Abstract
Recent literature suggests that extracellular vesicles (EV), secreted from most cells and containing cell specific cargo of proteins, lipids and nucleic acids are major driver of intracellular communication in normal physiology and pathological conditions. The recent evidence on stem/progenitor cell EVs as potential therapeutic modality mimicking their parental cell function is exciting since EVs could possibly be used as a surrogate for the stem cell based therapy and this regimen may overcome certain roadblocks identified with the use of stem/progenitor cell themselves. This review provides a comprehensive update on our understanding on the role of EVs in cardiac repair and emphasizes the applications of stem/progenitor cell derived EVs as therapeutics and discusses the current challenges associated with the EV therapy.
Keywords: Extracellular vesicles, exosomes, cardiovascular, biogenesis, stem/progenitor, biomarker, cardiac repair
Introduction
Cardiovascular disease (CVD) remains one of the major health issues in the US, with greater than 1 in 3 American adults suffering from at least one form of CVD1. Current therapies for CVD do prolong patient’s life, however they do not actually regenerate the damaged cardiac muscle tissue. From this perspective, stem/progenitor cells have shown great promise in cardiac repair and regeneration. In recent times, a consensus has emerged that adult stem/progenitor cells largely exert their therapeutic benefits via paracrine factors. Several stem/progenitor cell derived paracrine factors have been studied as a therapeutic module for cardiac repair including conditioned medium, cytokines, growth factors, extracellular vesicles including exosomes and micro vesicles2–7. Of note, the current paradigm in cardiac regenerative medicine is to understand potential stem cell derived factors that might enhance cardiac repair process and is of immense biological and therapeutic importance. This review provides a comprehensive update on our understanding on the role of EVs in cardiac repair and emphasizes the applications of stem/progenitor cell derived EVs as therapeutics and also discusses different cardiac cell derived exosomes as potential biomarkers for cardiovascular disease and the current challenges associated with the EV therapy. EV targeting and uptake by specific recipient cells as well as the importance of systems biology and computational modeling tools in EV therapy is also discussed in this review.
EV nomenclature, biogenesis and isolation
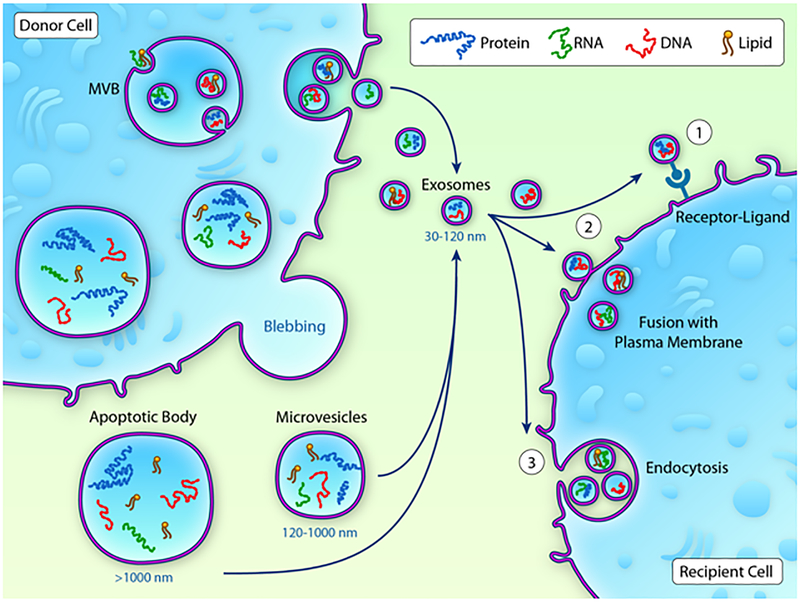
EVs can be isolated from wide range of sample sources such as blood, plasma, pericardial fluid, cell culture conditioned media, etc.; however, the cell type specific isolation of EVs in vivo is still technically challenging. So far there is a lack of consensus on the EVs nomenclature. Typically, the current classification has divided EVs into multiple subtypes: I) EVs with diameters ranging 30–120 nm are the smallest vesicles that are formed by exocytosis of multi-vesicular bodies (MVBs) and are defined as exosomes. Inward budding of membrane forms early endosome which further results in the accumulation of intraluminal vesicles (ILVs). ILVs are formed by endosomal sorting complex required for transport (ESCRT, four protein complexes that guide intracellular cargo) or non-ESCRT mechanisms including lipid rafts and tetraspanins8–10. ILVs consisting of cytoplasmic molecules such as proteins, lipids and nucleic acids within large multi vesicular bodies (MVB). These formed MVBs can be directed towards lysosomes for degradation or directed towards plasma membrane, where MVBs fuse with the plasma membrane to release the exosomes into the extra cellular space (depicted in Fig-1). This process of exosomes release has a set of mechanisms proposed, including Rab GTPases (Rab11/35/27)11, 12, SNARE (soluble N-ethylmaleimide-sensitive attachment protein receptor)13 complex and aforementioned tetraspanins10. Also, genotoxic stress has been reported to augment exosome secretion in tumor cells via p53 activation14. Based on the physiological/pathological state of a given source, the number and content of exosomes can change impacting the cellular functions of the recipient cells15. II) Microvesicles, also called micro particles, are intermediate size of EVs with their diameters ranging 0.1–1μm. These vesicles which bud directly from the plasma membrane of various cell types (including cardiomyocytes), carry cell surface markers and cytosolic contents similar to their cell of origin. These MVs are formed by increased cytosolic calcium leading to the activation of a calcium sensitive protease called calpain, which cleaves cytoskeletal proteins and remodels cytoskeleton facilitating the blebbing to occur16, 17 III) Apoptotic bodies which are the largest vesicles include particles >1μm formed by blebbing of apoptotic cells. These vesicles are formed during the last steps of apoptosis, typically by caspase dependent cleavage mediated activation of Rho-associated kinase-15, 18, carry cellular organelles and other cytosolic contents including degrading proteins, RNAs, and DNA. Another type of MVs include argosomes, which are released from the basolateral membrane of morphogen synthesizing cells ofthe fly (Drosophila melanogaster). Argosomes carry morphogens, which governs tissue patterning during development in multicellular organisms19. Examples of the characteristics of exosomes, microvesicles and apoptotic bodies are shown in Table-1.
Fig-1: Exosome biogenesis:
Inward budding of membrane forms early endosome, which further results in accumulation of intraluminal vesicles (ILVs) consisting of cytoplasmic molecules such as proteins, lipids and nucleic acids within large multi vesicular bodies. Conversely, microvesicles and apoptotic bodies are formed by the outward budding of the plasma membrane. Both exosomes and microvesicles carry proteins, lipids and nucleic acids and transfer to recipient cells either by receptor ligand mediated transfer or directing fusing with the plasma membrane or through endocytosis.
Table-1:
Exosomes, microvesicles and apoptotic bodies characteristics. MVB: Multi Vesicular Bodies.
| Characteristics | Exosomes | Microvesicles | Apoptotic bodies |
|---|---|---|---|
| Morphology | Spherical-shaped | Heterogeneous | Heterogeneous |
| Origin | MVB | Plasma membrane | Plasma membrane |
| Protein markers | CD81, CD63, Alix, TSG-101 | Selectins, integrins, CD40 | Caspase-3, histones |
| Cargo | Proteins, lipids, RNA, DNA | Proteins, lipids, RNA, DNA | Cell organelles, Proteins, lipids, RNA, DNA |
Different biochemical and biophysical methods are used for the isolation of EVs. These methods isolate EVs mainly based on their size, shape, charge and surface markers. Here, we explain some of the most applied EV isolation methods and cover their pros and cons: Differential centrifugation (DC) is an easy yet time-consuming method for EV isolation which mainly isolates exosomes and microvesicle particles20, 21. Given the complexity associated with different subtypes of EVs, Thery et al demonstrated sedimentation of EVs based on the ultra-centrifugation speed22, 23 and suggested particles sediment at 100,000g are small EVs (including exosomes), the ones sediment at 20,000g are intermediate EVs (including microvesicles), and particles that sediment at 2000g are large EVs (includes apoptotic vesicles)22, 24, 25. Therefore, in broader terms, different types of experimentally isolated vesicles have been recommended to be collectively called as “extracellular vesicles”. The presence of lipoprotein and ribonucleoprotein contaminations such as AGO2 has been reported to be co-precipitated with EVs upon ultracentrifugation. Overall, the isolation of same-sized particles (like virus contamination) and the formation of EV aggregates are the pitfalls of DC method20. To overcome this issue, recent reports recommend to perform a density gradient centrifugation (DGC) (sucrose or preferably iodixanol)22. Similar to differential centrifugation, DGC is also an easy but time-consuming isolation method. DGC is widely used for the isolation of exosomes. Lipoproteins such as HDL and LDL molecules have been reported to be co-isolated along with EVs20, 26, 27. Other concerns also include damage of EVs upon ultracentrifugation and aggregation of exosomes. Aggregation of exosomes pose a new layer of complexity, which might give false positive results of increased EV size. Size exclusion chromatography (SEC) is another method that separates EVs based on their size on a single chromatography column and has recently gained interest for EV isolation. This exciting option can be utilized to separate EVs from bulk and soluble proteins27. However, since EVs are separated based solely on the size of the particles, the contamination with the same size particles is still a concern and the chances of co-isolating LDL or HDL molecules along with EVs is high28, 29. Overall, SEC is a fast technique with relatively a high recovery rate. Using this technique, most of the isolated EVs are biophysically intact20. Alternatively, ultrafiltration (UF) method separates EVs from soluble components through a filter while applying an external force like ultracentrifugation. Although, EV particles may aggregate during UF, this method is fast with a high recovery rate of EVs. Immuno-affinity capture assay (IC) is another alternative method that uses monoclonal antibodies on a surface to bind to and isolate subpopulations of EVs. Despite being a very time-consuming method, IC can yield pure population of different EV subtypes but the success relies majorly on the choice of the affinity reagent and ligand density on different EV types30. Alternatively, microfluidic devices have been recently shown to enrich exosomes size ranging between 30–200nm using polycarbonate membranes31. The captured EVs can be analyzed using on-chip ELISA, although future studies rigorously testing this new technology are needed. Finally, precipitation assay, which is mainly a concentration method, uses precipitation kits to isolate EV particles. In this method, various commercial kits, based on volume excluding polymers (for example poly ethylene glycol) are being used to rapidly precipitate EVs. Although precipitation assay is fast with a high recovery rate, but protein contamination and the formation of EV aggregates are the concerns of this method20. In this technology, co-precipitation of lipoproteins and residual matrix as well as soluble proteins might affect the function of EVs.
Fetal bovine serum (FBS) or fetal calf serum (FCS) represent a potential source of exosomes contamination in the cell culture supernatant. FBS and FCS contain large number of bovine/calf serum exosomes and lipoproteins, which can be co-isolated with the EVs and influence EVs biological effects on recipient cells. Bovine/calf serum exosomes can be essentially removed by ultracentrifugation of FBS/FCS at 100000 g for 18h32fFB. In this regard, several companies market exosome free FBS. However, the method for removal of exosomes from FBS/FCS is not detailed. Alternatively, considering EVs for clinical use, future work on isolation of EVs using synthetic serum or serum free conditions might be beneficial.
