Abstract
Despite optimal current therapies, cardiovascular disease (CVD) remains the leading cause for death worldwide. Importantly, advances in peptide engineering have accelerated the development of innovative therapeutics for diverse human disease states. Additionally, the advancement of bispecific therapeutics targeting more than one signaling pathway represents a highly innovative strategy for the treatment of CVD. We therefore engineered a novel designer peptide which simultaneously targets the particulate guanylyl cyclase A (pGC-A) receptor and the Mas-receptor (MasR), potentially representing an attractive cardiorenoprotective therapeutic for CVD. We engineered a novel, bispecific receptor activator, NPA7 that represents the fusion of a 22-amino acid sequence of B-type natriuretic peptide (an endogenous ligand of pGC-A) with Angiotensin 1-7 (ANG1-7), the 7-amino acid endogenous activator of MasR. We assessed NPA7’s dual receptor activating actions in vitro (second messenger production and receptor interaction). Further, we performed an intravenous peptide infusion comparison study in normal canines to study its biological actions in vivo, including in the presence of a MasR antagonist. Our in vivo and in vitro studies demonstrate the successful synthesis of NPA7 as a bispecific receptor activator targeting pGC-A and MasR. In normal canines, NPA7 possesses enhanced natriuretic, diuretic, systemic and renal vasorelaxing and cardiac unloading properties. Importantly, NPA7s actions are superior to that of the individual native pGC-A or MasR ligands. These studies advance NPA7 as a novel bispecific designer peptide with potential cardiorenal therapeutic benefit for the treatment of CVD such as hypertension and heart failure.
Keywords: cardiovascular disease, bispecific peptide, cardiorenal therapeutics, multivalent therapy, Mas-receptor, particulate guanylyl cyclase A receptor
INTRODUCTION
With an estimated cost of $207 billion each year, cardiovascular disease (CVD) such as hypertension (HTN) and heart failure (HF) is regarded as a major health burden worldwide. In the United States alone, CVD is the leading cause of mortality in men and women from different ethnic backgrounds, accounting for 1 in every 4 deaths despite current optimal therapies and prevention strategies1,2. Moreover, current drug therapies, such as ACE inhibitors and angiotensin receptor blockers (ARB)s, have been shown to have relative efficacy in preventing the occurrence of CV events3. Thus, the development of innovative new drugs with novel modes of action is therefore a priority of great importance4.
An emerging strategy in drug discovery has been the design and development of bispecific therapeutics. Bispecific drugs target two independent signaling pathways, and aim to achieve therapeutic synergy that transcends the effects of single-pathway activation. As an example, this concept has been supported by the approval of the small molecule sacubitril/valsartan for chronic as well as acute HF which has also demonstrated efficacy in HTN5,6,7.
An increasingly recognized molecular target in cardiorenal therapeutics is the Mas-receptor (MasR). Studies have established that the MasR possesses anti-apoptotic, anti-inflammatory, vasodilatory, anti-thrombotic and AT1R antagonizing actions by activation via its ligand ANG1–7 and its second messenger cAMP8–11. This ANG1–7/MasR axis has also been reported to be cardiorenal protective in models of HTN, HF, diabetic nephropathy and obesity12–15. The therapeutic development of MasR has been limited however by the rapid in vivo degradation of ANG1–716.
A second molecular target that is well recognized to mediate cardiorenal protection in CVD, is the particulate guanylyl cyclase A (pGC-A) receptor and its second messenger cGMP, for which the cardiac hormones atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) are its endogenous ligands17,18. Indeed, activation of pGC-A/cGMP pathway mediates a number of key biological properties and includes natriuresis, diuresis, blood pressure (BP) lowering, inhibition of cardiomyocyte hypertrophy and fibroblast proliferation, browning of white adipocytes with enhanced energy utilization, suppression of inflammatory cytokines and T cells and inhibition of aldosterone19–24. Furthermore, chronic activation of pGC-A by adenoviral BNP gene delivery in spontaneously hypertensive rats reduced BP, protected against cardiac hypertrophy and diastolic dysfunction, while BNP knockout rats are characterized by HTN and end organ damage25,26. Moreover in humans with stable HF, chronic pGC-A augmentation with twice daily subcutaneously BNP injections improved cardiorenal function and clinical symptoms27. Together, these experimental and human studies continue to support the therapeutic benefit of long-term pGC-A enhancement for cardiorenal protection.
Based upon exploratory studies, we hypothesized that replacing the 9 amino acid N-terminus of BNP1–32 with the MasR agonist ANG1–7 would result in a novel peptide, we call NPA7, that successfully co-actives both pGC-A and MasR. We also hypothesized that NPA7 would possess enhanced systemic and renal vasodilating, natriuretic, diuretic and cardiac unloading properties that go beyond that mediated by either ANG1–7 or BNP alone. Thus, we report the design, synthesis, in vitro validation of NPA7, and in vivo biological actions of a first-in-class, bispecific designer peptide with potentially beneficial efficacy for the treatment of CVD such as HTN and HF.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files.
Design and synthesis of NPA7: first-in-class bispecific pGC-A receptor/MasR peptide
NPA7 was designed as a first-in-class bispecific peptide that fuses a 22 amino acid (AA) residue cassette of the human pGC-A receptor activator BNP with the 7 AA cassette sequence of the MasR agonist ANG1–7 (Supplemental figure S1). NPA7 was synthesized by Fmoc solid phase methods on preloaded Nα-Fmoc-Nim-trityl-L-Histidine Wang resin (Anaspec, Inc). The peptide chain was assembled from Nα-Fmoc protected AA on a Liberty Blue Microwave-Assisted Peptide Synthesizer (CEM Corp) according to the manufacturer’s synthesis protocols. The peptide and its side chain protecting groups were removed from the resin support by acid cleavage with a solution of 90% aqueous trifluoroacetic acid (TFA) containing 2.5% triisopropylsilane (v/v) for 2h at room temperature. The crude linear NPA7 peptide was purified by preparative RP-HPLC using an aqueous/acetonitrile gradient containing 0.1 % TFA (v/v) on a reverse-phase C18 column (Phenomenex Jupiter 15µ; 250 × 21.2 mm). The average mass of the purified linear NPA7 peptide was verified by ESI-TOF mass spectrometry analysis on an Agilent 6224 TOF LC/MS instrument (See Supplemental Figure S2A-B).
