ABSTRACT
We introduce a new concept and potentially general platform for antibody (Ab) purification that does not rely on chromatography or specific ligands (e.g., Protein A); rather, it makes use of detergent aggregates capable of efficiently capturing Ab while rejecting hydrophilic impurities. Captured Ab are then extracted from the aggregates in pure form without co-extraction of hydrophobic impurities or aggregate dissolution. The aggregates studied consist of conjugated “Engineered-micelles” built from the nonionic detergent, Tween-20; bathophenanthroline, a hydrophobic metal chelator, and Fe2+ions. When tested in serum-free media with or without bovine serum albumin as additive, human or mouse IgGs were recovered with good overall yields (70–80%, by densitometry). Extraction of IgGs with 7 different buffers at pH 3.8 sheds light on possible interactions between captured Ab and their surrounding detergent matrix that lead to purity very similar to that obtained via Protein A or Protein G resins. Extracted Ab preserve their secondary structure, specificity and monomeric character as determined by circular dichroism, enzyme-linked immunosorbent assay and dynamic light scattering, respectively.
KEYWORDS: Antibody purification, IgG, Protein A, Protein G, Chromatography
Introduction
Antibodies (Ab) are frequently encountered in scientific research as detection and quantitation agents and in medicine as vehicles for delivering drugs, enzymes and isotopes to target cells.1,2 Obtaining pure Ab preparations is obviously essential for these applications, and this is most commonly achieved by column chromatography with the ligand Protein A (ProA). ProA binds strongly3 and specifically4 to diverse Ab types, making it possible to efficiently capture Ab from complex media and reach high purity (>98%) within a single chromatographic step.5 Such remarkable features have made ProA chromatography the gold standard in Ab purification.6 Nevertheless, Ab purification without the need for ProA or chromatographic steps represents an appealing alternative, primarily due to: 1) the high cost of ProA resins and the potential leaching of ProA (or its fragments) into the purified Ab;6 2) the limited binding capacities of ProA affinity columns which may pose a problem at high Ab concentrations;7 3) deamidation of ProA asparagines during column sanitation,8 leading to lower binding efficiency; and 4) the high maintenance costs associated with the use of high-performance liquid chromatography instrumentation. These experimental challenges have been the driving force behind the development of potentially more economical, non-chromatographic strategies for Ab purification. They include: 1) novel precipitation methods;9 2) aqueous two-phase extraction systems;10,11 and 3) ultrafiltration with charged membranes.12 To the best of our knowledge, none of these approaches have been widely implemented.
Several publications demonstrating purification of diverse monoclonal Ab (mAb) by hydrophobic interaction chromatography (HIC)13 or hydrophobic membrane interaction chromatography14 are of particular relevance to the present study. These reports imply that mAb may have a tendency to bind more strongly to hydrophobic resins (or surfaces) compared to water-soluble proteins that are not immunoglobulins (IgGs). We therefore considered whether Ab would bind to detergent aggregates composed of conjugated Tween-20 micelles. Such aggregates are generated in a simple two-step sequence. First, the hydrophobic chelator, bathophenanthroline (batho), is added to a dispersion of Tween-20 micelles and transform the latter into what we have called ‘Engineered-micelles’.15–17 These contain, in addition to the detergent, a hydrophobic chelator that positions itself at the micelle/water interface (Figure 1). In the following step, Fe2+ ions are added as FeSO4. These ions serve as mediators between the engineered-micelles because they can bind with high affinity up to three batho molecules simultaneously,18 and, hence, lead to Tween-20 aggregates as a distinct hydrophobic phase (Figure 1).
Figure 1.
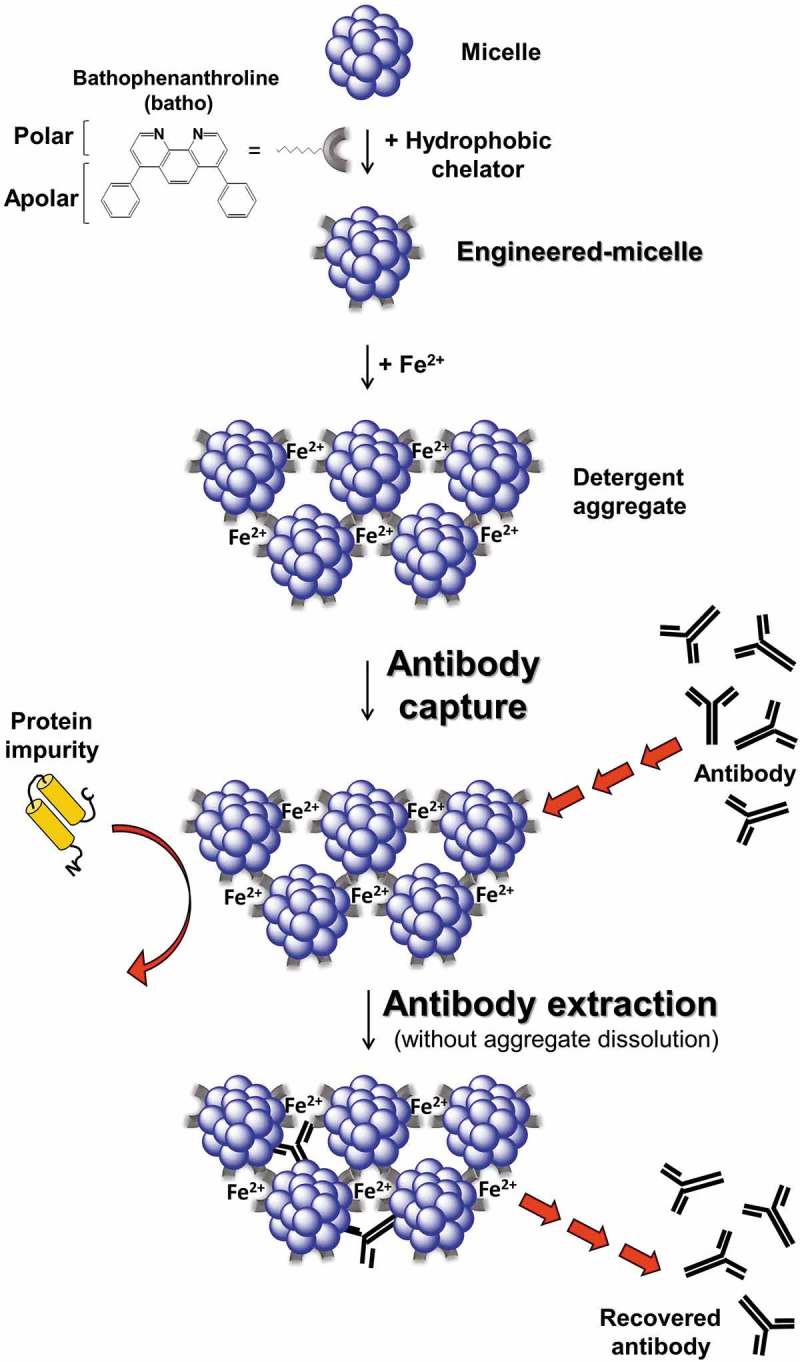
Illustration of antibody (Ab) purification using engineered-micelles. Non-ionic detergent micelles are transformed into engineered-micelles upon incubation with a hydrophobic chelator capable of positioning itself at the micelle:water interface. Specific conjugation of engineered-micelles is induced once Fe2+ ions (FeSO4) are added. Fe2+ ions bind several chelators in parallel leading to detergent aggregates. Ab are captured by the detergent aggregates whereas more hydrophilic protein impurities, are rejected. Pure Ab are obtained by extraction from the aggregates without concomitant aggregate dissolution.