Almost none of the EV isolation methods extract a pure sample of EV and each method has its advantages and limitations. Despite the challenges of each of the methods, the technical aspects of EV isolation are rapidly growing with the possibility of standardizing universal protocol (s). To overcome the lack of consensus on the most effective method for EV isolation, and to centralize all EV related methods, Van Deun et al. initiated EV-TRACK website (http://evtrack.org/) with the goal of encouraging researchers to share their experimental techniques and guidelines with their peer scientists33. On the other hand, the International Society for Extracellular Vesicles (ISEV) which also focuses on EV studies, provides researchers an updated and comprehensive source of EV studies34.
EV Cargo
Most of the EVs constitutively express certain molecules including tetraspanins (CD9, CD63, CD81 and CD82), heat shock proteins (Hsc70, Hsp90), Rab GTPases, flotillin, Alix, TSG101, MHC-class I and class II, regardless of the source24, 35. However, recent studies on exosomes characterization recommends co-expression of tetraspanins (CD63, CD9 and CD81) and endosomal proteins (syntenin-1 and TSG101)24. Furthermore, EV subtypes can be identified based on the markers enriched with endosomal proteins or plasma membrane proteins such as selectins, integrin’s and CD40 expression36. Immune cells, such as B cells, T cells and dendritic cells have been demonstrated to mediate MHC-I and MHC-II dependent immune responses upon EV secretion37, 38. Additionally, a comprehensive database on a growing list of EV contents can help identifying potential markers (www.exocarta.org/). Secondly, cargo molecules (including proteins, RNA, miRNAs, and lipids) within EVs may vary with a specific cell type or physiological or pathological condition. For instance, Gyorgy et al. analyzed exosome and microvesicle-associated protein contents by Ingenuity Pathway Analysis (IPA) and reported that both types of vesicles are highly involved in cellular movement, cell-to-cell signaling, tissue development and cancer. However, unlike exosomes, microvesicles were shown to be more associated with inflammation and cell death biological functions21. Furthermore, we have recently shown that bone marrow endothelial progenitor cells secrete dysfunctional exosomes with drastically altered exosomal cargo (RNAs and proteins) under inflammatory stimulus39. Also, recent reports indicate active sorting of specific miRNAs in to the exosomes distinct to the cytoplasmic RNAs, suggesting exosomal miRNA mediated regulation of their target genes in the recipient cells40–43. In addition to miRNA, we and others have shown several other RNA species including lncRNAs, tRNA, scaRNA, piRNA, rRNA in the exosomes15, 44. A recent report shows miRNAs constitute a small portion of the RNA present in the CDC-exosomes (~7%), whereas ~18% is comprised of Y RNA45. It will be interesting to explore in depth whether these less studied RNA species exhibit similar gene regulatory functions as demonstrated by miRNAs in EVs. However, little is known about the underlying mechanisms how specific RNA species are packaged with in the EVs or whether different subtypes of EVs from a given source have similar distribution of these RNAs remains an open question.
EVs visualization and characterization
Characterization of EVs is required for studying the functional role of each single EV. EVs can be visualized and characterized by a variety of different methods including electron microscopy, flow cytometry, mass spectrometry, and western blot assays. Other novel methods like nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), resistive pulse sensing (RPS), and nano-plasmonic exosome (nPLEX) assay are also applied for EV detection and characterization. Electron microscopy, mainly transmission electron microscopy (TEM), is the standard method for EV visualization that can detect even the smallest EVs. One method to visualize EVs by TEM is Immuno-gold labeling. In this approach, proteins and lipids on the surface of EVs are labeled with gold particles and detected by TEM46. Electron microscopy detection techniques give high-resolution images of EVs and are capable of distinguishing EV particles from same-sized non-EV contaminants. Overall, EVs morphology can be detected by transmission electron microscopy47, cyro-electron microscopy48 and atomic absorption microscopy.49 Furthermore, flow cytometry is a more general technique that is used for the detection of all different types of EVs (including exosomes, microvesicles, and apoptotic bodies). Flow cytometry is fast and can help to identify/sort subpopulation of EVs based on specific membrane markers20, 21, 50–52. Moreover, consensus is building towards using NTA or DLS to evaluate the quality or quantity of EVs. However, NTA do not distinguish the EVs associated contaminants such as LDL and ribonucleoproteins26, 53, 54, due to its limitation of identifying particles beyond 50nm including LDL. Whereas, DLS is a method used for determination of the size distribution of particles, ranging in size from 1 nm to 6 μm, therefore can detect LDL particles (10–20nm). Nevertheless, NTA distinguishes the size and concentration of EV particles by tracking their Brownian motion, which is important to evaluate the EVs yield from a given source. Therefore, other approaches which intend to quantify EVs purity, such as particle number or protein concentration to lipid content can be performed29, 55–57.57.58. Further, resistive pulse sensing (RPS) is another technique that determines the size and concentration of EV particles by Coulter principle59. Similar to nanoparticle tracking assay, this method is also not capable of distinguishing EVs’ associated contaminants20. On the other hand, nano-plasmonic exosome (nPLEX) assay is a novel, high-throughput, sensitive technique that has been used for quantitative analysis of exosomes for the detection of cancer biomarkers including ovarian cancer. nPLEX assay is based on transmission surface plasmon resonance which can detect exosomal surface proteins and lysate components by the application of specific antibodies. This technique can be expanded and applied for the characterization of exosomes originating from cells other than cancer cells60.
EV-Mediated Intercellular Communication
Several mechanisms of EV uptake and transfer to recipient cells have been proposed. EVs can deliver their cargo through endocytosis (including clathrin or micropinocytosis or raft domain mediated endocytosis) or they can directly fuse with the recipient cell membrane and deliver the cargo with or without specific receptors. Alternatively, exosomes could enter the cells through the actin-rich filopodia protruding the cells61. Lipid rafts in the plasma membrane could also play a role in the EV uptake. EVs may also release their cargo in the extracellular space to activate the recipient cells. Furthermore, EVs can trigger the cellular signaling in recipient cells by receptor-ligand interaction62. Thus, EV cargo (depicted in Fig-2) participate in the intracellular communication between different cardiac cell types including cardiomyocytes, fibroblasts, endothelial cells and macrophages in both physiological and pathological conditions. The effect of EV on cardiovascular recipient cells has been widely reported and is summarized in Table-2. In the following section, we will discuss the examples of the mechanisms of the communication between the cardiac cells in vitro, as isolation and function of EVs in vivo is still challenging and is currently an active area of investigation.
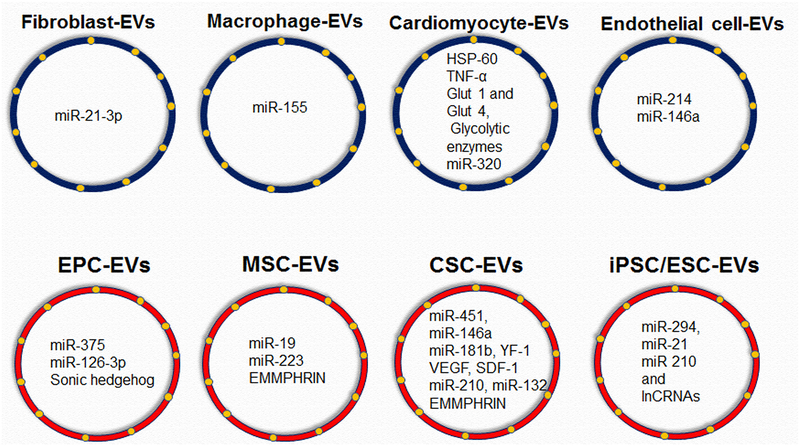
Fig-2: EVs cargo of different cardiac cells and different stem/progenitor cells:
(A) cardiac cells including fibroblasts, macrophages, cardiomyocytes and endothelial cells EV cargo enriched with RNA/proteins etc.; (B) stem/progenitor cells including endothelial progenitor cells, mesenchymal stem cells, cardiac stem/progenitor cells, induced pluripotent stem cells and embryonic stem cells EV cargo enriched with RNA/proteins etc.
Table-2:
Effects of EVs on different cardiac cells.
| EV- donor cells | Size | Functional unit | EV-Recipient cell | Function | Reference |
|---|---|---|---|---|---|
| Cardiomyocytes | ND | HSP60 | ND | ND | 64 |
|
Cardiomyocytes Rat NRVM |
50–80 nm | TNF-α | NRVM | Inflammation | 65 |
| Cardiomyocytes | 60–65 nm | AT1R | Cardiomyocytes | Modulate vascular responses to neurohormonal stimulation | 174 |
|
Cardiomyocytes Rat NRVM |
<70 nm | Glut 1 and Glut 4, Glycolytic enzymes | Endothelial cells | regulate glycolytic flux in endothelial cells | 175 |
| Cardiomyocytes | ND | miRNAs/did not specify | Endothelial cells | Promotes angiogenesis | 176 |
|
Cardiomyocytes (Healthy and Goto-Kakizaki diabetic) |
~ 77–78 nm | miR-320 | Endothelial cells | Vascular dysfunction | 177 |
| Fibroblasts | 50–100 nm | ND | Cardiomyocyte | Cardiomyocyte hypertrophy | 178 |
| Fibroblasts | 50–100 nm | miR-21 | Cardiomyocyte | Cardiomyocyte hypertrophy | 73 |
| Macrophages | 40–100 nm | miR-155 | Cardiac fibroblasts | Fibrosis and inflammation | 74 |
| Endothelial cells | 120 nm | miR-214 | Endothelial cells | Enhanced endothelial cell function | 70 |
| Endothelial cells | 100 nm | 146a | Endothelial cells and cardiomyocytes | Anti-angiogenesis and impair cardiac function | 71 |
| Endothelial cells | 100 nm | Altered RNA and protein composition | ND | Hypoxia and inflammation alters mRNA and protein composition of exosomes | 40 |
| Endothelial progenitor cells | 100 nm | miR-375 | Endothelial | Endothelial cell survival | 15 |
(ND, not determined; nm, nanometers; HSP, heat shock protein; TNF, tumor necrosis factor; NRVM, Neonatal rat ventricular myocytes; AT1R, Angiotensin II receptor type 1; Glut, glucose transporter)
Cardiomyocyte-derived EVs
Cardiomyocytes constitute the important cells in the heart responsible for generating contractile force63. EVs secreted from neonatal cardiomyocytes has been recently studied. Gupta et al has shown that HSP60 is released from cardiac myocytes through exosomes64. Further, a study has shown the possibility of hypoxia triggering TNF-α packaging in exosomes in the cardiomyocytes which may lead to the stimulation of inflammatory response65. Also, mechanical stretch of cardiomyocytes secretes exosomes enriched with angiotensin II type-1 receptors (AT1Rs) and modulate vascular responses to neurohormonal stimulation by targeting cardiac muscles66. β-arrestin-2 G-protein coupled receptor plays an essential role in the packaging of AT1R cargo in the exosomes. Further, AT1R-enriched exosomes administration improved blood pressure induced by angiotensin II in AT1R-KO mice. These findings propose that EV surface proteins may affect tissue-specific targeting66.
Garcia et al demonstrated that neonatal cardiomyocytes under glucose deprivation release exosomes carrying glucose transporters and glycolytic enzymes to recipient endothelial cells to increase glucose uptake, glycolytic activity and pyruvate synthesis67. Further the same group has shown that glucose deprivation in rat cardiomyocyte cell line releases exosomes that enhance cell proliferation and angiogenesis of the recipient endothelial cells68. Overall these studies shed light on the role of exosomes in intracellular communication between CM and ECs under glucose deprivation and nutrient stress.
