Abstract
Rationale:
Acute kidney injury (AKI) has a high prevalence and mortality in critically ill patients. It is also a powerful risk factor for heart failure (HF) incidence driven by hemodynamic changes and neurohormonal activation. However, no drugs have been approved by the FDA. Endogenous particulate guanylyl cyclase A receptor (pGC-A) activators were reported to preserve renal function and improve mortality in AKI patients, while hypotension accompanied by pGC-A activators have limited their therapeutic potential.
Objective:
We investigated the therapeutic potential of a non-hypotensive pGC-A activator, CRRL269 in a short-term, large animal model of ischemia induced AKI and also investigated the potential of urinary C-type natriuretic peptide (uCNP) as a biomarker for AKI.
Methods and Results:
We first showed CRRL269 stimulated cGMP generation, suppressed plasma angiotensin II and reduced cardiac filling pressures without lowering blood pressure in the AKI canine model. We also demonstrated CRRL269 preserved glomerular filtration rate (GFR), increased renal blood flow (RBF) and promoted diuresis and natriuresis. Further, CRRL269 reduced kidney injury and apoptosis as evidenced by ex vivo histology and tissue apoptosis analysis. We also showed, compared to native pGC-A activators, CRRL269 is a more potent inhibitor of apoptosis in renal cells and induced less decreases in intracellular Ca2+ concentration in vascular smooth muscle cells. The renal anti-apoptotic effects were at least mediated by cGMP/PKG pathway. Further, CRRL269 inhibited pro-apoptotic genes expression using a PCR gene array. Additionally, we demonstrated AKI increased uCNP levels.
Conclusions:
Our study supports developing CRRL269 as a novel renocardiac protective agent for AKI treatment.
Keywords: natriuretic peptide, particulate guanylyl cyclase A receptor, acute kidney injury, urinary CNP, apoptosis, calcium, heart failure, drug research, cardio-renal syndrome
Subject Terms: Cardiorenal Syndrome, Heart Failure, Ischemia, Nephrology, Kidney
INTRODUCTION
Acute kidney injury (AKI) is a syndrome of impaired renal function, characterized by a reduction of glomerular filtration rate (GFR) or urine output (UV) 1, 2. It has an overall frequency of 6% in community acquired and hospital acquired subjects and is especially common in critically ill patients admitted to intensive care units, with a frequency of 57% 3, 4. Notably, AKI is a common complication after cardiovascular (CV) surgery such as coronary artery bypass grafting and aortic repair, ranging from 13% to 43%, depending on the definition of AKI 5–7. Further, hypotension and decreased renal perfusion also plays a key role in initiating AKI in the surgical setting. In a contemporary matched cohort of 146,941 hospitalized adults, 31,245 (21%) experienced AKI. Importantly, the study observed that AKI was independently associated with a higher risk for heart failure (HF) after discharge 2. Despite increasing prevalence of AKI with high mortality and high risk for HF, no drugs for AKI have been approved by the FDA.
Both ischemia and hypovolemia have been implicated in mediating AKI through activation of pathways of cellular injury and death. Indeed, one mechanism for AKI as well as the increased risk for HF is activation of intrarenal and circulating inflammatory cytokines and deleterious hormones such as angiotensin II (ANGII). Notably, experimental AKI and transition to chronic kidney disease is reduced with ANGII receptor type 1 (AT1R) blockade prior to the onset of ischemic AKI 8. Moreover, recent studies have also reported that ANGII and the AT1R mediate intrarenal inflammation in models of AKI 8, 9. Additionally, renal cell apoptosis has emerged as an underlying final pathway contributing to AKI initiation and associated decline in GFR 10.
CRRL269 is a novel designer natriuretic peptide (NP) which was engineered at the Mayo Clinic as an innovative peptide targeting the renocardiac protective particulate guanylyl cyclase A receptor (pGC-A) which synthesizes the second messenger guanosine 3’,5’ cyclic monophosphate (cGMP) 11. Importantly, CRRL269 is a selective pGC-A activator with minimal particulate guanylyl cyclase B receptor (pGC-B) activity 11.Activation of pGC-A is associated with inhibition of cell apoptosis, suppression of inflammatory cytokines, diuresis and natriuresis, induction of endothelial regeneration, anti-remodeling with inhibition of the renin-angiotensin system (RAS) 12–18. Specifically, CRRL269 represents a chimeric peptide that integrates key amino acids (AAs) from URO (urodilatin, a renal-derived peptide) with key AAs from BNP (B-type NP, a cardiac derived peptide) 11. CRRL269 possesses enhanced renoprotective properties such as diuresis, natriuresis and increase in GFR compared with native pGC-A activators such as BNP or URO while retaining RAS-suppressing properties with less hypotension. Indeed, CRRL269 mediated superior renal pGC-A activation in vitro and renal protective properties in vivo compared to the two respective NPs. Mechanisms for its enhanced renal actions are due to increased resistance to neprilysin degradation and RAS suppression 11. The mechanism of BP preservation was due to reduced arterial relaxation and greater cardiomyocyte cAMP generation 11. Although it is known that NPs induce vasorelaxation via suppression of intracellular Ca2+ in vscular smooth muscle cells (VSMC) 19, 20, the action of CRRL269 on Ca2+ levels in VSMC remains undefined.
Given the renoprotective properties of CRRL269 and the need for FDA-approved therapies targeting AKI, here we investigated for the first time the renocardiac protective and RAS suppressing actions of CRRL269 in a large animal model of ischemic AKI produced by the clinically relevant maneuver of aortic cross clamping (ACC) simulating surgical aortic repair 21. This included assessing CRRL269 mediated increases in plasma, urinary and renal cGMP. We also sought to define in vitro the role of renal apoptosis in ischemic AKI and the ability of CRRL269 to attenuate cell death compared with BNP and atrial NP (ANP). Additionally, we defined the role of PKG as a mediator of the anti-apoptotic actions of CRRL269. We also explored the molecular mechanism of CRRL269’s BP preservation through the actions of CRRL269 on intracellular Ca2+ levels in vascular smooth muscle cells. Lastly, we assessed urinary C-type natriuretic peptide (uCNP) as a biomarker for AKI, based upon our previous findings in human HF cohorts and aged animals 22–24.
METHODS
Studies were performed in accordance with the Animal Welfare Act and with approval of the Mayo Clinic Institutional Animal Care and Use Committee (IACUC protocol number: A00002722).
The authors declare that all supporting data are available within the article (and its online supplementary files in the Online Data Supplement).
Ischemic acute kidney injury in canines.