We studied the potential of such hydrophobic aggregates to serve as a general purification platform for IgGs, regardless of the IgG origin. It was obvious that a practical IgG purification strategy would require a demonstration that Tween-20 aggregates: 1) bind IgGs efficiently; 2) exclude the majority of impurities; 3) allow IgG extraction from the aggregates without concomitant aggregate dissolution or co-extraction of hydrophobic impurities; and 4) lead to highly pure, monomeric and functional Ab molecules (Figure 1) Complying with these criteria is mandatory, highly challenging and represents the focus of our work.
Results
Preparation of conjugated Tween-20 micelles and their characterization by light microscopy and cryo–transmission electron microscopy
As described in the Material and Methods section, the preparation of Tween-20 aggregates requires only the addition of the chelator (batho) and Fe2+ ions to an aqueous medium containing Tween-20 micelles. These two additives trigger immediate formation of precipitates containing the red-colored, hydrophobic [(batho)3:Fe2+] complex (Figure 2(a)). Following centrifugation (2 min., 14K), well-defined, red pellets and colorless supernatants are generated, indicating that most of the hydrophobic red complex had precipitated (Figure 2(a) – inset). Control experiments show that the presence of the chelator and the metal are both mandatory: in the absence of chelator, no aggregates, nor any form of phase separation, are observed, whereas in the absence of Fe2+, the chelator itself crystallizes as long, elongated rods (data not shown). To characterize the architecture of Tween-20 aggregates in more detail, cryo-transmission electron microscopy (cryo-TEM) was employed.19 Whereas cryo-TEM images of control samples, containing non-conjugated Tween-20 micelles, revealed a homogenous distribution of small micelles (Figure 2(b): white arrows), the aggregates appeared as a mixture of detergent clusters (Figure 2(c): white arrows) and elongated hydrophobic crystals. (Figure 2(c): blue arrows) These findings are consistent with our previous studies,16 and demonstrate the ability of the [(batho)3:Fe2+] hydrophobic complex to conjugate non-ionic detergent micelles and to lead to condensed detergent aggregates.
Figure 2.
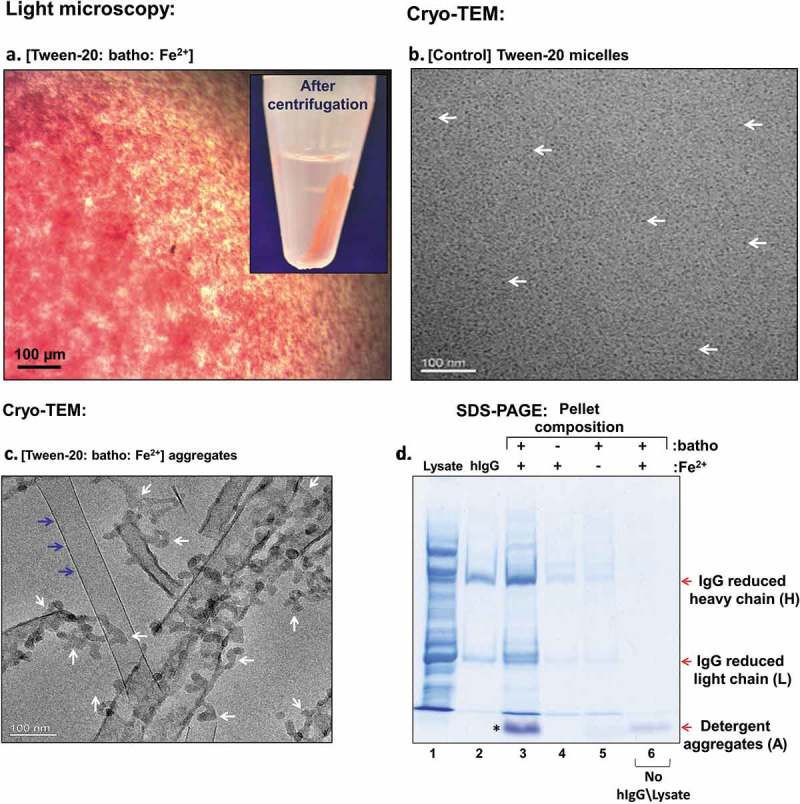
(a) Light microscopy images of Tween-20 aggregates comprising Tween-20 micelles, the hydrophobic chelator bathophenanthroline (batho) and Fe2+ ions. The red color derives from the [(batho)3:Fe2+] complex. The white arrow points to the red pellet generated by brief (14K, 2 min.) centrifugation (inset). (b) and (c). Cryo-TEM images of Tween-20 micelles White arrows point to individual micelles (dark black dots). Tween-20 aggregates as in (a). White arrows point to Tween-20 aggregates, while blue arrows points to an elongated crystal of excess hydrophobic chelator (batho). (d) SDS-PAGE analysis of Tween-20 pellets and process dependence on the chelator (batho) and Fe2+ Lane 1: E. coli lysate – serving as an artificial contamination background; Lane 2: target human (hIgG); Lane 3: pellet composition obtained after incubating Tween-20 aggregates comprising [Tween-20:batho:Fe2+] with a mixture of [E. coli lysate:hIgG], followed by centrifugation and supernatant removal as described in Experimental Section; Lanes 4–5: as in lane 3, but in the absence of batho or of Fe2+, respectively; Lane 6: Detergent aggregates devoid of added protein. H, L denote the reduced heavy and light chains of the target antibody, respectively. A points to the detergent aggregate band, migrating at the front of the gel.
