Abstract
Background:
The amygdala represents a core node in the human brain’s emotional signal processing circuitry. Given its critical role, both the typical and atypical functional connectivity patterns of the amygdala have been extensively studied in adults. However, the development of amygdala functional connectivity development during infancy is less well-studied, thus our understanding of the normal growth trajectory of key emotion-related brain circuits during a critical period is limited.
Methods:
In this study we used resting-state fMRI (N=233 subjects with 334 datasets) to delineate the spatiotemporal dynamics of amygdala functional connectivity development during the first two years of life. Their relationships with 4-year emotional (i.e., anxiety and inhibitory self-control parent report measures) and cognitive behavioral outcomes (i.e., IQ) were also assessed using multivariate modeling.
Results:
Our results revealed non-linear growth of amygdala functional connectivity during the first two years featuring dramatic synchronization during the first year followed by moderate growth or fine tuning during the second year. Interestingly, functional connectivity growth during the second year had important behavioral implications exemplified by multiple significant predictions of 4-year-old emotional and cognitive developmental outcomes.
Conclusions:
The delineation of the spatiotemporal dynamics of amygdala functional connectivity development during infancy and their associations with 4-year behavioral outcomes may provide new references on the early emergence of both typical and atypical emotion processing capabilities.
Keywords: Functional Connectivity, Amygdala, Anxiety, Inhibitory Control, Emotion Development, Early Brain Development
Introduction
The amygdala is a key node in human emotion processing (1) that is critical for normal behavior and mental health. Atypical emotion processing associated with the amygdala is implicated in multiple mood (e.g., depression, anxiety disorder) (2-4) and psychiatric disorders (e.g., schizophrenia) (5, 6) thus a better understanding of the emotion processing circuitry centered on the amygdala represents a high priority. In adult functional magnetic resonance imaging (fMRI) studies, activations of the amygdala, insula, hippocampus, parahippocampus, medial/lateral prefrontal cortex, and medial parietal cortex have been frequently reported during emotional stimuli processing, indicating widely distributed emotion processing circuitry (7). Similarly, functional connectivity (FC) studies of the amygdala revealed positive connectivity of the amygdala with insula and medial prefrontal cortices that typically assess the affective state of the stimuli and negative connectivity with lateral prefrontal and dorsal parietal regions that are more involved in cognitive processes and emotion regulation (8, 9). Together, these two streams of connectivity are thought to reflect the contemporary understanding of emotion processing involving complex interactions between bottom-up emotion encoding/appraisal and top-down regulatory processes (10).
Adult amygdala FC patterns and associated disease-related alterations are well-studied (8, 9), however the development of amygdala FC during the first years of life remains poorly understood despite compelling evidence that emotion regulation strategies emerge during infancy (11, 12). Specifically, while neonates demonstrate an almost complete reliance on caregivers’ care for emotional relief (13, 14), a range of self-regulatory behaviors (e.g. self-comforting and social referencing) emerge in toddlers (15). The development of emotion processing/regulation strategies during infancy have been documented to have far-reaching and enduring effects on cognitive development (14, 16, 17), academic achievement (17, 18), quality of life (19), and psychopathology (15). Therefore, it is important to elucidate the development of amygdala FC patterns during infancy in order to better understand the brain basis for the emergence of both typical and atypical emotion processing/regulation strategies during this critical period. Consistent with this goal, previous research has shown that positive neonatal amygdala connectivity with bilateral anterior insula and ventral striatum is associated with higher fear at 6-months (20). However, no longitudinal characterization has been conducted, thus the normal growth trajectory of amygdala FC during infancy remains elusive.
In this study, we sought to characterize the spatiotemporal dynamics of amygdala FC during the first two years of life. Based on behavioral observations, we hypothesize incomplete functional connections of the amygdala in neonates (13, 14) compared with adults followed by dramatic maturation during the first two years of life (21). Moreover, we also aimed to determine the behavioral importance of amygdala FC growth during infancy by testing its predictive power of 4-year emotional (i.e., parent-reported anxiety and emotional regulation) and cognitive (i.e., IQ) outcomes. Given previous reports on the association between amygdala-related development and both emotional processing capabilities (20, 22) and cognitive outcomes (17, 18), we hypothesized significant correlations between amygdala FC development during infancy and 4-year anxiety, emotion regulation, and IQ measures.
Materials and Methods
Participants.
Typically developing infant participants were part of the UNC Early Brain Development Study, characterizing early childhood brain and behavior development (23-26). We retrospectively identified 223 subjects (107 males) with at least one successful resting-state fMRI scan during the first two years of life. Participant characteristics (Table 1) of interest included: sex, age [gestational age at birth, age at scan, postnatal age (age at scan – age at birth), maternal education and twin status (N = 93 twins, N = 38 twin-pairs). Exclusion criteria included gestational age at birth < 37 weeks and any neonatal illness requiring greater than a 24 hour stay at a neonatal intensive care unit (NICU). Study protocols were approved by the University of North Carolina at Chapel Hill Institutional Review Board. Subjects were fed, swaddled, and fitted with ear protection prior to imaging. All subjects were in a natural sleep state during the imaging session.
Table 1:
Summary of Participant Characteristics
| Subjects (N = 223, 107 males) | Mean | Stan. Dev. | Min | Max | |
| Birth weight (ounces) | 3126.73 | 506.94 | 1960 | 4562 | |
| Birth age (days) | 272.29 | 9.66 | 259 | 295 | |
| Maternal Education (years) | 15.56 | 3.24 | 3 | 24 | |
| Cross-sectional | Scan age (days) | ||||
| N | Mean | Stan. Dev. | Min | Max | |
| Neonate (NEO) | 152 | 296.83 | 11.84 | 274 | 348 |
| 1-Year (1YR) | 105 | 657.79 | 20.37 | 605 | 714 |
| 2-Year (2YR) | 77 | 1026.04 | 31.33 | 973 | 1144 |
| Total | 334 | ||||
| 4-year (4YR) Behavior | |||||
| N | Mean | Stan. Dev. | Min | Max | |
| ANX | 125 | 47.64 | 10.12 | 32 | 78 |
| ISC | 126 | 49.35 | 10.14 | 34 | 80 |
| IQ | 129 | 108.88 | 13.17 | 73 | 139 |
Imaging.
