Abstract
Background
The diagnosis of bronchiectasis is defined by abnormal dilation of the airways related to a pathological mechanism of progressive airway destruction that is due to a 'vicious cycle' of recurrent bacterial infection, inflammatory mediator release, airway damage, and subsequent further infection. Antibiotics are the main treatment option for reducing bacterial burden in people with exacerbations of bronchiectasis and for longer‐term eradication, but their use is tempered against potential adverse effects and concerns regarding antibiotic resistance. The comparative effectiveness, cost‐effectiveness, and safety of different antibiotics have been highlighted as important issues, but currently little evidence is available to help resolve uncertainty on these questions.
Objectives
To evaluate the comparative effects of different antibiotics in the treatment of adults and children with bronchiectasis.
Search methods
We identified randomised controlled trials (RCTs) through searches of the Cochrane Airways Group Register of trials and online trials registries, run 30 April 2018. We augmented these with searches of the reference lists of published studies.
Selection criteria
We included RCTs reported as full‐text articles, those published as abstracts only, and unpublished data. We included adults and children (younger than 18 years) with a diagnosis of bronchiectasis by bronchography or high‐resolution computed tomography who reported daily signs and symptoms, such as cough, sputum production, or haemoptysis, and those with recurrent episodes of chest infection; we included studies that compared one antibiotic versus another when they were administered by the same delivery method.
Data collection and analysis
Two review authors independently assessed trial selection, data extraction, and risk of bias. We assessed overall quality of the evidence using GRADE criteria. We made efforts to collect missing data from trial authors. We have presented results with their 95% confidence intervals (CIs) as mean differences (MDs) or odds ratios (ORs).
Main results
Four randomised trials were eligible for inclusion in this systematic review ‐ two studies with 83 adults comparing fluoroquinolones with β‐lactams and two studies with 55 adults comparing aminoglycosides with polymyxins.
None of the included studies reported information on exacerbations ‐ one of our primary outcomes. Included studies reported no serious adverse events ‐ another of our primary outcomes ‐ and no deaths. We graded this evidence as low or very low quality. Included studies did not report quality of life. Comparison between fluoroquinolones and β‐lactams (amoxicillin) showed fewer treatment failures in the fluoroquinolone group than in the amoxicillin group (OR 0.07, 95% CI 0.01 to 0.32; low‐quality evidence) after 7 to 10 days of therapy. Researchers reported that Pseudomonas aeruginosa infection was eradicated in more participants treated with fluoroquinolones (Peto OR 20.09, 95% CI 2.83 to 142.59; low‐quality evidence) but provided no evidence of differences in the numbers of participants showing improvement in sputum purulence (OR 2.35, 95% CI 0.96 to 5.72; very low‐quality evidence). Study authors presented no evidence of benefit in relation to forced expiratory volume in one second (FEV₁). The two studies that compared polymyxins versus aminoglycosides described no clear differences between groups in the proportion of participants with P aeruginosa eradication (OR 1.40. 95% CI 0.36 to 5.35; very low‐quality evidence) or improvement in sputum purulence (OR 0.16, 95% CI 0.01 to 3.85; very low‐quality evidence). The evidence for changes in FEV₁ was inconclusive. Two of three trials reported adverse events but did not report the proportion of participants experiencing one or more adverse events, so we were unable to interpret the information.
Authors' conclusions
Limited low‐quality evidence favours short‐term oral fluoroquinolones over beta‐lactam antibiotics for patients hospitalised with exacerbations. Very low‐quality evidence suggests no benefit from inhaled aminoglycosides verus polymyxins. RCTs have presented no evidence comparing other modes of delivery for each of these comparisons, and no RCTs have included children. Overall, current evidence from a limited number of head‐to‐head trials in adults or children with bronchiectasis is insufficient to guide the selection of antibiotics for short‐term or long‐term therapy. More research on this topic is needed.
Plain language summary
How do different antibiotics compare in terms of safety and treatment effectiveness for people with bronchiectasis?
Background Bronchiectasis is defined as abnormal widening of the airways in the lungs. It is usually caused by repeated bacterial chest infections, which damage the airways. Antibiotics are the main option for treating these infections and are used to prevent repeated infections over the longer term. However, use of antibiotics must be weighed against potential side effects and concerns over the development of bacterial resistance to treatment with antibiotics that reduces their effectiveness. Only a small number of studies have focused on antibiotics for people with bronchiectasis. Further clarity about how different antibiotics compare with one another is urgently needed.
Study characteristics In April 2018, we looked for studies including adults or children with bronchiectasis that randomly allocated participants to receive one antibiotic or another by the same method of administration. We found only four studies, and they were very small. In total, they included 138 participants. This small sample makes it very difficult to draw clear conclusions.
Key results Four randomised trials were eligible for inclusion in this systematic review. None of the included studies reported information on flare‐ups (exacerbations). Included studies reported no deaths and no serious adverse events. Treatment failures were fewer with fluoroquinolone antibiotics than with amoxicillin antibiotics.
Quality of the evidence Reviewers considered the quality of the evidence provided by the four small included studies to be low or very low.
Key message
Fluoroquinolone antibiotics may be more successful than amoxicillin antibiotics in treating exacerbations, but this finding is based on low‐quality evidence. More evidence from high‐quality clinical trials of short‐term and long‐term treatment with antibiotics is needed if clear conclusions are to be reached on the benefits of one antibiotic over another for people with bronchiectasis.
Summary of findings
Background
Description of the condition
Bronchiectasis is characterised by abnormal dilation of the airways that is associated with a pathological mechanism of progressive airway destruction that is due to the 'vicious cycle' of recurrent bacterial infection, inflammatory mediator release, airway damage, and subsequent further infection (Cole 1986). The airways show chronic inflammation with various features, including loss of ciliated epithelium and mucous gland hypertrophy. Bacterial colonisation is facilitated by this loss of an integral epithelial structure (host defence), which, in turn, triggers further immune responses and continuation of the inflammatory process. An understanding of this cycle is central to the management of bronchiectasis, as strategies to arrest both inflammatory and bacterial components are required to limit the progression of lung injury. Typically microbiology for patients with bronchiectasis includes Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Pseudomonas aeruginosa, although the microbiological profile of the latter differs between adults and children, with P aeruginosa more common in adults and prevalent in only 0% to 6% of children. P aeruginosa colonisation often occurs later in the natural progression of the condition and may infer a worse prognosis in terms of symptoms, exacerbations, and loss of lung function (Evans 1996). In severe cases, the cycle of lung infection may lead to repeated hospitalisations, chronic respiratory failure, and death.
Most adult cases of bronchiectasis are idiopathic or are the result of a previous severe lung infection. However, treatable causes, such as immune deficiency, allergic bronchopulmonary aspergillosis, mycobacterial infection, and recurrent aspiration, may be identified in a minority of cases (Goeminne 2012; Pasteur 2010; Wilson 2013). One study found that a proportion of cases were associated with chronic obstructive pulmonary disease (COPD) and connective tissue diseases (Loni 2015). Underlying causes can be determined in up to 70% of paediatric cases (Eastham 2004; Twiss 2005). Diagnosis is based on a combination of clinical symptoms and high‐resolution computerised tomography (HRCT) showing one or more abnormally dilated bronchi (Chang 2010; Pasteur 2010). Symptoms may include chronic productive cough, wheeze, and breathlessness, together with recurrent lower respiratory tract infections. One study estimated the prevalence of P aeruginosa in bronchiectasis to be 15% (Araújo 2018). Colonisation with P aeruginosa and frequent exacerbations are associated with accelerated decline in lung function (Evans 1996; Martínez García 2007) and, along with impaired exercise capacity and respiratory symptoms, reduced quality of life and increased hospitalisations (Finch 2015; Wilson 1997).
Management of bronchiectasis requires careful attention to sputum clearance, bronchodilator therapy, and the prescription of antibiotics (Welsh 2015). In the short term, the main aim is to reduce microbial load to reduce the severity and frequency of exacerbations, thereby ameliorating symptoms and improving quality of life (Pasteur 2010), with the longer‐term aim of breaking the infection cycle, slowing the decline in lung function, and reducing mortality rates. Antibiotics have traditionally been reserved for the treatment of acute infection/exacerbation, although prophylactic strategies may have a role in some cases. Use of macrolides is attracting further interest, and trials have explored their prescription for patients with bronchiectasis (Wu 2014).
Global prevalence estimates are unclear because of variable diagnostic strategies (Weycker 2005), along with higher prevalence rates in low‐ and middle‐income countries (Habesoglu 2011). Mortality rates in England and Wales rose by 3% per year between 2001 and 2007 (Roberts 2010), and hospitalisations increased by 3% per year over a nine‐year period in the USA (Seitz 2010). Higher prevalence rates were associated with people over 60 years of age and with women, and they varied by ethnicity (Chang 2003; Seitz 2012). Recent data from a UK study suggest that incidence and prevalence may be higher than previously estimated (Quint 2016). Over a nine‐year period to 2013, point prevalence rates per 100,000 rose from 350.5 to 566.1 in women, and from 301.2 to 485.5 in men. This reflects an increase of more than 60%, with almost 263,000 adults living with bronchiectasis in 2013. Similarly, incidence rates per 100,000 person‐years rose from 21.2 to 35.2 in women, and from 18.2 to 26.9 in men, representing an approximate increase in new cases of 63% to over 15,000 in 2013. Bronchiectasis is also associated with higher age‐adjusted mortality rates, with estimates 2.26 times higher in women and 2.14 times higher in men compared to the general population (Quint 2016). The disease has a significant impact on paediatric populations, and quality of life is worse for younger children and those with a more frequent annual exacerbation rate (Kapur 2012). Global prevalence estimates are variable, ranging from conservative estimates of 17.2 in the North‐East of England ‐ as reported in Eastham 2004 ‐ to 33.5 in New Zealand ‐ presented in Twiss 2005 ‐ per 100,000 children under 15 years of age. Rates may be higher in children from indigenous populations, with estimates of 1 per 625 (160 per 100,000) in children from the Pacific Islands (Twiss 2005), 15 per 1000 (1500 per 100,000) in native central Australian Aborginal children, and 16 per 1000 (1600 per 100,000) in Native Alaskan children (Chang 2002; Singleton 2000). However, these increases in bronchiectasis may reflect improved detection rates through high‐quality computerised tomography (CT) scans, rather than a true rise in prevalence (Goeminne 2016).
The economic burden of bronchiectasis may be considerable, but little information is available. Data collected in 2001 in the USA showed an additional two days in hospital, six more outpatient encounters, and 27.2 more days of antibiotic therapy associated with bronchiectasis (Weycker 2005). Estimates of overall additional annual costs of bronchiectasis range from USD 5681 to USD 7827, based on data collected between 2001 and 2009 (Joish 2013; Seitz 2010; Weycker 2005).
Description of the intervention
Bronchiectasis is characterised by daily coughing, sputum expectoration, and recurrent respiratory infection. Serial infections often culminate in bacterial colonisation with dilation and inflammation of the airways. Whilst abnormalities may be pan‐lobar (i.e. throughout both lungs), bronchiectasis may be limited to a single lung lobe or may manifest in a patchy distribution. Antibiotics are used to reduce bacterial burden to tackle the cycle of infection and tissue damage (Cole 1984; Pasteur 2010). They may be administered on a short‐term basis (less than four weeks) to treat acute exacerbations, or over a longer‐term (≥ four weeks). Longer durations of antibiotics are used to eradicate pathogens, to suppress bacterial load, or to enhance anti‐inflammatory properties (e.g. macrolides). Several routes of administration are available, including oral, inhaled, and parenteral routes, with analysis of sputum bacteriology informing the specific choice of antibiotic (Polverino 2017). Prescribing is also informed by clinical context, and bacteriology and sputum purulence are considered reliable indicators of the need for treatment (Hill 1988). Antibiotics may therefore be prescribed before the results of sputum bacteriology are obtained. Antibiotics are a frontline therapy for the management of bacterial load in bronchiectasis, but their use is tempered against adverse effects and increasing concerns over antibiotic resistance (Pasteur 2010).
How the intervention might work
A range of antibiotic strategies have been used to reduce bacterial load and re‐infection rates in people with bronchiectasis, including short‐term prescriptions for acute exacerbations and longer‐term prophylactic use for frequent exacerbations in which chronic sputum purulence is a common feature (Chalmers 2012; Evans 2003). Longer‐term use of antibiotics is not currently recommended as part of routine treatment (Valery 2012; Wu 2014), but it may be considered for patients with frequent exacerbations (three or more per year requiring antibiotic therapy) (Pasteur 2010). Antibiotic choice is usually guided by sputum microbiology and patterns of local antibiotic resistance, but treatment is often started empirically with a broad‐spectrum oral or intravenous antibiotic until the specific pathogen has been isolated (Pasteur 2010). If more than one culture is positive, an antibiotic is selected to cover both. Macrolide antibiotics may be prescribed for their potential anti‐inflammatory properties as well as for their antibacterial effects.
Why it is important to do this review
Evidence for the effectiveness of a range of treatment strategies in bronchiectasis is limited by the number and quality of clinical trials, including those on antibiotics, and the need for evidence based on head‐to‐head comparisons of antibiotics has been highlighted as a key priority (Welsh 2015). The comparative effectiveness, cost‐effectiveness, and safety of within‐class antibiotics (e.g. from different manufacturers) remain unclear, but this type of evidence could be used to inform choice of antibiotic, particularly in developing countries, where use of cheaper antibiotics may be more prevalent than in developed countries.
