1. Introduction
On the basis of family, twin, and adoption studies, it is well established that common psychiatric disorders like schizophrenia (SZ), bipolar disorder (BD), and major depressive disorder (MDD) have a genetic basis (Bargmann & Gilliam, 2013). For the past few decades it has been thought that such disorders are not simple single gene (Mendelian) disorders, and are instead polygenic disorders, and influenced by environmental factors (Bargmann & Gilliam, 2013). Polygenic disorders are thought to follow various modes of transmission. According to one model, many genetic mutations in the population can contribute to the disorder, but each genetic mutation is individually rare and has a strong effect. This is called the “heterogeneity model” (Yang et al. 2005). Another model posits that a small number of relatively common genetic mutations, each of which individually has a small effect on risk for the disorder, interact to cause the disorder. This model is sometimes called the “common disease-common variant model” (Yang et al. 2005). Recently, Boyle at al. (2017) proposed a new model for complex traits which they termed the “omnigenic model”. This model has generated widespread interest and discussion. Here we discuss the possible role of the omnigenic model in relation to psychiatric disorders like SZ, BD, and MDD, invoking epigenetic mechanisms of gene expression to support this model for these disorders.
2. The Omnigenic model of Complex Traits
The omnigenic model of complex traits proposed by Boyle et al. (2017) was based on datasets of the inheritance pattern of height and three disorders which show complex patterns of inheritance: two autoimmune disorders, Crohn’s disease and rheumatoid arthritis, and one disorder of the central nervous system, SZ. Based on their analyses, the authors came to the following conclusions: 1. There is an extremely large number of causal variants with tiny effect sizes on height as well as Crohn’s disease, rheumatoid arthritis and SZ, and these causal variants are spread very widely across the genome. 2. The genetic contribution to disease is markedly concentrated in regions that are transcribed or marked by active chromatin in relevant cell types and tissues. 3. For several traits the largest effect variants are modestly enriched in specific genes or pathways that play direct roles in disease. However, the single nucleotide polymorphisms (SNPs) that contribute the most to heritability of disease tend to be spread across the genome and are not close to genes with disease-specific functions.
These conclusions led the authors to formulate the omnigenic model of complex traits according to which most complex traits are directly affected by a modest number of genes or gene pathways with specific roles in the etiology of disease, as well as their direct regulators. These are “core genes” and have biological roles in disease. The core genes generally contribute only a little to total heritability, and most “peripheral genes” expressed in relevant cell types could also contribute to heritability. The peripheral genes greatly outnumber the core genes and together contribute to most of the heritability of the traits. For this to be possible, the authors suggest that cell regulatory networks are highly interconnected so that any expressed gene regulates the function of core genes. The regulatory networks likely include all layers of interactions among cellular molecules, including transcriptional networks, post-translational modifications, protein-protein interactions, and intercellular signalling.
The authors mention that there are great challenges for fully understanding the implications of the omnigenic model for complex traits. They suggest several questions and tests for future work on the omnigenic model. The authors also suggest that some complex disorders may not even have core genes, and instead, in such disorders the global activity (including gene expression) of all genes may set cellular system states that determine cellular function and disease risk.
3. Epigenetic Mechanisms of Gene Expression
Epigenetics, above, or, in addition to genetics, is presently an active area of biomedical research. It involves interacting molecular mechanisms including DNA methylation, histone modifications, and noncoding RNA (ncRNA)-mediated regulation of gene expression (Allis et al. 2015). Epigenetic regulation of gene expression is influenced by environmental factors and can result in either activation or repression of gene expression (Allis, et al., 2015). Epigenetic changes are known to display tissue-and cell type-specificity in response to environmental stimuli (Bettscheider et al., 2012). DNA methylation occurs due to the conversion of cytosine to 5-methylcytosine (5mC) by the addition of a methyl group and is associated with gene silencing. This reaction is catalyzed by DNA methyltransferases using S-adenosylmethionine as the source of the methyl group. The 5mC can be further modified to 5-hydroxymethylcytosine (5hmc) by the action of the ten eleven translocation (TET) family of enzymes (Allis et al. 2015). Histone modifications like acetylation, methylation, and phosphorylation occur mainly on the tails protruding from the globular domains of the histones.
