Abstract
Background
Brain metastases occur when cancer cells spread from their original site to the brain and are a frequent cause of morbidity and death in people with cancer. They occur in 20% to 40% of people during the course of their disease. Brain metastases are also the most frequent type of brain malignancy. Single and solitary brain metastasis is infrequent and choosing the most appropriate treatment is a clinical challenge. Surgery and stereotactic radiotherapy are two options. For surgery, tumour resection is performed using microsurgical techniques, while in stereotactic radiotherapy, external ionising radiation beams are precisely focused on the brain metastasis. Stereotactic radiotherapy may be given as a single dose, also known as single dose radiosurgery, or in a number of fractions, also known as fractionated stereotactic radiotherapy. There is uncertainty regarding which treatment (surgery or stereotactic radiotherapy) is more effective for people with single or solitary brain metastasis.
Objectives
To assess the effectiveness and safety of surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3, March 2018), MEDLINE and Embase up to 25 March 2018 for relevant studies. We also searched trials databases, grey literature and handsearched relevant literature.
Selection criteria
We included randomised controlled trials (RCTs) comparing surgery versus stereotactic radiotherapy, either a single fraction (stereotactic radiosurgery) or multiple fractions (fractionated stereotactic radiotherapy) for treatment of single or solitary brain metastasis.
Data collection and analysis
Two review authors screened all references, evaluated the quality of the included studies using the Cochrane tool for assessing risk of bias, and performed data extraction. The primary outcomes were overall survival and adverse events. Secondary outcomes included progression‐free survival and quality of life . We analysed overall survival and progression‐free survival as hazard ratios (HRs) with 95% confidence intervals (CIs), and analysed adverse events as risk ratios (RRs). For quality of life we used mean difference (MD).
Main results
Two RCTs including 85 participants met our inclusion criteria. One study included people with single untreated brain metastasis (n = 64), and the other included people with solitary brain metastasis (22 consented to randomisation and 21 were analysed). We identified a third trial reported as completed and pending results this may be included in future updates of this review. The two included studies were prematurely closed due to poor participant accrual. One study compared surgery plus whole brain radiotherapy (WBRT) versus stereotactic radiosurgery alone, and the second study compared surgery plus WBRT versus stereotactic radiosurgery plus WBRT. Meta‐analysis was not possible due to clinical heterogeneity between trial interventions. The overall certainty of evidence was low or very low for all outcomes due to high risk of bias and imprecision.
We found no difference in overall survival in either of the two comparisons. For the comparison of surgery plus WBRT versus stereotactic radiosurgery alone: HR 0.92, 95% CI 0.48 to 1.77; 64 participants, very low‐certainty evidence. We downgraded the certainty of the evidence to very low due to risk of bias and imprecision. For the comparison of surgery plus WBRT versus stereotactic radiosurgery plus WBRT: HR 0.53, 95% CI 0.20 to 1.42; 21 participants, low‐certainty evidence. We downgraded the certainty of the evidence to low due to imprecision. Adverse events were reported in both trial groups in the two studies, showing no differences for surgery plus WBRT versus stereotactic radiosurgery alone (RR 0.31, 95% CI 0.07 to 1.44; 64 participants) and for surgery plus WBRT versus stereotactic radiosurgery plus WBRT (RR 0.37, 95% CI 0.05 to 2.98; 21 participants). Most of the adverse events were related to radiation toxicities. We considered the certainty of the evidence from the two comparisons to be very low due to risk of bias and imprecision.
There was no difference in progression‐free survival in the study comparing surgery plus WBRT versus stereotactic radiosurgery plus WBRT (HR 0.55, 95% CI 0.22 to 1.38; 21 participants, low‐certainty evidence). We downgraded the evidence to low certainty due to imprecision. This outcome was not clearly reported for the other comparison. In general, there were no differences in quality of life between the two studies. The study comparing surgery plus WBRT versus stereotactic radiosurgery plus WBRT found no differences after two months using the QLQ‐C30 global scale (MD ‐10.80, 95% CI ‐44.67 to 23.07; 14 participants, very low‐certainty evidence). We downgraded the certainty of evidence to very low due to risk of bias and imprecision.
Authors' conclusions
Currently, there is no definitive evidence regarding the effectiveness and safety of surgery versus stereotactic radiotherapy on overall survival, adverse events, progression‐free survival and quality of life in people with single or solitary brain metastasis, and benefits must be decided on a case‐by‐case basis until well powered and designed trials are available. Given the difficulties in participant accrual, an international multicentred approach should be considered for future studies.
Plain language summary
Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis
Background Brain metastases are cancer cells that spread to the brain from the place where the disease first started (primary tumour) to form one or more tumours. In most cases, brain metastases are multiple lesions that are diagnosed in later stages of the disease. However, some can appear as the only deposit detected, either as the only known metastasis of a tumour in the whole body which happens to be localised in the central nervous system (a solitary brain metastasis) or as a single cerebral metastasis with additional metastases in other organ systems (a single brain metastasis).
Surgery and stereotactic radiotherapy are two of the treatments currently available for single and solitary brain metastasis. Surgery consists of either a biopsy (an extraction of a small piece of the tumour through a small hole (burr hole) to be examined under the microscope) or an attempted complete removal of the metastasis through a more extensive surgical operation (craniotomy). Steroetactic radiotherapy is a type of external radiation therapy where ionising radiation beams are precisely focused on the brain metastasis. This can be via a single fraction treatment (stereotactic radiosurgery) or through multiple smaller fractions (fractionated stereotactic radiotherapy).
Review question What is the effectiveness and safety of surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis?
Study characteristics We searched relevant databases up to 25 March 2018. We found two clinical trials with a total of 85 participants with either single or solitary brain metastasis. One trial included 64 participants with a single brain metastasis, and the other included participants with a solitary brain metastasis (22 of these consented to randomisation and 21 were analysed). Both studies were prematurely closed due to difficulties in finding participants meeting the inclusion criteria or agreeing to participate. One trial compared surgery plus whole brain radiotherapy (WBRT) versus stereotactic radiosurgery alone, and the second trial compared surgery plus WBRT versus stereotactic radiosurgery plus WBRT.
Key results Due to the small number of people included in the studies, neither study had sufficient power to detect differences in the effects of surgery versus stereotactic radiotherapy on overall survival, adverse events, progression‐free survival or quality of life in participants with single or solitary brain metastasis.
Certainty of the evidence The certainty of the evidence was low or very low mainly because of imprecision and risk of bias since the number of people in each trial was very small and participants and researchers were aware of the trial intervention (not blinded studies), so this could have affected how the participants evaluated outcomes, such as some adverse events and quality of life. Even though blinding of participants is difficult due to the nature of the intervention, study authors did not mention other ways of reducing the risk of bias, such as blinding during data analysis.
Summary of findings
Background
Description of the condition
The development of brain metastases is a frequent complication in people with cancer. In brain metastases cancer cells migrate from the place where they first formed (primary tumour) and travel, mainly through the blood, to the brain and form one or more tumours. Brain metastases are the most commonly diagnosed type of central nervous system tumour, affecting up to 30% of adult patients with cancer (Pruitt 2017). Incidence rates range from 8.3 to 14.3 per 100,000 according to population‐based studies (Nayak 2012), and it is increasing as survival after primary diagnosis for cancer patients has increased due to new cancer therapies, advanced imaging, and improved screening (Nolan 2018). Although many different malignant tumours have the ability to infiltrate the central nervous system, the most common primary tumours responsible for brain metastases are lung cancer, breast cancer, renal cell carcinoma and melanoma. In contrast, other carcinomas, for instance prostate, oesophageal, oropharyngeal or non‐melanoma skin cancers, rarely infiltrate the brain (Barnholtz‐Sloan 2004; Bouffet 1997; Nayak 2012; Sundermeyer 2005). Brain metastases occur in more than 64% of people with lung cancer, and approximately 20% of those with breast cancer (Lassman 2003).
Haematogenous spread (when cancer cells are transported through the blood to distant sites of the body) is the most common mechanism of metastasis to the brain (Gavrilovic 2005), and as a consequence, the junction of the grey matter and white matter is the most frequent location, probably because blood vessels have a narrow diameter, acting as a trap for clumps of tumour cells (Delattre 1988).
Brain metastases, in the majority of cases, are multiple lesions that are diagnosed in later stages of the disease. However, in some cases brain metastases appear as the only deposit detected, either as a solitary brain metastasis, defined as "the only known metastasis of a tumour in the whole body which happens to be localised in the central nervous system" or as a single (also named singular) brain metastasis, defined as "a single cerebral metastasis with additional metastases in other organ systems" (Westphal 2003).
Description of the intervention
The most widely used therapeutic modalities for single or solitary brain metastasis are surgery and also some forms of stereotactic radiotherapy. Surgery consists of either a biopsy (an extraction of a tissue sample to be examined under the microscope) or a resection of the metastasis by means of a neurosurgical technique in the operating theatre. Resection can be either partial or complete, as confirmed by postoperative imaging. Stereotactic radiotherapy is a type of external radiation therapy where ionising radiation beams are precisely focused on selected areas of the brain; in this case, to the brain metastasis (Pannullo 2011).The stereotactic radiotherapy may be given as a single dose, sometimes named stereotactic radiosurgery, or in a number of fractions, also known as fractionated stereotactic radiotherapy. This treatment may be given using different technical options, for example, robotic delivery of radiation, multiple convergent sources of cobalt and other technical devices adapted to linear accelerators (LINACs) (Flickinger 1994; Joseph 1996; Suh 2010). The selection of the technique (type of radiation and device) depends on many factors including the location, size and type of lesion.
How the intervention might work
The outcome for patients with brain metastases is generally poor, and cranial radiotherapy is mostly palliative intended. In people with multiple brain metastases whole brain radiotherapy or steroids, or both, are the treatment of choice (Bradley 2004; Mulvenna 2016; Patchell 2003). In people with solitary or single brain metastasis a more radical approach with surgery or stereotactic radiotherapy has been applied in order to improve outcomes. A Cochrane Review with three included clinical studies (Patil 2012), concluded from one of the studies that people with only one brain metastasis may live longer when they receive stereotactic radiosurgery in addition to whole brain radiotherapy (WBRT) versus WBRT alone (Andrews 2004). Another Cochrane Review (Hart 2005), with three included randomised controlled trials (RCTs) (Mintz 1996; Patchell 1990; Vecht 1993), concluded that surgery and WBRT may reduce the proportion of deaths due to neurological cause and functionally independent survival. However, it did not show a clear improvement in overall survival.