Therefore this Cochrane Review will include studies that directly compare the effectiveness of antibiotics and consider issues related to duration of treatment and mode of delivery. We will endeavour to draw together existing evidence showing their effectiveness for bronchiectasis against key outcomes identified by Welsh 2015. We are conducting this as a Cochrane Review and are employing established methods in accordance with the recent evaluation of these standards versus alternative approaches (Page 2016). This Cochrane Review is being conducted alongside four other closely related reviews: "Macrolide antibiotics for bronchiectasis" (Kelly 2018); "Dual antibiotics for bronchiectasis" (Felix 2018); "Oral versus inhaled antibiotics for bronchiectasis" (Spencer 2018); and "Continuous versus intermittent antibiotics for bronchiectasis" (Donovan 2018).
Objectives
To evaluate the comparative effects of different antibiotics in the treatment of adults and children with bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported as full‐text articles, those published as abstracts only, and unpublished data.
Types of participants
We included adults and children (less than 18 years of age) with a diagnosis of bronchiectasis by bronchography or high‐resolution computed tomography who reported daily signs and symptoms, such as cough, sputum production, or haemoptysis, and those with recurrent episodes of chest infection. We excluded studies in which patients received continuous or high‐dose antibiotics during the four weeks before the start of the study, if they had received a diagnosis of traction bronchiectasis due to pulmonary fibrosis, or if they had received a diagnosis of cystic fibrosis.
Types of interventions
We included studies that compared one antibiotic versus another when they were administered by the same delivery method (e.g. nebulised vs nebulised) to isolate the effect of the antibiotic rather than the delivery device. We considered short‐term use (less than four weeks) for treating acute exacerbations and longer‐term use as a prophylactic (≥ four weeks) separately. We also planned to analyse generational comparisons (e.g. third‐ vs fourth‐generation fluoroquinolones) separately from between‐class comparisons (e.g. penicillin vs fluoroquinolones).
Types of outcome measures
Primary outcomes
We included the following primary outcomes.
Exacerbation (e.g. frequency during follow‐up, time to first exacerbation).
Serious adverse events, defined according to Hansen 2015.
Secondary outcomes
We included the following secondary outcomes for both short‐ and long‐term therapy.
Frequency of hospitalisations due to exacerbations of bronchiectasis.
Response rates as defined by study authors (e.g. diary cards of physician global assessment).
Sputum volume and purulence.
Measures of lung function (e.g. forced expiratory volume in one second (FEV₁)).
Systemic markers of infection (e.g. leucocyte count, C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR)).
Adverse events (e.g. cardiac arrhythmias, gastrointestinal symptoms, hearing impairment).
Deaths, all‐cause and respiratory.
Emergence of resistance to antibiotics.
Exercise capacity (e.g. six‐minute walk distance (6MWD)).
Quality of life (e.g. St George Respiratory Questionnaire (SGRQ), QoL‐B).
Reporting one or more of the outcomes listed here was not an inclusion criterion for studies in this Cochrane Review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE Ovid SP 1946 to date.
Weekly searches of Embase Ovid SP 1974 to date.
Monthly searches of PsycINFO Ovid SP 1967 to date.
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date.
Monthly searches of AMED EBSCO (Allied and Complementary Medicine).
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. We have provided details of these strategies, as well as a list of handsearched conference proceedings, in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov), as well as the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) portal (www.who.int/ictrp/en/). We searched all databases from their inception to 30 April 2018, and we imposed no restriction on publication language.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We also searched relevant manufacturers' websites for trial information.
We searched for errata or retractions from included studies published in full text on PubMed on 30 November 2017 (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (ES and LF) independently screened titles and abstracts of all studies identified by the literature search and coded them as 'retrieve' (eligible or potentially eligible/unclear studies) or 'do not retrieve'. We retrieved the full‐text study reports/publications for all articles in the 'retrieve' category. The same review authors independently screened the full‐text articles, identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion and consulted a third review author (SJM) to clarify the inclusion of two similar reports. We planned to identify and exclude duplicates and to collate multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We recorded the selection process in detail in a PRISMA flow diagram and in the Characteristics of excluded studies tables (Moher 2009).
Data extraction and management
We used a data collection form that was pilot‐tested on one included study to record study characteristics and outcome data. One review author (LF) extracted the following characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study settings, withdrawals, dates of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant medications, excluded medications.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (AK and LF) independently extracted outcome data from the included studies and noted in the Characteristics of included studies table when outcome data were not reported in a usable way. We resolved disagreements by consensus or by consultation with a third review author (SS or SJM). One review author (LF) transferred data into Review Manager 5 (RevMan 5) (Review Manager 2014), and a second review author (AK) verified the data by spot‐checking study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (AK and LF) independently assessed the risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (SS or SJM). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to the outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Kaehne 2017), and we reported deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios and continuous data as mean differences or standardised mean differences. We entered data presented as a scale with a consistent direction of effect.
We planned to undertake meta‐analyses only when this was meaningful (i.e. if treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
We narratively described skewed data reported as medians and interquartile ranges.
When a single trial had multiple trial arms, we included only relevant trial arms. If two comparisons (e.g. drug A vs drug B and drug C vs drug B) were combined in the same meta‐analysis, we planned to halve the comparison group to avoid double‐counting.
Unit of analysis issues
In all included studies, the unit of analysis was the participant. We planned to analyse exacerbation rates as rate ratios if data had been available.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and we believed that the missing data may have introduced serious bias, we planned to explore the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We planned to use the I² statistic to measure heterogeneity among the trials in each meta‐analysis. When we identified substantial heterogeneity (i.e. I² > 50%) (Deeks 2011), we reported this and explored possible causes by conducting pre‐specified subgroup analysis.
Assessment of reporting biases
If we had pooled more than 10 studies, we planned to create and examine a funnel plot to explore possible small‐study effects and evidence of publication bias.
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis and to perform sensitivity analyses using a random‐effects model.
'Summary of findings' table
We created a 'Summary of findings' table using the following primary and secondary outcomes: exacerbations, serious adverse events, response rates, deaths, and quality of life. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence from studies contributing data to meta‐analyses of pre‐specified outcomes. We used methods and recommendations described in Section 8.5 ‐ in Higgins 2011 ‐ and in Chapter 12 ‐ Schünemann 2011 ‐ of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro software (GRADEpro GDT). We provided justification for our decisions to downgrade or upgrade the quality of studies by using footnotes and made comments to aid the reader's understanding of the Cochrane Review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Adults versus children.
Dose or schedule, or both.
Duration (prophylactic antibiotics).
Type of antibiotic.
We planned to use the following outcomes in subgroup analyses.
Exacerbation duration (short‐term therapy).
Exacerbation frequency (long‐term therapy).
Hospitalisation.
Adverse events.
We planned to use the formal test for subgroup interactions in RevMan 5 (Review Manager 2014).
Sensitivity analysis
If we had found sufficient studies, we planned to evaluate the impact of methodological study quality by removing studies at high or unclear risk of bias according to the following risk of bias domains: random sequence generation and allocation concealment. We planned to use a fixed‐effect model, as well as a random‐effects model, in performing our sensitivity analysis.
Results
Description of studies
Results of the search
A systematic search, conducted on 30 April 2018, identified 295 unique records of potentially relevant studies. Of these, we considered 262 records irrelevant following inspection of their titles and abstracts. We obtained the full texts of the remaining 33 records and scrutinised them for selection. Four studies met the Review selection criteria (Lam 1989; Chan 1996; Dimakou 2014; Kaponi 2017), and we included them in the Review (see Characteristics of included studies); we formally excluded 26 records (documented in Excluded studies). Two records were protocols for an ongoing study ‐ Chang 2013 (documented in Characteristics of ongoing studies), and for one study that is awaiting classification ‐ Lam 1986 ‐ as we could not ascertain whether it is a separate study from Lam 1989 (documented in Characteristics of studies awaiting classification). We have summarised the study selection process in the study flow diagram (Figure 1).
1.
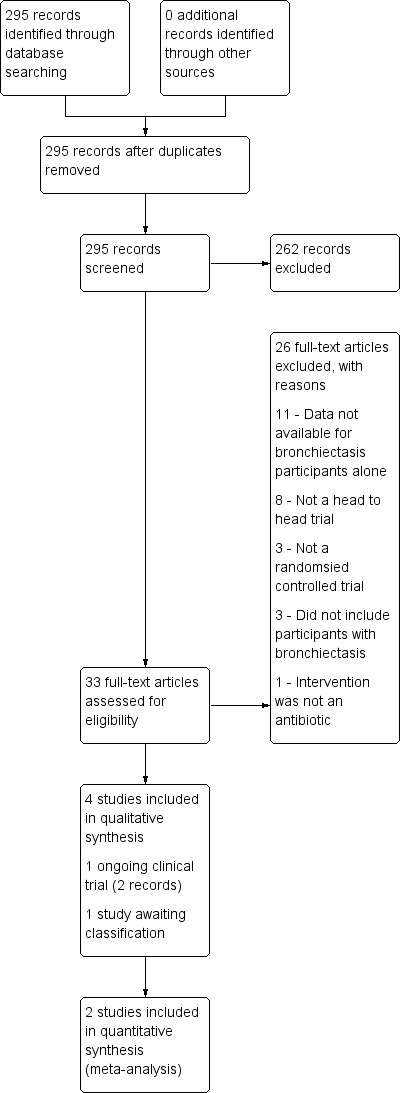
Study flow diagram.
Included studies
Methods
All four included studies were reported as RCTs (Chan 1996; Dimakou 2014; Kaponi 2017; Lam 1989). Two studies were two‐arm, double‐blind, placebo‐controlled trials using a double‐dummy design, each conducted at a single centre in Hong Kong (Chan 1996; Lam 1989). Dimakou 2014 was a three‐arm (tobramycin vs colistin vs saline) trial, and Kaponi 2017 was also a three‐arm trial (tobramycin vs colistin vs placebo). Both studies were available only as conference abstracts and did not report study details such as design methods, study setting, or methods of participant recruitment. We contacted trial authors for further information, but at the time of publication, we have received no reply. Both Chan 1996 and Lam 1989 worked with patients admitted to the hospital. Chan 1996 reported no withdrawals. No patients withdrew from Lam 1989, although therapy was suspended for two patients with adverse reactions to the intervention. No patients withdrew from Dimakou 2014 or Kaponi 2017.
Participants
The four studies included a total of 164 adults, aged 18 years and older. Dimakou 2014 was a three‐arm study that included a placebo group with nine control group participants. Kaponi 2017 was a three‐arm study that included a placebo group of 17 participants. Therefore 138 participants were eligible for inclusion in the Review. Two studies based a diagnosis of bronchiectasis on clinical and radiological criteria (Chan 1996; Lam 1989), but diagnostic criteria in Dimakou 2014 were unclear. Chan 1996 and Lam 1989 included participants hospitalised with infective exacerbation of bronchiectasis, confirmed by sputum purulence or volume. Dimakou 2014 and Kaponi 2017 included participants with sputum cultures with > 10⁴ colony‐forming units (CFUs) of P aeruginosa per millilitre. Of the three studies that reported gender, 64% of participants in Chan 1996 (F 27; M 42), 44% in Lam 1989 (F 18; M 23), and 63% in Kaponi 2017 (F 33; M 19) were female. The mean age of participants was 64 years in Chan 1996; 56 years in Dimakou 2014; 53 years in Lam 1989; and 59 years in Kaponi 2017, representing a total age range of 22 to 74 years.
Two studies reported that data showed no baseline imbalances between intervention groups (Chan 1996; Lam 1989). Chan 1996 reported a mean baseline FEV₁ of 69%, and Lam 1989 91%, of predicted.
Two studies reported smoking history, with non‐smokers representing 62% of participants in Chan 1996 and 48% in Lam 1989. In Chan 1996, one participant in the ciprofloxacin group was a current smoker and 15 were former smokers (six ciprofloxacin, nine amoxicillin). In Lam 1989, two participants in each of the intervention groups were current smokers, and a total of 17 were former smokers (eight ofloxacin, nine amoxicillin). Dimakou 2014 and Kaponi 2017 did not provide data on smoking history.
Interventions
Researchers compared the following two types of antibiotics: fluoroquinolone versus β‐lactam in Chan 1996 and Lam 1989, and aminoglycoside versus polymyxin in Dimakou 2014 and Kaponi 2017. The two fluoroquinolone versus β‐lactam studies delivered antibiotics orally (Chan 1996; Lam 1989), and the two aminoglycoside versus polymyxin studies by inhalation (Dimakou 2014; Kaponi 2017). Studies also differed by duration, with antibiotics administered for seven and 10 days (Chan 1996; Lam 1989), respectively, in the two short‐term studies, and for four weeks and three months (Dimakou 2014;Kaponi 2017), respectively, in the two long‐term studies.
Table 3 shows the characteristics of interventions in each study including numbers of participants, types of antibiotics, and dose, duration, and frequency of administration.