Longitudinal resting-state fMRI data were acquired from the cohort of typically developing infants (N=223, 107 males) at ~3 weeks (neonates, NEO), 1-year (1YR), and 2-years (2YR) of age. The distribution of available datasets for functional connectivity analyses is shown in Figure 1. Infant images were acquired using a single scanner (3T head-only Siemens Allegra with circular polarization head coil). Functional images were acquired using a T2*-weighted EPI sequence: time repetition (TR) = 2 s, time echo (TE) = 32 ms, 33 slices, voxel size = 4 mm3, 150 volumes. Structural images were acquired using a 3D MPRAGE sequence: TR = 1820 ms, TE = 4.38 ms, inversion time = 1100 ms), voxel size = 1 mm3.
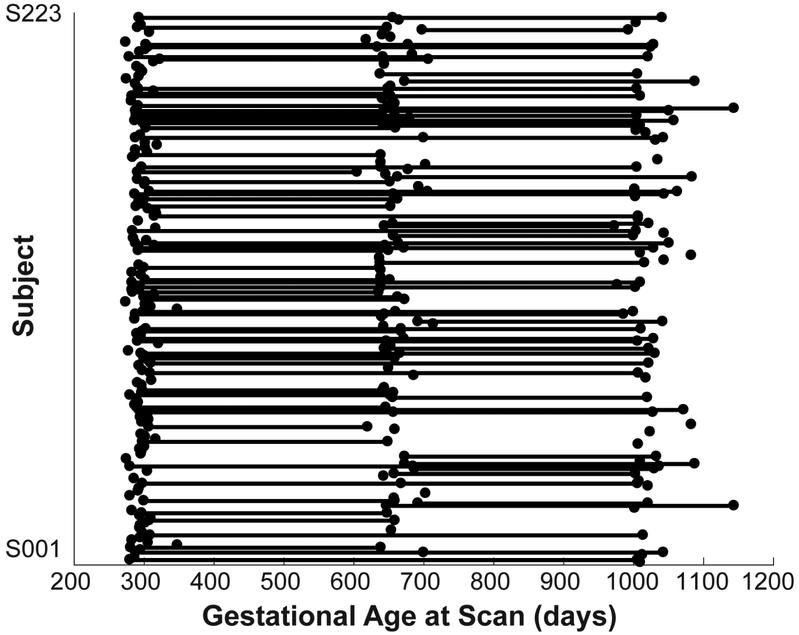
Figure 1: Data distribution.
The distribution of gestational age at scan for all included infant subjects (N = 223 totaling 334 datasets; neonate (NEO), 1-year (1YR), and 2-year (2YR) old) whose image quality passed the quality control procedures. Each dot represents a successful rsfMRI scan and dots along each line represent all the available longitudinal scans for a given subject; NEO: N = 152, 1YR: N = 105, 2YR: N = 77, NEO & 1YR: N = 57, 1YR & 2YR: N = 44, NEO & 2YR: N = 10, and NEO & 1YR & 2YR: N = 25.
Functional connectivity (FC) and related statistical analyses.
After standard image preprocessing (see Supporting Materials: Image preprocessing), including discarding the first 10 volumes, slice-timing correction, rigid-body motion correction, bandpass filtering (0.01– 0.08 Hz), nuisance signal regression and data scrubbing, we used the anatomical automatic labeling (AAL) atlas (Table S1) and seed-based technique (27) to characterize whole-brain FC associated with left or right amygdala. Correlation measures were normalized using Fisher’s Z-transformation and analyzed at the regional (i.e. voxel-wise) and network levels. Specifically, t-tests and log-linear mixed-effect (LME) models were used to quantify cross-sectional (NEO, 1YR, and 2YR) and longitudinal (NEO→1YR and 1YR→2YR) effects, respectively. Log-transformation of age was used in the LME modeling given previous work showing log-linear growth trends of FC during the first two years of life (28-30). The LME models included random intercept and slope terms with the effect estimate associated with post-natal age at scans (“growth”) being the principal variable of interest. Other participant characteristics were included as covariates in the LME models: sex, birth weight, motion, maternal education, and twin status. For the regional analyses, significance was defined using a clustering approach (AFNI: 3dClustsim). We used conservative settings (31-33) to achieve the desired correction rate of α=0.05. Specifically, we imposed a voxel-wise cutoff of P < 0.001 and generated smoothness estimates from the pre-processed data using the mixed model autocorrelation function. The following cluster sizes (bi-sided, edge or face connectivity, i.e. NN = 1) were established for each sub-sample (in voxels); NEO = 16, 1YR = 10, 2YR = 6, NEO→1YR = 12, and 1YR→2YR = 8. For network-level analyses we used the average left or right amygdala FC within predefined resting-state networks (RSN). RSN masks were defined using adult templates (34) warped into 2-year-old template space (35) – Figure S1.
Emotional and Cognitive Assessment at 4 years of age.
We obtained both parent-reported and task-based laboratory assessments of children’s behaviors at 4 years. The primary emotion-related outcome variables were ratings of anxiety (4YR-ANX) and inhibitory self-control (4YR-ISC) assessed using Behavior Assessment System for Children – 2nd edition (BASC-2) (36) and Behavior Rating Inventory of Executive Function – Preschool version (BRIEF-P) (37), respectively. Moreover, 4-year IQ (4YR-IQ) was also assessed using Stanford-Binet Intelligence Scales – 5th edition (38) as an index of cognitive development. More details are included in supporting materials (see Supporting Materials: Behavioral assessment information and subject breakdown).
Brain-behavior analyses.
We used linear regression to characterize the associations between infant amygdala FC measures and 4-year behavior. Specifically, for each detected cluster or RSN, regression models were generated using cross-sectional (NEO, 1YR, 2YR) or longitudinal (NEO→1YR, or 1YR→2YR) FC measures and 4-year behavioral outcomes in subjects with both measures (Table S1). For cross-sectional relationships we used the average FC within each significant cluster or RSN and included covariates in the analysis. Similarly, for longitudinal characterizations we extracted individual average growth estimates at the cluster or RSN level using the aforementioned LME models. Significance was defined at the P<0.01 level.