Management of people newly diagnosed with single or solitary brain metastasis varies widely with location and extension of the primary tumour, histological subtype of the tumour (i.e. microscopic characteristics of the tumour), location of the metastasis and treatment facilities of the referred centre being the most relevant factors (Bradley 2004; Patchell 2003). People having two or more brain metastases (oligometastasis) are not generally considered candidates for surgery, therefore we will not consider this subgroup in this review.
Some studies have described a significant survival extension when single brain metastasis are managed aggressively using surgery or stereotactic radiotherapy of the lesion with or without whole brain radiotherapy (WBRT) (Andrews 2004; Aoyama 2003; Patchell 1998).
Why it is important to do this review
Single and solitary brain metastasis is infrequent. However, as mentioned before, the incidence of brain metastases is increasing as more people are living longer with a primary diagnosis (Nolan 2018).
Choosing the most appropriate treatment for people with brain metastases is always a clinical challenge. It is imperative to balance the risks and benefits due to the incurable nature of the vast majority of metastatic cancer patients, even with a single brain deposit. The best‐described treatment strategies in the literature are surgery and stereotactic radiotherapy given as a single dose (stereotactic radiosurgery) or fractionated stereotactic radiotherapy, with or without WBRT.
Surgery can be a reasonable option for some people with a solitary/single brain metastasis. However, morbidity and mortality associated with the procedure have to be taken into account. Complications include the increasing or onset of focal motor or sensory deficit, seizures and surgical wound and bone flap infection. In people with solitary and single brain metastasis, survival with surgery has been reported to be better than with stereotactic radiosurgery (Bougie 2015; Muacevic 1999), although treatment‐related complications have been shown to be higher with surgery Bougie 2015.
Stereotactic radiosurgery has shown comparable local control compared to surgery, and survival rates may be similar to surgery if patients receive equally aggressive treatment of the primary tumour. Since stereotactic radiosurgery is a non‐invasive technique, complications are expected to be lower. Complications may include cerebral oedema (i.e. an excessive accumulation of fluid inside brain cells), seizures and nausea (Muacevic 1999).
This review aims to assess the effectiveness and safety of surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis. It is also possible that the meta‐analysis approach may overcome the limitations of small individual studies regarding rare conditions like solitary/single brain metastasis.
Objectives
To assess the effectiveness and safety of surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies (RCTs).
Types of participants
Population
Adults (as defined by the study authors) with the following characteristics.
Single or solitary brain metastasis of any size.
Biopsy‐proven malignancy (any tumour histology).
No previous cranial radiation.
Any chemotherapy or target therapy must have been administered before the study intervention.
Types of interventions
Intervention
Surgery (any neurosurgical technique). We included trials where complete resection was intended. Postoperative confirmation of metastasis resection with magnetic resonance imaging (MRI) was desirable, but not necessary for inclusion. We described and analysed partial resections with the intention‐to‐treat principle, as a potential outcome of surgery.
Comparison
Any type of stereotactic radiotherapy including stereotactic radiosurgery: robotic delivery radiation; multiple convergent sources of cobalt or other technical devices adapted to linear accelerators (LINACs), for example, any form of LINAC stereotactic radiotherapy (with or without relocatable frame) using single or multiple fractions or Gamma Knife stereotactic radiosurgery.
The number of fractions was not a criterion of eligibility in the review, therefore we included studies using either a single (stereotactic radiosurgery) or a multifraction treatment (fractionated stereotactic radiotherapy), as long as they met the remaining eligibility criteria.
Cointervention
Studies with or without whole brain radiotherapy (WBRT) as cointervention were eligible as long as they compared surgery with stereotactic radiotherapy.
Studies with or without chemotherapy or target therapy as cointervention for the treatment of single or solitary brain metastasis were eligible as long as they compared surgery with stereotactic radiotherapy.
Studies where postintervention management varied between study groups, including non‐standardised postintervention management, were eligible for inclusion. We carefully analysed variations in postintervention management between study groups.
Types of outcome measures
Primary outcomes
Overall survival: length of time (in days, weeks or months) until death from any cause. We assessed survival from the time when participants were randomised.
Adverse events: untoward medical events that may present during treatment with the study intervention, with or without a causal relationship with this treatment (Nebeker 2004). Examples of adverse events that we considered in this review are: headache; nausea; vomiting; fatigue and seizures or other neurological toxicities; wound complications such as infection or dehiscence; other infections different from wound infection; haematoma or cerebrospinal fluid leak. We described separately the proportion of participants with moderate and severe adverse events.
Secondary outcomes
Progression‐free survival: survival from time of randomisation to time of disease progression. We considered disease progression to be an increase in the size of any lesion, development of new lesions, decline in performance status, worsening of symptoms or death.
Quality of life: assessed through validated questionnaires of health‐related quality of life (e.g. Karnofsky performance status (KPS), QLQ‐BN20, QLQ‐C15‐PAL, QLQ‐C30 or FACT‐G with Brain sub scale).
Search methods for identification of studies
Electronic searches
We carried out electronic searches in the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 3) in the Cochrane Library (searched 25 March 2018).
Ovid MEDLINE (1950 to 25 March 2018).
Embase (1980 to 25 March 2018).
We did not apply any restrictions regarding language, publication status or date of publication.
The search strategies for MEDLINE, Embase and CENTRAL are listed in Appendix 1.
Searching other resources
We searched the following clinical trials registries: ClinicalTrials.gov (www.clinicaltrials.gov), the UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk), the EU Clinical Trials register (www.clinicaltrialsregister.eu) and the World Health Organization (WHO) International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch).
For identifying grey literature, we searched Open Grey (www.opengrey.eu), and we also checked the reference lists of all included trials and other systematic reviews identified in the electronic searches (Appendix 2).
As part of handsearching, we searched the following journals from the fields of oncology, neuro‐oncology, neurosurgery and radiotherapy to identify articles of trials published in the last three years.
Radiotherapy and Oncology
Journal of Clinical Oncology
Seminars in Radiation Oncology
Journal of Neurosurgery
Neurosurgery
Neuro‐Oncology
Journal of Neuro‐Oncology
World Journal of Surgical Oncology
Journal of Radiotherapy in Practice
Journal of Neurology, Neurosurgery and Psychiatry
Cancer and Metastasis Reviews
In order to identify newly published studies, we used the PubMed email alert service “My NCBI[A1] ” (National Center for Biotechnology Information), applying the search strategy described in Appendix 1.
We contacted at least one of the study authors by e‐mail when we identified conference communications or when we had no access to a study report. We also contacted principal researchers asking them about possible unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (DO and MJM) independently screened all references identified in the search for potential eligibility, by reading the title and abstract. At this point, none of the outcomes listed were required as part of the eligibility criteria for including studies. We reviewed the full texts of all potentially relevant articles and assessed studies for eligibility, irrespective of their publication status or language of publication. A third review author (RF or JEH) resolved any disagreements about which articles were eligible. We created a Preferred Reporting Items for Systematic Reviews andMeta‐Analyses (PRISMA) flow chart (Moher 2009) to illustrate our study selection process Figure 1.
1.
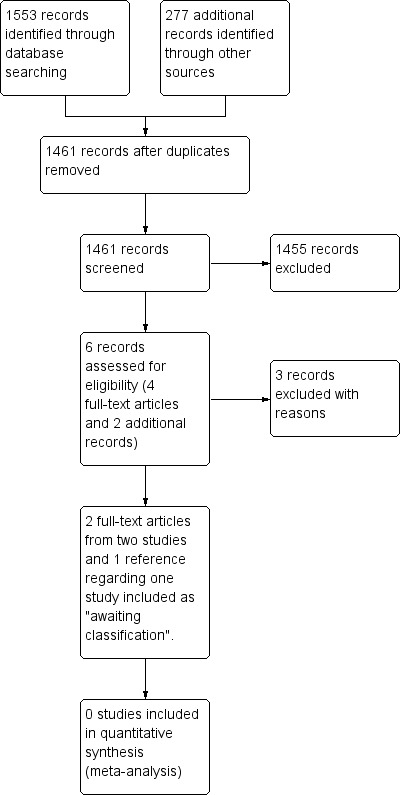
Study (PRISMA) flow diagram.
Data extraction and management
Using a pretested data collection form (Appendix 3), two review authors (DO and MJM) independently collected the following information from individual studies: study eligibility; study design (e.g. randomisation and blinding) and setting; participant characteristics; interventions and comparisons of interest; outcomes; loss to follow‐up; risk of bias (see section below); type of analysis (intention‐to‐treat, per protocol); study funding source; and any conflicts of interest stated by the investigators. Information was collected in sufficient detail to complete Characteristics of included studies tables. We classified interventions as:
surgery alone;
surgery and whole brain radiotherapy (WBRT);
stereotactic radiotherapy alone, including any technique: stereotactic radiosurgery using either Gamma Knife, robotically‐assisted radiation delivery system, linear accelerator (LINAC), stereotactic radiosurgery using LINAC and stereotactic radiotherapy using any kind of relocatable frame;
any stereotactic radiotherapy technique plus WBRT.
We extracted data in order to analyse data following the intention‐to‐treat principle. We analysed participants in the groups to which they were randomised, regardless of whether or not they received the treatment they had been assigned to, or whether or not they had been observed until the completion of the follow‐up period.
We resolved any disagreements about the data extraction by discussion. We provided the reasons why we excluded a potentially relevant study in the Characteristics of excluded studies tables. We did not identify any ongoing study; however, we will provide relevant information regarding ongoing trials that may be included in future versions of the review in the 'Characteristics of ongoing studies' table.
Assessment of risk of bias in included studies
Two review authors (DO and MJM) independently assessed the risk of bias using the Cochrane tool for assessing the risk of bias (Higgins 2011), and judged the following domains in each included study: adequate sequence generation; allocation concealment; participants' blinding; provider blinding; data collector blinding; outcome assessor blinding; analyst blinding; percentage of follow‐up and whether incomplete outcome data were addressed; whether the trial was free of selective reporting; and whether the trial was stopped early for benefit (i.e. stop recruiting participants before reaching the sample size or as soon as they find a positive result) (Guyatt 2012). We also considered other bias reported by the study authors or identified by the review authors.
Review authors judged the risk of bias in each domain as 'low risk', 'high risk' or 'unclear risk'. We resolved any disagreements by discussion or by consulting a third review author (RF or JEH).
Review authors explained their risk of bias judgements for individual studies in the 'Risk of bias' tables. We presented judgements about each methodological quality item as percentages across all included studies in a 'Risk of bias' graph, and summarised judgements about each methodological quality item for each included study in a 'Risk of bias' summary chart.
