Abstract
Background
Alzheimer's disease is the most common cause of dementia in older people. One approach to symptomatic treatment of Alzheimer's disease is to enhance cholinergic neurotransmission in the brain by blocking the action of the enzyme responsible for the breakdown of the neurotransmitter acetylcholine. This can be done by a group of drugs known as cholinesterase inhibitors. Donepezil is a cholinesterase inhibitor.
This review is an updated version of a review first published in 1998.
Objectives
To assess the clinical efficacy and safety of donepezil in people with mild, moderate or severe dementia due to Alzheimer's disease; to compare the efficacy and safety of different doses of donepezil; and to assess the effect of donepezil on healthcare resource use and costs.
Search methods
We searched Cochrane Dementia and Cognitive Improvement’s Specialized Register, MEDLINE, Embase, PsycINFO and a number of other sources on 20 May 2017 to ensure that the search was as comprehensive and up‐to‐date as possible. In addition, we contacted members of the Donepezil Study Group and Eisai Inc.
Selection criteria
We included all double‐blind, randomised controlled trials in which treatment with donepezil was administered to people with mild, moderate or severe dementia due to Alzheimer's disease for 12 weeks or more and its effects compared with those of placebo in a parallel group of patients, or where two different doses of donepezil were compared.
Data collection and analysis
One reviewer (JSB) extracted data on cognitive function, activities of daily living, behavioural symptoms, global clinical state, quality of life, adverse events, deaths and healthcare resource costs. Where appropriate and possible, we estimated pooled treatment effects. We used GRADE methods to assess the quality of the evidence for each outcome.
Main results
Thirty studies involving 8257 participants met the inclusion criteria of the review, of which 28 studies reported results in sufficient detail for the meta‐analyses. Most studies were of six months' duration or less. Only one small trial lasted 52 weeks. The studies tested mainly donepezil capsules at a dose of 5 mg/day or 10 mg/day. Two studies tested a slow‐release oral formulation that delivered 23 mg/day. Participants in 21 studies had mild to moderate disease, in five studies moderate to severe, and in four severe disease. Seventeen studies were industry funded or sponsored, four studies were funded independently of industry and for nine studies there was no information on source of funding.
Our main analysis compared the safety and efficacy of donepezil 10 mg/day with placebo at 24 to 26 weeks of treatment. Thirteen studies contributed data from 3396 participants to this analysis. Eleven of these studies were multicentre studies. Seven studies recruited patients with mild to moderate Alzheimer's disease, two with moderate to severe, and four with severe Alzheimer's disease, with a mean age of about 75 years. Almost all evidence was of moderate quality, downgraded due to study limitations.
After 26 weeks of treatment, donepezil compared with placebo was associated with better outcomes for cognitive function measured with the Alzheimer's Disease Assessment Scale‐Cognitive (ADAS‐Cog, range 0 to 70) (mean difference (MD) ‐2.67, 95% confidence interval (CI) ‐3.31 to ‐2.02, 1130 participants, 5 studies), the Mini‐Mental State Examination (MMSE) score (MD 1.05, 95% CI 0.73 to 1.37, 1757 participants, 7 studies) and the Severe Impairment Battery (SIB, range 0 to 100) (MD 5.92, 95% CI 4.53 to 7.31, 1348 participants, 5 studies). Donepezil was also associated with better function measured with the Alzheimer's Disease Cooperative Study activities of daily living score for severe Alzheimer's disease (ADCS‐ADL‐sev) (MD 1.03, 95% CI 0.21 to 1.85, 733 participants, 3 studies). A higher proportion of participants treated with donepezil experienced improvement on the clinician‐rated global impression of change scale (odds ratio (OR) 1.92, 95% CI 1.54 to 2.39, 1674 participants, 6 studies). There was no difference between donepezil and placebo for behavioural symptoms measured by the Neuropsychiatric Inventory (NPI) (MD ‐1.62, 95% CI ‐3.43 to 0.19, 1035 participants, 4 studies) or by the Behavioural Pathology in Alzheimer's Disease (BEHAVE‐AD) scale (MD 0.4, 95% CI ‐1.28 to 2.08, 194 participants, 1 study). There was also no difference between donepezil and placebo for Quality of Life (QoL) (MD ‐2.79, 95% CI ‐8.15 to 2.56, 815 participants, 2 studies).