Purification of human and mouse IgGs using Tween-20 aggregates
Our initial purification trials were aimed at assessing process viability and mandatory dependence on both the chelator (batho) and Fe2+ ions. As described in the Material and Methods section, a mixture of polyclonal human IgG (hIgG) and E. coli lysate (serving as an artificial contamination background) were added to preformed Tween-20 aggregates. A brief incubation (5 min.) was followed by centrifugation (2 min., 14K), the supernatant was discarded and the presence of the target IgG within the pellet was detected by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The heavy and light chains of the target IgG were clearly observed (Figure 2(d): lane 3) and provided direct evidence for Tween-20 aggregates’ ability to bind hIgG. Interestingly, the majority of bacterial proteins that were present in the system, together with the hIgG, were rejected from the aggregates (Figure 2(d): lane 1 vs. lane 3). Moreover, repetition of the same protocol in the absence of only the chelator (batho) (Figure 2(d): lane 4) or in the absence of only the Fe2+ (Figure 2(d): lane 5) led to a dramatic reduction in the intensity of the heavy and light chain bands of the target IgG in the aggregate. These results provided strong evidence for the participation and contribution of the hydrophobic chelator and metal ion (Fe2+) in the purification process. The appearance of a band at the front of lane 3 (see asterisk), was an unexpected finding since it did not appear in the contamination background (Figure 2(d): lane 1) nor in the target IgG (Figure 2, lane 2). This puzzle was solved when Tween-20 aggregates devoid of any protein (i.e., bacterial lysate or hIgG) were analyzed as well. This analysis showed that Tween-20 aggregates containing the [(batho)3:Fe2+] hydrophobic complex (but not proteins) are stained by the Coomassie brilliant blue dye (R-250) and migrate to the front of the gel as observed (Figure 2, lane 6).
Purification of human and mouse IgGs in serum-free media
Process optimization trials in the presence of E. coli lysate were followed by implementation of the method in hybridoma serum-free media. Both hIgG and mouse IgG were detected by SDS-PAGE in the Tween-20 pellets (Figure 3(a) or (b): lane 2). Inclusion of bovine serum albumin (BSA) (or human serum albumin (HSA), data not shown) at concentrations higher than 0.5 mg/ml (in addition to the target IgG at 1 mg/mL), was found to progressively suppress IgG binding, concomitant with an increase of the albumin concentration within the aggregates (Figure 3(a) or (b): lanes 2–10). The finding that human and mouse IgGs can be extracted from Tween-20 aggregates with 50 mM isoleucine (pH 3.8) without significant co-extraction of BSA nor aggregate dissolution, (Figure 3(c) or (d): lanes 3–4) was of particular importance. At higher BSA concentrations (≥0.5 mg/mL), albumin was observed along with the extracted IgG (Figure 3(a,b): lanes 5–10). Similar results were observed when BSA was replaced by HSA (data not shown).
Figure 3.
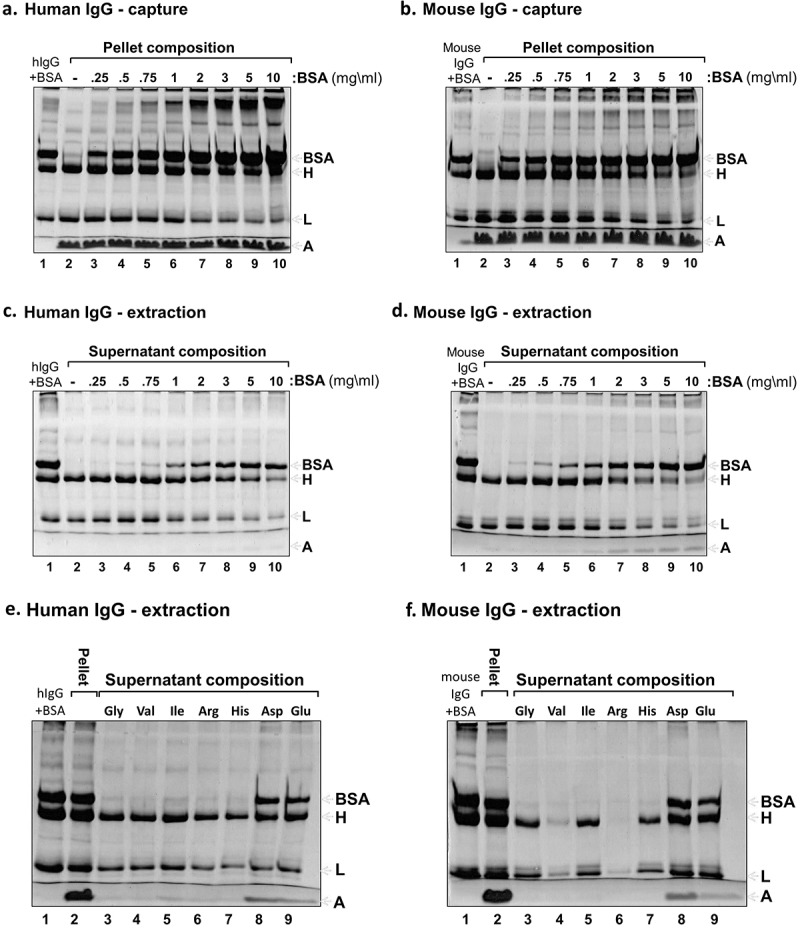
IgG purification in serum-free media with or without added BSA. IgG capture: (a). Human IgG (hIgG) capture: Lane 1: hIgG and BSA; lanes 2–10: Pellet composition obtained after incubating Tween-20 aggregates with hIgG (1 mg/mL) and the indicated BSA concentrations in serum-free media as described in Experimental. (b). Gel B, as described in A, but with mouse IgG rather than human. (c-d). Supernatant composition after incubating the pellets with 50 mM isoleucine at pH 3.8 as described in Experimental. (e). Effect of buffer composition on IgG extraction. Lane 1: hIgG and BSA; lane 2: Pellet composition obtained after incubating Tween-20 aggregates with hIgG (1 mg/mL) and BSA (0.5 mg/mL) in serum free-media as described in Experimental; lanes 3–9: Supernatant composition after extracting hIgG from pellets generated under conditions shown in lane 2 with buffers containing the amino acids indicated at pH 3.8 as described in Experimental. (f). As in E, but in the presence of mouse IgG. H, L denote the reduced heavy and light chains of the target antibody, respectively. A points to the detergent aggregate band. Gels are stained with Coomassie blue.
