Abstract
Background:
The mouse is the most widely used mammal in experimental biology. While many clinically relevant in vivo cardiac stressors are employed, one that has eluded translation is long-term cardiac pacing. Here we present the first method to chronically simulate and simultaneously record cardiac electrical activity in conscious mobile mice. We then apply it to study right ventricular (RV)-pacing induced electro-mechanical dyssynchrony, and its reversal (resynchronization).
Methods and Results:
The method includes a custom implantable bipolar stimulation/ and recording lead, and flexible external conduit and electrical micro-commutator linked to a pulse generator/recorder. This achieved continuous pacing for at least 1 month in 77% of implants. Mice were then subjected to cardiac ischemia/reperfusion (I/R) injury to depress heart function, followed by 4 weeks pacing at the RV (dyssynchrony), right atrium (synchrony), or for 2 weeks RV and then 2 weeks normal sinus (resynchronization). RV-pacing induced dyssynchrony substantially reduced heart and myocyte function compared to the other groups, increased gene expression heterogeneity (>10 fold) comparing septum to lateral walls, and enhanced growth and metabolic kinase activity in the late-contracting lateral wall. This was ameliorated by restoring contractile synchronization.
Conclusions:
The new method to chronically pace conscious mice yields stable atrial and ventricular capture and a means to dissect basic mechanisms of electro-mechanical physiology and therapy. The data on dyssynchrony and resynchronization in I/R hearts is the most comprehensive to date in ischemic heart disease, and its similarities to non-ischemic canine results supports the translational utility of the mouse.
Keywords: Heart Failure, Pacemaker, Dyssynchrony, Animal Model
Subject codes: mouse, pacemaker, left ventricle, methods, cardiac resynchronization
INTRODUCTION
Mice are the most tractable and widely utilized mammalian model of disease, primarily due to their amenability to genetic engineering to probe molecular pathways. For cardiac disorders, clinically inspired mouse models of pressure overload, myocardial infarction, hormone hyper-stimulation, and cardiotoxic stress are well established, and have been widely combined with genetically modified backgrounds to explore mechanisms. Another common intervention is cardiac pacing, which is clinically used to regulate heart rhythm and function. In 2006, we reported the first effort to chronically pace mice that used a custom implantable battery-powered stimulator and lead system1. While effective in the short term, it often suffered battery run down and lost capture after 10 days. A second effort reported in 2015 2 surrounded mice in an electromagnetic field to induce current wirelessly in an implanted lead. This system, however, was limited to acute studies in sedated mice. In late 2018, Hulsmans3 et al used a newly developed clinical miniature pacemaker (Micra™, Medtronic, Minneapolis) customized with a unipolar lead to provide chronic (4-week) fixed rate pacing. This method, however, does not provide continuous monitoring, making overdrive pacing at near the sinus-rate unattractive given the risk of capture loss. In addition, the pacemaker is expensive, and though small, is 7% of the weight of an average mouse.
In addition to regulating heart rate, another use of pacing is to improve cardiac function in failing hearts with electrical conduction delay and corresponding intraventricular contractile dyssynchrony. Dyssynchrony results from early electrical activation at one region of the ventricle that is followed by late activation of the opposite region. Cardiac resynchronization therapy (CRT) offsets this by simultaneously stimulating both regions, enhancing chamber mechanics and energetic efficiency4, 5, and is now standard therapy in appropriate patients6. CRT also disproves the theory that any means of stimulating ventricular systolic function and work in a failing heart will worsen long-term outcome, as CRT improves both morbidity and mortality6. This aspect spawned basic research to identify cellular and molecular effects of dyssynchrony and CRT, and these revealed that CRT enhances myocyte survival7, β-adrenergic signaling8–10, mitochondrial ATP synthesis11, sarcomere calcium sensitivity12, and calcium homeostasis13–15. All of these findings were obtained in large mammals (mostly tachypaced dogs)16, and adoption to mice for more mechanistic studies remains absent.
The current study aimed to develop a method for chronic pacing of the mouse atrium or ventricle with simultaneous electrocardiogram recording in conscious minimally constrained mice. Our second goal was to use the method to model cardiac dyssynchrony and resynchronization with a background of ischemic heart disease. Rather than pursue implantable stimulators, we employ an externalized custom combination bipolar pacing and ECG recording lead, that passes through a spring-conduit and near-frictionless electrical commutator to an external pulse generator/recorder system. We then applied the method to assess cardiac dyssynchrony and resynchronization in mice, and reveal chamber, cellular, and molecular changes that are very concordant with results previously only obtained in large mammals. The method and application opens up new approaches to study chronic electro-mechanical cardiac disease and interventions.
METHODS
All individual data and analysis will be made available to other researchers upon reasonable request to the corresponding author, for the purpose of replicating the procedures. While it is not feasible for us to provide all the specific equipment used for the chronic pacing system, all of the components except for the pacing leads are commercially available, and we will assist anyone who wishes to develop the system for their own use.
Mouse Pacemaker System
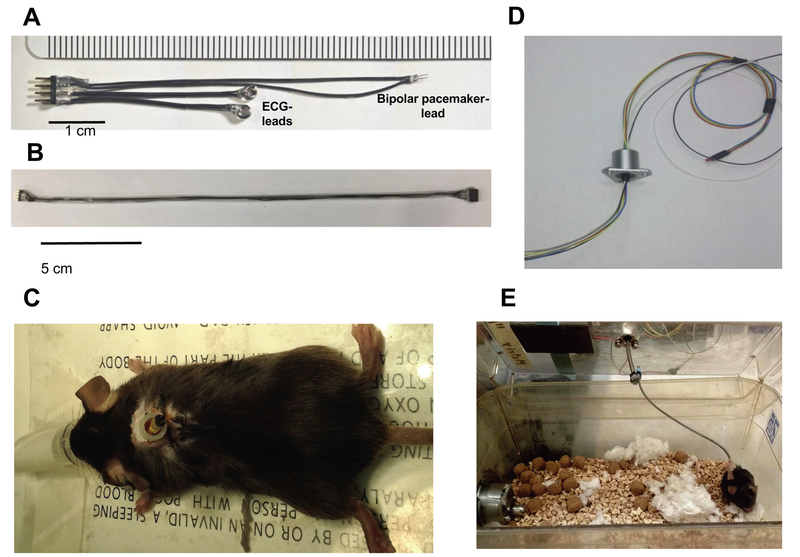
Figure 1 shows components of the mouse pacemaker system. It is comprised of a custom pacemaker/ECG lead (Fig. 1A, construction details in Supplemental Methods, and Supplemental Video-1), a spring-conduit containing four wires (Fig. 1B) terminating with 4-pin connectors at each end to link the externalized lead (Fig. 1C) to a miniature electrical slip-ring commutator (Model SL-88–10, Dragonfly R&D Inc, Ridgeley, WV) (Fig. 1D). The latter is mounted centrally atop a standard rat-cage (Fig. 1E), allowing the mouse to move with minimal constraint while maintaining continuous electrical contact for pacing and ECG recording. The pacing lead has a micro-pitchfork shape, with anode and cathode made from 38 gauge (0.1 mm) platinum wire. The commutator transmits up to 6 independent electrical signals while simultaneously rotating 360° with minimal torque. The externalized pacing wires are connected to a pulse generator (SD9, Grass Instruments, Quincy, MA) and ECG-leads to a data-acquisition system (LabChart, PowerLab, ADInstruments, AU) for continuous display and signal analysis.