Results
3.1. Voxel-wise and network-level amygdala functional connectivity (FC) during the first two years of life.
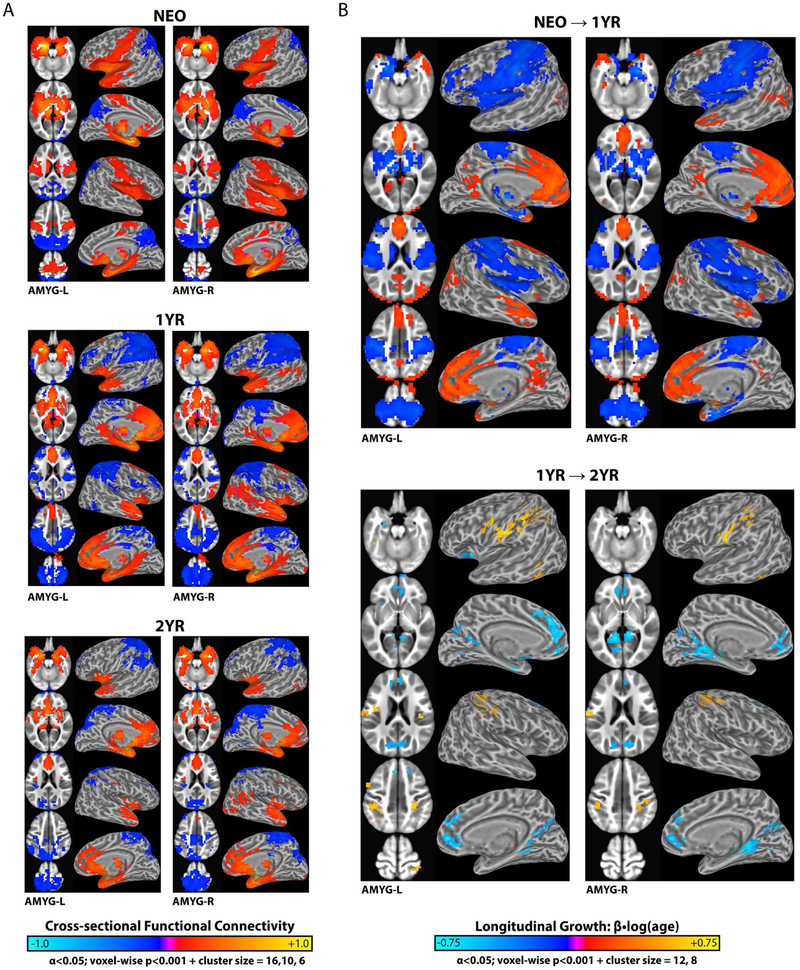
FC maps for the left and right amygdala in neonates (NEO), 1-year-olds (1YR), and 2-year-olds (2YR) are shown in Figure 2a, with corresponding longitudinal-effects (log(age); NEO→1YR & 1YR→2YR) presented in Figure 2b. The full results are summarized in Tables S3-6. Besides the highly similar FC patterns between the left and right amygdala (Supporting Material – Figure S2), there are four notable features. First, consistent with adult patterns (8, 9), amygdala FC showed significant positive connections in adjacent hippocampus, parahippocampus, and subcortical areas [e.g. caudate, putamen, thalamus, etc.] for all three age groups. Second, unlike adult patterns, positive amygdala FC with medial prefrontal cortex and negative FC with lateral prefrontal/parietal cortices was largely absent in neonates while, conversely, prominent positive FC in primary auditory cortex [e.g., middle temporal gyrus] and sensorimotor regions [e.g., pre-/postcentral gyrus and supplemental motor area], that are not typically observed in adults, was present in neonates. Third, during the first year (i.e. NEO→1YR) there was significant growth of amygdala FC towards adult-like patterns, including the emergence of positive connectivity within medial prefrontal areas, negative connectivity with lateral prefrontal/parietal areas, and the diminishing positive connectivity within primary auditory and sensorimotor regions (Figure 2a). These cross-sectional observations are highly consistent with statistically significant FC growth during this period (Figure 2b). In one-year-olds, amygdala FC was largely adult-like, featuring positive FC in ventral/medial cortices and negative clusters in the dorsal/lateral brain areas. Fourth, one and two-year amygdala FC patterns were qualitatively similar, however quantitative growth (i.e. 1YR→2YR) was evident (Figure 2b). Specifically, the primary sensorimotor regions showed positive growth (warm clusters in Figure 2b) whereas the medial prefrontal cortex, cuneus, precuneus and neighboring visual areas exhibited negative growth (cool clusters in Figure 2b). Interestingly, these relationships were predominantly in the opposite direction compared to NEO→IYR trends, signifying non-monotonic changes during infancy. Finally, three sex-related effects were detected (Supporting Material – Figure S3).
Figure 2: Regional functional connectivity (FC) and corresponding longitudinal changes for the left and right amygdala (AMYG-L/R) in neonates (NEO), 1-year (1YR), and 2-year old (2YR).
(a) Cross-sectional patterns of significant positive (warm colors) and negative (cool colors) amygdala FC. (b) Patterns of significant positive and negative amygdala FC growth: NEO→1YR and 1YR→2YR. Growth or age-dependent changes [β•log(age)] characterized using linear-mixed effect modeling (random intercept + slope) controlling for other participant characteristics (i.e. sex, twin status, motion, maternal education, and birth weight). See Tables S3-6 for full breakdown of significant clusters.