Such modifications cause changes in the accessibility of DNA to the transcription machinery leading to changes in gene expression. Regarding ncRNA-mediated regulation of gene expression, there are several types of ncRNAs, including short ncRNAs which are less than 200 base pairs long and long ncRNAs which are more than 200 base pairs long (Allis et al., 2015). There are several thousand genes encoding ncRNAs in humans and they are located throughout the human genome (Esteller, 2011). The most widely studied among the ncRNAs are the microRNAs (miRNAs), and there are about 2500 miRNAs that have been catalogued in humans (RNAcentral Consortium, 2015). Each miRNA can bind to hundreds of different messenger RNAs (mRNAs) which collectively results in the regulation of more than 60% of protein-coding genes in humans (Esteller, 2011).
4. Relevance of Epigenetics to the Omnigenic Model of Psychiatric Disorders
Despite a huge effort by genetic mapping studies, to date, no genetic mutations or polymorphisms predisposing to SZ, BD, and MDD have been definitively identified, although some associations have been found (Gebicke-Haerter, 2016). However, there is increasing evidence that there are abnormalities of epigenetic mechanisms of gene expression underlying these disorders (Lee & Avramopoulos, 2014; Gebicke-Haerter, 2016). Such epigenetic abnormalities include changes in DNA methylation, histone modifications, and ncRNA-mediated regulation of gene expression.
An epigenetic basis for psychiatric disorders like SZ, BD, and MDD supports the omnigenic model of Boyle and colleagues for complex traits in many ways (Fig. 1): 1. According to the omnigenic model predisposing genes are spread across the genome. In this regard, there is accumulating evidence that there are genome-wide epigenetic abnormalities in the brain underlying these disorders. Viana et al. (2017) conducted a genome-wide study of DNA methylation changes in the post-mortem brain of patients with SZ in comparison to controls (41 SZ patients and 47 controls without any psychiatric disorder). Multiple brain regions (prefrontal cortex, striatum, hippocampus, and cerebellum) were assessed for DNA methylation changes across the genome. The authors found that more than 50 loci in each brain region in patients with SZ showed significant DNA methylation changes when compared with controls. These findings suggest that DNA methylation abnormalities are spread across the genome in the brain of patients with SZ. Hannon et al. (2016) used an integrated epigenetic-genetic approach to provide evidence for co-localization of genetic associations and differential DNA methylation across the epigenome in patients with SZ. 2. According to the omnigenic model for complex traits, there are an extremely large number of genes that are implicated in these traits. In this context, as mentioned above, Viana et al. (2017) detected DNA methylation abnormalities at more than 50 loci in multiple brain regions in SZ patients in comparison to controls. Ruzicka et al. (2015) studied DNA methylation levels at 1308 glutamic acid decarboxylase1 (GAD1) regulatory network-associated CpG loci in post-mortem hippocampus in patients with SZ and BD, and normal controls (8 subjects in each of the 3 groups). A total of 146 differentially methylated positions with a false detection rate lower than 0.05 were found. Thus, it appears that large numbers of genes are epigenetically associated with SZ and BD. 3. According to the omnigenic model of complex traits, there are highly interconnected networks involved in the development of complex traits. In this context, several ncRNAs have been implicated in the pathogenesis of SZ, BD, and MDD. Kim et al. (2010) studied the expression of 667 miRNAs in the post-mortem prefrontal cortex of 35 SZ patients and 35 BD patients in comparison to control subjects. They found that 22 miRNAs were differentially expressed in the patients in comparison with controls. As mentioned in Section 3, epigenetics involves interacting molecular mechanisms, and miRNAs regulate the expression of hundreds of genes and together they regulate more than 60% of protein-coding genes in humans. 4. Some genes are consistently modified epigenetically in patients with psychiatric disorders in comparison to controls. In SZ and BD, there are DNA methylation abnormalities in the GAD1 gene encoding GAD67 and the reelin (RELN) gene that encodes a protein involved in brain development and synaptogenesis (Guidotti & Grayson, 2014). In MDD there are DNA methylation abnormalities in the brain-derived neurotrophic factor (BDNF) gene (Mitchelmore & Gede, 2014 ). The miRNA miR-137, which is thought to play a major role in brain development, is dysregulated in SZ (Mahmoudi and Cairns, 2017). The gene MIR137, encodes this miRNA. MIR137 is known to be subject to epigenetic changes and changes in DNA sequence, leading to dysregulation of the activity of miR-137 (Mahmoudi and Cairns, 2017). 5. According to the omnigenic model for complex traits, the predisposing genes are markedly concentrated in active chromatin in relevant cells and tissues. In this context, it is well known that epigenetic mechanisms of gene expression play a major role in regulating the structure of chromatin. The chromatin can be epigenetically modified to a compacted form less amenable to transcription, and a less compacted form more amenable to transcription (Allis et al. 2015). Moreover, these changes are cell-type specific and occur to differing extents in different brain structures (Bettscheider et al. 2012). 6. Transcriptome studies of post-mortem brain tissues of patients with common psychiatric disorders have revealed abnormalities of gene expression across the genome in comparison to controls.