Figure 1. Experimental components of mouse pacemaker system.
(A) Combined pacemaker/ECG lead; (B) flexible spring conduit terminating in a male and female 4-pin connector; (C) externalized lead exiting the mid-scapulae of a mouse within a protective plastic housing; (D) a six-wire micro-electronic commutator; (E) overall attachment set up, with the commutator attached to the center of the cage top, the flexible wire linked to the commutator and mouse, allowing mobility of the mouse with minimal constraint.
Pacemaker- and ECG lead implantation
The procedure for constructing the pacing/recording lead and its implantation are provided in Supplemental Methods and Videos. Mice are anesthetized with isoflurane, intubated and placed on a mechanical ventilator. Anesthesia is maintained with 1.8–2% isoflurane. A small incision is made in the mid-scapular region with the mouse in a prone position, and serves as the exit site for pacing/recording lead. With the mouse on its back, a small skin incision is made in the left lateral thorax. Next, the mouse is rotated to a right lateral decubitus position, and the lead tunneled subcutaneously to the skin incision in the left lateral thorax. After performing a limited left lateral thoracotomy into the second intercostal space, the pericardium is removed, right ventricle (RV) identified, and 100% platinum pacemaker electrodes pushed gently into the wall at a ~45 ° angle to the mid-RV free wall. The wires are sufficiently small and firm to pass through the myocardium easily without causing any noticeable collateral tissue damage. The same procedure is used for atrial implants, but access is through the third intercostal space. Once the electrodes are implanted, 10µL corticosteroid (triamcinolone acetonide nasal spray, Geiss, Destin & Dunn, Peachtree City, GA) is applied to the surrounding epicardium and secured with 1 μL tissue adhesive (Vetbond, 3M, St. Paul, MN) (~ volume of single drop at tip of standard gel electrophoresis loading pipette). Initial capture thresholds are generally <1.5 V at 0.5 ms pulse width. The majority (75 %) of thresholds remain <2.5 V with only 18 % rising above 5 V within the 28-day period (Supplemental Figure). The thoracotomy is closed with 6–0 prolene sutures, the mouse placed in a prone position, and the ECG electrodes tunneled subcutaneously into each upper proximal limb and sutured to the superficial muscle layer. The externalized 4-pin connector is placed within a plastic protector and secured closing the initial mid-scapular incision. Buprenorphine (0.1 mg/kg s.c.) is provided for peri- and post-operative analgesia. Antibiotics were not employed.
Heart Failure model and Pacing Protocol
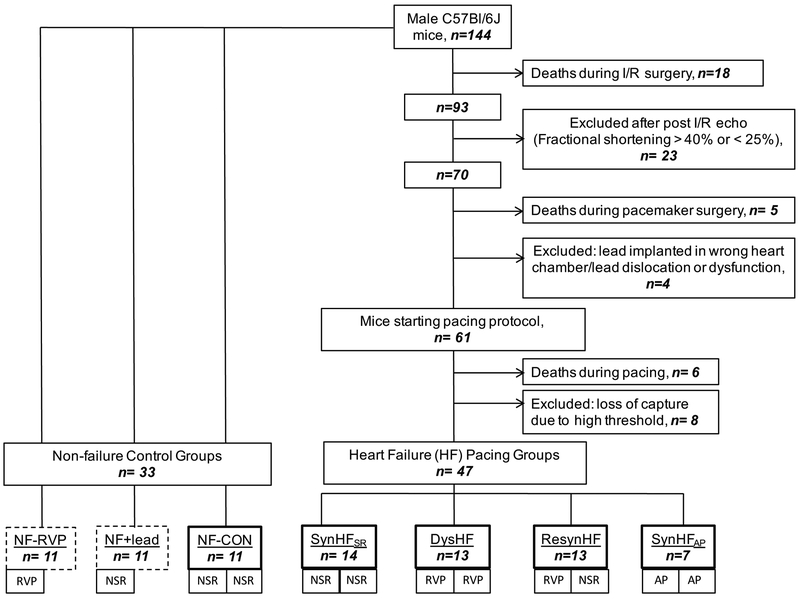
Figure 2 displays the flow chart for the study. All of the protocols were reviewed and approved by the JHU Institutional Animal Care and Use Committee. The study involved 144 C57Bl6/J adult mice (17–24 wk old), of which 33 were allocated to control groups, and the remainder to undergo ischemia-reperfusion (I/R) injury (60 min proximal left anterior descending artery total constriction, followed by reperfusion) prior to receipt of the pacemaker. Based on prior studies, this yields a risk area of ≈55% of the LV wall, and infarct size of 25%. Mice surviving I/R (84%) were screened within 24 hours by echocardiography, and those with reduced fractional shortening falling between 25–40% (normal 57%) received pacing/recording leads one week later (n=70). Successful implants (87%) were then randomized to four pacing groups: 1) Synchronous heart failure with 4 weeks of sinus rhythm (SynHFSR, n=14); 2) Dyssynchronous heart failure (DysHF, n=13) with 4 weeks of RV pacing at the lowest rate needed to assure capture; 3) Synchronous heart failure with 4 weeks atrial pacing (SynHFAP, n=7) serving as a rate-matched control for DysHF; and 4) resynchronized heart failure (ResynHF, n=13) with 2 weeks RV pacing followed by 2 weeks normal sinus rhythm (NSR). We used this approach rather than bi-ventricular stimulation since it greatly simplified the instrumentation. Importantly, the effects of CRT stem principally from its re-establishment of coordinated contractions, which is generated by restoring normal His-Purkinje excitation as with bi-ventricular pacing8. Lastly, we studied non-failing controls (NF-CON, n=11) with NSR and no lead implant, and two NF control groups with lead implants: NF+Lead, without pacing, and NF+RVP with RV pacing-induced dyssynchrony (n=11/both). The latter groups were studied with a 2 week protocol.