An ischemic AKI model in normal mongrel canines (male, 25–30kg, age 1–2 years old, n=5 per group, Twin Valley Kennel) was created with high dose intravenously indomethacin infusion (10mg/kg with NaHCO3 [5mg/kg] dissolved in 50ml distilled water) followed by suprarenal aortic cross clamping (ACC), as described previously 21. The number of animals in each group was defined by a priori power calculation considering a statistical power of 80% and α=0.05. We used male canines only to minimize the experimental variation of the outcomes of ischemic acute kidney injury and we plan to recruit both male and female patients in our future clinical trials. Briefly, canines were anesthetized with intravenous pentobarbital (15–30 mg/kg) and fentanyl (4–12 µg/kg), intubated and mechanically ventilated on air with supplemental oxygen, 3 L/min, 12 cycles/min (Harvard Apparatus, Millis, MA). Both femoral artery and carotid artery were accessed for mean arterial pressure (MAP) monitoring and blood sampling. The femoral vein was cannulated for continuous inulin infusion and saline/indomethacin/drug infusions. The left renal artery was attached with a probe for renal blood flow (RBF) measurement and a catheter was placed in the left ureter for passive urine collection. A flow-directed balloon-tipped thermodilution catheter was advanced to the pulmonary artery through the external jugular vein for measurement of cardiac filling pressures and cardiac output (CO). After 60 min equilibration, baseline (BL) characteristics were established. A 45 min infusion of indomethacin (INDO) was then started. A Blalock clamp was applied at the level of the suprarenal aorta (ACC) to occlude blood flow to two kidneys. ACC was maintained for 60 min and 16.3 pmol/kg/min CRRL269 or vehicle (0.9% saline) infusion was initiated 5 min before releasing the clamp. Dogs were randomized to receive CRRL269 or vehicle infusion. Investigators were not blinded to treatments but biochemical analysis was performed by technicians who were blinded to treatment. The dose of CRRL269 was chosen based upon ANP dose used in our previous AKI study 21 and it is a medium dose between low and high doses we investigated in our previous study in normal canines 11. Drug infusion lasted for 120 min and data/samples were collected every 30 min, termed as Clearance 1 (CL1), Clearance 2 (CL2), Clearance 3 (CL3) and Clearance 4 (CL4). Immediately after completion of CL4 the animal was sacrificed, left kidney was harvested, sliced and preserved in 10% formalin solution or slices were snap frozen with liquid nitrogen. GFR values were calculated based on inulin clearance. Urinary sodium was measured with pHOx Ultra (Nova Biomedical, Waltham, MA). Dogs were included in the study based upon the effectiveness of renal aortic clamping which was demonstrated by the marked elevation of MAP and lack of urine output. One dog was euthanized early as a result of severe blood loss after unclamping of the aorta, which was torn by the surgical clamp, and therefore was excluded from the analysis.
Hormonal measurements.
Plasma and urinary cGMP were measured with commercially available ELISA kits (Enzo Life Sciences, Farmingdale, NY). Ex vivo renal parenchyma cGMP levels were measured with the same ELISA kits. Approximately 0.1g frozen canine renal cortex or medulla (n=5 Veh, n=4 CRRL269) were homogenized with 2mL 0.1N HCl by a homogenizer (Polytron PT1200E, Fisher Scientific, Waltham, MA). Homogenates were subjected to 600g 10 min centrifugation and supernatants were collected for cGMP assay. Final cGMP levels were adjusted by protein concentration measured by BSA method. Plasma angiotensin II (ANGII) was measured by radioimmunoassay (RIA) (Phoenix pharmaceuticals, Burlingame, CA). Plasma and urinary CNP was measured by CNP RIA (Phoenix pharmaceuticals, Burlingame, CA). Both ELISA and RIA were performed as instructed by the manufacturers’ protocols.
Histology and TUNEL analysis.
Fixed renal tissues were embedded with paraffin and sliced. For histology analysis, paraffin-embedded slides in vehicle (n=5) and CRRL269 (n=5) were stained with hematoxylin and eosin (H&E). A renal pathologist who is blinded to the study examined the H&E staining slides. Renal morphologic injury such as tubular dilation and vacuolization was evaluated. Renal cortical apoptosis in vehicle (n=5) and CRRL269 (n=5) dogs were assessed with TUNEL assay (Promega, Madison, WI) followed the protocol provided by the manufacturer. Normal canines (n=4, same background as the canines used in the current study) which did not undergo AKI or any other surgeries were added as an additional group to evaluate the renal apoptosis. DAPI (Vectashield, Vector Laboratories, Burlingame, CA) was used to label the nucleus. TUNEL results were analyzed by confocal fluorescence microscopy (Nikon A50R, Melville, NY).
Oil immersion induced hypoxia/reoxygenation in vitro.
Human primary renal proximal tubular epithelial cells (HRPTC, ScienCell Research Laboratories, Carlsbad, CA) were seeded in 96-well plates and cultured for 1 day until 70–80% confluency before experiments. Passages 3–5 were used. Caspase 3/7 green apoptosis assay agent (Essen BioScience, Ann Arbor, MI) was added to culture medium for the detection of apoptosis. Plates were then transferred to a time-lapsed, live imaging system IncuCyte (Essen BioScience, Ann Arbor, MI) for apoptosis monitoring for a total of 24 hours. Hypoxia/reoxygenation (H/R) in cells was mimicked with mineral oil (Sigma, St Louis, MO) immersion 25. Cells were immersed with oil for 2 hours, washed twice and replaced with fresh medium including vehicle (H/R group) or drugs. ANP, BNP, CRRL269 at concentrations of 10−8, 10−7 or 10−6 M were used. Protein kinase G (PKG, important cGMP binding protein) activating analogue 8-pCPT-cGMP (specific for PKG and low affinity to phosphodiesterases, Sigma, St Louis, MO) at concentrations of 10−7, 10−6 or 10−5 M were used. Cells were treated with 10−6, 5X10−6 or 1.5X10−5 M PKG inhibitor KT-5823 (Abcam, Cambridge, MA) for 30 min before 10−6 M CRRL269 was added. Cells without H/R stimulation were labeled as negative control (Neg Ctrl) group. Apoptosis signals (e.g. apoptosis object counts) were analyzed with the software provided by IncuCyte.
Apoptosis Pathway PCR Array and Caspase7 Real Time PCR.
Two groups of HRPTC (vehicle and 10−6 M CRRL269) which underwent oil induced H/R stress as described above were grown in 6-well plates and total RNA were collected with the Trizol method (Thermo Fisher Scientific, Waltham, MA). RNA was then reverse transcribed for cDNA for PCR RT2 array experiment. Apoptosis pathway PCR array (Qiagen, Germantown, MD) was then conducted with the SYBR green (Bio-Rad Laboratories, Hercules, CA) method in Lightcycler 480 (Roche Diagonostics, Indianapolis, IN). Raw data was analyzed with online Qiagen RT2 PCR analysis program. The Caspase7 gene was up-regulated by H/R and down-regulated by CRRL269 both at least two-fold and was selected for RT-PCR verification. Caspase7 RT-PCR primer (PPH00110C-200, Qiagen, Germantown, MD) was obtained and RT-PCR reactions were performed with SYBR green method. B2M gene (PPH01094E, Qiagen, Germantown, MD) was used for normalization.
Measurements of intracellular calcium in vascular smooth muscle cells.
Human primary aortic vascular smooth cells (HASMC, ScienCell Research Laboratories, Carlsbad, CA) were cultured and maintained as instructed by the manufacturer and cells at passage 4 or 5 were used. Calcium imaging was performed as previously described 26. Briefly, cells were seeded on a 25mm round cover glass (Fisher Scientific, Pittsburgh, PA) with a density of 5X104 cells per slide. These glass slides were pretreated with 0.5N NaOH for 8 hours, rinsed with water and sterilized before use. After overnight culturing, the glass with the cells was assembled in a MS-502 Chamber (ALA Scientific Instrument, Farmingdale, NY) and loaded with 3 uM fura-2 AM (Invitrogen, Carlsbad, CA) for 30 min at a 37°C incubator. The chamber was then placed on the stage of an inverted Olympus IX71 microscope (Olympus America Inc, Waltham, MA). The cells were maintained in 1 mL HASMC medium and pretreated with or without ANP, BNP or CRRL269 at concentration of 10−9 or 10−7 M for 5 min before perfused with 5 mL medium containing ANGII (10−8 or 10−7 M). The intracellular Ca2+ fluorescence signals were measured as a ratio of fluorescence intensities at 510 nm from excitations of 340/380 nm using a Hamamatsu ORCA-R2 CCD camera with a Sutter LB-LS/17 light source. Baseline fluorescence was recorded after 5 min of pretreatment and fluorescence images were acquired continuously during the ANGII perfusion. Background fluorescence was subtracted and the calcium signal (F) was normalized to baseline fluorescence (F0) and expressed as a ratio (F/F0) using MetaFluor software (Olympus America Inc, Waltham, MA).