1. Study intervention characteristics.
| Study |
Adults/ Children (N) |
Arm 1 | Arm 2 | Arm 3 | Duration | ||||||
| Antibiotic (N) | Dose/ Frequency | Mode of delivery | Antibiotic (N) | Dose/ Frequency | Mode of delivery | Comparison (N) | Dose/ Frequency | Mode of delivery | |||
| Fluoroquinolones vs β‐lactams (amoxicillin) | |||||||||||
| Chan 1996 | Adults (42) | Ciprofloxacin (plus amoxicillin placebo) (21) | 500 mg 3 times daily |
Oral | Amoxicillin (plus ciprofloxacin placebo) (21) |
1000 mg 3 times daily |
Oral | ‐ | ‐ | ‐ | 7 days |
| Lam 1989 | Adults (41) | Ofloxacin (plus amoxicillin placebo) (20) |
200 mg 3 times daily |
Oral | Amoxicillin (plus
ofloxacin placebo) (21) |
1000 mg 3 times daily |
Oral | ‐ | ‐ | ‐ | 10 days |
| Aminoglycosides vs polymyxins | |||||||||||
| Dimakou 2014 | Adults (29) | Tobramycin (10) |
300 mg twice daily |
Inhalation by nebuliser | 1 MU colistimethate sodium (10) |
300 mg twice daily |
Inhalation by nebuliser | Saline solution (9) |
4 mL of 0.9% solution | Inhalation by nebuliser | 4 weeks |
| Kaponi 2017 | Adults (52) | Tobramycin (17) |
300 mg twice daily |
Inhalation by nebuliser | 1 MU colistimethate sodium (18) |
300 mg twice daily |
Inhalation by nebuliser | Saline solution (17) |
4 mL of 0.9% solution | Inhalation by nebuliser | 3 months |
N: number of participants.
Outcomes
Primary outcomes
Exacerbation
The included studies did not report this outcome.
Serious adverse events
None of the included studies formerly reported serious adverse events, but these are implied from the reporting of adverse events.
Secondary outcomes
Response rate ‐ treatment failure
Chan 1996 defined treatment failure as 'poor' improvement in sputum purulence (assessed at day 7).
Response rate ‐ microbiological response
Chan 1996 reported microbiological response, defined as elimination of bacterial organisms amongst those who tested positive for bacteriological culture at day 0. This was assessed on day 7. Kaponi 2017 defined microbiological response rate as the proportion of participants in each group showing eradication of P aeruginosa at the end of treatment.
Response rate ‐ improvement in sputum purulence
Three included studies reported this outcome (Chan 1996; Dimakou 2014; Lam 1989). Both Chan 1996 and Lam 1989 categorised improvement in sputum purulence as follows: excellent ‐ mucoid; fair ‐ pale yellow or pale green; and poor ‐ dark green or dark yellow. Chan 1996 assessed improvement from baseline at day 7, and Lam 1989 at day 10. In addition, Lam 1989 assessed relapse of sputum purulence at three‐month follow‐up in participants classified as excellent or fair at 10 days. Dimakou 2014 did not include classification of sputum purulence.
Sputum volume
All four studies reported sputum volume (Chan 1996; Dimakou 2014; Kaponi 2017; Lam 1989). Chan 1996 reported changes in sputum volume between baseline and follow‐up at five and 10 days. Lam 1989 reported changes between baseline and seven days. Dimakou 2014 and Kaponi 2017 measured changes in sputum volume before and after treatment but did not report further details and direct comparisons between intervention groups in the abstracts.
Measures of lung function
Lam 1989 reported improvement in FEV₁ % predicted on day 10. Chan 1996 reported FEV₁ % predicted and FEV₁/forced vital capacity (FVC) % predicted only at baseline. Dimakou 2014 and Kaponi 2017 measured changes in spirometry before and after treatment but did not include further details and comparisons between intervention groups in the abstracts.
Adverse events
Three studies reported this outcome (Chan 1996; Dimakou 2014; Lam 1989). Both Chan 1996 and Lam 1989 reported the frequency of event types by group and did not report the proportion of participants in each group who experienced at least one adverse event. Dimakou 2014 reported the number of participants with adverse events but did not include further details in the abstract. Kaponi 2017 did not report adverse events.
Deaths
The four included studies did not formally report this outcome, but it was inferred based on the number of participants who completed each study.
Frequency of hospitalisations due to exacerbations of bronchiectasis; systemic markers of infection: C‐reactive protein (CRP); emergence of resistance to antibiotics; exercise capacity; quality of life
None of the included studies reported any of the above secondary outcomes.
Notes
Only two studies provided information about the source of study funding (Chan 1996; Lam 1989). Both reported that Daiichi Seiyaku Co Ltd provided active and dummy placebo tablets for the intervention groups. No included studies provided other information such as power calculations and declarations of conflicts of interest.
Excluded studies
We recorded in Characteristics of excluded studies reasons for exclusion of 26 studies from the 287 reports. We excluded 11 studies of mixed populations because data were not available for bronchiectasis participants alone (Begg 2000; Brambilla 1992; Finegold 1981; Garcia‐Rodriguez 1984; Jia 2010; Kobayashi 1984; Nakamura 2007; Pines 1964; Pines 1967; Pines 1981; Ramer 1981). Eight studies were not head‐to‐head comparisons of antibiotics using the same mode of delivery (Alberto 1968; Allen 1988; Bevilacqua 1973; Bilton 2006; Ip 1998; Liu 2012; NCT03093974; NCT03058718). Three studies did not include bronchiectasis participants (Khan 2003; Kobayashi 1984; Kobbernagel 2016), a further three studies were not randomised controlled trials (Cherniack 1959; Lioberes 1990; Mehta 1991), and the intervention in one study was not an antibiotic (Bradley 2011).
We endeavoured to contact study authors for missing data, but at the time of publication, these data remain unavailable.
Risk of bias in included studies
Two review authors (AK and LF) assessed the risk of bias in each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. We have presented an overview of our judgements in Figure 2.
2.
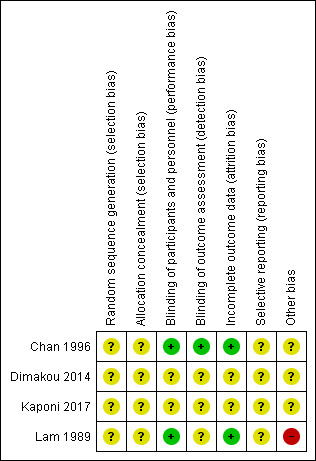
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The four included studies did not report information on sequence generation or allocation concealment. Therefore, we classified the risk of allocation bias as unclear.
Blinding
Two studies were double‐blinded using a double‐dummy design (Lam 1989; Chan 1996); therefore we considered them to be at low risk of performance bias. Chan 1996 used blinded outcome assessors, and we classified this study as having low risk of detection bias, but Lam 1989 did not report information on outcome assessors, and we judged this study as having unclear risk. Dimakou 2014 and Kaponi 2017 did not report information on blinding of the intervention or outcome assessments in the abstracts; we therefore assessed risk of performance and detection bias in these studies as unclear.
Incomplete outcome data
Two studies assessed all randomised participants at the end of treatment (Lam 1989; Chan 1996); we judged these studies to be at low risk of attrition bias. Although Dimakou 2014 and Kaponi 2017 reported assessment of all participants before and after treatment, it is not clear whether this was done at the end of the study. We therefore judged risk of attrition bias as unclear.
Selective reporting
We judged risk of selective reporting to be unclear in all four studies because pre‐specified protocols were not available and therefore it was not clear whether researchers reported all planned study outcomes (Chan 1996; Dimakou 2014; Kaponi 2017; Lam 1989).
Other potential sources of bias
Researchers in Chan 1996 withdrew amoxicillin from two participants who developed a rash but reported no further in terms of how long treatment was suspended and whether it was restarted. We therefore judged this study to be at unclear risk of other sources of bias. The authors of Lam 1989 noted that high levels of H influenzae, K pneumoniae, and P aeruginosa may be attributable to previous exposure to ampicillin and other antibiotics; we therefore judged this study to be at high risk of other bias. Dimakou 2014 and Kaponi 2017 provided insufficient study information in the abstracts to inform a clear judgement; we therefore classified this study as being at unclear risk of other bias.
Effects of interventions
Summary of findings for the main comparison. Fluoroquinolones compared to amoxicillin for bronchiectasis.
| Fluoroquinolones compared to amoxicillin for bronchiectasis: short‐term studies (< 4 weeks) | ||||||
| Patient or population: adults aged 18 years and above with diagnosis of non‐cystic fibrosis bronchiectasis Setting: hospital, Hong Kong Intervention: fluoroquinolones (Chan 1996: ciprofloxacin, 500 mg, oral, twice daily, 7 days; Lam 1989: ofloxacin, 200 mg, oral, thrice daily, 10 days) Comparison: amoxicillin (Chan 1996: 1000 mg, oral, 3 times per day, 7 days; Lam 1989: 1000 mg, oral, 3 times per day, 10 days) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with amoxicillin | Risk with fluoroquinolones | |||||
| Exacerbations | ‐ | ‐ | Not estimable | ‐ | ‐ | Outcome not reported in included studies |
| Serious adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 83 (2 RCTs) |
⊕⊕⊝⊝ LOWa,b | Evidence graded on the overall quality of the study |
| Response rate ‐ treatment failure | 429 per 1000 | 50 per 1000 (7 to 194) | OR 0.07 (0.01 to 0.32) | 83 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | |
| Response rate ‐ microbiological response | 2 out of 8 participants responded. | 8 out of 8 participants responded. | Peto OR 20.09 (2.83 to 142.59) | 16 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Small single study. Peto OR used owing to 100% response in intervention arm |
| Response rate ‐ improvement in sputum purulence (excellent) | 357 per 1000 | 566 per 1000 (348 to 761) | OR 2.35 (0.96 to 5.72) | 83 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | |
| Deaths | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 83 (2 RCTs) |
⊕⊕⊝⊝ LOWa,b | No deaths reported. Evidence graded on the overall quality of the study |
| Quality of life | ‐ | ‐ | Not estimable | ‐ | ‐ | Outcome not reported in included studies |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOne point deducted in relation to design and implementation of available studies suggesting likelihood of bias (unclear generation of randomisation sequence, potential selective reporting bias, and risk of other bias in Lam 1989).
bOne point deducted for imprecision (small sample size and few events).
cOne point deducted for imprecision (wide confidence interval).
Summary of findings 2. Polymyxins compared to aminoglycosides for bronchiectasis.
| Polymyxins compared to aminoglycosides for bronchiectasis: long‐term studies (≥ 4 weeks) | ||||||
| Patient or population: adults aged 18 years and above with diagnosis of bronchiectasis Setting: not reported Intervention: polymyxins (Dimakou 2014: 300 mg, inhalation using Pari LC Plus jet nebulizer, twice daily, 4 weeks; Kaponi 2017: 300 mg, inhalation using Pari LC Plus jet nebulizer, twice daily, 3 months) Comparison: aminoglycosides (Dimakou 2014: 1 MU, inhalation using Pari LC Plus jet nebulizer, twice daily, 4 weeks; Kaponi 2017: 1 MU, inhalation using Pari LC Plus jet nebulizer, twice daily, 3 months) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with aminoglycosides | Risk with polymyxins | |||||
| Exacerbation | ‐ | ‐ | Not estimable | ‐ | ‐ | Outcome not reported in included studies |
| Serious adverse events | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 20 (1 RCT) |
⊕⊝⊝⊝ VERY LOWa,b,c | Evidence graded on the overall quality of the study |
| Response rate ‐ improvement in sputum purulence | 800 per 1000 | 390 per 1000 (38 to 939) |
OR 0.16 (0.01 to 3.85) | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,d | Definition of improvement not reported |
| Response rate ‐ P aeruginosa eradication | 471 per 1000 | 554 per 1000 (242 to 826) |
OR 1.40 (0.36 to 5.35) | 35 (1 RCT) |
⊕⊝⊝⊝ VERY LOWa,b,d | Head‐to‐head comparison not reported directly |
| Deaths | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | No deaths reported. Evidence graded on the overall quality of the study |
| Quality of life | ‐ | ‐ | Not estimable | ‐ | ‐ | Outcome not reported in included studies |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; P aeruginosa: Pseudomonas aeruginosa; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOne point deducted in relation to design and implementation of available studies suggesting likelihood of bias (all study methods unclear).
bOne point deducted for indirectness (no direct head‐to‐head comparisons).
cOne point deducted for imprecision (small sample size and few events).
dOne point deducted for imprecision (wide confidence intervals).
Fluoroquinolone versus β‐lactam (amoxicillin): short‐term studies (< 4 weeks)
Primary outcomes
Exacerbation
The included studies did not report this primary outcome.
Serious adverse events
The two included studies did not explicitly report the number of participants who had at least one serious adverse event. However, none of the adverse events reported in Chan 1996 and Lam 1989 were considered serious adverse events and no randomised participants withdrew; we therefore concluded that no serious adverse events occurred in these two studies.
According to GRADE criteria, we judged this evidence as low quality.
Secondary outcomes
Response rate ‐ treatment failure
Two studies with 83 adults reported sputum purulence using the same classification criteria of excellent, fair, and poor (Chan 1996; Lam 1989).Chan 1996 defined treatment failures as those with poor improvement after seven days of oral treatment, and we used this definition of treatment failure for the two studies. The pooled analysis showed clear differences between groups in the numbers of participants with poor improvement in sputum purulence, with significantly fewer treatment failures in the fluoroquinolone group than in the group receiving amoxicillin (odds ratio (OR) 0.07, 95% confidence interval (CI) 0.01 to 0.32; Analysis 1.1).
