Abstract
The demographics of patients undergoing heart transplantation in the United States have shifted over the last ten years, with an increasing number of racial and ethnic minorities undergoing heart transplant. Multiple studies have shown that survival of African American patients after heart transplantation is lower when compared to other ethnic groups. Here we review the data supporting the presence of this outcome disparity and examine the multiple mechanisms that contribute. With an increasingly diverse population in the United States, knowledge of these disparities, their mechanisms, and ways to improve outcomes is essential.
Keywords: Cardiomyopathy, Heart transplantation, Health disparities
Introduction
Heart transplantation (HT) remains the therapy of choice for patients with end-stage heart failure (HF), offering the best quality of life and long-term survival. However, the benefit of HT is not uniform, with outcomes data demonstrating that certain racial/ethnic minorities have inferior post-transplant survival when compared to Caucasians.1–3 The disparity in outcomes in minority patients is not unique to HT, as minority patients undergoing kidney and liver transplantation also have inferior survival at 3 years post-transplant.4 The exact mechanisms for these disparities remain unclear; however, suggested explanations include socioeconomic, immunologic, and pharmacogenetic factors. Understanding the risk factors associated with worse survival for racial/ethnic minorities after transplantation is imperative to improve outcomes and eliminate disparities.
The use of race as a proxy for disease risk
The concept of categorizing persons on the basis of race is relatively recent in human history, originating from European exploration during the 16th century.5 Recent application of modern molecular genetic techniques has shown that there is more genetic variation within persons of the same self-reported ethnic group than between persons of different ethnic groups.6,6 Thus, the concept of race as a general classifier for genetic differences has been invalidated, and represents a social construct, not a biological concept.7, 8 However, persons of similar ethnogeographic origin do share specific genetic markers, including microsatellites and single nucleotide polymorphisms (SNP), all of which interact with the environment to contribute to susceptibility to certain diseases.9, 10 Consequently, race/ethnicity is widely used as a marker for examining differences in the prevalence of medical conditions, including cardiometabolic diseases that contribute to the development of end-stage HF. For example, African Americans have an increased risk of HF in the setting of hypertension, but have a markedly decreased risk of developing aortic stenosis.11, 12
The United States (US) Office of Management and Budget recognizes five racial categories: Caucasian, Black or African American, American Indian or Alaska Native, Asian, and Native Hawaiian or Other Pacific Islander. Hispanic/Latino background is considered to be an ethnicity. Within the US, there is marked regional variation in the prevalence of minority racial/ethnic groups (Figure 1). Furthermore, important demographic changes continue to impact the US, with Hispanic-Latinos becoming the most populous racial/ethnic minority group between the 2000 and 2010 US census.13 The increasing diversity in the US population mandates that research efforts reflect this diversity, and address methods to improve outcomes for all racial/ethnic groups after HT. Here we comprehensively review the evidence for racial/ethnic disparities in outcomes after HT, we examine the multiple mechanisms that contribute to these disparities, and discuss potential targets for new interventions and research priorities.
Figure 1. States with greater than average racial/ethnic minority population.
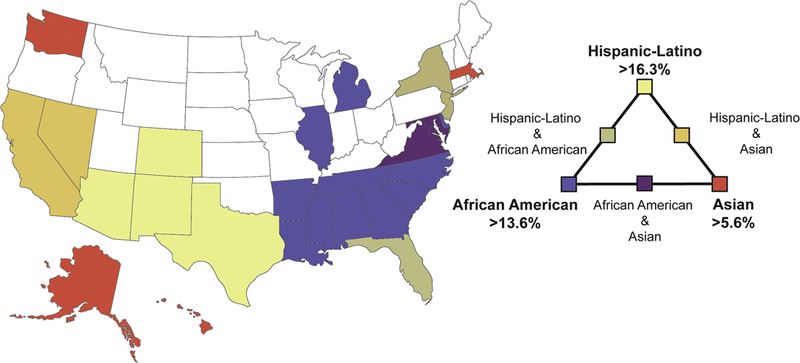
In this map of the US, states are colored if their population of Hispanic/Latino, African American, or Asian persons is greater than the national mean for that group (mean for Hispanic/Latino 16.3%, African American 13.6%, and Asian 5.6%). States with intermediate colors contain two groups at greater than the national average.
Racial differences in HT waiting list characteristics and mortality
Differential access to health care and differential care within the health system are significant, as minority groups are less likely to receive counseling regarding transplant options and to be referred for transplant evaluation.4 Despite these disadvantages, the 2012 United Network for Organ Sharing (UNOS) annual data report confirms that the number of racial/ethnic minorities awaiting HT continues to increase, with the proportion of non-Caucasians increasing from 22.9% to 31.6% over the past decade.14 African Americans accounted for 21.7% of patients on the waiting list for HT in 2012, while Hispanic-Latinos and Asians accounted for 7.1% and 2.1%, respectively. Prior studies have identified important clinical characteristics that differ between Caucasian and non-Caucasian patients listed for HT. Non-Caucasian recipients tend to be younger, are more frequently women, are more likely to have non-ischemic HF etiology, and have higher rates of comorbid conditions including diabetes and renal failure.15, 16 African American and Hispanic-Latino patients are also more likely to be sicker at listing, with higher urgency status (UNOS status 1A/1B) and are more likely to be supported on intravenous inotropes at listing. In an analysis of 10,377 patients awaiting primary HT, wait-list mortality was 50% higher for Hispanic-Latino and 13% higher for African American patients compared to Caucasian patients, even after adjusting for factors such as cardiac diagnosis, hemodynamic support, and end-organ function.16 Notably, the higher risk was present despite a shorter waiting time for receiving a HT in Hispanic-Latino patients.
