ABSTRACT
Reprogrammed lipid metabolism and persistent androgen receptor signaling commonly mark aggressive prostate cancer. We describe that targeting de-novo lipogenesis deprives prostate cancer cells of substrates and fuel, while inhibiting androgen receptor signaling. Our study uncovers the interplay between lipogenesis and androgen receptor and proposes novel combinatorial therapeutic approaches.
KEYWORDS: Metastatic prostate cancer, fatty acid synthesis, androgen receptor, lipid metabolism, reticulum endoplasmic stress response
The number of publications proposing therapeutic strategies targeting cancer metabolic pathways has dramatically increased due to regained awareness of metabolic reprogramming as a major player in the processes of tumorigenesis and tumor progression.1 Thus far, however, only a limited number of drugs targeting metabolic pathways has reached the clinical setting due to toxicity, non-specificity, redundant or compensatory mechanisms put in place by tumor cells or provided by the tumor microenvironment and external factors. The identification of metabolic vulnerabilities that are truly limiting for cancer cell growth remains crucial for the success of therapies that target metabolism.
Increased de-novo fatty acid synthesis represents a unique vulnerability for prostate cancer cells. Despite the abundance of circulating fatty acids, which are preferentially utilized by normal cells, an increased rate of de-novo fatty acid synthesis characterizes transformed prostate cells, especially in the advanced stages of disease.2 This unique metabolic vulnerability of prostate cancer cells creates a therapeutic window that can be exploited in the clinical setting. Increased expression of genes for fatty acid synthesis has been reported to contribute to the development of castration resistance,3 a stage where prostate cancer cells are no longer responsive to therapies targeting the androgen receptor (AR) signaling. Different mechanisms are responsible for resistance to such therapies, including the emergence of AR splice variants (AR-Vs) such as AR-V7. AR-V7 lacks the C-terminal ligand-binding domain of full-length AR (AR-FL) and functions as a constitutively active, ligand-independent transcription factor that drives the growth of metastatic castration-resistant prostate cancer (mCRPC) cells in vitro and in vivo.4
We specifically looked at the interplay between lipid metabolism and AR signaling.5 While it is well established that androgens increase the expression of lipogenic genes and promote de-novo lipogenesis, the consequences of interfering with lipid metabolism on AR signaling were unknown. We leveraged IPI-9119, a novel, specific and selective inhibitor of fatty acid synthase (FASN), a key enzyme in fatty acid synthesis, to investigate the metabolic and molecular effects of inhibiting