Abstract
Objective:
Concomitant treatment with angiotensin-converting enzyme (ACE) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors is increasingly common. Pharmacological studies have suggested a potential adverse drug interaction between ACE inhibitors and DPP-4 inhibitors resulting in unfavorable hemodynamic changes; very few studies have examined such an interaction between angiotensin II receptor blockers (ARBs) and DPP-4 inhibitors. We investigated blood pressure (BP) and heart rate (HR) during treatment with the DPP-4 inhibitor linagliptin in individuals receiving either ACE inhibitors or ARBs in the MARLINA-T2D trial.
Methods:
In this study, 360 individuals with type 2 diabetes and albuminuria receiving unchanged doses of ACE inhibitors or ARBs were randomized to linagliptin or placebo. Twenty-four-hour ambulatory BP monitoring, an exploratory endpoint, was conducted at baseline and after 24 weeks.
Results:
Ambulatory BP monitoring data were available for 208 individuals (linagliptin: n = 111; placebo: n = 97). Baseline mean ± SD 24-h SBP and DBP were 132.5 ± 12.4 mmHg and 75.9 ± 9.4 mmHg, respectively; mean 24-h HR was 76.3 ± 10.1 bpm. At week 24, no overall effect of the DPP-4 inhibitor versus placebo was seen on mean 24-h SBP, DBP, or HR. Furthermore, in the subgroups receiving either an ACE inhibitor or an ARB, no effect on these hemodynamic parameters was seen as a result of concomitant DPP-4 inhibitor treatment.
Conclusion:
Adding linagliptin to treatment with ACE inhibitors or ARBs was not associated with any hemodynamic changes, supporting their concomitant use in individuals with type 2 diabetes and albuminuria.
Keywords: ambulatory, angiotensin-converting enzyme inhibitors, AT1 antagonists, blood pressure, blood pressure monitoring, dipeptidyl-peptidase IV inhibitors, drug interaction, heart rate
INTRODUCTION
Diabetes affects an estimated 30 million people in the United States [1] and 415 million individuals globally [2], most commonly type 2 diabetes (T2D) (90–95% of cases [1]). T2D is frequently associated with conditions such as hypertension, chronic kidney disease and heart failure [3–5], which are often treated with drugs that interrupt the renin–angiotensin system (RAS), including angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs).
Consequently, as a result of the epidemic of T2D and its associated comorbidities, a large number of individuals currently receive concomitant treatment with RAS blockers such as ACE inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors, a class of frequently prescribed oral glucose-lowering drugs [6]. DPP-4 inhibitors reduce blood glucose levels mainly by inhibiting enzymatic degradation of glucagon-like peptide-1, a major substrate of DPP-4 that stimulates glucose-dependent insulin secretion. However, DPP-4 also has other peptide substrates, some of which are vasoactive such as substance P. Furthermore, DPP-4 is largely responsible for the inactivation of substance P when ACE is inhibited [7,8].
An adverse hemodynamic interaction between DPP-4 inhibitors and ACE inhibitors has been postulated based on pharmacological studies in which increases in blood pressure (BP) and heart rate (HR) manifested during concomitant treatment with ACE inhibitors and experimental (P32/98) or licensed DPP-4 inhibitors (sitagliptin) [9,10]. The need for further studies in this area was highlighted recently [11]. Furthermore, there appears to be little data on potential hemodynamic interactions between DPP-4 inhibitors and ARBs. Consequently, we explored the potential hemodynamic effects of another licensed DPP-4 inhibitor, linagliptin, in individuals with T2D and renal disease as reflected by the presence of microalbuminuria or macroalbuminuria who were receiving concomitant treatment with ACE inhibitors or ARBs.
METHODS
This was a prespecified, exploratory analysis of a 24-week, randomized, double-blind, placebo-controlled, multinational clinical trial of linagliptin conducted in individuals with T2D and renal dysfunction (MARLINA-T2D; ClinicalTrials.gov: NCT01792518).