Measures of treatment effect
We analysed time‐to‐event outcomes (i.e. overall survival and survival free of brain relapses) as hazard ratios (HRs), and adverse events as dichotomous data using risk ratios (RRs). For quality of life, we used either mean differences (MDs) or standardised mean differences (SMDs), depending on whether the outcomes are reported using the same or different scales. We reported all effect measures with 95% CIs (CIs).
Unit of analysis issues
The unit of analysis for all predefined outcomes was individual participants, except for the outcome, 'adverse events', which we analysed by number of adverse events. When analysing outcomes, we took into account the level at which randomisation occurred.
Dealing with missing data
Where data were missing, we contacted study authors to request the necessary information. If data were still missing, after attempts to retrieve information had been exhausted, we reported the available results. We did not impute missing outcome data for any of the outcomes in the review. For future versions of this review, we will consider missing outcome data of 20% or higher for any outcome as high risk of attrition bias.
Assessment of heterogeneity
We assessed heterogeneity of effect sizes by visual inspection of forest plots and we measured heterogeneity using the I2 statistic (Higgins 2003). If substantial heterogeneity was detected, we attempted to explain the differences between studies based on the clinical characteristics (i.e. setting, characteristics of participants, comorbidity and treatments) and the methodological characteristics (i.e. randomisation, study quality and analytical method). When included studies were different, we did not combine their results; we presented the results using a narrative approach instead.
Assessment of reporting biases
We assessed potential reporting bias of the review using a funnel plot when 10 or more studies were available (Sterne 2011). The funnel plot illustrates variability among trials. If asymmetry is detected, other causes such as the methodological quality of studies, the influence of small studies or the presence of heterogeneity are checked.
Data synthesis
In this review we could not combine the included studies. In future versions of the review we will undertake a meta‐analysis of the results for all primary and secondary outcomes.
We will combine the results with a random‐effects model (Higgins 2003), using inverse variance weighting in Review Manager 5 (Review Manager 2014). We will analyse differences among studies based on clinical characteristics and methodological characteristics of included studies. If the included studies are too diverse for a meta‐analysis, we will present the results using a narrative approach.
To interpret the findings and to rate the certainty of evidence we used the GRADE approach (Guyatt 2011). First, we analysed the overall certainty of evidence for each outcome individually, downgrading the evidence from 'high certainty' to 'moderate', 'low' or 'very low' depending on the risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates and potential publication bias. We took this analysis into account in our conclusions. We used the GRADEpro Guideline Development Tool to produce two 'Summary of findings' tables with the results of this analysis (GRADEpro GDT 2015; Table 1; Table 2).
Summary of findings for the main comparison. Surgery plus whole brain radiotherapy (WBRT) compared to stereotactic radiosurgery for people with single or solitary brain metastasis.
| Surgery plus whole brain radiotherapy (WBRT) compared to stereotactic radiosurgery for adults with single or solitary brain metastasis | ||||||
| Patient or population: people with single or solitary brain metastasis Setting: hospital Intervention: surgery plus WBRT Comparison: stereotactic radiosurgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with stereotactic radiosurgery | Risk with surgery plus WBRT | |||||
| Overall survival follow‐up: mean 12 months | Study population | HR 0.92 (0.48 to 1.77) | 64 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa | ||
| 515 per 1000 | 486 per 1000 (294 to 720) | |||||
| Adverse events follow‐up: mean 12 months | Study population | RR 0.31 (0.07 to 1.44) | 64 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb | ||
| 194 per 1000 | 60 per 1000 (12 to 277) | |||||
| Adverse events (moderate) follow‐up: mean 12 months | Study population | RR 0.62 (0.11 to 3.50) | 64 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb | ||
| 61 per 1000 | 38 per 1000 (7 to 212) | |||||
| Adverse events (severe) follow‐up: mean 12 months | Study population | RR 0.13 (0.00 to 2.50) | 64 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Progression‐free survival: not measured | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life (HRQoL) assessed with: QLQ‐C30 follow‐up: mean 6 months | Even though there was an improvement in scores for the domains 'role functioning' and 'quality of life' six weeks after stereotactic radiosurgery (reported only as P < 0.05), the difference was lost six months after treatment. Numeric results were not reported | ‐ | (1 RCT) | ⊕⊝⊝⊝ VERY LOWb | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by two levels due to risk of bias issues (high risk of selection bias) and imprecision. bDowngraded by two levels due to risk of bias issues (high risk of selection, performance and attrition bias) and imprecision.
Summary of findings 2. Surgery plus whole brain radiotherapy (WBRT) compared to stereotactic radiosurgery plus WBRT for people with single or solitary brain metastasis.
| Surgery plus whole brain radiotherapy (WBRT) compared to stereotactic radiosurgery plus WBRT for adults with single or solitary brain metastasis | ||||||
| Patient or population: people with single or solitary brain metastasis Setting: hospital Intervention: surgery plus WBRT Comparison: stereotactic radiosurgery plus WBRT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with stereotactic radiosurgery plus WBRT | Risk with surgery plus WBRT | |||||
| Overall survival follow‐up: mean 16 months | Study population | HR 0.53 (0.20 to 1.42) | 21 (1 RCT) | ⊕⊕⊝⊝ LOWa | ||
| 400 per 1000 | 237 per 1000 (97 to 518) | |||||
| Adverse events (moderate) follow‐up: mean 16 months | Study population | RR 0.37 (0.05 to 2.98) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb | ||
| 100 per 1000 | 37 per 1000 (5 to 298) | |||||
| Progression‐free survival follow‐up: mean 16 months | Study population | RR 0.55 (0.22 to 1.38) | 21 (1 RCT) | ⊕⊕⊝⊝ LOWa | ||
| 100 per 1000 | 55 per 1000 (22 to 138) | |||||
| Quality of life (HRQoL) assessed with: QLQ‐C30 global scale from: 0 to 100 follow‐up: mean 2 months | The mean quality of life was 51.4 points | MD 10.8 points lower (44.67 lower to 23.07 higher) | ‐ | 14 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; HRQoL: health‐related quality of life; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level due to imprecision. bDowngraded two levels due to risk of bias issues (performance bias) and imprecision.
Subgroup analysis and investigation of heterogeneity
Since we could not combine the included studies in this version of the review, in future versions of the review and when possible, we will carry out subgroup analyses by the location of the primary tumour (lung cancer, breast cancer, renal cell carcinoma, melanoma, and others), by the clinical presentations (solitary or single), by number of fractions in the stereotactic radiosurgery treatment, and by the use of WBRT or chemotherapy, or both as co interventions.
Sensitivity analysis
In future versions of the review, if meta‐analyses are performed, we will conduct sensitivity analyses to assess the effect on the primary outcome, based on the methodological quality of included studies: studies classified as 'overall low' risk of bias versus those classified as 'overall high' or 'unclear' risk of bias'.
Any special sensitivity analysis will take into account possible variations in postintervention management between study groups and studies.
Results
Description of studies
Results of the search
Figure 1 shows the review PRISMA flow chart. The search completed on 25 March 2018 yielded 1830 records: we identified 1553 through database searching and retrieved 277 references from other sources. We excluded 369 duplicated references, leaving 1461 unique references. The unique references were screened by reading titles and abstracts. From these 1461 references, we identified six potentially eligible references that we reviewed in full‐text, and from which we excluded three studies with reasons. From the included references, we identified three studies comparing surgery with stereotactic radiotherapy for people with single or solitary brain metastasis meeting the inclusion criteria: two studies were published in two articles. One trial, registered in ClinicalTrials.gov as completed, has no related publications and we classified this under 'Characteristics of studies awaiting classification'. We have contacted the study authors by e‐mail, but have not yet received a response.
Included studies
We included two studies (85 participants) in the review (Muacevic 2008; Roos 2011). Both used stereotactic radiosurgery. See also Characteristics of included studies tables.
Study design
The two studies were parallel randomised controlled trials (RCTs). The Muacevic 2008 study included 64 participants and compared surgery plus whole brain radiotherapy (WBRT; n = 33) versus stereotactic radiosurgery (n = 33). In the Roos 2011 study, 22 consented to randomisation and 21 were analysed. This study compared stereotactic radiosurgery plus WBRT (n = 11) versus surgery plus WBRT (n = 10).
Participants
The Muacevic 2008 study took place in four hospitals in Germany and included 64 adults. The mean age was 58.3 years (standard deviation (SD) 9.6) in the surgery group and 54.3 years (SD 11.7) in the stereotactic radiosurgery group; 58% were women. The Roos 2011 study took place in Australia; 22 consented to randomisation and 21 were analysed; one patient in the surgery plus WBRT group was found to be ineligible due to a lesion invisible on repeat imaging without treatment and was subsequently excluded. The mean age was 58 in the surgery group and 63 in the stereotactic radiosurgery group; 50% were women. In both studies the age of participants ranged from 32 to 84 years old.
The Muacevic 2008 study included people with single untreated brain metastasis smaller than 3 cm, while the Roos 2011 study included participants with solitary brain metastasis smaller than 4cm (see Characteristics of included studies). Most participants had lung cancer as the primary tumour in the two studies. In the Muacevic 2008 study, 36% of participants in the surgery group and 32% in the stereotactic radiosurgery group had non‐small cell lung cancer. In the Roos 2011 study, this proportion was 50% and 45%, respectively. The rest of the participants in the Muacevic 2008 study had genito‐urinary tract tumours (12% in the surgery group and 19% in the stereotactic radiosurgery group), gastrointestinal tract tumours (9% in the surgery group and 3% in the stereotactic radiosurgery group), melanoma (15% in the surgery group and 13% in the stereotactic radiosurgery group), breast cancer (15% in the surgery group and 19% in the stereotactic radiosurgery group), liver cancer (3% in the surgery group and 3% in the stereotactic radiosurgery group) and unknown primary tumour (9% in the surgery group and 10% in the stereotactic radiosurgery group). In the Roos 2011 study, 20% of participants had colorectal cancer in each study group and 30% in the surgery group and 40% in the stereotactic radiosurgery group had other tumours.
In most of the cases, the location of the brain metastasis was supratentorial. The median of the maximum brain metastasis diameter ranged between 17 mm and 24 mm. The median of time to metastasis was two months for surgery and nine months for stereotactic radiosurgery in one trial (Roos 2011), and 13 months for surgery versus 15 months for stereotactic radiosurgery in the other trial (Muacevic 2008).
Setting
Both studies took place in a hospital setting, one in Germany and the other in Australia.
Interventions
Surgery was carried out using standard stereotactic‐guided neurosurgical techniques in both studies (Muacevic 2008; Roos 2011).