FASN and its therapeutic potential in mCRPC. We demonstrated that fat metabolism and AR signaling are mutually regulated. Inhibiting de-novo fatty acid synthesis causes a metabolic catastrophe that triggers endoplasmic reticulum (ER) stress response, cell cycle arrest, and apoptosis. A closer look at ER stress response revealed the involvement of the PERK/eiF2α arm in AR protein reduction, linking lipid metabolism to the modulation of AR signaling (Figure 1).
Figure 1.
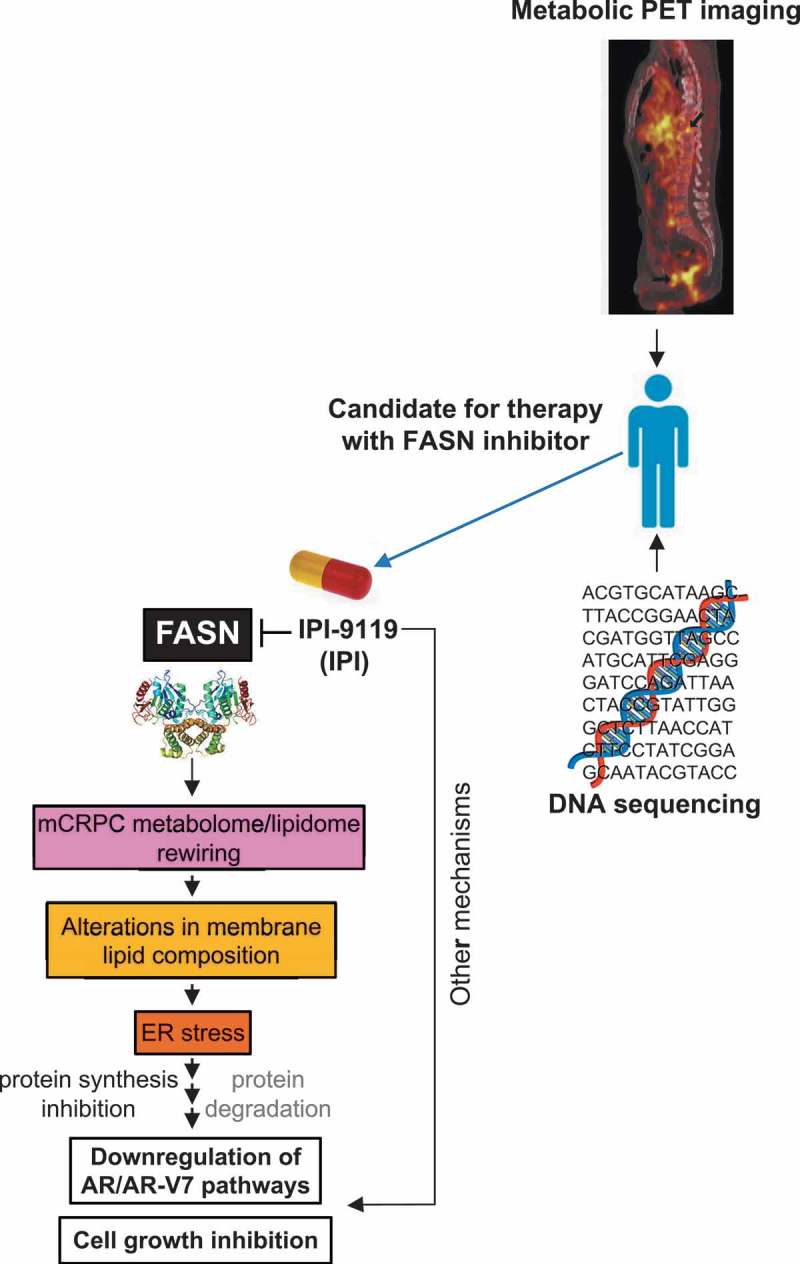
Therapeutic effects of FASN inhibition.
The FASN inhibitor IPI-9119 (IPI) induces a metabolic reprogramming that affects lipid synthesis and membrane composition resulting in the induction of ER stress response [i.e., Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) arm]. This is associated with global protein synthesis and more specifically AR pathway deregulation. Precision medicine approaches to select the best candidates for treatment can be based on metabolic imaging (e.g., 11C-acetate) or DNA sequencing to identify genetic alterations that induce dependence on de-novo lipid synthesis. The PET/computerized tomography fused image included in the figure was kindly provided by Prof. Umar Mahmood (Massachusetts General Hospital, Boston, MA). Black arrows indicate metastatic sites. AR = androgen receptor, AR-V7 = androgen receptor, splicing variant V7, ER = endoplasmic reticulum, FASN = fatty acid synthase, mCRPC = metastatic castration-resistant prostate cancer, PET = positron emission tomography.
From a clinical perspective, our study demonstrates that IPI-9119 is effective in mCRPC human organoids. We reported that almost all human CRPC metastases express FASN in association with AR-FL. AR-V7 was detected in 39% of the bone metastases and consistently co-expressed with FASN as well. These data suggest that FASN could be targeted in the majority of mCRPC, independent of AR-V7 status. However, patients with AR-V7-positive mCRPC could gain additional benefit from FASN inhibitors in multiple ways. In AR-V7-positive, treatment-naïve mCRPC patients, which are resistant to the second-generation AR signaling inhibitors Enzalutamide and Abiraterone, IPI-9119 may be administered along with chemotherapy. Promising results have been reported for the combination of FASN inhibitors and taxanes in PCa preclinical models.6 In the setting of AR-V7 expression as a mechanism of resistance to Enzalutamide/Abiraterone exposure, IPI-9119 could be administered in combination with these agents to delay/overcome resistance. Alternatively, FASN inhibition could be undertaken once Enzalutamide/Abiraterone resistance has emerged and no options of targeted therapy are left. Thus, carefully designed clinical trials are required to establish the therapeutic timing and the suitable population for treatment with FASN inhibitors.