Statistics and data analysis.
Data were expressed as means±SEM and significance analysis was performed with Prism 7 (Graphpad, La Jolla, LA). Data normality was assessed with Shapiro-Wilk test. For normally distributed data, one-way ANOVA and two-way ANOVA was used to in the in vivo and in vitro studies in which multiple time points were involved, and two-tailed t-tests were used for comparisons between two groups. Non-parametric Mann-Whitney test was applied for data that were not normally distributed.
RESULTS
Ischemic AKI hormonal and renal function in vivo in canines.
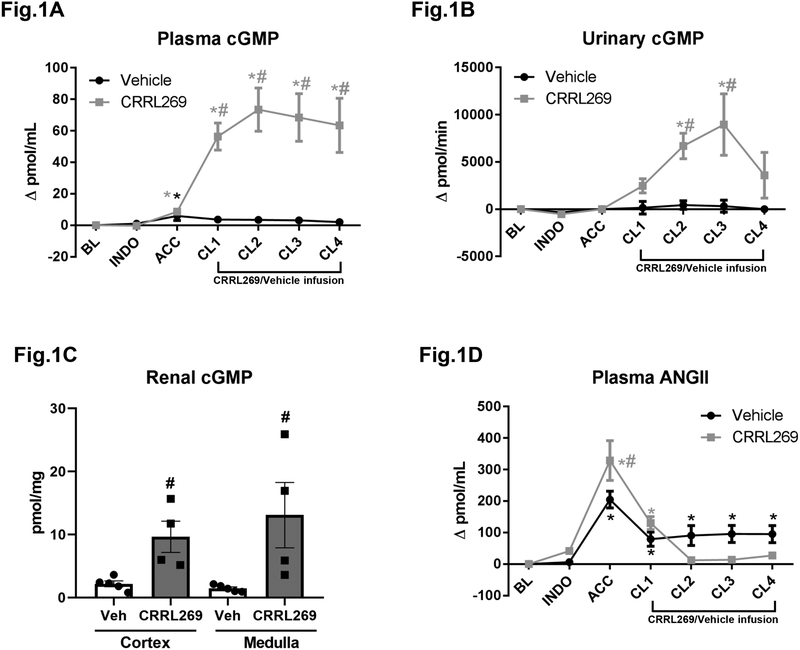
In vivo, continuous infusion for 2 hours with CRRL269 increased plasma, urinary and renal parenchyma cGMP levels consistent with pGC-A activation (Figure 1A, B, C and D). Results in vivo were presented as changes from baseline. Compared to vehicle, CRRL269 treatment markedly elevated cGMP values from CL1 to CL4. Notably, AKI induced by ACC greatly stimulated plasma ANGII levels compared to baseline. CRRL269 inhibited plasma ANGII levels (Figure 1D). Specifically, in vehicle group, ANGII levels remained high during post-ischemia phases from CL1 to CL4, while CRRL269 infusion significantly reduced circulating ANGII levels.
Figure 1. Plasma, urinary, renal cGMP and plasma angiotensin II (ANGII) values modulated by CRRL269.
(A) plasma cGMP, n=5 each group, # p<0.05 vs vehicle using two-way ANOVA followed by Bonferroni’s post-hoc test, * p<0.05 vs BL using one-way ANOVA followed by Dunnett’s post-hoc test (B) urinary cGMP, n=5 each group, same statistical methods as plasma cGMP (C) renal cortical and medullary cGMP, n=5 Veh and n=4 CRRL269, # p<0.05 vs Veh with unpaired t-test, and (D) plasma ANGII value changes from baseline by CRRL269 or vehicle. n=5 each group, same statistical methods as plasma cGMP. AKI protocol in canines was consisted of BL, INDO, ACC, CL1, CL2, CL3 and CL4. After 60 min equilibration, a baseline (BL) was recorded. Then 45 min infusion of indomethacin (INDO) was initiated, and this was followed by 60 min aortic cross clamping (ACC). 16.3 pmol/kg/min CRRL269 or vehicle (0.9% saline) infusion started after ACC and it lasted for 120 min. Data were recorded at BL, INDO, ACC, clearance 1 (CL1), clearance 2 (CL2), clearance 3 (CL3) and clearance 4 (CL4) during CRRL269/vehicle infusion.
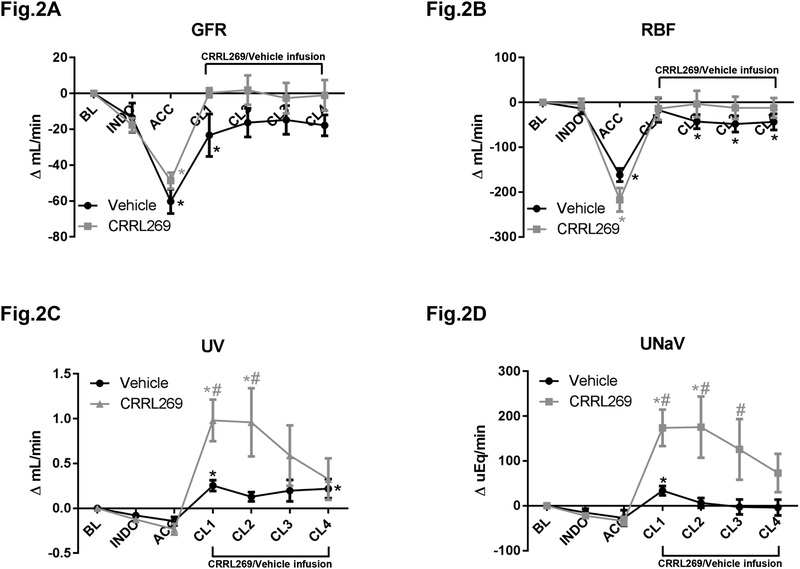
With ACC occluding suprarenal aorta, GFR was markedly reduced during ACC and changes from baseline results are showed in Figure 2A. A marked reduction of RBF was also observed as reported in Figure 2B during ACC. Importantly during post-ischemia phases, CRRL269 maintained GFR and RBF while vehicle did not preserve these two renal hemodynamic parameters (Figure 2A and B). Similarly, urine output (UV) and urinary sodium excretion (UNaV) were reduced by ACC while CRRL269 significantly induced diuresis and natriuresis compared to vehicle, which is consistent with the renal protective actions observed with GFR and RBF (Figure 2C and D).
Figure 2. Renal function parameters by CRRL269 in AKI.
(A) glomerular filtration rate (GFR), (B) renal blood flow (RBF), (C) urinary output (UV) and (D) urinary sodium excretion rate (UNaV) in CRRL269 or vehicle infusion group. Data were presented as absolute changes from baseline. Experimental schedule was described in Figure 1 legend. n=5 each group, # p<0.05 vs vehicle using two-way ANOVA followed by Bonferroni’s post-hoc test, * p<0.05 vs BL using one-way ANOVA followed by Dunnett’s post-hoc test.
Cardiovascular function in ischemic AKI canines.