Following synthesis of NPA7, the HPLC purified linear NPA7 was oxidized with clear-ox™ resin (Peptides International) to form the cyclized ring between cysteine residues 8 and 24. Briefly, the peptide was mixed with the clear-ox™ resin at room temperature according to the manufacturer’s protocol for 12 h or until the resin developed a dark orange color indicating complete oxidation. The cyclized peptide was then repurified by RP-HPLC as described above. The average mass of the cyclized NPA7 peptide (obs. 3472.8 Da; calc. 3473.1 Da) was verified by ESI-TOF mass spectrometry (Supplemental Figure S2). Analytical RP-HPLC indicated that the purified cyclized peptide was greater than 96% in homogeneity.
In vitro experiments
To assess activation of MasR and pGC-A, we utilized HEK293 cells that stably overexpressed either the human pGC-A or human MasR.
pGC-A and MasR interaction with NPA7 by AlphaScreen luminescence receptor assay
NPA7 interaction with pGC-A and MasR was determined by the AlphaScreen luminescence proximity receptor assay as previously described with modifications28–30. The membrane protein fractions of transfected HEK cells and parental (non-transfected) cells were isolated by ProteoExtract Native Membrane Protein Extraction Kit (Calbiochem, San Diego, CA). Interaction of NPA7 with pGC-A was determined using reaction mixtures consisting of biotinylated NPA7 peptides (10−6 M), 1.25ug membrane protein fraction, 20ug/ml anti-GFP antibody-coated acceptor beads (PerkinElmer Life Sciences, Waltham MA) and 20ug/ml streptavidin-coated donor beads (PerkinElmer Life Sciences, Waltham MA) in a 0.1% BSA, 0.1% Tween-20 PBS buffer. Interaction of NPA7 to MasR was determined using reaction mixtures that consisted of NPA7 peptides (10−6 M), 1ug membrane protein fraction, 5 nM of rabbit anti-BNP polyclonal antibody (abCam, Cambridge MA), 10ug/ml anti-rabbit IgG antibody coated acceptor beads (PerkinElmer Life Sciences, Waltham MA) and 10ug/ml streptavidin-coated donor beads (PerkinElmer Life Sciences, Waltham MA).
pGC-A and MasR activation in transfected HEK293 cells
Cells overexpressing human pGC-A were treated with or without the pGC-A activator BNP or NPA7 (10−10, 10−8, and 10−6M) for 10 min to determine cGMP production (pGC-A second messenger). Cells overexpressing human MasR with or without the MasR activator ANG1–7 or NPA7 (10−7, 10−6, and 10−5M) for 10 min to determine cAMP production (MasR second messenger). Note concentrations of peptides used in the MasR overexpressing cells were higher than concentrations used in pGC-A cells as cAMP levels are lower intracellularly and higher concentrations of MasR activators are required for cAMP generation. 70–80% confluent cells at passages 2 through 6 were used for experiments. Briefly, cells were lysed and sonicated for 10 minutes after incubation with ligands. Cell lysates were centrifuged and supernatants were extracted, dried and reconstituted in 300 µL cGMP or cAMP were assayed using a RIA cGMP or RIA cAMP kit, as previously described31.
In vivo experiments
Cardiorenal actions of NPA7 in normal canines
Male mongrel canine studies were performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee. Sixteen male mongrel dogs (weight 22.0 – 30.0 kg) were assigned to infusion with one of the following: the MasR ligand ANG1–7 (n=5), the pGC-A ligand BNP (n=5) or NPA7 (n=6). Study drugs in each group were infused in equimolar doses, 8.66 pmol x kg−1 x min−1 (based upon previous studies from our laboratory infusing BNP in healthy canines32). Investigators were not blinded to treatments but biochemical analysis was performed by technicians who were blinded to treatment. Groups were not randomized but performed in serial fashion. Normal mongrel dogs were included if deemed healthy by the Institutional Veterinarian Department.
On the evening before the acute study, canines were fasted with ad libitum access to water. On the day of the experiment, canines were anesthetized with intravenous Pentobarbital (15–30 mg x kg−1) and Fentanyl (4–12 µg x kg−1), intubated and mechanically ventilated on air with supplemental oxygen, 3 L x min−1, 12 cycles x min−1 (Harvard Apparatus, Millis, MA). The right femoral artery was cannulated for measurement of mean arterial pressure (MAP) and for blood sampling. The right femoral vein was cannulated for intravenous (IV) infusion of inulin and normal saline or peptide. The left kidney was exposed through a left flank incision. The left ureter was cannulated for continuous urine collection. An electromagnet flow probe (Carolina Medical Electronics Inc., East Bend, NC) was placed on the left renal artery for measurement of renal blood flow (RBF). The right jugular vein was cannulated, and a Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA) was introduced for measurement of intracardiac pressures. Normal saline was infused at 1 mL x min−1 (infusion was temporarily discontinued during peptide infusion). After instrumentation, a 60-minutes equilibrium period followed, and a 30-minutes pre-infusion (baseline, BL) period was performed. BL was followed by a 45-minutes continuous infusion of Ang1–7, BNP or NPA7 [15 minutes lead-in drug infusion, 30 minutes clearance during drug infusion (DRUG)]. Directly after discontinuation of peptide infusion, a 30-minute wash-out (WO) clearance was started, followed by two 30-minute recovery clearances (REC1, REC2, respectively). During all clearances, hemodynamic parameters (IX/408 Data Acquisition System hardware, iWorks System, Inc., Dover, NH), intracardiac pressures were measured and urine was collected. Blood was drawn half-way through each clearance and replaced with equal volume of normal saline.
Cardiorenal actions of NPA7 in normal canines with MasR blockade
To better understand the biological effects of NPA7’s dual-receptor activating properties with a special focus on MasR, we performed a intravenous co-infusion study of NPA7 with the MasR antagonist (MasR-i) A779 (Sigma-Aldrich, St. Louis, MO). Six male mongrel canines (weight 22.5 – 30 kg) received co-infusion of 8.66 pmol x kg−1 x min−1 NPA7 and 5.83 microgram x kg−1 x hr−1 A779 with comparison to the NPA7 alone infusion canines as described above. Fasting of dogs, experimental set-up of acute study and data collection was done according to the abovementioned protocol. MasR-i was infused using a saphenous vein (0.2 mL/min).
Analyses of neurohumoral and renal function
All urine and blood samples were stored immediately on ice after collection. Following centrifugation (2500 rpm, at 4ºC for 10 minutes) plasma and serum were aliquoted and stored at −80°C until analysis. Plasma and urine cGMP were determined by radioimmunoassay (RIA) as described previously31,32. Plasma and urine electrolytes were analyzed on a flame photometer (model 1L943; Instrumentation Laboratory, Lexington, MA)31. Renal generation of cGMP was calculated as urine excretion of cGMP – (plasma cGMP x GFR).