Left ventricular assist devices (LVAD) are required in 20% to 40% of patients awaiting HT in the US.17 Despite their higher urgency status at listing, Hispanic-Latino patients are less likely to be supported on a LVAD or other mechanical support at listing than other ethnic groups.16 Potential explanations for this disparity include a higher proportion of Hispanic-Latino patients with Medicaid insurance, and that Hispanic-Latino patients are more likely to be listed at low-volume transplant centers compared to Caucasian patients.16 In a single center analysis in which bridge to transplantation was the indication for LVAD implant in 21 African Americans (65.6%) and 39 Caucasians (69.6%), Tsiouris et al. determined there were no differences in survival between African Americans and Caucasians at 6 months or 1 year.18
Racial/ethnic differences in outcomes after transplantation
Previous analyses have identified clear disparities in outcomes after HT based on race and ethnicity. Liu et al. retrospectively evaluated post-transplant mortality in 39,075 adult primary HT recipients from 1987 to 2009.15 During a median follow-up of 1815 days (interquartile range, 478 to 3304 days), the unadjusted 1- and 5-year mortality rates were 13.2% and 26.5% in Caucasian recipients, 13.6% and 29.4% in Hispanic-Latino recipients, 15.8% and 36.7% in African American recipients, and 12.4% and 26.2% in other recipients. After adjusting for recipient, transplant, and socioeconomic variables, only African American recipients had an increased risk of death (hazard ratio [HR] 1.34, 95% confidence interval [CI] 1.21 – 1.47; P<0.001). African Americans were more likely to die of graft failure or a cardiovascular cause (57.9%) than were Caucasians (37.8%) or other non-Caucasians (44.1%; P<0.001), but were also less likely to die of infection or malignancy.
Although overall survival after HT has progressively improved in recent eras19, Singh et al. examined post-transplant outcomes in 36,748 HT recipients to determine whether these improvements have been equal across racial/ethnic groups.2 Early (6-month) post-transplant survival improved with time in all racial/ethnic groups from 86.3% in the earliest era (1987–1992) to 90.8% in the most recent era (2005–2008). However, the risk of death or retransplant within the first 6 months was higher in African American recipients than in Caucasian recipients (HR 1.15, 95% CI 1.05–1.26; P=0.004), even after adjusting for patient factors and era of transplant. Furthermore, longer-term survival (after 6 months) improved across eras in Caucasian recipients but not in African American or Hispanic-Latino recipients, suggesting that racial/ethnic disparities in long-term survival after HT have actually worsened with time (Figure 2).
Figure 2. Disparities in outcome for African American and Hispanic/Latino patients have increased over time.
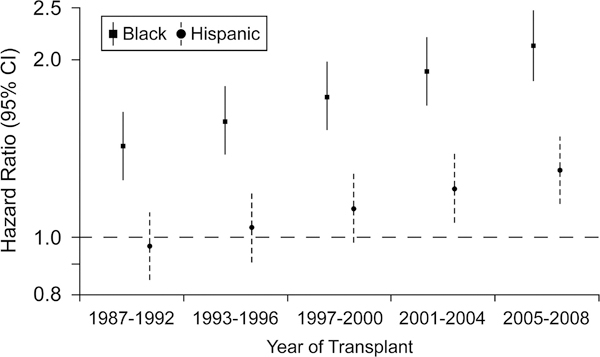
Racial disparities for the risk of death or retransplantation, conditional on surviving the first 6 months after heart transplant, are widening over progressive eras. The reference group is white heart transplant recipients. The hazard ratios and 95% confidence intervals are adjusted for baseline risk factors. Source, Singh et al.2
Factors contributing to racial/ethnic disparities in post-transplant outcomes
The exact etiologies underlying inferior transplant outcomes in African Americans and Hispanic-Latinos are unclear. Previous studies have confirmed the impact of socioeconomic status as a major contributor to poor outcomes after HT, particularly in minority populations. However, additional data suggest immunologic mechanisms contribute to a higher risk of acute and chronic rejection, and ultimately allograft loss in minority HT recipients. In the following section, we review potential causes for disparate outcomes after HT.
Socioeconomic status.
Due to the significant expense of transplant care, socioeconomic status (SES) and access to care are major factors that influence post-transplant outcomes. . For example, the cost of the standard immunosuppressive (IS) regimens averages $13,400 per year, and additional costs are incurred for travel and lodging whenever the patient must return for follow-up evaluations at the transplant center.20 Multiple studies have shown that African American and Hispanic-Latino HT recipients are more likely to have Medicare or Medicaid as their primary payer as opposed to Caucasian and Asian recipients who are more likely to have private insurance.2, 3 Morris et al. found that 35% of Caucasian, 48% of African American, 51% of Hispanic-Latino, and 27% of Asian HT recipients had Medicare or Medicaid as their primary payer.3 Singh et al. examined the impact of both SES and race/ethnicity on post-transplant outcomes in 520 pediatric and adult HT recipients and found that both non-Caucasian race and low SES were risk factors for a higher incidence of rejection, after controlling for gender, ventricular assist device placement, and era of HT.21
There is little data that specifically addresses how racial differences in access to care impact HT outcomes. However, Chakkera et al. hypothesized that racial disparities in kidney transplant would be less pronounced among patients inside the US Department of Veterans Affairs (VA) compared to patients outside of the VA system, since the VA provides comprehensive medical care and prescription coverage to eligible veterans.22 Among 79,361 patients (77,715 non-VA users and 1646 VA users), African-American race was associated with a 30% higher risk for graft failure and 10% higher risk for death, even after adjustment for a wide range of recipient and donor characteristics. Furthermore, the relative risk of graft failure by race was remarkably similar among VA users and non-VA users, and among VA users who received a transplant within and outside the VA. Thus, racial disparities in transplant outcomes persist even in a universal access-to-care system such as the VA.
Donor and cardiac allograft related factors.
Multiple donor-, surgery, and transplant center-specific parameters impact long-term survival after HT.23, 24 Prior data have suggested there may be an interaction between donor race/ethnicity and outcome after HT. Weiss et al. found that mismatch between the recipient and donor for race/ethnicity was a risk factor for 1-year mortality after HT (adjusted OR 1.13, 95% CI 1.02−−1.24; P=0.02).25 In contrast, Allen et. al demonstrated that donor race/ethnicity did not affect survival and furthermore, that matching the recipient and donor for race/ethnicity had no impact on survival.1
Allograft quality is a potentially novel source of transplant disparity that has recently been shown to impact outcomes in liver transplantation. Mathur et al. assessed the donor risk index in over 19,000 liver transplant recipients.26 They found that both Hispanic-Latino and female recipients were more likely to receive a low-quality allograft (adjusted HR 1.21 and 1.24, respectively) after adjusting for comorbidities, age, diagnosis, MELD score at transplant, and donation service area. The presence of disparities in allograft quality by recipient race/ethnicity or gender is unexplored in HT.