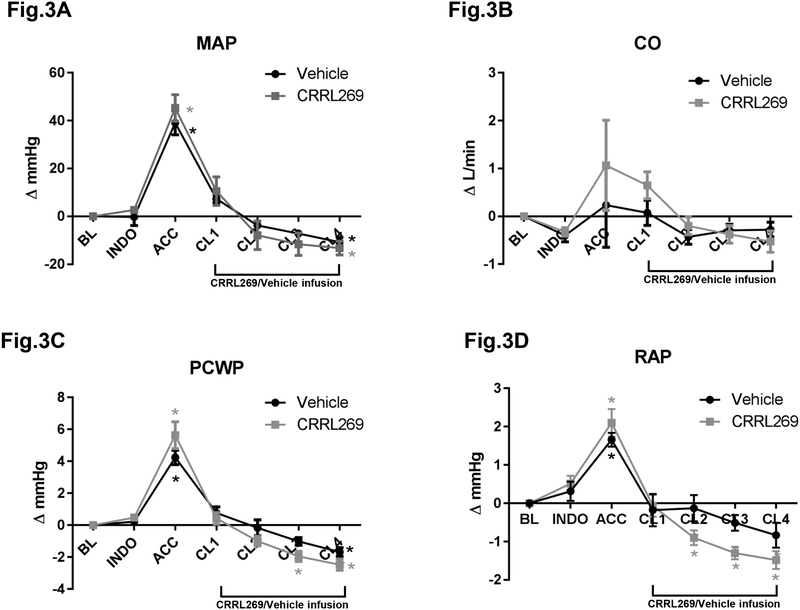
ACC resulted in a marked elevation of mean arterial pressure (MAP) while during post-ischemia reperfusion periods, MAP returned to baseline levels. Notably, CRRL269 induced similar BP effects compared with vehicle infusion (Figure 3A), indicating CRRL269 is not a hypotensive agent and a similar trend was observed in cardiac output (CO) (Figure 3B). Right atrial pressure (RAP) and pulmonary capillary wedge pressure (PCWP) were also markedly elevated during renal ischemia, of which CRRL269 reduced RAP and PCWP after ischemia (Figure 3C and D). The results of CV function parameters support CRRL269 reduced cardiac filling pressure without a hypotensive response.
Figure 3. Cardiovascular function parameters by CRRL269 in AKI.
(A) mean arterial pressure (MAP), (B) cardiac output (CO), (C) right atrial pressure (RAP) and (D) pulmonary capillary wedge pressure (PCWP) in CRRL269 or vehicle infusion group. Data were presented as absolute changes from baseline. Experimental schedule was described in Figure 1 legend. n=5 each group, * p<0.05 vs BL using one-way ANOVA followed by Dunnett’s post-hoc test.
Renal injury ex vivo analysis.
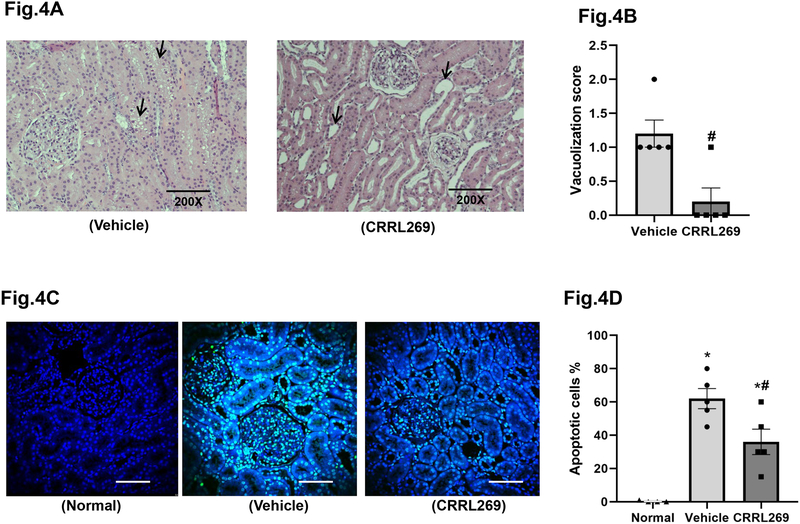
H&E staining indicated that CRRL269 group presented with less vacuolization compared to vehicle consistent with less renal injury (Figure 4A and B). Additionally, ischemia/reperfusion markedly increased renal cortical apoptotic cell death compared to normal canine kidneys, as illustrated by TUNEL assay using confocal microscopy (Figure 4C and D). Apoptotic cells were mostly tubular epithelial cells and glomerular mesangial cells. Importantly, CRRL269 treatment reduced apoptosis in the renal cortex in comparison with vehicle.
Figure 4. H&E staining and TUNEL apoptosis results ex vivo from AKI canine kidneys.
(A) Representative images of H&E staining of kidney cortex in vehicle and CRRL269 groups. Arrow heads indicate vacuolization or tubular dilation. (B) Quantification of renal cortex vacuolization score. n=5 each group, # p<0.05 vs vehicle with unpaired t-test (C) Representative images and (D) quantification of kidney cortex apoptosis assessed by TUNEL assay in normal, vehicle and CRRL269 groups. n=4 normal, n=5 vehicle and n=5 CRRL269, * p<0.05 vs normal, # p<0.05 vs vehicle, with unpaired t-test. TUNEL results were analyzed by confocal fluorescence microscopy. Green fluorescence indicates DNA fragmentation and blue fluorescence labels the nucleus. Scale bars=100um.
H/R mediated apoptosis in HRPTC.
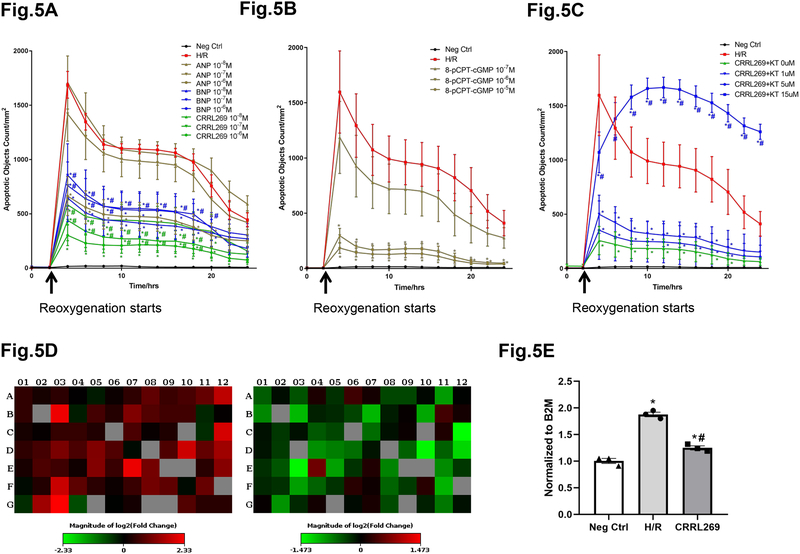
Recognizing the anti-apoptosis observed in renal tubular cells ex vivo by CRRL269, we employed human renal proximal tubular epithelial cells (HRPTC) to investigate hypoxia/reoxygenation (H/R) induced apoptosis in vitro with a time-lapsed and live imaging system. Here, H/R markedly stimulated apoptosis in HRPTC as evidenced by the sharp increase of apoptotic signals 2 hours after reoxygenation initiated, in comparison with the negative control group (Figure 5A, B and C). CRRL269 at concentrations 10−7 and 10−6 M significantly reduced apoptotic signals compared to the H/R group alone. More importantly, CRRL269 induced more robust anti-apototic effects compared to ANP and a trend of superiority was observed in comparison with BNP (Figure 5A). We next found PKG specific analog, 8-pCPT-cGMP, at concentrations of 10−6 and 10−5 M mimicked the apoptosis inhibition observed with pGC-A activators (Figure 5B). Furthermore, PKG inhibitor KT-5823 abrogated the anti-apoptotic effects induced by CRRL269 (Figure 5C). In the human apoptosis pathway gene array, 33 out of 84 genes in the apoptosis pathway were up-regulated at least 2 fold by H/R stimulation. In contrast, 8 out of 84 genes (CASP7, FADD, CRADD, DAPK1, HGDC, BCL2L11, BIK, TNFRSF1A) were down-regulated at least 2 fold by CRRL269 treatment (Figure 5D). Caspase7 gene (top gene, up-regulated in H/R and down-regulated in CRRL269) was chosen to be verified by RT-PCR due to its critical function mediating apoptosis and our results support that H/R increased Caspase7 gene by 1.89 fold and CRRL269 treatment significantly reduced its expression level to 1.25 (Figure 5E) 27.
