Abstract
Background
Several psychoactive medications are known to cause QTc prolongation. Patient factors also increase the risk for QTc prolongation, including bradycardia, female sex, older age, metabolic abnormalities, and polypharmacy. Donepezil, a cholinesterase inhibitor, prolongs the QTc interval through a multimodal mechanism.
Patient History
A 26-year-old African American female was admitted to the inpatient psychiatric hospital following a suicide attempt that was not an overdose. Past medical history was significant for major depression, traumatic brain injury, seizures, hemiplegia, gastroesophageal reflux disease, and tachycardia. Two baseline electrocardiograms (EKGs) were obtained showing normal QTc intervals. After several weeks, donepezil (5 mg by mouth once daily) was initiated for cognitive rehabilitation and titrated over 3 weeks to a dose of 20 mg. An EKG performed after the last dose change showed a prolonged QTc of 463 ms. Another follow-up EKG performed 9 days later showed further prolongation to 528 ms. Laboratory values were within normal limits during her hospital stay. Donepezil was discontinued completely, leading to normalization of the QTc interval.
Discussion
QTc prolongation and torsades de pointes have been identified in postmarketing case reports of donepezil. Instances of QTc prolongation have predominantly been documented in the geriatric population, primarily in those with additional risk factors. Additionally, current literature does not support the use of donepezil for neurocognitive rehabilitation in daily doses exceeding 10 mg. A temporal and causal relationship was observed between the initiation and titration of donepezil and development of QTc prolongation.
Keywords: donepezil, QTc prolongation, electrocardiogram, neurocognitive rehabilitation
Background
QTc prolongation can increase the risk of torsades de pointes (TdP), which may lead to the development of ventricular fibrillation, cardiac arrest, and sudden death. A prolonged QTc interval is defined as >480 ms in women and >460 ms in men although this definition varies by source.1 Risk of developing TdP increases significantly with QTc intervals >500 ms.1 Patient risk factors for QTc prolongation include bradycardia, structural heart disease, female sex, older age (>65 years), metabolic abnormalities, traumatic brain injury (TBI), and concomitant QTc-prolonging agents.1
Drug-induced QTc prolongation is the most common cause of QTc prolongation.2 Medications that increase the QTc interval are thought to do so through their ability to inhibit or interfere with delayed rectifier potassium channels.3 Several psychoactive medications are known to cause QTc prolongation, including antidepressants, antipsychotics, and cholinesterase inhibitors.3,4
Since its approval in 1996 for use in Alzheimer disease (AD), there have been postmarketing reports of QTc prolongation and development of TdP with donepezil use.5 However, most published cases6-11 have been in older adults with additional risk factors, including structural heart disease and concomitant QTc-prolonging drugs. We report a case of suspected donepezil-induced QTc prolongation in a 26-year-old female patient with a history of TBI. Currently available case reports6-11 are limited to individuals over the age of 65. Additionally, use of donepezil for cognitive rehabilitation following TBI is considered off label. To our knowledge, no case reports of QTc prolongation with donepezil use have been documented in the TBI population or those of younger age.
Case Report
The patient was a 26-year-old African American female admitted to the inpatient psychiatric hospital after a suicide attempt by means that were not an overdose. Past medical history was significant for major depressive disorder, TBI, seizures, asthma, dysarthria, hemiplegia, gastroesophageal reflux disease, constipation, and tachycardia. Her social history was noncontributory. She was initially continued on her previous outpatient medications, including quetiapine 100 mg in the morning, 200 mg at noon, and 300 mg at bedtime for mood stabilization; divalproex sodium extended-release 500 mg twice daily for mood stabilization and history of seizures; metoprolol extended-release 25 mg daily for tachycardia; montelukast 10 mg daily for asthma; polyethylene glycol-3350 17 g daily for constipation; calcium with vitamin D supplement daily for nutritional deficiency; pantoprazole 40 mg daily for gastroesophageal reflux disease; and cephalexin 500 mg 4 times daily for cellulitis. Two baseline electrocardiograms (EKGs) were obtained on admission. The first showed a QTc of 425 ms with T-abnormality in the inferior lead. The second showed a QTc of 438 ms. She was noted to be in sinus tachycardia with a heart rate of 112 beats/min (bpm) during both reads.