MARLINA-T2D was designed to investigate the glycemic and albuminuric effects of linagliptin; the full study methodology and main results are described in detail elsewhere [12,13]. In brief, individuals with T2Ds were eligible to participate if they were aged 18–80 years with glycated hemoglobin A1c levels of 6.5–10.0%, BMI of 40 kg/m2 or less, estimated glomerular filtration rate (eGFR) at least 30 ml/min per 1.73 m2, and were either drug-naïve or receiving up to two oral glucose-lowering drugs and/or basal insulin. Individuals were also required to have albuminuria, manifesting as urinary albumin-to-creatinine ratio (UACR) of 30–3000 mg/g, or albuminuria more than 30 mg/l of urine, or urinary albumin excretion rate more than 30 μg/min clearly documented in the previous 12 months or detected at screening. Albuminuria had to be confirmed prior to randomization with a geometric mean UACR of 30–3000 mg/g from three consecutive morning urine samples collected 14–16 days before randomization. Eligible individuals must also have been receiving a stable (unchanged) dose of an ACE inhibitor or ARB for at least the ten preceding weeks.
Following a two-week placebo run-in period, participants were randomized to receive double-blind, once-daily oral treatment with linagliptin 5 mg or placebo for 24 weeks. In an exploratory analysis, changes from baseline in mean 24-h SBP, mean 24-h DBP, and mean 24-h HR – all recorded using ambulatory BP monitoring (ABPM) – were assessed at baseline and after 24 weeks of treatment. These endpoints were analyzed in the treated set of participants (those who received at least one dose of study drug) using an analysis of covariance. ABPM values measured after the start of glycemic rescue therapy or after a change in antihypertensive treatment were not included in the analysis. Post-hoc analyses were conducted for changes in ethnic subgroups (Asian/non-Asian) and changes in the day or night separately; for the latter, day and night were defined as 0601–2159 h and 2200–0600 h, respectively.
Safety analyses were performed as previously described [13]. Adverse events of relevance to the current analysis were defined as those in the vascular disorders system organ class from the Medical Dictionary for Regulatory Activities (MedDRA) version 18.1, as well as hypersensitivity reactions (MedDRA 18.1 Standardized MedDRA Query), and cardiovascular events adjudicated by a blinded, external clinical event committee.
RESULTS
Of the 360 participants in the treated set, ABPM data were available at baseline for 298 individuals (linagliptin: n = 155; placebo: n = 143). At baseline, mean ± SD 24-h SBP, 24-h DBP, and 24-h HR were 132.7 ± 12.9 mmHg, 75.9 ± 9.1 mmHg and 77.3 ± 10.4 bpm, respectively (Table 1). These and other mean baseline characteristics were similar in the 208 participants (linagliptin: n = 111; placebo: n = 97) with ABPM data at both baseline and week 24 (Table 1).
TABLE 1.
Baseline demographic and clinical characteristics
| Treated seta | 24-h ABPM populationb | |||
| Linagliptin, n = 182 | Placebo, n = 178 | Linagliptin, n = 111 | Placebo, n = 97 | |
| Age (years) | 61.0 ± 10.0 | 60.1 ± 9.3 | 61.2 ± 9.7 | 60.7 ± 9.6 |
| Male, n (%) | 116 (63.7) | 113 (63.5) | 74 (66.7) | 63 (64.9) |
| Race, n (%) | ||||
