Abstract
Extremes of sleep duration and obstructive sleep apnea (OSA) are both associated with hypertension. We aimed to explore whether sleep duration modifies the relationship between OSA and prevalent hypertension, using both objective and subjective measures of total sleep duration. A total of 7107 OSA patients and 1118 primary snorers were included in the study. Hypertension was defined based either on direct blood pressure measures or on diagnosis by a physician. Objective sleep duration was derived by polysomnography and subjective sleep duration was self-reported. Logistic regression models were used to estimate the associations between objective/subjective sleep duration and hypertension prevalence in OSA and primary snorers. Compared to primary snorers, OSA combined with objective sleep duration of 5–6 h increased the odds of hypertension by 45% (odds ratio=1.45, 95% confidence interval=1.14–1.84), whereas OSA combined with objective sleep duration<5 h further increased the odds of hypertension by 80% (odds ratio=1.80, 95% confidence interval=1.33–2.42). These results were independent of major confounding factors frequently associated with OSA or hypertension. In stratified analysis by sleep duration, risk of hypertension in those with extremely short sleep (<5 h) was not significantly different between OSA and primary snorers, while odds were significant for OSA in the other four sleep duration strata (5–6, 6–7, 7–8 and >8 h). No significance was evident using subjective sleep duration. We conclude that objective short sleep duration is associated with hypertension in OSA patients. Extremely short sleep duration in itself may actually be even more detrimental than OSA in terms of hypertension risk.
Keywords: Obstructive sleep apnea, sleep duration, hypertension, high blood pressure, polysomnography
Introduction
Obstructive sleep apnea (OSA) is a common disease that affects approximately 34% of men and 17% of women aged 30 to 70 years1. Cross-sectional and longitudinal studies have consistently identified OSA as an important cause of hypertension2, 3. Hypertension is a well-established risk factor for various adverse health outcomes, such as stroke, cardiovascular disease (CVD), and mortality, and may mediate the heightened CVD risk associated with OSA4.
Individual variations in sleep duration are influenced by both biological and environmental factors. Recent studies in humans suggest that habitual sleep duration is a stable trait under genetic regulation5, 6. Tang et al.7 also found that the rodents under well habituated conditions exhibited sleep duration patterns (short, intermediate, or long sleep time) that were consistent within individual animals across days. However, the emerging trend of reduction in sleep duration worldwide over the past decades, has led to a growing sleep debt on a population level8, 9, and uncertain implications for risk of systemic disease.
There is increasing evidence indicating a link between sleep duration and blood pressure (BP). Sleep deprivation in experimental studies may cause significant increases in BP both in normotensive and hypertensive subjects10, 11. In addition, epidemiological studies examining the link between sleep duration and hypertension support an independent association between short sleep duration and higher risk for prevalent and incident hypertension12, 13. On the other hand, there are studies showing that also long sleep may be associated with increased risk for hypertension14–16. However, the majority of previous studies assessed sleep duration solely based on subjective reports, which are often at variance with objective measures of sleep duration17–19, and those findings may not necessarily be corroborated by objective sleep indicators.
Since both OSA and extremes of sleep duration are individually associated with hypertension, the co-occurrence of inadequate sleep duration in OSA patients may potentially contribute to elevate BP in this population and modify the relationship between OSA and hypertension20. To our knowledge, this hypothesis was only explored previously by Priou and colleagues21. They found that patients with OSA and short sleep duration, as assessed by polysomnography (PSG), had a substantially higher risk of having hypertension compared to those with OSA but normal sleep duration. However, in this aforementioned paper short sleep was only defined as a binary variable. The implications of long sleep duration, as well as the role of subjective versus objective sleep duration, remain unknown.
We evaluated a large sample of 8225 patients with and without OSA, and sought to assess: 1) whether there is a U-shaped relationship between objective sleep duration, as derived from PSG, and risk of hypertension; 2) whether the risk of hypertension differs when using a subjective definition of sleep duration, based on self-report measures; and 3) whether the presence of OSA modulates such risk.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
This was a cross-sectional study of consecutive patients seen at the Sleep Medicine Center, West China Hospital. The study procedure was approved by the University’s Institutional Review Board and informed consent was obtained from each participant.
All participants were Chinese adults (age>18 years), evaluated at the Sleep Center for suspected OSA. All potential research subjects were interviewed with a comprehensive questionnaire to collect information on general health, medication use, and history of sleep complaints.
To qualify for the present study, patients with OSA had to meet an apnea/hypopnea index (AHI) criterion of ≥5 events/ hour, whereas individuals with an AHI <5 events/ hour were classified as the primary snoring group. We excluded subjects who had (1) a chronic sleep-disrupting medical condition (e.g., pain, heart disease); (2) a current major psychiatric condition (e.g., depression); (3) current or recent (within the past 3 months) use of hypnotics, anxiolytics, antidepressants, and any other antipsychotics; (4) any other comorbid sleep disorder (e.g., insomnia, central sleep apnea). Additional information on eligibility criteria is provided in the online data supplement. Please see http://hyper.ahajournals.org.
Blood pressure measures
Following standard methods22, BP was measured on two different occasions: in the evening about 2 hours before starting PSG (i.e., 20:00–21:00), and in the morning after the end of the overnight sleep study (i.e., 06:00–07:00), when the patient was still recumbent. We used a pneumoelectric microprocessor–controlled instrument (Nissei, DS-1902, Japan) with cuffs of appropriate size. The accuracy of this monitor is reported to be ± 3 mm Hg; in addition, internal calibration was performed before each use and the machine was checked against a mercury sphygmomanometer at least once a year. Recorded BP was the average of 3 consecutive readings during a 5-minute period following at least 10 minutes of rest in the supine position. Hypertension was defined as (1) diastolic BP (DBP) ≥90 mmHg and/or systolic BP (SBP) ≥140 mmHg at either evening or morning measurement; (2) use of anti-hypertensive medication; or 3) physician-diagnosed hypertension as per clinical history23. We used the average of evening and morning BP for analysis.