Figure 2. Study protocol.
Figure shows the study design and allocation of animals to each model. The results for each phase of the procedure are provided as well as the ultimate composition of the primary pacing groups and controls. The groups identified with a dark solid box around them are all included in the primary pacing analysis. The two groups with dashed line-boxes represent additional controls +/− RV pacing for 2 weeks. RVP – right ventricular pacing; NSR – normal sinus rhytym; AP – atrial pacing.
For groups 1–4, acute 3-lead ECG and echocardiography studies were obtained following pacemaker implantation to assess the effects of pacing on electromechanical dyssynchrony. Mice were housed in a temperature-controlled room (27.8 °C and a 12 hour light-dark cycle, 8 am – 8 pm). At the end of study, mice were anesthetized, and hearts rapidly removed. Both atria and right ventricle were dissected from the LV. The LV was then divided into five segments: the distal apex, septum (early activated in DysHF), lateral wall (late activated in DysHF), anterior wall (peri-infarct zone), and posterior wall (remote). Myocardium was rapidly frozen in liquid nitrogen and stored at −80 °C until analyzed. In 7 studies, the LV was subjected to a myocyte isolation protocol for cell studies.
Echocardiography
Cardiac short-axis wall thickness, left ventricular dimensions and fractional shortening were measured by 2-D echocardiography (Vevo 2100, VisualSonics, Inc. Toronto Canada). Data were obtained before and 24 hours after I/R, 24 hours after pacemaker implantation, and at completion of the pacing protocol. In ResynHF mice, additional images were obtained after 2-weeks RV pacing, and 1 and 7 days after return to NSR (resynchronization). Studies were performed under normal sinus rhythm, with ≥15 minute suspension of pacing in conscious, acclimated, manually constrained mice.
Heart rate analysis, capture verification, threshold testing and pacemaker settings
Electrocardiograms were continuously recorded throughout the study. In ten SynHFSR, the native heart rate was analyzed daily over four ten-second segments every 6 hours, and then averaged over the four-week study. In mice receiving pacing, the percent of captured heartbeats was calculated from the ratio of paced- to total heart-beats during similar ten-second segments. The pacemaker capture threshold at 0.5 ms and 2.5 ms pulse duration was checked daily and the stimulator output set to twice threshold. Mice with early capture threshold rise (> 5 V at 2.5 ms pulse width in the first three weeks, Supplemental Figure) were removed from the study. Pacing rate was set to 720 beats/min during the first week and 650 beats/min thereafter. To assure capture, small adjustments in pacing rate were made daily based on individual mouse rates.
Cardiomyocyte function studies
Myocytes were isolated from NF-CON, SyncHFSR, DysHF and ResynHF groups (n= 18 – 50 cells, mean 37 cells/group from 7 hearts), using retrograde perfusion of the isolated heart with zero calcium buffer and collagenase17. The septum was then cut out, placed in buffer within a shallow dish, and myocytes gently disaggregated. We focused on this territory as it was less impacted by post-MI scar and provided greater yield of viable cells. Myocyte sarcomere shortening and whole-cell calcium transients were measured using 1.0 mM Ca2+ in buffer solution17, at a stimulation rate of 0.5 Hz and 37°C, before and after incubation with isoproterenol (10 nM). Data was obtained using the IonOptix system (MyoCam, IonOptix, Milton, MA) on an inverted microscope (Nikon Eclipse TE2000).
Protein kinase phosphorylation assay
To evaluate post-translational modification/activation of multiple signaling kinases associated with growth, mitogen activation, survival and metabolic signaling, and other pathways, we used a kinase-activity array (Proteome Profiler Array, ARY003B, R&D Systems, Minneapolis, MN) which simultaneously assesses phosphorylation of a panel of 43 relevant kinases, using tissue extract from the LV lateral wall (the region of highest wall stress in a dyssynchronous heart)7, 9 and following manufacturer instructions.
RNAseq Analysis
To assess differential gene expression between early contracting (septum) and late contracting (lateral) wall in HF-LV left ventricles with and without RV-pacing-induced dyssynchrony, RNA was extracted from myocardial samples using Qiagen RNeasy Mini kit and used for cDNA library synthesis using Illumina TruSeq RNA Library Prep kit v2 following manufacturer’s instructions. Libraries were sequenced on an Illumina HiSeq 4000 machine that produced 396 million 100-bp single-end reads (W. M. Keck Biotechnology Center at University of Illinois at Urbana-Champaign). RNA-seq reads were trimmed and mapped to the Ensembl mouse reference genome (mm10) using HISAT2 version 2.0.5 (http://ccb.jhu.edu/software/hisat2/index.shtml). Transcript counts were quantified using RSEM v1.3.0 with default parameters18. Differential expression analysis was performed using R package DESeq2 v1.18.119. Pathway analysis of differentially expressed genes with adjusted p<0.05 (false discovery rate <5%) employed the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and R package KEGG.db v3.2.3 (http://bioconductor.org/packages/release/data/annotation/html/KEGG.db.html.)
Statistical analysis
Data are presented as mean ± SD unless specifically indicated. Between group comparisons were generally performed using one-way ANOVA, with post-hoc multiple pairwise comparisons testing by Tukey test.. If indicated, a non-parametric Kruskal-Wallis test with Dunn’s test for multiple comparisons was employed. Time-course data were analyzed by repeated measures ANOVA. Individual tests are noted in each figure legend, as is sample size. Statistical analysis was performed using GraphPad Prism7 (GraphPad Software, La Jolla, CA, USA). P values are provided in each figure, and p<0.05 considered statistically significant.