Statistical analyses
Data from in vitro experiments are presented as mean±SEM. For in vitro studies, treatment-induced differences were analyzed using Student’s-t-test. For in vivo studies, differences between groups were analyzed using two way repeated measures analyses of variance (rANOVA) with Bonferroni’s test for multiple comparisons, and each group (ANG1–7, BNP, NPA7) was compared to the other two groups. Statistical comparison between NPA7 and NPA7 + MasR-i groups was also analyzed using two-way rANOVA with Bonferroni’s test for multiple comparisons. Within group differences were assessed with one-way rANOVA followed by Dunett’s test for multiple comparisons. Analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). A p-value of less than 0.05 was considered significant for all statistical analyses.
RESULTS
NPA7 interaction with pGC-A and MasR in HEK293 cells
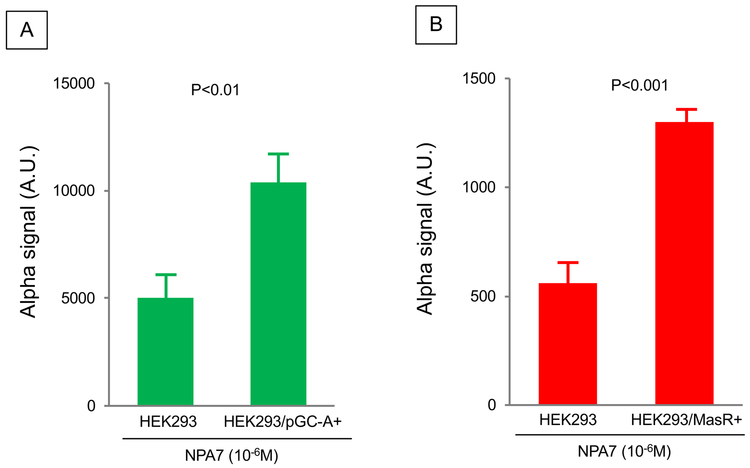
Figure 1A-B reports in vitro receptor interaction of NPA7 with pGC-A (and MasR in pGC-A and MasR overexpressing HEK293 cells, respectively. Background signal was observed in the control when adding NPA7 to parental (non-transfected) HEK293 cells. Importantly, there was significant increase from control in both pGC-A and MasR overexpressing cells with NPA7 thus demonstrating that NPA7 successfully interacts with pGC-A and MasR.
Figure 1.
In vitro receptor interaction of NPA7 with pGC-A and MasR in A) pGC-A++ HEK293 cells; B) MAS++ HEK293 cells.
NPA7 generates the second messengers cGMP and cAMP in HEK293 cells
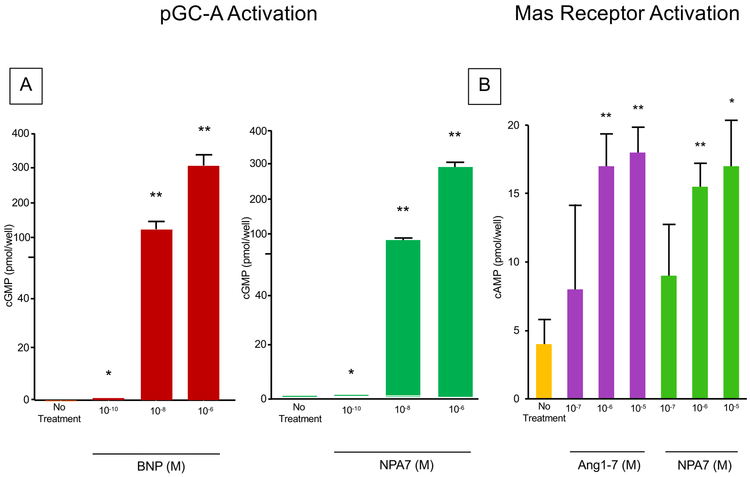
Figure 2A reports the action of pGC-A activation with no treatment and increasing concentrations of BNP or NPA7. Compared to no treatment, both BNP and NPA7 followed similar trends with significantly increasing concentrations of cGMP production with both BNP or NPA7 supporting the ability of both BNP and NPA7 to activate pGC-A.
Figure 2.
A. In vitro action of BNP and NPA7 on generation of the second messenger of the pGC-A receptor (cGMP). *P<0.05 vs No Treatment; **P<0.01 vs No Treatment;
B. In vitro action of ANG1-7 and NPA7 on generation of the second messenger of MasR (cAMP). *P<0.05 vs No Treatment; **P<0.01 vs No Treatment.
Figure 2B reports the action of MasR activation with no treatment and increasing concentrations of ANG1–7 or NPA7. Compared to no treatment, both ANG1–7 and NPA7 followed similar trends with significantly increasing concentrations of cAMP production with both ANG1–7 or NPA7 supporting the ability of both ANG1–7 and NPA7 to activate MasR.
NPA7 has superior cGMP, hemodynamic, natriuretic, and diuretic actions in normal canines as compared to pGC-A or MasR activation alone in vivo
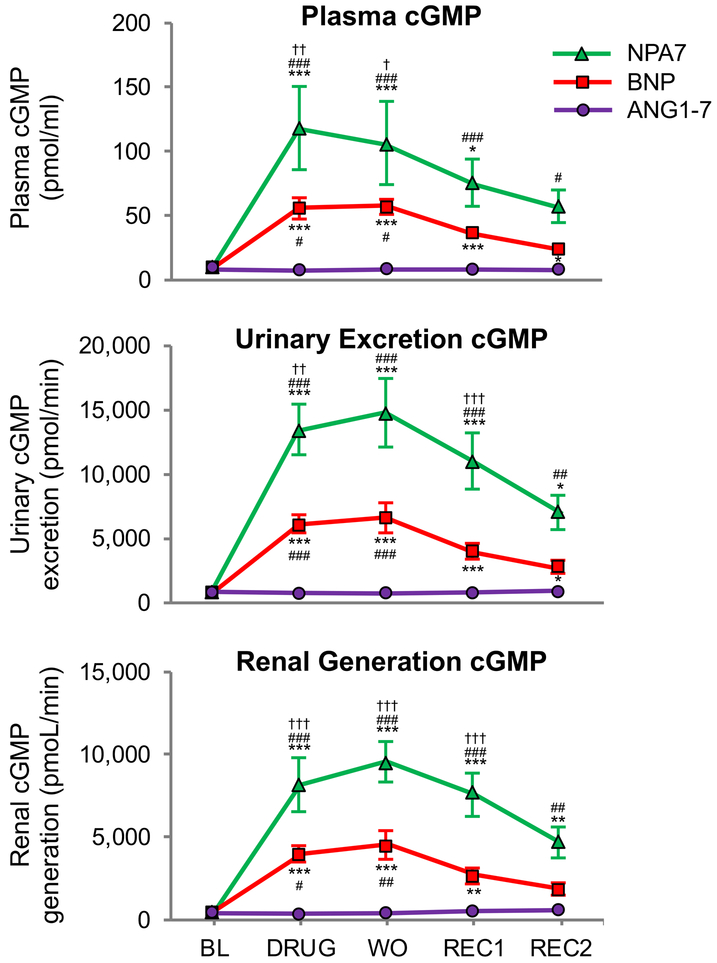
We measured cGMP production in vivo resulting from MasR activation with ANG1–7, pGC-A activation with BNP and dual activation with NPA7 infusion. At a dose of 8.66 pmol x kg−1 x min−1, both BNP and NPA7 significantly activated plasma cGMP, urinary cGMP excretion and renal cGMP generation. Importantly, NPA7 was the most potent generator of cGMP in normal canines, increasing cGMP production to the greatest magnitude compared to an equimolar dose of the endogenous pGC-A activator BNP (Figure 3). This was observed for plasma cGMP, urinary cGMP excretion and renal generation of cGMP.