Figure 5. CRRL269 inhibits apoptosis induced by H/R in human primary proximal tubular epithelial cells (HRPTC).
(A) Apoptotic signals were quantified by IncuCyte software and comparisons were made between negative control, H/R and H/R with 3 concentrations (10−8, 10−7, or 10−6 M) of ANP, BNP or CRRL269. n=8, * p<0.05 vs H/R, # p<0.05 vs ANP using two-way ANOVA followed by Dunnett’s post-hoc test. (B) Apoptosis by control, H/R and H/R with 3 concentrations (10−7, 10−6, or 10−5 M) of PKG-specific (low affinity to phosphodiesterases) cGMP analogue 8-pCPT-cGMP. n=8, * p<0.05 vs H/R, two-way ANOVA followed by Dunnett’s post-hoc test. (C) Apoptosis by control, H/R and H/R with 4 concentrations (0, 1, 5, 15 uM) of PKG inhibitor KT-5823 (KT) with 10−6 M CRRL269. n=8, * p<0.05 vs H/R, # p<0.05 vs 0 uM KT-5823, two-way ANOVA followed by Dunnett’s post-hoc test. (D) Apoptosis pathway genes expression were compared between H/R and negative control (left panel), 10−6 M CRRL269 and H/R (right panel). (E) Caspase7 gene was verified with RT-PCR in negative control, H/R and H/R with 10−6 M CRRL269. n=3, * p<0.05 vs negative control, # p<0.05 vs H/R, unpaired t-test.
Intracellular Ca2+ levels inhibited by pGC-A activators under ANGII stimulation.
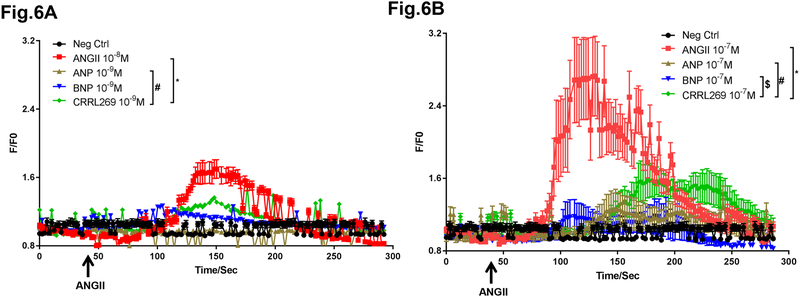
Intracellular Ca2+ levels in human aortic smooth muscle cells (HASMC) were increased under the stimulation by the vasoconstrictor ANGII at 10−8 or 10−7 M (Figure 6A and B). Pretreatment with pGC-A activator ANP, BNP or CRRL269 at 10−9 or 10−7 M suppressed Ca2+ enhancement by ANGII. Notably, CRRL269 presented less Ca2+ suppression in comparison with ANP and BNP.
Figure 6. Intracellular Ca2+ levels in human primary aortic smooth muscle cells (HASMC).
(A) Relative Ca2+ levels were calculated based upon recorded fluorescence signals in Fura-2 loaded HASMC cells. Fold difference F/F0 was used as relative Ca2+ concentration values. Cells were pretreated with 10−9 M ANP, BNP or CRRL269 for 5 min before 10−8 M ANGII was perfused. Perfusion lasted for about 4 min. (B) Cells were pretreated with 10−7 M ANP, BNP or CRRL269 for 5 min before 10−7 M ANGII was perfused. n=9–11,* p<0.05 vs ANGII, # p<0.05 vs ANP, $ p<0.05 vs BNP, two-way ANOVA followed by Dunnett’s post-hoc test.
CNP as a compensatory biomarker for AKI.
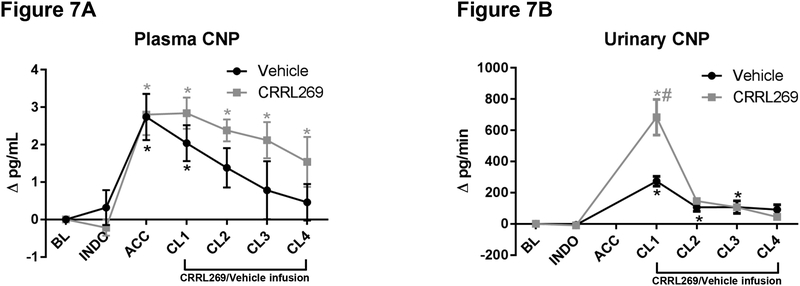
We found plasma and urinary CNP levels increased starting from the AKI reperfusion phase with elevation in urinary CNP being more pronounced (Figure 7A and B). Infusion with CRRL269 increased urinary CNP levels compared to vehicle.
Figure 7. C-type natriuretic peptide (CNP) changes by CRRL269 in AKI.
(A) plasma CNP, n=5 each group, # p<0.05 vs vehicle using two-way ANOVA followed by Bonferroni’s post-hoc test, * p<0.05 vs BL using one-way ANOVA followed by Dunnett’s post-hoc test (B) urinary CNP, statistics same as plasma CNP. Data were presented as absolute changes from baseline. Experimental schedule was described in Figure 1 legend.
Furthermore, with RT-PCR, cortical NPPC gene expression levels, although lower compared to medullary expression, were elevated by CRRL269 treatment (Supplemental Figure IA). In vitro, human glomerular microvascular endothelial cells (HGMEC) presented considerably lower NPPC mRNA levels than tubular cells HRPTC (Supplemental Figure IB). Additionally, CNP treatment at 10−7 M suppressed human renal fibroblast (HRF) proliferation (Supplemental Figure IC).
DISCUSSION
The current study was designed to advance the therapeutic potential of CRRL269, a novel pGC-A activator, for the treatment of AKI with a special focus on studies in a large animal model with high clinical relevance. The major findings of our study are that CRRL269 exerted renal protective actions in canine model of AKI post-I/R for 2 hours with GFR and RBF preservation, enhanced diuresis and natriuresis, reduced renal cortical vacuolization and suppression of ANGII without inducing hypotension. This occurred in the setting of CRRL269 mediated increases in plasma, urinary and renal tissue cGMP. Moreover, CRRL269 reduced cardiac filling pressures such as RAP and PCWP. Further, CRRL269 reduced apoptosis in the kidney cortex in our AKI model. In vitro, CRRL269 exerted more robust anti-apoptotic effects in human renal tubular cells and less intracellular Ca2+ suppression in arterial vascular smooth muscle cells, compared to native pGC-A activators and also attenuated H/R-mediated gene up-regulation of apoptotic pathways. Additionally, we established that urinary CNP may be a novel biomarker for AKI. Taken together, our results support CRRL269 as a promising novel AKI drug.