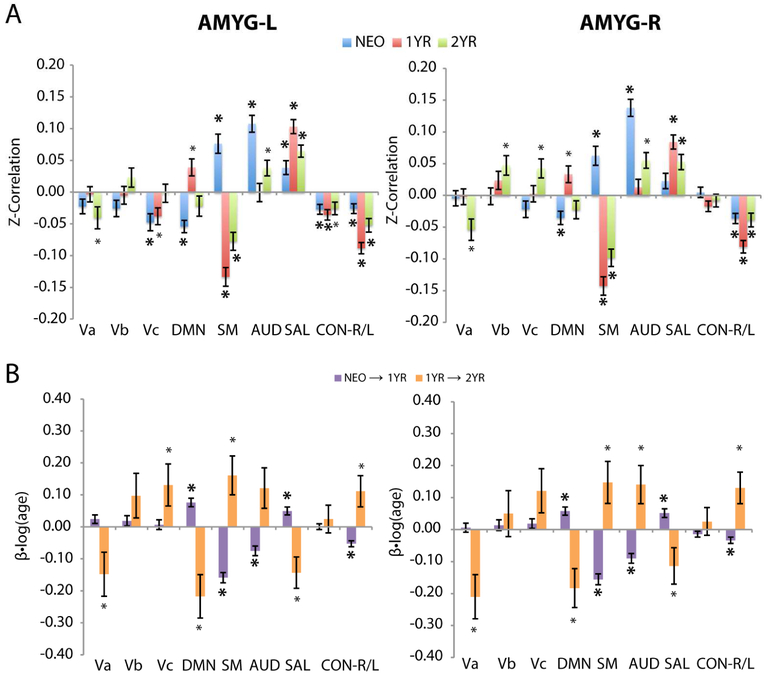
When examined at the network-level, the nine canonical resting-state functional networks [RSNs (34); visual (Va,b,c – primary, middle, lateral), default-mode (DMN), sensorimotor (SM), auditory (AUD), salience (SAL), and right/left executive control (CON-R/L) – see Fig. S1] showed distinct cross-sectional (Figure 3a) and growth (Figure 3b) patterns that were highly consistent with the regional observations. First, during year one, the trend of emerging connectivity (both positive and negative) with higher-order RSNs combined with regression in primary RSNs was apparent. Specifically, amygdala FC with the DMN was negative in neonates, likely driven by negative connectivity within the posterior parietal regions, while that for SAL was slightly positive, although no significant positive clusters were detected (Figure 2). During the first year, both left and right amygdala FC with the DMN and SAL showed statistically significant positive growth (p <= 0.001) and ultimately strong positive FC at the end of the first year. For CON-L, the slightly negative left and right amygdala FC in neonates also experienced significant negative growth (p <= 0.001) resulting in stronger negative connectivity by one year of age. In contrast, the two primary RSNs of AUD and SM, both showed strong positive left and right amygdala FC in neonates but significant negative growth (p <= 0.001) after that resulting in negative FC for SM and close-to-zero FC for AUD by one year of age. Interestingly, similar “reversals” in growth at the network level were observed when comparing the second and first year’s trend (8 out of 9 RSNs for the left amygdala and 7 out of 9 RSNs for the right amygdala).
Figure 3: Network-level functional connectivity (FC) and corresponding longitudinal changes for the left or right amygdala (AMYG-L/R) in neonates (NEO), 1-year (1YR), and 2-year old (2YR).
(a) Cross-sectional effects: NEO (blue), 1YR (red) , and 2YR (green). (b) Longitudinal effects or growth: NEO→1YR (purple) and 1YR→2YR (orange). Resting-state networks (RSNs) based on Smith networks (34) warped into infant template space – Figure S1. Primary, middle, and lateral visual (Va,b,c), default-mode network (DMN), sensorimotor (SM), auditory (AUD), salience (SAL), and right/left executive control (CON-R/L). Bars correspond to group means with error bars denoting standard error. * P <= 0.05 and * P <= 0.001.
3.2. Relationship between amygdala FC development during infancy and behavioral outcomes at 4-years of age.
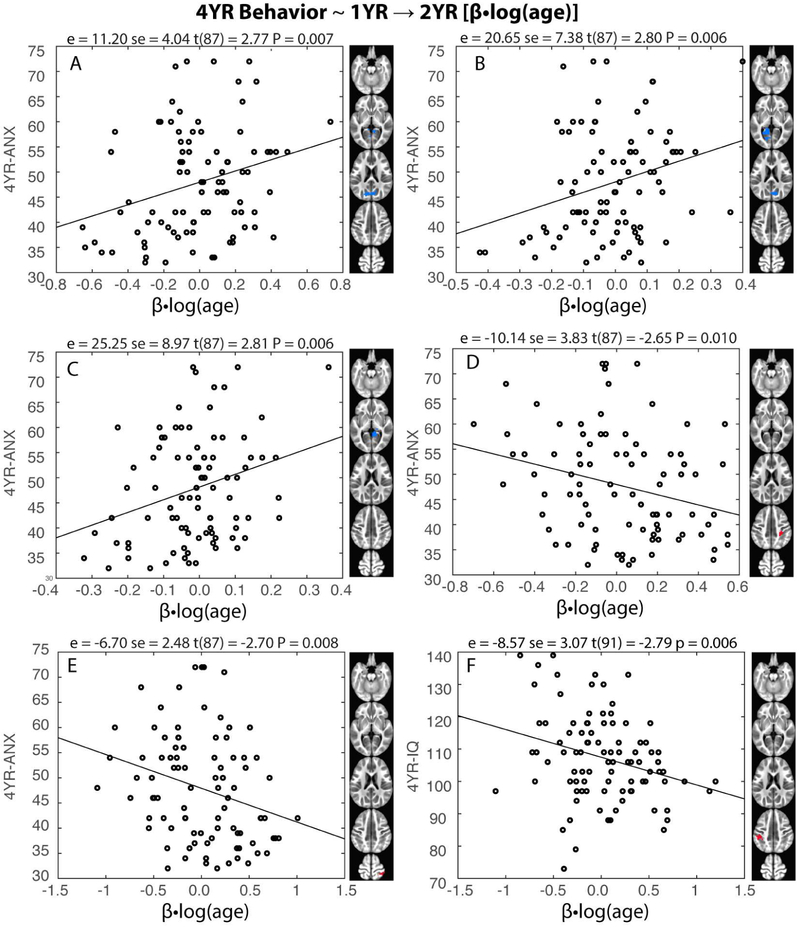
Having characterized amygdala FC development during the first two years of life, we asked if these measures could predict emotion-related parent-reported behavioral outcomes at 4 years of age. At the regional level, five significant predictions were detected (p<0.01) and all five involved 1YR→2YR growth rates and 4-year anxiety measures (4YR-ANX; Figure 4a-e). Specifically, 1YR→2YR left and right amygdala FC growth estimates (negative in sign) in the posterior regions of the DMN (PCUN, CUN) and neighboring visual areas (LING and CAL) positively predicted 4YR-ANX. Conversely, 1YR→2YR left amygdala FC growth estimates (positive in sign) in the right SM cortices (SMG-R, IPL-R, PoCG-R) negatively predicted 4YR-ANX. Note the signs between the FC growth estimates and FC ~ 4YR-ANX relationships were always opposite, indicating that the more FC growth towards the group trend, the lower the anxiety ratings were at 4 years of age. These relationships remained largely unchanged after including cognitive development (4YR-IQ) as an additional explanatory variable (Table S7). When directly assessing the relationships between regional level amygdala FC and 4YR-IQ (Figure 4f): 1YR→2YR left amygdala FC growth in left SM regions (IPL, PoCG) negatively predicted 4YR-IQ (P<0.001). There were fifteen additional marginally significant (P < 0.05, uncorrected) emotion-related relationships (Table S8). Overall, the significant/marginally significant predictions (N=20) were evenly split between anxiety and emotion regulation scores (i.e., 10/10 relationships with 4YR-ANX/4YR-ISC). Moreover, these relationships were predominantly associated with the left amygdala (n = 13 or 70%) and involved more longitudinal growth estimates (n = 13 or 65%, five associated with NEO→1YR and eight associated with 1YR→2YR). There were six other marginally significant regional relationships associated with 4YR-IQ (Table S9).