Participants receiving donepezil were more likely to withdraw from the studies before the end of treatment (24% versus 20%, OR 1.25, 95% CI 1.05 to 1.50, 2846 participants, 12 studies) or to experience an adverse event during the studies (72% vs 65%, OR 1.59, 95% 1.31 to 1.95, 2500 participants, 10 studies).
There was no evidence of a difference between donepezil and placebo for patient total healthcare resource utilisation.
Three studies compared donepezil 10 mg/day to donepezil 5 mg/day over 26 weeks. The 5 mg dose was associated with slightly worse cognitive function on the ADAS‐Cog, but not on the MMSE or SIB, with slightly better QoL and with fewer adverse events and withdrawals from treatment. Two studies compared donepezil 10 mg/day to donepezil 23 mg/day. There were no differences on efficacy outcomes, but fewer participants on 10 mg/day experienced adverse events or withdrew from treatment.
Authors' conclusions
There is moderate‐quality evidence that people with mild, moderate or severe dementia due to Alzheimer's disease treated for periods of 12 or 24 weeks with donepezil experience small benefits in cognitive function, activities of daily living and clinician‐rated global clinical state. There is some evidence that use of donepezil is neither more nor less expensive compared with placebo when assessing total healthcare resource costs. Benefits on 23 mg/day were no greater than on 10 mg/day, and benefits on the 10 mg/day dose were marginally larger than on the 5 mg/day dose, but the rates of withdrawal and of adverse events before end of treatment were higher the higher the dose.
Keywords: Humans, Alzheimer Disease, Alzheimer Disease/drug therapy, Cholinesterase Inhibitors, Cholinesterase Inhibitors/therapeutic use, Cognition, Cognition/drug effects, Cognition Disorders, Cognition Disorders/drug therapy, Donepezil, Indans, Indans/therapeutic use, Nootropic Agents, Nootropic Agents/therapeutic use, Piperidines, Piperidines/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Donepezil for people with dementia due to Alzheimer's disease
Review question
What effects (benefits or harms) does donepezil have on people with dementia due to Alzheimer's disease?
Background
Alzheimer's disease is the most common cause of dementia. As the disease progresses, people lose the ability to remember, communicate, think clearly and perform the activities of daily living. Their behaviour may also change. In severe Alzheimer's disease people lose the ability to care for themselves.
The most commonly used treatment for Alzheimer's disease are medicines known as acetylcholinesterase inhibitors. Donepezil is one of these medicines. It is taken as a pill once a day.
In Alzheimer's disease, one of the changes in the brain is a reduced number of nerve cells called cholinergic neurones. These are nerve cells that signal to other cells using a chemical called acetylcholine. Acetylcholinesterase inhibitors, such as donepezil, work by preventing acetylcholine from being broken down. This may improve the symptoms of dementia. However, acetylcholine is also found elsewhere in the body and so drugs of this type may have unwanted effects.
Review methods
In this review we examined evidence about benefits and harms from studies that compared donepezil, taken for at least 12 weeks, to placebo (a dummy pill), or that compared different doses of donepezil. The studies had to be double‐blind and randomised, that is, the decision whether people taking part got donepezil or placebo had to be made randomly and neither they nor the researchers should have known which treatment they were getting while the trial was going on. This was to make the comparison as unbiased, or fair, as possible. We searched for studies up to May 2017. We assessed the quality of all the studies we included. When it was sensible to do so, we analysed the results of studies together to get an overall result.
Key results
We included 30 studies with 8257 participants. Most of the people in the studies had mild or moderate dementia due to Alzheimer's disease, but in nine studies they had moderate or severe dementia. Almost all of the studies lasted six months or less. The majority of the studies were known to have been funded by the manufacturer of donepezil.
We found that people with Alzheimer's disease who took 10 mg of donepezil a day for six months did slightly better than people taking placebo, on scales measuring their cognitive function (e.g. thinking and remembering), how well they could manage their daily activities, and the overall impression of a trained researcher. We did not find any effect on behaviour or quality of life.
People taking donepezil were more likely than those taking placebo to report side effects and to drop out of the studies. Most side effects were described as mild. Nausea, vomiting and diarrhoea were most common.
Comparing 5 mg of donepezil a day with 10 mg/day, people on 5 mg had fewer side effects, but did slightly less well on cognitive function tests. A higher dose (23 mg/day) offered no advantages and was associated with more side effects.