| Asian | 117 (64.3) | 122 (68.5) | 79 (71.2) | 71 (73.2) |
| White | 56 (30.8) | 53 (29.8) | 29 (26.1) | 24 (24.7) |
| Black/African-American | 8 (4.4) | 3 (1.7) | 2 (1.8) | 2 (2.1) |
| Hawaiian/Pacific Islander | 1 (0.5) | 0 (0.0) | 1 (0.9) | 0 (0.0) |
| BMI (kg/m2) | 28.3 ± 4.8 | 28.6 ± 4.9 | 27.8 ± 4.5 | 28.6 ± 4.9 |
| Weight (kg) | 78.1 ± 18.6 | 77.9 ± 19.3 | 76.4 ± 17.5 | 77.5 ± 18.4 |
| HbA1c, % (mmol/mol) | 7.81 ± 0.87 (61.9 ± 9.5) | 7.87 ± 0.88 (62.5 ± 9.6) | 7.83 ± 0.80 (n/a) | 7.88 ± 0.82 (n/a) |
| Time since diagnosis of diabetes, n (%) | ||||
| ≤1 year | 11 (6.0) | 8 (4.5) | 4 (3.6) | 3 (3.1) |
| >1–5 years | 26 (14.3) | 41 (23.0) | 17 (15.3) | 23 (23.7) |
| >5–10 years | 47 (25.8) | 56 (31.5) | 27 (24.3) | 32 (33.0) |
| >10 years | 98 (53.8) | 73 (41.0) | 63 (56.8) | 39 (40.2) |
| eGFR (CKD-EPI, cystatin C) (ml/min per 1.73 m2) | 102.8 ± 49.7 | 94.4 ± 43.3 | 104.17 ± 52.53 | 92.32 ± 44.37 |
| eGFR (CKD-EPI, cystatin C) (ml/min per 1.73 m2), n (%) | ||||
| ≥90 | 98 (53.8) | 88 (49.4) | 58 (52.3) | 47 (48.5) |
| 60–<90 | 54 (29.7) | 48 (27.0) | 35 (31.5) | 28 (28.9) |
| 30–<60 | 25 (13.7) | 39 (21.9) | 15 (13.5) | 20 (20.6) |
| <30 | 5 (2.7) | 3 (1.7) | 3 (2.7) | 2 (2.1) |
| UACR (mg/g) gMean ± gCV | 120.8 ± 152.9 | 131.8 ± 165.8 | 117.1 ± 147.5 | 150.1 ± 163.9 |
| UACR (mg/g), n (%) | ||||
| <30 | 11 (6.0) | 10 (5.6) | 6 (5.4) | 4 (4.1) |
| 30–<300 | 134 (73.6) | 128 (71.9) | 85 (76.6) | 68 (70.1) |
| ≥300 | 35 (19.2) | 37 (20.8) | 20 (18.0) | 25 (25.8) |
| Missing data | 2 (1.1) | 3 (1.7) | 0 (0) | 0 (0) |
| SBP (mmHg) | 135.1 ± 13.8 | 134.6 ± 13.7 | 134.5 ± 13.6 | 134.3 ± 12.9 |
| Mean 24-h SBP (mmHg) | 131.8 ± 12.6c | 133.7 ± 13.1d | 131.6 ± 12.4 | 133.5 ± 12.3 |
| DBP (mmHg) | 77.3 ± 9.0 | 78.6 ± 8.3 | 76.0 ± 9.2 | 79.0 ± 7.6 |
| Mean 24-h DBP (mmHg) | 74.8 ± 9.1c | 77.0 ± 9.0d | 74.4 ± 9.4 | 77.5 ± 9.2 |
| Heart rate (bpm) | 75.0 ± 10.9 | 75.6 ± 10.9 | 74.1 ± 11.0 | 76.1 ± 9.7 |
| Mean 24-h heart rate (bpm) | 76.9 ± 10.4c | 77.8 ± 10.3d | 76.0 ± 10.9 | 76.6 ± 9.2 |
| Antihypertensive therapy, n (%) | 182 (100.0) | 178 (100.0) | 111 (100.0) | 97 (100.0) |
| ARBs | 120 (65.9) | 120 (67.4) | 81 (73.0) | 69 (71.1) |
| ACE inhibitors | 62 (34.1) | 58 (32.6) | 30 (27.0) | 28 (28.9) |
| Calcium antagonists | 79 (43.4) | 88 (49.4) | 54 (48.6) | 50 (51.5) |
| Diuretics | 52 (28.6) | 54 (30.3) | 29 (26.1) | 29 (29.9) |
| β-Blockers | 40 (22.0) | 47 (26.4) | 25 (22.5) | 25 (25.8) |
| Other | 11 (6.0) | 15 (8.4) | 7 (6.3) | 9 (9.3) |
| Oral glucose-lowering monotherapy, n (%) | 66 (36.3) | 67 (37.6) | 41 (36.9) | 35 (36.1) |
| Metformin | 61 (33.5) | 64 (36.0) | 37 (33.3) | 33 (34.0) |
| Oral glucose-lowering combination therapy without insulin, n (%) | 42 (23.1) | 48 (27.0) | 23 (20.7) | 25 (25.8) |
| Insulin, n (%) | 64 (35.2) | 49 (27.5) | 41 (36.9) | 27 (27.8) |
Data are mean ± SD unless otherwise stated. ABPM, ambulatory blood pressure monitoring; ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; gCV, geometric coefficient of variation; gMean, geometric mean; HbA1c, glycated hemoglobin A1c; n/a, not available; UACR, urinary albumin-to-creatinine ratio.
aAll randomized participants who received at least one dose of study drug.
bAll participants in the treated set with ABPM data at both baseline and week 24.
cn = 155.
dn = 143.