Polysomnography (PSG)
All subjects underwent one night of attended PSG recording in a sound-attenuated, light- and temperature-controlled room. All the sleep studies were full-night diagnostic studies. During this evaluation, subjects were allowed to follow their habitual sleep time, with the recording time ranging from 21:00–22:00 to 6:00–7:00. Sleep data were collected and scored via the Alice 5 Diagnostic Sleep System (Philips Respironics, Bend, OR, USA). All sleep parameters and stages (N1, N2, N3, and rapid eye movement (REM) sleep stages) were scored by senior PSG technicians according to the American Academy of Sleep Medicine criteria24. An apnea was defined as more than 90% reduction in airflow for at least 10s; whereas hypopnea was defined as 50% or more reduction of airflow for at least 10s associated with 3% or more reduction in SpO2. The AHI was calculated as the average number of apneas and hypopneas per hour of sleep. T90% was the percentage of time spent in sleep below 90% oxygen saturation.
Sleep duration measures
Subjective sleep duration was assessed during the face-to-face clinical interview as follows: “On average, how many hours of sleep do you usually get per day?”. Objective sleep duration was defined as total sleep time (TST) as recorded during overnight PSG. Five categories of sleep duration were then derived (>8 h, 7–8 h, 6–7 h, 5–6 h, and <5 h).
Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables and percent for categorical variables. Comparisons between groups were conducted using independent-sample t-tests/variance analysis or Mann–Whitney U tests for normally and skewed distributed continuous variables, respectively, and with the χ2 test for categorical variables.
Logistic regression models were used to assess the independent associations between OSA and subjective and objective sleep duration with hypertension, as well as interaction effects. In a first step, we separately studied the association of hypertension with OSA alone and with short objective and subjective sleep duration alone. In a second step, we determined adjusted odds ratio (OR) (95% confidence intervals [CI]) for hypertension considering the joint effect of OSA and objective sleep duration and using primary snorers as a reference group. Then, we calculated the adjusted OR (95%CI) for hypertension associated with OSA in different strata of objective sleep duration. In all models, we sequentially controlled for multiple covariates expected to affect the relationships of interest, including age, sex, BMI, tobacco, alcohol drinking, coffee use, diabetes, total time in bed, time spent in N3 sleep stage (%TST), ESS, T90% and AHI (or TST, depending on the primary exposure).
In order to further characterize the association between different objective sleep duration groups and frequency of hypertension and BP values, we conducted an analysis of variance. Post hoc Dunnett tests were used to control for type I errors in multiple comparisons. Linear regression models were used to explore the association between BP values and objective and subjective sleep duration in patient with and without OSA, respectively. The covariates we adjusted for included age, sex, BMI, tobacco, alcohol drinking, coffee use, diabetes, anti-hypertensive medications, total time in bed, time spent in N3 sleep stage (%TST), ESS, T90% and AHI.
Data were analyzed using SPSS 19.0. Comparisons with p-values <0.05 were considered statistically significant.
Additional information on statistical analysis is provided in the online data supplement. Please see http://hyper.ahajournals.org.
Results
A total of 8225 individuals, 7107 OSA and 1118 primary snoring patients were included in the present study. Table S1 in the online-only Data Supplement presents the demographic, clinical, and sleep characteristics of primary snoring and OSA patients. Hypertension was found in 27.3% of those in the primary snoring group versus in 53.8% of OSA patients.