Extraction buffers, circular dichroism and dynamic light scattering
The relative efficiency of extraction buffers (pH 3.8), containing different, individual amino acids, was studied. Highest overall yields of hIgG were obtained with Gly, Val or Ile buffers (Figure 3(e): lanes 3–5), while Arg or His buffers were found to be less efficient (Figure 3(e): lanes 6–7). The use of Asp or Glu buffers promoted partial aggregate dissolution, (Figure 3(e): lanes 8–9, viz., bands at the front of the gel). Incubation at 32 °C led to the highest extraction yields compared to lower temperatures (4–19 °C, data not shown); this was not of concern because therapeutic mAb were only reported to undergo chemical modification at higher temperatures (e.g., 37 °C, pH 4.5) and significantly longer incubation times (1–4 days).20
Implementation of the same extraction protocol on mouse IgGs showed that Gly or Ile buffers were the most efficient (Figure 3(f): lanes 3 and 5); His buffer was moderately efficient (Figure 5(e): lane 7); and Val or Arg buffers were inefficient (Figure 3(f): lanes 4 and 6). The tendency of Asp or Glu buffers to dissolve Tween-20 aggregates was again observed (Figure 3(f): lanes 8–9).
Figure 5.
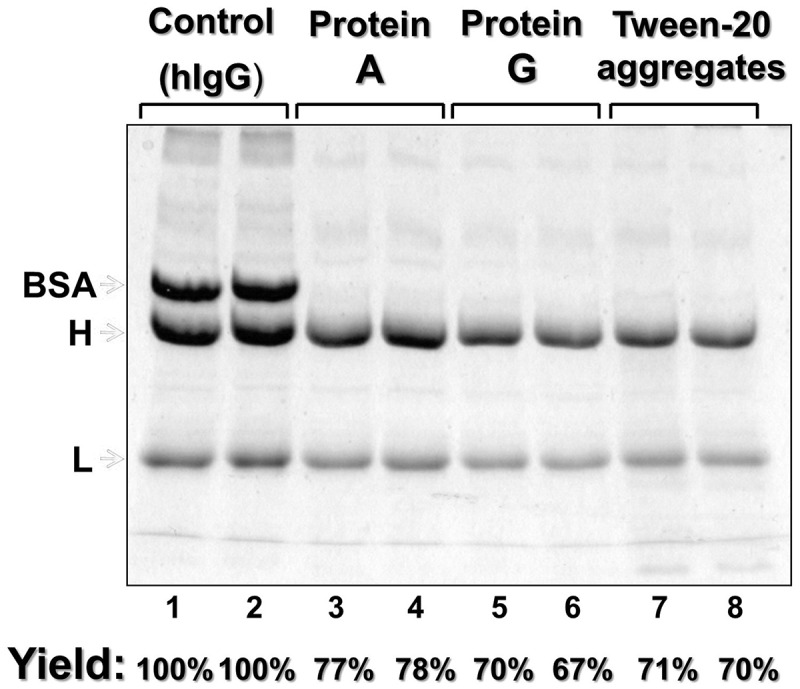
Yield of the engineered-micelle platform for Ab purification compared to ProA or Protein G (ProG) resins. Lanes 1–2: hIgG (control); lanes 3–4 and 5–6: supernatant composition obtained after purification of hIgG with ProA or Protein G spin columns as described in Experimental Section; Lanes 7–8: supernatant composition after purification of hIgG with Tween-20 detergent aggregates as described in the Material and Methods section. BSA, H & L are bovine serum albumin and the reduced heavy and light chains of the target antibody, respectively. Gels are Coomassie blue stained.
Comparison of the circular dichroism (CD) spectrum of hIgGs that were subjected to purification with Tween-20 aggregates and extracted in Gly buffer with that of untreated, control hIgGs, showed that the spectra are very similar. Both reveal the prominent secondary structure of IgGs (i.e., anti-parallel beta-sheets21) with strong negative ellipticity band at 218 nm,22 and are in agreement with previous reports in the literature (Figure 4(a)).23 Since similar spectra were also obtained with mouse IgGs (Figure 4(b)), we conclude that micelle-based purification is mild and is capable of preserving IgGs secondary structure.
Figure 4.