1.1. Analysis.
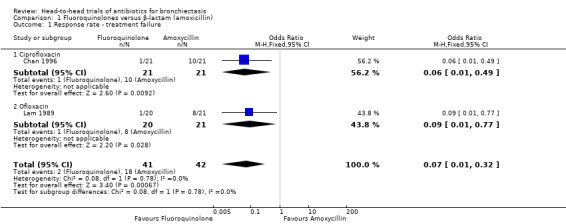
Comparison 1 Fluoroquinolones versus β‐lactam (amoxicillin), Outcome 1 Response rate ‐ treatment failure.
According to GRADE criteria, we judged this evidence as low quality.
Response rate ‐ microbiological response
One study with 42 adults reported elimination of bacterial organisms in more participants receiving seven days' oral treatment with ciprofloxacin versus seven days' oral treatment with amoxicillin (Peto OR 20.09, 95% CI 2.83 to 142.59; Analysis 1.2), although the effect estimate was based on only 16 participants who tested positive following baseline sputum culture (Chan 1996).
1.2. Analysis.
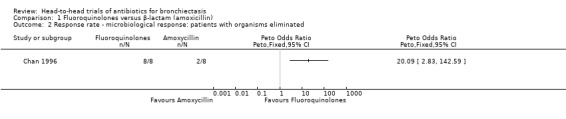
Comparison 1 Fluoroquinolones versus β‐lactam (amoxicillin), Outcome 2 Response rate ‐ microbiological response: patients with organisms eliminated.
According to GRADE criteria, we judged this evidence as low quality.
Response rate ‐ improvement in sputum purulence
Two studies with 83 adults reported no clear differences between groups in the number of participants with excellent improvement in sputum purulence (OR 2.35, 95% CI 0.96 to 5.72; Analysis 1.3) (Chan 1996; Lam 1989). According to GRADE criteria, we judged this evidence as very low quality. The same studies reported no differences in the number of participants with fair improvement in sputum purulence (OR 2.30, 95% CI 0.88 to 6.00; I² = 53%; Analysis 1.4). We noted substantial heterogeneity between studies but no clear evidence of subgroup differences (test for subgroup differences: Chi² = 2.12, df = 1 (P = 0.15), I² = 52.9%). Separate analysis of the two studies indicated a greater proportion of participants with fair improvement after seven days' oral treatment with ciprofloxacin versus seven days' oral treatment with amoxicillin (OR 4.67, 95% CI 1.17 to 18.69; Analysis 1.4). Data show no differences between participants after 10 days' oral treatment with ofloxacin versus amoxicillin (OR 1.07, 95% CI 0.26 to 4.44; Analysis 1.4).
1.3. Analysis.
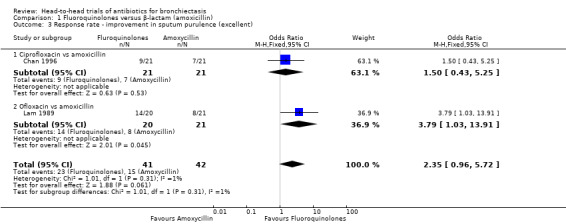
Comparison 1 Fluoroquinolones versus β‐lactam (amoxicillin), Outcome 3 Response rate ‐ improvement in sputum purulence (excellent).
1.4. Analysis.
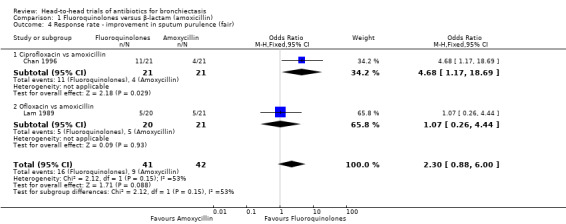
Comparison 1 Fluoroquinolones versus β‐lactam (amoxicillin), Outcome 4 Response rate ‐ improvement in sputum purulence (fair).
Relapse of sputum purulence
One study with 41 adults reported relapse of sputum purulence at three‐month follow‐up among 32 adults with excellent improvement at the end of treatment (10 days) (Lam 1989). Results showed no clear difference in relapse of sputum purulence between study groups (OR 1.04, 95% CI 0.23 to 4.77; Analysis 1.5).
1.5. Analysis.
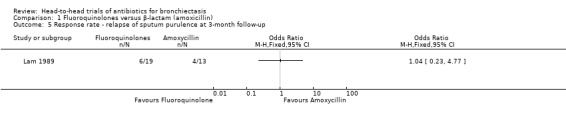
Comparison 1 Fluoroquinolones versus β‐lactam (amoxicillin), Outcome 5 Response rate ‐ relapse of sputum purulence at 3‐month follow‐up.
Sputum volume
A single study with 42 adults reported greater reduction in sputum volume after seven days' oral treatment with ciprofloxacin versus amoxicillin (mean difference (MD) ‐13.00 mL, 95% CI ‐18.44 to ‐7.56; Analysis 1.6) (Chan 1996). Lam 1989 reported more rapid reduction in sputum volume at day 5 and at day 10 (end of treatment) in the ofloxacin group than in the amoxicillin group (P < 0.05), but these study authors did not report mean values for each group.
1.6. Analysis.
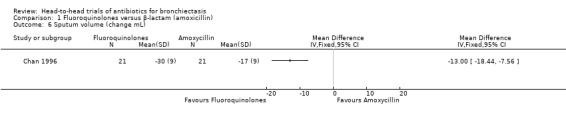
Comparison 1 Fluoroquinolones versus β‐lactam (amoxicillin), Outcome 6 Sputum volume (change mL).
Measures of lung function
One study with 41 adult participants reported no clear differences between groups in the change in FEV₁ % predicted from baseline to end of treatment (10 days) (MD ‐2.70, 95% CI ‐17.01 to 11.61; Analysis 1.7) (Lam 1989).
1.7. Analysis.
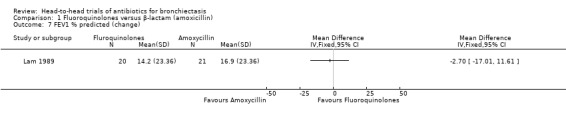
Comparison 1 Fluoroquinolones versus β‐lactam (amoxicillin), Outcome 7 FEV1 % predicted (change).
Adverse events
Chan 1996 and Lam 1989 did not report the number of participants who experienced at least one adverse event. Chan 1996 reported the frequency of each adverse event as follows: gastrointestinal upset: 0 ciprofloxacin, 2 amoxicillin; dizziness/vertigo: 2 ciprofloxacin, 2 amoxicillin; headache: 1 ciprofloxacin, 1 amoxicillin; and rash: 0 ciprofloxacin, 2 amoxicillin. Amoxicillin was discontinued in the two participants who developed a rash, but study authors did not report further details. Lam 1989 reported the frequency of each adverse event as follows: nausea/epigastric pain: 2 ofloxacin, 5 amoxicillin; dizziness/vertigo: 1 ofloxacin, 1 amoxicillin; and peripheral eosinophilia: 0 ofloxacin, 1 amoxicillin.
Deaths
All randomised participants completed both of the included studies (Chan 1996; Lam 1989); we therefore concluded that no deaths occurred during these trials.
Based on the overall study, we graded the quality of this evidence as low.
Fluoroquinolone versus β‐lactam (amoxicillin): long‐term studies (≥ four weeks)
We did not find any studies that performed this comparison.
Polymyxins versus aminoglycosides: short‐term studies (< four weeks)
We did not find any studies that performed this comparison.
Polymyxins versus aminoglycosides: long‐term studies (≥ four weeks)
Primary outcomes
Exacerbation
The included studies did not report this primary outcome.
Serious adverse events
One study with 20 adults reported that six participants who had adverse events did not require discontinuation of treatment and no randomised participants withdrew (Dimakou 2014). We therefore concluded that no serious adverse events occurred after four weeks of treatment. Kaponi 2017 did not report this outcome.
Based on GRADE criteria, we judged the quality of this evidence to be very low.
Secondary outcomes
Response rate ‐ improvement in sputum purulence
One study with 20 adults reported no differences between groups in the number of participants with improvement in sputum purulence after four weeks of nebulised treatment (OR 0.16, 95% CI 0.01 to 3.85; Analysis 2.1), although study authors did not include criteria for improvement in the abstract (Dimakou 2014). The study reported improvement in sputum purulence in all 10 participants receiving nebulised colistin compared with eight out of 10 participants receiving nebulised tobramycin for 4 weeks.
2.1. Analysis.
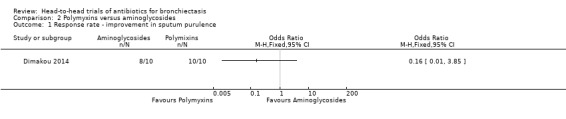
Comparison 2 Polymyxins versus aminoglycosides, Outcome 1 Response rate ‐ improvement in sputum purulence.
According to GRADE criteria, we judged this evidence as very low quality.
Response rate ‐ P aeruginosa eradication
Kaponi 2017 reported no differences between groups in terms of the number of participants with P aeruginosa eradication after three months of nebulised treatment (OR 1.40. 95% CI 0.36 to 5.35; Analysis 2.2). This study reported that P aeruginosa had been eradicated in 47% of 17 participants receiving tobramycin and in 39% of 18 participants receiving colistin.
2.2. Analysis.
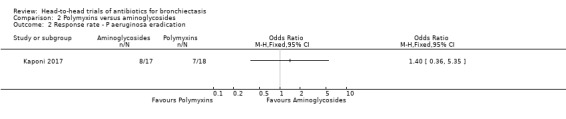
Comparison 2 Polymyxins versus aminoglycosides, Outcome 2 Response rate ‐ P aeruginosa eradication.
According to GRADE criteria, we judged this evidence as very low quality.
Sputum volume
Dimakou 2014 reported a significant reduction in sputum volume among participants receiving nebulised tobramycin (P = 0.005) and nebulised colistin (P = 0.009) but did not report mean values and comparisons between study groups in the abstract. Kaponi 2017 reported a reduction in sputum volume of 11.2 mL with tobramycin and 11.4 mL with colistin but included direct comparisons only for each antibiotic against placebo and did not include standard deviations in the abstract.
Sputum purulence
Kaponi 2017 reported mean reductions in sputum purulence scores with each antibiotic compared to placebo but reported no differences in mean improvement in scores between the group receiving tobramycin and the group receiving colistin (MD ‐0.20, 95% CI 0.80 to 0.40; Analysis 2.3).
2.3. Analysis.
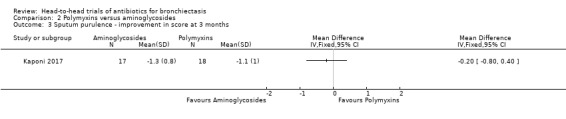
Comparison 2 Polymyxins versus aminoglycosides, Outcome 3 Sputum purulence ‐ improvement in score at 3 months.
Measures of lung function
Dimakou 2014 and Kaponi 2017 reported that spirometry tests were not significantly different between groups but did not provide further details in the abstracts.
Adverse events
Dimakou 2014 reported no clear differences between groups in the number of participants experiencing adverse events (OR 2.67, 95% CI 0.36 to 19.71; Analysis 2.4) after four weeks of nebulised treatment.
2.4. Analysis.
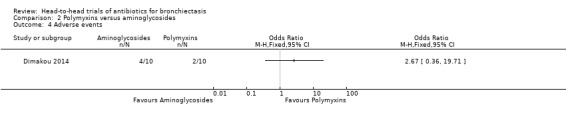
Comparison 2 Polymyxins versus aminoglycosides, Outcome 4 Adverse events.
Deaths
All randomised participants completed the Dimakou 2014 study; we therefore concluded that no deaths occurred during this trial. Kaponi 2017 did not explicitly report deaths, and we were not able to infer this outcome because study authors did not report the number of participants who reached the end of the study.
Based on the overall study, we graded the quality of this evidence as very low.
Discussion
Summary of main results
Four randomised trials met the inclusion criteria for this systematic review (Chan 1996; Dimakou 2014; Kaponi 2017; Lam 1989). All four studies included adults with a diagnosis of bronchiectasis. Two trials with a total of 83 participants were conducted at a single centre in Hong Kong and assessed the effectiveness of fluoroquinolones compared with amoxicillin administered orally for ten and seven days, respectively (Chan 1996; Lam 1989). The Dimakou 2014 and Kaponi 2017 trials were available only as conference abstracts and compared nebulised polymyxins (colistin) versus nebulised aminoglycosides (tobramycin) in 55 adults for four weeks and three months, respectively.
None of the included studies reported our primary outcome ‐ exacerbations. No serious adverse events and no deaths were reported in any of the included studies, but we considered the quality of this evidence to be low or very low. Two studies compared oral amoxicillin with oral fluoroquinolones; Chan 1996 used ciprofloxacin and Lam 1989 used ofloxacin. Treatment failure rates were lower with fluoroquinolones than with amoxicillin, but this finding was based on low‐quality evidence, leading to uncertainty in the results. Researchers found no evidence of differences between groups in sputum purulence (two studies) nor relapse in sputum purulence during follow‐up (one study), although again this was based on evidence of low or very low quality. We found limited evidence of reduced sputum volume with fluoroquinolones, but this was based largely on one study (Chan 1996), with limited data available from the other study. Microbiological response reported in one study showed greater elimination of infective organisms with ciprofloxacin, but again this finding was based on low‐quality evidence. Evidence from one study shows no differences in lung function. The included studies did not report the number of participants who had an adverse event.