Tables 1 and 2 show demographic and clinical characteristics and PSG-determined sleep parameters of all patients stratified according to objective and subjective sleep duration, respectively. Patients with shorter objective or subjective sleep duration were older, with lower AHI, higher prevalence of hypertension and higher SBP. The correlation between subjective and objective sleep duration in the entire sample was significant but very modest (R=0.125, P<0.001).
Table 1.
Demographic, clinical and sleep characteristics of all patients stratified by objective sleep duration categories.
| Characteristics | >8 h (n=2053) |
7–8 h (n=2960) |
6–7 h (n=1959) |
5–6 h (n=805) |
<5 h (n=448) |
P |
|---|---|---|---|---|---|---|
| Demographic and clinical characteristics | ||||||
| Men, n (%) | 1800 (87.7) | 2422 (81.8) | 1512 (77.2) | 612 (76.0) | 319 (71.2) | < 0.001 |
| Age (years) | 42.50 ± 10.49 | 43.34 ± 11.50 | 46.32 ± 12.37 | 48.61 ± 12.97 | 49.27 ± 13.58 | < 0.001 |
| BMI (kg/m2) | 27.39 ± 3.91 | 26.64 ± 3.90 | 26.19 ± 3.63 | 25.88 ± 3.84 | 25.85 ± 3.84 | < 0.001 |
| Hypertension, n (%) | 823 (48.7) | 1415 (47.8) | 951 (48.5) | 427 (53.0) | 268 (59.8) | < 0.001 |
| SBP (mmHg) | 126.28 ± 15.35 | 125.70 ± 15.09 | 126.41 ± 15.52 | 127.88 ±15.91 | 127.82 ± 15.21 | 0.002 |
| DBP (mmHg) | 83.11 ± 11.67 | 82.05 ± 11.38 | 81.44 ± 11.24 | 81.52 ± 10.58 | 81.70 ± 10.56 | < 0.001 |
| Diabetes mellitus, n (%) | 93 (4.5) | 137 (4.6) | 115 (5.9) | 50 (6.2) | 41 (9.2) | < 0.001 |
| Tobacco use, n (%) | 933 (45.4) | 1176 (39.7) | 757 (38.6) | 275 (34.2) | 149 (33.3) | < 0.001 |
| Alcohol consumption, n (%) | 1031 (50.2) | 1314 (44.4) | 818 (41.8) | 301 (37.4) | 150 (33.5) | < 0.001 |
| Coffee consumption, n (%) | 618 (30.1) | 825 (27.9) | 498 (25.4) | 175 (21.7) | 97 (21.7) | < 0.001 |
| Subjective sleep duration (min) | 430.56 ± 89.79 | 417.16 ± 87.41 | 406.53 ± 91.45 | 397.63 ± 100.22 | 396.00 ± 108.95 | < 0.001 |
| ESS | 10.34 ± 6.30 | 8.95 ± 6.73 | 8.16 ± 5.88 | 8.11 ± 5.70 | 7.10 ± 5.68 | < 0.001 |
| ESS>10, n (%) | 955 (46.5) | 1111 (37.5) | 622 (31.8) | 263 (32.7) | 118 (26.3) | < 0.001 |
| Polysomnography | ||||||
| Sleep onset latency (min) | 7.53 ± 9.06 | 11.13 ± 12.40 | 18.29± 20.70 | 24.31 ± 28.38 | 42.35 ± 55.99 | < 0.001 |
| Total sleep duration (min) | 514.58 ± 27.78 | 450.40 ± 17.40 | 394.11 ± 16.86 | 335.05 ± 16.97 | 240.45 ± 63.12 | < 0.001 |
| Total time in bed (min) | 558.70 ± 34.95 | 512.86 ± 35.66 | 495.30 ± 44.27 | 488.78 ± 54.18 | 457.57 ± 108.59 | < 0.001 |
| Sleep efficiency (%) | 92.26 ± 4.34 | 88.17 ± 5.95 | 80.19 ± 7.73 | 69.40 ± 8.52 | 54.49 ± 16.59 | < 0.001 |
| Wake after sleep onset (min) | 36.59 ± 25.33 | 51.33 ± 34.24 | 82.91 ± 46.79 | 129.43 ± 58.41 | 174.80 ± 100.47 | < 0.001 |
| N1 (% TST) | 35.78 ± 22.33 | 31.22 ± 20.01 | 31.22 ± 19.47 | 31.13 ± 19.83 | 34.10 ± 22.15 | < 0.001 |
| N2 (% TST) | 42.20 ± 18.45 | 45.14 ± 16.71 | 44.98 ± 16.35 | 45.81 ± 16.87 | 43.18 ± 18.79 | < 0.001 |
| N3 (% TST) | 5.84 ± 6.21 | 7.40 ± 7.10 | 8.24 ± 7.77 | 8.80 ± 8.37 | 10.09 ± 10.60 | < 0.001 |
| REM (% TST) | 16.18 ± 6.41 | 16.23 ± 6.28 | 15.56 ± 6.46 | 14.27 ± 6.87 | 12.63 ± 8.92 | < 0.001 |
| AHI (events/h) | 49.34 ± 30.17 | 39.83 ± 29.03 | 35.11 ± 27.26 | 31.02 ± 25.71 | 30.30 ± 27.86 | < 0.001 |
| T90% (%) | 24.69 ± 25.89 | 16.62 ± 22.17 | 11.55 ± 17.50 | 11.33 ± 19.06 | 15.48 ± 34.93 | < 0.001 |
| Lowest- SpO2 (%) | 64.36 ± 20.57 | 70.18 ± 19.35 | 74.59 ± 16.85 | 75.74 ± 16.68 | 76.40 ± 19.20 | < 0.001 |
| OSA, n (%) | 1857 (90.5) | 2558 (86.4) | 1669 (85.2) | 671 (83.4) | 352 (78.6) | < 0.001 |
| Mild OSA, n (%) | 232 (11.3) | 422 (14.3) | 350 (17.9) | 161 (20.0) | 87 (19.4) | < 0.001 |
| Moderate OSA, n (%) | 221 (10.8) | 486 (16.4) | 341 (17.4) | 166 (20.6) | 83 (18.5) | < 0.001 |
| Severe OSA, n (%) | 1404 (68.4) | 1650 (55.7) | 978 (49.9) | 344 (42.7) | 182 (40.8) | < 0.001 |
Percent for categorical and other variables are presented with mean ± SD. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AHI, apnea-hypopnea index; T90%, percentage of sleep time spent below 90% oxygen saturation; Lowest- SpO2, the lowest oxygen saturation, ESS, Epworth Sleepiness Scale.
Table 2.
Demographic, clinical and sleep characteristics of all patients stratified by different subjective sleep duration categories.