The lack of use of large animal models of AKI has, in part, limited successful drug discovery in AKI as rat and mice models, compared to canine or porcine models, lack the high functional similarity and hemodynamics profiles common to humans. Further, use of small animal models is also complicated by the difficulty in measuring key hemodynamic data during and after induction of AKI 28, 29. We incorporated a large animal model in our study, which highly mimics ischemia induced AKI in the clinical setting resulting from suprarenal aortic cross clamping (ACC) that is highly relevant to vascular surgical reconstruction of the aorta and/or renal arteries. In this model, CRRL269 infusion was started after ischemia and before reperfusion, and resulted in renoprotection which corroborated with the in vitro apoptosis experiments thus confirming and extending previous studies in large animal models 21, 30, 31. Specifically, ACC in canines in the presence of inhibition of vasodilating prostaglandins induced reductions in GFR in association with renal vasoconstriction with tubular vacuolization and marked increase in apoptotic cell death. Though the duration of the study was short and physiological parameters were only collected 2 hour post-ischemia, we observed moderate loss of GFR, which is in line with published studies in AKI long-term studies such as post-ischemia for 48 hours 32, 33. We believe the promising results obtained from this short-term study will guide us in the design of more long-term studies, such as 2 or 7 days post-ischemia AKI, to evaluate CRRL269’s therapeutic potential more extensively.
Importantly, CRRL269 treatment improved renal function highlighted by GFR preservation and enhanced renal excretory actions in vivo. The enhanced renal actions occurred in the absence of hypotension. The maintenance of BP is especially useful for the safety, therapeutic potential and renal functional preservation in human clinical studies in AKI patients 34. Indeed, it was demonstrated that a reduction in renal perfusion pressure leads to abolished natriuretic effects stimulated by ANP administration in rodents, implicating the importance of maintaining BP in pGC-A therapeutics 35. A decrease of cardiac filling pressures without significant hypotension would lead to an increase in transrenal perfusion pressure and consequently improve renal function. CRRL269 retains its ability to reduce cardiac filling pressures, consistent with our previous report in normal canines 11. The reduction of RAP and PCWP could be due to the decrease of intravascular volume in view of the markedly greater increase in diuresis and natriuresis with CRRL269 treatment in the presence of AKI. Importantly and in contrast to endogenous pGC-A activators such as BNP, CRRL269 was not associated with hypotension 11. Here we report that a key mechanism of the more preserved BP is, at least partially, mediated by less intracellular Ca2+ suppression in vascular smooth muscle cells stimulated by ANGII in the presence of CRRL269. This data complements the potential enhanced positive cardiac inotropic effects secondary to our findings of cAMP increases in cardiomyocytes and reduced arterial relaxing effects observed previously 11. However, future in-depth studies are warranted to elucidate this novel CRRL269 modulation of intracellular Ca2+ levels.
In AKI, neurohormonal pathways such as RAS activation are suggested to induce cardiac structural changes and dysfunction 36. The pathophysiological consequences by RAS activation include cardiac hypertrophy, fibrosis, inflammation and apoptosis, which further result in cardiac dysfunction and HF. Studies reported that plasma ANGII was significantly increased in ischemic AKI and more importantly angiotensin converting enzyme inhibitor (ACEi) administration was shown to attenuate the initiation and progression of myocardial dysfunction and remodeling in animal models and patients 37–39. Thus these previous results were not surprising given the enormous cardioprotective effects induced by ACEi and anti-RAS drugs in the treatment of HF. Thus a promising therapeutic agent suppressing RAS especially ANGII activation is critical for the protection of cardiac function in AKI. In our current study, CRRL269 suppressed plasma ANGII levels, hence supporting the potential of cardioprotection in the setting of AKI.
Lines of evidence support that the pGC-A/cGMP signaling pathway plays a critical role maintaining renal physiological integrity and protecting the kidney from injury 40–44. One of the key pathophysiological pathways targeted by pGC-A is apoptosis. Repeated studies have established that apoptosis is a key feature of AKI although drugs targeting apoptosis have not been effective in preventing AKI 45. Recognizing the critical role of I/R induced apoptosis in tubular cells in our in vivo AKI model, we employed an in vitro a model of acute H/R mediated apoptosis in tubular cells to investigate the molecular mechanism of CRRL269’s renal protective action. CRRL269 showed superior anti-apoptotic actions compared to ANP and we demonstrated that this property is mediated by the cGMP/PKG pathway illustrated by a PKG-specific cGMP analogue and a PKG inhibitor. Further, CRRL269 suppressed apoptotic genes with PCR array analysis. Moreover RT-PCR demonstrated down-regulation of Caspase7, which is important in cell apoptosis execution, by CRRL269 in HRPTC thus providing useful evidence for inhibition of renal cell apoptosis through activation of pGC-A/cGMP signaling by CRRL269 in vivo. Indeed, in vivo we observed robust increases in plasma, urinary and renal tissue cGMP. The superior anti-apoptotic effects of CRRL269 observed in vitro support its direct cytoprotective effect, which is independent of its beneficial effects on renal hemodynamics. Further studies on the underlying molecular mechanism of CRRL269 renoprotective effects such as PKG substrates (e.g. cell survival related protein–Akt) phosphorylation are warranted.
Biomarkers such as NGAL and KIM-1 have been used for AKI early prediction and prognosis 46, 47. However, the use of current biomarker profiles in the clinic has been limited by the specificity and predictive power signified by the average or low AUCs 48. Novel biomarkers providing an early detection of AKI immediately after injury insult are needed. In our laboratory, we previously documented that CNP is present in the kidney and is predominantly distributed in proximal, distal tubules and collecting ducts, and not in glomeruli 24, 49, 50. Urinary CNP was previously reported to predict patient outcomes in acute decompensated HF which was superior compared to KIM-1 or NGAL 22. Furthermore, it was suggested in subjects with impaired renal function, uCNP was increased 51. In the current study we explored, for the first time, the biomarker potential of uCNP in AKI and we showed that uCNP was markedly elevated by I/R. Notably, CRRL269 treatment increased uCNP and renal CNP gene expression levels, which is consistent with report of in vitro CNP mRNA and protein increases by native pGC-A activators 52. We also confirmed CNP’s renal protective effects in which CNP suppressed renal fibroblast proliferation in vitro. It is thus interesting to speculate if the renoprotective actions of CRRL269 may, in part, be also secondary to actions of CNP.
Our study is a pilot, short-term study assessing the renocardiac protective actions of CRRL269 in AKI canines. The current study also advances the understanding of pathophysiological changes caused by AKI, such as apoptosis and renal dysfunction. Our study has limitations as we only assessed the acute effects of CRRL269 in AKI. Specifically, its renoprotective effects in intermediate and long-term periods were not investigated in the current study. Thus, further studies in more long-term model models of AKI are warranted with the use of CRRL296 to evaluate if its renal protective actions are sustained. As described above, renal and cardiac function assessment conducted in 2 day and 7 days post-I/R in AKI model to further confirm CRRL269’s sustained renocardiac protection and rule out the possibility of a merely transient improvement by CRRL269 observed in the present study is warranted. Additionally, a preventive strategy with CRRL269 infusion prior to ischemic insult is warranted to explore its potential as a drug for AKI prevention. Moreover, it is important to assess the protective actions of CRRL269 in other models of AKI such as nephrotoxic drug induced AKI to explore its potential in a wider scope given the heterogeneity observed in the AKI patient population.