Two studies comparing polymyxins with aminoglycosides did not demonstrate differences between groups in terms of microbiological response, improvement in sputum purulence or volume, or adverse events, and data on lung function provided in the abstracts were insufficient to inform clear conclusions. Only 55 participants contributed to the effect estimates in this comparison, and we judged the evidence to be of very low quality.
The wide confidence intervals and low‐ or very low‐quality evidence contribute to overall uncertainty in the results.
None of the included studies reported our other secondary outcomes ‐ systemic markers of infection, emergence of resistance to antibiotics, exercise capacity, and quality of life.
Overall completeness and applicability of evidence
All four studies potentially suffer from methodological issues, as the risk of bias for almost all domains was unclear. Moreover, the studies were not adequately powered to detect clinically important differences in treatment effects between intervention groups. The antibiotic comparisons in this review included only two classes of antibiotics; the comparison between fluoroquinolones and amoxicillin included 83 adults, and the comparison between polymyxins and aminoglycosides included only 55 adults. All studies were small; two were conducted at the same centre, and settings for the third and fourth studies were not reported. Our primary outcome ‐ frequency of hospitalisation due to exacerbations of bronchiectasis ‐ was not reported in any of the included studies, but this outcome may be less relevant for the one‐week trials conducted by Chan 1996 and Lam 1989, wherein all randomised participants had an active chest infection that could be defined as an exacerbation. These studies may have limited clinical relevance, as exacerbations treated with oral antibiotics would not normally require admission to hospital. The definition of treatment failure used in Chan 1996 is of limited value, as it is based solely on a poor sputum purulence response, and, as noted by the study authors, the extent of baseline infection in Lam 1989 may have been influenced by previous treatment with ampicillin. A definition of exacerbations was not provided in the studies that compared polymyxins with aminoglycosides. None of the studies reported our secondary outcomes ‐ systemic markers of infection, emergence of resistance to antibiotics, exercise capacity, or quality of life. Some of the differences in outcomes between groups, such as sputum volume, were reported only as P values, which limited our opportunity to conduct pooled data analyses. Most of the data in trial abstracts for Dimakou 2014 and Kaponi 2017 were reported narratively or with P values alone.
We did not identify any completed studies that included children, although our search identified two protocols (phase 1 and phase 2) of an ongoing head‐to‐head study ‐ the BEST trial, which includes children from Australia and New Zealand (Chang 2013). Contact with the principal investigator confirmed that the study concluded and the research group is currently analysing the data.
We excluded 11 head‐to‐head trials with mixed populations of participants (lower respiratory conditions) that did not explicitly provide data for bronchiectasis patients alone. We attempted to contact four of the study authors to obtain this information, but at the time of publication, these data were not available. We were unable to contact the authors of seven other studies.
Quality of the evidence
The overall quality of the evidence ranged from low to very low for outcomes included in the GRADE assessment. Data were available for only three of the five pre‐specified outcomes that we had planned to include in the summary of findings table. For the comparison of fluoroquinolones versus amoxicillin, we included three outcomes for response rate from two studies, but only one study contributed data on microbiological response. The quality of evidence for both of these outcomes was low. We downgraded quality by one level for serious design limitations owing to unclear sequence generation and allocation concealment, and by another level for small sample sizes with few events. Evidence for excellent improvement in sputum purulence was of very low quality, downgraded by one level for serious design limitations, one level for wide confidence intervals, and one level for small sample size with few events. Similarly, quality of the evidence for improvement in sputum purulence for the comparison of polymyxins with aminoglycosides was very low. Unclear reporting of the study design, indirectness of comparisons (not designed as head‐to‐head trials), imprecision in the effect estimate, and small sample size contributed to downgrading of the quality of evidence.
Potential biases in the review process
We used a comprehensive systematic search, conducted by a highly experienced information specialist, to identify potentially eligible studies. We searched multiple resources including electronic databases, journals, conference proceedings, reference lists of included studies, citations of included studies, and trial registries. Nevertheless, we recognise the possibility of publication bias in this review, which could either overestimate or underestimate effects of the intervention in terms of the different outcomes included in this review. Trials showing no, or negative, effects are less likely to be offered for publication, and if offered are less likely to be accepted, resulting in a biased set of data available for review. As only a small number of studies with few participants were included for each outcome, we were unable to assess publication bias by using formal tests.
Furthermore, it is possible that some papers were misclassified as not eligible for inclusion in the Review. All studies were independently assessed by two review authors, and verified by a third, and we are confident that studies excluded from the analyses were assessed on the basis of consistent and appropriate criteria. For some full‐text reports, it is possible that data could have been incorrectly entered into analyses, although all data were double‐checked to avoid data extraction errors.
We contacted the investigator of two of the included studies to request further information on study characteristics and other numerical outcome data, as the reports were available only as conference abstracts, but at the time of publication, we have not received a response.
Owing to the small number of included studies, we were unable to conduct sensitivity or subgroup analyses as planned.
Agreements and disagreements with other studies or reviews
The EMBARC Working Group recently provided a comprehensive and explicit definition of pulmonary exacerbations for bronchiectasis that includes "three or more of the following key symptoms for at least 48 hours: cough; sputum volume and/or consistency; sputum purulence; breathlessness and/or exercise tolerance; fatigue and/or malaise; haemoptysis" (Hill 2017). However, the definition of an exacerbation used as an entry criterion in our four included studies was based on only sputum purulence and/or sputum volume. The duration of antibiotic therapy in two of the four included studies ‐ Lam 1989 and Chan 1996 ‐ could be considered suboptimal compared with guideline‐recommended therapy of at least 14 days (Pasteur 2010; Polverino 2017). Although evidence to inform optimal choice of antibiotic is limited, current guidelines recommend amoxicillin, 1 g three times a day, for those with no previous bacteriology, or clarithromycin, 500 mg twice daily, for those allergic to penicillin, as the primary treatment for exacerbations (Pasteur 2010). Furthermore, high‐dose oral regimens such as amoxicillin 1 g three times a day or amoxicillin 3 g twice daily are recommended for patients with severe bronchiectasis chronically colonised with Haemophilus influenzae (Pasteur 2010).
Authors' conclusions
Implications for practice.
This systematic review identified a small amount of low‐quality evidence favouring oral fluoroquinolones over beta‐lactams based on 83 adult patients hospitalised with an exacerbation, but found no evidence of this comparison for long‐term use or with a nebulised route of administration. Very low‐quality evidence from 55 adults suggests no benefit from long‐term use of polymyxins compared with aminoglycosides, but again we found no evidence for short‐term use or other routes of administration for this comparison. We found no evidence for either of these comparisons in children. Based on the limited number of studies included in this review, evidence is insufficient to guide the choice of antibiotic therapy for exacerbations of bronchiectasis in adults or children, although we found no evidence of significant adverse events. Overall we have low or very low confidence in the reported outcomes. Recommendations for the general use of antibiotics are provided in the European guidelines for bronchiectasis (Polverino 2017).
Implications for research.
In view of the remarkable paucity of evidence that met our pre‐defined inclusion criteria, there is clearly a need for sufficiently powered high‐quality trials on this topic. All four trials that met our inclusion criteria were very small (with a combined total of just 138 participants), and none of them provided robust evidence. Our review stresses the need for further work with adults and children with a diagnosis of bronchiectasis by bronchography or high‐resolution computed tomography to compare one antibiotic versus another, administered by the same delivery method. New trials must consider this comparison in the short term and over the longer term. We encourage researchers to incorporate the outcome measures pre‐specified in our review, and in particular, our primary outcome measures of exacerbation (e.g. frequency during follow‐up, time to first exacerbation) and serious adverse events,
Acknowledgements
We thank Edge Hill University and the Cochrane Airways Group for their support.
We also thank Haley Harrison (HH) for searching trial registries. HH was an NIHR intern based at the Postgraduate Medical Institute, Edge Hill University.
Rebecca Normansell was the Editor for this protocol and commented critically on the document.
The Background and Methods section of this protocol are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Search frequency |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
Bronchiectasis search
1. exp Bronchiectasis/
2. bronchiect$.mp.
3. bronchoect$.mp.
4. kartagener$.mp.
5. (ciliary adj3 dyskinesia).mp.
6. (bronchial$ adj3 dilat$).mp.
7. or/1‐6
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the Cochrane Airways Trials Register
#1 BRONCH:MISC1
#2 MeSH DESCRIPTOR Bronchiectasis Explode All
#3 bronchiect*
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Anti‐Bacterial Agents Explode 1
#6 antibiotic* or anti‐biotic*
#7 anti‐bacteri* or antibacteri*
#8 *cillin
#9 *mycin or micin*
#10 *oxacin
#11 *tetracycline
#12 macrolide*
#13 quinolone*
#14 trimethoprim
#15 ceph*
#16 sulpha*
#17 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #4 and #17
[In search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, bronchiectasis]
Data and analyses
Comparison 1. Fluoroquinolones versus β‐lactam (amoxicillin).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response rate ‐ treatment failure | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.32] |
| 1.1 Ciprofloxacin | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.49] |
| 1.2 Ofloxacin | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.77] |
| 2 Response rate ‐ microbiological response: patients with organisms eliminated | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Response rate ‐ improvement in sputum purulence (excellent) | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.96, 5.72] |
| 3.1 Ciprofloxacin vs amoxicillin | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.43, 5.25] |
| 3.2 Ofloxacin vs amoxicillin | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.79 [1.03, 13.91] |
| 4 Response rate ‐ improvement in sputum purulence (fair) | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.88, 6.00] |
| 4.1 Ciprofloxacin vs amoxicillin | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.68 [1.17, 18.69] |
| 4.2 Ofloxacin vs amoxicillin | 1 | 41 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.26, 4.44] |
| 5 Response rate ‐ relapse of sputum purulence at 3‐month follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Sputum volume (change mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 FEV1 % predicted (change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Polymyxins versus aminoglycosides.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response rate ‐ improvement in sputum purulence | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Response rate ‐ P aeruginosa eradication | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Sputum purulence ‐ improvement in score at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chan 1996.
| Methods |
Aims: to compare the effectiveness of oral amoxicillin and ciprofloxacin for treatment of infective exacerbations of patients with bronchiectasis Design: randomised, double‐blind, placebo‐controlled trial. Each arm received the active intervention plus a dummy of the comparator intervention (i.e. double dummy). Total study duration: not reported Number of study centres and locations: single, Hong Kong Study setting: hospital Methods of recruitment: not reported Withdrawals: no participants withdrew from the study, but amoxicillin treatment was withdrawn from 2 participants who developed a rash. Study start and end dates: not reported Analysis by intent‐to‐treat: yes |
|
| Participants | 42 adults were randomised. Inclusion criteria: hospitalised patients with an infective exacerbation of bronchiectasis, defined by a change in sputum colour (i.e. becoming purulent) or an increase in sputum volume Exclusion criteria: history of hypersensitivity to study antibiotics and those on antibiotic treatment within 2 weeks before admission Mean age: ciprofloxacin group 1 (CG): 64.5 years; amoxicillin group (AG): 63.4 years; range: 34 to 89 years Gender: CG: 14 females, 7 males; AG: 13 females, 8 males Bronchiectasis diagnosis: based on clinical and radiological criteria (Luce JM: Bronchiectasis. In Murray JF, Nadal JA (eds): Textbook of Respiratory Medicine. Philadelphia: Saunders, 1988, pp 1107‐1125) Definition of acute exacerbation: change in sputum colour (i.e. becoming more purulent) or increase in volume of sputum Severity of condition: All participants had moderate to severe airflow obstruction. Most had chronic sputum production between exacerbations. Almost all participants had cystic changes on chest radiographs. History of bronchiectasis, years (mean): CG: 12.7 years; AG: 12.5 years Mean exacerbation episodes per participant requiring antibiotics in previous year: CG: 4.3; AG: 3.8 Patients producing daily sputum between exacerbations (n): CG: 20; AG: 18 Baseline lung function (mean ± SD): FEV₁ % predicted: CG: 0.69 ± 0.34; AG: 0.72 ± 0.51; FEV₁/FVC: CG: 58.6 ± 20.6; AG: 58 ± 15.3 Smoking history: current: CG: 1; AG: 0; former: CG: 6; AG: 9; non‐smoker: CG: 14; AG: 13 Baseline imbalances: none |
|
| Interventions | Treatment started on the day of admission to hospital CG: ciprofloxacin (n = 21) Dose: 500 mg; delivery mode: oral; frequency: twice daily; duration: 7 days Co‐intervention: amoxicillin placebo AG: amoxicillin (n = 21) Dose: 1000 mg; delivery mode: oral; frequency: 3 times daily; duration: 7 days Co‐intervention: ciprofloxacin placebo Both groups: Other treatments such as physiotherapy and bronchodilators were prescribed as required. Two participants in AG developed a rash, and treatment was stopped, although it is unclear at what stage of treatment and whether treatment was restarted. |
|
| Outcomes | Temperature, sputum volume, sputum purulence, haemoptysis, dyspnoea and cough, FEV₁, FVC, blood count, biochemistry | |
| Notes |
Power calculation: not reported Trial registration: not reported Conflict of interest: not reported Funders: Daiichi Seiyaku Co Ltd provided active and matched placebo tablets. Role of the sponsors: not reported Ethical approval: obtained from the Chinese University of Hong Kong Conclusions: Ciprofloxacin is an effective treatment for infective exacerbations of bronchiectasis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded using a double‐dummy design. The appearance of active tablets of ciprofloxacin and amoxicillin was identical to the placebo tablets of ciprofloxacin and amoxicillin, so that neither patients nor the physicians responsible for treatment and evaluation were aware of the treatment each individual patient was receiving. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were made by blinded observers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were assessed at the end of treatment. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | Unclear risk | Amoxicillin was withdrawn from 2 patients who developed a rash, but no further details were reported. |
Dimakou 2014.