RESULTS
Cardiac Pacing System in Mice with I/R injury
Of the 61 mice subjected to the pacing protocol, six died before completing the full protocol. An additional eight were withdrawn due to a high pacing-threshold with loss of ventricular or atrial capture (Figure 2). Intrinsic heart rate was 517 bpm (25%−75% quartiles: 434–598, from 952 recordings)in the SynHFSR group and the pacing rate was 650 bpm (650–720, from 281 recordings). This rate was similar among the different pacing groups, and myocardial capture was achieved in 92±5.7% of paced beats for atrial and ventricular pacing.
Electromechanical dyssynchrony and resynchronization in mice
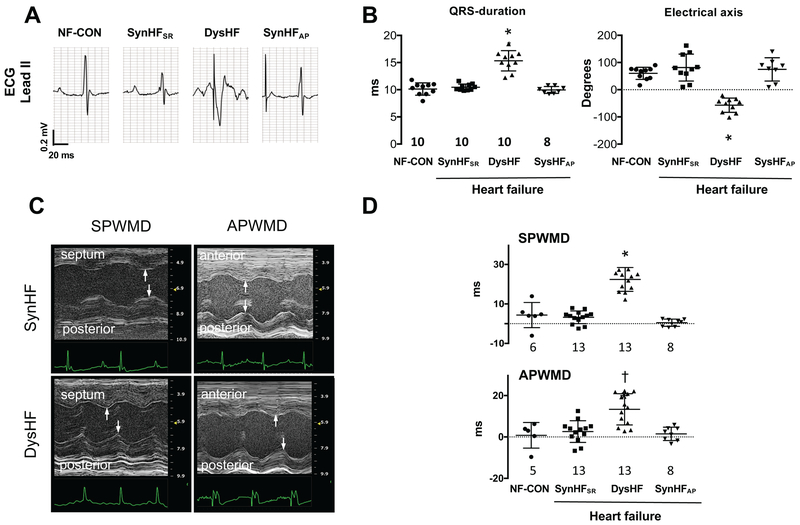
Representative electrocardiogram tracings and group data for QRS-duration and electrical axis are provided in Figure 3A-B. QRS-duration and electrical axis were unaltered between normal-sinus non-failing controls (NF-CON) and I/R-HF mice with normal sinus or atrial pacing (SyncHFSR, SyncHFAP). QRS widened by 50% and axis shifted on average −130° in DysHF versus the other groups (both p<0.0001). Wall motion dyssynchrony indexed by the time delay between septum-to-posterior wall motion or anterior-posterior wall motion20 (Figures 3C, 3D) only increased in the DysHF (p≤0.002).
Figure 3. Electromechanical dyssynchrony in mice.
(A) Representative electrical tracings from non-failing mice in sinus rhythm (NF-CON) and after I/R with normal sinus rhythm (SyncHFSR), RV over-drive pacing (DysHF), or atrial over-drive pacing (SyncHFAP). (B) Average QRS-duration and electrical axis for the four groups. (C) M-mode echocardiograms showing increased septum-to-posterior wall motion delay (SPWMD) and anterior-to-posterior wall motion delay (APWMD) in post I/R mice at SR (SyncHFSR) or with RVP (DysHF). The delay increases with RVP. (D) Summary data for both delay indexes. Summary analysis by 1-Way ANOVA, Tukey post-hoc pairwise comparisons: *<0.0001 versus all other groups, † p=0.002 vs NF-CON, p=0.0003 vs SynHFSR, p=0005 vs SynHFAP.
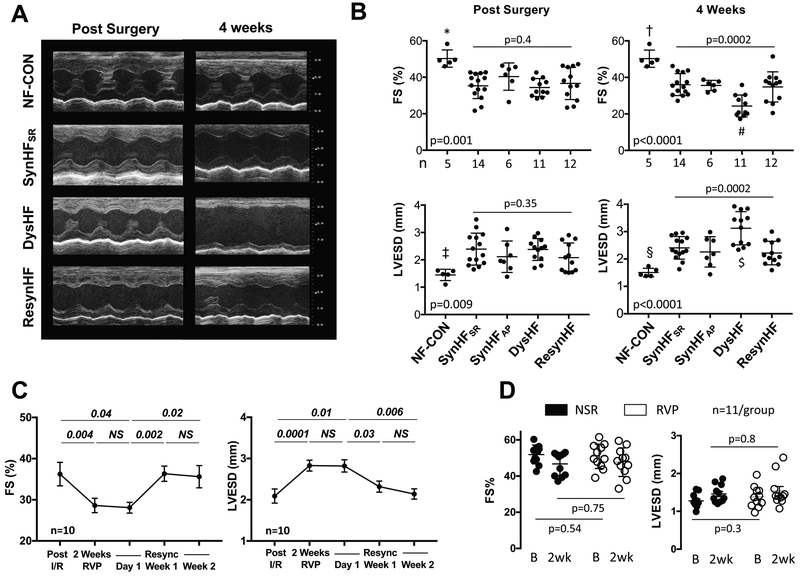
Individual and group echocardiographic results are displayed in Figure 4, with all measurements obtained at normal sinus rhythm, with pacing temporarily suspended. Following I/R, fractional shortening (FS) declined and LV end-systolic diameter (LVESD) increased similarly among the different pacing groups (Fig. 4A, 4B). In SynHFSR and SynHFAP hearts, there was minimal further change 4 weeks later, and data for both groups were similar. However, FS declined further in DysHF (from 36±6 to 24±6%) and LVESD increased (2.4 ± 0.4 to 3.1±0.6 mm), becoming significantly different than that of the other groups (p≤0.007, individual p-values in legend). LV function in ResynHF was similar to both SynHF groups, reversing the changes in DysHF. In ResynHF, FS declined from 36.2±2.8 to 28.6±1.8 (p=0.004) and LVESV rose (2.1±0.2 to 2.8±0.1) after only 2 weeks of RV pacing, values similar to those after 4-weeks RVP in DysHF. These were unaltered 1 day after stopping pacing, confirming they were intrinsic to the heart and did not require active RV pacing. However, after 1 week of restored synchronous contraction, LV function improved to post-I/R levels, and this persisted thereafter. We also tested the impact of RV-pacing dyssynchrony on LV function in normal hearts (Figure 4D), finding minimal effects after 2 weeks unlike the response in the 2-week RVP phase of ResynHF mice (Figure 4C). This supports the importance of underlying LV disease to observe meaningful pathobiology from dyssynchrony.
Figure 4. Effect of chronic dyssynchrony and resynchronization on LV function in mice following I/R injury.