Figure 4: Longitudinal changes in infant amygdala functional connectivity (FC) during the second year (1YR→2YR) predict four-year outcome measures.
(a-c) 1YR→2YR left and right amygdala FC growth estimates [(β•log(age)] in the posterior regions of the default mode network [DMN: precuneus (PCUN) and cuneus (CUN) sub-regions] and neighboring visual areas [lingual gyrus (LING) and calcarine cortex (CAL)] positively predicted parent-reported anxiety at four years (4YR-ANX), (d-e) 1YR→2YR left amygdala FC growth estimates in the right SM cortices [supramarginal gyrus (SMG), inferior parietal lobule (IPL), and post-central gyrus (PoCG-R)] negatively predicted 4YR-ANX. (f) 1YR→2YR left amygdala FC growth in left SM regions (IPL, PoCG) negatively predicted four-year intelligence (4YR-IQ). Statistics; e = effect estimate, se = standard error, t-statistic (degrees of freedom) and P-values.
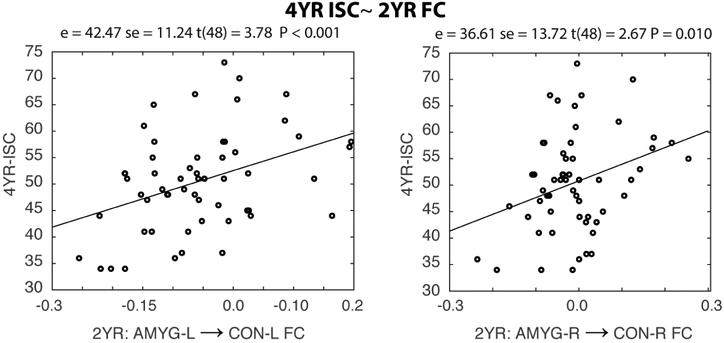
At the network level, two symmetric predictions of 4YR-ISC were detected: the mean FC between the left/right amygdala and the left/right executive control (CON-L/R) network at 2 years of age positively predicted 4YR-ISC (p<=0.01, Figure 5), indicating the more negative the within-hemisphere amygdala-CON network connectivity in 2-year-olds, the better the emotion regulation capability at 4 years of age. Potentially related, 2YR FC and 1YR→2YR FC growth between the left amygdala and CON-L/R also negatively predicted 4YR-IQ scores (P<=0.01 Table S10). However, when 4YR-IQ was included as an additional control variable both 4YR-ISC relationships remained marginally significant (p = 0.01/0.052 for the left and right amygdala, respectively). Finally, eleven other marginally significant network level brain-behavior relationships were identified (see Table S10)
Figure 5: Amygdala functional connectivity (FC) with the executive control network (CON) at two years (2YR) predicts four-year inhibitory self-control (4YR-ISC).
Mean FC between the left/right amygdala and the left/right executive control network (CON-L/R; see Figure S1) at 2-years of age positively predicted parent-reported 4YR-ISC. Statistics; e = effect estimate, se = standard error, t-statistic (degrees of freedom) and P-values.
Discussion
In this study, we have delineated the spatiotemporal dynamics of amygdala functional connectivity (FC) development during infancy. Specifically, the neonatal amygdala connectivity pattern featured adult-like positive connections with nearby subcortical (e.g., thalamus, caudate, and putamen) and limbic areas (e.g., hippocampus, parahippocampus) but was missing positive connections with medial prefrontal areas and negative connectivity with lateral prefrontal/parietal regions. Interestingly, long-range positive connectivity with primary auditory and sensorimotor areas were observed in neonates. During the first year of life, both positive and negative connectivity with medial prefrontal and lateral prefrontal/parietal cortices emerged while the neonate-specific long-range connections to primary auditory and sensorimotor areas regressed to zero or become negative. The amygdala connectivity patterns in 2-year-olds were qualitatively like those in 1-year-olds but statistically detectable changes revealed a reversed direction of growth during the second year compared with that during the first year. Importantly, our results also highlighted the behavioral significance of infantile amygdala FC development on 4-year emotional and IQ measures. Specifically, at the regional level, 1YR→2YR growth of amygdala FC with clusters within the precuneus/cuneus positively predicted 4-year anxiety while that with sensorimotor cortices negatively predicted 4-year anxiety scores. At the network level, within-hemisphere connectivity between the left/right amygdala and left/right executive control network (CON-L/R) positively predicted 4-year emotional regulation scores. FC between the amygdala and CON-L/R, as well as sensorimotor-related regions, also significantly correlated with 4-year IQ scores. Taken together, our results improve our understanding of the brain basis of emerging emotion processing/regulation capabilities in infants and may serve as the first step towards imaging-based biomarkers for identification of risks for atypical emotional development.