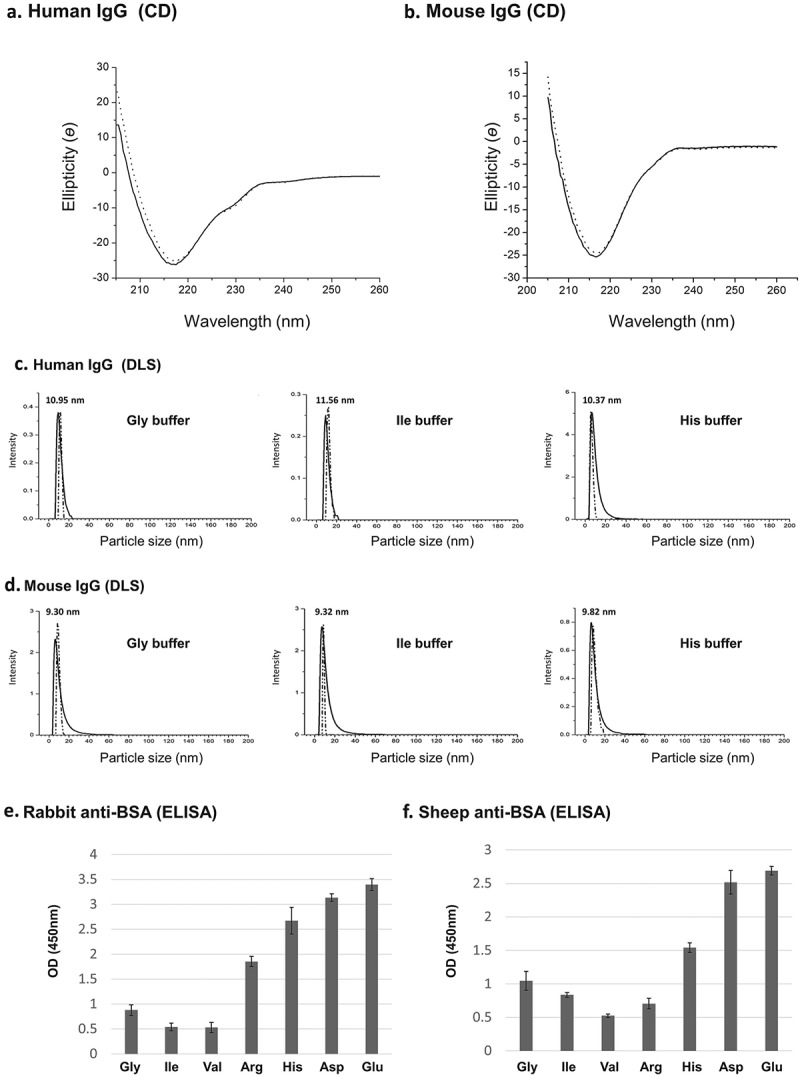
(a-b) CD spectra. Human and mouse IgGs were subjected to purification with Tween-20 aggregates and extracted with Gly buffer at pH 3.8. CD spectra of control IgGs (i.e., untreated IgGs – solid line) and IgGs that were subjected to purification (dotted line) are shown. (c-d) Dynamic light scattering (DLS). Human and mouse IgGs were purified as described in A-B and extracted with indicated buffers at pH 3.8. Control untreated IgGs – solid line, purified IgGs – dotted line. (e-f) ELISA analysis of extracted IgGs. Polyclonal anti-BSA IgGs originating from rabbit (naked) or sheep (biotinylated) were subjected to the micelle-based purification method and extracted at 32 °C (5 min.) from Tween-20 aggregates with the amino acid buffers indicated (50 mM) at pH 3.8. The ability of the purified Ab to bind target epitopes on BSA was determined by ELISA assays as described in the Material and Methods section. The data presented are the results of at least 12 independent experiments.
Dynamic light scattering (DLS) measurements did not demonstrate any significant differences in the size distribution of purified hIgG or mouse IgG extracted with each of three buffers (50 mM Gly, Ile or His, pH 3.8) from untreated, control IgGs (Figure 4(c,d)). No evidence for IgG aggregates was observed. Still, a slight increase in size (~10%) of the purified IgGs was detected, which may represent bound Tween-20 detergent monomers (Figure 4(c,d)).
Preservation of IgG specificity
Preservation of IgG specificity upon completion of the purification process was studied with two types of polyclonal antibodies (sheep and rabbit) that recognize BSA. Each of these Ab was purified with Tween-20 aggregates (containing only HSA in order to eliminate BSA from the system); extracted with each of the 7 buffers studied; and finally, tested for their ability to bind target BSA in an enzyme-linked immunosorbent assay (ELISA). Differences in the ELISA signals observed (Figure 4(e,f)) reflect differences in extraction efficiency as had been observed with hIgG and mouse IgG (Figure 3(e,f)). The strongest signals were obtained when extraction buffers were composed of Asp or Glu. These findings are consistent with those described earlier, where it was shown that Asp and Glu buffers induce partial aggregate dissolution (Figure 3(e) or (f): lanes 8–9) and lead to higher IgG concentration in the extraction buffer (i.e., supernatant) (Figure 4(e,f)).
Discussion
The development of a non-chromatographic platform for Ab purification, one not requiring the use of the gold standard ligand, ProA, has proven to be a true challenge. However, an alternative approach seemed possible when detergent aggregates comprising Tween-20 and the hydrophobic [(batho)3:Fe2+] red complex demonstrated an ability to specifically capture human IgG, while excluding the large majority of impurities present in a E. coli lysate included as an artificial background. We observed similar efficiency in hybridoma serum-free media, i.e., the common environment for mAb production.24 Interestingly, inclusion of BSA, an albumin that contributes to the successful culture of mammalian cells24 and to stimulation of hybridoma cell growth,25 showed that it competes with IgGs for binding to the Tween-20 aggregates. However, when equimolar amounts of target IgGs and BSA were present, IgG capturing efficiency was not affected and remained quantitative (by densitometry). Consistent with the above, analysis of the supernatant after IgG capture confirmed that no IgG (human or mouse) are present in the supernatant when the molar concentration of BSA is not higher than that of the IgG (Supplementary, S1: A and B lanes 4–5). Under these conditions, analysis of the pellet under non-reducing conditions, also showed that only the pellets contained intact IgGs, but not the supernatant (Supplementary, S1: C lanes 3–4 for hIgG; lanes 8–9 for mouse IgG).