Transplant center performance is another measure that impacts post-transplant outcomes. Kilic et al. examined 18,710 HT recipients, and found that African-Americans were more likely to be transplanted at centers with higher-than-expected mortality, even after adjusting for insurance and education level.27 Moreover, African-Americans were at increased risk of 1-year mortality as compared with whites in poor performing centers (OR 1.37, 95% CI 1.12–1.69; P=0.002) as well as at excellent performing centers (OR 1.42, 95% CI, 0.99–2.02; P=0.06).
Immunologic mechanisms:
Rejection.
Multiple prior analyses have documented a higher rate of acute rejection in African American HT recipients.2, 3, 28, 29 Kilic et al. derived a simple score to assess the risk of rejection, and found that recipient African American race independently increased the risk for rejection during the first post-transplant year (OR 1.21, 95% CI 1.10–1.33; P<0.001), while both Hispanic-Latinos (OR 0.87, 95% CI 0.76–1.01; P=0.06) and Asians (OR 0.56, 95% CI 0.42 – 0.74; P<0.001) had decreased risk as compared to Caucasians.29 Morris et al. found that African American recipients (31%) were more commonly treated for rejection within 1 year of HT than non-sensitized Caucasian (27%), Hispanic-Latino (27%), or Asian (22%) recipients.3 African American race has also been demonstrated to predict the severity of acute rejection. Girnita et al. assessed the frequency of rejection with hemodynamic compromise in 532 pediatric HT recipients.30 Five years after transplant, African American recipients had a higher rate of hemodynamically significant rejection compared with non-African American recipients (36.1% vs. 22.2%; P=0.08), and African American recipients were also more likely to require inotropic support (20% vs 7.7%; P=0.007).
Differences in human leukocyte antigen (HLA) matching may account for some of the increased frequency and severity of rejection in minorities after HT, as higher degrees of HLA mismatch have consistently been associated with an increased risk of rejection.28, 29, 31 However, the class I and class II genes of the major histocompatibility complex are highly polymorphic and HLA matching is not performed as precisely in HT as in kidney transplantation due to limitations related to donor supply and ischemic time. As a result, HLA mismatch between HT donor and recipient is common, with a median of five mismatched serological antigens regardless of the donor or recipient racial/ethnic group.3 To add to the complexity of achieving adequate matching, HLA haplotypes are strongly associated with ethnicity. On average, HLA polymorphism is greatest among African American and lowest among Native Americans, with Asian, Caucasian and Hispanic-Latino populations falling at intermediate frequencies (Figure 3).32, 33 Thus, minority HT recipients are more likely to have a higher degree of HLA mismatch regardless of whether the donor is of the same or a different race or ethnicity.34–36
Figure 3. African American and Hispanic/Latino persons have a higher HLA haplotypic diversity as compared to Caucasian and Asian persons.
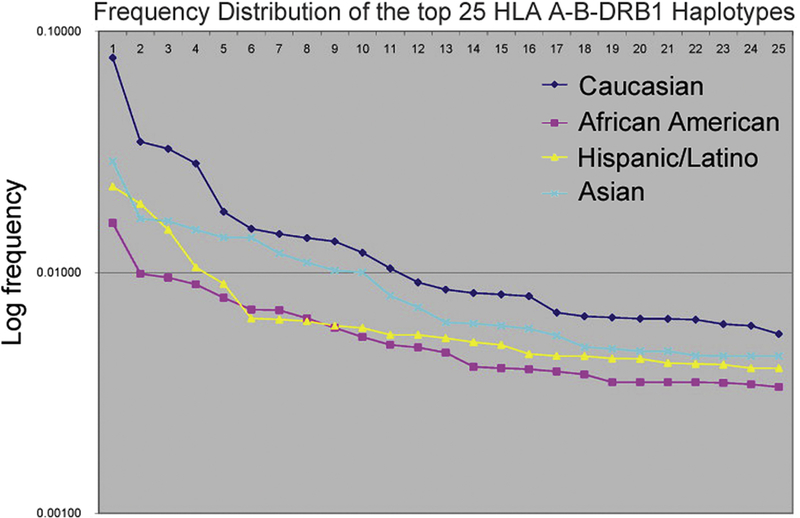
The first 25 ranked haplotypes in each racial/ethnic group are graphed against the frequency of that haplotype on a logarithmic scale. The frequency of each HLA haplotype is higher for Caucasians and Asians, indicating that the HLA haplotypes of African Americans and Hispanic/Latinos are overall less frequent and therefore more diverse. Source: Maiers et al.33
Differences in immune responsiveness may also contribute to inferior post-transplant outcomes in racial-ethnic minorities. Kerman et al. demonstrated that African American kidney transplant recipients have stronger responses in immune function assays (i.e. active T cells, helper T cell:suppressor T-cell ratio, mixed lymphocyte culture alloantigen response) compared to white kidney transplant recipients37, 38, correlating with inferior 1-year allograft survival in African Americans (67% vs. 80%, P<0.01).37 Moreover, Hutchings et al. confirmed increased expression of the CD80 and CD86 costimulatory molecules, and increased T cell costimulation during mitogen activation, by antigen-presenting cells from African American subjects.39 The costimualtion process is critical to T cell activation and is in fact the target of the novel immunosuppressant belatacept has been approved by the US Food and Drug Administration for use in kidney transplantation (REF: Kinnear et al, Transplantation 2013)
Immunologic mechanisms:
Graft Failure.