The amygdala is among the brain’s earliest areas to develop and becomes structurally and cytoarchitectually adult-like before birth (39). Therefore, it is not surprising to observe significant, adult-like FC of the amygdala with adjacent subcortical/limbic areas (9), including the hippocampus, parahippocampus, thalamus, caudate, and putamen, in neonates. Note these areas are key nodes in the “bottom-up” emotional appraisal system, so their synchronization at birth may enable neonates to generate critical emotional signals associated with life-essential endogenic (e.g., hunger) or exogenic stimuli (e.g., temperature, noise, smell, etc.). However, unlike adults, our results also revealed direct amygdala functional connections to primary sensorimotor cortices (i.e., middle temporal and pre/post-central gyrus) that were unique in neonates; these connections disappeared by age 1 and were not present in 2 year-olds. Moreover, the positive connections with medial prefrontal regions and negative connectivity with lateral prefrontal/parietal areas that are typically observed in adults were absent in neonates but emerged in 1- and 2-year-olds. Taken together, these findings may suggest that in the absence of top-down emotional regulation connections (e.g., the negative connectivity with lateral prefrontal/parietal areas), neonates could rely on positive connections with sensorimotor areas for direct motor output (e.g., crying, kicking) after the initial emotional valence appraisal enabled by the amygdala-subcortical-limbic circuit. This is highly in line with behavioral findings highlighting emotional impulsivity and lack of self-regulation as hallmark behaviors of newborns (13, 14).
During the first year, significant positive connectivity with medial prefrontal areas and negative connectivity with lateral prefrontal/parietal regions emerged while those positive connections with primary sensorimotor cortices largely regressed. At the network level, these patterns resulted in significant increase in amygdala connectivity with the default-mode (DMN) and the salience (SAL) networks and significant decrease in connectivity with the executive control (CON), and primary auditory (AUD)/sensorimotor (SM) networks. These dramatic changes resulted in a largely adult-like amygdala FC topology by the end of the first year, featuring positive connectivity with subcortical and ventral medial prefrontal areas and negative connectivity with lateral prefrontal and dorsal parietal regions (8, 9). These observations, particularly the negative connections with lateral prefrontal/parietal regions within the CON network, provide strong support for the emergence of emotion regulation circuits during the first year of postnatal development. Our findings are in line with a body of research documenting the development of emotion-regulatory behaviors within the first 12 months of life (14). For example, infant self-soothing behaviors and attentional distraction strategies emerge during the first year as effective ways leading to decreased negative affect or anger (21, 40, 41). The emergence of negative connectivity between CON regions and the amygdala during the first year, as observed in this study, is consistent with this previous research and supports the early development of top-down emotion regulation strategies. This emergence, together with the disappearance of direct amygdala connectivity with sensorimotor areas perhaps allows the infants to regulate their emotional responses by shifting attention away from distressing stimuli and reduce impulsive motor output.
Although qualitatively similar connectivity patterns were observed in 2-year-olds compared with 1-year-olds, statistically detectable changes do occur during the second year of life. Interestingly, most of the growth during the second year is sign-inversed compared with corresponding growth during the first year and this trend is consistent at both regional and network levels. These findings may suggest important “corrections” or fine tuning of first-year connectivity growth that may have important behavioral implications. Indeed, most of our significant brain-emotional behavioral relationships were either related to second year growth of amygdala FC (Figure 4) or FC strength at 2 years of age (Figure 5). Of the seven significant predictions (p<0.01) detected, five of them are between negative connections of the amygdala with high-order executive control regions/networks (i.e., left/right amygdala-precuneus in Figure 4 and left/right amygdalae-CON L/R in Figure 5) and 4-year anxiety/inhibitory control scores, supporting the importance of the emotion regulation circuits development during the second year on anxiety management and inhibitory control outcomes at 4 years of age. Consistent with our findings, behavioral studies have documented significant growth of emotion regulation strategies during the second year of life (13, 14, 42). For example, Parritz (43) found that 18-month-olds engaged in greater directing, information seeking, and social referencing of mothers than did 12-month-olds in distress regulation. Similarly, Grolnick et al. (44) found that active self-distraction (e.g., engagement with substitute toys) was more frequently used by 2-year-olds than by 1-year-olds as the main behavioral strategy for emotion regulation. These observations, together with our findings of the significant prediction power of infant amygdala FC on 4-year emotional behavioral outcomes, highlight the importance of infantile emotional circuit development on long-term behavioral outcomes.
Besides emotional outcomes, our results also revealed significant prediction power of infantile amygdala FC on 4-year IQ (Figure 4f, Table S9-10). Consistent with this finding, one recent study showed that infant temperament, specifically positive affectivity and emotion regulation capacity, at 4 months of age, was predictive of school readiness in preschool-aged children (18). Although school readiness is only partially explained by IQ, it represents a set of important cognitive abilities that also includes self-regulation and inhibitory control (e.g. (45)). Therefore, our findings with both emotional regulation and IQ are in line with this previous work. The results of this study provide strong support for the importance of infantile emotional circuitry and related behavioral development on later cognitive and academic outcomes.
In this study, the FC growth patterns were largely left-right symmetric (Fig. S2) but most of the detected brain-behavior relationships were associated with the left amygdala (30 out of 39). This finding is consistent with previous reports of the functional bias of the left amygdala toward sustained emotional processing (46, 47) and lateralized amygdala connectivity abnormalities associated with different psychiatric disorders (48, 49) and prenatal drug exposure (50). These findings imply more significant emotional behavior relevance of the left amygdala during infancy but more focused studies are needed to formally test this hypothesis.