IgG purity was achieved by defining conditions under which captured IgGs were extracted from the Tween-20 aggregates without concomitant pellet dissociation or co-extraction of hydrophobic impurities. Notably, low salt concentration (30 mM NaCl) was required in order to prevent pellet dissolution. Extraction was performed at pH 3.8 to avoid co-extraction of BSA, which occurs under more acidic conditions.2626 . To characterize the interactions between captured IgGs and their detergent surroundings, seven different buffers, each containing 50 mM of individual amino acids, potential competitors for IgG side chain interactions, were studied to access: 1) hydrophobic interactions (Val, Ile); 2) ionic and or H-bond interactions (Asp, Glu, Arg); or 3) metal chelation (His). These buffers were compared to the Gly buffer, commonly used for IgG elution from ProA columns.26 For hIgG, the most efficient extraction buffers were Gly, Val or Ile, suggesting that hIgGs may be involved in van der Waals interactions with the surrounding detergent matrix. By contrast, partial aggregate dissolution was observed when Asp or Glu buffers were used. That it was in fact possible to define conditions under which albumin molecules (BSA, HSA) do not elute together with the IgGs, suggested that this tighter binding may derive from interaction between high affinity metal binding sites of albumins27–29 and bound Fe2+ ions embedded in the detergent matrix. Repetition of the above buffer survey with mouse IgGs led to a similar but not identical extraction profile.
IgGs subjected to the Tween-20 micelle-based purification process preserve their secondary structure: anti-parallel beta-sheet and the characteristic ellipticity band at ~218 nm21–23 are both clearly observed in CD spectra. Additional measurements in the near UV (260–380 nm) showed that the tertiary structure of both human and mouse IgGs is also preserved (Supplementary, S1: D and E). This stability may derive from the multiple (≥14) disulfide bonds present in all IgG isotypes.30 Extracted IgGs were found to be monomeric by DLS independent of the extraction buffer used. This is of paramount importance in the drug industry where preparation of therapeutic grade mAb must avoid protein aggregation.31 Moreover, anti-BSA rabbit or sheep IgGs that were subjected to the micellar purification platform were found to bind to BSA, thus providing direct evidence of the preservation of the Fab domain structure.
Given identical starting conditions (i.e., IgG:BSA mixtures), column chromatography with ProA resin does lead to somewhat higher yields of purified IgGs than those obtained via Tween-20 aggregates, although purity was essentially identical for all samples (Figure 5). These findings are notable because they demonstrate that high purity IgG can in fact be recovered at good yields (70–80%, by densitometry) even in the absence of specific ligands and chromatographic system. Moreover, our non-chromatographic, ligand-free antibody purification strategy has at least four inherent advantages that enhance its potential for becoming a practical, cost-effective platform for antibody purification. First, ProA is not required. This is expected to significantly reduce production costs because ProA resins account for more than 35% of total raw material costs for industrial-scale purification.32 Second, the combined binding/extraction stages are rapid, ~15–20 min. total. This finding, together with the granular nature of Tween-20 aggregates, suggests that removal/exclusion of impurities during IgG binding, followed by IgG recovery from the aggregates, can both be accomplished rapidly via filtration. If that indeed proves to be the case on a large scale, then our purification strategy may be integrated into continuous flow industrial processes. Third, unlike ProA columns, our purification platform has the potential to be efficient with cell cultures containing high IgG concentrations. Since the volume of Tween-aggregates introduced into the system is theoretically unlimited, we expect that efficient IgG capture will occur regardless of the antibody concentration. Such a scenario is in obvious contrast to ProA columns, where the IgG concentration is limited by: 1) orientation of ProA relative to its surrounding polymeric resin; 2) accessibility of ProA to the aqueous phase, which is dependent on resin pore size; and obviously 3) ligand density, i.e., the number of ProA molecules per gram of resin.3,6 In this regard, it has been argued that when IgG expression levels reach 8–10 g/L, ProA resins exhibiting binding capacities greater than 30 g/L at linear flow velocities of 200 cm/h and residence time of 3 minutes would lead to processing times that would be extremely long.6 Fourth and finally, the constituent raw materials are not costly. The cost of Tween-20, PEG-6000, Fe2+, bathophenanthroline, does not present economic difficulty: our calculations show that approximately 9 grams of dry Tween-20 aggregates will be required to produce 1 gram of relatively pure (~95%) IgG.
In conclusion, Tween-20 aggregates containing the hydrophobic [(batho)3:Fe2+] complex appear to represent a general platform for IgG purification that may potentially replace ProA columns in downstream processing of therapeutic grade mAb. Similar, or improved, results are expected to be achieved with other non-ionic detergents when implemented on IgGs or other antibody classes (e.g., IgA, IgM).
Materials and methods
Materials: BSA (Sigma, A7906), HSA (Sigma, A8763), Protein A HP Spin-Trap (Sigma, 28903132), Protein AB Spin-Trap (Sigma, 28408347), isoleucine (Sigma, W527602), valine (Sigma, V0500), leucine (Sigma, L8000), arginine (Sigma, A5006), aspartic acid (Sigma, A9256), glutamic acid (Sigma, G1251), iron (II) sulfate heptahydrate (Sigma, F7002), sodium chloride (Sigma, S7653), zinc chloride (Sigma, 208086), polysorbate 20 (Sigma, 44112), rabbit anti-bovine albumin antibody (Sigma, B1520), Ex-CELL 610-HSF medium (Sigma, 14610C), anti-rabbit antibody (Sigma, A9169), glycine (Bio-lab 07132391), histidine (Fluka, 53319), bathophenanthroline (GFS chemicals, C038446), sheep anti-bovine albumin antibody (Bethyl Lab, A10-113B), streptavidin-horseradish peroxidase (HRP) conjugate (RD system, 321894), TMB solution (eBioscience,:00–4201), phosphate-buffered saline (PBS) 10X (Bio-Lab, 00162323G500), human IgG (Lee-Biosciences, 340–21), mouse IgG (Equitech, SLM66).
Preparation of Tween-20 aggregates (Tween-20: bathophenanthroline: Fe2+)
Tween-20 aggregates were obtained by mixing equal volumes of medium A and B as follows: medium A was prepared by the addition of 30 μL of the hydrophobic chelator bathophenanthroline (20 mM in methanol) to 270 μL of 0.25 mM Tween-20 in DDW with vigorous vortexing to a final volume of 300 μL. An equal volume of medium B, containing 1 mM FeSO4 in 20 mM NaCl was then added to medium A with vigorous vortexing.