There is some evidence that use of donepezil is neither more nor less expensive than placebo when total health care costs are taken into account.
Quality of the evidence
In general, we thought that the quality of the evidence was moderate. The main factor reducing our confidence was concern that the results of some studies might have been biased by the way they were done. We cannot be sure that the results apply to treatment longer than six months.
Conclusions
After six months of treatment, there are benefits of donepezil that are large enough to measure in studies. It is associated with side effects that are mainly mild, but that may cause people to stop treatment.
Being able to stabilise cognitive performance or ability to maintain activities of daily living may be important clinically. In terms of total healthcare costs the use of donepezil appears cost neutral. However, there does not appear to be an effect on quality of life. More data are still required from longer‐term clinical studies examining measures of disease progression or time to needing full time care.
Summary of findings
Summary of findings for the main comparison. Donepezil 10 mg/day compared with placebo for dementia due to Alzheimer's disease.
| Donepezil 10 mg/day compared with placebo for dementia due to Alzheimer's disease | ||||||
|
Patient or population: people with Alzheimer's disease Settings: worldwide Intervention: donepezil 10 mg/day for 24 to 26 weeks Comparison: placebo for 24 to 26 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Donepezil 10 mg/day | |||||
|
Cognitive function (change from baseline at 26 weeks using ADAS‐Cog)1 |
The mean score in the donepezil group was 2.67 lower (3.31 to 2.02 lower) than in the placebo group | 1130 (5 studies) | ⊕⊕⊕⊝ moderate2 | ADAS‐Cog score has a maximum of 70 points, the lower score in the donepezil group indicates greater improvement | ||
|
Cognitive function (change from baseline at 26 weeks using MMSE)1 |
The mean score in the donepezil group was 1.05 higher (0.73 to 1.37 higher) than in the placebo group | 1757 (7 studies) | ⊕⊕⊕⊝ moderate2 | MMSE has a maximum score of 30 points, a lower score indicates greater impairment. Treatment effect was in favour of donepezil. | ||
| Activities of daily living (change from baseline at 26 weeks measured using the ADCS)1 | The mean score in the intervention group was 1.03 higher (0.21 to 1.85 higher) than in the placebo group | 733 (3 studies) | ⊕⊕⊕⊝ moderate2 | The higher score indicates greater improvement. | ||
|
Clinician‐rated global impression tests (improved compared with baseline, measured using CIBIC‐plus at 24‐26 weeks)1 |
331 per 1000 | 487 per 1000 (432 to 542) | OR 1.92 (1.54 to 2.39) | 1674 (6 studies) | ⊕⊕⊕⊝ moderate2 | |
|
Behavioural symptoms (change from baseline at 26 weeks measured using the NPI)1 |
The mean score in the intervention group was 1.62 lower ( 3.43 lower to 0.19 higher) than in the placebo group | 1035 (4 studies) |
⊕⊕⊕⊝ moderate2 | A lower score indicates greater improvement. There was no significant difference between the 2 groups | ||
|
Acceptability of treatment (as measured by withdrawals from trial before end of treatment at 26 weeks)1 |
248 per 1000 | 291 per 1000 (256 to 331) | OR 1.25 (1.05 to 1.50) | 2846 (12 studies) |
⊕⊕⊕⊝ moderate2 | Withdrawals were significantly more frequent in the donepezil group compared with placebo group |
|
Incidence of adverse events (at least one adverse event by 26 weeks)1 |
780 per 1000 | 849 per 1000 (822 to 874) | OR 1.59 (1.31 to 1.95) | 2500 (10 studies) | ⊕⊕⊕⊝ moderate2 | Adverse events were significantly more frequent in the donepezil group compared with placebo group |
|
Quality of life of participants (change from baseline at 26 weeks)1 |
The mean score was 2.79 lower (8.15 lower to 2.56 higher) than in the placebo group | 815 (2 studies) | ⊕⊕⊕⊝ moderate2 | A higher svcore indicates greater improvement.There was no significant difference between the 2 groups | ||
| *The assumed risk is the weighted average across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer's Disease Assessment Scale‐Cognitive; ADCS: Alzheimer's Disease Cooperative Study; CI: confidence interval; CIBIC: Clinician's Interview‐Based Impression of Change; MMSE: Mini‐Mental State Examination; NPI: Neuropsychiatric Inventory; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1All statistics are based on the analyses of the intention to treat last observation carried forward (ITT‐LOCF) population. Although using the ITT population in the analyses for studies in degenerative conditions can be criticised as substitution of the LOCF when a patients is lost before end point may enhance the outcome, in this review the results of the analyses of the population who completed the study were similar to the ITT results and did not alter our conclusions. 2Downgraded one level due to the risk of bias due to lack of information on allocation concealment and on the blinding of outcome assessment.