Wang et al mimicked diabetic cardiomyopathy in vitro by subjecting the cardiomyocytes to hyperglycemic and hypoxia stress and observed that exosomes secreted by these cells and taken up by recipient endothelial cells, exerted anti-angiogenic functions via miR-320 targeting GF-1, Hsp20 and Ets269. This study provides a novel mechanism of diabetes induced vascular dysfunction mediated via cardiomyocyte exosomes. These observations emphasize the importance of cardiomyocyte EVs as potential biomarkers in pathological stress.
Endothelial cell and fibroblast derived EVs
Vascular endothelial cells which line the entire circulatory system secrete vesicles that exchange biological messages with multiple cardiac cells. A recent report suggested that endothelial cells subjected to hypoxia and inflammation can influence the exosomal mRNA and protein cargo suggesting cellular stress effects on the exosome content40. Further, it has been shown that endothelial cells secreting exosomes enriched with miR-214 suppress senescence and promote angiogenesis and migration targeting ataxia telangiectasia mutatedprotein70. Halkein et al reported miR-146a as a critical mediator of the antiangiogenic effects in endothelial cells in a model of post-partum cardiomyopathy. Further this study also reported that endothelial cell exosomes loaded miR-146a can be transferred to cardiomyocytes and impair their metabolism by targeting erb-b2 receptor tyrosine kinase 4 (ERBB4), NOTCH1, and interleukin-1 receptor-associated kinase 1 (IRAK1). Further, in a mouse model of peripartum cardiomyopathy, locked nucleotide acid (LNA) modified antagomiR-146a treatment attenuated heart failure phenotype71. In contrast, cardiosphere derived cell derived exosomes enriched miR-146a has been shown to exhibit cardio-protection in a mouse model of MI, downregulating IRAK-1 levels. Thus, beneficial or deleterious role of exosomal miR-146 appears to be dependent upon cells and stimuli and warrants further investigation.
Cardiac fibroblasts constitute the major proportion of the heart. Activated fibroblasts play an important role in fibrosis. Cardiac fibroblasts in response to angiotensin-II release exosomes into cardiomyocytes to induce cardiomyocyte hypertrophy via activating AT1R and AT2R72 and suppression of exosomes release by GW4869 and dimethyl amiloride (DMA) inhibited cardiomyocyte hypertrophy. Further it has been shown that, cardiac fibroblasts EV enriched with elevated levels of miR-21 mediate cardiomyocyte hypertrophy. Authors found that cardiac fibroblasts exosome derived miR-21* as a key paracrine mediator targeting sorbin and SH3 domain-containing protein 2 (SORBS2) and PDZ and LIM domain 5 (PDLIM5) in cardiomyocytes to induce hypertrophy. Furthermore, therapeutic inhibition of miR-21* attenuated Ang-II induced cardiac hypertrophy pathology in mice73. These results suggest that specific targeting of cardiac fibroblasts exosomes release or CFs enriched exosomal miRs may be a potential novel therapeutic approach for the treatment of pathological hypertrophy or heart failure.
Macrophage derived EVs
Macrophages are an important mediator of inflammation in cardiac injury. Lyu et al has shown that during the cardiac injury, stimulated macrophages increased miR-155 enrichment in exosomes and act as a major paracrine mediator of fibroblast proliferation by downregulating Son of Sevenless 1 expression and fibroblast inflammation by decreasing suppressor of cytokine signaling-1 expression74. These effects were reversed by miR-155 antagomiR. Using miR-155 KO mice, authors have further demonstrated that transplantation of wild-type macrophage exosomes into miR-155 KOmice aggravated cardiac rupture and adverse cardiac remodeling after MI. Thus, macrophage-enriched exosomal miR-155 appears to impair cardiac repair after AMI and targeting miR-155 may be a potential therapeutic approach after MI.
Plasma-derived EVs
Treatment with plasma exosomes isolated from rat and healthy human volunteer was reported to augment cardio-protective signaling in myocytes in vitro and ex vivo and in vivo models of ischemia-reperfusion (I/R)60. Authors identified that TLR-4 mediated interaction of cardiomyocytes with HSP70 on plasma exosomes led to activation of HSP27 and ERK 1/2 signaling pathway, playing a key role in cardio protection. Further, cardiomyocyte survival was inhibited by a neutralizing antibody against HSP70 on exosomes or TLR4 in cardiomyocytes, suggesting plasma exosomes as novel therapeutics for cardiac repair62. However, the same group has recently reported that plasma exosomes derived from rats or humans with type II diabetes could not activate ERK1/2 and therefore did not offer cardio-protection75. How diabetes affects plasma exosome function is yet to be determined.
On a whole, above studies demonstrated that exosomes derived from different cells subjected to pathological stress have detrimental effects on the post- cardiac injury repair. These observations suggest that therapeutic targeting of exosomes release or exosomal content could represent a novel avenue for the treatment of cardiovascular diseases.
Pericardial fluid derived EVs
Pericardial fluid (PF)harbors heart secreted hormones and has been well known to reflect the cardiac function76. In this regard, Cristina et al showed that PF exosomes derived from aortic valve replacement (AVR) patients showed enriched let-7b-5p levels. PF exosomes treatment improved survival, proliferation, and tube formation ability of endothelial cells (ECs) in vitro. Importantly, PF exosomes treatment improved blood flow recovery and angiogenesis after hind limb ischemia in mice compared to the plasma exosomes. Mechanistically, PF exosomes enriched with let-7b-5p regulates its target gene transforming growth factor beta receptor-1 (TGFBR1) expression to mediate aforementioned functional benefits77. Further, one other study by Eleonora et al, using shot-gun proteomics approach identified clusterin in PF exosomes derived from acute MI patients. Further, authors have shown using a mouse model of MI that exogenous delivery of clusterin activated epicardial cells, enhanced arteriogenesis and reduced apoptosis leading to improved cardiac function78. Collectively suggesting PF exosomes as a novel therapeutic regimen for the treatment of cardiovascular disease.
Stem Cell EV mediated cardiac repair
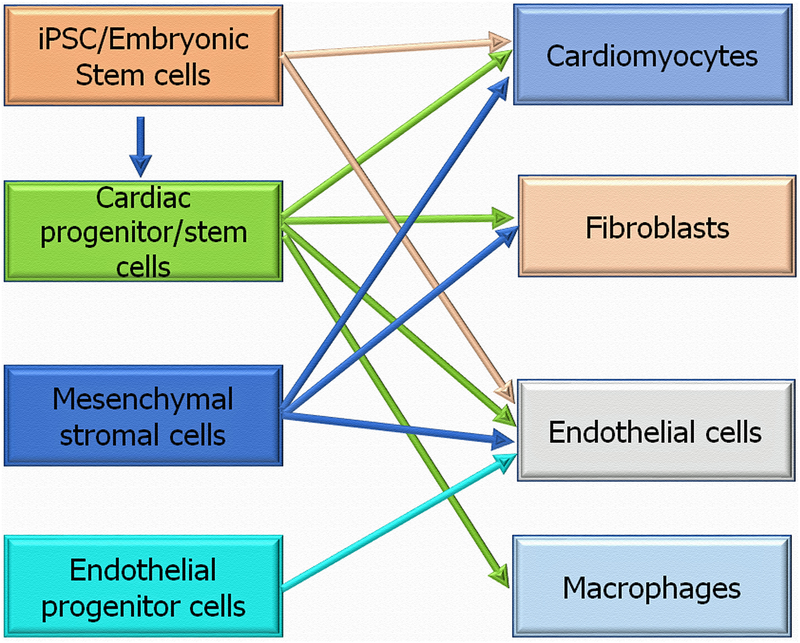
Stem cells hold great promise for potential clinical therapeutic strategies. Increasing evidence from animal models of experimental ischemic injuries, including those from ours and other independent labs79–87 suggest that stem/progenitor cells participate tissue repair and regeneration of the ischemic myocardium. Furthermore, clinical trials involving different stem /progenitor cell transplantation for ischemic myocardium has confirmed this possibility88–92. However, despite successful evidence from several pre-clinical studies, the therapeutic efficacy observed in clinical trials remains modest. Low retention and viability as well as diminished function of transplanted autologous stem cells obtained from patients with existing heart disease and associated co-morbid conditions like diabetes and systemic inflammation remains critically unresolved problems that limit the realization of full functional benefits of cell therapy and warrants alternate strategies to enhance efficiency of cell based therapies79, 80, 93. Stem/progenitor cell derived exosomes provide one such alternate cell-free therapeutic modality. Novel, non-traditional use of cell-free components of stem/progenitor cells such as exosomes, loaded with stem cell-specific miRs and proteins (depicted in Fig-2) may allow for harnessing the regenerative power of these cells to augment and modulate endogenous protection by transferring the cargo to various cardiac cells (depicted in Fig-3) and stimulate repair processes in the ischemic myocardium3, 6, 45, 94–97. Examples of stem/progenitor cells EVs communication with different cardiac cells in the injured heart are shown in Table-3 and discussed below.
Fig-3: Stem/progenitor cells EVs communication with different cardiac cells in the injured heart:
Stem progenitor cells secreted exosomes transfer RNA and proteins to recipient cardiac cells (cardiomyocytes, fibroblasts, endothelial cells and macrophages) to repair the injured heart.
Table-3:
Stem/progenitor cells EVs mediated cardiac repair
| EV Source |
Experimental model | Functional unit | Function | Reference |
|---|---|---|---|---|
| ESC/iPSC | MI | miRNA/lnCRNA | Cardio protective | 44 |
| ESC | MI | miR-294 | Improved cardiac function. Enhanced angiogenesis and cardiomyocyte survival and stimulation of the survival and proliferation of CPCs | 94 |
| iPSC | MI | miR-21 and miR 210 | Cardiomyocyte survival | 99 |
| CD34 cells | HLI | miR-126–3p | Enhanced angiogenesis in vitro and in vivo | 97 |
| CD34 cells | Matrigel plug assay Corneal angiogenesis | ND | Enhanced angiogenesis in vitro and in vivo | 6 |
| CD34 cells | MI | Sonic hedgehog (Shh) | Restored cardiac function and enhanced neovascularization. Shh was enriched in exosomes and transferred to recipient cells | 96 |
| Rat MSC | MI | miR-19 | Reduction in infarct size and improved cardiac function | 112 |
| Human MSC | MI | ND | Reduction in infarct size, improved cardiac function and enhanced angiogenesis | 179 |
| Mouse MSC | MI | miR-223 | Anti-apoptoticand anti-inflammatory | 180 |
| Human umbilical cord MSC | MI | ND | Reduce cardiomyocyte apoptosis and enhance neovascularization | 109 |
| Human MSC | Matrigel plug assay | EMMPHRIN | Enhanced angiogenesis | 110 |
| Mouse CPC | MI | miR-451 | Suppression of apoptosis | 110 |
| Human CDC | MI | miR-146a | Inhibit cardiomyocyte apoptosis, enhance neovascularization, cardiomyocyte proliferation | 3 |
| Human CDC | I/R injury | miR-181b | Cardio protection | 120 |
| Human CDC | I/R injury | EV-YF-1 | Reduce infarct size and improve cardiac function | 45 |
| Human CDC | MI | VEGF and SDF1-α | Primed fibroblasts stimulated angiogenesis and cardio protection | 117 |
| Neonatal human CPC | MI | HSF-1 | Enhanced neovascularization and improved cardiac function | 124 |
| Human CPC | MI | Cluster of miRNAs | Improved cardiac function | 95 |
| Human CPC | MI | miR-210, miR-132, and miR-146a-3p | Reduced apoptosis, enhanced angiogenesis and improved cardiac function | 110 |
| Human plasma | RIC | HSP70 | TLR4 mediated activation of MAPK/ERK1/2 signaling leading to cardio protection | 181 |
(ESC, embryonic stem cell; iPSC, inducible pluripotent stem cell; CPC, cardiac progenitor cell; HSP, heat shock protein; MSC, mesenchymal stem cell; CDC, cardiosphere derived cell; MI, myocardial infarction; HLI, hind limb ischemia; I/R, ischemia/reperfusion injury; RIC, remote ischemic preconditioning; ND, not determined).