| Characteristics | > 8 h (n=938) |
7–8 h (n=2001) |
6–7 h (n=2456) |
5–6 h (n=1158) |
<5 h (n=1672) |
P |
|---|---|---|---|---|---|---|
| Demographic and clinical characteristics | ||||||
| Men, n (%) | 805 (85.8) | 1643 (82.1) | 2056 (83.7) | 893 (77.1) | 1268 (75.8) | < 0.001 |
| Age (years) | 42.66 ± 12.60 | 42.81 ± 11.62 | 43.56 ± 11.57 | 46.69 ± 11.80 | 48.30 ± 11.67 | < 0.001 |
| BMI (kg/m2) | 27.06 ± 3.94 | 26.61 ± 3.82 | 26.48 ± 3.70 | 26.62 ± 3.96 | 26.51 ± 4.03 | 0.003 |
| Hypertension, n (%) | 476 (50.7) | 957 (47.8) | 1187 (48.3) | 610 (52.7) | 862 (51.6) | 0.010 |
| SBP (mmHg) | 125.42 ± 14.60 | 125.92 ± 15.28 | 126.33 ± 15.20 | 126.89 ± 15.66 | 126.99 ± 15.84 | 0.057 |
| DBP (mmHg) | 82.37 ± 11.34 | 82.15 ± 11.56 | 82.25 ± 11.26 | 82.08 ± 11.24 | 81.67 ± 11.13 | 0.503 |
| Diabetes mellitus, n (%) | 52 (5.5) | 98 (4.9) | 115 (4.7) | 65 (5.6) | 106 (6.3) | 0.129 |
| Tobacco use, n (%) | 415 (44.2) | 797 (39.8) | 967 (39.4) | 453 (39.1) | 658 (39.4) | 0.096 |
| Alcohol consumption, n (%) | 433 (46.2) | 920 (46.0) | 1114 (45.4) | 486 (42.0) | 661 (39.5) | 0.001 |
| Coffee consumption, n (%) | 266 (28.4) | 564 (28.2) | 642 (26.1) | 324 (28.0) | 417 (24.9) | 0.204 |
| Subjective sleep duration (min) | 553.61 ± 58.97 | 473.16 ± 12.73 | 414.05 ± 10.33 | 357.66 ± 7.61 | 256.12 ± 62.82 | < 0.001 |
| ESS | 9.96 ± 6.42 | 8.98 ± 7.35 | 8.50 ± 5.65 | 8.92 ± 5.71 | 8.93 ± 6.31 | < 0.001 |
| ESS>10, n (%) | 408 (43.5) | 711 (35.5) | 850 (34.6) | 438 (37.8) | 662 (39.6) | < 0.001 |
| Polysomnography | ||||||
| Sleep onset latency (min) | 14.47 ± 19.96 | 14.70 ± 20.72 | 13.88± 20.28 | 16.30 ± 23.55 | 16.02 ± 26.82 | 0.006 |
| Total sleep duration (min) | 445.47 ± 75.49 | 436.70 ± 73.86 | 432.63 ± 71.17 | 421.77 ± 75.33 | 416.57 ± 80.70 | < 0.001 |
| Total time in bed (min) | 522.95 ± 56.62 | 514.13 ± 57.51 | 511.49 ± 52.31 | 513.15 ± 53.93 | 516.79 ± 52.82 | < 0.001 |
| Sleep efficiency (%) | 85.19 ± 11.15 | 84.94 ± 11.49 | 84.57 ± 11.35 | 82.29 ± 12.57 | 80.67 ± 13.80 | < 0.001 |
| Wake after sleep onset (min) | 63.01 ± 54.34 | 62.73 ± 55.57 | 64.99 ± 53.16 | 75.08 ± 60.79 | 84.21 ± 64.74 | < 0.001 |
| N1 (% TST) | 36.57 ± 22.78 | 33.62 ± 20.82 | 31.19 ± 19.66 | 31.19 ± 19.82 | 31.73 ± 21.00 | < 0.001 |
| N2 (% TST) | 41.11 ± 18.62 | 43.52 ± 17.34 | 45.39 ± 16.31 | 45.32 ± 16.49 | 44.87 ± 17.97 | < 0.001 |
| N3 (% TST) | 6.79 ± 7.37 | 7.30 ± 7.32 | 7.56 ± 7.21 | 7.60 ± 7.54 | 7.94 ± 8.18 | 0.003 |
| REM (% TST) | 15.54 ± 6.23 | 15.56 ± 6.64 | 15.85 ± 6.47 | 15.89 ± 6.77 | 15.46 ± 7.05 | 0.217 |
| AHI (events/h) | 46.40 ± 30.27 | 42.70 ± 29.61 | 38.56 ± 28.34 | 37.69 ± 28.56 | 35.41 ± 28.93 | < 0.001 |
| T90 % (%) | 22.61 ± 26.51 | 18.21 ± 23.92 | 15.04 ± 21.52 | 15.73 ± 22.62 | 15.40 ± 23.13 | < 0.001 |
| Lowest- SpO2 (%) | 66.72 ± 20.92 | 69.33 ± 19.44 | 71.97 ± 18.06 | 71.16± 19.14 | 72.20 ± 19.73 | < 0.001 |
| OSA, n (%) | 831 (88.6) | 1764 (88.2) | 2139 (87.1) | 998 (86.2) | 1375 (82.2) | < 0.001 |
| Mild OSA, n (%) | 111 (11.8) | 286 (14.3) | 378 (15.4) | 184 (15.9) | 293 (17.5) | < 0.001 |
| Moderate OSA, n (%) | 116 (12.4) | 269 (13.4) | 442 (18.0) | 200 (17.3) | 270 (16.1) | < 0.001 |
| Severe OSA, n (%) | 604 (64.4) | 1209 (60.5) | 1319 (53.7) | 614 (53.0) | 812 (48.6) | < 0.001 |
Percent for categorical and other variables are presented with mean ± SD. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AHI, apnea-hypopnea index; T90%, percentage of sleep time spent below 90% oxygen saturation; Lowest- SpO2, the lowest oxygen saturation, ESS, Epworth Sleepiness Scale.
As indicated in Table 3, OSA showed a significant association with hypertension (fully adjusted OR = 1.60, 95% CI 1.36–1.89, P<0.001). Objective sleep duration was associated with hypertension in a dose-response fashion. As compared to objective sleep duration 7–8 h (reference), 5–6 h and <5 h of sleep increased the odds of hypertension by 39% (OR = 1.39, 95% CI 1.16–1.66, P<0.001) and by 96% (OR = 1.96, 95% CI 1.54–2.49, P<0.001), respectively. On the contrary, objective sleep duration >8 h was associated with decreased odds of hypertension (OR = 0.81, 95% CI, 0.70–0.93, P=0.02). However, no significant relationships were found when subjectively determined sleep duration was considered.
Table 3.
Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the association of hypertension with OSA and objective and subjective sleep duration.
| OR (95% CI) | ||||
|---|---|---|---|---|
| Predictors | n | Model 1 | Model 2 | Model 3 |
| Primary snoring | 1118 | Reference | Reference | Reference |
| OSA | 7107 | 1.92 (1.64—2.25) | 1.76 (1.49—2.07) | 1.60 (1.36—1.89) |
| Objective sleep duration | ||||
| > 8 h | 2053 | 1.02 (0.91—1.16) | 0.93 (0.79—1.03) | 0.81 (0.70—0.93) |
| 7–8 h | 2960 | Reference | Reference | Reference |
| 6–7 h | 1959 | 0.98 (0.86—1.11) | 1.05 (0.92—1.19) | 1.10 (0.97—1.26) |
| 5–6 h | 805 | 1.11 (0.94—1.32) | 1.28 (1.07—1.52) | 1.39 (1.16—1.66) |
| < 5 h | 448 | 1.52 (1.22—1.89) | 1.85 (1.46—2.35) | 1.96 (1.54—2.49) |
| P for linear trend | 0.018 | < 0.001 | < 0.001 | |
| Subjective sleep duration | ||||
| > 8 h | 938 | 1.07 (0.91—1.27) | 1.05 (0.89—1.25) | 1.02 (0.86—1.22) |
| 7–8 h | 2001 | Reference | Reference | Reference |
| 6–7 h | 2456 | 1.00 (0.89—1.14) | 1.03 (0.90—1.17) | 1.09 (0.95—1.24) |
| 5–6 h | 1158 | 1.04 (0.89—1.22) | 1.09 (0.93—1.27) | 1.16 (0.99—1.37) |
| < 5 h | 1672 | 0.96 (0.84—1.11) | 1.02 (0.88—1.18) | 1.10 (0.95—1.28) |
| P for linear trend | 0.410 | 0.874 | 0.117 | |
Model 1 was adjusted for age, sex and BMI; Model 2 was adjusted for variables included in Model 1 and tobacco use, alcohol and coffee consumption, diabetes mellitus, total time in bed, N3(%TST) and ESS; Model 3 was adjusted for variables included in Model 2 and T90% and AHI (or TST).
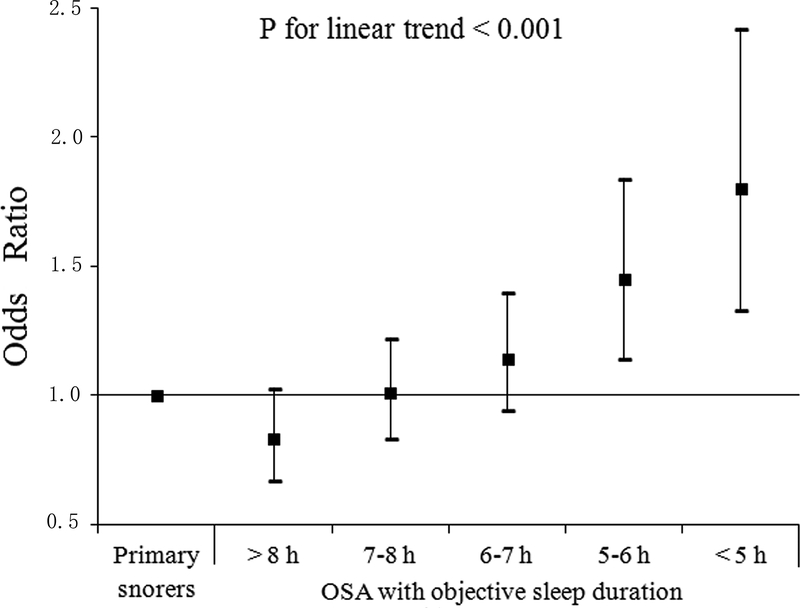
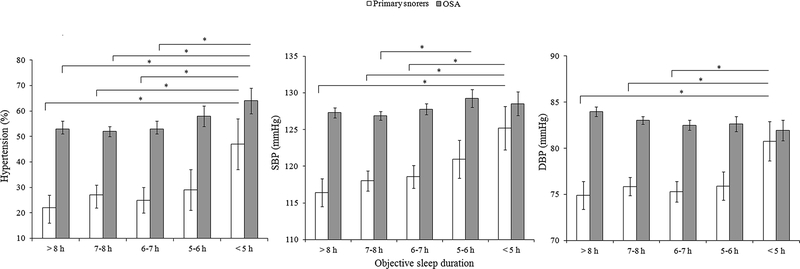
Figure 1 illustrates the joint effect of OSA and objective sleep duration on hypertension, reflecting the significant interaction between AHI and objective sleep duration (P=0.016). Compared to primary snoring, OSA with objective sleep duration 5–6 h and <5 h increased the odds of hypertension by approximately 45% (OR = 1.45, 95% CI 1.14–1.84, P=0.002) and 80% (OR = 1.80, 95% CI 1.33–2.42, P<0.001), respectively. In contrast, OSA patients with objective sleep duration >8 h, 7–8 h and 6–7 h did not have significantly higher likelihood of having hypertension than primary snorers. Figure 2 depicts the frequency of hypertension and BP levels in patients with and without OSA stratified by objective sleep duration. Compared to primary snoring, OSA patients had significantly higher prevalence of hypertension and higher levels of SBP and DBP within different sleep duration strata. However, the relationships between sleep time and prevalence of hypertension and BP level were similar within each group of patients – hypertension was more frequent and BP was higher in those exhibiting 5–6 h or <5 h of sleep compared to those sleeping 6–7 h, 7–8 or >8 h.
Figure 1: Multivariable adjusted odds ratios and 95% confidence intervals for hypertension associated with different categories of objective sleep duration in OSA patients.
All data were adjusted for age, sex, BMI, tobacco, alcohol and coffee consumption, diabetes mellitus, total time in bed, N3 (%TST), ESS, T90% and AHI.*P<0.05.
Figure 2: Frequency of hypertension and SBP and DBP levels across different categories of objective sleep duration in primary snoring and OSA groups.