(A), Example echocardiograms early post I/R and after 4-weeks pacing protocol in non-failing controls (NF-CON), synchronous HF (SynHFSR), dyssynchronous HF (DysHF), and resynchronized HF (ResynHF). (B), Summary scatter plots with mean±SD for percent fractional shortening (FS) and LV end-systolic dimension (LVESD) for the same two time points. P-values displayed are for 1-WANOVA; symbols are for pairwise comparisons by Tukey Test. *p=0.0007,†p=0.0004,‡p=0.003,§p=0.003 vs SynHFSR; *p=0.0005, †p=0.0001,‡p=0.004, §p=0.0001 vs DysHF; *p=0.0025,†p=0.0001,‡p=0.07,§p=0.0001 vs ResynHF; *p=0.08, †p=0.0015,‡p=0.09,§p=0.03 vs SynHFAP; # p<0.008; $ p<0.004 vs other pacing groups. (C), Time-plots (mean ± SEM) for full protocol for FS and LVESD in RsynHF mice (n=10). Data analyzed by repeated measures 1W-ANOVA, followed by Sidak’s multiple comparisons test (p-values shown). (D) FS and LVESD from NF-Lead and NF-RVP control groups at initial baseline (B) and after 2-weeks (2wk). Data analyzed by 1WANOVA, with Sidak’s multiple comparisons test. There was no significant change in either parameter with RVP in the otherwise normal ventricle.
Isolated myocyte studies
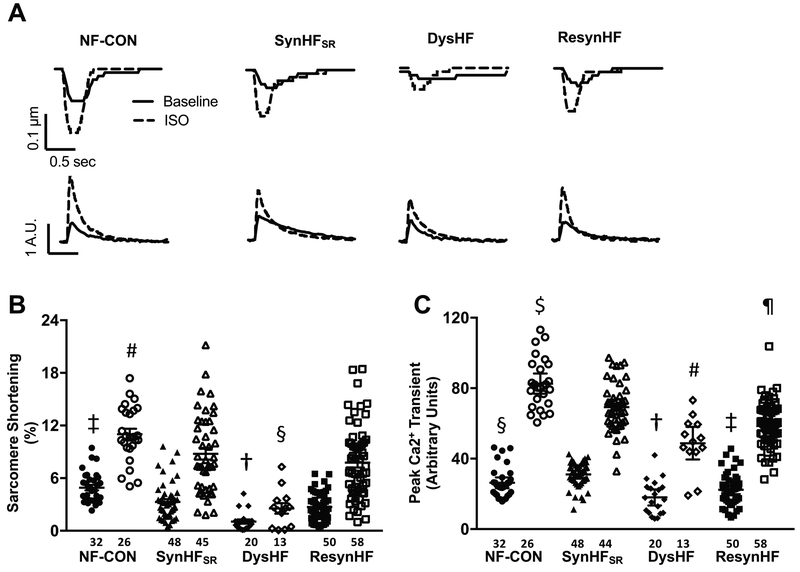
Individual example time-tracings of myocyte sarcomere shortening and calcium transients at baseline (control) and during isoproterenol stimulation from the various experimental groups are shown in Figure 5A. Summary data are provided in Figures 5B, 5C. Despite the variance among cells, mean sarcomere shortening in the basal state was substantially lower in all HF groups versus control, and DysHF was significantly below all the other pacing groups (p=0.001 vs ResynHF, p<0.0001 vs NF-CON and SynHFSR, Tukey test, 1-way ANOVA). Isoproterenol-stimulated shortening maintained these disparities. DysHF peak calcium transients were significantly reduced both at rest and upon ISO stimulation compared to NF-CON and SynHFSR, but they did not differ from peak transients in ResynHF. Thus, restoring synchrony enhanced rest and isoproterenol-stimulated shortening, though peak calcium transients remained depressed.
Figure 5. Left ventricular myocyte function and whole cell calcium in synchronous, dyssynchronous and resynchronized mouse hearts post I/R injury.
(A), Individual tracings of sarcomere shortening and whole cell calcium transients at baseline (solid line) and with isoproterenol (ISO, 10 nM, dotted line), from four experimental groups. Data are from the anterio-septal region (early activated), and similar results were obtained in the lateral wall (late activated; not shown). (B,C), Group results (mean ± SEM) for % sarcomere shortening and peak calcium transient for the same four groups. Kruskal Wallis test performed for resting and ISO-stimulated data separately, and pairwise comparisons are shown based on Dunn’s test: B): Resting Data: † p<0.0001 vs NF-CON, SynHFSR, p=0.0012 vs ResynHF; ‡ p<0.0001 vs ResynHF, p=0.002 vs SynHFSR; ISO Stimulated Data: § p<0.0001 vs NF-CON, SynHFSR, p=0.0007 vs ResynHF; # p=0.004 vs ResynHF, p=0.002 vs SynHFSR. C): Resting Data: †p<0.0001 vs SynHFSR, p=0.04 vs NF-CON, p=NS vs ResynHF; ‡ p<0.0001 vs SynHFSR; § p 0.04 vs SynHFSR; ISO Stimulated Data: # p<0.0001 vs NF-CON, p=0.0009 vs SynHFSR, p=NS vs ResynHF; $ p=0.02 vs SynHFSR, p<0.0001 vs ResynHF; ¶ p=0.003 vs SynHFSR.