Pluripotent stem cell EVs
Since iPSC discovery by Yamanaka, iPSCs revolutionized the research field as somatic cells can now be reprogrammed to pluripotent stem cells, thus circumvent the immune rejection and ethical issues associated with embryonic stem cells98. As iPSC therapy might induce teratomas, differentiation of iPSC to cardiomyocytes offers new possibilities and significant advantages in regenerative medicine98.
Post I/R injury, iPSC-derived exosome therapy exhibited cardio protective effects via miR-21 and miR 210 targeting Nanog and HIF-1α, respectively99. Further, a recent study also proposed exosomes carrying miRNAs and lncRNAs in iPSC and ESC derived exosomes and iPSC and ESC derived cardiomyocytes transplantation exhibited reparative abilities post myocardial ischemia in mice44. Thus, autologous iPSC derived cardiomyocyte exosome therapy might be envisaged as a potential option for cardiovascular regenerative medicine. EV transfer of miRNAs to the recipient cells should be carefully interpreted, because there might be a possibility that a component of EVs induce endogenous expression of a specific miR rather than by the EV enclosed miRNA transfer. For instance, we have shown that embryonic stem cell exosomes transfer miR-294 to CPC which lack this miRNA (as this miR is not expressed in adult cells/animals). Further, we have shown that mouse embryonic stem cell exosomes secreting miR-294 enhanced myocardial repair by augmenting resident CPC proliferation and differentiation as well as activating cardiomyocyte cell cycle re-entry94. It has been shown that hiPSC-derived microvesicles (MV) could also effectively transfer their cargo to cardiac mesenchymal stromal cells (cMSC) and stimulate cardiac and endothelial differentiation in these cells. Therefore, similar to hiPSC-derived exosomes, hiPSC-derived MV effects could be exerted in cardiac tissue repair and stem cell therapy medicine100. On the other hand, Adamiak et al has shown that, mouse iPSC-EVs compared to mouse iPSCs do not form teratomas and more efficacious in cardiac repair after myocardial ischemia/reperfusion (I/R) injury in mice, mediated via transfer of multiple cardio protective miRNAs viz. miR-145, let-7 family and miR-302a-5p, miR-290–295, miR-19b, miR-20a, miR-126–3p, miR-130a-3p, miR-210–3p and miR-17–92 involved in cell proliferation, differentiation, angiogenesis, anti-apoptosis, adaptation to hypoxic stress, regulation of cell cycle, self-renewal and pluripotency. Similarly, several proteins involved in angiogenesis and remodeling were identified as nodal proteins in the network analysis. Thus, iPSC-EVs therapy has the potential for cardiac repair is extremely encouraging and may have future clinical implications for cardiovascular regenerative medicine.
Endothelial progenitor cell EVs
Increasing evidence from animal models of experimental ischemic injuries including those from our labs15, 79–81, suggest that EPCs participate in the process of neo-vascularization and tissue repair leading to enhanced recovery of ischemic myocardium. However, ample evidence from the literature indicated that the transplanted cells secrete angiogenic growth factors and orchestrate ischemic tissue neovascularization and repair by paracrine mechanisms6.
In a very first demonstration that stem/progenitor cell derived exosomes can mimic angiogenesis responses just like their parent cell of origin, we reported that exosomes from human CD34+ hematopoietic cells augment angiogenesis both in vitro and in vivo101. Furthermore, we demonstrated ex vivo modification of human CD34+ cells with sonic hedgehog79 can preserve cardiac function in face of ischemic injury. Importantly, the cardio protective effect of CD34+ cells are mediated by sonic hedgehog (Shh) packaging in exosomes produced by the CD34+ cells that not only protected existing cardiomyocytes but enhanced angiogenic response of the heart. Further, a recent study has shown that, human CD34-Exo enriched miR-126 transfer to endothelial cells targeting SPRED1 enhance angiogenic and therapeutic benefits following ischemic insult102. Since content and function of exosomes mirror the physical and genetic state of the cell, it is unclear if exosomes from dysfunctional stem cells would be therapeutically functional. In these lines, we have recently shown that EPC exosomes under inflammatory stimulus (IL-10 null EPC) exhibit altered exosome cargo especially via increased levels of miR-375 in exosomes from IL-10KO EPCs103. Interestingly, miR-375 modulation in the IL-10KOex partly rescued endothelial cell protection activity subjected to H202 stress104. These exciting results suggest that stem/progenitor cell-specific dysfunction observed in their exosomes can be rescued by modulation of their contents.
Mesenchymal stem/stromal cell EVs
Mesenchymal stem/stromal cells (MSC) represent the most extensively studied cell types for paracrine signaling in therapeutic applications105–107. MSC-exosomes preconditioned cardiac stem cells transplantation enhanced neovascularization, reduced fibrosis and improved cardiac function post-MI. Further miRNA profiling revealed exosomes were enriched with miR‐147, let‐7i‐3p, miR‐503‐5p, and miR‐362‐3p associated with targets genes involved in cell proliferation, differentiation, migration and angiogenesis. Thus suggesting MSC-exo pretreatment can be a potential strategy to improve survival and angiogenic ability of transplanted CSCs108. Further, it has been shown that MSC-Exo enhance myocardial viability and adverse remodeling by increasing ATP levels, reducing oxidative stress and promoting AKT activation in a myocardial infarction model109. Interestingly, a recent study has shown that extracellular matrix metalloproteinase inducer (EMMPRIN) enriched MSC-exo transplantation in mice induced blood vessel growth in matrigel plugs was mediated through ERK/AKT axis validating the angiogenic effects of MSC-Exo110 . MSCs preconditioning with hypoxia has been shown to enhance angiogenesis. Gonzalez-King et al studied the contribution of HIF-1α-overexpressing MSC-exo. Intriguingly, exosomes derived from MSCs overexpressing HIF-1α demonstrated increased angiogenic capacity of human umbilical vein endothelial cells (HUVEC) in part via elevated levels of Jagged1 in the HIF1-α overexpressing MSC-Exo111. Similarly, exosomes secreted by GATA-4 overexpressing MSCs restored cardiac function and cardiomyocyte survival by transferring miR-19a to cardiomyocytes and activating ERK/AKT survival signaling112. Mesenchymal stem cells (MSCs) have been shown to be cardio-protective effects in sepsis models, but mechanistic insights were unclear. In this regard, a recent study evidenced miR-223 contribution to MSC-elicited protective effects against sepsis through the exosome-mediated transfer of miR-223 to macrophages and cardiomyocytes leading to reduced inflammatory responses and cell survival, respectively, by down-regulating its targets semaphorin 3A and signal transducer and activator of transcription-3 expression. These results suggest a new role of exosomes mediated cardio protection in sepsis113. Very interestingly, Luo et al recently introduced synthetic MSC (synMSC), where human MSC secreted factors were packaged into poly (lactic-co-glycolic acid) micro particles and MSC membrane coated on to them. This exciting synMSC exhibited superior cryo stability and transplantation of synMSC attenuated cardiac dysfunction post-MI in mice. Importantly, this strategy might offer a uniform “off the shelf” therapy114.
Cardiac stem/progenitor cell EVs
Cardiac stem cells, both cardiac progenitor cells (CPCs) and cardiosphere derived cells (CDCs), sparkled remarkable interest in cardiac regenerative medicine, owing to their source of origin. CPCs transplantation led to augmentation of cardiac function in animal models of cardiac disease115, 116. Two recent clinical trials using CSC demonstrated their efficacy in patients with heart failure89, 90. Recent consensus suggests of like other stem/progenitor cells CSC therapeutic benefits are also attributed to its paracrine factors.
Recent studies demonstrated that exosomes released from CDCs or cardiac progenitor cells (CPCs) are cardio protective. Very interestingly, Ibrahim et al showed that miR-146a which is highly enriched in CDC-EVs promotes cardiac regeneration in vivo. Further, authors demonstrated that inhibition of exosome secretion in CDCs renders them ineffective for myocardial repair process3. Further, the same group has shown that CDC-EVs are anti-fibrotic and angiogenic117 and promote cardiomyocyte survival and proliferation following MI3. While identifying miR-146a as a highly-enriched miRNA within CDC-EVs that promotes cardiac regeneration in vivo3. One independent report also confirmed that miR-146a to be highly enriched in CPC exosomes and promotes cardiac repair post-MI118. A recent study suggested that intracoronary transplantation of EV-YF-1 following I/R injury reduced infarct size via elevated IL-10 secretion correlating with CDC reparative potential in vivo. This study provides a novel direction and highlights the importance of better characterization of exosomal cargo beyond miR and proteins45. Importantly, CDC exosomes transplantation following myocardial ischemia and reperfusion reduced infarct size and improved cardiac function 4 weeks later119.
It has also been recently demonstrated that, CDC exosomes drastically altered the secretory profile of dermal fibroblasts. Further, transplantation of these CDC-exosome-primed fibroblasts, reduced scar mass, improved neovascularization and cardiac function in rat hearts post- chronic MI120. Thus, CDC-derived exosomes transformed dermal fibroblasts to therapeutic cells. Further a recent study has shown that miR-181b transfer from CDCs-Exo into macrophage reduces PKCδ transcript levels, thus renders cardio protective effects post-I/R injury120 .
Similarly, exosomes secreted by CPCs were reported to stimulate migration of endothelial cells and protect ischemic myocardium via EMMPHRIN121. Further, in vivo administration of CPC exosomes inhibited cardiomyocyte apoptosis by 53% in an ischemia/reperfusion mouse model122 validating the cardio protective effect of stem cell derived exosomes. Furthermore, Barile etal. demonstrated administration of human CPC exosomes inhibited cardiomyocyte apoptosis, enhanced neovascularization and attenuated left ventricular dysfunction post-MI in mice118 mediated via enriched miR-210, miR-132, and miR-146a-3p in CPC exosomes leading to reducedcardiomyocyte apoptosis118. A recent study suggested that hypoxia preconditioned nCPCs exosomes restore cardiac function via enriched angiogenic genes, anti-fibrotic genes and a cluster of exosomal miRNA in the exosomes123 . However, results from another study by Sharma et al support the synergistic role of both-total conditioned media and exosomes derived from neonatal cardiac progenitor cells in augmenting cardiac repair post-MI124, suggesting developmental stages affects CPCs function and utilizing young or healthy donors CPC exosomes as therapeutics might be beneficial, thereby avoiding CPC dysfunction associated with advancement of age. These results collectively support that CDC and CPC derived EVs mediate cardiac repair and regeneration.