Figure 3.
Second messenger cGMP generation in normal canines treated with ANG1-7, BNP and NPA7; † P<0.05 vs BNP, #P<0.05 vs ANG1-7, *P<0.05 vs baseline; ††P<0.01 vs BNP, ##P<0.01 vs ANG1-7, **P<0.01 vs baseline ; †††P<0.001 vs BNP, ###P<0.001 vs ANG1-7, ***P<0.001 vs baseline BL= Baseline; DRUG= drug-infusion; WO= wash-out (15 min after DRUG); REC1= recovery 1 (45 min after DRUG); REC2= recovery 2 (75 min after DRUG).
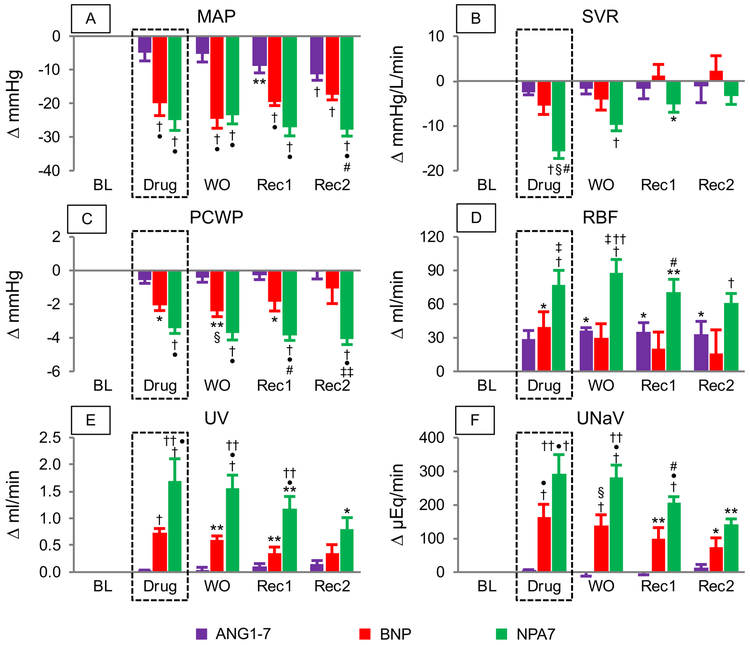
At baseline, hemodynamic and renal functions were comparable among all 3 treatment groups (Supplemental Table S1). During drug infusion, ANG1–7 did not reduce MAP, and during recovery clearances this MasR-activating peptide had a moderate MAP lowering effect. Moreover, as compared to BNP and NPA7 the reduction in MAP was modest (Figure 4A). However pGC-A activation with BNP reduced MAP significantly, while the effect of NPA7 on MAP was prolonged compared to BNP infusion (Figure 4A). In normal canines, NPA7 was the only peptide that showed a significant decrease in systemic vascular resistance (SVR) (Figure 4B). In addition, NPA7 possessed stronger and longer lasting cardiac unloading actions than ANG1–7 or BNP on pulmonary capillary wedge pressure (PCWP) (Figure 4C).
Figure 4.
Hemodynamic and renal actions of equimolar doses of ANG1-7, BNP and NPA7 infusion in normal canines; *P<0.05 baseline (BL) change; **P<0.01 BL; †P<0.001 BL change; ‡P<0.05 vs ANG1-7; §P<0.01 vs ANG1-7; •P<0.001 vs ANG1-7; #P<0.05 vs BNP; ††P<0.01 vs BNP; ‡‡P<0.001 vs BNP. BL= Baseline; DRUG= drug-infusion; WO= wash-out (1-30 min after DRUG); REC1= recovery 1 (31-60 min after DRUG); REC2= recovery 2 (61-90 min after DRUG).
Despite the observed reduction in MAP, infusion with NPA7 significantly increased RBF during infusion, wash-out and recovery phase (Figure 4D). Moreover, NPA7 possessed potent natriuretic and diuretic actions, which were significantly stronger than observed in single ANG1–7 or BNP infusion (Figure 4E-F). GFR was unchanged from baseline in all groups.
MasR antagonism with the MasR-i A779 attenuates NPA7 mediated blood pressure lowering, cardiac unloading, systemic vasorelaxation, natriuresis and diuresis
Addition of A779 to NPA7 infusion significantly reduced plasma cGMP levels, urinary cGMP excretion and renal cGMP generation (Table 1). Figure 5 reports the in vivo hemodynamic responses to MasR-i infusion in normal canines. A779 significantly attenuated responses in MAP and SVR (Figure 5A and B) and also resulted in less cardiac unloading, as represented by significantly less reduction in right atrial pressure (RAP, Figure 5C) and PCWP (Figure 5D). MasR blockade also attenuated NPA7 induced diuresis and natriuresis (Figure 5E-F).
Table 1.