RNAseq Analysis – Regional Gene Expression Variability
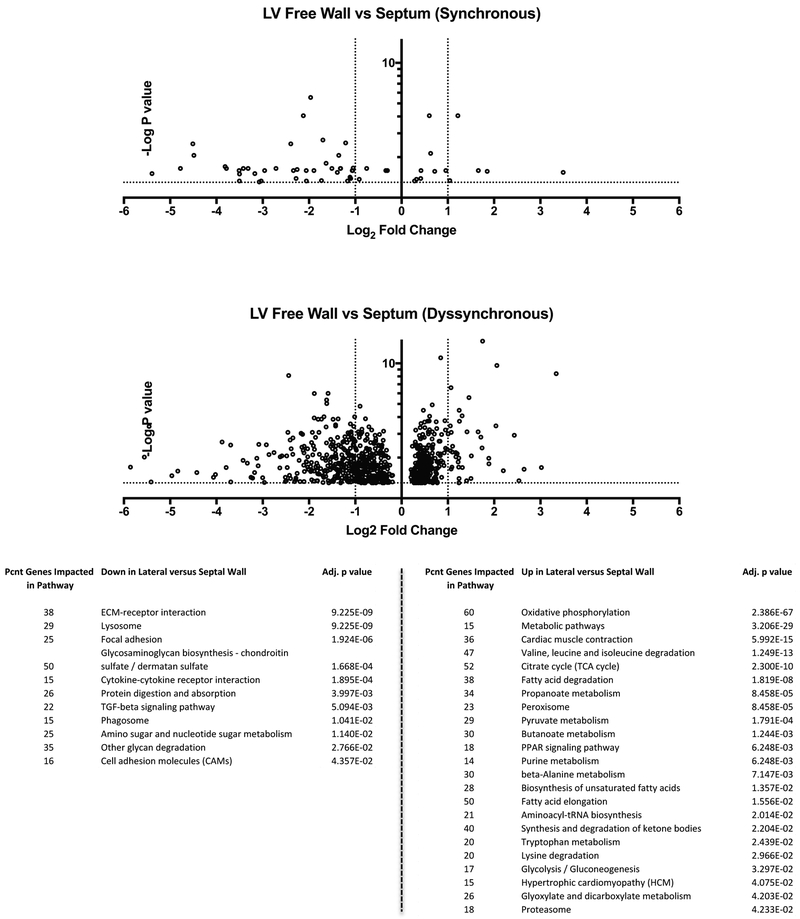
We next tested the extent to which myocardial I/R injury induced regional heterogeneity of gene expression itself (comparing non-infarcted lateral wall and septum) and if this was altered by superimposition of RV pacing-induced dyssynchrony. Figure 6 displays RNA-seq data as volcano plots of genes with significant expression disparities between septal and lateral wall (p<0.05, -log(p) > 1.3). The results show that despite I/R injury, synchronously contracting hearts (SynHFSR) displayed only 62 genes with regional expression disparity between regions. However, with dyssynchrony this number increased to 804 genes (Supplemental Table 1 and 2). KEGG pathway analysis of differentially-expressed genes found many upregulated in the lateral versus septal wall were associated with energetics and metabolism, whereas relative down-regulated genes were predominantly within cytoskeletal and matrix-signaling pathways.
Figure 6. RV pacing induced dyssynchrony in post I/R mouse heart markedly augments regional gene expression differences between lateral and septal wall.
Volcano plots of heterogeneous gene expression comparing lateral to septal wall in hearts after I/R injury with or without superimposition of sustained RVP-induced dyssynchrony. Y-axis shows –log of p-value and data are shown accepting a 10% false discovery rate. There is very little heterogeneity in the post MI heart, but this is markedly increased after 4 weeks of RVP. This remains true even if a more stringent false discovery rate (1–5%) is used. The KEGG analysis reveals pathways that are significantly disparate between sides of the chamber, based on the number of genes modified within each pathway. Results for up versus down-regulated genes are listed below the figure; pcnt: Percent; adjusted p value (q-value, with false discovery rate - FDR <0.05).
Regional Disparity in Protein Kinase Activation
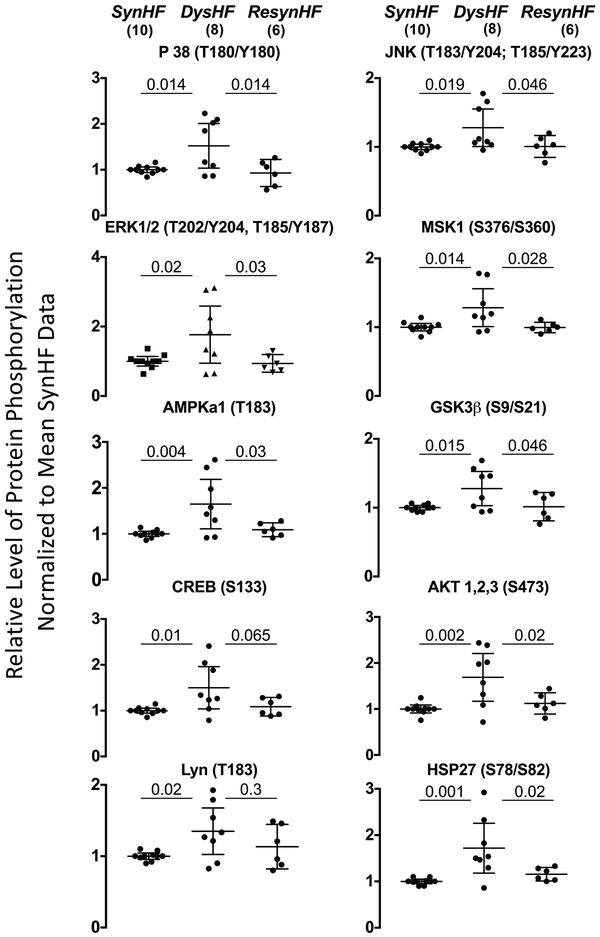
Dyssynchronous HF generates both global molecular changes that impact cell myocyte behavior (e.g. depressed adrenergic signaling9) and regional differences in kinase activation7, 21. Figure 7 shows results of the kinase-phosphorylation assay, showing all modified proteins in lateral wall myocardial extracts from DysHF versus SyncHFSR. These include mitogen activated protein kinases p38, ERK1/2, and JNK, mitogen stress-activated protein kinase 1 (MSK1) and its downstream target CREB (cyclic AMP response element binding protein), Lyn (tyrosine protein kinase), AMP stimulated kinase α1-subunit, and heat shock protein HSP27. Phosphorylation of GSK-3β also increased (kinase is thereby inhibited), consistent with prior findings in dyssynchronous failing canine ventricles7. These changes were significantly reversed with resynchronization in all but two proteins (CREB and Lyn).
Figure 7. Regional disparity of stress-stimulated kinase phosphorylation in late activated left ventricular myocardium from dyssynchrony in post I/R hearts.
Dot plots with 95% confidence intervals for kinase phosphorylation changes associated with DysHF, SynHFSR and ResynHF from myocardium obtained from the mid-left lateral wall. Results are shown normalized to SynHFSR, which served as common controls for different assay plates. Data analyzed by 1W-ANOVA, with Holms-Sidak’s test for multiple comparisons (p-values are shown).