The pre-treatment prediction of responsive patients remains the key to success for targeted therapies tailored to individual patients. Non-invasive metabolic imaging using positron emission tomography (PET) with the labeled lipid precursor 11C-acetate may identify prostate tumors with a high rate of de-novo lipogenesis.7 In addition, sequencing approaches using liquid biopsies can inform non-invasively on alterations in oncogene and tumor suppressors that drive addictions on lipid metabolism [e.g., overexpression of the oncogene c-MYC or loss of the tumor suppressor Phosphatase and tensin homolog (PTEN)].8,9 Metabolic profiling in the serum may also be a non-invasive biomarker reflective of intra-tumor metabolic activities (Figure 1). The inclusion of approaches of metabolism-based precision medicine in the clinical practice might, therefore, affect treatment decision-making by targeting cancer metabolic vulnerabilities.
Conclusions and perspectives
Our study demonstrates that targeting de-novo fatty acid synthesis represents a clinically exploitable vulnerability in the setting of mCRPC. Pharmacological suppression of FASN not only rewires the metabolome, but also affects AR and its splice variants, and represents, importantly, a “non-canonical” approach for targeting AR signaling. Our study sets the stage and provides the rationale for clinical trials to test FASN inhibitors in mCRPC.
We are driven by the enthusiasm of the promising results for the recent/ongoing clinical trials using FASN inhibitors in advanced solids tumors, including breast, astrocytoma, and colon.10
It is a great era for FASN inhibitors, which are finally entering the clinical setting. We hope they will contribute to improve the clinical outcomes for patients with advanced prostate cancer.
Funding Statement
This study was supported by the following grants to Massimo Loda: IMPACT Award from U.S. Department of Defense [PC160357], Synergistic Award from U.S. Department of Defense [W81XWH1410405], R01-CA131945 from National Institutes of Health. Giorgia Zadra is a recipient of Idea Development Award for New Investigators from U.S. Department of Defense [PC150263] and a Claudia Adams Barr Award in Innovative Basic Cancer Research from Dana-Farber Cancer Institute.
Disclosure of Potential Conflicts of Interests
No potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 PMID: 21376230 [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Graner E, Febbo P, Weinstein L, Bhattacharya N, Onody T, Bubley G, Balk S, Loda M.. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1(10):707–715. PMID: 12939396 [PubMed] [Google Scholar]
- 3.Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, Gleave ME, Nelson CC. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64(6):2212–2221. PMID: 15026365 [DOI] [PubMed] [Google Scholar]
- 4.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594 PMID: 18593950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zadra G, Ribeiro CF, Chetta P, Ho Y, Cacciatore S, Gao X, Syamala S, Bango C, Photopoulos C, Huang Y, et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2019;116(2):631–640. doi: 10.1073/pnas.1808834116 PMID: 30578319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuer TS, Ventura R, Mordec K, Lai J, Fridlib M, Buckley D, Kemble G. FASN inhibition and taxane treatment combine to enhance anti-tumor efficacy in diverse Xenograft Tumor models through disruption of tubulin palmitoylation and microtubule organization and FASN inhibition-mediated effects on oncogenic signaling and gene expression. EBioMedicine. 2017. February;16:51–62 . doi: 10.1016/j.ebiom.2016.12.012 PMID: 28159572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spick C, Herrmann K, Czernin J. Evaluation of prostate cancer with 11C-acetate PET/CT. J Nucl Med. 2016;57(Suppl3):30S–37S. doi: 10.2967/jnumed.115.169599 PMID: 27694168 [DOI] [PubMed] [Google Scholar]
- 8.Priolo C, Pyne S, Rose J, Regan ER, Zadra G, Photopoulos C, Cacciatore S, Schultz D, Scaglia N, McDunn J, et al. AKT1 and MYC induce distinctive metabolic fingerprints in human prostate cancer. Cancer Res. 2014;74(24):7198–7204. doi: 10.1158/0008-5472.CAN-14-1490 PMID: 25322691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez-Billalabeitia E, Liu XS, Lee YR, Fung J, Katon JM, et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat Genet. 2018;50(2):206–218. doi: 10.1038/s41588-017-0027-2 PMID: 29335545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical Trials Using FASN Inhibitor TVB-2640 https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/fasn-inhibitor-tvb-2640.