Second Messenger Cyclic-GMP Activation with NPA7 or NPA7 + MasR-inhibitor A779
| Pre- Infusion |
Infusion | Post-infusion | |||
|---|---|---|---|---|---|
| DRUG | |||||
| BL | DRUG | WO | REC1 | REC2 | |
| Plasma cGMP, pmol/mL−1 | |||||
| NPA7 | 9±1 | 119±32* | 107±33* | 77±18† | 58±13 |
| NPA7 + A779 | 7±1 | 15±2*§ | 19±3*∥ | 12±1† | 9±1 |
| Urinary cGMP excretion, pmol/mL−1 | |||||
| NPA7 | 846±121 | 13477±1943* | 14801±2392* | 11003±2006* | 7032±1198‡ |
| NPA7 + A779 | 706±80 | 1783±316†§ | 2039±388†§ | 1646±399‡§ | 1206±357‡ |
| Renal cGMP generation, pmol/mL−1 | |||||
| NPA7 | 469±96 | 8233±1605* | 9630±1119* | 7612±1199* | 4677±845‡ |
| NPA7 + A779 | 453±59 | 1133±196§ | 1269±254‡§ | 1123±306§ | 854±210¶ |
NPA7 and NPA7 + MasR-inhibitor A779 groups were analyzed using two-way rANOVA with Bonferroni’s test for multiple comparisons. Within group differences were assessed with one-way rANOVA followed by Dunett’s test for multiple comparisons.
p<0.001 baseline (BL) change;
p<0.01 BL change;
p<0.05 BL change;
p<0.001 vs NPA7;
p<0.01 vs NPA7;
p<0.05 vs NPA7. BL= Baseline; DRUG= drug-infusion; WO= washout; REC1= recovery 1; REC2= recovery 2.
REC2= recovery 2 (61-90 min after DRUG).
Figure 5.
In vivo responses to NPA7 and NPA7+MasR-i infusion in normal canines; *P<0.05 baseline (BL) change; **P<0.01 BL change; †P<0.001 BL change; ‡P<0.05 vs NPA7; §P<0.01 vs NPA7; •P<0.001 vs NPA7. BL= Baseline; DRUG= drug-infusion; WO= wash-out (1-30 min after DRUG); REC1= recovery 1 (31-60 min after DRUG);
DISCUSSION
In this study we report, for the first time, the design, synthesis, in vitro receptor interaction and the generation of its second messenger as well as the in vivo cardiorenal actions of a novel, bispecific receptor activating peptide, NPA7. Here, we validated receptor interaction and activation of NPA7 to both pGC-A and MasR, and showed in vivo that NPA7 is biologically active with potent and more sustained cardiorenal actions that go beyond MasR or pGC-A alone. Moreover, blockade of MasR attenuated NPA7 mediated cGMP activation, as well as the hemodynamic, natriuretic and diuretic responses in vivo. The present findings are the first results of a novel and unique MasR activator that possesses ANG1–7 properties, hence generating in vitro and in vivo actions that represent alternative renin-angiotensin system (RAS) activation. Our findings also report a MasR activating peptide that also functions as a pGC-A activator.
In this study we demonstrate receptor interaction in vitro with the alpha luminesce assay of NPA7 in HEK293 cells overexpressing either human pGC-A or human MasR. These receptor interaction studies confirm NPA7’s dual receptor system activating properties. Further validation of activation of both receptors in vitro by was investigated by quantifying generation of each receptor’s second messenger with NPA7. Specifically, the cGMP and cAMP generating actions of NPA7 followed similar trends to increasing concentrations of the native ligands BNP for pGC-A and ANG1–7 for MasR reinforcing the bispecific properties of NPA7 to co-activate both receptors. Further studies will be needed to further establish that NPA7 does not also bind to AT1 and/or AT2 based upon the structure of NPA7 which includes the ANG1–7 sequence in the N-terminus.
In normal canines, IV infusion with NPA7 resulted in superior diuretic and natriuretic effects, as well as systemic and renal vasodilation and cardiac unloading together with greater cGMP production than either pGC-A or MasR activation alone. Importantly, this favorable cardiorenal profile is NPA7-specific and transcended the effects of single pGC-A activation with BNP or MasR stimulation with ANG1–7 treatment alone, underscoring the importance of unique design and ability of NPA7 to activate two protective receptor systems. Indeed, after addition of the MasR-i A779, NPA7 lost its unique cardiorenal profile with significant blunting of its vasodilatory, cardiac unloading, and diuretic and natriuretic actions. The remaining effects were less effective than observed during BNP infusion, suggesting a synergistic manner of NPA7’s receptor activation, again emphasizing the significance of the unique mode of action of this innovative designer peptide. Indeed, the demonstration of the potent in vivo cardiorenal actions of NPA7 linked to MasR, based upon MasR blockade, suggests that NPA7 may serve as a carrier or chaperone for ANG1–7 possibly preventing rapid degradation as occurs with ANG1–7 alone, thus resulting in robust MasR activation and actions. An additional potential mechanism warranting further studies is the possibility of dimerization of pGC-A and MasR with synergistic actions during co-activation. Indeed, this concept is supported by studies by Leonhardt and co-workers, which provided evidence for heterodimerization and functional interaction of the AT2 receptor, and MasR33. While the enhanced cardiorenal actions of NPA7 compared to BNP and ANG1–7 alone are novel and important, one must emphasize that more chronic studies are needed to establish that such actions are sustained over time.
The unique and striking generation in vivo of cGMP in plasma, urine and the kidney warrants comments. Cyclic GMP has emerged as an important target for CV therapeutics based upon its pleotropic properties in multiple organs and cells. Its generation results from stimulation of membrane bound pGC-A that we believe is activated by NPA7. Nevertheless, the mechanism(s) of the more sustained increases in cGMP with NPA7 warrants further investigations to examine its full potential as novel therapeutic.
For decades, treatment of CVD such as HTN, HF and most recently metabolic disease, has relied upon the beneficial effects of targeting a single-receptor system associated with the underlying pathology. However, the remarkable results with sacubitril/valsartan, a small molecule that inhibits NEP and AT1R, has provided rationale for the design and development of innovative therapeutics that work on two different receptor systems5,34. Further, recent elegant studies have reported that neprilysin (NEP) is the principal pathway generating ANG1–7 in the murine and human kidney and thus inhibition of NEP could reduce the generation of ANG1–7 and limit its renoprotection35. Nonetheless, the promising results of sacubitril/valsartan that antagonizes the AT1R and NEP support the concept of bispecific therapeutics that targets multiple receptor systems has significant efficacy, especially related to the natriuretic peptide system and RAS. In the current study we have successfully engineered a first-in-class bispecific peptide that directly targets the both of these pathways.