In the Muacevic 2008 study, stereotactic radiosurgery was performed with Gamma Knife (Elekta, Stockholm, Sweden), using stereotactic magnetic resonance imaging (MRI) guidance. The treatment was performed on an outpatient basis. The mean dose applied to the tumour margin was 21 Gray (range: 14 Gray to 27 Gray). In radioresistant tumours (i.e. melanoma, hypernephroma), the prescribed tumour dose was in the range of 20 Gray to 27 Gray; while in radiosensitive tumours (i.e. breast cancer) the dose was in the range of 14 Gray to 20 Gray. The mean maximum dose was 41 Gray (range: 28 Gray to 54 Gray), and on average, the 50% isodose (range: 35% to 85%) was used to irradiate the tumour margin. Conformal multiple isocenter Gamma Knife surgery was performed in all participants, with a mean number of isocentres per participant of seven.
In the Roos 2011 study, stereotactic radiosurgery was planned on a Fischer‐Leibinger system and delivered on a Varian 600C/D linear accelerator using multiple arcs with circular collimators. A single fraction stereotactic radiosurgery dose was prescribed to the 70% to 90% isodose envelope and was based upon lesion size: 20 Gray, 18 Gray and 15 Gray marginal dose for maximum tumour diameter 20 mm, 21 to 30 mm and 31 to 40 mm, respectively.
Whole brain radiotherapy (WBRT) was used as adjuvant therapy in the surgery group in the Muacevic 2008 study, while in the Roos 2011 study it was used in both groups. In the Muacevic 2008 study, participants received 40 Gray over four weeks. The dose in the Roos 2011 study was 30 Gray in 10 fractions over two weeks. Corticosteroids were used at the clinician's discretion in both studies.
Funding sources
The Muacevic 2008 study was funded by the Elekta Research Foundation, while the Roos 2011 study was funded by the Royal Australian and New Zealand College of Radiologists and by the Royal Adelaide Hospital through research grants.
Outcomes
Both studies assessed overall survival. However, in the Muacevic 2008 study it was measured from the date of surgery/stereotactic radiosurgery (time from randomisation to surgery/stereotactic radiosurgery was not reported), while in the Roos 2011 study it was measured from randomisation. The hazard ratio (HR) was not reported in the Muacevic 2008 paper, and we could not obtain this information from the study authors; therefore we calculated it from the available information (see Differences between protocol and review).
Treatment‐related toxicity (including acute and late toxicities) and survival free of relapse, taking into account both, local and systemic disease, were also reported in the two studies. The two studies assessed quality of life using the same instruments: the European Organization for Research and Treatment of Cancer quality of life Questionnaire (EORTC‐QLQ‐C30), including the Brain Cancer Module 20 (BCM20). However, they were measured at different moments. The Muacevic 2008 study used the instruments on day one after randomisation, at six weeks, and at six months after treatment, while the Roos 2011 study assessed the quality of life at randomisation, two and three months after starting treatment, then three monthly thereafter until relapse. The Roos 2011 study also assessed the Karnofsky performance status (KPS) and neurological function.
Excluded studies
We excluded three studies. The Fogarty 2014 study was a pilot study to assess the feasibility of a trial on whole brain radiotherapy (WBRT). On the other hand, in the Kocher 2011 study, randomisation was carried out after surgery or stereotactic radiosurgery. A third study was excluded because no results were available; it was stooped prior to enrolment due to accrual difficulties. See the table Characteristics of excluded studies for further details.
Studies awaiting classification
We identified one trial in clinicaltrials.gov that we may include in future updates of this review (NCT00460395). According to the registry, the trial aims to compare the survival (overall, systemic, and neurological) of participants with single cerebral metastasis treated with either conventional surgical resection or stereotactic radiosurgery. It also aims to compare rates of recurrence, complications, cognitive ability, functional status, and quality of life. However, we have not found any related publication. We have contacted the trial's lead author and the applicant in order to obtain more information; a response is pending (see Characteristics of studies awaiting classification).
Ongoing studies
We did not identify any further ongoing studies.
Risk of bias in included studies
The risk of bias in terms of allocation, blinding, outcome, reporting, and other criteria is summarised in Figure 2 and Figure 3. The two included studies were prematurely closed due to poor participant accrual (Muacevic 2008; Roos 2011).
2.
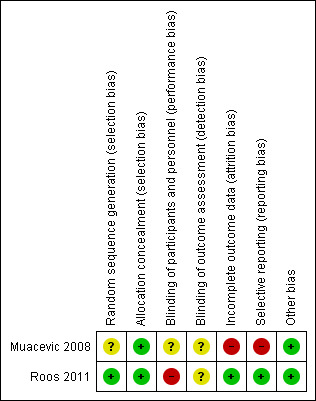
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
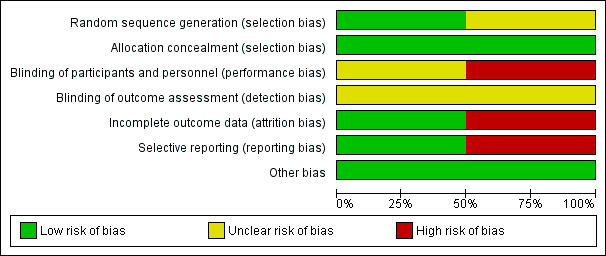
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Only the Roos 2011 study used random sequence generation to minimise selection bias. Randomisation was based on a permuted block design with randomly varied block sizes of four or six, not stratified by prognostic factors; therefore we considered this study to have a low risk of bias. Even though the Muacevic 2008 study mentioned randomisation, the authors did not specify the sequence generation process, making the assessment of selection bias difficult (unclear risk of bias).
Allocation concealment
The two studies explicitly reported how the allocation was concealed. In both studies (Muacevic 2008; Roos 2011), randomisation was performed centrally and treatments were allocated by telephoning a trial centre. We considered both studies to have a low risk of bias in this domain.
Blinding
The Muacevic 2008 study did not provide adequate data to allow assessment of blinding, therefore we considered this study to have an unknown risk of bias. On the other hand, we rated the Roos 2011 study as at high risk of bias because the blinding methods for participants and personnel were not appropriated; lack of blinding may affect subjective outcomes such as adverse events and quality of life.
Incomplete outcome data
We considered the Muacevic 2008 study to have a high risk of bias for this domain because results for quality of life were not available for 25% of participants. In the Roos 2011 study, even though one participant randomised did not receive an intervention, we considered the trial to have a low risk of bias.
Selective reporting
The two studies reported outcomes that are typically reported for this condition. However, 95% confidence intervals (CIs) were not reported in the Muacevic 2008 study; therefore, we rated this study at high risk of selective reporting.
Other potential sources of bias
We did not identify any other sources of bias. Both studies reported characteristics of the research design, such as sample size calculation and sources of funding, therefore we considered them as having a low risk of bias.
Effects of interventions
See also Table 1 and Table 2. We were unable to perform a meta‐analysis due to clinical heterogeneity between trial interventions.
Comparison 1. Surgery plus whole brain radiotherapy (WBRT) versus stereotactic radiosurgery
Overall survival
In the Muacevic 2008 study, the median survival (measured from the date of surgery/stereotactic radiosurgery) was 9.5 months (95% confidence interval (CI) not reported) after surgery plus WBRT and 10.3 months after stereotactic radiosurgery alone. There was no clear evidence of a difference in overall survival with surgery plus WBRT when compared with stereotactic radiosurgery (hazard ratio (HR 0.92, 95% CI 0.48 to 1.77; 64 participants; very low‐certainty evidence). We considered the certainty of the evidence to be very low due to risk of bias issues (high risk of selection bias) and imprecision.
Adverse events
In the Muacevic 2008 study overall, there were no differences in the adverse events rate; eight adverse events were reported, two in the surgery group and six in the stereotactic radiosurgery group (6.1% versus 19%; risk ratio (RR) 0.31, 95% CI 0.07 to 1.44; 64 participants; very low‐certainty evidence). We considered the two adverse events in the surgery plus WBRT group to be moderate: one participant developed pulmonary embolism and another suffered from pneumonia. In the stereotactic radiosurgery group, three participants (9.6%) developed moderate adverse events due to transient oedema related to the stereotactic radiosurgery, which were treated successfully with steroids in all cases (RR for moderate adverse events was 0.62, 95% CI 0.11 to 3.50; 64 participants; very low‐certainty evidence). Another three patients (9.6%) developed severe adverse events: one participant experienced hemiparesis Radiation Therapy Oncology Group (RTOG) neurological function status Grade III, which was associated with intratumoural bleeding after treatment; another participant suffered from seizures; and a third died six months after stereotactic radiosurgery due to a generalised convulsive status epilepticus (RR for severe adverse events was 0.13, 95% CI 0.00 to 2.50; 64 participants; very low‐certainty evidence). No participant had space occupying radionecrotic lesions requiring decompressive surgery. Participants receiving radiosurgical re treatment did not exhibit radiogenic complications. We considered the certainty of the evidence to be very low due to risk of bias issues (high risk of selection and performance bias) and imprecision.
Progression‐free survival
This outcome was not reported in the Muacevic 2008 study and we could not calculate it from the available information.
Quality of life
In the Muacevic 2008 study participants' quality of life significantly decreased after tumour progression, mostly due to systemic disease progression. Even though there was an improvement in scores for the domains 'role functioning' and 'quality of life' six weeks after stereotactic radiosurgery (reported only as P < 0.05), the difference was lost six months after treatment. Numeric results were not reported. We considered the certainty of the evidence to be very low due to risk of bias issues (high risk of selection, performance and attrition bias) and imprecision.
Comparison 2. Surgery plus WBRT versus stereotactic radiosurgery plus WBRT
Overall survival
In the Roos 2011 study, survival (measured from randomisation) was 2.8 months (95% CI 1.3 to 20.6) after surgery plus WBRT, and 6.2 months (95% CI 2.1 to 63.7) after stereotactic radiosurgery plus WBRT. There was no clear evidence of a difference in overall survival with surgery plus WBRT when compared with stereotactic radiosurgery plus WBRT (HR 0.53, 95% CI 0.20 to 1.42; 21 participants; low‐certainty evidence). We considered the certainty of the evidence to be low for this comparison due to very serious imprecision.
Adverse events
In the Roos 2011 study there were four moderate acute radiation toxicities. One occurred in the surgery plus WBRT group (severe hearing loss) and three occurred in the stereotactic radiosurgery plus WBRT group (severe fatigue: 9% versus 27%; RR 0.37, 95% CI 0.05 to 2.98; 21 participants very low‐certainty evidence). Regarding other complications, three participants had transient onset or worsening of neurological symptoms after surgery. We considered the certainty of the evidence to be very low due to risk of bias issues (performance bias) and imprecision. The RR was not reported by the study authors, and so we calculated it from the available information (see Differences between protocol and review).