Error bars indicate 95% confidence intervals. SBP, systolic blood pressure; DBP, diastolic blood pressure.*P<0.05.
Notably, logistic regression analysis conducted on stratified data showed that, compared to primary snoring within the same sleep duration stratum, odds of prevalent hypertension in those with OSA and extremely short sleep (<5h) (OR = 1.16, 95% CI 0.67–2.01) were not significant, while odds were significant in the other four sleep duration strata (5–6, 6–7, 7–8 and >8 h) (Table 4).
Table 4.
Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the association of hypertension with OSA across different objective sleep durations.
| OR (95% CI) | ||||
|---|---|---|---|---|
| Objective sleep duration | n | Model 1 | Model 2 | Model 3 |
| > 8 h | ||||
| Primary snoring | 196 | Reference | Reference | Reference |
| OSA | 1857 | 2.07 (1.40—3.07) | 1.78 (1.19—2.67) | 1.57 (1.05—2.36) |
| 7–8 h | ||||
| Primary snoring | 402 | Reference | Reference | Reference |
| OSA | 2558 | 1.82 (1.40—2.36) | 1.67 (1.27—2.19) | 1.42 (1.08—1.87) |
| 6–7 h | ||||
| Primary snoring | 290 | Reference | Reference | Reference |
| OSA | 1669 | 2.25 (1.64—3.11) | 2.22 (1.60—3.10) | 1.97 (1.41—2.74) |
| 5–6 h | ||||
| Primary snoring | 134 | Reference | Reference | Reference |
| OSA | 671 | 2.48 (1.60—3.86) | 2.18 (1.38—3.44) | 1.99 (1.25—3.17) |
| < 5 h | ||||
| Primary snoring | 96 | Reference | Reference | Reference |
| OSA | 352 | 1.27 (0.76—2.13) | 1.20 (0.69—2.08) | 1.16 (0.67—2.01) |
Model 1 was adjusted for age, sex and BMI; Model 2 was adjusted for variables included in Model 1 and, tobacco use, alcohol and coffee consumption, diabetes mellitus, total time in bed, N3(%TST) and ESS; Model 3 was adjusted for variables included in Model 2 and T90%.
The associations between different objective sleep duration categories and hypertension risk in subgroups defined according to OSA severity, sex, age, and obesity (BMI ≥28 kg/m2)23 are presented in Tables S3 and S4. The association between hypertension and short objective sleep duration was present in all levels of OSA severity, both genders, younger ages (<60 years old), and in non-obese patients. We also applied linear regression models to examine the association between sleep duration values and BP within primary snoring and OSA patients, respectively (see Online Data Supplements and Tables S5 and S6). Objective sleep duration was significantly associated with BP in both primary snorers and OSA patients. Subjective sleep duration was significantly but modestly associated with BP only in OSA patients.
Discussion
We examined the relationship between objective and subjective sleep duration and risk of hypertension in a large population of primary snoring and OSA patients. Our findings suggest that, in patients with OSA, objective short sleep duration, as measured by PSG, is associated with hypertension risk in a linear, dose-response manner. Notably, a similar association was not found when subjective sleep duration was used.
The average sleep duration has decreased dramatically during the past decades, from 9 hours in 1910 to 7.5 hours in 1975 and 6.8 hours in 200513. Multiple cross-sectional and longitudinal epidemiological studies have documented a significant relationship between short sleep duration and hypertension. As summarized by two recent meta-analyses, decreased sleep duration is associated with 21% increased risk of prevalent hypertension and 23% increased risk of incident hypertension, and the relationship between short sleep and high BP appears to be more pronounced in middle-aged and female subjects12, 13, 25. Nevertheless, in the vast majority of these studies, sleep duration was self-reported as only few investigations have evaluated the association between objective sleep duration, as measured by PSG, and hypertension.
Little is known about the association of sleep duration with the risk for hypertension in clinical populations of OSA. In a retrospective study of 312 patients who underwent PSG, Ucar et al26 found shorter total sleep time in patients with hypertension than in those without hypertension. However, when the analysis was restricted to OSA patients (150 patients), sleep duration <6 h was not significantly associated with hypertension. Another case-control study found that OSA patients with resistant hypertension had shorter sleep duration than subjects with either controlled hypertension or normotension after controlling for confounders, including OSA severity27. Furthermore, Priou et al21 found a cumulative association between OSA severity and short sleep duration for hypertension, as those with OSA and decreased objective sleep duration exhibited the highest risk of hypertension. Our study corroborates these previous findings, and further adds to the literature by showing a dose-response relationship between objective sleep duration and risk of hypertension in this population. Furthermore, our data also suggest that normalization of sleep duration should be prioritized specifically in some subsets of OSA patients as subgroup analyses further showed that short objective sleep duration had stronger associations with hypertension, including in patients aged below 60 years and non-obese patients. This is further reinforced by our findings on the role of long sleep. Although a number of studies have found long sleep to be associated with heightened probability of hypertension15, 16, the bulk of the evidence points towards a stronger relation with short sleep, as summarized by recent meta-analyses13, 25. Our results are in line with the latter observation, as OSA patients sleeping >8 h exhibited reduced likelihood of having high BP, suggesting that, in this population, prolonged sleep time may be protective against such risk.
Our study failed to find a significant relationship between subjective short sleep duration and hypertension in individuals with OSA, which is in accordance with a prior study in insomnia patients28. Objective and self-reported sleep are known to be only moderately correlated17, 19. Estimations of sleep duration are often imprecise due to a variety of factors including fewer years of education, age, lower self-rated health, and work stress, to name a few17, 18. In addition, objective and subjective sleep measures may be associated with distinct psychological and biological outcomes. Patel et al29 found that objective sleep duration was linked to tumor necrosis factor alpha while self-reported sleep duration was not. Our results are in line with these concepts, showing a modest relationship between objective and subjective sleep measures and favoring the use of objective sleep measures to determine sleep duration, rather than subjective measures, when attempting to detect comorbidity risk associated with OSA. On the other hand, subjective sleep has been identified as a marker for psychological issues, such as depression and anxiety, and for poor perceived health, but no such associations were found with objective sleep measures18, 30, 31.