Summary of findings 2. Donepezil 23 mg/day compared with donepezil 10 mg/day for dementia due to Alzheimer's disease.
| Donepezil 23 mg/day compared with donepezil 10 mg/day for dementia due to Alzheimer's disease | ||||||
|
Patient or population: people with Alzheimer's disease Settings: worldwide Intervention: donepezil 23 mg/day for 24 weeks Comparison: donepezil 10 mg/day for 24 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Donepezil 10 mg/day | Donepezil 23 mg/day | |||||
|
Cognitive function (change from baseline at 24 weeks using SIB)1 |
The mean score in the donepezil 23 mg/day group was 1.05 higher (0.15 lower to 2.25 higher) than in the donepezil 10 mg/day group | 1704 (2 studies) |
⊕⊕⊕⊝ moderate2 | SIB has a maximum score of 100 points, a lower score indicates greater impairment.There was no significant difference between the 2 groups | ||
|
Cognitive function (change from baseline at 24 weeks using MMSE)1 |
The mean score in the donepezil 23 mg/day group was 0.20 higher (0.33 lower to 0.73 higher) than in the donepezil 10 mg/day group | 1370 (1 study) |
⊕⊕⊕⊝ moderate2 | MMSE has a maximum score of 30 points, a lower score indicates greater impairment. There was no significant difference between the 2 groups | ||
|
Activities of daily living (change from baseline at 24 weeks using the ADCS‐ADL‐sev)1 |
The mean score in the donepezil 23 mg/day group was 0 higher (1.18 lower to 1.18 higher) than in the donepezil 10 mg/day group | 1396 (1 study) |
⊕⊕⊕⊝ moderate2 | There was no significant difference between the 2 groups | ||
|
Clinician‐rated global impression test (improved compared with baseline assessed using CIBIC‐plus at 24 weeks)1 |
212 per 1000 | 210 per 1000 (173 to 253) | OR 0.99 (0.78 to 1.26) | 1704 (2 studies) | ⊕⊕⊕⊝ moderate2 | There was no significant difference between the 2 groups |
|
Acceptability of treatment (as measured by withdrawals from trial before end of treatment at 24 weeks)1 |
172 per 1000 | 296 per 1000 (248 to 348) | OR 2.02 (1.59 to 2.57) | 1818 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
|
Incidence of adverse events (at least one adverse event by 24 weeks)1 |
624 per 1000 | 732 per 1000 (690 to 771) | OR 1.65 (1.34 to 2.03) | 1785 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADCS‐ADL‐sev: Alzheimer's Disease Cooperative Study‐Activities of Daily Living Scale (severe version); CI: confidence interval; CIBIC: Clinician's Interview‐Based Impression of Change; MMSE: Mini‐Mental State Examination; OR: odds ratio; SIB: Severe Impairment Battery | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1All statistics are based on the analyses of the intention to treat last observation carried forward (ITT‐LOCF) population. Although using the ITT population in the analyses for studies in degenerative conditions can be criticised as substitution of the LOCF when a patients is lost before end point may enhance the outcome, in this review the results of the analyses of the population who completed the study were similar to the ITT results and did not alter our conclusions. 2Downgraded one level due to the risk of bias due to lack of information on the blinding of outcome assessment.