On a whole, EVs derived from different stem cell populations promote cardiac repair. However, the heterogeneity with in EV population represents a major challenge in distinguishing the characteristics, composition and functional vesicle type within all the EVs secreted by a donor stem/progenitor cell125. Therefore, future studies might focus on improving existing technologies and development of novel high-resolution methodologies for EV- subpopulations isolation. Also, use of serum may introduce contaminant RNA/lipoproteins typically co-isolated with the EVs, which may influence EVs biological effects on recipient cells126. Therefore, reproducible stem/progenitor EVs isolation using serum-free/pre-defined serum conditions may have potential future clinical applications to accelerate cardiac repair127. Furthermore, a recent study on quantitative and stoichiometric analysis of the miRNA content of exosomes revealed that over 100 exosomes would be required to fuse to a recipient cell to transfer a single copy of a highly abundant endogenous miRNA, suggesting a significant amount of miRNA or EV component needs to be transferred to have a biological effect128. Therefore, it would be interesting to investigate if certain miRNA/s or other bio-active molecules aforementioned in EVs are abundant and more important than others? Additionally, comparison and integration of the growing number of RNA/proteome data sets from different stem cell secretome will further help to obtain an entire picture of the critical regulators of cardiac regeneration.
EV targeting and uptake
As mentioned, EVs play important roles in cell to cell communication by carrying a range of different proteins and nucleic acids to their recipient cells. EVs target their recipient cells, release their cargo and affect the phenotype and function of these cells. To demonstrate EVs function, reports suggest EVs can be fluorescently labelled with lipophilic dyes such as PKH26, PKH67 etc. However, unbound dyes can also co-label recipient cells and may give false positive results. For this purpose, recent efforts suggest removal of free dye can be achieved by ultracentrifugation or by using viva spin filters94, 129. Specific protein-protein interactions between EVs and target cells could play an important role in specific cell targeting and vesicular uptake. Although exosomes are generated from different sources of cells, they all share similar structural characteristics including similar protein and lipid contents in their plasma membranes. There are a wide variety of proteins associated with exosome membranes including transmembrane proteins and adhesion molecules of tetraspanins, integrin, and membrane receptors130–132. These are the most prominent proteins associated with exosomes which play important roles in cell targeting and association133–136. Specific exosomal cell targeting occurs through a highly specific ligand-receptor interaction. This mainly happens when matching combinations of ligands and receptors are expressed on the surface of EVs and target cells respectively137. Subsequently, attached EVs exert their effects on the target cells by the activation of specific intracellular signaling pathways. Various reports reveal mechanisms involved in cell targeting and exosome uptake. Tumor-derived exosomes and their targeting mechanisms are among the most studied EVs. It was recently shown that integrin (ITG) adhesion molecules expressed on tumor-derived exosomes are sufficient to guide exosomes to specific pre-metastatic niche and initiate metastasis138. In this study, it was revealed that tumor exosomes expressing ITG αvβ5 are guided to liver cells and specifically bind to Kupffer cells138,139. Similarly, exosomal integrins α6β4 and α6β1 are lung-tropic factors associated with lung fibroblasts and epithelia cells. Furthermore, the same group showed the presence of ITGβ3 in exosomes fused with brain endothelial cells. The uptake of EVs by these specific cells activates Src signaling in the recipient cells which is followed by the upregulation of pro-migratory/pro-inflammatory factor of S100. These findings shed light on the role of exosomes in mediating organ-specific metastasis and showed that specific subtypes of integrins are important in mediating the binding of exosomes to the target cells138.
Moreover, Liu et al showed that the activation of CD4+ T cells plays a major role in healing the myocardial wounds after myocardial infarction (MI). It was shown that the uptake of dendritic cell-derived exosomes is increased by CD4+ T cells after myocardial infarction140. Subsequently, these exosomes induce upregulation of chemokines and inflammatory cytokines such as IFN-Ƴ and TNF-α in CD4+ cells and improve cardiac function post-MI. However, the mechanisms through which the specific triggering of CD4+ T cells occur is not discussed in this study140.
Another example of specific cell targeting is studied in the exosome membrane of cardiac cell lines. Cx43 is a gap junction transmembrane protein embedded in the membrane of cardiac cell exosomes. In vitro studies revealed that Cx43 mediates the interaction of exosomes with their target cells and regulates the transfer of exosomal cargo between exosomes and their target cells141.
The formation of circulating EVs is increased in cardiovascular diseases and the interaction between these vesicles and their target cells could play an important role in the development of the diseases142. In atherosclerotic lesions, CD40L+ vesicles bind to their receptor CD40 on endothelial cells and induce in vivo angiogenesis143. Moreover, in the presence of sonic hedgehog (shh), T cell-derived vesicles induce the activation of Patched/Smoothened receptors and stimulate angiogenesis in their corresponding recipient cells144, 145. In a different study, it was shown that circulating EVs expressing heparin-binding EGF-like growth factor (HB-EGF) promote pro-oxidative and pro-inflammatory responses by binding to EGFR+ endothelial cells146. Additionally, Rautou et al. showed that ICAM-1+ EVs derived from atherosclerotic human plaques can interact with endothelial cells in a phosphatidylserine dependent way147. This interaction leads to the increased expression of adhesion molecules on the endothelial cells which consequently recruits inflammatory cells such as monocytes in to the place and promotes atherosclerotic plaque progression147. These results demonstrate that EVs isolated from human atherosclerotic plaques exacerbates the progression of atherosclerotic formation. Therefore, as some of the circulating EVs are potentially proinflammatory, their immediate clearance from the circulatory system is necessary to avoid the development of thrombotic diseases.
The mechanism for clearing the harmful circulating exosomes is discussed by Happonen et al108. They showed that upon activation, platelets release plasma membrane-derived vesicles expressing phosphatidylserine on their surface. The protein-protein interaction of GAS6 to tyrosine-protein kinase receptor AXL is responsible for stimulating the uptake of these EVs by aortic endothelial cells and human umbilical vein endothelial cells. This is followed by subsequent phagocytosis of these EVs by both of these endothelial cells148. However, it is noteworthy that even though these circulating vesicles interact with their recipient cells through specific molecules, but whether the target of EVs is a specific cell type or a random cell remains to be determined.
As mentioned earlier, in a recent study, CD34+ stem cell exosomes and their role in mediating ischemic tissue repair in patients with myocardial and critical limb ischemia was investigated. CD34+ stem cell exosomes promote angiogenesis when applied to ischemic hind limbs. Interestingly, it was shown that these exosomes are internalized by endothelial cells to a greater extent than smooth muscle cells and fibroblasts, which suggests that CD34 transmembrane protein on the stem cell derived exosomes specifically targets endothelial cells and binds to its matching proteins on these cells149.
Similarly, in another recent study, it was shown that isolated CPC exosomes are also taken up at varying levels by cardiac target cells (fibroblast, endothelial, and cardiomyocytes)120. It was shown that exosomes are internalized by fibroblast cells at maximum extent, while the minimum uptake was detected by cardiac myocytes. The different levels of uptake suggests the existence of cell-specific differences between cardiac cells which its mechanism has remained to be determined150. Although the knowledge on mechanisms regulating cardiac exosomal targeting is limited and the exact mechanisms by which exosomes internalized in cardiac target cells is not fully understood yet, but certain assumptions can be made based on the knowledge obtained from studies on exosomes derived from other cells. Overall, unraveling the mechanisms involved in the exosomal targeting and uptake is beneficiary in designing strategies for selective delivery of therapeutic molecules.
Mode of EVs action in vivo
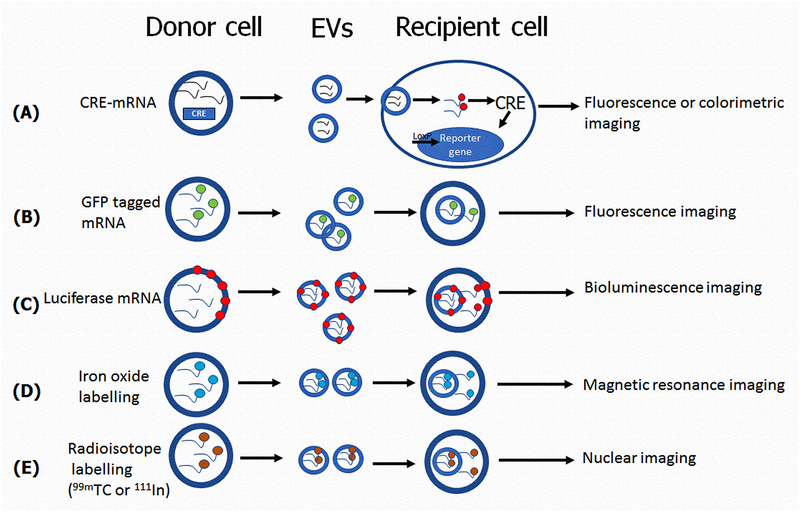
Several techniques for EV labelling and tracking has been reported in vivo. For example, an elegant approach for EV transfer has been demonstrated using transgenic mice expressing CRE recombinase and a LacZ reporter gene. Riddler et al demonstrated thatinjection of Cre mRNA-in EVs can induce recombination in the cerebellum151. This exciting finding suggests that Cre mRNA in EVs leads to excision of loxP sites in recipient cells can serve as an important tool for understanding the physiological or pathological role of EVs in cardiovascular diseases. Another study used green fluorescence protein (EGFP) and tandem dimer Tomato (tdTomato) reporters fused with a palmitoylation sequence for EV membrane labeling to visualize and track multiple tumor EV populations release, uptake and exchange between cell populations bothin vitroandin vivo152. However, it should be noted that fluorescent protein tags may affect the normal function and trafficking of the EVs. Also, one other study reported a combinedluciferase and biotinylation EV reporter for bioluminescence imagingin vivo153. Further, the possibility of exosome tracking has been demonstrated by labelling exosomes with iron-oxide nanoparticles or radioisotopes and can be imaged using MRI or nuclear imaging modalities.154, 155,156,157 (depicted in Fig-4) However, our understanding of EV bio distribution and clearance is still lacking.
Fig-4: Mode of EVs action in vivo:
(A)To visualize specific expression of EV associated mRNA, Cre mRNA labelled with fluorescent or enzymatic reporter gene in EVs leads to excision of loxP sites in recipient cells in vivo, can be detected by fluorescence or colorimetry.(B) Florescence protein (GFP) reporters labelled EVs mRNA can be visualized and tracked in the recipient cells in vivo using florescence imaging.(C) Fluorescence protein (EGFP) and tandem dimer Tomato (tdTomato) reporters fused with a palmitoylation sequence for EV membrane labeling to visualize and track EVs uptake by recipient cells in vivo, can be detected by bioluminescent imaging. (D) EVs can be tracked by labelling EVs with iron-oxide nanoparticles and can be imaged using Magnetic resonance imaging. (E) EVs can be tracked by labelling EVs with radioisotopes and can be imaged using nuclear imaging.
Several studies suggest exogenous delivery of EVs into circulation, is very short. It has been shown that, rabbit labelled EVs were cleared in ~10 min, when delivered into their circulation158. Similarly, different sources including splenocyte supernatants, red blood cell-derived EVs and EVs from melanoma cells all were cleared from circulation in 30 mins159–161. However, human platelet concentrate-derived EVs remained in the circulation with a half-life of 5.5 h. These results suggest EVs half-life depends on the cell source and availability of target cells to uptake the circulating EVs162. Future studies comparing the donor source (healthy vs diseased), number of exosomes, site of injection are warranted to establish uptake of the different EV populations. Nevertheless, bio distribution, pharmacokinetics, and possibility of targeted cell/tissue delivery of EVs are not fully known. Thus, imaging techniques may help to recognize the aforementioned issues and promote clinical translation of EVs for cardiac repair.
