say that different AtPEPRs and AtPROPEPs might have different roles and functions in addition to their role in immunity, in development31 and also other biological process from germination to flowering and seed production. As an internal alarm, they might control the activation or repression of other genes. Cumulatively, they also might have specific functions such as a role in defense and also in development. In addition, it seems that their activity is distinct from each other in different tissues. As an example, AtPEPR2 promoter is highly active in the root even in the root tips but AtPEPR1 is more active in the leaves but not in the root tips. Therefore, it seems that the activation of the Pep family genes is regulated under special physiological and environmental circumstances. Hence, subsequent studies are needed to determine their multiple functions.
Materials and methods
Plant material and growth conditions
All Arabidopsis accessions were derived from the wild-type accession Columbia-0 (Col-0). Nine transgenic lines containing different promoter-GUS-reporter constructs and a resistance gene against BASTA were obtained from S. Bartels,8 namely: pAtPEPR1::GUS, pAtPEPR2::GUS, pAtPROPEP1::GUS, pAtPROPEP2::GUS, pAtPROPEP3::GUS, pAtPROPEP4::GUS, pAtPROPEP5::GUS, pAtPROPEP7::GUS, and pAtPROPEP8::GUS. Seedlings and mature leaves of Arabidopsis were used for GUS staining. For the preparation of sterile seedlings, Arabidopsis seeds were first washed with 99% ethanol supplemented with 0.5% Triton for 1 min; then washed with 50% ethanol supplemented with 0.5% Triton for 1 min, and finally with 100% ethanol for 2 min. Then, seeds were sown on Murashige and Skoog (MS) salt solid medium containing 1% sucrose and 0.8% Phytagel (Sigma-Aldrich) at pH 5.7. The plates were stratified for at least 2 d at 4°C, and then germinated at 21°C in the continuous light (MLR-350; Sanyo chamber). For mature Arabidopsis plants, seeds were sown in soil and after stratification for at least 2 d, pots were placed in short-day conditions (10 h light at 21°C and 14 h dark at 18°C with 60% humidity) for 4–5 weeks.
Peptides
Peptides used as elicitors were flg22 (QRLSTGSRINSAKDDAAGLQIA), and AtPep1 (ATKVKAKQRGKEKVSSGRPGQHN), which were obtained from EZBiolabs (http://www.ezbiolab.com/) and were dissolved in a BSA solution (containing 1 mg/mL bovine serum albumin and 0.1 M NaCl) and were kept in −20°C. The BSA treatment is used as the control and regarded as the mock-treated control.
Elicitor treatment and GUS staining
For seedlings, 1 week old Arabidopsis plants were used. Two seedlings were placed in each well of a 24-well plate containing 200μl ddH2O. For leaf staining, leaves of 4–5 weeks old Arabidopsis plants were harvested. The leaves were treated with elicitors AtPep1 and flg22 (1 μM); MeJA (1 μM, 10 μM, 100 μM), NaCl (100 mM NaCl and 250 mM) for different times in order to produce a time course including: Zero, 1 h after treatment, 6 h after treatment, and 24 h after treatment. The experiment has been done two times with four replicates for each time point. As a control for AtPep1 and flg22 treatment, the solvent for the peptides was used, i.e. a BSA solution and regarded as the mock-treated control. As a control for the MeJA treatment, a solvent control consisting of DMSO was used, and as a control for the NaCl treatment, the same amount of ddH2O was added. GUS staining was done as described previously by Bartels et al.9 Briefly, first the water containing elicitors was discarded and the tissue was fixed using ice-cold 90% acetone for 20 min. Then, GUS staining buffer (1 mM 5-bromo-4-chloro-3-indolyl β-d-glucuronide cyclohexyl ammonium salt monohydrate (Biosynth AG, Switzerland), 100 mM sodium phosphate (pH 7.5), 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 10 mM EDTA, and 0.1% (v/v) Triton X-100) was added to each tube and placed at 37°C for 24 h. Plant tissue was washed and cleared with 75% (v/v) ethanol for two times then photographed with Olympus SZX12 binocular in attached with an Olympus DP72 camera and the CellSens imaging software (Olympus America, Pennsylvania, USA).
Comparison consensus of the AtPROPEPs
In order to generate sequence logos of AtPROPEPs, 24 amino acid sequences from C-terminal region of AtPROPEPs (AtPROPEP1-AtPROPEP8) were sent to the WeLogo (http://www.weblogo.berkeleky.edu/) and graphical representations of consensus sequences were visualized.