Pre-transplant screening for panel reactive antibody (PRA) is used during transplant evaluation to determine the presence of circulating antibodies to HLA antigens in order to assess the likelihood of finding a suitable donor, and to mitigate the risk of post-transplant hyperacute and antibody mediated rejection. The higher the PRA value, the greater the likelihood of a positive cross-match with a random donor, such that patients with higher PRA values tend to have extended waiting times while listed for solid organ transplant, lower rates of transplantation, higher rates of rejection, and worse allograft survival.40, 41 Morris et al. confirmed that African American HT recipients had higher peak PRA than Caucasian, Hispanic-Latino and Asian recipients.3 Over 1207 median days of follow-up, sensitized (PRA ≥10%) African American recipients had the lowest rate of graft survival, while non-sensitized Asians had the highest rate of graft survival (Figure 4). After adjustment for covariates, African American race (adjusted HR 1.3, 95% CI 1.2 – 1.4; P<0.001), Hispanic-Latino ethnicity (adjusted HR 1.2, 95% CI 1.0 – 1.4; P=0.035), and sensitization (adjusted HR 1.2, 95% CI 1.04 – 1.3; P=0.004) remained significant predictors of higher risk of graft failure.
Figure 4. Graft survival in heart transplant recipients by recipient ethnicity and PRA level.
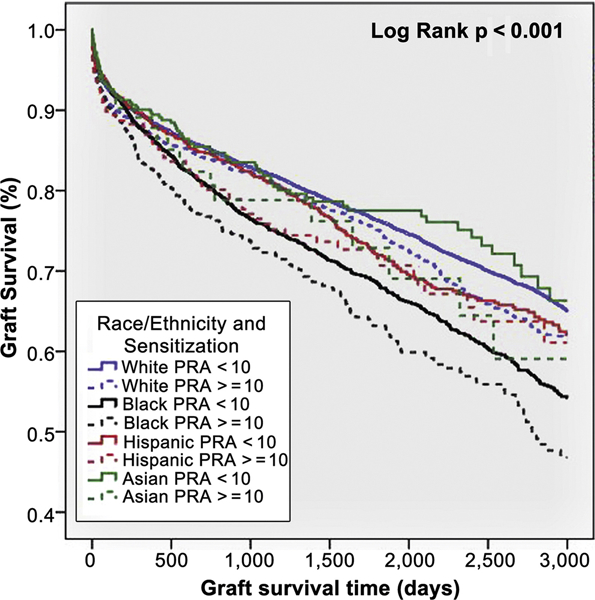
Kaplan-Meier curve showing graft survival in heart transplant recipients stratified by recipient ethnicity and PRA level (<10% or ≥10%). African American recipients with PRA ≥10% had the lowest graft survival, whereas Asian recipients with PRA <10% had the highest graft survival. Source Morris et al.3
Cardiac allograft vasculopathy.
The development of cardiac allograft vasculopathy (CAV) accounts for major morbidity and mortality after HT, accounting for 10–12% of deaths after the first year of HT.42 The development of CAV is partly immune-mediated, as endothelial cells express both HLA and non-HLA antigens that appear to be primary targets of cell-mediated and humoral immune responses.43 African American HT recipients are at increased risk for the development of CAV, and appear to have a shorter time to diagnosis of CAV compared to Hispanic-Latino and Caucasian recipients.2, 44 Kobayashi et al. similarly found that African American race was associated with a 55% lower CAV-free survival after adjustment for other variables in 5,211 pediatric HT recipients.45 The higher risk of CAV in African Americans is likely related to the increased burden of acute cellular rejection, which is a known risk factor for the earlier development of CAV.46
Inflammatory gene SNPs associated with the risk of rejection.
Previous analyses have investigated the role of genetic polymorphisms in the risk of rejection after HT. Girnita et al. found an association between SNPs in the interleukin 10 (IL-10), Fas cell surface death receptor (Fas), and angiotensin I converting enzyme (ACE) genes and the risk of rejection with hemodynamic compromise in a population of pediatric HT recipients.30 They found that the high expressing “GG” genotype of IL-10 and the low expressing “II” genotype of ACE protected recipients against the development of rejection with hemodynamic compromise, whereas the high expressing “AA” genotype of Fas led to a higher risk of rejection. The effect of the IL-10 and Fas SNPs was similar for non-African American and African American recipients; however, the protective effect of ACE appeared to be limited to non-African American recipients.
Gene expression profiling associated with the risk of rejection.
Noninvasive gene expression profiling (GEP) of peripheral blood has been used in lieu of endomyocardial biopsy (EMB) as surveillance testing for HT recipients who have a low probability of rejection.47 In the Invasive Monitoring Attenuation through Gene Expression (IMAGE) study, HT recipients at least 6 months post-transplant were randomized to either surveillance with routine EMB or GEP testing.48 The cohort (N=602) was predominantly Caucasian (77.7%), but did include African American (11.7%), Hispanic-Latino (6.5%), and Asian (2.2%) patients. The rate of the primary outcome (first occurrence of rejection with hemodynamic compromise, graft dysfunction due to other causes, death, or retransplantation) was higher among African American and non-Caucasian patients compared to Caucasian patients (18.3% and 22.2% vs. 8.5% respectively). However, monitoring for rejection with GEP was noninferior to EMB with respect to prevention of the primary outcome in all groups irrespective of race/ethnicity. Follow-up studies have demonstrated that the variability of GEP scores within individuals predicts future risk of allograft dysfunction or death.49 Interestingly, African American recipients taking cyclosporine had higher GEP scores than Caucasians on cyclosporine, despite similar trough levels.50
Future directions and strategies to improve post-transplant outcomes in racial/ethnic minorities
Given the increasing diversity of the US population, and recent data showing that race/ethnic disparities in HT outcomes may be worsening, future efforts must be focused on eliminating these disparities. Although improving social inequities and access to care are often targets for equalizing outcomes, focusing on the unique immunologic contributors to race/ethnic disparities may also provide an avenue to further improve transplant outcomes. In fact, recent data suggest that immunologic factors confer the greatest risk for incident GF for all race/ethnic groups, with a slightly higher risk in African American HT recipients.51 Future research examining strategies for both induction and maintenance immunosuppressive regimens may be particularly beneficial in high risk minority HT recipients. With improved IS regimens in recent eras, the incidence of any rejection has decreased from 32% in 2004 to 25% in 2010 in the first year after HT.52 Both induction and maintenance IS regimens are used to induce immune quiescence, and individualization of the IS strategy has been established as a priority for improving post-HT outcomes.53
Induction immunosuppression.