| Methods |
Aims: to evaluate the effect of inhaled tobramycin (Bramitob, Chiesi) and colistimethate sodium (Colistin, Norma) in patients with non‐CF bronchiectasis andP aeruginosa in sputum Design: randomised, placebo‐controlled trial Total study duration: not reported Number of study centres and locations: not reported Study setting: not reported Methods of recruitment: not reported Withdrawals: not reported Study start and end dates: not reported Analysis by intent‐to‐treat: not reported |
|
| Participants | 29 adults were randomised. Inclusion criteria: non‐CF bronchiectasis with > 10⁴ CFUs of P aeruginosa/mL in sputum culture. Exclusion criteria: not reported Mean age: 56.06 years; range: 24 to 80 (not reported per group) Gender: 19 women, 10 men (not reported per group) Bronchiectasis diagnosis: not reported Definition of acute exacerbation: not reported Severity of condition: not reported Baseline lung function: not reported Smoking history: not reported Baseline imbalances: not reported |
|
| Interventions |
Tobramycin (n = 10) Dose: 300 mg; delivery mode: inhalation using Pari LC Plus jet nebulizer; frequency: twice daily; duration: 4 weeks Colistin (n = 10) Dose: 1 MU; delivery mode: inhalation using Pari LC Plus jet nebulizer; frequency: twice daily; duration: 4 weeks Saline solution (n = 9) Dose: 0.9% 4 mL; delivery mode: inhalation using Pari LC Plus jet nebulizer; frequency: twice daily; duration: 4 weeks |
|
| Outcomes | Symptoms, sputum purulence and culture, spirometry, SaO₂ before and after treatment | |
| Notes |
Power calculation: not reported Trial registration: not reported Conflict of interest: not reported Funders: not reported Role of the sponsors: not reported Ethical approval: not reported Conclusions: "Data indicate that inhaled antibiotics, tobramycin and colistin may be effective in improving symptoms and reducing P aeruginosa load in bronchiectatic patients. Further investigation is necessary". Significance values are given for improvements over 4 weeks, and both antibiotics are described as showing greater improvement compared with saline solution. Comparisons between the 2 antibiotics are not reported in this conference abstract, and we were unable to contact the study authors for further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
Kaponi 2017.
| Methods |
Aims: to evaluate the effect of inhaled tobramycin and colistin on eradication of P aeruginosa in patients with NCFB [Abstract] Design: randomised trial Total study duration: 3 months Number of study centres and locations: not reported Study setting: not reported Methods of recruitment: not reported Withdrawals: not reported Study start and end dates: not reported Analysis by intent‐to‐treat: not reported |
|
| Participants | 52 adults were randomised. Inclusion criteria: non‐CF bronchiectasis with > 10⁴ CFUs of P aeruginosa/mL in sputum culture. Exclusion criteria: not reported Mean age: 58.6 years; standard deviation: 15.2 (not reported per group) Gender: 33 women, 19 men (not reported per group) Bronchiectasis diagnosis: not reported Definition of acute exacerbation: not reported. Severity of condition: not reported Baseline lung function: not reported Smoking history: not reported Baseline imbalances: not reported |
|
| Interventions | All patients received ciprofloxacin 750 mg bid for 14 days before randomisation. Tobramycin (n = 17) Dose: 300 mg; delivery mode: inhalation using Pari LC Plus jet nebulizer; frequency: twice daily; duration: 3 months Colistin (n = 18) Dose: 1 MU; delivery mode: inhalation using Pari LC Plus jet nebulizer; frequency: twice daily; duration: 3 months Saline solution (n = 17) Dose: 0.9% 4 mL; delivery mode: inhalation using Pari LC Plus jet nebulizer; frequency: twice daily; duration: 3 months |
|
| Outcomes | Sputum culture, volume and purulence, dyspnoea (MRC scale), spirometry, and SaO₂ were estimated before and after treatment | |
| Notes |
Power calculation: not reported Trial registration: not reported Conflict of interest: not reported Funders: not reported Role of the sponsors: not reported Ethical approval: not reported Conclusions: "Our findings indicate that inhaled antibiotics, tobramycin and colistin may be effective in eradicating P aeruginosa, reducing bacterial load and improving symptoms of bronchiectatic patients. Further investigation is required". Significance values are given for improvements over 3 months, and both antibiotics are described as showing greater improvements compared to saline solution. Direct comparisons between the 2 antibiotics are not reported in this conference abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
Lam 1989.
| Methods |
Aims: to compare the effectiveness of oral amoxicillin and oral ofloxacin for infective exacerbations in non‐cystic fibrosis bronchiectasis Design: randomised, double‐blind, placebo‐controlled trial. Each arm received the active intervention plus a dummy of the comparator intervention (i.e. double dummy). Total study duration: not reported Number of study centres and locations: single, Hong Kong Study setting: hospital Methods of recruitment: not reported Withdrawals: none Study start and end dates: not reported Analysis by intent‐to‐treat: not reported |
|
| Participants | 41 adults were randomised. Inclusion criteria: hospitalised adults with an infective exacerbation of bronchiectasis, as evidenced by sputum turning from mucoid or mucopurulent to frankly purulent Exclusion criteria: past history of allergy to antibiotics, hepatic or renal dysfunction, or pregnancy Mean age: ofloxacin (OG): 53.1 years; range: 22 to 74; amoxicillin (AG): 52.8 years; range: 28 to 65 Gender: OG: 9 females, 11 males; AG: 9 females, 12 males Bronchiectasis diagnosis: in most patients (34), diagnosis of bronchiectasis was confirmed by clinical and radiological evidence of bronchial wall thickening or cystic changes; in 7 patients, it was confirmed clinically and by bronchogram. Definition of acute exacerbation: sputum turning from mucoid or mucopurulent to frankly purulent Severity of condition: All participants had moderate to severe airflow obstruction. Almost all participants had cystic changes on chest radiographs. History of bronchiectasis, years (mean): OG: 14.1 years; range: 3 to 60; AG: 14.7 years; range: 2 to 50 Mean episodes of exacerbations per participant in previous year requiring antibiotics: OG: 3.3; AG: 3.8 Sputum production daily between exacerbations (n): OG: 15; AG: 17 Baseline lung function mean (SD): FEV₁ (L): OG: 0.97 (0.57); AG: 0.91 (0.49); FEV₁ (% predicted): OG: 42.1%; AG: 39.8%; FEV₁/FVC: OG: 59.5 (16.9); AG: 58.0 (19.5) Smoking history: current: OG: 2; AG: 2; former: OG: 8; AG: 9; non‐smoker: OG: 10; AG: 10 Baseline imbalances: none reported |
|
| Interventions | Treatment started from the day of admission to hospital OG: ofloxacin plus amoxicillin placebo tablets (n = 20) Dose: 200 mg; delivery mode: oral; frequency: 3 times daily; duration: 10 days Co‐intervention: postural drainage and other prescribed treatment including bronchodilators as required AG: amoxicillin plus ofloxacin placebo tablets (n = 21) Dose: 1000 mg; delivery mode: oral; frequency: 3 times daily; duration: 10 days Co‐intervention: postural drainage and other prescribed treatment including bronchodilators as required |
|
| Outcomes | Temperature, sputum appearance and volume, haemoptysis, cough and dyspnoea: daily Spirometry and chest radiology: days 0 and 10 Haematological and biochemical tests, and sputum for gram smears and cultures: days 0, 5, and 10. Antibiotic levels in serum and sputum at 2 hours post dosage determined by a disc‐plate bioassay: day 5. Sputum purulence: day 10 Side effects: daily |
|
| Notes |
Power calculation: not reported Trial registration: not reported Conflict of interest: not reported Funders: Daiichi Seiyaku Co Ltd provided the active and matched placebo tablets. Role of the sponsors: not reported Ethical approval: not reported Conclusions: The role of oral ofloxacin in infective episodes of bronchiectasis appears to be promising. It would be a useful alternative antibiotic for empirical initial treatment on an outpatient basis. Further studies are required to define the optimal dosage ad duration of therapy and to define its role as compared to high‐dose or nebulised amoxicillin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were assessed at the end of treatment. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Other bias | High risk | Study authors note that high levels of H influenzae, K pneumoniae, and P aeruginosa may be attributable to previous exposure to ampicillin and other antibiotics. |
AG: amoxicillin group.
CF: cystic fibrosis.
CFUs: colony‐forming units.
CG: ciprofloxacin group.
FEV1: forced expiratory volume in one second.
FVC: forced vital capacity.
H influenzae: Haemophilus influenzae.
K pneumoniae: Klebsiella pneumoniae.
NCFB: non cystic fibrosis bronchiectasis
OG: ofloxacin group.
P aeruginosa: Pseudomonas aeruginosa.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alberto 1968 | Not a head‐to‐head trial of antibiotics. Comparison of mucolytic acetylcysteine vs nothing against background antibiotic therapy |
| Allen 1988 | Not a head‐to‐head trial of antibiotics. Comparison of probenecid, a uricosuric agent, vs nothing against background therapy |
| Begg 2000 | Not restricted exclusively to patients with bronchiectasis. We endeavoured to contact the study authors to ascertain availability of data from bronchiectasis participants, but at the time of publication of this review, the data were unavailable. |
| Bevilacqua 1973 | Not a head‐to‐head trial of antibiotics. Different tetracycline doses |
| Bilton 2006 | Not a head‐to‐head trial of antibiotics. Comparison of dual antibiotic vs single antibiotic alone (included in separate review of dual antibiotics) |
| Bradley 2011 | Intervention was not an antibiotic. |
| Brambilla 1992 | Not restricted exclusively to patients with bronchiectasis, and no separate subgroup analysis for patients with bronchiectasis. We endeavoured to contact the study authors to ascertain availability of data from bronchiectasis participants, but at the time of publication of this review, the data were unavailable. |
| Cherniack 1959 | Not a randomised controlled trial |
| Finegold 1981 | Not restricted exclusively to patients with bronchiectasis. Although distribution of initial diagnosis and treatment response was provided in Table 2, study authors provided no data on the total number of patients with bronchiectasis in each group. We were unable to contact the study authors for further information. |
| Garcia‐Rodriguez 1984 | Not restricted exclusively to patients with bronchiectasis. We were unable to contact the study authors for further information. |
| Ip 1998 | Not a head‐to‐head trial of antibiotics. Same antibiotic was administered by different delivery methods. |
| Jia 2010 | Not restricted exclusively to patients with bronchiectasis, and no separate subgroup analysis for patients with bronchiectasis. We endeavoured to contact the study authors to ascertain availability of data from bronchiectasis participants, but at the time of publication of this review, the data were unavailable. |
| Khan 2003 | Does not include bronchiectasis patients |
| Kobayashi 1984 | Not restricted exclusively to patients with bronchiectasis. We were unable to contact the study authors for further information. |
| Kobbernagel 2016 | Does not include bronchiectasis patients |
| Krawczyk 1981 | Does not include bronchiectasis patients |
| Lioberes 1990 | Not a randomised controlled trial |
| Liu 2012 | Not a head‐to‐head trial of antibiotics. Single antibiotic vs control |
| Mehta 1991 | Not a randomised controlled trial |
| Nakamura 2007 | Not restricted exclusively to patients with bronchiectasis. We endeavoured to contact the study authors to ascertain availability of data from bronchiectasis participants, but at the time of publication of this review, the data were unavailable. |
| NCT03058718 | Not a head‐to‐head trial. Procalcitonin‐guided antibiotic therapy |
| NCT03093974 | Not a head‐to‐head trial. Single antibiotic vs placebo |
| Pines 1964 | Not restricted exclusively to patients with bronchiectasis. We were unable to contact the study authors for further information. |
| Pines 1967 | Not restricted exclusively to patients with bronchiectasis. We were unable to contact the study authors for further information. |
| Pines 1981 | Not restricted exclusively to patients with bronchiectasis. We were unable to contact the study authors for further information. |
| Ramer 1981 | Not restricted exclusively to patients with bronchiectasis. We were unable to contact the study authors for further information. |
Characteristics of studies awaiting assessment [ordered by study ID]
Lam 1986.
| Methods |
Aims: to compare the effectiveness of oral amoxicillin and oral ofloxacin for infective exacerbations in non‐cystic fibrosis bronchiectasis Design: randomised, double‐blind, double‐dummy placebo‐controlled trial Total study duration: not reported Number of study centres and locations: single, Hong Kong Study setting: hospital Methods of recruitment: not reported Withdrawals: none Study start and end dates: not reported Analysis by intent‐to‐treat: not reported |
| Participants | 32 adults were randomised. Inclusion criteria: hospitalised adult patients with an infective exacerbation of bronchiectasis Exclusion criteria: past history of allergy to antibiotics, hepatic or renal dysfunction, or pregnancy Mean age: ofloxacin (OG): 53.6 years; range: 29 to 74; amoxicillin (AG): 54.7 years; range: 43 to 65 Gender: OG: 7 females, 8 males; AG: 7 females, 10 males Bronchiectasis diagnosis: not reported Severity of condition: not reported History of bronchiectasis, years (mean): OG: 15.3 years; range: 3 to 60; AG: 16.2 years; range: 2 to 50 Mean episodes of exacerbations per patient in previous year requiring antibiotics: not reported Sputum production daily between exacerbations (n): not reported Baseline lung function mean (SD): not reported Smoking history: current: OG: 2; AG: 2; former: OG: 5; AG: 7; non‐smoker: OG: 8; AG: 8 Baseline imbalances: none reported |
| Interventions | Treatment started from the day of admission to hospital OG: ofloxacin plus amoxicillin placebo tablets (n = 15) Dose: 200 mg; delivery mode: oral; frequency: 3 times daily; duration: 10 days Co‐intervention: postural drainage and other prescribed treatment including bronchodilators as required AG: amoxicillin plus ofloxacin placebo tablets (n = 17) Dose: 1000 mg; delivery mode: oral; frequency: 3 times daily; duration: 10 days Co‐intervention: postural drainage and other prescribed treatment including bronchodilators as required |
| Outcomes | Temperature, sputum appearance and volume, haemoptysis, cough and dyspnoea: daily Spirometry and chest radiology: days 0 and 10 Haematological and biochemical tests, and sputum for gram smears and cultures: days 0, 5, and 10. Antibiotic levels in serum and sputum at 2 hours post dosage determined by a disc‐plate bioassay: day 5. Sputum purulence: day 10 |
| Notes |
Power calculation: 170 children (85 per arm), providing 90% power (α = 0.05, 1‐sided) with 20% non‐inferiority margin to detect 80% resolution rate by day 21 Trial registration: ANZCTR; ACTRN12612000010897 Funders: Daiichi Seiyaku Co Ltd provided the active and matched placebo tablets. Role of the sponsors: not reported Ethical approval: not reported Conclusions: the role of oral ofloxacin in infective episodes of bronchiectasis appears to be promising. If confirmed in a larger number of patients, ofloxacin may prove to be a useful antimicrobial in bronchiectasis on an outpatient basis. |
AG: amoxicillin group.