System biology and computational genomics in cardiac stem cell exosome therapy
Congenital heart defects are among the leading cause of death in the world, therefore the application of efficient therapeutic systems in reducing the size of the damaged tissues in cardiovascular patients is of interest. The application of cardiac stem/progenitor cells (including CPCs and CDCs) as well as the stem/progenitor cell-derived exosomes in the treatment of cardiac dysfunction is relatively new163. Computational modeling is a new powerful tool that can potentially play an important role in the therapeutic fields. Computational analysis can be used to evaluate large dataset including the miRNA content of the exosomes and link this dataset to the corresponding regenerative effects123, 150, 164, 165. Exosomes are miRNA-enriched vesicles that carry their cargo to their final target, release them, and mediate the functionality of the target cells based on the characteristic of the contents they are carrying. Using microarray data, computational modeling determines the signals (miRNA levels) that are mostly associated with the specific functional outcome and creates a predictive model. Principal component analysis (PCA) and partial least squares regression analysis (PLSR) are two computational tools used for creating predictive models based on miRNA content of the exosomes. Using these models, the functional outcome of other exosomes from different conditions and even different cell types can be predicted.
PCA analysis and PLSR are used to establish relationships between covariant miRNAs within exosomes as well as with their regenerative functional outcomes in an unbiased way. PCA along with PLSR determines clusters of genes and miRNAs that are associated with specific phenotypes and functional outcomes. For example, Agarwal et al. showed that CPC-derived exosomes from different age groups cluster together in the first PC analysis165. They also showed that exosomes derived from younger CPCs (neonate) mediate cardiac repair and regeneration more efficiently than child and adult exosomes. Interestingly, the same effect of neonate exosomes on the cardiac functionality had been predicted by the computational modeling. In a different study from the same group, CPC exosomes with similar reparative effects clustered together. It was shown that exosomes derived from neonatal normoxia, neonatal hypoxia, infant hypoxia, and child hypoxia which significantly improved cardiac functions, clustered together in the PC space. The calculated predictability for the functional outcome was also high for these groups150.
Nowadays, the application of computational modeling is being increased by investigators in different areas of research. For example, in a recent study, the integrated experimental and computational analysis was used to reveal the effects of hMSC exosomes on human cardiac contractility and arrhythmogenicity. In this study, the paracrine effects of hMSC on cardiac contractility was also predicted and verified in these cells166. Additionally, Platt et al. also successfully investigated and predicted the monocyte-derived-macrophage differentiation and its subsequent proteolytic activity by computational modeling164.
Overall, the application of computational tools is beneficiary in the field of stem cell therapy and should be expanded and used more commonly by investigators. In the stem cell context, the data obtained by computational modeling from a specific cell type could be used to predict the potential functional outcomes in other cell types. Altogether, exosomal stem cell prediction analysis along with experimental approaches could provide insights into the development of therapeutic strategies in congenital heart defects.
EVs as biomarkers
Recently it has been shown that plasma levels of exosomes containing miR-1 and miR-133 were significantly elevated in patients undergoing CABG and were positively correlated with cardiac troponin-I cTnI167. One other study reported, that elevated miR-126 and miR-199a only in the EVs predict the occurrence of cardiovascular events in patients with stable coronary artery disease168. Further it has been reported that miRNA-208a was significantly elevated in the serum exosomes of acute coronary syndrome patients169. Similarly, exosomal miRNAs miR-192, miR-194 and miR-34a have been identified as biomarkers for the development of heart failure170. Interestingly, a recent report showed heart failure patient’s pericardial fluid as a new source of exosomes containing miR-21–5p, miR-451a, miR-125b-5p, let-7b-5p and miR-16–5p171. These studies highlight the importance of EVs carrying miRNAs as prognostic markers for cardiovascular diseases. Even though aforementioned findings are encouraging, going forward it would be important to validate the observations in more number of patients at different time point’s post-cardiovascular disease with diverse racial backgrounds to establish miRNA or other RNA species in the EVs as potential diagnostic markers.
Future Perspectives:
EVs are interesting entities for cardiovascular research because of three important things. Firstly, for understanding their role in intracellular communication in physiological and pathological conditions. Secondly, EVs may serve as biomarkers in pathological conditions. Finally, the recent evidence on stem/progenitor cell exosomes as potential therapeutic regimen is exciting since EVs could possibly be used as a surrogate for the stem cell based therapy and this regimen may overcome the roadblocks identified with the use of stem/progenitor cell themselves.
Importantly, exosomes as therapeutics have already made their way to clinical trials172. Phase-I trails with dendritic cell-exosomes carrying tumor antigens has been tested as anti-tumor therapy and found to be safe173. Recently therapeutic efficacy of autologous plasma exosomes has been initiated for wound repair in patients with intractable cutaneous ulcers (NCT02565264). Further, MSC derived exosomes therapy effect on β-mass of type-I diabetes has also been initiated (NCT02138331). These trials suggest the possibility/hope of stem/progenitor cell exosome therapy in patients with cardiovascular diseases.
When combined with available stem cell-exosome studies for myocardial repair in literature, two things become amply evident: −1) stem cell exosomes mimic reparative properties of their parental stem cells and 2) inhibition of exosome secretion renders stem cell ineffective suggesting exosomes as major paracrine mediators of stem cell function.
However a number of important questions need to be answered before stem cell exosomes could potentially be employed in clinic includes: [1] standardizing robust techniques for isolation of homogenous EV population [2], better understanding of immunogenicity of autologous stem/progenitor cell exosomes [3], comprehensive characterization of exosome cargo [4], establishment of pharmacokinetics of EVs [5], standardizing optimal dose and route of administration [6], Improving strategies for scaling up reproducible EVs isolation methods for clinical use. Importantly EV therapy for ischemic cardiac diseases should consider background diseases such as aging, diabetes hypercholesterolemia and hypertension which might affect the number and function of EVs.
Sources of Funding:
This work was supported in part by funding from the National Institute of Health grants HL091983, HL126186 and HL134608 to RK and HL12438 to MED.
Disclosures: None
Abbreviations:
- CPCs
Cardiac progenitor cells
- CDC
Cardiosphere derived cells
- DLS
Dynamic light scattering
- ESC
Embryonic stem cells
- EPCs
Endothelial progenitor cells
- iPSCs
Induced pluripotent stem cells
- ILVs
Intraluminal vesicles
- MSCs
Mesenchymal stromal cells
- miRNA
MicroRNA
- MVBs
Multivesicular bodies
- MV
Microvesicles
- NTA
Nanosight tracking analysis
REFERENCES
- 1.Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360 [DOI] [PubMed] [Google Scholar]
- 2.Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016;118:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishore R, Garikipati VN, Gumpert A. Tiny shuttles for information transfer: Exosomes in cardiac health and disease. J Cardiovasc Transl Res. 2016;9:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.S ELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357 [DOI] [PubMed] [Google Scholar]
- 6.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human cd34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L, Tulapurkar ME, Taylor BS, Yang P, Karathanasis SK, Goodlett DR, Kaushal S. A deep proteome analysis identifies the complete secretome as the functional unit of human cardiac progenitor cells. Circ Res. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247 [DOI] [PubMed] [Google Scholar]
- 9.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of escrt functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565 [DOI] [PubMed] [Google Scholar]
- 10.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin cd63 regulates escrt-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30; sup pp 11–13 [DOI] [PubMed] [Google Scholar]
- 12.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-alix regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685 [DOI] [PubMed] [Google Scholar]
- 13.Zylbersztejn K, Galli T. Vesicular traffic in cell navigation. FEBS J. 2011;278:4497–4505 [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: A novel function of the p53 protein. Cancer Res. 2006;66:4795–4801 [DOI] [PubMed] [Google Scholar]
- 15.Yue Y, Garikipati VN, Verma SK, Goukassian DA, Kishore R. Interleukin-10 deficiency impairs reparative properties of bone marrow-derived endothelial progenitor cell exosome function. Tissue Eng Part A. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: Two sides of the coin. Physiology (Bethesda). 2005;20:22–27 [DOI] [PubMed] [Google Scholar]
- 17.Pasquet JM, Dachary-Prigent J, Nurden AT. Calcium influx is a determining factor of calpain activation and microparticle formation in platelets. Eur J Biochem. 1996;239:647–654 [DOI] [PubMed] [Google Scholar]
- 18.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289 [DOI] [PubMed] [Google Scholar]
- 19.Greco V, Hannus M, Eaton S. Argosomes: A potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106:633–645 [DOI] [PubMed] [Google Scholar]
- 20.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mager I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O, Nieuwland R. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632–1648 [DOI] [PubMed] [Google Scholar]
- 21.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3 22 [DOI] [PubMed] [Google Scholar]
- 23.Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mateescu B, Kowal EJ, van Balkom BW, Bartel S, Bhattacharyya SN, Buzas EI, Buck AH, de Candia P, Chow FW, Das S, Driedonks TA, Fernandez-Messina L, Haderk F, Hill AF, Jones JC, Van Keuren-Jensen KR, Lai CP, Lasser C, Liegro ID, Lunavat TR, Lorenowicz MJ, Maas SL, Mager I, Mittelbrunn M, Momma S, Mukherjee K, Nawaz M, Pegtel DM, Pfaffl MW, Schiffelers RM, Tahara H, Thery C, Tosar JP, Wauben MH, Witwer KW, Nolte-’t Hoen EN. Obstacles and opportunities in the functional analysis of extracellular vesicle rna - an isev position paper. J Extracell Vesicles. 2017;6:1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, Palinkas Z, Kumar V, Nagy P, Kittel A, Buzas EI, Ferdinandy P, Giricz Z. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10:e0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welton JL, Webber JP, Botos LA, Jones M, Clayton A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J Extracell Vesicles. 2015;4:27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scientific program 2012 isev meeting wednesday 18th april. J Extracell Vesicles. 2012;1:18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang LG, Kong MQ, Zhou S, Sheng YF, Wang P, Yu T, Inci F, Kuo WP, Li LJ, Demirci U, Wang S. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci Rep. 2017;7:46224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sluijter JPG, Davidson SM, Boulanger CM, Iren Buzas E, de Kleijn DPV, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Van Laake LW, Ytrehus K, Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position paper from the working group on cellular biology of the heart of the european society of cardiology. Cardiovasc Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Consortium E-T, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L, Berx G, Boere J, Boukouris S, Bremer M, Buschmann D, Byrd JB, Casert C, Cheng L, Cmoch A, Daveloose D, De Smedt E, Demirsoy S, Depoorter V, Dhondt B, Driedonks TA, Dudek A, Elsharawy A, Floris I, Foers AD, Gartner K, Garg AD, Geeurickx E, Gettemans J, Ghazavi F, Giebel B, Kormelink TG, Hancock G, Helsmoortel H, Hill AF, Hyenne V, Kalra H, Kim D, Kowal J, Kraemer S, Leidinger P, Leonelli C, Liang Y, Lippens L, Liu S, Lo Cicero A, Martin S, Mathivanan S, Mathiyalagan P, Matusek T, Milani G, Monguio-Tortajada M, Mus LM, Muth DC, Nemeth A, Nolte-’t Hoen EN, O’Driscoll L, Palmulli R, Pfaffl MW, Primdal-Bengtson B, Romano E, Rousseau Q, Sahoo S, Sampaio N, Samuel M, Scicluna B, Soen B, Steels A, Swinnen JV, Takatalo M, Thaminy S, Thery C, Tulkens J, Van Audenhove I, van der Grein S, Van Goethem A, van Herwijnen MJ, Van Niel G, Van Roy N, Van Vliet AR, Vandamme N, Vanhauwaert S, Vergauwen G, Verweij F, Wallaert A, Wauben M, Witwer KW, Zonneveld MI, De Wever O, Vandesompele J, Hendrix A. Ev-track: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232 [DOI] [PubMed] [Google Scholar]