Progression‐free survival
In the Roos 2011 study, the mean progression‐free survival was 1.7 months (95% CI 1.3 to 7.7) in the group of surgery plus WBRT and 3.1 months (95% CI 2.1 to 63.7) in the group of stereotactic radiosurgery plus WBRT. There was no clear evidence of a difference in progression‐free survival with surgery plus WBRT when compared with stereotactic radiosurgery plus WBRT (HR 0.55, 95% CI 0.22 to 1.38; 21 participants; low‐certainty evidence). We considered the certainty of the evidence to be low due to imprecision.
Quality of life
In the Roos 2011 study, 14 participants (8 in the stereotactic radiosurgery plus WBRT group and 6 in the surgery plus WBRT group) completed health‐related quality of life questionnaires about two months (41 to 77 days) after starting treatment. The global health status in the QLQ‐C30 global scale showed no differences between surgery plus WBRT and stereotactic radiosurgery plus WBRT at baseline (mean difference (MD) ‐0.70, 95% CI ‐24.69 to 23.29; 18 participants). After two months, authors found no differences between stereotactic radiosurgery plus WBRT and surgery plus WBRT in the QLQ‐C30 global scale (MD ‐10.80, 95% CI ‐44.67 to 23.07; 14 participants). There were no differences for nausea and vomiting, insomnia and appetite loss, also for visual disorder and communication deficit (BN20 symptom scale) or for the neurological function according to the RTOG neurological function status. However, none of the differences between study arms were different when adjusted for multiple comparisons. We considered the certainty of the evidence to be very low due to risk of bias issues (high risk of performance and attrition bias) and imprecision.
Discussion
Summary of main results
We retrieved 1830 articles through our literature searches. Two studies met our inclusion criteria. Both studies stopped prematurely due to poor participant accrual.
The two included studies (n = 64 and n = 21 participants, respectively) were underpowered to detect differences in overall survival and progression‐free survival in the stereotactic radiosurgery groups. It was not possible for us to perform a meta‐analysis due to clinical heterogeneity between study interventions, since in one trial participants received whole brain radiotherapy (WBRT) only in the surgery group; while in the other trial, both study groups received WBRT.
Acute adverse events were present in the two included studies, both in the stereotactic radiosurgery group as well as in the surgery group. In one of the studies, participants in both groups received WBRT and there was a tendency to have more adverse events in the stereotactic radiosurgery group. However, results were imprecise. In the other trial, participants in the stereotactic radiosurgery group did not receive WBRT, but they showed a tendency to have more adverse events. Again, these results were quite imprecise. Quality of life was similar in both groups.
Overall completeness and applicability of evidence
The overall completeness of the evidence is poor. The included studies provided insufficient evidence to draw reliable conclusions on the effects of stereotactic radiosurgery compared to surgery on overall survival, adverse events, progression‐free survival and quality of life. The two studies had accrual difficulties and none of them reached the calculated study sample. Therefore, neither of them had statistical power to draw precise results. Furthermore, the certainty of the evidence was low or very low, meaning that further research is likely to change the estimate of effect found in the studies.
These trials recruited people with a variety of cancer types; mainly lung cancer and genito‐urinary and gastrointestinal tract tumours with single or solitary brain metastasis of less than 4 cm in diameter. However, the limitations in the applicability of evidence are related to two aspects. The first relates to clinical scenarios where either surgery or stereotactic radiosurgery are feasible; while stereotactic radiosurgery can be considered in the presence of more than one brain metastasis, surgery usually is feasible in the presence of a single brain metastasis; therefore, the applicability of results in clinical practice should be based on this consideration. The second aspect is related to the ability of patients to perform ordinary tasks, since the Karnofsky performance status (KPS) score of participants in the studies ranged from 70 to 100.
Certainty of the evidence
The two trials provide low or very low‐certainty of the evidence for the outcomes overall survival, adverse events, progression‐free survival and quality of life. We downgraded the certainty of the evidence mainly due to high risk of bias and imprecision.
One of the reasons to consider that the studies have a high risk of bias is because of lack of blinding. This bias is especially important for the outcomes, adverse events and quality of life. Lack of blinding may have a strong influence on how participants and researchers rate these two outcomes. Even though blinding of interventions is not possible when comparing these two procedures, researchers did not use other ways to reduce the risk of bias; for instance, blinding during data analysis.
Finally, imprecision is explained by the small sample size in the two studies; neither could reach the calculated sample size. Accrual difficulties shown in the two studies, as well as in other studies comparing surgery versus stereotactic radiosurgery in a clinical trial, might be explained by several reasons: the facts that single and solitary brain metastasis is not frequent; because few people are candidates for both surgery and stereotactic radiosurgery; because of physicians' preferences for either surgical resection or stereotactic radiosurgery; or because of patients' values and preferences. For instance, in one of the included studies 17 out of 40 participants declined to participate in the trial because they did not want either surgery or stereotactic radiosurgery.
Potential biases in the review process
We conducted this review according to Cochrane methodology in order to minimise the risk of bias by assessing eligibility, evaluating risk of bias and carrying out data extraction independently by more than one review author; also, by a narrative account of results due to clinical heterogeneity. However, despite our effort to include all published studies comparing surgery to stereotactic radiotherapy for people with single or solitary brain metastasis, it is possible that we did not identify all relevant data. The small number of trials identified in our review raises concerns about publication bias. We could not carry out funnel plot analysis in order to identify this bias since we included fewer than 10 studies in the review. We contacted trial authors during the identification of trials in order to clarify questions related to eligibility criteria (Appendix 4), to complete data extraction and analysis and to discover if they knew other trials that addressed our review question. We took into account published and unpublished data during the review process. We received no reply from researchers of one of the included studies, therefore we considered the missing information as reporting bias.
Agreements and disagreements with other studies or reviews
A clinical practice guideline (CPG) based on a systematic review analysed the role of surgical resection in the management of newly diagnosed brain metastases (Kalkanis 2010). The review included surgery versus stereotactic radiosurgery studies, among other comparisons. For the comparison, surgical resection plus WBRT versus stereotactic radiosurgery, the CPG authors concluded that it is difficult to offer firm guidelines based upon a prematurely closed study, referring to the Muacevic 2008 trial. For the comparison of surgical resection plus WBRT versus stereotactic radiosurgery plus WBRT the CPG authors recommend stereotactic radiosurgery for single surgically inaccessible lesions measuring less than 3 cm in maximum diameter based on four retrospective studies (N = 368). The randomised controlled trial (RCT) from Roos 2011 was not available when the Kalkanis 2010 CPG was published.
New strategies are emerging regarding the management of brain metastases, including the use of stereotactic radiotherapy of the surgical bed after surgical metastasectomy or the avoidance of WBRT after stereotactic radiosurgery of brain metastases under certain circumstances. Two RCTs were recently published exploring the value of stereotactic radiosurgery after surgical resection for brain metastasis. One study (Mahajan 2017), focused on local control, concluding that stereotactic radiosurgery of the resection cavity of 1 to 3 brain metastases, decreases local recurrence after 11 months of follow‐up compared with observation. The second study (Brown 2017), focused in cognitive deterioration and overall survival, when stereotactic radiosurgery is compared with WBRT after one metastasis resection. The stereotactic radiosurgery group (98/194 patients) showed significantly better cognitive evolution, although overall survival was similar.
Authors' conclusions
Implications for practice.
Currently, solitary or single brain metastasis is relatively infrequent, however this is likely to change due to advances in diagnosis, often leading to longer survival times. Clinicians may consider that more radical treatment is indicated, when possible, where patients have single or solitary brain metastasis. Surgery or fractionated stereotactic radiotherapy/stereotactic radiosurgery with or without WBRT may be considered. From this review it is concluded that evidence on effectiveness and safety of surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis in terms of overall survival, progression‐free survival or quality of life is weak. Nevertheless, as radical treatment for single or solitary brain metastasis is usually offered to patients the benefits must be determined on a case‐by‐case basis. Well designed and powered studies are needed.
Implications for research.
The main issue regarding studies on single and solitary brain metastasis is participant accrual therefore future studies should have a multicentred, and ideally, multinational base which would result in a more reliable and comprehensive view of the interventions available.
Acknowledgements
The review authors wish to thank all the members of Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers for editorial guidance. We also wish to thank to Víctor Andrés Velásquez Rimachi, student of Medicine in San Fernando's Faculty of Medicine of Universidad Nacional Mayor de San Marcos (Lima, Perú) for participating in the handsearching screening, data extraction and assessment of risk of bias.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health, UK.
Appendices
Appendix 1. Search strategy
MEDLINE search strategy (Ovid)
1 Radiosurgery/ 2 (radiosurg* or stereotactic or linear accelerator or cyberknife or gamma knife or linac).mp. 3 1 or 2 4 surgery.fs. 5 exp Neurosurgical Procedures/ 6 (surg* or neurosurg* or excis*).mp. 7 4 or 5 or 6 8 exp Brain Neoplasms/ 9 ((brain or cerebral or cerebellum) adj5 (tumor* or tumour* or neoplas* or cancer* or carcinoma* or malignan* or metast*)).mp. 10 8 or 9 11 3 and 7 and 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 clinical trials as topic.sh. 17 randomly.ab. 18 trial.ti. 19 12 or 13 or 14 or 15 or 16 or 17 or 18 20 11 and 19 21 exp animals/ not humans.sh. 22 20 not 21
key:
mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, fs = floating subheading, ab = abstract, sh = subject heading, pt = publication type.