Human genetic epidemiology studies underscore potential important beneficial effects for both ANP and BNP which activate pGC-A since individuals with genetic variants associated with higher circulating levels of ANP and BNP have lower BP, reduced risk of HTN, protection from metabolic syndrome and increased survival36–38. Further, we recently reported the use of AAV9 vector as a gene delivery strategy that allowed long-term (9 months) BNP overexpression in spontaneously hypertensive rats25. Specifically, chronic increases in circulating BNP reduced BP and improved myocardial structure and function. Chen et al also reported that chronic subcutaneously administered BNP in human HF improved symptoms and myocardial structure and function27. Thus, targeting of the pGC-A receptor pathway remains therefore an important therapeutic target for development of novel CV therapeutics.
The MasR activator ANG1–7 is a RAS modulating peptide with an extremely short half-life time, which has limited clinical utility because of its rapid in vivo degradation16. This heptapeptide counter-regulates the deleterious effects of ANG II, and induces vasodilatation and enhances sodium excretion, while promoting anti-apoptotic, anti-inflammatory, anti-thrombotic and anti-fibrotic actions10,39,40. Recently, favorable metabolic actions of ANG1–7/MasR have been reported which include protection from obesity-induced HTN in rodents and induction of brown adipocyte differentiation with upregulation of thermogenesis and improved metabolic profile in diet-induced obesity15. The production of ANG1–7 is highly dependent on degradation of ANG II by ACE2, although ANG1–7 can also be formed in an ANG I / ANG1–9 -dependent manner, that in part is also dependent upon NEP42–45. Importantly, ANG1–7 specifically binds to the G-protein coupled MasR through which it exerts G-protein coupled receptor (GPCR) specific actions such as PKA/cAMP pathway activation46 and NO production47. In addition, ANG1–7 may exert cardioprotective effects via inhibition of cardiodeleterious ANG II/AT1R-dependent G-protein signaling and concomitant selective activation of the cardiorenoprotective β-arrestin pathway48,49. Current CV therapeutic treatment largely focuses on inhibition of the RAS and targeting ANG II or AT1R. However, inhibition of the hypertensive axis of this system has only limited efficacy, and it has therefore been suggested that activation of the alternative, protective arm of the RAS with ANG1–7 may render an attractive next generation therapeutic intervention50–52. We now present the first results of a novel therapeutic MasR activator that possesses ANG1–7 properties, hence generating in vitro and in vivo actions that represent activation of the so called “alternative RAS” with synergistic properties when molecularly integrated with a pGC-A activator.
The current study has implications for the treatment of CVD, especially for those disease states in which RAS activation plays a pivotal role (i.e. HTN and HF). Patients with HTN, and also patients with HF, have disturbed neurohumoral balance, which is characterized by NP-deficiency and RAS activation53–55. Treatment with drugs that target both the pGC-A receptor system and MasR may thus have the potential to restore neurohumoral balance in HTN and HF. Importantly, several recent studies have shown that chronic pGC-A receptor activation, even in preclinical HF, is associated with improved cardiorenal function, left ventricular function and/or structure, and overall clinical outcomes27,56,57. Other studies showed that long-term treatment with ANG1–7 significantly improved cardiac function in diabetic mice, and prevented cardiomyocyte hypertrophy, apoptosis and fibrosis in diabetic mice and rats overexpressing the mouse Ren2-gene13,14,58,59. Hence, NPA7 may not only improve hemodynamic function, diuresis and natriuresis in the short term, but may have wide potential application in CV, renal and metabolic diseases when chronically used. Thus, future studies are needed to address the full therapeutic potential of this first in class peptide and the concept of optimizing therapy with bivalency.
This study has both strengths and limitations. The strengths include: 1) the use of human amino acid sequences of both human BNP (pGC-A ligand) and human ANG1–7 (MasR ligand) to design and synthesize NPA7 as a bispecific peptide; 2) in vitro binding and second messenger studies supporting bispecific receptor activating properties and; 3) in vivo studies demonstrating cardiorenal actions of NPA7 that extend beyond BNP or ANG1–7 alone and attenuation of these actions with a MasR blocker. There are important limitations as well, which should lay the foundation for future studies. Such studies should include validating the lack of binding and absence of activation of AT1 and AT2 receptor by NPA7 recognizing amino acid homologies with ANGII. Moreover, additional in-depth receptor pharmacological experiments are warranted, which can provide intriguing insights on the cross talk between the pGC-A receptor and the angiotensin receptor family.
In conclusion, we report the design, synthesis, in vitro pharmacology and in vivo cardiorenal actions of the innovative, first-in-class, bispecific peptide NPA7. This chimera represents a pGC-A receptor activator together with a MasR activator that directly potentiates two different receptor systems. NPA7 has potent and sustained cGMP generating properties, including superior vasorelaxation, cardiac unloading and natriuretic and diuretic actions. Our findings are significant and demonstrate the successful fusion of two different receptor-activators into one peptide structure that now possesses favorable and clinically relevant cardiorenal actions. Altogether, NPA7 may address the high and unmet need of a novel, bispecific cardiorenoprotective therapeutic for treatment of CVD such as HTN and HF.
Supplementary Material
PERSPECTIVES:
NPA7 represents the first-in-class bispecific designer peptide which simultaneously targets the particulate guanylyl cyclase A (pGC-A) receptor and the Mas-receptor (MasR).
In vivo, NPA7s actions are superior to that of single pGC-A or MasR ligands.
NPA7 has cardiorenal therapeutic treatment potential in CVD, especially hypertension and heart failure.
NOVELTY AND SIGNIFICANCE:
What Is New:
Design and biological actions of a new first in class drug called NPA7
This first in class novel drug is a bispecific which targets two molecular signaling pathways
What Is Relevant:
NPA7 targets the receptors (pGC-A and MasR) of both the natriuretic peptide system and the alternative renin-angiotensin system respectively which are important in blood pressure regulation
This novel drug called NPA7 may represent an innovative drug to treat hypertension
Summary:
NPA7 is a novel new bispecific peptide therapeutic for CVD which targets the pGC-A receptor and MasR which may represent a potential innovative new therapeutic for hypertension.
ACKNOWLEDGEMENTS
We would like to thank M. Durik for his excellent help and support.
SOURCES OF FUNDING
L.M.G.M. received a research grant from ICIN - Netherlands Heart Foundation. J.C.B has received research grants from NIH (RO1 HL36634 and HL134688), Minnesota Regeneration Medicine Program and the Harrington Discovery Institute.
Footnotes
DISCLOSURES
There are no conflicts of interest.