During the few weeks following admission, several medication changes were made, including a significant dose reduction of quetiapine to 50 mg 3 times daily due to daytime sedation and a 7-day trial of sertraline 50 mg, which was discontinued due to increased agitation. On hospital day (HD) 35, donepezil 5 mg daily was initiated for cognitive rehabilitation due to TBI. An EKG performed 5 days after donepezil initiation (HD 40) showed sinus rhythm, a normal QTc of 423 ms and a heart rate of 97 bpm. Relevant scheduled medications at this time included quetiapine 50 mg 3 times daily, donepezil 5 mg at bedtime, and pantoprazole 40 mg in the morning. Approximately 7 days after donepezil initiation (HD 42), donepezil was increased to 5 mg twice daily. Five days later (HD 47), the bedtime dose was titrated to 10 mg for a total of 15 mg daily. The donepezil dose was further titrated to 10 mg twice daily 8 days later (HD 56). There was no documentation of gastrointestinal side effects during this time. A repeat EKG performed on HD 68 showed sinus rhythm, an increased QTc of 463 ms, and heart rate of 70 bpm. At the time of this EKG, she was taking donepezil 10 mg twice daily, pantoprazole 40 mg in the morning, and quetiapine 50 mg twice daily. Notable as needed (PRN) medications during the week prior to this EKG included quetiapine 25 mg (4 doses total with 2 doses given on HD 66), which was administered for agitation and aggression.
A follow-up EKG on HD 77 revealed a prolonged QTc of 528 ms with a heart rate of 81 bpm and sinus rhythm noted. Her quetiapine dose was increased back to 50 mg 3 times daily the day prior. Donepezil 10 mg twice daily and pantoprazole 40 mg in the morning had been continued at the same doses. Notable PRN medications during the week prior to this EKG included quetiapine 25 mg (5 doses total with 2 given on HD 76) and 1 PRN dose of both quetiapine 100 mg and olanzapine 10 mg. At this point, donepezil was reduced to 10 mg in the morning, and she continued to take scheduled quetiapine 50 mg 3 times daily.
On HD 81 an EKG showed the patient was in sinus rhythm; however, QTc remained prolonged at 496 ms, heart rate 95 bpm. At this time, an adverse drug reaction was reported to the pharmacy. Notable PRN medications in the days preceding this EKG included a single dose of oral quetiapine 25 mg and intramuscular diphenhydramine 50 mg for acute agitation. Donepezil was discontinued completely. She continued to take quetiapine and pantoprazole during this time. Due to the half-life of donepezil, a follow-up EKG was performed 15 days later with a normal QTc of 416 ms. From available labs, there was no evidence of electrolyte abnormalities during her hospital admission. Potassium levels remained within normal limits; however, there were no magnesium concentrations on record. A hospital course summary is outlined in the Figure.
FIGURE.
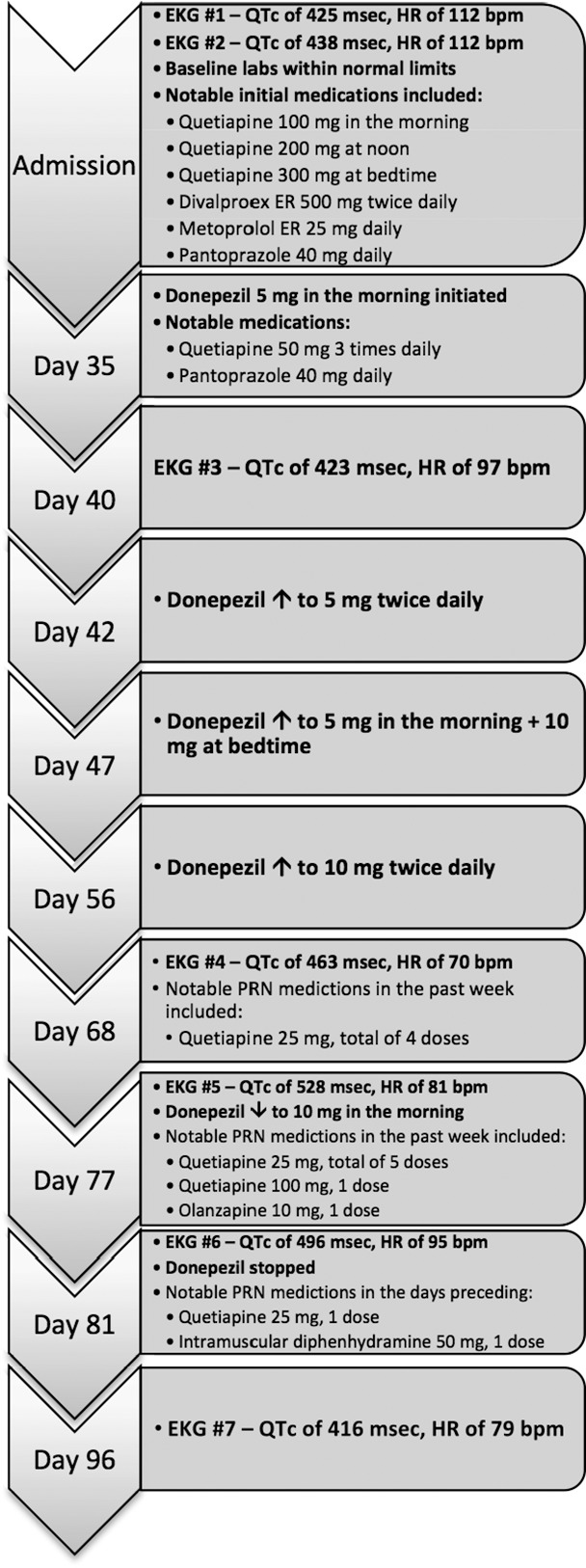
Hospital course and timeline of donepezil-induced QTc prolongation (bpm = beats/min; EKG = electrocardiogram; ER = extended release; HR = heart rate; PRN = as needed)
Discussion
A literature search was conducted to identify current reports of QTc prolongation associated with donepezil use. A PubMED search was conducted using the keywords donepezil, QTc prolongation, cardiac, torsades de pointes, and arrhythmia. Approval by the facility privacy officer was obtained.