Fig. 1.
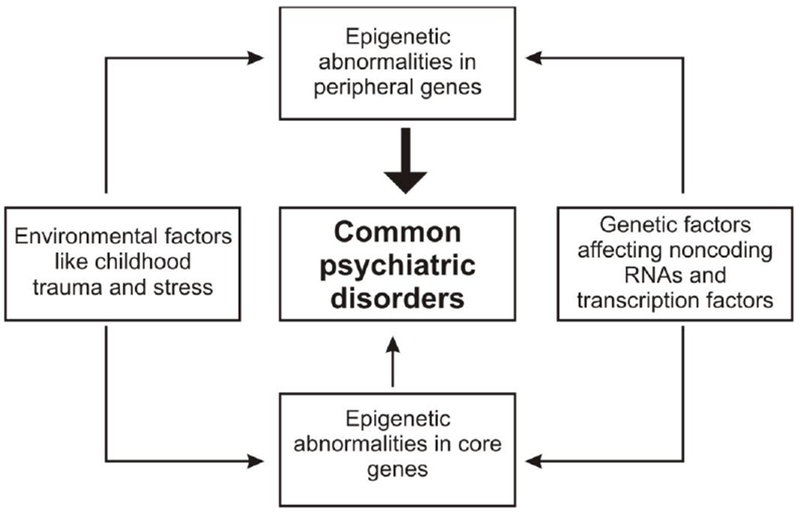
Schematic diagram demonstrating that epigenetic abnormalities in core and peripheral genes predispose to common psychiatric disorders. Peripheral genes are much greater in number than core genes and have a greater effect on the disorders (represented by a thick arrow). Environmental and genetic factors influence the expression of core and peripheral genes.
Chang et al. (2017) used RNA sequencing to investigate transcriptome-wide changes in expression of mRNAs in post-mortem amygdala tissues from 22 SZ patients and 24 controls. They found that 569 mRNAs (75%) were up-regulated and 192 mRNAs (25%) were down-regulated in the amygdala of SZ patients compared to controls. Akula et al. (2014) used RNA-seq to survey the brain transcriptome in post-mortem dorsolateral prefrontal cortex from 11 BD patients and 11 controls. A total of 1225 genes were found to be differentially expressed between the patients and controls with a nominal meta-analysis P-value of <0.05. Labonte et al (2017) used RNA-seq to examine the transcriptome of six post-mortem brain regions (ventromedial prefrontal cortex, orbitofrontal cortex, dorsolateral prefrontal cortex, anterior insula, nucleus accumbens, and ventral subiculum) in 26 MDD patients and 22 controls. They found significant transcriptional differences between patients and controls in each of the six brain regions studied.