DISCUSSION
We developed a method to chronically and continuously stimulate and concomitantly record electrical activity of the heart in conscious and minimally constrained mice. This was then applied to model LV disease with electromechanical dyssynchrony or resynchronization. The new technology is the first to enable both pacing and continuous electrophysiological monitoring in conscious mice, and has a low (13%) combined procedural failure or mortality rate. It utilizes custom leads and commercially available electronic components allowing for scalability without excessive cost. By employing an external pulse generator, a broad range of stimulation protocols are feasible all the while providing simultaneous pacing and recording.
There are several methodological differences between the recent mouse pacing study from Hulsmans et al3 and our approach. The implantable pacemaker used in their study was first clinically tested in 2013, and their modified version enables chronic fixed pacing at high rates. However, it is not amenable to more complex pacing protocols, whereas ours is flexible as the stimulator is externalized. While the miniature pacemaker is small and light for humans, it is still nearly 2 grams, representing ~7% of the weight of a typical mouse, or 5.2 kg for a 75 kg human (weight of ~2.5 standard bags of flour). By contrast, we require implantation of just a light pacing lead. Admittedly, our lead is not currently commercially available and requires some training to make; but we have achieved this with multiple individuals and the supplemental video is intended to make the process clear. A potential disadvantage of externalizing the lead is morbidity from infection, though we found this to be very rare. Each component of the externalized conduits is designed to facilitate freedom of movement while still protecting the wires from being chewed by the mouse. Lastly, the implantable pacemaker requires a unit/mouse, and based on their clinical cost $7,000–10,000 U.S., scaling up could be expensive.
The results of the present dyssynchrony and resynchronization model are the first such analysis in the mouse, and first to assess cellular and molecular modifications from either intervention when superimposed on a model of pre-existing ischemic heart disease. Virtually all prior data derives from a canine model of tachypacing induced heart failure16, where the evolution of failure and pacing-dyssynchrony or CRT effects were coupled. The data reveal striking similarities at many levels to what is reported in the dog7, 8, 22, and in some limited studies in humans23, revealing the mouse to be a viable model system to explore this pathophysiology. This was not a forgone conclusion, given the mouse heart is small so there is less distance to be traveled from early to late stimulated muscle at myocardial conduction velocities similar to those found in humans24. Hulsmans et al3 subjected mice to RV-apex or atrial tachypacing (at 1200 bpm), and the former but not latter resulted in LV dysfunction. However, 1:2 atrial-paced beats induced ventricular excitation, so the effective rate was ~600 bpm. While this suggests LV disease from tachypacing occurs in mice as in larger mammals, it might also reflect the impact of dyssynchrony from RV pacing. Indeed, we show RV pacing at ~600 bpm also worsens LV and myocyte function so long as underlying cardiac disease is present, whereas atrial pacing at the same rate does not. In this regard, the present model also differs from most prior work in the dog that used tachypacing as a background to generate heart failure. That the results are similar is reassuring and opens up new ways to study this behavior.
The present study did not aim to recapitulate the nearly 15 years of work conducted in the canine model which revealed how CRT modifies adrenergic and cell survival signaling, gene expression heterogeneity, and myocyte function and calcium homeostasis. Rather, we focused on generating some key new data sets, specifically as this was performed in an ischemic disease model, and the RNAseq results provide fodder for future research. The activation of MAP kinase activity by dyssynchrony and its resolution by resynchronization recapitulates a number of prior canine findings 7, 21, but also adds many more pathways to the effected list. The finding that GSK3β inhibition (e.g. increased S9 phosphorylation)7 is observed in mouse dyssynchrony as in the canine heart is interesting, as this was previously shown in dogs to contribute to myofilament dysfunction, and be reversed by CRT12, 25. This may in part explain the improved myocyte function in ResynHF despite persisting depressed calcium transients. Future work utilizing genetically modified mice to dissect out the role of specific protein changes can now be performed.
There are some limitations to our study. We did not sense or inhibit pacing based on intrinsic electrical stimulation, but paced continuously; however, this could be modified by using a more sophisticated external pulse-stimulator. We required pacing rates 25% above normal sinus to assure >93% capture. While this could introduce a rate effect, mean HR was identical for SyncHFAP and DysHF groups. Moreover, though HR was lower at normal sinus rhythm than with pacing, both SyncHFAP and SyncHFNS displayed very similar results. The Resyn-HF model did not employ bi-ventricular pacing, as is used with CRT, though resynchronization of contraction was similar. While I/R itself induces regional changes in myocardial biology, it did not induce significant dyssynchrony by image analysis, and was common to all the pacing models. Despite this, we observed differences at chamber, cellular, and molecular levels from pacing-induced dyssynchrony. Furthermore, reversibility of the RV-pacing related abnormalities upon restoring normal conduction indicates that the pathobiology related to superimposed dyssynchrony and not the pre-existing I/R injury.
In summary, the current study presents a detailed method for chronic mouse pacing and applies it to generate a model of dyssynchrony and resynchronization. The full utility of this platform for dissecting critical molecular signaling relevant to this or other electromechanical interventions remains to be explored. The distinction between altering myocardial biology by pharmaceuticals, biologicals, or devices such as pacemakers is becoming increasingly blurred. More and more evidence shows electro-mechanical stimulation that alters conduction and associated ventricular contraction is also a potent tool to vary underlying cell structure and function26. The present methodology provides a new means to employ this in mice that should facilitate future mechanistic studies of these interactions.
Supplementary Material
What is new?
We present a novel method for long-term (four-week) continuous atrial and ventricular pacing in conscious mice with simultaneous ECG recording.
RV pacing induced cardiac dyssynchrony superimposed on mouse hearts with ischemia reperfusion injury worsens ventricular and myocyte function, and this is improved following electromechanical resynchronization. RNAseq shows broad expression heterogeneity from dyssynchrony, and stress-response kinase changes induced by dyssynchrony are largely reversed by resynchronization.
Murine cardiac dyssynchrony and resynchronization results share similarities with larger mammal and human findings, providing a translational platform to dissect molecular signaling of this and other stimulation-based heart therapies.
What are the clinical implications?
The chronic mouse heart pacing/recording system opens up new avenues of research into cardiac stimulation physiology and therapeutics. The dyssynchrony and resynchronization model may facilitate discoveries of new molecular targets for heart failure therapy.
ACKNOWLEDGMENTS
The authors thank Dr. Andrew Posner for his key efforts in the early testing of precursor implantable pacemaker systems, and George Coles (JHU Applied Physics Laboratory) for assistance in advancing the pacemaker lead design.