Higgins et al. assessed the impact of induction therapy in 5,897 patients transplanted from 1990 to 2001.54 Induction with anti-thymocyte preparations conferred a survival benefit in high risk recipients, particularly in African American recipients ≤25 years of age with ≥4 HLA mismatches, and recipients on VAD support with ≥4 HLA mismatches (non-African American recipients <30 years of age, African American recipients ≤35 years of age). More recently, Coleman et al. found that younger African American recipients (age 21 to 39) had improved survival after treatment with anti-thymocyte globulin (HR 0.67, 95% CI 0.68– 1.04; P=0.03), whereas patients age 40 to 59 did not have a significant benefit; patients ≥ 60 years of age may have had decreased survival (HR 1.35, 95% CI 0.89–2.04; P=0.1).55 Given that induction IS with anti-thymocyte globulin has also been shown to delay the development of CAV56, induction IS with anti-thymocyte globulin may be considered in African American patients who do not have a contraindication.
Maintenance immunosuppression.
Maintenance IS regimens have also improved over time, with most patients currently receiving tacrolimus and mycophenolate mofetil.42 The literature supports tacrolimus as the preferred calcineurin-inhibitor (CNI) for African American patients. Mehra et al. compared outcomes at 1-year for 21 Caucasian and 20 African American patients treated with tacrolimus, and 22 African American control patients treated with cyclosporine.57 Tacrolimus-based IS was associated with better 1-year survival in African Americans compared to cyclosporine (95% vs. 73%; P=0.04); tacrolimus-treated Caucasian HT recipients achieved a similar end point (95% 1-year survival). Usage of tacrolimus has increased in all patients over time irrespective of race/ethnicity, with associated decrease in the incidence of rejection in the first post-transplant year.2
Racial/ethnic diversity also influences important differences in the pharmacogenetic profile of tacrolimus. Mehra et al. noted that African American patients require significantly higher doses of tacrolimus than Caucasians, to achieve similar trough levels.57 Similarly elevated dose requirements have also been noted in African American renal transplant patients.58 African Americans are more likely to be expressers of the CYP3A5*1 genotype, which has been associated with higher clearance and lower bioavailability of tacrolimus. In fact, the CYP3A5*1 polymorphism is present in 65% of African American kidney transplant recipients compared with 8% of non-African American recipients.58 Despite these observations, few clinical studies have incorporated this information in a prospective fashion, nor have guidelines been issued that might guide transplant centers how to systematically utilize pharmacogenetic information in their clinical practice.
Corticosteroids are used universally within the early post-HT period, although many centers currently wean corticosteroids within the first year after HT. The 2012 report of the International Society for Heart and Lung Transplantation confirms that ~50% of patients are steroid-free at 5 years after HT.19 In a cohort of 72 patients treated with azathioprine and cyclosporine, Felkel et al. found that African American patients were less likely to be successfully weaned from corticosteroids.59 However, a more recent study of patients treated with tacrolimus and mycophenolate mofetil did not find a difference in the number of African American patients that could be weaned from steroids.60
Significant clinical interest exists regarding the use of the novel proliferation-signal inhibitors sirolimus or everolimus, given their beneficial effects on progression of CAV and post-transplant renal insufficiency. In a randomized clinical trial of 116 HT patients with chronic kidney disease, conversion from CNI to sirolimus improved renal function at 1 year versus continuing CNI.61, 62 However, multivariate analysis confirmed that non-white race (OR 15.3, 95% CI 1.35 – 172.7; P = 0.06) was independently associated with acute cellular rejection in patients converted to sirolimus. In patients who have developed CAV, both sirolimus when used as the primary IS agent, and everolimus when used in conjunction with mycophenolate mofetil, have been shown to reduce the progression of CAV.63, 64 Therefore use of these agents may be beneficial in African American HT recipients who are prone to earlier development of CAV, but extreme caution and careful monitoring are required when converting African American patients from a CNI to sirolimus to avoid development of acute cellular rejection.
Gene expression profiling and other tools.
The IMAGE study enrolled patients who were at a lower risk for rejection because the relative safety of GEP had not yet been confirmed, and the investigators did not want to expose the study participants to an undue risk of adverse events.48 In fact, only 20% of potentially eligible patients were enrolled in the study and patients who had received a HT less than 3 years previously were recruited preferentially. Despite these limitations of the original study, GEP may be a novel tool that can be used to monitor high-risk populations such as race/ethnic minorities for future risk of rejection. Future studies will need to be performed in higher risk HT recipients in conjunction with EMB, to determine if GEP provides additional information that can be used to surveil high-risk HT recipients for future risk of allograft rejection or death.
Conclusions
Outcomes in patients listed for and undergoing HT are not uniform, with Hispanic-Latino patients experiencing an increased risk of death on the waiting list, and African American HT recipients experiencing an increased risk of rejection and death post-transplant. In contrast, Asian recipients experience a significantly lower risk of rejection and death post-transplant. These disparities in outcomes appear to be worsening in the current era despite the overall improvements for survival after HT. With the increasing diversity of the US population as a whole, and the increasing diversity of patients wait-listed for HT, addressing inequities in pre- and post-transplant care for minority patients must become a priority among transplant professionals. Potential areas for future research include greater focus on immunologic factors, including use of GEP and clinical algorithms for rational use of pharmacogenetic data to tailor IS regimens. Ultimately, as our knowledge of how to incorporate data from genetic sequencing into transplantation medicine improves, we may be able to eliminate race/ethnicity disparities. Public policy efforts should also continue to address timely referral for transplantation for minorities, as well as optimizing insurance coverage for IS regimens after transplantation since inability to pay for healthcare services remains a barrier to equal access and delivery of transplant care. Continued vigilance and further prospective studies will be required to fully understand the mechanisms underlying the impact of modifiable risk factors in HT outcomes in diverse populations.