Regarding sex effects, only three small clusters were detected (Fig. S3) indicating minor sex-related differences, which is consistent with other FC studies during infancy (29, 51). However, the three detected clusters are potentially interesting given reported sex differences in amygdala FC in adolescents (52, 53), adults (54, 55) and affective regulation behaviors in 6-month infants (56). While it’s tantalizing to hypothesize that the observed FC differences may underlie these findings, future studies are needed to rigorously test these hypotheses.
There are several limitations deserving further discussion. First, caution must be used when directly comparing our infant results with adult-based findings since the infant rsfMRI data were acquired during natural sleep while most adult reports are based on data collected during the awake state (57). Moreover, different sleep-stages (i.e. fluctuating wakefulness) represents another protentional confound in resting-state fMRI (58) but objective monitoring using simultaneous electrophysiology has proven operationally challenging in this population. In older populations, an interesting shift from positive to negative amygdala-MPFC connectivity has been reported (59), and this shift has been shown to be sensitive to social adversity (i.e., maternal deprivation) (48). However, one has to be cautious when attempting to extrapolate our results to connect the dots between infancy and childhood/adolescence findings since the later results were based on emotional task states rather than natural sleep. In addition, non-linear changes, that are similar as shown here between the first and second year of development, likely exist beyond infancy so future longitudinal studies covering a large age span are needed to delineate the longterm developmental trend of amygdala FC. Previous studies have shown risk-related alterations of amygdala FC development (e.g., prenatal drug exposure (60) and maternal depression (61, 62)) so future studies of different risk factors and related behavioral implications are needed to better translate amygdala-based imaging findings into practical use. An additional limitation concerns the use of parent-report outcome data. Several factors contribute to how parents rate their children’s behaviors, including, but not limited to, their own emotional states. This study does not have information about parent mood states or diagnoses that could affect how they rate their children on measures such as anxiety. Given the complicated interactions between parent emotions and behaviors, child emotions and behaviors, and how parents report on their children, unravelling significant relations can be difficult. Additional research should consider parents’ mood status as a factor and/or should utilize task-based assessments to measure child emotion-related behaviors in attempt to clarify these associations.
In conclusion, this study provides the first set of results delineating the spatiotemporal dynamics of amygdala functional connectivity development during infancy. The non-linear developmental trends together with significant predictions of 4-year behavioral outcomes in both emotional and cognitive domains highlight the importance of emotional circuit development during infancy on long-term outcomes. If independently validated, these results may provide important candidates for imaging-based biomarkers for early identification of risks related not only to emotional problems but also cognitive development delays.
Supplementary Material
Acknowledgement:
This work was supported by National Institutes of Health (R01DA DA042988, R21NS088975, R21DA043171, R03DA036645 to WG; T32-MH106440 to RLS ; U01MH110274 to WL; and R01MH064065 and R01HD05300 to JHG), and Cedars-Sinai Precision Medicine Initiative Award and institutional support to WG. We are grateful to the research assistants who collected and scored the 4-year-old cognitive data and/or scored the BASC-2 and/or the BRIEF over the years for this study: Haley Parrish Black, Sadie Hasbrouck, Monica Ferenz Guy, Kassidy Jezierski, Margaret Hamilton Fox, Molly McGinnis , Mallory Turner, Emma Brink, Emily Bostwick, Margo Williams, Neha Patel, Portia Henderson, and Jenna Obitko.
Footnotes
Financial Disclosure:
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Gallagher M, Chiba AA (1996): The amygdala and emotion. Curr Opin Neurobiol. 6:221–227. [DOI] [PubMed] [Google Scholar]
- 2.Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL (2013): Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 73:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis M (1992): The role of the amygdala in fear and anxiety. Annual review of neuroscience. 15:353–375. [DOI] [PubMed] [Google Scholar]
- 4.Drevets WC (2003): Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 985:420–444. [DOI] [PubMed] [Google Scholar]
- 5.Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, et al. (2006): Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Archives of general psychiatry. 63:139–149. [DOI] [PubMed] [Google Scholar]
- 6.Anticevic A, Repovs G, Barch DM (2012): Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 38:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin LM, Liberzon I (2010): The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. (2007): A validated network of effective amygdala connectivity. Neuroimage. 36:736–745. [DOI] [PubMed] [Google Scholar]
- 9.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochsner K, Gross J (2007): The Neural Architecture of emotion regulation. New York: Guilford Press. [Google Scholar]
- 11.Posne MI, Rothbart MK (2000): Developing mechanisms of self-regulation. Dev Psychopathol. 12:427–441. [DOI] [PubMed] [Google Scholar]
- 12.Rothbart MK, Ziaie H, O”Boyle C (1992): Self-regulation and emotion in infancy. San Francisco: Jossey-Bass Publishers. [DOI] [PubMed] [Google Scholar]
- 13.Cole PM, Martin SE, Dennis TA (2004): Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Dev. 75:317–333. [DOI] [PubMed] [Google Scholar]
- 14.Rothbart MK, Sheese BE, Rueda MR, Posner MI (2011): Developing Mechanisms of Self-Regulation in Early Life. Emot Rev. 3:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole PM, Deater-Deckard K (2009): Emotion regulation, risk, and psychopathology. J Child Psychol Psychiatry. 50:1327–1330. [DOI] [PubMed] [Google Scholar]
- 16.Sarason IG (1984): Stress, anxiety, and cognitive interference: reactions to tests. J Pers Soc Psychol. 46:929–938. [DOI] [PubMed] [Google Scholar]
- 17.Blair C (2002): School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children's functioning at school entry. Am Psychol. 57:111–127. [DOI] [PubMed] [Google Scholar]
- 18.Gartstein MA, Putnam S, Kliewer R (2016): Do Infant Temperament Characteristics Predict Core Academic Abilities in Preschool-Aged Children? Learn Individ Differ. 45:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole PM, Michel MK, Teti LO (1994): The development of emotion regulation and dysregulation: a clinical perspective. Monogr Soc Res Child Dev. 59:73–100. [PubMed] [Google Scholar]