REFERENCES
- 1.Heron M Deaths: Leading causes for 2008. Natl Vital Stat Rep. 2012;60:1–94. [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 3.Reyes S, Varagic J, Ahmad S, VonCannon J, Kon ND, Wang H, Groban L, Cheng CP, Dell’Italia LJ, Ferrario CM. Novel cardiac intracrine mechanisms based on Ang-(1–12)/chymase axis require a revision of therapeutic approaches in human heart disease. Curr Hypertens Rep. 2017;19(2):16.1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meems LMG, Burnett JC Jr. Innovative therapeutics: Designer natriuretic peptides. JACC: Basic to Translational Science. 2016;1:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: Rationale for and design of the prospective comparison of arni with acei to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15:1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomized, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–1266. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCaque K, Rocha R, Braunwald E, PIONEER-HF Investigators. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2018;November 11: DOI: 10.1056/NEJMoa1812851. [DOI] [Google Scholar]
- 8.Ferrario CM, Brosnihan KB, Diz DI, Jaiswal N, Khosla MC, Milsted A, Tallant EA. Angiotensin-(1–7): A new hormone of the angiotensin system. Hypertension. 1991;18:III126–133. [DOI] [PubMed] [Google Scholar]
- 9.Trask AJ, Ferrario CM. Angiotensin-(1–7): Pharmacology and new perspectives in cardiovascular treatments. Cardiovasc Drug Rev. 2007;25:162–174. [DOI] [PubMed] [Google Scholar]
- 10.Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the g protein-coupled receptor mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraga-Silva R, Pinheiro S, Goncalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1–7) involves Mas-mediated NO release from platelets. Mol Med. 2008;14(1–2):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Twist DJ, Kroon AA, de Leeuw PW. Angiotensin-(1–7) as a strategy in the treatment of hypertension? Curr Opin Nephrol Hypertens. 2014. September;23 (5):480(5):480–486. [DOI] [PubMed] [Google Scholar]
- 13.Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Angiotensin 1–7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail. 2014;7:327–339. [DOI] [PubMed] [Google Scholar]
- 14.Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, Lopaschuk GD, Oudit GY. Angiotensin 1–7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol. 2014;306:F812–821. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto H, Mori J, Kawabe Y, Tsuma Y, Fukuhara S, Kodo K, Ikoma K, Matoba S, Oudit GY, Hosoi H. Angiotensin 1–7 stimulates brown adipose tissue and reduces diet induced obesity. Am J Physiol Endocrinol Metab. 2017;doi: 01152/ ajpendo .00192.2017. [DOI] [PubMed] [Google Scholar]
- 16.Iusuf D, Henning RH, van Gilst WH, Roks AJ. Angiotensin-(1–7): Pharmacological properties and pharmacotherapeutic perspectives. Eur J Pharmacol. 2008;585:303–312. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn M Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev. 2016;96:,751–804. [DOI] [PubMed] [Google Scholar]
- 18.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. [DOI] [PubMed] [Google Scholar]
- 19.Burnett JC Jr, Granger JP, Opgenorth TJ. Effects of synthetic atrial natriuretic factor on renal function and renin release. Am J Physiol. 1984;247(5 Pt 2):F863–F866. [DOI] [PubMed] [Google Scholar]
- 20.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111(9):1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuruda T, Boerrigter G, Huntley BK, Noser JA, Cataliotti A, Costello-Boerrigter LC, Chen HH, Burnett JC Jr. Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;13;91(12):1127–1134. [DOI] [PubMed] [Google Scholar]
- 22.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzhani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vellaichamy E, Kaur K, Pandey KN. Enhanced activation of pro-inflammatory cytokines in mice lacking natriuretic peptide receptor-A. Peptides. 2007;28(4):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L, Li J, Wang G, Gong S, Zhang L, Li K, Ji X, Liu Y, Chen P, Xiang X. Atrial natriuretic peptide suppresses Th17 development through regulation of cGMP-dependent protein kinase and PI3K–Akt signaling pathways. Reg Peptides. 2013;181:9–16. [DOI] [PubMed] [Google Scholar]
- 25.Cataliotti A, Tonne JM, Bellavia D, Martin FL, Oehler EA, Harders GE, Campbell JM, Peng KW, Russell SJ, Malatino LS, Burnett JC Jr, Ikeda Y. Long-term cardiac pro-b-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation. 2011;123:1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holditch SJ, Schreiber CA, Nini R, Tonne JM, Peng KW, Geurts A, Jacob HJ, Burnett JC, Cataliotti A, Ikeda Y. B-type natriuretic peptide deletion leads to progressive hypertension, associated organ damage, and reduced survival: novel model for human hypertension. Hypertension. 2015;66(1):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC Jr., Novel protein therapeutics for systolic heart failure: Chronic subcutaneous b-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhi X, Zhou XE, Melcher K, Motola DL, Gelmedin V, Hawdon J, Kliewer SA, Mangelsdorf DJ, Xu HE. Structural conservation of ligand binding reveals a bile acid-like signaling pathway in nematodes. J Biol Chem. 2012;287:4894–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Choi M, Suino K, Kovach A, Daugherty J, Kliewer SA, Xu HE. Structural and biochemical basis for selective repression of the orphan nuclear receptor liver receptor homolog 1 by small heterodimer partner. Proc Natl Acad Sci U S A. 2005;102:9505–9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou XE, Suino-Powell KM, Xu Y, Chan CW, Tanabe O, Kruse SW, Reynolds R, Engel JD, Xu HE. The orphan nuclear receptor tr4 is a vitamin a-activated nuclear receptor. J Biol Chem. 2011;286:2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichiki T, Huntley BK, Sangaralingham SJ, Burnett JC Jr., Pro-atrial natriuretic peptide: A novel guanylyl cyclase-a receptor activator that goes beyond atrial and b-type natriuretic peptides. JACC Heart Fail. 2015;3:715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Huntley BK, Cataliotti A, Lapp H, Burnett JC Jr. B-type natriuretic peptide 8–32, which is produced from mature BNP 1–32 by the metalloprotease meprin a, has reduced bioactivity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonhardt J, Villela DC, Teichmann A, Münter LM, Mayer MC, Mardahl M, Kirsch S, Namsolleck P, Lucht K, Benz V, Alenina N, Daniell N, Horiuchi M, Iwai M, Multhaup G, Schülein R, Bader M, Santos RA, Unger T, Steckelings UM. Evidence for heterodimerization and functional interaction of the angiotensin type 2 receptor and the receptor MAS. Hypertension. 2017. June;69(6):1128–1135. [DOI] [PubMed] [Google Scholar]