- 34.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the international society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belov L, Matic KJ, Hallal S, Best OG, Mulligan SP, Christopherson RI. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J Extracell Vesicles. 2016;5:25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of mhc class ii-peptide complexes on b cell exosomes. EMBO J. 2007;26:4263–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quah BJ, O’Neill HC. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis. 2005;35:94–110 [DOI] [PubMed] [Google Scholar]
- 39.Yue Y, Garikipati VNS, Verma SK, Goukassian DA, Kishore R. Interleukin-10 deficiency impairs reparative properties of bone marrow-derived endothelial progenitor cell exosomes. Tissue Eng Part A. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of micrornas from multiple cell types. BMC Genomics.13:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squadrito ML, Baer C, Burdet F, Maderna C, Gilfillan GD, Lyle R, Ibberson M, De Palma M. Endogenous rnas modulate microrna sorting to exosomes and transfer to acceptor cells. Cell Rep.8:1432–1446 [DOI] [PubMed] [Google Scholar]
- 42.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nsmase2)-dependent exosomal transfer of angiogenic micrornas regulate cancer cell metastasis. J Biol Chem.288:10849–10859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sanchez-Madrid F. Sumoylated hnrnpa2b1 controls the sorting of mirnas into exosomes through binding to specific motifs. Nat Commun.4:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee WH, Chen WY, Shao NY, Xiao D, Qin X, Baker N, Bae HR, Wei TT, Wang Y, Shukla P, Wu H, Kodo K, Ong SG, Wu JC. Comparison of non-coding rnas in exosomes and functional efficacy of human embryonic stem cell- versus induced pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cambier L, de Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, Kreke M, Smith RR, Marban L, Marban E. Y rna fragment in extracellular vesicles confers cardioprotection via modulation of il-10 expression and secretion. EMBO Mol Med. 2017;9:337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brisson AR, Tan S, Linares R, Gounou C, Arraud N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets. 2017;28:263–271 [DOI] [PubMed] [Google Scholar]
- 47.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22 [DOI] [PubMed] [Google Scholar]
- 48.Arraud N, Linares R, Tan S, Gounou C, Pasquet JM, Mornet S, Brisson AR. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–627 [DOI] [PubMed] [Google Scholar]
- 49.Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S. Atomic force microscopy: A novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8:315–323 [DOI] [PubMed] [Google Scholar]
- 50.Arraud N, Gounou C, Turpin D, Brisson AR. Fluorescence triggering: A general strategy for enumerating and phenotyping extracellular vesicles by flow cytometry. Cytometry A. 2016;89:184–195 [DOI] [PubMed] [Google Scholar]
- 51.Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259–272 [DOI] [PubMed] [Google Scholar]
- 52.Groot Kormelink T, Arkesteijn GJ, Nauwelaers FA, van den Engh G, Nolte-’t Hoen EN, Wauben MH. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometry A. 2016;89:135–147 [DOI] [PubMed] [Google Scholar]
- 53.Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filipe V, Hawe A, Jiskoot W. Critical evaluation of nanoparticle tracking analysis (nta) by nanosight for the measurement of nanoparticles and protein aggregates. Pharm Res. 2010;27:796–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osteikoetxea X, Balogh A, Szabo-Taylor K, Nemeth A, Szabo TG, Paloczi K, Sodar B, Kittel A, Gyorgy B, Pallinger E, Matko J, Buzas EI. Improved characterization of ev preparations based on protein to lipid ratio and lipid properties. PLoS One.10:e0121184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sodar BW, Kittel A, Paloczi K, Vukman KV, Osteikoetxea X, Szabo-Taylor K, Nemeth A, Sperlagh B, Baranyai T, Giricz Z, Wiener Z, Turiak L, Drahos L, Pallinger E, Vekey K, Ferdinandy P, Falus A, Buzas EI. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep. 2016;6:24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, Sturk A, van Leeuwen TG, Nieuwland R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12:1182–1192 [DOI] [PubMed] [Google Scholar]
- 59.Coumans FA, van der Pol E, Boing AN, Hajji N, Sturk G, van Leeuwen TG, Nieuwland R. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. J Extracell Vesicles. 2014;3:25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, Lee H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, Genoud C, Martin K, Pizzato N, Voshol J, Morrissey DV, Andaloussi SE, Wood MJ, Meisner-Kober NC. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the er. J Cell Biol. 2016;213:173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–1536 [DOI] [PubMed] [Google Scholar]
- 63.Woodcock EA, Matkovich SJ. Cardiomyocytes structure, function and associated pathologies. Int J Biochem Cell Biol. 2005;37:1746–1751 [DOI] [PubMed] [Google Scholar]
- 64.Gupta S, Knowlton AA. Hsp60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–3056 [DOI] [PubMed] [Google Scholar]
- 65.Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, Liao Y. Mechanism of tnf-alpha autocrine effects in hypoxic cardiomyocytes: Initiated by hypoxia inducible factor 1alpha, presented by exosomes. J Mol Cell Cardiol. 2012;53:848–857 [DOI] [PubMed] [Google Scholar]
- 66.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin ii type 1 receptors. Circulation.131:2120–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of glut transporters and glycolytic enzymes. Cardiovasc Res.109:397–408 [DOI] [PubMed] [Google Scholar]
- 68.Garcia NA, Ontoria-Oviedo I, Gonzalez-King H, Diez-Juan A, Sepulveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One.10:e0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of mir-320 into endothelial cells. J Mol Cell Cardiol.74:139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Wurdinger T, Verhaar MC. Endothelial cells require mir-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood.121:3997–4006, S3991–3915 [DOI] [PubMed] [Google Scholar]
- 71.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. Microrna-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest.123:2143–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol.89:268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T. Cardiac fibroblast-derived microrna passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang C, Zhang C, Liu L, A X, Chen B, Li Y, Du J. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther. 2017;25:192–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davidson SM, Riquelme JA, Takov K, Vicencio JM, Boi-Doku C, Khoo V, Doreth C, Radenkovic D, Lavandero S, Yellon DM. Cardioprotection mediated by exosomes is impaired in the setting of type ii diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J Cell Mol Med. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watanabe M, Kawaguchi S, Nakahara H, Hachimaru T. The roles of natriuretic peptides in pericardial fluid in patients with heart failure. Clin Cardiol. 2009;32:159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beltrami C, Besnier M, Shantikumar S, Shearn AI, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E, Angelini GD, Emanueli C. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed micrornas and promotes therapeutic angiogenesis. Mol Ther. 2017;25:679–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foglio E, Puddighinu G, Fasanaro P, D’Arcangelo D, Perrone GA, Mocini D, Campanella C, Coppola L, Logozzi M, Azzarito T, Marzoli F, Fais S, Pieroni L, Marzano V, Germani A, Capogrossi MC, Russo MA, Limana F. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following mi through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int J Cardiol. 2015;197:333–347 [DOI] [PubMed] [Google Scholar]
- 79.Garikipati VN, Krishnamurthy P, Verma SK, Khan M, Abramova T, Mackie AR, Qin G, Benedict C, Nickoloff E, Johnson J, Gao E, Losordo DW, Houser SR, Koch WJ, Kishore R. Negative regulation of mir-375 by interleukin-10 enhances bone marrow-derived progenitor cell-mediated myocardial repair and function after myocardial infarction. Stem Cells. 2015;33:3519–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joladarashi D, Garikipati VN, Thandavarayan RA, Verma SK, Mackie AR, Khan M, Gumpert AM, Bhimaraj A, Youker KA, Uribe C, Babu S Suresh, Jeyabal P, Kishore R, Krishnamurthy P. Enhanced cardiac regenerative ability of stem cells after ischemia-reperfusion injury: Role of human cd34+ cells deficient in microrna-377. J Am Coll Cardiol.66:2214–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajasingh J, Lambers E, Hamada H, Bord E, Thorne T, Goukassian I, Krishnamurthy P, Rosen KM, Ahluwalia D, Zhu Y, Qin G, Losordo DW, Kishore R. Cell-free embryonic stem cell extract-mediated derivation of multipotent stem cells from nih3t3 fibroblasts for functional and anatomical ischemic tissue repair. Circ Res. 2008;102:e107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rajasingh J, Bord E, Hamada H, Lambers E, Qin G, Losordo DW, Kishore R. Stat3-dependent mouse embryonic stem cell differentiation into cardiomyocytes: Analysis of molecular signaling and therapeutic efficacy of cardiomyocyte precommitted mes transplantation in a mouse model of myocardial infarction. Circ Res. 2007;101:910–918 [DOI] [PubMed] [Google Scholar]
- 84.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marban E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human cardiac progenitor cells engineered with pim-i kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams AR, Suncion VY, McCall F, Guerra D, Mather J, Zambrano JP, Heldman AW, Hare JM. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J Am Heart Assoc. 2013;2:e000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA.308:2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet.378:1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet.379:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Maat AP, Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42:2063–2069 [DOI] [PubMed] [Google Scholar]
- 92.Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, Frohwein S, Henry TD, Schatz RA, Dib N, Toma C, Davidson CJ, Barsness GW, Shavelle DM, Cohen M, Poole J, Moss T, Hyde P, Kanakaraj AM, Druker V, Chung A, Junge C, Preti RA, Smith RL, Mazzo DJ, Pecora A, Losordo DW. Preserve-ami: A randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous cd34+ cells in patients with left ventricular dysfunction post stemi. Circ Res.120:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary cd34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res.112:165–173 [DOI] [PubMed] [Google Scholar]
- 94.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, Davis ME. Experimental, systems and computational approaches to understanding the microrna-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ Res. 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A, Kamide CE, Liu T, Gupta R, Sahoo S, Misener S, Kishore R, Losordo DW. Sonic hedgehog-modified human cd34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ, Sahoo S. Angiogenic mechanisms of human cd34+ stem cell exosomes in the repair of ischemic hindlimb. Circ Res. 2017;120:1466–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshida Y, Yamanaka S. Induced pluripotent stem cells 10 years later: For cardiac applications. Circ Res.120:1958–1968 [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective mirnas and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bobis-Wozowicz S, Kmiotek K, Sekula M, Kedracka-Krok S, Kamycka E, Adamiak M, Jankowska U, Madetko-Talowska A, Sarna M, Bik-Multanowski M, Kolcz J, Boruczkowski D, Madeja Z, Dawn B, Zuba-Surma EK. Human induced pluripotent stem cell-derived microvesicles transmit rnas and proteins to recipient mature heart cells modulating cell fate and behavior. Stem Cells. 2015;33:2748–2761 [DOI] [PubMed] [Google Scholar]
- 101.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human cd34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res.109:724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ, Sahoo S. Angiogenic mechanisms of human cd34+ stem cell exosomes in the repair of ischemic hindlimb. Circ Res.120:1466–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garikipati VNS, Verma SK, Jolardarashi D, Cheng Z, Ibetti J, Cimini M, Tang Y, Khan M, Yue Y, Benedict C, Nickoloff E, Truongcao MM, Gao E, Krishnamurthy P, Goukassian DA, Koch WJ, Kishore R. Therapeutic inhibition of mir-375 attenuates post-myocardial infarction inflammatory response and left ventricular dysfunction via pdk-1-akt signalling axis. Cardiovasc Res. 2017;113:938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yue Y, Garikipati VNS, Verma SK, Goukassian DA, Kishore R. Interleukin-10 deficiency impairs reparative properties of bone marrow-derived endothelial progenitor cell exosomes. Tissue Eng Part A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsushita K, Dzau VJ. Mesenchymal stem cells in obesity: Insights for translational applications. Lab Invest. [DOI] [PubMed] [Google Scholar]