Purification of hIgG and mouse IgG with Tween-20 aggregates
Freshly prepared Tween-20 aggregates were resuspended in 100 μL serum-free medium (Ex-CELL 610-HSF) containing: 5% PEG-6000, the target IgG (1 mg/mL) and BSA or HSA (0–10 mg/mL). After 5–10 minutes incubation at room temperature, centrifugation (14K, 2 min.) was applied, the supernatant discarded, and pellets were briefly washed with 100 μL of cold 20 mM NaCl. An additional centrifugation step followed (14K rpm, 2 min.), the supernatant was removed, and pellets were analyzed by SDS-PAGE. Similarly, human and mouse IgGs were purified from E. coli lysate in the presence of 20 mM NaCl.
Extraction of IgGs from Tween-20 aggregates
Tween-20 pellets containing target IgG were incubated with 100 μL of either: 50 mM Gly, Val, Ile, Arg, His, Asp or Glu (pH 3.8), 30 mM NaCl for 5 minutes at 32 °C. Centrifugation followed (13K rpm, 2 min.) and the supernatant was carefully removed for further analysis.
Comparison study against ProA and Protein G spin columns
The IgG:BSA sample, prepared as described above, was applied to commercial ProA or Protein G spin columns and purification was performed according to manufacturer instructions (General Electric). Eluted IgGs were then compared to those purified using Tween-20 aggregates.
Cryo-TEM analysis
Tween-20 aggregates were prepared as described above and aliquots (10 µL) were used for cryo-TEM analysis. Samples were prepared in a controlled environment vitrification system (CEVS),19,33 equilibrated at 25 °C and at saturation. Vitrified specimens were examined in an FEI T12 G2 TEM operating at 120 kV. Images were recorded under low dose conditions as described previously.19,33
Dynamic light scattering
IgG (0.5–1.0 mg/mL), treated and untreated, was solubilized in one of three buffers: 50 mM glycine + 30 mM NaCl, pH 3.8: 50 mM isoleucine + 30 mM NaCl, pH 3.8; and 50 mM histidine + 30 mM NaCl pH 3.8. Samples were centrifuged at 13,000 rpm for 20 min and the supernatant collected for analysis. The intensity-weighted size distributions of human and mouse IgG samples were determined using the auto correlation spectroscopy protocol of the Nanophox instrument (Sympatec GmbH, Germany).
Circular dichroism spectroscopy
Antibodies that were extracted from Tween-20 aggregates as described above were subjected to CD analysis using a Chirascan CD spectrometer (Applied Photophysics). CD spectra report ellipticity (θ), proportional to the difference in absorbance of left and right circularly polarized light [θ = 3300°] (AL−AR) as a function of wavelength. A quartz cell with dimensions 10 × 10 mm; light path of 10 mm, was used. The CD spectra were recorded with 1 nm bandwidth resolution in 1 nm steps at 20°C. The CD spectra were corrected for baseline distortion by subtracting a reference spectrum of the corresponding buffer.
Enzyme-linked immunosorbent assay
Nunc-Immuno Microwell plates (F96 Maxisorp) were first coated with 2% BSA (200 µL), left for overnight incubation at 4 °C, and excess of BSA was removed with three aliquots of PBS (200 µL). Purified anti-BSA IgGs, i.e., IgGs that had been captured by Tween-20 aggregates and extracted with amino acid buffer, were then added to the washed wells. Accordingly, 100 µL of purified and diluted (1:250) biotinylated anti-BSA IgG (sheep) or diluted (1:1200) naked anti-BSA IgG (rabbit) were added to the BSA coated wells, incubated for 2 hours at room temperature (RT) and unbound anti-BSA IgGs were then removed with PBS (3 x 200 µL). To wells containing the biotinylated anti-BSA IgG, a diluted (1:200) streptavidin-HRP conjugate was added (100 µL), whereas a diluted (1:15,000) anti-rabbit IgG-HRP conjugate was introduced into wells containing the naked IgG. In both cases, the system was further incubated for 1 hour at RT and excess HRP-conjugates (either streptavidin or IgG) were excluded with PBS (3 x 200 µL). Addition of the HRP substrate (1XTMB solution) was followed by 10 minutes of incubation at RT and the reaction was stopped upon addition of 2 N H2SO4 (50 µL). The intensity of the yellow color in the wells was measured at 450 nm using an ELISA reader (Tecan infinite M200).
Densitometry
Bands present in Coomassie-stained gels were quantified using the EZQuant program. http://www.ezquant.com/en/
Light microscopy
Images were obtained using an Olympus CX-40 light microscope equipped with an Olympus U-TV1X-2 digital camera.