Donepezil, pantoprazole, and quetiapine are known to potentially contribute to QTc prolongation. QTc prolongation and TdP have been identified in postmarketing reports of donepezil.5,12-14 Donepezil is on the CredibleMeds list of medications12 with known risk of TdP, and pantoprazole and quetiapine are listed as a conditional risk, meaning that there is no convincing evidence that these medications cause TdP or pathological QTc prolongation unless certain clinical conditions are present. Furthermore, diphenhydramine and olanzapine are also listed as medications with a conditional risk for TdP.12 Quetiapine has a moderate-to-high risk of QTc prolongation compared with most antipsychotics with the exception of thioridazine, iloperidone, and ziprasidone.13 Studies and case series have demonstrated that risk of QTc prolongation with quetiapine is minimal when used within recommended therapeutic doses.14 However, this minimal risk has only been demonstrated in patients without other risk factors for QTc prolongation.14 There is limited evidence regarding effects on the QTc interval with intermittent doses of quetiapine, olanzapine, or diphenhydramine.
Donepezil is a cholinesterase inhibitor prescribed for the treatment of AD.5 The donepezil package insert5 recommends that patients tolerate the 5 mg dose daily for 4 to 6 weeks before titrating to 10 mg daily due to the incidence of adverse gastrointestinal events. It is not known if the rate at which donepezil is titrated correlates with cardiovascular side effects. Furthermore, the package insert5 recommends patients tolerate donepezil 10 mg daily for 3 months before increasing the dose further (up to 23 mg). Donepezil is currently only approved for the treatment of AD.1 Because acetylcholine pathways play an important role in neurological recovery following injury, donepezil has also been studied for neurocognitive rehabilitation following TBI.15 Per our literature review, the results are variable for the available studies. Donepezil has only been studied up to 10 mg daily for this use, and dosing regimens were diverse between case reports and studies.15
There is limited information regarding the cardiovascular adverse effects of donepezil; however, donepezil is known to cause bradycardia through its cholinergic effect on vagal tone at the sinoatrial and atrioventricular nodes. Donepezil has also demonstrated inhibition of the rapid delayed rectifier potassium channel in addition to its ability to interfere with potassium channel trafficking.3,4 Case reports on donepezil use in the elderly population have demonstrated significant prolongations of the QTc interval even at recommended doses. Three cases of elderly female patients documented prolonged QTc interval with use of donepezil 5 mg daily with 2 of the 3 cases progressing to TdP.8,10 Two additional case reports7,9 of QTc prolongation occurred in an elderly male and female after donepezil was increased from 5 mg to 10 mg daily with TdP and atrioventricular block occurring in each case, respectively. The Table summarizes published reports on QTc prolongation with donepezil use.6-11
TABLE.