Embase search strategy (Ovid)
1 exp Radiosurgery/ 2 (radiosurg* or stereotactic or linear accelerator* or cyberknife or gamma knife or linac*).mp. 3 1 or 2 4 su.fs. 5 exp Neurosurgery/ 6 (surg* or neurosurg* or excis* or resect*).mp. 7 4 or 5 or 6 8 exp Brain tumor/ 9 ((brain or cerebral or cerebellum) adj5 (tumor* or tumour* or neoplas* or cancer* or carcinoma* or malignan* or metast*)).mp. 10 8 or 9 11 3 and 7 and 10 12 crossover procedure/ 13 double‐blind procedure/ 14 randomized controlled trial/ 15 single‐blind procedure/ 16 random*.mp. 17 factorial*.mp. 18 (crossover* or cross over* or cross‐over*).mp. 19 placebo*.mp. 20 (double* adj blind*).mp. 21 (singl* adj blind*).mp. 22 assign*.mp. 23 allocat*.mp. 24 volunteer*.mp. 25 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 11 and 25
CENTRAL search strategy
#1MeSH descriptor: [Radiosurgery] explode all trees #2(radiosurg* or stereotactic or linear accelerator* or cyberknife or gamma knife or linac*).mp. #3#1 or #2 #4MeSH descriptor: [General Surgery] this term only #5 Any MeSH descriptor with qualifier(s): [Surgery ‐ SU] #6MeSH descriptor: [Neurosurgery] this term only #7 (surg* or neurosurg* or excis* or resect*).mp. #8#4 or #5 or #6 or #7 #9MeSH descriptor: [Brain Neoplasms] explode all trees #10 ((brain or cerebral or cerebellum) near/5 (tumor* or tumour* or neoplas* or cancer* or carcinoma* or malignan* or metast*)).mp. #11 #9 or #10 #12 #3 and #8 and #11
Appendix 2. Other databases search strategy
ClinicalTrials.gov (www.clinicalTrials.gov)
Radiosurgery AND "brain metastases" | Interventional Studies cyberknife AND "brain metastases" "gamma knife" AND brain
UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk)
brain AND metastases AND radiosurgery brain AND cyberknife "gamma knife" AND brain
EU Clinical Trials register (www.clinicaltrialsregister.eu)
Radiosurgery AND "brain metastases" cyberknife AND "brain metastases" "gamma knife" AND brain
World Health Organization (WHO) International Clinical Trials Registry Platform Search Portal (apps.who.int/trialsearch)
"brain metastases" AND Radiosurgery cyberknife AND brain "gamma knife" AND brain
Open Grey (www.opengrey.eu)
Brain metastas*
Appendix 3. Data collection form
| Review: Surgery versus radiosurgery for participants with single or solitary brain metastasis | |
|
Study ID (surname of first author and year first full report of study was published e.g. Smith 2001) | |
| Report ID | |
| General Information | |
| Date form completed | (dd/mm/yyyy) |
| Name/ID of person extracting data | |
| Reference citation | |
| Study author contact details | |
| Publication type | (e.g. full report, abstract, letter) |
| Study eligibility | |
| 1. Type of study (Randomised controlled trials) |
(Yes or no) |
| 2. Participants (Adults aged 18 years or more, with single or solitary brain metastasis of any size, a biopsy‐proven malignancy of any tumour histology, no previous cranial radiation, and any chemotherapy or target therapy administered before the study intervention) |
(Yes or no) |
| 3. Types of intervention (Any neurosurgical technique where complete resection was intended) |
(Yes or no) |
| 4. Types of comparison (Any type of radiosurgery: robotic delivering of radiation, multiple convergent sources of cobalt or other technical devices adapted to linear accelerators e.g. any form of LINAC stereotactic radiotherapy with or without relocatable frame using single or multiple fractions or Gamma Knife radiosurgery |
(Yes or no) |
| Inclusion (Do not proceed if the study does not meet the four eligibility criteria) |
(Included or excluded) |
| Reason for exclusion | |
| Notes(any other information you consider important) | |
| Methods (descriptions as stated in report/paper) | |
| Country | (where the study was conducted) |
| Design | (e.g. parallel, cluster) |
| Was the study multicentred? | (if yes, state No. of centres) |
| Funders of the trial | |
| Duration of trial | (state start date and end date of trial) |
| Duration of participation | (from start of recruitment to last follow‐up) |
| Ethical approval needed/obtained for study | (Yes, no, unclear) |
| Notes (any other information you consider important) | |
| Participants (Include comparative information for each intervention or comparison group if available) | |
| Population description | (Describe any risk factors, and criteria for diagnosing the metastasis) |
| Setting | (From where were participants enrolled?) |
| Inclusion criteria | |
| Exclusion criteria | |
| Method of recruitment of participants | (e.g. phone, mail, clinic patients) |
| Total no. randomised | |
| No. of participants assigned to each group | |
| No. of participants receiving the intended treatment | |
| No. of participants analysed | |
| Withdrawals and exclusions | (If not provided below by outcome) |
| Age | |
| Sex | |
| Race/ethnicity | |
| Characteristics of the primary tumour and metastasis | (Time to diagnosis, time to metastasis, characteristics of brain metastasis, solitary versus single?) |
| Notes (any other information you consider important) | |
| Intervention group | |
| Intervention name | |
| No. randomised to group | (Specify whether no. people or clusters) |
| Details of the intervention | (Type of surgery, details of the technique) |
| (Was a postoperative confirmation of metastasis resection with MRI done?) | |
| Cointerventions |
(Any additional interventions given e.g. WBRT, chemotherapy or other target therapy. Has the cointervention been used equally in both study groups) |
| Notes (any other information you consider important) | |
| Comparator group | |
| Intervention name |
E.g. robotic delivering of radiation, LINAC stereotactic radiotherapy, gamma knife radiosurgery or other multiple convergent sources of cobalt or other technical devices adapted to linear accelerators |
| No. randomised to group | |
| Details of the intervention | (Brand, doses, frequency, duration) |
| Cointerventions | (Any additional interventions given e.g. WBRT, chemotherapy or other target therapy. Has the cointervention been used equally in both study groups) |
| Notes (any other information you consider important) | |
| Outcome characteristics | |
| Outcome 1 name | Overall survival: survival from the intervention (surgery or radiosurgery) until death from any cause |
| Time points measured |
(specify whether from start or end of intervention) (How long was the follow‐up for this outcome?) |
| Time points reported | |
| Person measuring/reporting | |
| Imputation of missing data | (e.g. assumptions made for ITT analysis) |
| Notes (any other information you consider important) | |
| Outcome 2 name | Any adverse event |
| Time points measured |
(Specify whether from start or end of intervention) (How long was the follow‐up for this outcome?) |
| Person measuring/reporting | |
| Notes (any other information you consider important) | |
| Outcome 3 name | Survival free of brain relapses: survival from the intervention until the diagnosis of a new brain metastasis by imaging, either by computed tomography (CT) or by magnetic resonance (MRI) |
| Time points measured |
(Specify whether from start or end of intervention) (How long was the follow‐up for this outcome?) |
| Person measuring/reporting | |
| Imputation of missing data | (e.g. assumptions made for ITT analysis) |
| Notes (any other information you consider important) | |
| Outcome 4 name | Quality of life: assessed through validated questionnaires |
| Time points measured | |
| Time points reported | |
| Person measuring/reporting | |
| How was quality of life assessed? (measurement scale) |
|
| Scales: upper and lower limits (indicate whether high or low score is good) |
|
| Is outcome/tool validated? | |
| Imputation of missing data | (e.g. assumptions made for ITT analysis) |
| Notes (any other information you consider important) | |
| Data and analysis (Descriptions as stated in report/paper) | |
| Outcome 1. Overall survival: survival from the intervention until death from any cause | |
| Comparison | |
| Outcome | |
| Subgroups | |
| Time point | (Specify from start or end of intervention) |
| Results |
Intervention # Event Total in group Control # Event Total in group |
| Any other results reported | (e.g. odds ratio, risk difference, CI or P value) |
| No. participants moved from other group | # Reason |
| Unit of analysis | (By individuals, cluster/groups or body parts) |
| Statistical methods used and appropriateness of these | Was any adjustment done? |
| Notes (any other information you consider important) | |
| Outcome 2. Any adverse event | |
| Comparison | |
| Outcome | |
| Subgroups | |
| Time point | (Specify from start or end of intervention) |
| Results |
Intervention # Event Total in group Control # Event Total in group |
| Any other results reported | (e.g. odds ratio, risk difference, CI or P value) |
| No. missing participants | # Reason |
| No. participants moved from other group | # Reason |
| Unit of analysis | (By individuals, cluster/groups or body parts) |
| Statistical methods used and appropriateness of these | Was any adjustment done? |
| Notes (any other information you consider important) | |
| Outcome 3. Survival free of brain relapses | |
| Comparison | |
| Outcome | |
| Subgroups | |
| Time point | (Specify from start or end of intervention) |
| Results |
Intervention # Event Total in group Control # Event Total in group |
| Any other results reported | (e.g. odds ratio, risk difference, CI or P value) |
| No. participants moved from other group | # Reason |
| Unit of analysis | (By individuals, cluster/groups or body parts) |
| Statistical methods used and appropriateness of these | Was any adjustment done? |
| Notes (any other information you consider important) | |
| Outcome 4. Quality of life: assessed through validated questionnaires | |
| Comparison | |
| Outcome | |
| Subgroups | |
| Time point | (Specify from start or end of intervention) |
| Results |
Intervention Mean, median Standard deviation Total in group Control Mean, median Standard deviation Total in group |
| Any other results reported | (e.g. odds ratio, risk difference, CI or P value) |
| No. missing participants | # Reason |
| No. participants moved from other group | # Reason |
| Unit of analysis | (By individuals, cluster/groups or body parts) |
| Statistical methods used and appropriateness of these | Was any adjustment done? |
| Notes (any other information you consider important) | |
| Risk of bias assessment | |
| Random sequence generation (selection bias) | Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Allocation concealment (selection bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of participants and personnel for outcome 1 (overall survival) (performance bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of participants and personnel for outcome 2 (any adverse event) (performance bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of participants and personnel for outcome 3 (survival free of brain relapses) (performance bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of participants and personnel for outcome 4 (quality of life) (performance bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of outcome 1 assessment (overall survival) (detection bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of outcome 2 assessment (any adverse event) (detection bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of outcome 3 assessment (survival free of brain relapses) (detection bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Blinding of outcome 4 assessment (quality of life) (detection bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Incomplete outcome 1 data (overall survival) (attrition bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Incomplete outcome 2 data (any adverse event) (attrition bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Incomplete outcome 3 data (survival free of brain relapses) (attrition bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Incomplete outcome 4 data (quality of life) (attrition bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Selective outcome reporting? (reporting bias) |
Low risk High risk Unclear (Include direct quotes where available with explanatory comments) |
| Notes (any other information you consider important) | |
Appendix 4. Authors contacted
Friedrich W. Kreth, Frederick F. Lang, Alexander Muacevic, Ricardo A Nakamura, Daniel E. Roos, Gelareh Zadeh.
Data and analyses
Comparison 1. Surgery plus WBRT versus stereotactic radiosurgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Surgery plus WBRT versus stereotactic radiosurgery, Outcome 1 Overall survival.
1.2. Analysis.
Comparison 1 Surgery plus WBRT versus stereotactic radiosurgery, Outcome 2 Adverse events.