At week 24, there were no significant placebo-adjusted changes from baseline in mean 24-h SBP, 24-h DBP, or 24-h HR in individuals treated with linagliptin (Fig. 1). There was no significant difference between the predominant ethnic group (Asians) and other (non-Asian) participants in these parameters: P values for treatment interaction with race were 0.7035, 0.8149, and 0.4196 for mean change from baseline in 24-h SBP, 24-h DBP, and 24-h HR respectively (Fig. S1, Supplemental Digital Content). There was also no significant treatment difference in changes in mean 24-h SBP, 24-h DBP, or 24-h HR within the day or night periods separately (Fig. S2, Supplemental Digital Content).
FIGURE 1.
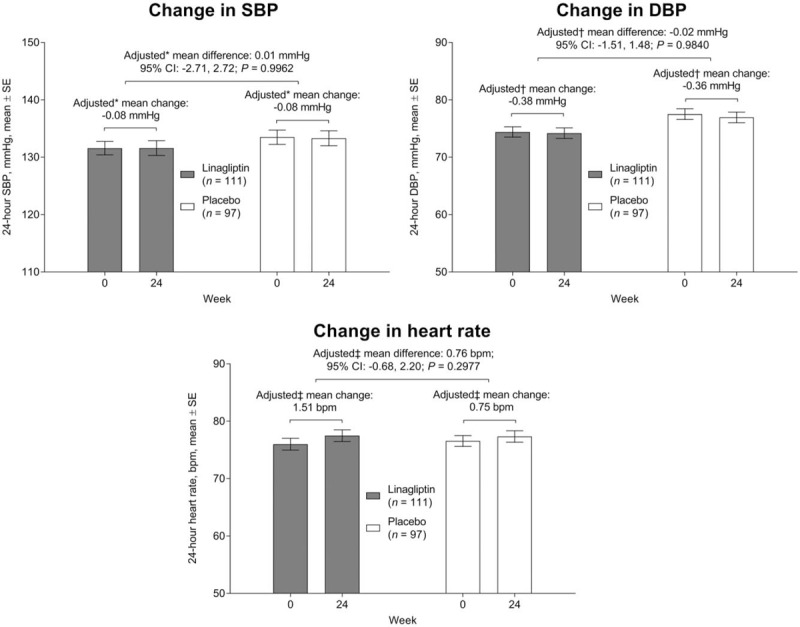
Change from baseline in mean 24-h SBP, DBP, and heart rate at week 24. ∗Analysis of covariance model includes baseline mean 24-h SBP, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment as a fixed effect, †analysis of covariance model includes baseline mean 24-h DBP, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment as a fixed effect, ‡analysis of covariance model includes baseline mean 24-h heart rate, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment as a fixed effect. CI, confidence interval.
Background antihypertensive medication was unchanged throughout the study in the large majority of participants: only 7.2 and 5.7% of the linagliptin and placebo groups, respectively, had a change in dose, and only 9.4 and 8.6%, respectively, started a new antihypertensive medication.
In subgroups based on the type of RAS blocker received, the placebo-adjusted change from baseline in mean 24-h SBP at week 24 with linagliptin was −2.30 mmHg [95% confidence interval (CI) −7.43, 2.84; P = 0.3789] in participants receiving ACE inhibitors and 0.90 mmHg (95% CI −2.32, 4.11; P = 0.5825) in those receiving ARBs (Fig. 2). For mean 24-h DBP, the placebo-adjusted change from baseline at week 24 was −0.61 mmHg (95% CI −3.41, 2.20; P = 0.6712) in participants taking ACE inhibitors and 0.20 mmHg (95% CI −1.57, 1.96; P = 0.8260) in those taking ARBs (Fig. 2). The placebo-adjusted change from baseline in mean 24-h HR at week 24 was −0.46 bpm (95% CI −3.16, 2.24; P = 0.738) in participants receiving ACE inhibitors and 1.29 bpm (95% CI −0.40, 2.98; P = 0.135) in those receiving ARBs (Fig. 2).