Available evidence for EKG changes with donepezil
|
Study |
Study Characteristics |
Donepezil Dose Intervention |
EKG Findings |
||
|
Study Design |
Patient Population |
Potential Patient Risk Factors |
|||
| Pourmad et al17 (2017) | Case report | 84-year-old male | Old age, possible structural heart disease | 35 mg (accidental ingestion) | QTc = 502 ms |
| Kitt et al7 (2015) | Case report | 80-year-old female | Old age, female, atrial fibrillation, polypharmacy | 5 mg increased to 10 mg for 2 wk | QTc = 490 ms TdP occurred despite dose reduction |
| Igeta et all11 (2014) | Prospective cohort | N = 18 (80% male) Mean age = 74 years old | Old age | 5-10 mg daily for an average of 4 mo | Prolonged PR interval; no change in QTc interval |
| Shinozaki8 (2012) | Case report | 80-year-old female | Old age, female, polypharmacy | 5 mg daily | QTc = 470 ms |
| Tanaka et al10 (2009) | Case report | 90-year-old male | Old age | 5 mg increased to 10 mg for 3 d | QTc = 514 ms Atrioventricular block |
| Takaya et al9 (2009) | Case report | 87-year-old female | Old age, female, possible structural heart disease, atrial fibrillation, bradycardia, polypharmacy | 5 mg daily | QTc #1 = 461 ms QTc #2 (1 mo later) = 594 ms TdP occurred |
| Case report | 83-year-old female | Old age, female, structural heart disease, atrial fibrillation, electrolyte abnormality | 5 mg daily | QTc = 645 ms TdP occurred | |
EKG = electrocardiogram; PR = P-R interval on an EKG; TdP = torsades de pointes.
The patient in this case had a QTc within normal range on admission while taking quetiapine 600 mg. Her quetiapine dose was reduced prior to initiation of donepezil. After initiation of donepezil at 5 mg in the morning, her QTc was within normal range. Following rapid titration of donepezil to a total daily dose of 20 mg (without documented gastrointestinal side effects), QTc prolongation was found on EKG. Her QTc improved following a dose reduction of donepezil to 10 mg daily, and her QTc eventually normalized following discontinuation of donepezil. The patient's QTc remained within normal range while taking quetiapine and pantoprazole.
The Naranjo Adverse Drug Reactions Probability Scale provides a score of 5, indicating this event is a probable adverse event resulting from donepezil use.16 Per the review of the data and this patient's history, it is possible that use of higher doses of donepezil in association with other patient risk factors for QTc prolongation (female sex, concomitant QTc-prolonging drugs, TBI, and use of medications that may cause bradycardia) contributed to this adverse event. Although there is no current data supporting that EKG changes are related to the rate of titration, it cannot be ruled out that a slower titration of donepezil may have helped prevent QTc prolongation despite concomitant use of quetiapine and pantoprazole. Although her heart rate at the time of the EKG finding was normal, it was significantly lower following donepezil titration compared with initial EKG readings on admission (a difference of 42 bpm). Furthermore, current data do not support donepezil doses greater than 10 mg for cognitive enhancement due to TBI.
Conclusion
Although there are potential confounding factors, including polypharmacy and inherent patient risk factors, such as TBI and female sex, we were able to identify a temporal and causal relationship between the initiation and titration of donepezil and the development of QTc prolongation. Reduction of donepezil dose resulted in shortening of the QTc interval and normalization with discontinuation. Clinicians should be aware that donepezil has the potential to cause life-threatening cardiac effects even at recommended doses. Providers and pharmacists should be cautious when initiating donepezil in individuals with risk factors for QTc prolongation, including patients with TBI. Additionally, practitioners should adhere to the recommended titration schedule when increasing beyond initial starting doses. Cardiac monitoring should be considered at baseline and following initiation or dose increases of donepezil in all patients but especially patients with multiple risk factors for QTc prolongation.