Funding Statement
This work was supported by the Ministry of Science Research and Technology, Tehran, Iran [14666-64891]; Nikolaus und Bertha Burckhardt-Buergin-Stiftung Foundation, Basel, Switzerland [22_March_2016].
Abbreviations
- DAMPs
Damage-associated molecular patterns;
- MAMPs
microbe-associated molecular patterns
- MeJA
methyl jasmonate
- PTI
pattern-triggered immunity
- PRR
pattern-recognition receptor
- PEPR
AtPep-receptor
Acknowledgments
The authors wish to thank Sebastian Bartels (University of Freiburg, Germany) for providing transgenic seeds and his very helpful comments and suggestions. We are grateful to Dominik Klauser (University of Basel; Syngenta Foundation for Sustainable Agriculture, Switzerland) for helping us to set up the experiments. Special thanks to Peter Palukaitis (Seoul women’s university, South Korea) for his critical review and proofreading of the manuscript. This research was partially supported by the Ministry of Science, Research and Technology of Iran and Niklaus und Bertha Burckhardt-Buergin-Stiftung Foundation Switzerland.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental Material footnote should be located in the CFN.
Highlight
The promoter activity of AtPROPEP and AtPEPR genes is characterized and classified based on their differential responses to biotic and abiotic stimuli.
References
- 1.Boller T, Felix G.. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. PMID:19400727. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 2.Macho AP, Zipfel C.. Plant PRRs and the activation of innate immune signaling. Mol Cell. 2014;54:263–272. PMID: 24766890. doi: 10.1016/j.molcel.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. PMID: 10911994. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 4.Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104(49):19613–19618. PMID:18042724. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan WL, Zhang L, Pruitt R, Zaidem M, Brugman R, Ma X, Krol E, Perraki A, Kilian J, Grossmann G, et al. Comparing Arabidopsis receptor kinase and receptor protein-mediated immune signaling reveals BIK1-dependent differences. New Phytol. 2018;25 PMID: 30252144. doi: 10.1111/nph.15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. PMID: 16713565. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA. 2006;103:10098–10103. PMID: 16785434. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels S, Boller T. Quo vadis, Pep? Plant elicitor peptides at the crossroads of immunity, stress and development. J Exp Bot. 2015;66:5183–5193. PMID: 25911744. doi: 10.1093/jxb/erv180. [DOI] [PubMed] [Google Scholar]
- 9.Bartels S, Lori M, Mbengue M, van Verk M, Klauser D, Hander T, Rainer B, Robatzek S, Boller T. The family of AtPeps and their precursors in Arabidopsis: differential expression and localization but similar induction of pattern-triggered immune responses. J Exp Bot. 2013;64:5309–5321. PMID: 24151300. doi: 10.1093/jxb/ert330. [DOI] [PubMed] [Google Scholar]
- 10.Newman MA, Sundelin T, Nielsen JT, Erbs G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front Plant Sci. 2013;16(139). PMID: 23720666. doi: 10.3389/fpls.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22(2):508–522. PMID:20179141. doi: 10.1105/tpc.109.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi Y, Huffaker A. Endogenous peptide elicitors in higher plants. Curr Opin Plant Biol. 2011;14(4):351–357. PMID:21636314. doi: 10.1016/j.pbi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA. 2010;7;107(49):21193–21198. PMID:21088220. doi: 10.1073/pnas.1000191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. J Exp Bot. 2014;65(5):1229–1240. PMID:24253197. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 15.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;28;415(6875):977–983. PMID:11875555. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 16.Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18(3):265–276. PMID:10377992. [DOI] [PubMed] [Google Scholar]
- 17.Ranf S, Eschen-Lippold L, Pecher P, Lee J, Scheel D. Interplay between calcium signalling and early signalling elements during defence responses to microbe- or damage associated molecular patterns. Plant J. 2011;68(1):100–113. PMID:21668535. doi: 10.1111/j.1365-313X.2011.04671.x. [DOI] [PubMed] [Google Scholar]