- 34.Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 35.Domenig O, Manzel A, Grobe N, Königshausen E, Kaltenecker CC, Kovarik JJ, Stegbauer J, Gurley SB, van Oyen D, Antlanger M, Bader M, Motta-Santos D, Santos RA, Elased KM, Säemann MD, Linker RA, Poglitsch M. Neprilysin is a mediator of alternative renin-angiotensin-system activation in the murine and human kidney. Sci Rep. 2016;21;6:33678..1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genetics. 2009;41:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cannone V, Boerrigter G, Cataliotti A, Costello-Boerrigter LC, Olson TM, McKie PM, Heublein DM, Lahr BD, Bailey KR, Averna M, Redfield MM, Rodeheffer RJ, Burnett JC Jr. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll Cardiol 2011;58:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seidelmann SB, Vardeny O, Claggett B, Yu B, Shah AM, Ballantyne CM, Selvin E, MacRae CA, Boerwinkle E, Solomon SD. An NPPB promoter polymorphism associated with elevated N-terminal pro-B-type natriuretic peptide and lower blood pressure, hypertension, and mortality. J Am Heart Assoc. 2017;6: e005257 DOI: 10.1161/JAHA.116.005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and mas: New players of the renin-angiotensin system. J Endocrinol. 2013;216:R1–R17. [DOI] [PubMed] [Google Scholar]
- 40.Ferrario CM. Importance of the renin-angiotensin-aldosterone system in the physiology and pathology of hypertension. an overview. Drugs. 1990;39 Suppl 2:1–8. [DOI] [PubMed] [Google Scholar]
- 41.Morimoto H, Mori J, Kawabe Y, Tsuma Y, Fukuhara S, Kodo K, Ikoma K, Matoba S, Oudit GY, Hosoi H. Angiotensin 1–7 stimulates brown adipose tissue and reduces diet induced obesity. Am J Physiol Endocrinol Metab. 2017;doi: 01152/ ajpendo .00192.2017. [DOI] [PubMed] [Google Scholar]
- 42.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ace2) converts angiotensin 1 to angiotensin 1–9. Circ Res. 2000;87:E1–9. [DOI] [PubMed] [Google Scholar]
- 44.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. [DOI] [PubMed] [Google Scholar]
- 45.Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. [DOI] [PubMed] [Google Scholar]
- 46.Tassone EJ, Sciacqua A, Andreozzi F, Presta I, Perticone M, Carnevale D, Casaburo M, Hribal ML, Sesti G, Perticone F. Angiotensin (1–7) counteracts the negative effect of angiotensin 2 on insulin signalling in HUVECS. Cardiovasc Res. 2013;99:129–136. [DOI] [PubMed] [Google Scholar]
- 47.Sampaio WO, Santos RA, Faria-Silva R, Machado LT, Schiffrin EL, Touyz RM. Angiotensin 1–7 through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent mechanisms. Hypertension. 2007;49:185–192. [DOI] [PubMed] [Google Scholar]
- 48.Galandrin S, Denis C, Boularan C, Marie J, M’Kadmi C, Pilette C, Dubroca C, Nicaise Y, Sequelas MH, N’Guyen D, Banères JL, Pathak A, Sénard JM, Galés C. Cardioprotective angiotensin-(1–7) peptide acts as a natural-biased ligand at the angiotensin 2 type 1 receptor. Hypertension. 2016;68:1365–1374. [DOI] [PubMed] [Google Scholar]
- 49.Kamal FA, Travers JG, Schafer AE, Ma Q, Devarajan P, Blaxall BC. G protein-coupled receptor-g-protein betagamma-subunit signaling mediates renal dysfunction and fibrosis in heart failure. J Am Soc Nephrol. 2017;8:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C. Angiotensin-converting enzyme 2 and angiotensin 1–7: Novel therapeutic targets. Nat Rev Cardiol. 2014;11:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicklin SA. A novel mechanism of action for angiotensin-(1–7) via the angiotensin type 1 receptor. Hypertension. 2016;68:1342–1343. [DOI] [PubMed] [Google Scholar]
- 52.Santos RA. Angiotensin-(1–7). Hypertension. 2014;63:1138–1147. [DOI] [PubMed] [Google Scholar]
- 53.Macheret F, Heublein D, Costello-Boerrigter LC, Boerrigter G, McKie P, Bellavia D, Mangiafico S, Ikeda Y, Bailey K, Scott CG, Sandberg S, Chen HH, Malatino L, Redfield MM, Rodeheffer R, Burnett J Jr, Cataliotti A. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J Am Coll Cardiol. 2012;60(16): 1558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cannone V, Buglioni A, Sangaralingham SJ, Scott C, Bailey KR, Rodeheffer R, Redfield MM, Sarzani R, Burnett JC Jr. Aldosterone, hypertension, and antihypertensive therapy: insights from a general population. Mayo Clin Proc. 2018;93(8):980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davogustto G, Wang TJ, Gupta DK. Untangling essential hypertension: the potential roles of aldosterone and atrial natriuretic peptide. Mayo Clin Proc. 2018;93(8);965–967. [DOI] [PubMed] [Google Scholar]
- 56.Wan SH, McKie PM, Schirger JA, Slusser JP, Hodge DO, Redfield MM, Burnett JC Jr, Chen HH. Chronic peptide therapy with b-type natriuretic peptide in patients with pre-clinical diastolic dysfunction (stage b heart failure). JACC Heart Fail. 2016;4:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKie PM, Schirger JA, Benike SL, Harstad LK, Slusser JP, Hodge DO, Redfield MM, Burnett JC Jr, Chen HH. Chronic subcutaneous brain natriuretic peptide therapy in asymptomatic systolic heart failure. Eur J Heart Fail. 2016;18:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kangussu LM, Guimaraes PS, Nadu AP, Melo MB, Santos RA, Campagnole-Santos MJ. Activation of angiotensin-(1–7)/mas axis in the brain lowers blood pressure and attenuates cardiac remodeling in hypertensive transgenic (mren2)27 rats. Neuropharmacology. 2015;97:58–66. [DOI] [PubMed] [Google Scholar]
- 59.Papinska AM, Soto M, Meeks CJ, Rodgers KE. Long-term administration of angiotensin (1–7) prevents heart and lung dysfunction in a mouse model of type 2 diabetes (db/db) by reducing oxidative stress, inflammation and pathological remodeling. Pharmacol Res. 2016;107:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.