Acknowledgments
Funding Sources: A.A.M is supported by funding from the NIH/NHLBI (K23 HL124287) and the Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program). B.L.C. is supported by funding from the National Center for Advancing Translational Sciences (NCATS), Grant UL1TR000124.
Abbreviations:
- ACE
angiotensin converting enzyme
- CI
confidence interval
- HF
heart failure
- HLA
human leukocyte antigen
- HR
hazard ratio
- HT
heart transplantation
- HTN
hypertension
- IS
immunosuppression
- PPCM
peripartum cardiomyopathy
- OR
odds ratio
- PRA
panel reactive antibody
- SES
socioeconomic status
- SNP
single nucleotide polymorphism
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
Relevant disclosures: None
References
- 1.Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV, Shah AS. The Impact of Race on Survival After Heart Transplantation: An Analysis of More Than 20,000 Patients. The Annals of Thoracic Surgery. 2010;89(6):1956–1964. [DOI] [PubMed] [Google Scholar]
- 2.Singh TP, Almond C, Givertz MM, Piercey G, Gauvreau K. Improved Survival in Heart Transplant Recipients in the United States: Racial Differences in Era Effect. Circulation: Heart Failure. 2011;4(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris AA, Cole RT, Veledar E, Bellam N, Laskar SR, Smith AL, Gebel HM, Bray RA, Butler J. Influence of Race/Ethnic Differences in Pre-Transplantation Panel Reactive Antibody on Outcomes in Heart Transplant Recipients. Journal of the American College of Cardiology. 2013;62(24):2308–2315. [DOI] [PubMed] [Google Scholar]
- 4.Higgins RSD, Fishman JA. Disparities in Solid Organ Transplantation for Ethnic Minorities: Facts and Solutions. American Journal of Transplantation. 2006;6(11):2556–2562. [DOI] [PubMed] [Google Scholar]
- 5.Smedley A, Smedley B. Race in North America: the Origin and Evolution of a Worldview. Fourth Edition ed: Westview Press; 2012. [Google Scholar]
- 6.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, Feldman MW. Genetic Structure of Human Populations. Science. 2002;298(5602):2381–2385. [DOI] [PubMed] [Google Scholar]
- 7.Keita SOY, Kittles RA, Royal CDM, Bonney GE, Furbert-Harris P, Dunston GM, Rotimi CN. Conceptualizing human variation. Nat Genet. 2004. [DOI] [PubMed] [Google Scholar]
- 8.Coleman B, Brown W. Multicultural considerations in providing genetic and genomic cancer care In: Calzone K, Masny A, Jenkins J, eds. Genetics and Genomics in Oncology Nursing Practice: Oncology Nursing Society; 2010. [Google Scholar]
- 9.Tang H, Quertermous T, Rodriguez B, Kardia SLR, Zhu X, Brown A, Pankow JS, Province MA, Hunt SC, Boerwinkle E, Schork NJ, Risch NJ. Genetic Structure, Self-Identified Race/Ethnicity, and Confounding in Case-Control Association Studies. The American Journal of Human Genetics. 2005;76(2):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6(4):287–298. [DOI] [PubMed] [Google Scholar]
- 11.Okin PM, Kjeldsen SE, Dahl√∂f Br, Devereux RB. Racial Differences in Incident Heart Failure During Antihypertensive Therapy. Circulation: Cardiovascular Quality and Outcomes. 2011;4(2):157–164. [DOI] [PubMed] [Google Scholar]
- 12.Patel D, Green K, Fudim M, Harrell F, Wang T, Robbins M. Racial differences in the prevalence of severe aortic stenosis. Journal of the American Heart Association. 2014;3(3):e000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States 2010 Census Data. Available at http://www.census.gov/2010census/data/ Accessed November 1, 2014.
- 14.Colvin-Adams M, Smithy JM, Heubner BM, Skeans MA, Edwards LB, Waller C, Schnitzler MA, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: Heart. American Journal of Transplantation. 2014;14(S1):113–138. [DOI] [PubMed] [Google Scholar]
- 15.Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent Racial Disparities in Survival After Heart Transplantation. Circulation. 2011;123(15):1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh TP, Almond CS, Taylor DO, Graham DA. Decline in Heart Transplant Wait List Mortality in the United States Following Broader Regional Sharing of Donor Hearts. Circulation: Heart Failure. 2012;5(2):249–258. [DOI] [PubMed] [Google Scholar]
- 17.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional Differences in Recipient Waitlist Time and Pre- and Post-Transplant Mortality After the 2006 United Network for Organ Sharing Policy Changes in the Donor Heart Allocation Algorithm. JACC: Heart Failure. 2014;2(2):166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: Racial disparities in outcomes. The Journal of Heart and Lung Transplantation. 2013;32(3):299–304. [DOI] [PubMed] [Google Scholar]
- 19.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: 29th Official Adult Heart Transplant Report -- 2012. The Journal of heart and lung transplantation. 2012;31(10):1052–1064. [DOI] [PubMed] [Google Scholar]
- 20.Kasiske B, Cohen D, Lucey M, Neylan J. Payment for immunosuppression after organ transplantation. Journal of the American Medical Association. 2000;283(18):2445–2450. [DOI] [PubMed] [Google Scholar]
- 21.Singh TP, Givertz MM, Semigran M, DeNofrio D, Costantino F, Gauvreau K. Socioeconomic Position, Ethnicity, and Outcomes in Heart Transplant Recipients. The American Journal of Cardiology. 2010;105(7):1024–1029. [DOI] [PubMed] [Google Scholar]
- 22.Chakkera HA, O’Hare AM, Johansen KL, Hynes D, Stroupe K, Colin PM, Chertow GM. Influence of Race on Kidney Transplant Outcomes within and outside the Department of Veterans Affairs. Journal of the American Society of Nephrology. 2005;16(1):269–277. [DOI] [PubMed] [Google Scholar]