- 106.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669 [DOI] [PubMed] [Google Scholar]
- 107.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA.311:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J Am Heart Assoc.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015;2015:761643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vrijsen KR, Maring JA, Chamuleau SA, Verhage V, Mol EA, Deddens JC, Metz CH, Lodder K, van Eeuwijk EC, van Dommelen SM, Doevendans PA, Smits AM, Goumans MJ, Sluijter JP. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via emmprin. Adv Healthc Mater. 2016;5:2555–2565 [DOI] [PubMed] [Google Scholar]
- 111.Gonzalez-King H, Garcia NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepulveda P. Hypoxia inducible factor-1alpha potentiates jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells. 2017;35:1747–1759 [DOI] [PubMed] [Google Scholar]
- 112.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M. Exosomes secreted from gata-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic micrornas for cardioprotection. Int J Cardiol. 2015;182:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B, Fan GC. Exosomal mir-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep. 2015;5:13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Luo L, Tang J, Nishi K, Yan C, Dinh PU, Cores J, Kudo T, Zhang J, Li TS, Cheng K. Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ Res. 2017;120:1768–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gallet R, de Couto G, Simsolo E, Valle J, Sun B, Liu W, Tseliou E, Zile MR, Marban E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (hfpef) in rats by decreasing fibrosis and inflammation. JACC Basic Transl Sci.1:14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Dembitsky W, Sussman MA. Rejuvenation of human cardiac progenitor cells with pim-1 kinase. Circ Res.113:1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G, Aminzadeh M, Middleton R, Valle J, Weixin L, Marban E. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J Am Coll Cardiol. 2015;66:599–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–541 [DOI] [PubMed] [Google Scholar]
- 119.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, Marban E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marban E. Exosomal microrna transfer into macrophages mediates cellular postconditioning. Circulation. 2017;136:200–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431:566–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microrna clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sharma S, Mishra R, Bigham GE, Wehman B, Khan MM, Xu H, Saha P, Goo YA, Datla SR, Chen L, Tulapurkar ME, Taylor BS, Yang P, Karathanasis S, Goodlett DR, Kaushal S. A deep proteome analysis identifies the complete secretome as the functional unit of human cardiac progenitor cells. Circ Res. 2017;120:816–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: New players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–71 [DOI] [PubMed] [Google Scholar]
- 126.Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Gorgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Kramer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lotvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Thery C, Rohde E, Giebel B. Applying extracellular vesicles based therapeutics in clinical trials - an isev position paper. J Extracell Vesicles. 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, Karnas E, Xuan YT, Skupien-Rabian B, Chen X, Jankowska U, Girgis M, Sekula M, Davani A, Lasota S, Vincent RJ, Sarna M, Newell KL, Wang OL, Dudley N, Madeja Z, Dawn B, Zuba-Surma EK. Induced pluripotent stem cell (ipsc)-derived extracellular vesicles are safer and more effective for cardiac repair than ipscs. Circ Res. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M. Quantitative and stoichiometric analysis of the microrna content of exosomes. Proc Natl Acad Sci U S A. 2014;111:14888–14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A, Sjostrand M, Gabrielsson S, Lotvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain rna: Uptake by macrophages . J Transl Med.9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266 [DOI] [PubMed] [Google Scholar]
- 131.Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barrès C, Blanc L, Bette-Bobillo P, André S, Mamoun R, Gabius HJ, Vidal M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705 [DOI] [PubMed] [Google Scholar]
- 133.Waldenström A, Ronquist G. Role of exosomes in myocardial remodeling. Circ Res. 2014;114:315–324 [DOI] [PubMed] [Google Scholar]
- 134.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human b-lymphocytes. J Biol Chem. 1998;273:20121–20127 [DOI] [PubMed] [Google Scholar]
- 135.Chaput N, Taïeb J, Schartz N, Flament C, Novault S, André F, Zitvogel L. The potential of exosomes in immunotherapy of cancer. Blood Cells Mol Dis. 2005;35:111–115 [DOI] [PubMed] [Google Scholar]
- 136.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004;31:114–121 [DOI] [PubMed] [Google Scholar]
- 137.Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal mirnas in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L, Ma L, Zhu J, Zou Y, Ge J. Exosomes derived from dendritic cells improve cardiac function via activation of cd4(+) t lymphocytes after myocardial infarction. J Mol Cell Cardiol. 2016;91:123–133 [DOI] [PubMed] [Google Scholar]
- 141.Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, Ferreira JV, Catarino S, Pinho MJ, Zuzarte M, Isabel Anjo S, Manadas B, P G Sluijter J, Pereira P, Girao H. Gap junctional protein cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep. 2015;5:13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353 [DOI] [PubMed] [Google Scholar]
- 143.Boulanger CM, Leroyer AS, Amabile N, Tedgui A. [circulating endothelial microparticles: A new marker of vascular injury]. Ann Cardiol Angeiol (Paris). 2008;57:149–154 [DOI] [PubMed] [Google Scholar]
- 144.Benameur T, Soleti R, Porro C, Andriantsitohaina R, Martínez MC. Microparticles carrying sonic hedgehog favor neovascularization through the activation of nitric oxide pathway in mice. PLoS One. 2010;5:e12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Agouni A, Mostefai HA, Porro C, Carusio N, Favre J, Richard V, Henrion D, Martínez MC, Andriantsitohaina R. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. FASEB J. 2007;21:2735–2741 [DOI] [PubMed] [Google Scholar]
- 146.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin ii is mediated via ang ii receptor type i/nadph oxidase/rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–1907 [DOI] [PubMed] [Google Scholar]
- 147.Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, Nalbone G, Castier Y, Leseche G, Lehoux S, Tedgui A, Boulanger CM. Microparticles from human atherosclerotic plaques promote endothelial icam-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–343 [DOI] [PubMed] [Google Scholar]
- 148.Happonen KE, Tran S, Mörgelin M, Prince R, Calzavarini S, Angelillo-Scherrer A, Dahlbäck B. The gas6-axl protein interaction mediates endothelial uptake of platelet microparticles. J Biol Chem. 2016;291:10586–10601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ, Sahoo S. Angiogenic mechanisms of human cd34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ Res. 2017;120:1466–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, Davis ME. Experimental, systems, and computational approaches to understanding the microrna-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ Res. 2017;120:701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Hoglinger G, Sultmann H, Altevogt P, Momma S. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, Breakefield XO. Visualization and tracking of tumour extracellular vesicle delivery and rna translation using multiplexed reporters. Nat Commun. 2015;6:7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Busato A, Bonafede R, Bontempi P, Scambi I, Schiaffino L, Benati D, Malatesta M, Sbarbati A, Marzola P, Mariotti R. Labeling and magnetic resonance imaging of exosomes isolated from adipose stem cells. Curr Protoc Cell Biol. 2017;75:3 44 41–43 44 15 [DOI] [PubMed] [Google Scholar]
- 155.O’Halloran SM, Heaf DP. Accident and emergency department attendance by asthmatic children. Thorax. 1989;44:700–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Garikipati VN, Jadhav S, Pal L, Prakash P, Dikshit M, Nityanand S. Mesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: An in vivo serial pinhole gated spect-ct study in rats. PLoS One. 2014;9:e100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. Cd169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rand ML, Wang H, Bang KW, Packham MA, Freedman J. Rapid clearance of procoagulant platelet-derived microparticles from the circulation of rabbits. J Thromb Haemost. 2006;4:1621–1623 [DOI] [PubMed] [Google Scholar]
- 160.Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, van den Bos AG, Bosman GJ, van Berkel TJ. Liver kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145 [DOI] [PubMed] [Google Scholar]
- 161.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma b16-bl6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84 [DOI] [PubMed] [Google Scholar]
- 162.Rank A, Nieuwland R, Crispin A, Grutzner S, Iberer M, Toth B, Pihusch R. Clearance of platelet microparticles in vivo. Platelets. 2011;22:111–116 [DOI] [PubMed] [Google Scholar]
- 163.Ishigami S, Ohtsuki S, Tarui S, Ousaka D, Eitoku T, Kondo M, Okuyama M, Kobayashi J, Baba K, Arai S, Kawabata T, Yoshizumi K, Tateishi A, Kuroko Y, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: The ticap prospective phase 1 controlled trial. Circ Res. 2015;116:653–664 [DOI] [PubMed] [Google Scholar]
- 164.Platt MO, Wilder CL, Wells A, Griffith LG, Lauffenburger DA. Multipathway kinase signatures of multipotent stromal cells are predictive for osteogenic differentiation: Tissue-specific stem cells. Stem Cells. 2009;27:2804–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Agarwal U, Smith AW, French KM, Boopathy AV, George A, Trac D, Brown ME, Shen M, Jiang R, Fernandez JD, Kogon BE, Kanter KR, Alsoufi B, Wagner MB, Platt MO, Davis ME. Age-dependent effect of pediatric cardiac progenitor cells after juvenile heart failure. Stem Cells Transl Med. 2016;5:883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mayourian J, Cashman TJ, Ceholski DK, Johnson BV, Sachs D, Kaji DA, Sahoo S, Hare JM, Hajjar RJ, Sobie EA, Costa KD. Experimental and computational insight into human mesenchymal stem cell paracrine signaling and heterocellular coupling effects on cardiac contractility and arrhythmogenicity. Circ Res. 2017;121:411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Emanueli C, Shearn AI, Laftah A, Fiorentino F, Reeves BC, Beltrami C, Mumford A, Clayton A, Gurney M, Shantikumar S, Angelini GD. Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac micrornas: An example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery . PLoS One.11:e0154274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning JM, Vasa-Nicotera M, Nickenig G, Werner N. Microrna expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc.3:e001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bi S, Wang C, Jin Y, Lv Z, Xing X, Lu Q. Correlation between serum exosome derived mir-208a and acute coronary syndrome. Int J Clin Exp Med.8:4275–4280 [PMC free article] [PubMed] [Google Scholar]
- 170.Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I. Circulating p53-responsive micrornas are predictive indicators of heart failure after acute myocardial infarction. Circ Res.113:322–326 [DOI] [PubMed] [Google Scholar]
- 171.Kuosmanen SM, Hartikainen J, Hippelainen M, Kokki H, Levonen AL, Tavi P. Microrna profiling of pericardial fluid samples from patients with heart failure. PLoS One. 2015;10:e0119646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in nsclc. Oncoimmunology.5:e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (dc) derived-exosomes: Results of thefirst phase i clinical trial. J Transl Med. 2005;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin ii type 1 receptors. Circulation. 2015;131:2120–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of glut transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397–408 [DOI] [PubMed] [Google Scholar]
- 176.Garcia NA, Ontoria-Oviedo I, Gonzalez-King H, Diez-Juan A, Sepulveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One. 2015;10:e0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of mir-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol. 2015;89:268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92:387–397 [DOI] [PubMed] [Google Scholar]
- 180.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting mecp2 via mir-22. PLoS One. 2014;9:e88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol.65:1525–1536 [DOI] [PubMed] [Google Scholar]