FIGURE 2.
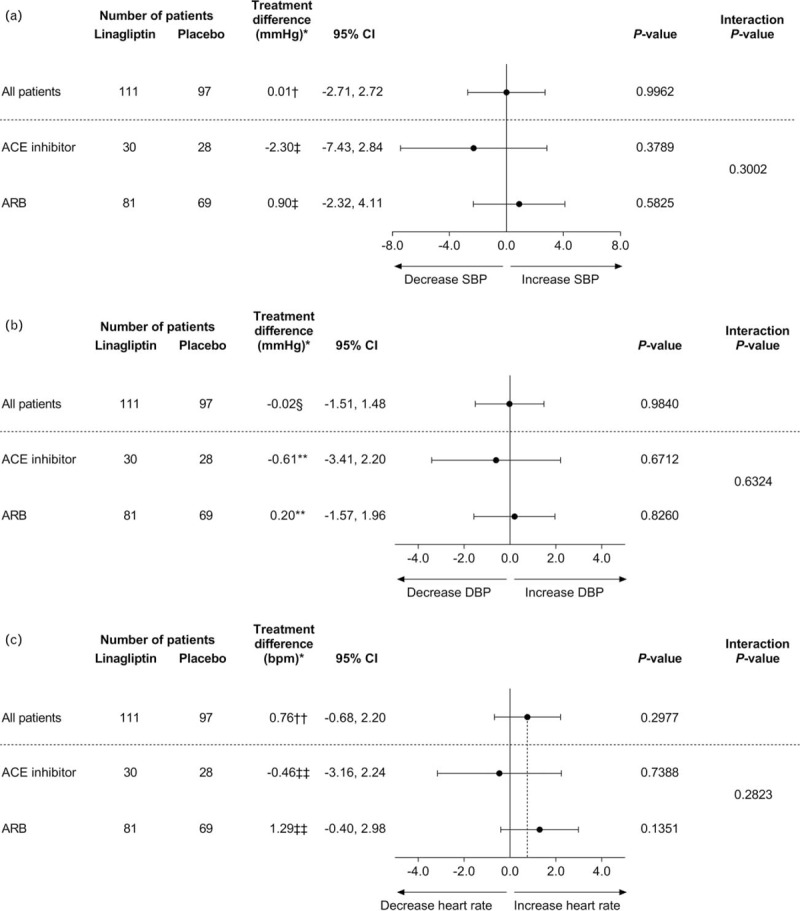
Treatment difference in adjusted mean change from baseline in mean 24-h SBP (a), 24-h DBP (b), and heart rate (c) at week 24 by antihypertensive background therapy. ∗Treatment difference: difference in the adjusted means for linagliptin versus placebo at week 24; †analysis of covariance model includes baseline mean 24-h SBP, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment as a fixed effect; ‡analysis of covariance model includes baseline mean 24-h SBP, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker background therapy at baseline, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker background therapy at baseline by treatment interaction as fixed effects; §analysis of covariance model includes baseline mean 24-h DBP, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment as a fixed effect; ∗∗analysis of covariance model includes baseline mean 24-h DBP, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker background therapy at baseline, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker background therapy at baseline by treatment interaction as fixed effects; ††analysis of covariance model includes baseline mean 24-h heart rate, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment as a fixed effect; ‡‡analysis of covariance model includes baseline mean 24-h heart rate, baseline log10 (urinary albumin-to-creatinine ratio), baseline glycated hemoglobin A1c as linear covariates and treatment, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker background therapy at baseline, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker background therapy at baseline by treatment interaction as fixed effects. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CI, confidence interval.
The overall incidence of adverse events was similar between the linagliptin and placebo groups, with few participants experiencing vascular disorders, hypersensitivity reactions or cardiovascular events (Table 2).
TABLE 2.