SOURCES OF FUNDING
Supported by National Institute of Health – NHLBI grants PO-HL077180, PO-HL107153, HL-135827 (DAK), The Swedish Society of Medicine and Swedish Research Council Grants (MS), post-doctoral fellowship from the American Heart Association (RN), and NHLBI T32 Fellowship grant HL-07227 (BJL).
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Bilchick KC, Saha SK, Mikolajczyk E, Cope L, Ferguson WJ, Yu W, Girouard S and Kass DA. Differential regional gene expression from cardiac dyssynchrony induced by chronic right ventricular free wall pacing in the mouse. Physiol Genomics. 2006;26:109–115. [DOI] [PubMed] [Google Scholar]
- 2.Laughner JI, Marrus SB, Zellmer ER, Weinheimer CJ, MacEwan MR, Cui SX, Nerbonne JM and Efimov IR. A fully implantable pacemaker for the mouse: from battery to wireless power. PloS one. 2013;8:e76291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulsmans M, Aguirre AD, Bonner MD, Bapat A, Cremer S, Iwamoto Y, King KR, Swirski FK, Milan DJ, Weissleder R and Nahrendorf M. A miniaturized, programmable pacemaker for long-term studies in the mouse. Circulation Research. 2018;123:1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B and Nevo E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–1573. [DOI] [PubMed] [Google Scholar]
- 5.Nelson GS, Berger RD, Fetics BJ, Talbot M, Spinelli JC, Hare JM and Kass DA. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053–9. [DOI] [PubMed] [Google Scholar]
- 6.Lalani GG and Birgersdotter-Green U. Cardiac resynchronisation therapy in patients with chronic heart failure. Heart. 2015;101:1008–14. [DOI] [PubMed] [Google Scholar]
- 7.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, Abraham TP, Tomaselli GF and Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117:1369–1377. [DOI] [PubMed] [Google Scholar]
- 8.Chakir K, Depry C, Dimaano VL, Zhu WZ, Vanderheyden M, Bartunek J, Abraham TP, Tomaselli GF, Liu SB, Xiang YK, Zhang M, Takimoto E, Dulin N, Xiao RP, Zhang J and Kass DA. Galphas-biased beta2-adrenergic receptor signaling from restoring synchronous contraction in the failing heart. Science translational medicine. 2011;3:100ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakir K, Daya SK, Aiba T, Tunin RS, Dimaano VL, Abraham TP, Jaques-Robinson KM, Lai EW, Pacak K, Zhu WZ, Xiao RP, Tomaselli GF and Kass DA. Mechanisms of enhanced beta-adrenergic reserve from cardiac resynchronization therapy. Circulation. 2009;119:1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderheyden M, Mullens W, Delrue L, Goethals M, Verstreken S, Wijns W, De Bruyne B and Bartunek J. Endomyocardial upregulation of beta1 adrenoreceptor gene expression and myocardial contractile reserve following cardiac resynchronization therapy. J Card Fail. 2008;14:172–178. [DOI] [PubMed] [Google Scholar]
- 11.Wang SB, Foster DB, Rucker J, O’Rourke B, Kass DA and Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res. 2011;109:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirk JA, Holewinski RJ, Kooij V, Agnetti G, Tunin RS, Witayavanitkul N, de Tombe PP, Gao WD, Van Eyk J and Kass DA. Cardiac resynchronization sensitizes the sarcomere to calcium by reactivating GSK-3beta. J Clin Invest 2014;124:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishijima Y, Sridhar A, Viatchenko-Karpinski S, Shaw C, Bonagura JD, Abraham WT, Joshi MS, Bauer JA, Hamlin RL, Gyorke S, Feldman DS and Carnes CA. Chronic cardiac resynchronization therapy and reverse ventricular remodeling in a model of nonischemic cardiomyopathy. Life Sci 2007;81:1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachse FB, Torres NS, Savio-Galimberti E, Aiba T, Kass DA, Tomaselli GF and Bridge JH. Subcellular structures and function of myocytes impaired during heart failure are restored by cardiac resynchronization therapy. Circ Res 2012;110:588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiba T, Hasketh GG, Barth AS, Liu T, Daya SK, Chakir K, Dimaano VL, Abraham TP, O’Rourke B, Akar FG, Kass DA and Tomaselli GF. Electrophysioloigcal consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirk JA and Kass DA. Cellular and Molecular Aspects of Dyssynchrony and Resynchronization. Heart failure clinics. 2017;13:29–41. [DOI] [PubMed] [Google Scholar]
- 17.Seo K, Rainer PP, Lee DI, Hao S, Bedja D, Birnbaumer L, Cingolani OH and Kass DA. Hyperactive Adverse Mechanical Stress Responses in Dystrophic Heart Are Coupled to Transient Receptor Potential Canonical 6 and Blocked by cGMP-Protein Kinase G Modulation. Circ Res. 2014;114:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B and Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love MI, Huber W and Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitzalis MV, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G, Guida P, Andriani A, Mastropasqua F and Rizzon P. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J AmCollCardiol. 2002;40:1615–1622. [DOI] [PubMed] [Google Scholar]
- 21.Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF and Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929–932. [DOI] [PubMed] [Google Scholar]
- 22.Barth AS, Aiba T, Halperin V, DiSilvestre D, Chakir K, Colantuoni C, Tunin RS, Dimaano VL, Yu W, Abraham TP, Kass DA and Tomaselli GF. Cardiac resynchronization therapy corrects dyssynchrony-induced regional gene expression changes on a genomic level. Circ Cardiovasc Genet 2009;2:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderheyden M, Mullens W, Delrue L, Goethals M, De Bruyne B, Wijns W, Geelen P, Verstreken S, Wellens F and Bartunek J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51:129–136. [DOI] [PubMed] [Google Scholar]
- 24.Kaese S and Verheule S. Cardiac electrophysiology in mice: a matter of size. Frontiers in physiology. 2012;3:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neubauer S and Redwood C. New mechanisms and concepts for cardiac-resynchronization therapy. N Engl J Med. 2014;370:1164–6. [DOI] [PubMed] [Google Scholar]
- 26.Kirk JA, Chakir K, Lee KH, Karst E, Holewinski RJ, Pironti G, Tunin RS, Pozios I, Abraham TP, de Tombe P, Rockman HA, Van Eyk JE, Craig R, Farazi TG and Kass DA. Pacemaker-induced transient asynchrony suppresses heart failure progression. Science translational medicine. 2015;7:319ra207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.