Transcriptome studies investigate genome-wide expression of genes (Chang et al., 2017). Gene expression is a complex process involving transcription of DNA, translation of mRNAs into proteins, and post-translational modifications of proteins (Gibney & Nolan, 2010), and is the link between the genotype and the phenotype in an organism (Papatheodorou et al., 2015). Gene expression not only varies from one organism to another, but within a given organism, varies from cell to cell, tissue to tissue, and organ to organ (Papatheodorou et al., 2015). Gene expression also varies temporally and is influenced by the environment (Gibney & Nolan, 2010). Variations in gene expression are both quantitative and qualitative (Papatheodorou et al., 2015). Gene expression is regulated not only by DNA sequence, but also epigenetically at the stages of transcription, translation, and post-translational modifications of proteins (Gibney & Nolan, 2010).
5. Environmental Factors and the Epigenetic-Based Omnigenic Model of Psychiatric Disorders
As mentioned in Section 1, environmental factors are known to be involved in the pathogenesis of common psychiatric disorders. As mentioned in Section 3, environmental factors are known to influence epigenetic mechanisms of gene expression. In this context, Labonté et al. (2012) investigated genome-wide epigenetic abnormalities caused by early life psychosocial trauma due to physical and sexual abuse. The authors studied DNA methylation abnormalities in gene promoters across the genome in post-mortem hippocampal tissue from 41 adult suicide completers (25 subjects with a history of severe childhood abuse and 16 control subjects with no such history). The authors identified 362 differentially methylated promoters in subjects with a history of childhood abuse in comparison to control subjects. Of these promoters, 248 showed DNA hypermethylation, and 114 showed DNA hypomethylation.
6. Concluding Remarks
The omnigenic model for complex traits was based on data-crunching of the inheritance patterns of height and three disorders that show complex patterns of inheritance (Crohn’s disease, rheumatoid arthritis, and SZ). Hence, there appears to be solid evidence supporting this model. Several clinical features of common psychiatric disorders like SZ, BD, and MDD suggest that epigenetic abnormalities underlie these disorders. Such features include a high degree of discordance of monozygotic twins for these disorders; difference between males and females in the rate and course of illness; and fluctuations in the course of illness of the disorders (Peedicayil, 2007). The study of epigenetic abnormalities in patients with common psychiatric disorders is in its early stages. A very high number of genes have been found to show epigenetic abnormalities in terms of DNA methylation, histone modifications, and ncRNAs across the genome in such disorders. However, to date a few genes like the GAD1, RELN, and BDNFgenes and the MIR137gene encoding miR-137 have shown relatively consistent abnormalities across studies. More genes showing consistent abnormalities across studies of patients with common psychiatric disorders are likely to be identified in the future.
This article has invoked abnormalities in epigenetic mechanisms of gene expression to support an omnigenic model of SZ, BD, and MDD (Section 4). If true, this model of psychiatric disorders like SZ, BD, and MDD will vindicate the suggestion of the noted American biologist Richard Strohman (1997) that common disorders involve interactive cellular epigenetic processes that are transcalculational (i.e., mind boggling). He suggested that these cellular processes involve open networks of genes, proteins, and environmental signals that may be coextensive with cells themselves. According to him, it is as if the cell has interposed between its genome and its behavior a second informational system able to integrate environmental and genetic information into its dynamic process, resulting in responses that are functional and adaptive (Strohman, 1997).
Highlights.