Comparison 2. Surgery plus WBRT versus stereotactic radiosurgery plus WBRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | 21 | Hazard Ratio (95% CI) | 0.53 [0.20, 1.42] |
| 2 Adverse events | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.05, 2.98] |
| 3 Progression‐free survival | 1 | 21 | Hazard Ratio (95% CI) | 0.55 [0.22, 1.38] |
| 4 Quality of life | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐4.07 [‐23.65, 15.50] |
| 4.1 Baseline | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐24.69, 23.29] |
| 4.2 At 2 months | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐10.80 [‐44.67, 23.07] |
2.1. Analysis.
Comparison 2 Surgery plus WBRT versus stereotactic radiosurgery plus WBRT, Outcome 1 Overall survival.
2.2. Analysis.
Comparison 2 Surgery plus WBRT versus stereotactic radiosurgery plus WBRT, Outcome 2 Adverse events.
2.3. Analysis.
Comparison 2 Surgery plus WBRT versus stereotactic radiosurgery plus WBRT, Outcome 3 Progression‐free survival.
2.4. Analysis.
Comparison 2 Surgery plus WBRT versus stereotactic radiosurgery plus WBRT, Outcome 4 Quality of life.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Muacevic 2008.
| Methods | Design: two‐group parallel RCT Unit of randomisation: 1:1 participants Unit of analysis: participants with brain metastasis who received any intervention Follow‐up: 12‐month follow‐up was after the last participant was enrolled Intention‐to‐treat: yes Overall study quality: high risk of bias |
|
| Participants | Number of centres: 4 Setting: University Duesseldorf, University Bonn, Klinikum Augsburg, University Hospital Munich Country: Germany Number of participants randomised: 64 Age (years; mean and SD)
Gender: women = 37 (58%) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Stereotactic radiosurgery (n = 31) Gamma Knife surgery alone (Elekta, Stockholm, Sweden). Gamma Knife surgery was administered using stereotactic MRI guidance. The treatment was performed on an outpatient basis. The mean dose applied to the tumour margin (prescribed tumour dose) was 21 Gy (range: 14 Gy to 27 Gy). The prescribed tumour dose was in the range of 20 Gy to 27 Gy for radioresistant tumours (such as melanoma, hypernephroma) and in the range of 14 Gy to 20 Gy for more radiosensitive tumours (such as breast cancer). The mean maximum dose was 41 Gy (range: 28 Gy to 54 Gy), and on average, the 50% isodose (range: 35% to 85%) was used to irradiate the tumour margin. Conformal multiple isocenter Gamma Knife surgery (mean number of isocentres per participant: 7) was performed in all participants. Surgery (n = 33) Microsurgical techniques plus WBRT. Navigational devices were applied according to the decision of the treating surgeon. WBRT was started within the first 14 days after tumour resection using lateral ports covering the brain and meninges to the foramen magnum. Patients received 40 Gy over 4 weeks (2 Gy x 20 fractions). Cointerventions The study protocol did not stipulate steroid management, but steroid dose prescriptions were recorded at each visit. |
|
| Outcomes |
Main outcome
Secondary outcomes
Assessed on day 1 after randomisation, 6 weeks, and 6 months after treatment
|
|
| Notes | Identifier number: not reported A priori sample estimation: yes (242 participants) Conflicts of interest: not reported Conduction trial date: October 1999 ‐ 1 November 2004 (stopped prematurely due to poor participant accrual). Funding/support: "Elekta Research Foundation. The sponsor had no role in study design, data collection, data interpretation, or writing of the report". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed centrally at the data center by telephone (Biometrisches Zentrum für Therapiestudien, München, Germany)." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding of participants is difficult due to the nature of the intervention. However, authors did not mention other ways to reduce the risk of bias, such as blinding during data analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: even though blinding of participants is difficult due to the nature of the intervention, study authors did not mention other ways to reduce the risk of bias, such as blinding during data analysis. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Health Related QoL (HRQoL) scores were available at baseline and post baseline for 48/64 participants". |
| Selective reporting (reporting bias) | High risk | Comment: 95% CIs are not reported |
| Other bias | Low risk | Quote: "The sponsor had no role in study design, data collection, data interpretation, or writing of the report." |
Roos 2011.
| Methods | Design: two‐group parallel RCT Unit of randomisation: 1:1 participants Unit of analysis: participants with brain metastasis who received any intervention Follow‐up: 5 years Intention‐to‐treat: yes Overall study quality: low risk of bias |
|
| Participants | Number of centres: 1 Setting: Royal Adelaide Hospital Country: Australia Number of participants: 22 consented to randomisation and 21 were analysed Age (years; mean and SD):
Gender: women = 11 (50%) Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Stereotactic radiosurgery (n = 11) Stereotactic radiosurgery was planned on a Fischer‐Leibinger system and delivered on a Varian 600C/D linear accelerator using multiple arcs with circular collimators. The dose was prescribed to the 70% to 90% isodose envelope and was based upon lesion size: 20 Gy, 18 Gy and 15 Gy marginal dose for maximum tumour diameter 20 mm, 21 mm to 30 mm and 31 to 40 mm, respectively. Surgery (n = 10) Surgery was carried out using standard stereotactic‐guided neurosurgical techniques. Cointerventions WBRT in both groups. Dose was 30 Gy in 10 fractions to the midplane using opposed lateral beams (clinical mark‐up) over 2 to 2.5 weeks. Corticosteroid use and treatment for extracranial disease and brain relapse were at the clinician’s discretion. Length of treatment: 6.5 weeks |
|
| Outcomes |
Primaries outcomes
Assessed at randomisation, 2 and 3 months after starting treatment, then 3 monthly thereafter until relapse Secondary outcomes
|
|
| Notes | Trial register number: NCT00124761 A priori sample estimation: yes (200 participants) Conflicts of interest: not reported Conduction trial date: between 16 February 2003 and 2 April 2009 Funding/support: Royal Australian and New Zealand College of Radiologists Research Grant and by Royal Adelaide Hospital research funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was based on a permuted block design with randomly varied block sizes of four or six, not stratified by prognostic factors" |
| Allocation concealment (selection bias) | Low risk | Quote: "were allocated by telephoning the trial centre" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding to trial arm will not be feasible." Comment: lack of blinding may affect one of the primary outcomes (QoL) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Blinding to trial arm will not be feasible." Comment: lack of blinding may affect one of the primary outcomes (QoL) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: one participant randomised did not receive an intervention |
| Selective reporting (reporting bias) | Low risk | Comment: the article exposes all findings in relation to the published primary and secondary outcomes of the protocol |
| Other bias | Low risk | Comment: the study included two groups in the same condition of cointervention |
CI: confidence interval BCM20: Brain Cancer Module 20 EORTC: European Organization for Research and Treatment of CancerGy: Gray HRQoL: health‐related quality of life KPS: Karnofsky Performance Score MRI: magnetic resonance imaging RCT: randomised controlled trial RTOG: Radiation Therapy Oncology Group QoL: quality of life WBRT: whole brain radiotherapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fogarty 2014 | Pilot study to assess the feasibility of a trial on whole brain radiotherapy (WBRT) following local treatment of intracranial melanoma metastases with neurosurgery and/or stereotactic radiosurgery. None of the review outcomes were measured in this trial. The study outcomes in the trial were: recruitment rate, number of participants included by centre, experience of the centre in trials, feasibility forecasts per centre, logistic issues. |
| Kocher 2011 | Randomisation was carried out after surgery or stereotactic radiosurgery |
| NCT01295970 | This study stopped prior to enrolment due to accrual difficulties. No results available |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00460395.
| Methods | Prospective, randomised trial Masking: open‐label |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Whole brain radiotherapy may be given to participants who demonstrate local or distant recurrence and can be given as the primary therapy or as adjunctive therapy. The WBRT dose will be 30 Gy delivered in 10 fractions. |
| Outcomes |
|
| Notes | Principal Investigator: Frederick F. Lang, M.D. Universtity of Texas MD Anderson Cancer Center Location: University of Texas MD Anderson Cancer Center, Houston, Texas, United States, 77030 Sponsors and Collaborators: MD Anderson Cancer Center This study has been completed. |
Gy: Gray KPS: Karnofsky Performance Score MRI: magnetic resonance imaging QoL: quality of life WBRT: whole brain radiotherapy
Differences between protocol and review
We made the following changes to the protocol (Fuentes 2016).
1. We modified the title from "Surgery versus radiosurgery for people with single or solitary brain metastases" to "Surgery versus stereotactic radiotherapy for people with single or solitary brain metastasis" to explain better the nature of the interventions. “Stereotactic radiotherapy” includes both radiosurgery given as a single dose (stereotactic radiosurgery) or in a number of fractions (fractionated stereotactic radiotherapy).
2. We added an additional author (Maria José Martínez‐Zapata).
3. We included two more appendices ‐ Appendix 4 contains the list of the authors that we contacted during the data collection and analysis stage.
4. We added the following text in the section 'Types of interventions (Comparison)': "The number of fractions was not a criterion of eligibility in the review and therefore the review also includes fractionated stereotactic radiotherapy. We included studies using either a single (stereotactic radiosurgery) or a multifraction treatment (fractionated stereotactic radiotherapy), as long as they met the remaining eligibility criteria".
5. Electronic searches; we did not search CINAHL.
6. The review authors were not blinded to the study author’s name, study institution, journal or study results during the selection of the studies.
7. Due to scarcity of evidence we were unable to carry out the following methods.
We will assess heterogeneity of effect sizes by visual inspection of forest plots and we will measure heterogeneity using the I2 statistic.
We will assess potential reporting bias of the review using a funnel plot when 10 or more studies are available.
Exploration of heterogeneity and sensitivity analyses.
Subgroup analysis.
8. Calculation of hazard ratio and relative risk: we calculated the hazard ratio for those studies not reporting this measure but reporting other information such as the number of deaths in each group and the P value of Kaplan–Meier curves. For this calculation we used the spreadsheet to facilitate the estimation of hazard ratios from published summary statistics or data extracted from Kaplan‐Meier curves created by Tierney et al (Tierney 2007).
When the risk ratio was not reported for dichotomous outcomes, we calculated it using the data analyses tool of the Review Manager software (Review Manager 2014).
9. In the section 'Subgroup analysis and investigation of heterogeneity' we added the following criteria for the subgroup analyses : "by the number of fractions in the stereotactic radiosurgery treatment" to those defined in the protocol: by the location of the primary tumour (lung cancer, breast cancer, renal cell carcinoma, melanoma, and others), by the clinical presentations (solitary or single), and by the use of WBRT or chemotherapy, or both as co interventions.
Contributions of authors
Rafael Fuentes (RF), Dimelza Osorio (DO), José Expóstio‐Hernández (JEH), Daniel Simancas‐Racines (DSR), Maria José Martinez‐Zapata (MJMZ), Xavier Bonfill Cosp (XBC) contributed to the following tasks.