OG: ofloxacin group.
SD: standard deviation.
Characteristics of ongoing studies [ordered by study ID]
Chang 2013.
| Trial name or title | BEST‐2 Trial |
| Methods |
Aims: is daily oral azithromycin non‐inferior (within a 20% margin) to oral amoxicillin‐clavulanate in achieving resolution of exacerbations by day 21 of treatment Design: randomised, double‐blind, double‐dummy, placebo‐controlled trial Total study duration: not yet published Number of study centres and locations: multi‐centre; Brisbane, Darwin, Melbourne, Perth, and Sydney in Australia, and Auckland in New Zealand Study setting: home Methods of recruitment: chest clinic Analysis by intent‐to‐treat: planned Power calculation: 170 children (85 per arm), providing 90% power (α = 0.05, 1‐sided) with 20% non‐inferiority margin to detect 80% resolution rate by day 21 Trial registration: ANZCTR; ACTRN12612000010897 Conflict of interest: Study authors declare that they have no financial competing interests related to this study. Funders: The study is funded by a 3‐year Australian NHMRC project grant (number 1019834) and is supported by a NHMRC Centre for Research Excellence in Lung Health of Aboriginal and Torres Strait Islander Children grant (number 1040830). A Chang (grant number 545216) is supported by NHMRC practitioner fellowship. KAF O'Grady is supported by funding from the Children's Health Foundation Queensland, Queensland Government Smart Futures fellowship (number 51008) and NHMRC Career Development Fellowship (number 1045157). Ethical approval: Human Research Ethics Committees of all participating institutions (Brisbane: Children's Health Queensland Hospital and Health Service (Royal Children's Hospital) and University of Queensland; Darwin: Department of Health and Families and Menzies School of Health Research; Melbourne: Royal Children's Hospital; Perth: Princess Margaret Hospital; Sydney: Sydney Children's Hospital Network Human Research ethics committee; and Auckland: Northern Ethics Committee, Ministry of Health and Starship Children's Health local ethics committee) |
| Participants |
Inclusion criteria: younger than 18 years; bronchiectasis, as defined by HRCT scan within the previous 5 years OR followed by a respiratory physician for treatment of bronchiectasis if diagnosed earlier; 2 or more respiratory exacerbations of bronchiectasis symptoms in the 18 months before study enrolment Exclusion criteria: cystic fibrosis (sweat chloride > 35 mmol/L or gene mutation); liver dysfunction; severe (hypoxia, dyspnoea, or hospitalisation required) or recent exacerbation (in the 4 weeks before study enrolment; known hypersensitivity to macrolides or penicillins; taking regular maintenance antibiotics of the same class as the investigational antibiotics (macrolides and penicillins); taking macrolides or penicillins within 3 weeks of study enrolment; current or recent (within 4 months before study enrolment) identification of P aeruginosa organism in the airways; current treatment for an oncology condition of any kind |
| Interventions |
Arm 1: daily oral azithromycin 5 mg/kg for 21 days Arm 2: twice‐daily oral amoxicillin‐clavulanic acid 22.5 mg/kg for 21 days These treatments are administered only for the first respiratory exacerbation after enrolment. |
| Outcomes | Detectable difference in PC‐QOL (Parent Cough‐specific Quality of Life) (minimum important difference between groups = 0.9) Detectable difference in serum laboratory assays: CRP, serum amyloid a, IL‐6, IL‐10, IP‐10 (this outcome is assessed only when possible, i.e. not in all participants) Duration of exacerbation symptoms (symptoms of exacerbation are considered increased or changed quality of cough, increased sputum production, or change in patient's normal sputum colour or purulence) Presence and prevalence of viral and bacterial respiratory pathogens and antibiotic (penicillin and macrolide) resistance of pathogens on nasal swabs and sputum samples Requirement for hospitalisation for respiratory exacerbations |
| Starting date | 1/10/2012 |
| Contact information | http://www.anzctr.org.au/ACTRN12612000010897.aspx |
| Notes | Status recorded as "recruitment completed"; details up‐to‐date on 12 June 2017 |
CRP: C‐reactive protein.
IL: interleukin.
IP: IFN‐γ‐induced protein .
NHMRC: Natgional Health and Medical Research Council.
P aeruginosa: Pseudomonas aeruginosa.
PC‐QOL: Patient Cough‐specific Quality of Life.
Contributions of authors
All protocol authors contributed to the Background section. SJM, SS, LF, and PM contributed to the Methods section.
ES and LF screened the searches; AK and LF extracted data and completed the risk of bias assessment; LF transferred data to Review Manager; LF and SS undertook data analysis; AK, LF, and SS completed the results section; and all review authors contributed to the Discussion and Authors' conclusions.
Sources of support
Internal sources
-
Edge Hill University, Other.
Funded SS to provide support for a series of reviews on bronchiectasis
External sources
The authors declare that no external funding was received for this systematic review, Other.
Declarations of interest
SS: lead applicant on a grant from Edge Hill University that provided support to LF for several bronchiectasis reviews. She is also an editor with the Cochrane Airways Group.
AK: none known.
SJM: none known.
LF: none known.
ES: none known.
PM: received lecture fees and conference accommodations/fees from industry unrelated to the current review.
New
References
References to studies included in this review
Chan 1996 {published data only}
- Chan TH, Ho SS, Lai CK, Cheung SW, Chan RC, Cheng AF, et al. Comparison of oral ciprofloxacin and amoxycillin in treating infective exacerbations of bronchiectasis in Hong Kong. Chemotherapy 1996;42(2):150‐6. [PUBMED: 8697891] [DOI] [PubMed] [Google Scholar]
Dimakou 2014 {published data only}
- Dimakou K, Triantafillidou C, Tsikritsaki K, Gousiou A, Dervas A, Toumbis M. Non‐CF bronchiectasis: the effect of inhaled antibiotics (tobramycin and colistin) in patients with Pseudomonas aeruginosa. European Respiratory Journal 2014;44(Suppl 58):P244. [Google Scholar]
Kaponi 2017 {published data only}
- Kaponi M, Karampitsakos T, Tzouvelekis A, Chrysikos S, Melachroinidou M, Gousiou A, et al. Eradication treatment in non‐CF bronchiectasis: the effect of inhaled antibiotics (tobramycin and colistin) on patients with Pseudomonas aeruginosa. European Respiratory Journal 2017;50(Suppl 61):OA1971. [Google Scholar]
Lam 1989 {published data only}
- Lam WK, Chau PY, So SY, Leung YK, Chan JC, Ip M, et al. Ofloxacin compared with amoxycillin in treating infective exacerbations in bronchiectasis. Respiratory Medicine 1989;83(4):299‐303. [PUBMED: 2692094] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alberto 1968 {published data only}
- Alberto S, Colongo PG, Brusasco L, Frigerio G. [Studies of the clinical and respiratory functional effects of a mucolytic‐antibiotic preparation in chronic bronchopulmonary diseases. Controlled double‐blind single code studies] [Studi sugli effetti clinici e sulla funzionalita respiratoria di un preparato mucolitico‐antibiotico nelle affezioni croniche broncopolmonari. Ricerca controllata doppio‐cieca a codifica singola.]. Minerva Medica 1968;59(53):2995‐3002. [PUBMED: 5671380] [PubMed] [Google Scholar]
Allen 1988 {published data only}
- Allen MB, Fitzpatrick R. Amoxycillin with and without probenecid in the management of patients with bronchiectasis. Clinical Science 1988;74(Suppl 18):56P. [Google Scholar]
Begg 2000 {published data only}
- Begg EJ, Robson RA, Saunders DA, Graham GG, Buttimore RC, Neill AM, et al. The pharmacokinetics of oral fleroxacin and ciprofloxacin in plasma and sputum during acute and chronic dosing. British Journal of Clinical Pharmacology 2000;49(1):32‐8. [PUBMED: 10606835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bevilacqua 1973 {published data only}
- Bevilacqua M, Gironi G, Marinai E, Ulivieri P, Frigerio G. [Comparative study of the clinical activity of tetracycline and a combination of tetracycline and thiamphenicol in respiratory tract infections. Controlled study of a cooperative type] [Studio comparativo sull'attivita clinica della tetraciclina in paragone ad una sua associazione con tiamfenicolo nelle infezioni respiratorie. Ricerca controllata di tipo cooperativo]. Minerva Medica 1973;64(10):466‐80. [PUBMED: 4571450] [PubMed] [Google Scholar]
Bilton 2006 {published data only}
- Bilton D, Henig N, Morrissey B, Gotfried M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006;130(5):1503‐10. [PUBMED: 17099030] [DOI] [PubMed] [Google Scholar]
Bradley 2011 {published data only}
- Bradley JM, Treacy K, O'Neill B, McCourt F, Green L, Gardner E, et al. S106 A randomised double blind 13 week crossover trial of hypertonic saline (HTS) (6%) vs isotonic saline (ITS) (0.9%) in patients with bronchiectasis. Thorax 2011;66(Suppl 4):A49. [Google Scholar]
Brambilla 1992 {published data only}
- Brambilla C, Kastanakis S, Knight S, Cunningham K. Cefuroxime and cefuroxime axetil versus amoxicillin plus clavulanic acid in the treatment of lower respiratory tract infections. European Journal of Clinical Microbiology and Infectious Diseases 1992;11(2):118‐24. [PUBMED: 1396725] [DOI] [PubMed] [Google Scholar]
Cherniack 1959 {published data only}
- Cherniack NS, Vosti KL, Dowling, HF, Lepper MH, Jackson GG. Long‐term treatment of bronchiectasis and chronic bronchitis; a controlled study of the effects of tetracycline, penicillin, and an oleandomycinpenicillin mixture. Archives of Internal Medicine 1959;103(3):345‐53. [PUBMED: 13626263] [DOI] [PubMed] [Google Scholar]
Finegold 1981 {published data only}
- Finegold SM, Gentry LO, Mogabgab W, Campbell SC, Skatrud J. Controlled comparative trial of bacampicillin and amoxicillin in therapy of bacterial infections of the lower respiratory tract. Reviews of Infectious Diseases 1981;3(1):150‐3. [PUBMED: 7012996] [DOI] [PubMed] [Google Scholar]
Garcia‐Rodriguez 1984 {published data only}
- Garcia‐Rodriguez JA, Gomez‐Garcia AC. A comparison of cefotaxime and cefoperazone in respiratory tract infections. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):301‐6. [PUBMED: 6094456] [DOI] [PubMed] [Google Scholar]
Ip 1998 {published data only}
- Ip M, Tsang K, Chan WM, Ho PL, Lam B, Lam WK. Randomised controlled study of levofloxacin versus ceftazidime in acute exacerbations of bronchiectasis. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A173. [Google Scholar]
Jia 2010 {published data only}
- Jia B, Lu P, Huang W, Li C, Huang A, Zhou X, et al. A multicenter, randomized controlled clinical study on biapenem and imipenem/cilastatin injection in the treatment of respiratory and urinary tract infections. Chemotherapy 2010;56(4):285‐90. [PUBMED: 20714145] [DOI] [PubMed] [Google Scholar]
Khan 2003 {published data only}
- Khan S, Javaid A, Ghori RA, Mahmood K, Anwer N, Khan SU, et al. Cefaclor AF vs Clarithromycin in acute exacerbation of chronic bronchitis (B3M‐PK‐AJBG). Journal of the Pakistan Medical Association 2003;53(8):338‐45. [PUBMED: 14558738] [PubMed] [Google Scholar]
Kobayashi 1984 {published data only}
- Kobayashi H, Takamura K, Kono K, Onodera S, Sasaki N, Nagahama F, et al. Comparison of DL‐8280 and amoxicillin in the treatment of respiratory tract infections. Journal of the Japanese Association for Infectious Diseases 1984;58(6):525‐55. [DOI] [PubMed] [Google Scholar]
Kobbernagel 2016 {published data only}
- Kobbernagel HE, Buchvald FF, Haarman EG, Casaulta C, Collins SA, Hogg C, et al. Study protocol, rationale and recruitment in a European multi‐centre randomized controlled trial to determine the efficacy and safety of azithromycin maintenance therapy for 6 months in primary ciliary dyskinesia. BMC Pulmonary Medicine 2016;16(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krawczyk 1981 {published data only}
- Krawczyk Z, Gornicka WM, Pilarska MA, Pruszynska S. Comparative evaluation of certain antibiotics and Biceptol preparation in the treatment of chronic bronchial disease exacerbations. Polski Tygodnik Lekarski 1981;36(25):927‐30. [PubMed] [Google Scholar]
Lioberes 1990 {published data only}
- Lioberes P, Barber E, Montserrat JM, Picado C. Intermittent antibiotic treatment in patients with stable bronchiectasis: a comparative study between amoxycillin and cefonicid. European Respiratory Journal 1990, (Suppl 10):88S. [Google Scholar]
Liu 2012 {unpublished data only}
- Liu JF, Zhong XN, He ZY, Zhong DJ, Bai J, Zhang JQ, et al. Impact of treatment with low dose roxithromycin on stable bronchiectasis. Chinese Journal of Tuberculosis and Respiratory Diseases 2012;35(11):824‐7. [PubMed] [Google Scholar]
Mehta 1991 {published data only}
- Mehta AP, Phutane L, Patel MH, Rupwate RU, Kamat SR. Comparative study of amoxycillin and amoxycillin/clavulanic acid in lower respiratory infections. Journal of the Association of Physicians of India 1991;39(3):251‐3. [PUBMED: 1880092] [PubMed] [Google Scholar]
Nakamura 2007 {unpublished data only}
- Nakamura M, Hasegawa N, Miyao N, Nakajima T, Terashima T, Sakamaki F, et al. Comparison of 5 day short‐course therapies for secondary infection in patients with chronic respiratory disease using gatifloxacin and levofloxacin. Japanese Journal of Chemotherapy 2007;55(6):451‐62. [Google Scholar]
NCT03058718 {published data only}
- NCT03058718. Effectiveness of procalcitonin‐guided antibiotic therapy in acute exacerbations of bronchiectasis: a randomized controlled trial. clinicaltrials.gov/show/NCT03058718 (first received 23 February 2017).