The intriguing finding that risk of hypertension in those with OSA and extremely short sleep duration (<5 h) was comparable to that of primary snorers reporting the same sleep duration suggested that very short sleep duration in itself may actually be even more detrimental than OSA, at least in terms of hypertension risk. Improving sleep duration and quality may result in the reduction of both daytime and nighttime BP32, 33.
The mechanisms underlying the association between sleep duration and hypertension remains unclear although several hypotheses have been proposed. Short sleep duration may increase BP through enhanced sympathetic activity, activation of inflammatory responses, endothelial dysfunction, insulin resistance, and/or blunted nocturnal BP decreases34–37. Moreover, oxidative stress may be implicated. DeMartino et al38 found that reduced PSG-TST was associated with elevated oxidative stress (myeloperoxidase levels) even after consideration of obesity and OSA severity. They further suggested a differential up-regulation of oxidative stress with objective versus habitual subjective reduced sleep duration, which may also partially explain the different effects of objective and subjective sleep duration on hypertension. In addition, short sleep duration may indirectly contribute to development of hypertension as it is often associated with unhealthy lifestyle habits39, 40, such as poor dietary regimens, sedentary living, and smoking, all factors known to predispose to BP elevation.
There are several strengths to this study, including a large sample size, and that measurements were not confounded by the use of sleep and other psychotropic medication, or presence of comorbid sleep and mental disorders. However, a number of limitations should be addressed. First, our sample consisted predominantly of middle-aged, overweight males, thus caution should be exercised in extrapolating our results to other populations. Second, as all participants in this study underwent sleep evaluation because of clinical suspicion of OSA, we cannot exclude a selection bias favoring symptomatic OSA, which may limit generalizability of our findings to asymptomatic patients. Third, objective sleep duration was derived from one full-night PSG and night to night variability and/or first night effects cannot be excluded. Actigraphy may constitute a simpler, cost-effective alternative to objectively measure sleep duration over a period of days and thus provide a better representation of habitual sleep duration in the home environment. However, a good level of agreement has been observed between actigraphy and PSG for sleep duration measurements in OSA41; Likewise, subjective sleep duration was determined solely in response to a single question during a clinical interview, while estimates from 1–2 week sleep diary may more comprehensively capture patients’ perceived sleep quantity. Fourth, due to the retrospective nature of this study and the population consisting of consecutive referrals to the sleep center due to high pretest probability of OSA, the sample sizes of the OSA and primary snoring groups are unequal. The imbalanced patient cohort may have led to undersampling or oversampling of one group and thus to biased prediction models42. Last, blood pressure measurements taken at two time points cannot approximate the entire 24 h blood pressure profile.
Perspectives
In conclusion, our study suggests that objective short sleep duration is independently associated with hypertension in OSA patients. Extremely short objective sleep duration (<5 h) in itself may actually be even more detrimental than OSA, at least for hypertension risk. Further studies with longitudinal designs are warranted to delineate the temporal association between objective short sleep duration and hypertension, and to determine whether interventions to optimize sleep time may also contribute to lower BP in patients with OSA.
Supplementary Material
Novelty and Significance
1). What Is New?
Our analysis showed that objective but not subjective sleep duration was associated with hypertension in a clear dose-response manner. Extremely short sleep duration in itself may actually be even more detrimental than OSA in terms of hypertension risk.
2). What Is Relevant?
Short sleep is prevalent in patients referred for evaluation in the sleep clinic. Physicians may consider sleep duration to estimate the biological severity of OSA in terms of cardiometabolic comorbidities.
3). Summary
Both OSA and short sleep are highly prevalent in the population. This study provides novel data indicating that in addition to AHI and nocturnal hypoxemia, objective sleep duration may be an important metric of hypertension risk in patients with OSA.
Acknowledgments
We would like to thank our technical staff (F. Lei, L. Du, L. Luo and L. Wu) for overnight sleep recording and scoring.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (81530002, 81629002) and the National Basic Research Program of China (2015CB856406). NC and VKS were supported by National Institute of Health HL65176, HL114676 and HL134808. NC is also supported by American Heart Association Scientific Development Grant16SDG27250156, and the Mayo Clinic CcATS Marie Ingalls Cardiovascular Research Career Development Fund in honor of Dr Alexander Schirger.