- 23.Smits JM, De Pauw M, de Vries E, Rahmel A, Meiser B, Laufer G, Zuckermann A. Donor scoring system for heart transplantation and the impact on patient survival. The Journal of heart and lung transplantation. 2012;31(4):387–397. [DOI] [PubMed] [Google Scholar]
- 24.Kransdorf EP, Stehlik J. Donor evaluation in heart transplantation: The end of the beginning. The Journal of Heart and Lung Transplantation. 2014;33(11):1105–1113. [DOI] [PubMed] [Google Scholar]
- 25.Weiss ES, Allen JG, Kilic A, Russell SD, Baumgartner WA, Conte JV, Shah AS. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. The Journal of Heart and Lung Transplantation. 2012;31(3):266–273. [DOI] [PubMed] [Google Scholar]
- 26.Mathur A, Schaubel D, Zhang H, Guidinger M, RM M. Disparities in Liver Transplantation: The Association between Donor Quality and Recipient Race/Ethnicity and Sex. Transplantation. 2014;97(8):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilic A, Higgins RSD, Whitson BA, Kilic A. Racial Disparities in Outcomes of Adult Heart Transplantation. Circulation. 2015;131(10):882–889. [DOI] [PubMed] [Google Scholar]
- 28.Jarcho J, Naftel D, Shroyer T, Kirklin J, Bourge R, Barr M, Pitts D, Starling R. Influence of HLA mismatch on rejection after heart transplantation: a multiinstitutional study. The Cardiac Transplant Research Database Group. The Journal of Heart and Lung Transplantation. 1994;13(4):583–595. [PubMed] [Google Scholar]
- 29.Kilic A, Weiss ES, Allen JG, Conte JV, Shah AS, Baumgartner WA, Yuh DD. Simple Score to Assess the Risk of Rejection After Orthotopic Heart Transplantation / Clinical Perspective. Circulation. 2012;125(24):3013–3021. [DOI] [PubMed] [Google Scholar]
- 30.Girnita DM, Ohmann EL, Brooks MM, Webber SA, Burckart GJ, Ferrell RE, Ranganathan S, Chinnock R, Canter C, Addonizio L, Bernstein D, Kirklin JK, Naftel DC, Zeevi A. Gene Polymorphisms Impact the Risk of Rejection With Hemodynamic Compromise: A Multicenter Study. Transplantation. 2011;91(12):1326–1332. [DOI] [PubMed] [Google Scholar]
- 31.Smith JD, Rose ML, Pomerance A, Burke M, Yacoub MH. Reduction of cellular rejection and increase in longer-term survival after heart transplantation after HLA-DR matching. The Lancet. 1995;346(8986):1318–1322. [DOI] [PubMed] [Google Scholar]
- 32.Gragert L, Madbouly A, Freeman J, Maiers M. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Human Immunology. 2013;74(10):1313–1320. [DOI] [PubMed] [Google Scholar]
- 33.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Human Immunology. 2007;68(9):779–788. [DOI] [PubMed] [Google Scholar]
- 34.Butts RJ, Scheurer MA, Atz AM, Moussa O, Burnette AL, Hulsey TC, Savage AJ. Association of Human Leukocyte Antigen Donor‚ÄìRecipient Matching and Pediatric Heart Transplant Graft Survival. Circulation: Heart Failure. 2014;7(4):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park M, Tolman D, Kimball P. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clinical transplantation. 1996;10(6):625–628. [PubMed] [Google Scholar]
- 36.Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplantation Proceedings. 1997;29(1‚Äì2):1460–1463. [DOI] [PubMed] [Google Scholar]
- 37.Kerman RH, Kimball PM, Van Buren CT, Lewis RM, Kahan BD. Possible contribution of pretransplant immune responder status to renal allograft survival differences of black versus white recipients. Transplantation. 1991;51(2):338–342. [DOI] [PubMed] [Google Scholar]
- 38.Suciu-Foca N, Reed E, Rohowsky C, Lewison A, King DW. Influence of race on the predictability of mixed lymphocyte culture identity by HLA-DR matching. Transplantation. 1983;35(1):35–39. [DOI] [PubMed] [Google Scholar]
- 39.Hutchings A, Purcell WM, Benfield MR. Peripheral blood antigen-presenting cells from African-Americans exhibit increased CD80 and CD86 expression. Clinical and Experimental Immunology. 1999;118(2):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray RA, Nolen JDL, Larsen C, Pearson T, Newell KA, Kokko K, Guasch A, Tso P, Mendel JB, Gebel HM. Transplanting the Highly Sensitized Patient: The Emory Algorithm. American Journal of Transplantation. 2006;6(10):2307–2315. [DOI] [PubMed] [Google Scholar]
- 41.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of Pretransplant Panel-Reactive Antibody on Outcomes in 8,160 Heart Transplant Recipients in Recent Era. Annals of Thoracic Surgery. 2007;84(5):1556–1563. [DOI] [PubMed] [Google Scholar]
- 42.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report -- 2013; Focus Theme: Age. The Journal of Heart and Lung Transplantation. 2013;32(10):951–964. [DOI] [PubMed] [Google Scholar]
- 43.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. The Journal of heart and lung transplantation. 2010;29(7):717–727. [DOI] [PubMed] [Google Scholar]
- 44.Costanzo M, Naftel D, Pritzker M, Heilman J, Boehmer J, Brozena S, Dec G, Ventura H, Kirklin J, Bourge R, Miller L. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. The Journal of Heart and Lung Transplantation. 1998;17(8):744–753. [PubMed] [Google Scholar]
- 45.Kobayashi D, Du W, L’Ecuyer TJ. Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients. Pediatric Transplantation. 2013;17(5):436–440. [DOI] [PubMed] [Google Scholar]
- 46.Raichlin E, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, Pereira NL, Daly RC, McGregor CG, Lerman A, Kushwaha SS. Acute Cellular Rejection and the Subsequent Development of Allograft Vasculopathy After Cardiac Transplantation. The Journal of Heart and Lung Transplantation. 2009;28(4):320–327. [DOI] [PubMed] [Google Scholar]