NCT03093974 {published data only}
- NCT03093974. Trial in non‐cystic fibrosis bronchiectasis patients with chronic lung infections treated with colistimethate sodium (PROMIS‐I) [A double‐blind placebo‐controlled multicentre clinical trial to investigate the efficacy and safety of 12 months of therapy with inhaled colistimethate sodium in subjects with non‐CF bronchiectasis chronically infected with P.Aeruginosa]. clinicaltrials.gov/ct2/show/record/NCT03093974 (first received 28 March 2017).
Pines 1964 {published data only}
- Pines A, Plucinski K, Greenfield JSB, Mitchell RC. Controlled comparison of lymecycline with tetracycline hydrochloride in exacerbations of chronic bronchitis. British Medical Journal 1964;2(5423):1495‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pines 1967 {published data only}
- Pines A, Raafat H, Plucinski K, Greenfield JSB, Linsell WD. Cephaloridine compared with penicillin and streptomycin in chronic purulent bronchitis: controlled trials of increasing dosage of cephaloridine. British Journal of Diseases of the Chest 1967;61(2):101‐10. [DOI: ] [DOI] [PubMed] [Google Scholar]
Pines 1981 {published data only}
- Pines A, Raafat H, Khorasani M, Mullinger BM. Cefuroxime and ampicillin compared in a double‐blind study in the treatment of lower respiratory tract infections. Chemotherapy 1981;27(6):459‐65. [PUBMED: 7028411] [DOI] [PubMed] [Google Scholar]
Ramer 1981 {published data only}
- Ramer P, Medici TC, Hatt RR, Unger S, Ronner B. [Trimethoprim‐sulfamethoxazole versus amoxycillin in the therapy of acute bacterial exacerbations in chronic non‐specific respiratory tract diseases. A controlled study] [Trimethoprim‐sulfamethoxazol versus amoxycillin in der therapie akuter bakterieller exazerbationen bei chronischen unspezifischen atemwegerkrankungen. eine kontrollierte Studie.]. Schweizerische Medizinische Wochenschrift 1981;111(48):1823‐9. [PUBMED: 7034198] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lam 1986 {published data only}
- Lam WK, Chau PY, So SY, Leung YK, Chan JC, Sham MK. A double‐blind randomized study comparing ofloxacin and amoxicillin in treating infective episodes in bronchiectasis. Infection 1986;14(Suppl 4):S290‐2. [PUBMED: 3546147] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Chang 2013 {published data only}
- Chang AB, Grimwood K, Robertson CF, Wilson AC, Asperen PP, O'Grady KAF, et al. Antibiotics for bronchiectasis exacerbations in children: rationale and study protocol for a randomised placebo‐controlled trial. Trials 2012;13(1):156‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AB, Grimwood K, Wilson AC, Asperen PP, Byrnes CA, O'Grady KAF, et al. Bronchiectasis exacerbation study on azithromycin and amoxycillin‐clavulanate for respiratory exacerbations in children (BEST‐2): study protocol for a randomized controlled trial. Trials 2013;14(1):53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Araújo 2018
- Araújo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon TC, et al. The independent contribution of Pseudomonas aeruginosa infection to long‐term clinical outcomes in bronchiectasis. European Respiratory Journal 2018;52(2):pii. [DOI] [PubMed] [Google Scholar]
Chalmers 2012
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. [DOI] [PubMed] [Google Scholar]
Chang 2002
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ, Working Group on Indigenous Paediatric Respiratory Health. Bronchiectasis in indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200‐4. [PUBMED: 12175325] [DOI] [PubMed] [Google Scholar]
Chang 2003
- Chang AB, Masel JP, Boyce NC, Wheaton G, Torzillo PJ. Non‐CF bronchiectasis: clinical and HRCT evaluation. Pediatric Pulmonology 2003;35(6):477‐83. [DOI] [PubMed] [Google Scholar]
Chang 2010
- Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes P, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. Medical Journal of Australia 2010;193(6):356‐65. [DOI] [PubMed] [Google Scholar]
Cole 1984
- Cole PJ. A new look at the pathogenesis and management of persistent bronchial sepsis: a Viscious Cycle hypothesis and its logical therapeutic connotations. In: Davies RJ editor(s). Strategies for the Management of Chronic Bronchial Sepsis. Oxford: Medicine Publishing Foundation, 1984. [Google Scholar]
Cole 1986
- Cole PJ. Inflammation: a two‐edged sword‐‐the model of bronchiectasis. European Journal of Respiratory Diseases 1986;147:6–15. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9. Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Donovan 2018
- Donovan T, Felix LM, Chalmers JD, Milan SJ, Mathioudakis AG, Spencer S. Continuous versus intermittent antibiotics for bronchiectasis. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD012733] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eastham 2004
- Eastham KM, Fall AJ, Mitchell L, Spencer DA. The need to re‐define non‐cystic fibrosis bronchiectasis in childhood. Thorax 2004;59(4):324‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1996
- Evans SA, Turner SM, Bosch BJ, Hardy CC, Woodhead MA. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. European Respiratory Journal 1996;9(8):1601‐4. [DOI] [PubMed] [Google Scholar]
Evans 2003
- Evans DJ, Greenstone M. Long‐term antibiotics in the management of non‐CF bronchiectasis ‐ do they improve outcome?. Respiratory Medicine 2003;97(7):851‐8. [DOI] [PubMed] [Google Scholar]
Felix 2018
- Felix LM, Grundy S, Milan SJ, Armstrong R, Harrison H, Lynes D, et al. Dual antibiotics for bronchiectasis. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD012514] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 2015
- Finch S, McDonnell MJ, Abo‐Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Annals of the American Thoracic Society 2015;12(11):1602‐11. [DOI] [PubMed] [Google Scholar]
Goeminne 2012
- Goeminne PC, Scheers H, Decraene A, Seys S, Dupont LJ. Risk factors for morbidity and death in non‐cystic fibrosis bronchiectasis: a retrospective cross‐sectional analysis of CT diagnosed bronchiectatic patients. Respiratory Research 2012;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goeminne 2016
- Goeminne PC, Soyza A. Bronchiectasis: how to be an orphan with many parents?. European Respiratory Journal 2016;47(1):10‐3. [DOI: 10.1183/13993003.01567-2015] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 28 July 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Habesoglu 2011
- Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clinical, radiologic, and functional evaluation of 304 patients with bronchiectasis. Annals of Thoracic Medicine 2011;6(3):131‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2015
- Hansen MP, Thorning S, Aronson JK, Beller EM, Glasziou PP, Hoffmann TC, et al. Adverse events in patients taking macrolide antibiotics versus placebo for any indication. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 1988
- Hill SL, Burnett D, Hewetson KA, Stockley RA. The response of patients with purulent bronchiectasis to antibiotics for four months. Quarterly Journal of Medicine 1988;66(250):163‐73. [PubMed] [Google Scholar]
Hill 2017
- Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. European Respiratory Journal 2017;49(6):1700051. [PUBMED: 28596426] [DOI] [PubMed] [Google Scholar]
Joish 2013
- Joish VN, Spilsbury‐Cantalupo M, Operschall E, Luong B, Boklage S. Economic burden of non‐cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Applied Health Economics and Health Policy 2013;11(3):299‐304. [DOI: 10.1007/s40258-013-0027-z] [DOI] [PubMed] [Google Scholar]
Kapur 2012
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non‐cystic fibrosis bronchiectasis. Chest 2012;141(4):1018‐24. [PUBMED: 21885727] [DOI] [PubMed] [Google Scholar]
Kelly 2018
- Kelly C, Chalmers JD, Crossingham I, Relph N, Felix LM, Evans DJ, et al. Art. No.: CD012406. DOI:. Macrolide antibiotics for bronchiectasis. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012406.pub2; CD012406] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loni 2015
- Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, Soyza A, et al. Etiology of non‐cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Annals of the American Thoracic Society 2015;12(12):1764‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martínez García 2007
- Martínez‐García MA, Soler‐Cataluña JJ, Perpiñá‐Tordera M, Román‐Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132(5):1565‐72. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2016
- Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: a cross‐sectional study. PLoS Medicine 2016;13(5):e1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasteur 2010
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non‐CF Guideline Group. British Thoracic Society guideline for non‐CF bronchiectasis. Thorax 2010;65(Suppl 1):i1–58. [DOI] [PubMed] [Google Scholar]
Polverino 2017
- Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE. Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. European Respiratory Journal 2017;50(3):1‐23. [DOI] [PubMed] [Google Scholar]
Quint 2016
- Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. European Respiratory Journal 2016;47(1):186‐93. [DOI: 10.1183/13993003.01033-2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2010
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine 2010;104(7):981‐5. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seitz 2010
- Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis‐associated hospitalizations in the United States, 1993‐2006. Chest 2010;138(4):944‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seitz 2012
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000‐2007. Chest 2012;142(2):432‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singleton 2000
- Singleton R, Morris A, Reading G, Poll J, Holck P, Martinez P, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatric Pulmonology 2000;29(3):182‐7. [DOI] [PubMed] [Google Scholar]
Spencer 2018
- Spencer S, Felix LM, Milan SJ, Normansell R, Geominne PC, Chalmers JD. Oral versus inhaled antibiotics for bronchiectasis. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012579] [DOI] [PMC free article] [PubMed] [Google Scholar]
Twiss 2005
- Twiss J, Metcalfe R, Edwards E, Byrnes C. New Zealand national incidence of bronchiectasis "too high" for a developed country. Archives of Disease in Childhood 2005;90(7):737‐40. [PUBMED: 15871981] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valery 2012
- Valery P, Morris P, Grimwood K, Torzillo P, Byrnes C, Masters IB, et al. Azithromycin for Indigenous children with bronchiectasis: study protocol for a multi‐centre randomized controlled trial. BMC Paediatrics 2012;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welsh 2015
- Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD010337.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weycker 2005
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205–9. [Google Scholar]
Wilson 1997
- Wilson CB, Jones PW, O'Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. European Respiratory Journal 1997;10(8):1754‐60. [DOI] [PubMed] [Google Scholar]
Wilson 2013
- Wilson R, Hansell DM, Loebinger MR. Definition and aetiology of non‐CF bronchiectasis. In: Blasi F, Miravitlles M editor(s). The Spectrum of Bronchial Infection. Sheffield: European Respiratory Society, 2013. [Google Scholar]
Wu 2014
- Wu Q, Shen W, Cheng H, Zhou X. Long‐term macrolides for non‐cystic fibrosis bronchiectasis: a systematic review and meta‐analysis. Respirology 2014;19(3):321–9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kaehne 2017
- Kaehne A, Milan SJ, Felix LM, Spencer S, Sheridan E, Marsden PA. Head‐to‐head trials of antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012590] [DOI] [PMC free article] [PubMed] [Google Scholar]