- 18.Mott GA, Middleton MA, Desveaux D, Guttman DS. Peptides and small molecules of the plant-pathogen apoplastic arena. Front Plant Sci. 2014;5:677 PMID: 25506352. doi: 10.3389/fpls.2014.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16(12):3386–3399. PMID:15539472. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huffaker A, Ryan CA. Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proc Natl Acad Sci USA. 2007;104(25):10732–10736. PMID:17566109. doi: 10.1073/pnas.0703343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross A, Yamada K, Hiruma K, Yamashita-Yamada M, Lu X, Takano Y, Tsuda K, Saijo Y. The Arabidopsis PEPR pathway couples local and systemic plant immunity. Embo J. 2014;33(1):62–75. PMID:24357608. doi: 10.1002/embj.201284303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan CA, Huffaker A, Yamaguchi Y. New insights into innate immunity in Arabidopsis. Cell Microbiol. 2007;9(8):1902–1908. PMID:17593247. doi: 10.1111/j.1462-5822.2007.00991.x. [DOI] [PubMed] [Google Scholar]
- 23.Logemann E, Birkenbihl RP, Rawat V, Schneeberger K, Schmelzer E, Somssich IE. Functional dissection of the PROPEP2 and PROPEP3 promoters reveals the importance of WRKY factors in mediating microbe-associated molecular pattern-induced expression. New Phytol. 2013;198(4):1165–1177. PMID:23496690. doi: 10.1111/nph.12233. [DOI] [PubMed] [Google Scholar]
- 24.Huffaker A, Dafoe NJ, Schmelz EA. ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol. 2011;155(3):1325–1338. PMID:21205619. doi: 10.1104/pp.110.166710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huffaker A, Pearce G, Veyrat N, Erb M, Turlings TC, Sartor R, Shen Z, Briggs SP, Vaughan MM, Alborn HT, et al. Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc Natl Acad Sci USA. 2013;110(14):5707–5712. PMID:23509266. doi: 10.1073/pnas.1214668110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tintor N, Ross A, Kanehara K, Yamada K, Fan L, Kemmerling B, Nürnberger T, Tsuda K, Saijo Y. Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc Natl Acad Sci USA. 2013;110:6211–6216. PMID:23431187. doi: 10.1073/pnas.1216780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J, Han Z, Sun Y, Zhang H, Gong X, Chai J. Structural basis for recognition of an endogenous peptide by the plant receptor kinase PEPR1. Cell Res. 2015;25(1):110–120. PMID:25475059. doi: 10.1038/cr.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert M. Peptides as triggers of plant defence. J Exp Bot. 2013;64(17):5269–5279. PMID:24014869. doi: 10.1093/jxb/ert275. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari S, Savatin DV, Sicilia F, Gramegna G, Cervone F, Lorenzo GD. Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci. 2013;4(49). PMID: 23493833. doi: 10.3389/fpls.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gully K, Hander T, Boller T, Bartels S. Perception of Arabidopsis AtPep peptides, but not bacterial elicitors, accelerates starvation-induced senescence. Front Plant Sci. 2015;6:14 PMID: 25667591. doi: 10.3389/fpls.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C, Guo J, Kang Y, Doman K, Bryan AC, Tax FE, Yamaguchi Y, Qi Z. AtPEPTIDE RECEPTOR2 mediates the AtPEPTIDE1-induced cytosolic Ca(2+) rise, which is required for the suppression of Glutamine Dumper gene expression in Arabidopsis roots. J Integr Plant Biol. 2014;56(7):684–694. PMID: 24450616. doi: 10.1111/jipb.12171. [DOI] [PubMed] [Google Scholar]
- 32.Poncini L, Wyrsch I, Dénervaud Tendon V, Vorley T, Boller T, Geldner N, Métraux JP, Lehmann S. In roots of Arabidopsis thaliana, the damage-associated molecular pattern AtPep1 is a stronger elicitor of immune signalling than flg22 or the chitin heptamer. PLoS One. 2017;12(10):e0185808 PMID:28973025. doi: 10.1371/journal.pone.0185808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klauser D, Desurmont GA, Glauser G, Vallat A, Flury P, Boller T, Turlings TC, Bartels S. The Arabidopsis Pep-PEPR system is induced by herbivore feeding and contributes to JA-mediated plant defence against herbivory. J Exp Bot. 2015;66(17):5327–5336. PMID:26034129. doi: 10.1093/jxb/erv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eulgem T. Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci. 2005;10(2):71–78. PMID:15708344. doi: 10.1016/j.tplants.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Moore JW, Loake GJ, Spoel SH. Transcription dynamics in plant immunity. Plant Cell. 2011;23(8):2809–2820. PMID:21841124. doi: 10.1105/tpc.111.087346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