- 20.Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, et al. (2015): Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stifter CA, Spinrad TL, Braungart-Rieker JM (1999): Toward a developmental model of child compliance: the role of emotion regulation in infancy. Child Dev. 70:21–32. [DOI] [PubMed] [Google Scholar]
- 22.Kagan J (1999): The concept of behavioral inhibition. . New York: Oxford University Press. [Google Scholar]
- 23.Gao W, Grewen K, Knickmeyer RC, Qiu A, Salzwedel A, Lin W, et al. (2018): A review on neuroimaging studies of genetic and environmental influences on early brain development. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmore JH, Knickmeyer RC, Gao W (2018): Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 19:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao W, Lin W, Grewen K, Gilmore JH (2016): Functional Connectivity of the Infant Human Brain: Plastic and Modifiable. Neuroscientist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, et al. (2012): Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 34:537–541. [DOI] [PubMed] [Google Scholar]
- 28.Alcauter S, Lin W, Keith Smith J, Gilmore JH, Gao W (2013): Consistent Anterior-Posterior Segregation of the Insula During the First 2 Years of Life. Cerebral cortex (New York, NY: 1991).1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W (2015): Development of human brain cortical network architecture during infancy. Brain Struct Funct. 220:1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pendl SL, Salzwedel AP, Goldman BD, Barrett LF, Lin W, Gilmore JH, et al. (2017): Emergence of a hierarchical brain during infancy reflected by stepwise functional connectivity. Human Brain Mapping.n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017): fMRI clustering and false-positive rates. Proc Natl Acad Sci US A. 114:E3370–E3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA (2017): FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, et al. (2011): Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 6:e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds C, Kamphaus R (2004): BASC-2 behavioral assessment system for children manual (2nd ed.). . Circle Pines, MN: AGS Publishing. [Google Scholar]
- 37.Gioia G, Espy K, Isquith P (2003): BRIEF-P: Behavior Rating Inventory of Executive Function--preschool Version: Professional Manual.: Psychological Assessment Resources. [Google Scholar]
- 38.Roid C (2003): Stanford-Binet Intelligence Scales: Fifth Edition. Ttasca, IL: Riverside Publishing. [Google Scholar]
- 39.Ulfig N, Setzer M, Bohl J (2003): Ontogeny of the human amygdala. Ann N Y Acad Sci. 985:22–33. [DOI] [PubMed] [Google Scholar]
- 40.Crockenberg SC, Leerkes EM (2004): Infant and Maternal Behaviors Regulate Infant Reactivity to Novelty at 6 Months. Developmental Psychology. 40:1123–1132. [DOI] [PubMed] [Google Scholar]
- 41.Ekas NV, Lickenbrock DM, Braungart-Rieker JM (2013): Developmental Trajectories of Emotion Regulation Across Infancy: Do Age and the Social Partner Influence Temporal Patterns? Infancy. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheese BE, Rothbart MK, Posner MI, White LK, Fraundorf SH (2008): Executive attention and self-regulation in infancy. Infant Behav Dev. 31:501–510. [DOI] [PubMed] [Google Scholar]
- 43.Parritz RH (1996): A descriptive analysis of toddler coping in challenging circumstances. Infant Behavior and Development. 19:171–180. [Google Scholar]
- 44.Grolnick WS, Bridges LJ, Connell JP (1996): Emotion regulation in two-year-olds: strategies and emotional expression in four contexts. Child Dev. 67:928–941. [PubMed] [Google Scholar]
- 45.Eisenberg N, Valiente C, Eggum ND (2010): Self-Regulation and School Readiness. Early Educ Dev. 21:681–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner G, Koch K, Reichenbach JR, Sauer H, Schlosser RG (2006): The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 44:2337–2347. [DOI] [PubMed] [Google Scholar]
- 47.Baas D, Aleman A, Kahn RS (2004): Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain research Brain research reviews. 45:96–103. [DOI] [PubMed] [Google Scholar]
- 48.Gee DG, Karlsgodt KH, van Erp TG, Bearden CE, Lieberman MD, Belger A, et al. (2012): Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr Res. 134:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL (2013): Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biological psychiatry. 73:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W (2015): Prenatal Drug Exposure Affects Neonatal Brain Functional Connectivity. The Journal of Neuroscience. 35:5860–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, et al. (2014): The Development of Human Amygdala Functional Connectivity at Rest from 4 to 23 Years: a cross-sectional study. Neuroimage. 95:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alarcón G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ (2015): Developmental sex differences in resting state functional connectivity of amygdala sub-regions. NeuroImage. 115:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin S, Young CB, Supekar K, Uddin LQ, Menon V (2012): Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences. 109:7941–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kogler L, Müller VI, Seidel E-M, Boubela R, Kalcher K, Moser E, et al. (2016): Sex differences in the functional connectivity of the amygdalae in association with cortisol. NeuroImage. 134:410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engman J, Linnman C, Van Dijk KRA, Milad MR (2016): Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology. 63:34–42. [DOI] [PubMed] [Google Scholar]
- 56.Weinberg MK, Tronick EZ, Cohn JF, Olson KL (1999): Gender differences in emotional expressivity and self-regulation during early infancy. Developmental Psychology. 35:175–188. [DOI] [PubMed] [Google Scholar]
- 57.Mitra A, Snyder AZ, Tagliazucchi E, Laufs H, Elison J, Emerson RW, et al. (2017): Resting-state fMRI in sleeping infants more closely resembles adult sleep than adult wakefulness. PLOS ONE. 12:e0188122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haimovici A, Tagliazucchi E, Balenzuela P, Laufs H (2017): On wakefulness fluctuations as a source of BOLD functional connectivity dynamics. Scientific Reports. 7:5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. (2013): A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salzwedel AP, Grewen KM, Vachet C, Gerig G, Lin W, Gao W (2015): Prenatal drug exposure affects neonatal brain functional connectivity. J Neurosci. 35:5860–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BF, et al. (2015): Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry. 5:e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, et al. (2016): Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry. 6:e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.