Given no drugs have been approved for AKI treatment by the FDA and the connection between the heart and kidney, there is an unmet need for the development of innovative drugs to preserve the kidney function directly and in the meantime protect the heart in the setting of AKI. The connection between the heart and kidney requires single agents targeting both organs thus treating cardiorenal syndrome. Recognizing the renocardiac protective effects of CRRL269 in AKI canines, we believe it may have translational potential for the treatment of AKI as well as HF prevention.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
To date, no drugs has been approved by the FDA for acute kidney injury (AKI) treatment.
Native particulate guanylyl cyclase A receptor (pGC-A) activators have potential beneficial actions in AKI, but may have limited use as they produce hypotension.
What New Information Does This Article Contribute?
A innovative designer pGC-A activator CRRL269 preserved renal function and exhibited cardiac protection, without causing hypotension in a large animal model of AKI.
Its beneficial actions were mediated, at least in part, by anti-apoptosis and reduced calcium suppression.
We also reported that urinary CNP (uCNP) may serve as a biomarker for identifying AKI.
Native pGC-A activators have the potential to treat AKI due to their renal and cardiac protective actions. However, native activators inevitably reduce blood pressure, which may reduce renal perfusion pressure and thereby compromise their renal function enhancement. Here we employed a novel pGC-A activator, CRRL269 which we previously documented possessed superior renal protective effects than native activators in a large animal model of AKI in canines. We reported that CRRL269 significantly improved renal function compared to vehicle treatment, with less kidney injury, renal apoptosis while importantly maintaining the blood pressure. We also reported for the first time that uCNP was elevated markedly during AKI and thus may represent a novel biomarker for AKI. Taken together, our results support the development of CRRL269 for the treatment of AKI.
ACKNOWLEDGMENTS
We are grateful for the insightful comments and suggestions provided by Dr. Karl A. Nath for this manuscript and the exceptional support received from Mayo Clinic Animal Histology Core Laboratory on preparing the histology slides.
SOURCES OF FUNDING
The work was supported by grants from the National Heart, Lung and Blood Institute (NHLBI) R01 HL36634 (J.C.B.), R01 HL132854 (S.J.S.) and Predoctoral Fellowship 16PRE30770009 (Y.C.) from the American Heart Association.
Nonstandard Abbreviations and Acronyms:
- ACC
aortic cross clamping
- ANGII
angiotensin II
- AKI
acute kidney injury
- BP
blood pressure
- cGMP
guanosine 3’,5’ cyclic monophosphate
- GFR
glomerular filtration rate
- I/R
ischemia/reperfusion
- HF
heart failure
- H/R
hypoxia/reoxygenation
- HRPTC
human renal proximal tubular epithelial cells
- NP
natriuretic peptide
- pGC-A
particulate guanylyl cyclase A receptor
- RAS
renin-angiotensin-system
- uCNP
urinary C-type natriuretic peptide
Footnotes
DISCLOSURES
Mayo Clinic, Y.C. and J.C.B. filed a patent for the use of CRRL269 for acute kidney injury treatment. Mayo Clinic, Y.C., S.J.S. and J.C.B. filed a patent for the use of urinary CNP as a potential biomarker for acute kidney injury diagnosis. The authors declare no conflicts of interests.
REFERENCES
- 1.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS. Kidney disease: Improving global outcomes (kdigo) acute kidney injury work group. Kdigo clinical practice guideline for acute kidney injury. Kidney international supplements. 2012;2:1–138 [Google Scholar]
- 2.Go AS, Hsu C-y, Yang J, Tan TC, Zheng S, Ordonez JD, Liu KD. Acute kidney injury and risk of heart failure and atherosclerotic events. Clinical Journal of the American Society of Nephrology. 2018;13:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired aki. Clin J Am Soc Nephrol. 2014;9:1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: The multinational aki-epi study. Intensive Care Med. 2015;41:1411–1423 [DOI] [PubMed] [Google Scholar]
- 5.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453 [DOI] [PubMed] [Google Scholar]
- 6.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, Schaff HV. Clinical accuracy of rifle and acute kidney injury network (akin) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care. 2011;15:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rydén L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation. 2014:CIRCULATIONAHA. 114.010622 [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Rudemiller NP, Patel MB, Wei Q, Karlovich NS, Jeffs AD, Wu M, Sparks MA, Privratsky JR, Herrera M. Competing actions of type 1 angiotensin ii receptors expressed on t lymphocytes and kidney epithelium during cisplatin-induced aki. Journal of the American Society of Nephrology. 2016:ASN. 2015060683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Romo R, Benítez K, Barrera-Chimal J, Pérez-Villalva R, Gómez A, Aguilar-León D, Rangel-Santiago JF, Huerta S, Gamba G, Uribe N. At1 receptor antagonism before ischemia prevents the transition of acute kidney injury to chronic kidney disease. Kidney Int. 2016;89:363–373 [DOI] [PubMed] [Google Scholar]
- 10.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in aki. Journal of the American Society of Nephrology. 2014:ASN. 2014030262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Harty GJ, Huntley BK, Iyer SR, Heublein DM, Harders GE, Meems L, Pan S, Sangaralingham SJ, Ichiki T, Burnett JC Jr. CRRL269: A novel designer and renal-enhancing pgc-a peptide activator. Am J Physiol Regul Integr Comp Physiol. 2018;314:R407–R414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn M Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev. 2016;96:751–804 [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Muraski J, Chen Y, Tsujita Y, Wall J, Glembotski CC, Schaefer E, Beckerle M, Sussman MA. Atrial natriuretic peptide promotes cardiomyocyte survival by cgmp-dependent nuclear accumulation of zyxin and akt. J Clin Invest. 2005;115:2716–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vellaichamy E, Kaur K, Pandey KN. Enhanced activation of pro-inflammatory cytokines in mice lacking natriuretic peptide receptor-a. Peptides. 2007;28:893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kook H, Itoh H, Choi BS, Sawada N, Doi K, Hwang TJ, Kim KK, Arai H, Baik YH, Nakao K. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. American Journal of Physiology-Heart and Circulatory Physiology. 2003;284:H1388–H1397 [DOI] [PubMed] [Google Scholar]
- 16.Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur Heart J. 2013;35:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa H, Somekawa S, Onoue K, Kumazawa T, Ueda T, Seno A, Nakada Y, Nakano T, Matsui M, Soeda T. Salt accelerates aldosterone-induced cardiac remodeling in the absence of guanylyl cyclase-a signaling. Life Sci. 2016;165:9–15 [DOI] [PubMed] [Google Scholar]
- 18.Kong Q, Blanton RM. Protein kinase gi and heart failure: Shifting focus from vascular unloading to direct myocardial antiremodeling effects. Circulation: Heart Failure. 2013;6:1268–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii K, Ishimatsu T, Kuriyama H. Mechanism of vasodilation induced by alpha‐human atrial natriuretic polypeptide in rabbit and guinea‐pig renal arteries. The Journal of physiology. 1986;377:315–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornwell TL, Lincoln TM. Regulation of phosphorylase a formation and calcium content in aortic smooth muscle and smooth muscle cells: Effects of atrial natriuretic peptide ii. J Pharmacol Exp Ther. 1988;247:524–530 [PubMed] [Google Scholar]