Recently, the omnigenic model for the inheritance of complex traits was proposed
There is increasing evidence that there are abnormalities of epigenetic mechanisms of gene expression underlying psychiatric disorders
This article invokes abnormalities of epigenetic mechanisms of gene expression to propose an omnigenic model for the inheritance of schizophrenia, bipolar disorder, and major depressive disorder
Acknowledgements
The authors acknowledge two anonymous Referees for their comments on the manuscript. This work was supported, in part, by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant P50AA022538 (Center for Alcohol Research in Epigenetics) to DRG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- Akula N, Barb J, Jiang X, Wendland JR, Choi KH, Sen SK, et al. 2014. RNA-sequencing of the brain transcriptome implicates dysregulation of neuroplasticity, circadian rhythms and GTPase binding in bipolar disorder. Mol. Psychiatry 19: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis CD, Caparros M-L, Jenuwein T, Lachner M, Reinberg D 2015. Overview and Concepts In: Allis CD, Caparros M-L, Jenuwein T, Reinberg D, Lachner M (Eds.), Epigenetics. Cold Spring Harbor Laboratory Press, New York, pp. 47–115. [Google Scholar]
- Bargmann CI, Gilliam TC, 2013. Genes and behavior In: Kandel ER, Schwartz JH, Jessell. TM, Siegelbaum SA, Hudspeth AJ (Eds.), Principles of Neural Science. McGraw-Hill, New York, pp. 39–65. [Google Scholar]
- Bettscheider M, Kuczynska A, Almeida O, Spengler D 2012. Optimized analysis of DNA methylation and gene expression from small, anatomically-defined areas of the brain. J. Vis. Exp., 65: e3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle EA, Li YI, Pritchard JK, 2017. An expanded view of complex traits: From polygenic to omnigenic. Cell 169: 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Liu Y, Hahn C-G, Gur RE, Sleiman PMA, Hakonarson H 2017. RNA-seq analysis of amygdala tissue reveals characteristic expression profiles in schizophrenia. Transl. Psychiatry 7:e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, 2011. Non-coding RNAs in human disease. Nat. Rev. Genet, 12: 861–874. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ, 2016. Systems psychopharmacology: A network approach to developing novel therapies. World J. Psychiatry 6: 66–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney ER, Nolan CM 2010. Epigenetics and gene expression. Heredity 105: 4–13. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Grayson DR 2014. DNA methylation and demethylation as targets for antipsychotic therapy. Dialogues Clin. Neurosci, 16: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon E, Dempster E, Viana J, Burrage J, Smith AR, Macdonald R et al. 2016. An integrated genetic-epigenetic analysis of schizophrenia: Evidence for co-localization of genetic associations and differential DNA methylation. Genome Biol, 17: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A,H, Reimers M, Maher B, Williamson V, McMichael O, McClay JL, 2010. microRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res, 124: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, et al. 2012. Genome-wide epigenetic regulation by early-life trauma. Arch. Gen. Psychiatry 69: 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. 2017. Sex-specific transcriptional signatures in human depression. Nat. Med, 23:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Avramopoulos D 2014. Introduction to Epigenetics in Psychiatry In: Peedicayil J, Grayson DR, Avramopoulos D (Eds.), Epigenetics in Psychiatry. Elsevier, Waltham, MA, pp. 3–25. [Google Scholar]
- Mahmoudi E, Cairns MJ 2017. miR-137: An important player in neural development and neoplastic transformation. Mol. Psychiatry 22: 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchelmore C, Gede L 2014. Brain-derived neurotrophic factor: Epigenetic regulation in psychiatric disorders. Brain Res, 1586: 162–172. [DOI] [PubMed] [Google Scholar]
- Papatheodorou I, Oellrich A, Smedley D 2015. Linking gene expression to phenotypes via pathway information. J. Biomed. Semantics 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peedicayil J 2007. The role of epigenetics in mental disorders. Indian J. Med. Res, 126:105–111. [PubMed] [Google Scholar]
- RNAcentral Consortium. 2015. RNAcentral: An international database of ncRNA sequences. Nucl. Acids Res, 43: D123–D129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka WB, Subburaju S, Benes FM 2015. Circuit-and diagnosis-specific DNA methylation changes at γ-aminobutyric acid-related genes in postmortem human hippocampus in schizophrenia and bipolar disorder. JAMA Psychiatry 72: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman RC 1997. Epigenesis and complexity: The coming Kuhnian revolution in biology. Nat. Biotechnol, 15: 194–200. [DOI] [PubMed] [Google Scholar]
- Viana J, Hannon E, Dempster E, Pidsley R, Macdonald R, Knox O et al. 2017. Schizophrenia-associated methylomic variation: Molecular signatures of disease and polygenic risk burden across multiple brain regions. Hum. Mol. Genet, 26: 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Khoury MJ, Friedman J, Little J, Flanders WD, 2005. How many genes underlie the occurrence of common complex diseases in the population? Int. J. Epidemiol, 34: 1129–1137. [DOI] [PubMed] [Google Scholar]