Adverse events
| Linagliptin, n = 182 | Placebo, n = 178 | |
| Any adverse event | 58.8 | 60.1 |
| Drug-related adverse event | 7.1 | 6.2 |
| Adverse event leading to discontinuation | 1.6 | 1.1 |
| Serious adverse event | 9.3 | 4.5 |
| Vascular disordersa | 3.8 | 3.9 |
| Hypertension | 2.2 | 2.2 |
| Hypotension | 0.5 | 1.1 |
| Aortic occlusion | 0.0 | 0.6 |
| Ischemic necrosis | 0.0 | 0.6 |
| Hematoma | 0.5 | 0.0 |
| Orthostatic hypotension | 0.5 | 0.0 |
| Hypersensitivity reactionsb | 4.4 | 3.4 |
| Eczema | 0.5 | 1.7 |
| Contact dermatitis | 1.1 | 1.1 |
| Eyelid edema | 0.0 | 0.6 |
| Dermatitis | 0.5 | 0.0 |
| Hypersensitivity | 0.5 | 0.0 |
| Rash | 0.5 | 0.0 |
| Allergic rhinitis | 0.5 | 0.0 |
| Face swelling | 0.5 | 0.0 |
| Cardiovascular eventsc | 1.6 | 0.6 |
| Transient ischemic attack | 0.0 | 0.6 |
| Nonfatal stroke | 0.0 | 0.0 |
| Nonfatal myocardial infarction | 0.5 | 0.0 |
| Hospitalization for unstable angina | 0.0 | 0.0 |
| Cardiovascular death | 1.1 | 0.0 |
| Acute myocardial infarction | 0.0 | 0.0 |
| Sudden death | 0.0 | 0.0 |
| Worsening of heart failure | 0.0 | 0.0 |
| Cardiogenic shock | 0.0 | 0.0 |
| Fatal stroke | 0.5 | 0.0 |
| Ischemic stroke | 0.0 | 0.0 |
| Hemorrhagic stroke | 0.5 | 0.0 |
| Not assessable | 0.0 | 0.0 |
| Other | 0.5 | 0.0 |
Data are % of participants in the treated set (all randomized participants who received at least one dose of study drug). Medical Dictionary for Regulatory Activities (MedDRA) version 18.1 used for reporting.
aSystem organ class from MedDRA 18.1.
bStandardized MedDRA Query from MedDRA 18.1.
cCardiovascular events confirmed after adjudication by an external clinical events committee.
DISCUSSION
In this prespecified exploratory analysis of the MARLINA-T2D study, treatment with the DPP-4 inhibitor linagliptin added to stable doses of ACE inhibitors or ARBs was not associated with changes in BP or HR in individuals with T2D and albuminuria.
A potential adverse hemodynamic interaction between DPP-4 inhibitors and ACE inhibitors was initially reported from pharmacological studies [9,10]. In the spontaneously hypertensive rat model, acute administration of an experimental DPP-4 inhibitor (P32/28) increased BP in animals pretreated with the ACE inhibitor captopril [9]. Conversely, in a randomized, placebo-controlled, cross-over study in 24 individuals with metabolic syndrome who were pretreated for 5 days with sitagliptin, a licensed DPP-4 inhibitor, the hypotensive response to an acute high dose (10 mg) of enalapril was attenuated, but not to a low dose (5 mg) of enalapril. Sitagliptin treatment alone led to a mild hypotensive response. Furthermore, only high-dose enalapril increased HR and plasma norepinephrine concentration [10]. The authors suggested that the mild hypotensive response to DPP-4 inhibition might be mediated in part by the attenuation of substance P metabolism. They further suggested that during combined high-dose ACE inhibition and DPP-4 inhibition, activation of the sympathetic nervous system by substance P and decreased degradation of neuropeptide Y (NPY)1–36 may offset the decreased degradation of vasodilatory peptides [10]. The vasodilatory substance P is normally inactivated via proteolytic cleavage by ACE in vivo – however, in the absence of ACE activity, substance P is inactivated by DPP-4 [7,8]. Subsequently, the same group reported that exogenous substance P increased sympathetic activity during acute treatment with both enalapril and sitagliptin in a randomized, double-blind, placebo-controlled, cross-over study in 12 healthy individuals [14]. The vasoconstrictive NPY1–36 is inactivated to NPY3–36 by DPP-4. In a study in the spontaneously hypertensive rat model, sitagliptin had prohypertensive effects that were mediated by NPY1–36, and the latter was shown to elicit potent vasoconstriction in the kidneys [15].