- 47.Kransdorf EP, Kobashigawa JA. Genetic and genomic approaches to the detection of heart transplant rejection. Personalized Medicine. 2012;9(7):693–705. [DOI] [PubMed] [Google Scholar]
- 48.Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Bogaev RC, Elashoff B, Baron H, Yee J, Valantine HA. Gene-Expression Profiling for Rejection Surveillance after Cardiac Transplantation. New England Journal of Medicine. 2010;362(20):1890–1900. [DOI] [PubMed] [Google Scholar]
- 49.Deng MC, Elashoff B, Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Cappola TP, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Bogaev RC, Shahzad K, Hiller D, Yee J, Valantine HA, for the ISG. Utility of Gene Expression Profiling Score Variability to Predict Clinical Events in Heart Transplant Recipients. Transplantation. 2014;97(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khush KK, Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Bogaev RC, Hlatky M, Elashoff B, Hiller D, Yee J, Valantine HA. Racial Disparities after Heart Transplant: Evidence from IMAGE. The Journal of heart and lung transplantation. 2013;32(4, Supplement):S102–S103. [Google Scholar]
- 51.Morris AA, Kalogeropoulos AP, Zhao L, Owen M, Raja Laskar S, David Vega J, Smith A, Butler J. Race and ethnic differences in the epidemiology and risk factors for graft failure after heart transplantation. The Journal of Heart and Lung Transplantation. 2015;34(6):825–831. [DOI] [PubMed] [Google Scholar]
- 52.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Official Adult Heart Transplant Report -- 2014; Focus Theme: Retransplantation. The Journal of Heart and Lung Transplantation. 2014;33(10):996–1008. [DOI] [PubMed] [Google Scholar]
- 53.Shah MR, Starling RC, Schwartz Longacre L, Mehra MR. Heart Transplantation Research in the Next Decade—A Goal to Achieving Evidence-Based Outcomes. National Heart, Lung, and Blood Institute Working Grou. Journal of the American College of Cardiology. 2012;59(14):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins R, Kirklin JK, Brown RN, Rayburn BK, Wagoner L, Oren R, Miller L, Flattery M, Bourge RC. To induce or not to induce: Do patients at greatest risk for fatal rejection benefit from cytolytic induction therapy? The Journal of heart and lung transplantation. 2005;24(4):392–400. [DOI] [PubMed] [Google Scholar]
- 55.Coleman B, Patel J, Czer L, Mirocha J, Kobashigawa J. 256 Use of Thymoglobulin after Heart Transplantation: Is There a Role in African American Patients? The Journal of Heart and Lung Transplantation. 2012;31(4):S92–S93. [Google Scholar]
- 56.Zhang R, Haverich A, Struber M, Simon A, Bara C. Delayed Onset of Cardiac Allograft Vasculopathy by Induction Therapy Using Anti-thymocyte Globulin. The Journal of Heart and Lung Transplantation. 2008;27(6):603–609. [DOI] [PubMed] [Google Scholar]
- 57.Mehra MR, Uber PA, Scott RL, Park MH. Ethnic Disparity in clinical outcome after heart transplantation is abrogated using tacrolimus and mycophenolate mofetil-based immunosuppression. Transplantation. 2002;74(11). [DOI] [PubMed] [Google Scholar]
- 58.Jacobson PA, Oetting WS, Brearley AM, Leduc R, Guan W, Schladt D, Matas AJ, Lamba V, Julian BA, Mannon RB, Israni A. Novel Polymorphisms Associated with Tacrolimus Trough Concentrations: Results from a Multicenter Kidney Transplant Consortium. Transplantation. 2011;91(3):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Felkel TO, Smith AL, Reichenspurner HC, LaFleur B, Lutz JF, Kanter KR, Gravanis MB, Johnston TS. Survival and incidence of acute rejection in heart transplant recipients undergoing successful withdrawal from steroid therapy. The Journal of Heart and Lung Transplantation. 2002;21(5):530–539. [DOI] [PubMed] [Google Scholar]
- 60.Teuteberg JJ, Shullo M, Zomak R, McNamara D, McCurry K, Kormos RL. Aggressive steroid weaning after cardiac transplantation is possible without the additional risk of significant rejection. Clinical Transplantation. 2008;22(6):730–737. [DOI] [PubMed] [Google Scholar]
- 61.Zuckermann A, Keogh A, Crespo‚ÄêLeiro M, Mancini D, Vilchez FGl, Almenar L, Brozena S, Eisen H, Tai S, Kushwaha S. Randomized controlled trial of sirolimus conversion in cardiac transplant recipients with renal insufficiency. American Journal of Transplantation. 2012;12(9):2487–2497. [DOI] [PubMed] [Google Scholar]
- 62.Zuckermann A, Eisen H, See Tai S, Li H, Hahn C, Crespo-Leiro M. Sirolimus conversion after heart transplant: risk factors for acute rejection and predictors of renal function response. American Journal of Transplantation. 2014;14(9):2048–2054. [DOI] [PubMed] [Google Scholar]
- 63.Topilsky Y, Hasin T, Raichlin E, Boilson BA, Schirger JA, Pereira NL, Edwards BS, Clavell AL, Rodeheffer RJ, Frantz RP, Maltais S, Park SJ, Daly RC, Lerman A, Kushwaha SS. Sirolimus as Primary Immunosuppression Attenuates Allograft Vasculopathy With Improved Late Survival and Decreased Cardiac Events After Cardiac Transplantation. Circulation. 2012;125(5):708–720. [DOI] [PubMed] [Google Scholar]
- 64.Kobashigawa JA, Pauly DF, Starling RC, Eisen H, Ross H, Wang S-S, Cantin B, Hill JA, Lopez P, Dong G. Cardiac allograft vasculopathy by intravascular ultrasound in heart transplant patients: substudy from the Everolimus versus mycophenolate mofetil randomized, multicenter trial. JACC: Heart Failure. 2013;1(5):389–399. [DOI] [PubMed] [Google Scholar]