Footnotes
Conflict of interest
None
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013; 177: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311: 507–520. [DOI] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 4.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360: 1903–1913. [DOI] [PubMed] [Google Scholar]
- 5.Marinelli M, Pappa I, Bustamante M, et al. Heritability and Genome-Wide Association Analyses of Sleep Duration in Children: The EAGLE Consortium. Sleep. 2016; 39: 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005; 28: 479–496. [DOI] [PubMed] [Google Scholar]
- 7.Tang X, Yang L, Sanford LD. Individual variation in sleep and motor activity in rats. Behav Brain Res. 2007; 180: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010; 33: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bin YS, Marshall NS, Glozier N. Sleeping at the limits: The changing prevalence of short and long sleep durations in 10 countries. Am J Epidemiol. 2013; 177: 826–833. [DOI] [PubMed] [Google Scholar]
- 10.Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996; 9: 503–505. [DOI] [PubMed] [Google Scholar]
- 11.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: A 24-h study. Am J Hypertens. 1999;12:63–68. [DOI] [PubMed] [Google Scholar]
- 12.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens. 2014; 27: 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, Sun Y. Epidemiological evidence for the link between sleep duration and high blood pressure: A systematic review and meta-analysis. Sleep Med. 2013; 14: 324–332. [DOI] [PubMed] [Google Scholar]
- 14.Shankar A, Charumathi S, Kalidindi S. Sleep duration and self-rated health: The national health interview survey 2008. Sleep. 2011; 34: 1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Mei H, Jiang YR, Sun WQ, Song YJ, Liu SJ, Jiang F. Relationship between duration of sleep and hypertension in adults: A meta-analysis. J Clin Sleep Med. 2015;11:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magee CA, Kritharides L, Attia J, McElduff P, Banks E. Short and long sleep duration are associated with prevalent cardiovascular disease in australian adults. J Sleep Res. 2012;21:441–447. [DOI] [PubMed] [Google Scholar]
- 17.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008; 19: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackowska M, Dockray S, Hendrickx H, Steptoe A. Psychosocial factors and sleep efficiency: Discrepancies between subjective and objective evaluations of sleep. Psychosom Med. 2011; 73: 810–816. [DOI] [PubMed] [Google Scholar]
- 19.Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, Hall MH, Loredo JS, Mossavar-Rahmani Y, Ramos AR, Reid KJ, Shah NA, Sotres-Alvarez D, Zee PC, Wang R, Patel SR. Comparison of self-reported sleep duration with actigraphy: Results from the hispanic community health study/study of latinos sueno ancillary study. Am J Epidemiol. 2016; 183: 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: An update. Hypertension. 2014; 63: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priou P, Le Vaillant M, Meslier N, Paris A, Pigeanne T, Nguyen XL, Alizon C, Bizieux-Thaminy A, Leclair-Visonneau L, Humeau MP, Gagnadoux F, group Isc. Cumulative association of obstructive sleep apnea severity and short sleep duration with the risk for hypertension. PloS one. 2014; 9: e115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Circulation. 2005; 111: 697–716. [DOI] [PubMed] [Google Scholar]
- 23.Ren R, Li Y, Zhang J, Zhou J, Sun Y, Tan L, Li T, Wing YK, Tang X. Obstructive sleep apnea with objective daytime sleepiness is associated with hypertension. Hypertension. 2016; 68: 1264–1270. [DOI] [PubMed] [Google Scholar]
- 24.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep M. Rules for scoring respiratory events in sleep: Update of the 2007 aasm manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J Clin Sleep Med. 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: A systematic review and meta-analysis. Hypertens Res. 2012; 35: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 26.Ucar ZZ, Cirak AK, Olcay S, Uysal H, Demir AU, Ozacar R. Association of duration of sleep and cardiovascular and metabolic comorbidities in sleep apnea syndrome. Sleep Disord. 2012; 2012: 316232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman O, Bradley TD, Ruttanaumpawan P, Logan AG. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. Am J Hypertens. 2010; 23: 174–179. [DOI] [PubMed] [Google Scholar]
- 28.Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016; 39: 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, Tracy R, Redline S. Sleep duration and biomarkers of inflammation. Sleep. 2009; 32: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackowska M, Ronaldson A, Brown J, Steptoe A. Biological and psychological correlates of self-reported and objective sleep measures. J Psychosom Res. 2016; 84: 52–55. [DOI] [PubMed] [Google Scholar]
- 31.Rao MN, Blackwell T, Redline S, Punjabi NM, Barrett-Connor E, Neylan TC, Stone KL, Osteoporotic Fractures in Men Study G. Association between sleep duration and 24-hour urine free cortisol in the mros sleep study. PloS one. 2013; 8: e75205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: A pilot study. Journal of sleep research. 2013;22:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004; 43: 192–197. [DOI] [PubMed] [Google Scholar]
- 34.Hall MH, Mulukutla S, Kline CE, Samuelsson LB, Taylor BJ, Thayer JF, Krafty RT, Frank E, Kupfer DJ. Objective sleep duration is prospectively associated with endothelial health. Sleep. 2017; 40: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson MD, Russell-Jones D, Umpleby AM, Dijk DJ. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism. 2013; 62: 204–211. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Haack M, Gautam S, Meier-Ewert HK, Mullington JM. Repetitive exposure to shortened sleep leads to blunted sleep-associated blood pressure dipping. J Hypertens. 2017; 35:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Souza SB, Irigoyen MC, Mostarda C, Borile S, Krieger EM, Moreno H, Jr., Lorenzi-Filho G. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol (1985). 2012; 113: 232–236. [DOI] [PubMed] [Google Scholar]
- 38.DeMartino T, El Ghoul R, Wang L, Bena J, Hazen SL, Tracy R, Patel SR, Auckley D, Mehra R. Oxidative stress and inflammation differentially elevated in objective versus habitual subjective reduced sleep duration in obstructive sleep apnea. Sleep. 2016; 39: 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Haifi AA, AlMajed HT, Al-Hazzaa HM, Musaiger AO, Arab MA, Hasan RA. Relative contribution of obesity, sedentary behaviors and dietary habits to sleep duration among kuwaiti adolescents. Glob J Health Sci. 2015; 8: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Hazzaa HM, Musaiger AO, Abahussain NA, Al-Sobayel HI, Qahwaji DM. Lifestyle correlates of self-reported sleep duration among saudi adolescents: A multicentre school-based cross-sectional study. Child Care Health Dev. 2014; 40: 533–542. [DOI] [PubMed] [Google Scholar]
- 41.Gagnadoux F, Nguyen XL, Rakotonanahary D, Vidal S, Fleury B. Wrist-actigraphic estimation of sleep time under ncpap treatment in sleep apnoea patients. Eur Respir J. 2004; 23: 891–895. [DOI] [PubMed] [Google Scholar]
- 42.Estabrooks A, ed. A combination scheme for inductive learning from imbalanced data sets Master thesis Computer Science, Dalhousie University; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.