There are several possible reasons for the divergent findings between these previous studies and our observations on the hemodynamic effects of combined DPP-4 and ACE inhibition. First, the previous studies only investigated the acute or short-term treatment effect, whereas individuals in the MARLINA-T2D study were treated with the combination of linagliptin and either an ACE inhibitor or ARB for 24 weeks. It is unclear whether transient initial increases in BP and HR arising from combined DPP-4 and ACE inhibition would have clinical relevance in the absence of long-term changes. Second, unlike the previous studies, many participants in MARLINA-T2D were receiving other antihypertensive agents as well as ACE inhibitors, which may have attenuated increases in BP and HR. For example, one-quarter of participants were receiving β-blockers. The antihypertensive polypharmacy seen in MARLINA-T2D is common in clinical practice, as most individuals with hypertension require two or more drugs to achieve target BP, thus highlighting the clinical generalizability of these findings [16,17]. Third, the increases in BP and HR observed previously during acute treatment with sitagliptin and enalapril occurred only with high doses of the latter agent [10] whereas individuals in our study were treated with lower, practice-based, ACE inhibitor doses. Fourth, there may be within-class heterogeneity for interactions between ACE inhibitors and DPP-4 inhibitors, as the latter comprise a chemically diverse class of compounds whose pharmacokinetic properties vary substantially [18]. Whereas the previous studies employed an experimental DPP-4 inhibitor, P32/98 [9], and a widely prescribed member of this drug class, sitagliptin [10], we evaluated another licensed DPP-4 inhibitor, linagliptin, with a different chemical structure. Furthermore, a recent post-hoc analysis of the EXAMINE cardiovascular outcomes study found no increase in cardiovascular events in individuals receiving combination treatment with alogliptin, another licensed DPP-4 inhibitor, and ACE inhibitors [19]. Alogliptin treatment was also not associated with increased HR or BP as assessed rather crudely by spot measurements. Mitigating against the possibility of heterogeneity in DPP-4 inhibitor interactions with ACE inhibitors, however, is the fact that all licensed compounds including sitagliptin, linagliptin and alogliptin have high affinity for DPP-4, with half-maximal inhibitory concentration values in the low nanomolar range [20].
The findings of this clinical trial are consistent with preclinical studies analyzing the combination of an ARB (telmisartan) and linagliptin in a hypertensive diabetic mouse model [21] and in the 2-kidney 1-clip rat model of renovascular hypertension [22]. Both studies showed that the BP-lowering effect of telmisartan was not affected by concomitant treatment with linagliptin [21,22].
Limitations of this study include the fact that treatment effects were not evaluated in subgroups based on BMI or eGFR level, due to low patient numbers in certain of these subgroups.
In conclusion, this prespecified exploratory analysis of the MARLINA-T2D clinical trial does not support the existence of an adverse hemodynamic interaction between DPP-4 inhibitors, in general, and ACE inhibitors or ARBs. The lack of an increase in BP or HR observed in our study suggests that concomitant use of linagliptin with either ACE inhibitors or ARBs in patients with albuminuric diabetic kidney disease is safe in this regard. Taken together with the lack of an increase in cardiovascular events seen in participants receiving alogliptin and ACE inhibitors in the EXAMINE study [19], our findings suggest that hemodynamic effects arising from the combination of a DPP-4 inhibitor and an ACE inhibitor are either limited to the acute setting or are not a class effect of DPP-4 inhibitors.
ACKNOWLEDGEMENTS
The authors thank the participants and staff involved in this study. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Giles Brooke, PhD, CMPP, of Envision Scientific Solutions during the preparation of this article.
Data from this study have previously been presented at the 52nd Annual Meeting of the European Association for the Study of Diabetes, Munich, Germany, 12–16 September, 2016.
The current study was supported by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance.
Conflicts of interest
M.E.C., V.P., P.-H.G., and B.H. have received fees for advisory services to Boehringer Ingelheim. U.H., T.M., A.K.-W., S.v.d.W., and M.v.E. are employees of Boehringer Ingelheim.