References
- 1.Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047–60. doi: 10.1161/CIRCULATIONAHA.109.192704. DOI: 10.1161/CIRCULATIONAHA.109.192704 PubMed PMID: 20142454 PubMed Central PMCID: PMC3056123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Weinacker A, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality. Crit Care Med. 2012;40(2):394–9. doi: 10.1097/CCM.0b013e318232db4a. DOI: 10.1097/CCM.0b013e318232db4a PubMed PMID: 22001585. [DOI] [PubMed] [Google Scholar]
- 3.Vieweg WVR. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Companion J Clin Psychiatry. 2003;5(5):205–15. doi: 10.4088/pcc.v05n0504. DOI: 10.4088/PCC.v05n0504 PubMed PMID: 15213787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubeddu L. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev. 2016;12(2):141–54. doi: 10.2174/1573403X12666160301120217. DOI: 10.2174/1573403x12666160301120217 PubMed PMID: 26926294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jublient Cadista Pharmaceuticals Inc. DailyMed [Internet] Bethesda (MD): National Library of Medicine (US); DONEPEZIL HYDROCHLORIDE (donepezil hydrochloride) tablet, film coated. 1996 [rev. 2016 Feb; cited 2018 Jun 19] Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0baef0fd-e6c0-4ee8-bb7e-1d2e89d6e0ec. [Google Scholar]
- 6.Pourmand A, Shay C, Redha W, Aalam A, Mazer-Amirshahi M. Cholinergic symptoms and QTc prolongation following donepezil overdose. Am J Emerg Med. 2017;35(9):1386.e1–1386.e3. doi: 10.1016/j.ajem.2017.06.044. DOI: 10.1016/j.ajem.2017.06.044 PubMed PMID: 28668178. [DOI] [PubMed] [Google Scholar]
- 7.Kitt J, Irons R, Al-Obaidi M, Missouris C. A case of donepezil-related torsades de pointes. BMJ Case Rep. 2015:bcr2015211900. doi: 10.1136/bcr-2015-211900. DOI: 10.1136/bcr-2015-211900 PubMed PMID: 26438681 PubMed Central PMCID: PMC4600768. [DOI] [PMC free article] [PubMed]
- 8.Shinozaki K. Shortening of donepezil-induced QTc prolongation with a change in the interacting drug, after electrocardiograph monitoring by community pharmacists: a case report. Yakugaku Zasshi. 2012;132(2):237–41. doi: 10.1248/yakushi.132.237. [DOI] [PubMed] [Google Scholar]
- 9.Takaya T, Okamoto M, Yodoi K, Hata K, Kijima Y, Nakajima H, et al. Torsades de pointes with QT prolongation related to donepezil use. J Cardiol. 2009;54(3):507–11. doi: 10.1016/j.jjcc.2009.03.011. DOI: 10.1016/j.jjcc.2009.03.011 PubMed PMID: 19944332. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka A, Koga S, Hiramatsu Y. Donepezil-induced adverse side effects of cardiac rhythm: 2 cases report of atrioventricular block and torsade de pointes. Intern Med. 2009;48(14):1219–23. doi: 10.2169/internalmedicine.48.2181. DOI: 10.2169/internalmedicine.48.2181 PubMed PMID: 19602789. [DOI] [PubMed] [Google Scholar]
- 11.Igeta H, Suzuki Y, Tajiri M, Someya T. Cardiovascular pharmacodynamics of donepezil hydrochloride on the PR and QT intervals in patients with dementia. Hum Psychopharmacol Clin Exp. 2014;29(3):292–4. doi: 10.1002/hup.2398. DOI: 10.1002/hup.2398 PubMed PMID: 24615803. [DOI] [PubMed] [Google Scholar]
- 12.Woosley RL, Romero KA. [Internet] Oro Valley (AZ): AZCERT; CredibleMeds: risk categories for drugs that prolong QT & induce torsades de pointes (TdP) 2013 [updated 2018 May 20; cited 2018 Jun 19]. Available from: https://www.crediblemeds.org/new-drug-list/ [Google Scholar]
- 13.Wenzel-Seifert K, Wittmann M, Haen E. QTc prolongation by psychotropic drugs and the risk of torsade de pointes. Dtsch Arztebl Int. 2011;108(41):687–93. doi: 10.3238/arztebl.2011.0687. DOI: 10.3238/arztebl.2011.0687 PubMed PMID: 22114630 PubMed Central PMCID: PMC3221427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasnain M, Vieweg WVR, Howland RH, Kogut C, Breden Crouse EL, Koneru JN, et al. Quetiapine, QTc interval prolongation, and torsade de pointes: a review of case reports. Ther Adv Psychopharmacol. 2014;4(3):130–8. doi: 10.1177/2045125313510194. DOI: 10.1177/2045125313510194 PubMed PMID: 25057346 PubMed Central PMCID: PMC4107702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballesteros J, Güemes I, Ibarra N, Quemada JI. The effectiveness of donepezil for cognitive rehabilitation after traumatic brain injury: a systematic review. J Head Trauma Rehabil. 2008;23(3):171–80. doi: 10.1097/01.HTR.0000319935.99837.96. DOI: 10.1097/01.HTR.0000319935.99837.96 PubMed PMID: 18520431. [DOI] [PubMed] [Google Scholar]
- 16.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