- 21.Sandok EK, Lerman A, Stingo AJ, Perrella MA, Gloviczki P, Burnett JC. Endothelin in a model of acute ischemic renal dysfunction: Modulating action of atrial natriuretic factor. Journal of the American Society of Nephrology. 1992;3:196–202 [DOI] [PubMed] [Google Scholar]
- 22.Zakeri R, Sangaralingham SJ, Sandberg SM, Heublein DM, Scott CG, Burnett JC. Urinary c-type natriuretic peptide: A new heart failure biomarker. JACC: Heart Failure. 2013;1:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakeri R, Burnett JC Jr., Sangaralingham SJ. Urinary c-type natriuretic peptide: An emerging biomarker for heart failure and renal remodeling. Clinica chimica acta; international journal of clinical chemistry. 2015;443:108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sangaralingham SJ, Heublein DM, Grande JP, Cataliotti A, Rule AD, McKie PM, Martin FL, Burnett JC Jr. Urinary c-type natriuretic peptide excretion: A potential novel biomarker for renal fibrosis during aging. Am J Physiol Renal Physiol. 2011;301:F943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meldrum K, Meldrum D, Hile K, Burnett A, Harken A. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. Journal of Surgical Research. 2001;99:288–293 [DOI] [PubMed] [Google Scholar]
- 26.Chai Q, Wang X-L, Zeldin DC, Lee H-C. Role of caveolae in shear stress-mediated endothelium-dependent dilation in coronary arteries. Cardiovasc Res. 2013;100:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holly TA, Drincic A, Byun Y, Nakamura S, Harris K, Klocke FJ, Cryns VL. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol. 1999;31:1709–1715 [DOI] [PubMed] [Google Scholar]
- 28.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieberthal W, Nigam SK. Acute renal failure. Ii. Experimental models of acute renal failure: Imperfect but indispensable. American Journal of Physiology-Renal Physiology. 2000;278:F1–F12 [DOI] [PubMed] [Google Scholar]
- 30.Shaw SG, Weidmann P, Hodler J, Zimmermann A, Paternostro A. Atrial natriuretic peptide protects against acute ischemic renal failure in the rat. J Clin Invest. 1987;80:1232–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein HG, Dimitrov D, Atanasova I, Hohenfellner RM, Schmausser U, Natcheff N, Thurau K. Atrial natriuretic factor infusion following acute renal ischemia in anesthetized dogs. Renal physiology and biochemistry. 1992;15:73–82 [DOI] [PubMed] [Google Scholar]
- 32.Montañés I, Badia A, Réngel MA, López-Novoa JM. Renal ammoniagenesis during the recovery phase of postischemic acute renal failure in the dog. Kidney and Blood Pressure Research. 1990;13:264–268 [PubMed] [Google Scholar]
- 33.Neumayer H-H, Blossei N, Seherr-Thohs U, Wagner K. Amelioration of postischaemic acute renal failure in conscious dogs by human atrial natriuretic peptide. Nephrology Dialysis Transplantation. 1990;5:32–38 [DOI] [PubMed] [Google Scholar]
- 34.Humes HD. Acute renal failure--the promise of new therapies. N Engl J Med. 1997;336:870–871 [DOI] [PubMed] [Google Scholar]
- 35.Davis CL, Briggs JP. Effect of reduction in renal artery pressure on atrial natriuretic peptide-induced natriuresis. American Journal of Physiology-Renal Physiology. 1987;252:F146–F153 [DOI] [PubMed] [Google Scholar]
- 36.McCullough PA, Kellum JA, Haase M, Mueller C, Damman K, Murray PT, Cruz D, House AA, Schmidt-Ott KM, Vescovo G. Pathophysiology of the cardiorenal syndromes: Executive summary from the eleventh consensus conference of the acute dialysis quality initiative (adqi) Adqi consensus on aki biomarkers and cardiorenal syndromes. Karger Publishers; 2013:82–98. [Google Scholar]
- 37.Rana I, Velkoska E, Patel SK, Burrell LM, Charchar FJ. Micrornas mediate the cardioprotective effect of angiotensin-converting enzyme inhibition in acute kidney injury. American Journal of Physiology-Renal Physiology. 2015;309:F943–F954 [DOI] [PubMed] [Google Scholar]
- 38.Efrati S, Berman S, Hamad RA, Siman-Tov Y, Ilgiyaev E, Maslyakov I, Weissgarten J. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrology Dialysis Transplantation. 2011;27:136–145 [DOI] [PubMed] [Google Scholar]
- 39.Gayat E, Hollinger A, Cariou A, Deye N, Vieillard-Baron A, Jaber S, Chousterman BG, Lu Q, Laterre PF, Monnet X. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-icu discharge outcome in patients with acute kidney injury. Intensive Care Med. 2018:1–8 [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Tong X, Maimaitiyiming H, Clemons K, Cao JM, Wang S. Overexpression of cgmp-dependent protein kinase i (pkg-i) attenuates ischemia-reperfusion-induced kidney injury. Am J Physiol Renal Physiol. 2012;302:F561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen K, Johnson DW, Gobe GC. The role of cgmp and its signaling pathways in kidney disease. American Journal of Physiology-Renal Physiology. 2016;311:F671–F681 [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Burnett JC. Particulate guanylyl cyclase A/cGMP signaling pathway in the kidney: Physiologic and therapeutic indications. International journal of molecular sciences. 2018;19:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hummel M, Kuhn M, Bub A, Mann B, Schneider B, von Eickstedt KW, Forssmann WG, Hetzer R. Urodilatin, a new therapy to prevent kidney failure after heart transplantation. J Heart Lung Transplant. 1993;12:209–217; discussion 217–208 [PubMed] [Google Scholar]
- 44.Staffel J, Valletta D, Federlein A, Ehm K, Volkmann R, Fuchsl AM, Witzgall R, Kuhn M, Schweda F. Natriuretic peptide receptor guanylyl cyclase-a in podocytes is renoprotective but dispensable for physiologic renal function. J Am Soc Nephrol. 2017;28:260–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devarajan P Update on mechanisms of ischemic acute kidney injury. Journal of the American Society of Nephrology. 2006;17:1503–1520 [DOI] [PubMed] [Google Scholar]
- 46.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (kim-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244 [DOI] [PubMed] [Google Scholar]
- 47.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology. 2003;14:2534–2543 [DOI] [PubMed] [Google Scholar]
- 48.de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: A narrative review on current status and future challenges. Clinical kidney journal. 2012;5:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattingly MT, Brandt RR, Heublein DM, Wei C-M, Nir A, Burnett JC Jr. Presence of c-type natriuretic peptide in human kidney and urine. Kidney Int. 1994;46:744–747 [DOI] [PubMed] [Google Scholar]
- 50.Nir A, Beers KW, Clavell AL, Wei CM, Heublein DM, Dousa TP, Burnett JC Jr. Cnp is present in canine renal tubular cells and secreted by cultured opossum kidney cells. Am J Physiol. 1994;267:R1653–1657 [DOI] [PubMed] [Google Scholar]
- 51.Gülberg V, Møller S, Henriksen J, Gerbes A. Increased renal production of c-type natriuretic peptide (cnp) in patients with cirrhosis and functional renal failure. Gut. 2000;47:852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nazario B, Hu RM, Pedram A, Prins B, Levin ER. Atrial and brain natriuretic peptides stimulate the production and secretion of C-type natriuretic peptide from bovine aortic endothelial cells. J Clin Invest. 1995;95:1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.