Supplementary Material
Footnotes
Abbreviations: ABPM, ambulatory blood pressure monitoring; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CI, confidence interval; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; MedDRA, Medical Dictionary for Regulatory Activities; NPY, neuropeptide Y; T2D, type 2 diabetes; UACR, urinary albumin-to-creatinine ratio
REFERENCES
- 1.Centers for Disease Control and Prevention. National diabetes statistics report, 2017. 2017; Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services, Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. [Accessed 25 July 2017]. [Google Scholar]
- 2.International Diabetes Federation. IDF diabetes atlas. 7th ed.2015; Brussels, Belgium: International Diabetes Federation, Available from: http://www.idf.org/diabetesatlas [Accessed 4 January 2017]. [Google Scholar]
- 3.National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60:850–886. [DOI] [PubMed] [Google Scholar]
- 4.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305:2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014; 2:843–851. [DOI] [PubMed] [Google Scholar]
- 6.Godinho R, Mega C, Teixeira-de-Lemos E, Carvalho E, Teixeira F, Fernandes R, et al. The place of dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapeutics: a ‘me too’ or ‘the special one’ antidiabetic class? J Diabetes Res 2015; 2015:806979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Wang L, Ward PE. Dipeptidyl(amino)peptidase IV and aminopeptidase M metabolize circulating substance P in vivo. J Pharmacol Exp Ther 1992; 260:1257–1261. [PubMed] [Google Scholar]
- 8.Russell JS, Chi H, Lantry LE, Stephens RE, Ward PE. Substance P and neurokinin A metabolism by cultured human skeletal muscle myocytes and fibroblasts. Peptides 1996; 17:1397–1403. [DOI] [PubMed] [Google Scholar]
- 9.Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase IV inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol 2008; 35:29–34. [DOI] [PubMed] [Google Scholar]
- 10.Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension 2010; 56:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson JR, Brown NJ. Examining EXAMINE for an interaction with angiotensin-converting enzyme inhibition. Hypertension 2016; 68:549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groop PH, Cooper ME, Perkovic V, Sharma K, Schernthaner G, Haneda M, et al. Dipeptidyl peptidase-4 inhibition with linagliptin and effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: Rationale and design of the MARLINA-T2D trial. Diab Vasc Dis Res 2015; 12:455–462. [DOI] [PubMed] [Google Scholar]
- 13.Groop PH, Cooper ME, Perkovic V, Hocher B, Kanasaki K, Haneda M, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab 2017; 19:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devin JK, Pretorius M, Nian H, Yu C, Billings FT, 4th, Brown NJ. Substance P increases sympathetic activity during combined angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibition. Hypertension 2014; 63:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson EK, Mi Z, Tofovic SP, Gillespie DG. Effect of dipeptidyl peptidase 4 inhibition on arterial blood pressure is context dependent. Hypertension 2015; 65:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan TO, Anderson AI, MacInnis RJ. ACE inhibitors, beta-blockers, calcium blockers, and diuretics for the control of systolic hypertension. Am J Hypertens 2001; 14:241–247. [DOI] [PubMed] [Google Scholar]
- 17.Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension 2015; 65:1372–1407. [DOI] [PubMed] [Google Scholar]
- 18.Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet 2012; 51:501–514. [DOI] [PubMed] [Google Scholar]
- 19.White WB, Wilson CA, Bakris GL, Bergenstal RM, Cannon CP, Cushman WC, et al. Angiotensin-converting enzyme inhibitor use and major cardiovascular outcomes in type 2 diabetes mellitus treated with the dipeptidyl peptidase 4 inhibitor alogliptin. Hypertension 2016; 68:606–613. [DOI] [PubMed] [Google Scholar]
- 20.Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylm ethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther 2008; 325:175–182. [DOI] [PubMed] [Google Scholar]
- 21.Alter ML, Ott IM, von Websky K, Tsuprykov O, Sharkovska Y, Krause-Relle K, et al. DPP-4 inhibition on top of angiotensin receptor blockade offers a new therapeutic approach for diabetic nephropathy. Kidney Blood Press Res 2012; 36:119–130. [DOI] [PubMed] [Google Scholar]
- 22.Chaykovska L, Alter ML, von Websky K, Hohmann M, Tsuprykov O, Reichetzeder C, et al. Effects of telmisartan and linagliptin when used in combination on blood pressure and oxidative stress in rats with 2-kidney-1-clip hypertension. J Hypertens 2013; 31:2290–2298. discussion 2299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