Abbreviations
- Ab
Antibody
- Arg
Arginine
- Asp
Aspartic acid
- Batho
Bathophenanthroline
- BSA
Bovine serum albumin
- CD
Circular dichroism
- Cryo-TEM
Cryo-transmission electron microscopy
- DLS
Dynamic light scattering
- E. coli
Escherichia coli
- ELISA
Enzyme-linked immunosorbent assay
- Glu
Glutamic acid
- Gly
Glycine
- His
Histidine
- hIgG
Human IgG
- HPLC
High-performance liquid chromatography
- HSA
Human serum albumin
- IgA
Immunoglobulin A
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- Ile
Isoleucine
- mAb
Monoclonal antibody
- ProA
Protein A
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Tween-20
Polysorbate 20
- UV
Ultraviolet
- Val
Valine
Acknowledgments
We thank the Kimmelman Center for Biomolecular Structure and Assembly at the Weizmann Institute of Science for its generous support (to M.S.). M. S. holds the Katzir-Makineni chair in chemistry. D.D. thanks the Israel Science Foundation and the Russell Berrie Nanotechnology Institute, Technion for their support. G. P. thanks Prof. M. Firer for the use of the ELISA reader and Ariel University for their support.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Alkan SS. Monoclonal antibodies: the story of a discovery that revolutionized science and medicine. Nat Rev Immunol. 2004;4(2):153–56. doi: 10.1038/nri1265. [DOI] [PubMed] [Google Scholar]
- 2.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15(6):361–70. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hober S, Nord K, Linhult M. Protein A chromatography for antibody purification. J Chromatogr B. 2007;848(1):40–47. doi: 10.1016/j.jchromb.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 4.DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–83. [DOI] [PubMed] [Google Scholar]
- 5.Azevedo AM, Gomes AG, Rosa PAJ, Ferreira IF, Pisco AMMO, Aires-Barros MR. Partitioning of human antibodies in polyethylene glycol–sodium citrate aqueous two-phase systems. Sep Purif Technol. 2009;65(1):14–21. doi: 10.1016/j.seppur.2007.12.010. [DOI] [Google Scholar]
- 6.Low D, O’Leary R, Pujar NS. Future of antibody purification. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848(1):48–63. doi: 10.1016/j.jchromb.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393–98. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 8.Linhult M, Gulich S, Graslund T, Simon A, Karlsson M, Sjoberg A, Nord K, Hober S. Improving the tolerance of a protein a analogue to repeated alkaline exposures using a bypass mutagenesis approach. Proteins. 2004;55(2):407–16. doi: 10.1002/prot.10616. [DOI] [PubMed] [Google Scholar]
- 9.McDonald P, Victa C, Carter-Franklin JN, Fahrner R. Selective antibody precipitation using polyelectrolytes: a novel approach to the purification of monoclonal antibodies. Biotechnol Bioeng. 2009;102(4):1141–51. doi: 10.1002/bit.22127. [DOI] [PubMed] [Google Scholar]
- 10.Azevedo AM, Rosa PA, Ferreira IF, Aires-Barros MR. Chromatography-free recovery of biopharmaceuticals through aqueous two-phase processing. Trends Biotechnol. 2009;27(4):240–47. doi: 10.1016/j.tibtech.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Mao LN, Rogers JK, Westoby M, Conley L, Pieracci J. Downstream antibody purification using aqueous two-phase extraction. Biotechnol Prog. 2010;26(6):1662–70. doi: 10.1002/btpr.477. [DOI] [PubMed] [Google Scholar]
- 12.van Reis R, Zydney A. Bioprocess membrane technology. J Memb Sci. 2007;297(1–2):16–50. doi: 10.1016/j.memsci.2007.02.045. [DOI] [Google Scholar]
- 13.Manzke O, Tesch H, Diehl V, Bohlen H. Single-step purification of bispecific monoclonal antibodies for immunotherapeutic use by hydrophobic interaction chromatography. J Immunol Methods. 1997;208:65–73. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh R, Wang L. Purification of humanized monoclonal antibody by hydrophobic interaction membrane chromatography. J Chromatogr A. 2006;1107(1):104–09. doi: 10.1016/j.chroma.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Patchornik G, Namboothiri INN, Nair DK, Wachtel E, Persky R. Tethered non-ionic micelles: a matrix for enhanced solubilization of lipophilic compounds. Soft Matter. 2012;8(32):8456–63. doi: 10.1039/c2sm25708d. [DOI] [Google Scholar]
- 16.Patchornik G, Wachtel E, Kesselman E, Danino D. Cryo-TEM structural analysis of conjugated nonionic engineered-micelles. Soft Matter. 2014;10(27):4922–28. doi: 10.1039/c4sm00462k. [DOI] [PubMed] [Google Scholar]
- 17.Dutta S, Nair DK, Namboothiri IN, Wachtel E, Friedman N, Sheves M, Patchornik G. Engineered-membranes and engineered-micelles as efficient tools for purification of halorhodopsin and bacteriorhodopsin. Analyst 2015;140(1):204–12. doi: 10.1039/c4an01423e. [DOI] [PubMed] [Google Scholar]
- 18.Martell AE, Smith RM. Critical stability constants. New York (NY): Plenum Press; 1974. [Google Scholar]
- 19.Danino D. Cryo-TEM of soft molecular assemblies. Curr Opin Colloid Interface Sci 2012;17(6):316–29. doi: 10.1016/j.cocis.2012.10.003. [DOI] [Google Scholar]
- 20.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A 2012;109(40):16101–06. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padlan EA. Anatomy of the antibody molecule. Mol Immunol 1994;31:169–217. [DOI] [PubMed] [Google Scholar]
- 22.Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 2006;1(6):2876–90. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demeule B, Lawrence MJ, Drake AF, Gurny R, Arvinte T. Characterization of protein aggregation: the case of a therapeutic immunoglobulin. Biochim Biophys Acta 2007;1774(1):146–53. doi: 10.1016/j.bbapap.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology 2010;62(1):1–16. doi: 10.1007/s10616-010-9263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glassy MC, Tharakan JP, Chau PC. Serum-free media in hybridoma culture and monoclonal antibody production. Biotechnol Bioeng 1988;32(8):1015–28. doi: 10.1002/bit.260320809. [DOI] [PubMed] [Google Scholar]
- 26.Narhi LO, Caughey DJ, Horan T, Kita Y, Chang D, Arakawa T. Effect of three elution buffers on the recovery and structure of monoclonal antibodies. Anal Biochem 1997;253(2):236–45. doi: 10.1006/abio.1997.2375. [DOI] [PubMed] [Google Scholar]
- 27.Carter DC, Ho JX. Structure of serum albumin. Adv Protein Chem 1994;45:153–203. [DOI] [PubMed] [Google Scholar]
- 28.Rozga M, Sokolowska M, Protas AM, Bal W. Human serum albumin coordinates Cu(II) at its N-terminal binding site with 1 pM affinity. J Bio Inorg Chem 2007;12(6):913–18. doi: 10.1007/s00775-007-0244-8. [DOI] [PubMed] [Google Scholar]
- 29.Bourdon E, Loreau N, Lagrost L, Blache D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic Res 2005;39:15–20. doi: 10.1080/10715760400024935. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. mAbs 2012;4(1):17–23. doi: 10.4161/mabs.4.1.18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts CJ. Therapeutic protein aggregation: mechanisms, design, and control. Trends Biotechnol 2014;32(7):372–80. doi: 10.1016/j.tibtech.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Follman DK, Fahrner RL. Factorial screening of antibody purification processes using three chromatography steps without protein A. J Chromatogr A 2004;1024:79–85. doi: 10.1016/j.chroma.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 33.Danino D, Bernheim-Groswasser A, Talmon Y. Digital cryogenic transmission electron microscopy: an advanced tool for direct imaging of complex fluids. Colloids Surf A 2001;183-185:113–22. doi: 10.1016/S0927-7757(01)00543-X. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