Conceiving the review: RF, DO, JEH, XBC
Designing the review: RF, DO, JEH, DSR, XBC
Co‐ordinating the review: DO
Screening search results: RF, DO, JEH, DSR, MJMZ
Organising retrieval of papers: DO
Screening retrieved papers against inclusion criteria: RF, DO, JEH, DSR, MJMZ
Appraising quality of papers: RF, DO, JEH, DSR, MJMZ
Abstracting data from papers: RF, DO, JEH, DSR, MJMZ
Writing to authors of papers for additional information: DO
Obtaining and screening data on unpublished studies: DO
Data management for the review: DO
Entering data into Review Manager (RevMan) (Review Manager 2014): DO, MJMZ
RevMan statistical data: DO, MJMZ
Other statistical analysis not using RevMan: none
Double‐entry of data: DO, MJMZ
Interpretation of data: RF, DO, JEH, DSR, MJMZ
Statistical inferences: RF, DO, JEH, DSR, MJMZ
Writing the review: RF, DO, JEH, DSR, MJMZ, XBC
Providing guidance on the review: MJMZ, XBC
Securing funding for the review: XBC
Guarantor for the review (one author): RF
Person responsible for reading and checking review before submission: RF, DO, JEH, DSR, MJMZ, XBC
All review authors drafted and approved the final version of the review.
Sources of support
Internal sources
Nil, Other.
External sources
Nil, Other.
-
Instituto de Salud Carlos III, Spain.
Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116).
Declarations of interest
Rafel Fuentes: no conflict of interest related to this review.
Dimelza Osorio: no conflict of interest related to this review.
José Expósito Hernandez: no conflict of interest related to this review.
Daniel Simancas‐Racines: no conflict of interest related to this review.
Maria José Martínez‐Zapata: no conflict of interest related to this review.
Xavier Bonfill Cosp: no conflict of interest related to this review.
New
References
References to studies included in this review
Muacevic 2008 {published data only}
- Muacevic A, Wowra B, Siefert A, Tonn JC, Steiger HJ, Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. Journal of Neuro‐oncology 2008;87(3):299‐307. [PUBMED: 18157648] [DOI] [PubMed] [Google Scholar]
Roos 2011 {published and unpublished data}
- Roos DE, Smith JG, Stephens SW. Radiosurgery versus surgery, both with adjuvant whole brain radiotherapy, for solitary brain metastases: a randomised controlled trial. Clinical Oncology (Royal College of Radiologists (Great Britain)) 2011;23(9):646‐51. [PUBMED: 21592754] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fogarty 2014 {published data only}
- Fogarty GB, Hong A, Jacobsen KD, Reisse CH, Shivalingam B, Burmeister B, et al. Accrual to a randomised trial of adjuvant whole brain radiotherapy for treatment of melanoma brain metastases is feasible. BMC Research Notes 2014;7:412. [PUBMED: 24981506] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocher 2011 {published data only}
- Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, et al. Adjuvant whole‐brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952‐26001 study. Journal of Clinical Oncology 2011;29(2):134‐41. [PUBMED: 21041710] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole‐brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality‐of‐life results. Journal of Clinical Oncology 2013;31(1):65‐72. [PUBMED: 23213105] [DOI] [PubMed] [Google Scholar]
NCT01295970 {published data only}
- NCT01295970. Surgery versus radiosurgery for the treatment of single brain metastases [Feasibility of a prospective, randomized trial comparing surgery versus radiosurgery for the treatment of single brain metastases]. clinicaltrials.gov/ct2/show/NCT01295970 (first received 15 February 2011).
References to studies awaiting assessment
NCT00460395 {unpublished data only}
- NCT00460395. Surgery Versus Stereotactic Radiosurgery in the Treatment of Single Brain Metastasis: A Randomized Trial. clinicaltrials.gov/ct2/show/study/NCT00460395 (first received16 April 2007).
Additional references
Andrews 2004
- Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363(9422):1665‐72. [PUBMED: 15158627] [DOI] [PubMed] [Google Scholar]
Aoyama 2003
- Aoyama H, Shirato H, Onimaru R, Kagei K, Ikeda J, Ishii N, et al. Hypofractionated stereotactic radiotherapy alone without whole‐brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. International Journal of Radiation Oncology, Biology, Physics 2003;56(3):793‐800. [PUBMED: 12788187] [DOI] [PubMed] [Google Scholar]
Barnholtz‐Sloan 2004
- Barnholtz‐Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. Journal of Clinical Oncology 2004;22(14):2865‐72. [PUBMED: 15254054] [DOI] [PubMed] [Google Scholar]
Bouffet 1997
- Bouffet E, Doumi N, Thiesse P, Mottolese C, Jouvet A, Lacroze M, et al. Brain metastases in children with solid tumors. Cancer 1997;79(2):403‐10. [PUBMED: 9010115] [DOI] [PubMed] [Google Scholar]
Bougie 2015
- Bougie E, Masson‐Cote L, Mathieu D. Comparison between surgical resection and stereotactic radiosurgery in patients with a single brain metastasis from non‐small cell lung cancer. World Neurosurgery 2015;83(6):900‐6. [PUBMED: 25659803] [DOI] [PubMed] [Google Scholar]
Bradley 2004
- Bradley KA, Mehta MP. Management of brain metastases. Seminars in Oncology 2004;31(5):693‐701. [PUBMED: 15497123] [DOI] [PubMed] [Google Scholar]
Brown 2017
- Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncology 2017;18(8):1049‐60. [PUBMED: 28687377] [DOI] [PMC free article] [PubMed] [Google Scholar]
Delattre 1988
- Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Archives of Neurology 1988;45(7):741‐4. [PUBMED: 3390029] [DOI] [PubMed] [Google Scholar]
Flickinger 1994
- Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi‐institutional experience with stereotactic radiosurgery for solitary brain metastasis. International Journal of Radiation Oncology, Biology, Physics 1994;28(4):797‐802. [PUBMED: 8138431] [DOI] [PubMed] [Google Scholar]
Gavrilovic 2005
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. Journal of Neuro‐oncology 2005;75(1):5‐14. [PUBMED: 16215811] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 20 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knotterus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [PUBMED: 15205295] [DOI] [PubMed] [Google Scholar]
Guyatt 2012
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ (Clinical research ed.) 2012;344:e3863. [PUBMED: 22705814] [DOI] [PubMed] [Google Scholar]
Hart 2005
- Hart MG, Walker M, Dickinson HO, Grant R. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD003292.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Joseph 1996
- Joseph J, Adler JR, Cox RS, Hancock SL. Linear accelerator‐based stereotactic radiosurgery for brain metastases: the influence of number of lesions on survival. Journal of Clinical Oncology 1996;14(4):1085‐92. [PUBMED: 8648361] [DOI] [PubMed] [Google Scholar]
Kalkanis 2010
- Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence‐based clinical practice guideline. Journal of Neuro‐oncology 2010;96(1):33‐43. [PUBMED: 19960230] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lassman 2003
- Lassman AB, DeAngelis LM. Brain metastases. Neurologic Clinics 2003;21(1):1‐23, vii. [PUBMED: 12690643] [DOI] [PubMed] [Google Scholar]
Mahajan 2017
- Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post‐operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single‐centre, randomised, controlled, phase 3 trial. Lancet Oncology 2017;18(8):1040‐8. [PUBMED: 28687375] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mintz 1996
- Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78(7):1470‐6. [PUBMED: 8839553] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS medicine 2009;6(7):e1000097. [PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muacevic 1999
- Muacevic A, Kreth FW, Horstmann GA, Schmid‐Elsaesser R, Wowra B, Steiger HJ, et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. Journal of Neurosurgery 1999;91(1):35‐43. [PUBMED: 10389878] [DOI] [PubMed] [Google Scholar]
Mulvenna 2016
- Mulvenna P, Nankivell M, Barton R, Faivre‐Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non‐small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non‐inferiority, randomised trial. Lancet 2016;388(10055):2004‐14. [PUBMED: 27604504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nayak 2012
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Current Oncology Reports 2012;14(1):48‐54. [PUBMED: 22012633] [DOI] [PubMed] [Google Scholar]
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795‐801. [PUBMED: 15148066] [DOI] [PubMed] [Google Scholar]
Nolan 2018
- Nolan C, Deangelis LM. Overview of metastatic disease of the central nervous system. Handbook of clinical Neurology 2018;149:3‐23. [PUBMED: 29307359] [DOI] [PubMed] [Google Scholar]
Pannullo 2011
- Pannullo S, Yama C, Wernicke AG. Stereotactic radiosurgery for brain tumors. In: Abujamra AL editor(s). Diagnostic Techniques and Surgical Management of Brain Tumors. InTech, 2011. [Google Scholar]
Patchell 1990
- Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New England Journal of Medicine 1990;322(8):494‐500. [PUBMED: 2405271] [DOI] [PubMed] [Google Scholar]
Patchell 1998
- Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280(17):1485‐9. [PUBMED: 9809728] [DOI] [PubMed] [Google Scholar]
Patchell 2003
- Patchell RA. The management of brain metastases. Cancer Treatment Reviews 2003;29(6):533‐40. [PUBMED: 14585263] [DOI] [PubMed] [Google Scholar]
Patil 2012
- Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006121.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruitt 2017
- Pruitt AA. Epidemiology, treatment, and complications of central nervous system metastases. Continuum (Minneap Minn) 2017;23(6):1580‐1600. [PUBMED: 29200112] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sterne 2011
- Sterne JA, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Suh 2010
- Suh JH. Stereotactic radiosurgery for the management of brain metastases. New England Journal of Medicine 2010;362(12):1119‐27. [PUBMED: 20335588] [DOI] [PubMed] [Google Scholar]
Sundermeyer 2005
- Sundermeyer ML, Meropol NJ, Rogatko A, Wang H, Cohen SJ. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clinical Colorectal Cancer 2005;5(2):108‐13. [PUBMED: 16098251] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vecht 1993
- Vecht CJ, Haaxma‐Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery?. Annals of Neurology 1993;33(6):583‐90. [PUBMED: 8498838] [DOI] [PubMed] [Google Scholar]
Westphal 2003
- Westphal M, Heese O, Wit M. Intracranial metastases: therapeutic options. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO 2003;14 Suppl 3:iii4‐10. [PUBMED: 12821532] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fuentes 2016
- Fuentes R, Osorio D, Expósito Hernandez J, Simancas‐Racines D, Bonfill Cosp X. Surgery versus radiosurgery for people with single or solitary brain metastases. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012086] [DOI] [PMC free article] [PubMed] [Google Scholar]