Summary of findings 3. Donepezil 10 mg/day compared with donepezil 5 mg/day for dementia due to Alzheimer's disease.
| Donepezil 10 mg/day compared with donepezil 5 mg/day for dementia due to Alzheimer's disease | ||||||
|
Patient or population: people with Alzheimer's disease Settings: worldwide Intervention: donepezil 10 mg/day Comparison: *donepezil 5 mg/day | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Donepezil 5 mg/day | Donepezil 10 mg/day | |||||
|
Cognitive function (change from baseline at 24 weeks using ADAS‐Cog)1 |
The mean score in the donepezil 10 mg/day group was 1.05 lower (1.80 lower to 0.30 lower) than in the donepezil 5 mg/day group | 818 (2 studies) | ⊕⊕⊕⊝ moderate2 | ADAS‐Cog score has a maximum of 70 points, the lower score in the donepezil 10 mg/day group indicates greater improvement | ||
|
Cognitive function (change from baseline at 24 weeks using MMSE)1 |
The mean score in the donepezil 10 mg/day group was 0.15 higher (‐0.55 to 0.85 higher) than in the donepezil 5 mg/day group | 303 (1 study) |
⊕⊕⊕⊝ moderate2 | MMSE has a maximum score of 30 points, a lower score indicates greater impairment. | ||
|
Clinician‐rated global impression test (improved compared with baseline assessed using CIBIC‐plus at 24 weeks)1 |
246 per 1000 | 291 per 1000 (235 to 353) | OR 1.26 (0.94 to 1.67) | 981 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
|
Acceptability of treatment (as measured by withdrawals from trial before end of treatment at 24 weeks)1 |
183 per 1000 | 272 per 1000 (217 to 333) | OR 1.67 (1.24 to 2.23) | 1052 (3 studies) |
⊕⊕⊕⊝ moderate2 | |
|
Incidence of adverse events (at least one adverse event by 24 weeks)1 |
785per 1000 | 851 per 1000 (796 to 893) | OR 1.56 (1.07 to 2.28) | 741 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer's Disease Assessment Scale‐Cognitive; CI: confidence interval; CIBIC: Clinician's Interview‐Based Impression of Change; MMSE: Mini‐Mental State Examination; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1All statistics are based on the analyses of the intention to treat last observation carried forward (ITT‐LOCF) population. Although using the ITT population in the analyses for studies in degenerative conditions can be criticised as substitution of the LOCF when a patients is lost before end point may enhance the outcome, in this review the results of the analyses of the population who completed the study were similar to the ITT results and did not alter our conclusions. 2Downgraded one level due to the risk of bias due to lack of information on the blinding of outcome assessment and of blinding of participants.
Summary of findings 4. Donepezil 10 mg/day compared with placebo for people with severe dementia due to Alzheimer's disease.
| Donepezil 10 mg/day compared with placebo for people with severe dementia due to Alzheimer's disease | ||||||
|
Patient or population: people with severe Alzheimer's disease Settings: worldwide Intervention: donepezil 10 mg/day for 24 weeks Comparison: placebo for 24 weeks | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Donepezil 10 mg/day | |||||
|
Cognitive function (change from baseline at 26 weeks using MMSE) |
The mean score in the donepezil group was 0.97 higher (0.56 higher to 1.38 higher) than in the placebo group | 1102 (4 studies) | ⊕⊕⊕⊝ moderate | MMSE has a maximum score of 30 points, a lower score indicates greater impairment. Treatment effect was in favour of donepezil. | ||
|
Cognitive function (change from baseline at 24 weeks using SIB)1 |
The mean score in the donepezil 10 mg/day group was 5.92 higher (4.53 higher to 7.31 higher) than in the placebo group | 1348 (5 studies) |
⊕⊕⊕⊝ moderate | SIB has a maximum score of 100 points, a lower score indicates greater impairment. | ||
|
Clinician‐rated global impression tests (improved compared with baseline, measured using CIBIC‐Plus at 24 weeks)1 |
274 per 1000 | 402 per 1000 (331 to 478) | OR 1.78 (1.31 to 2.43) | 755 (3 studies) | ⊕⊕⊕⊝ moderate | |
|
Activities of daily living (change from baseline at 24 weeks using the ADCS‐ADL‐sev)1 |
The mean score in the donepezil 10 mg/day group was 1.03 higher 0.21 higher to 1.85 higher) than in the placebo group | 733 (3 studies) | ⊕⊕⊕⊝ moderate | The higher score indicates greater improvement. | ||
|
Acceptability of treatment (as measured by withdrawals from trial before end of treatment at 24 weeks)1 |
187 per 1000 | 233 per 1000 (190 to 282) | OR 1.32 (1.02 to 1.71) | 1396 (5 studies) |
⊕⊕⊕⊝ moderate | Withdrawals significantly more frequent in the donepezil group compared with placebo group |
|
Incidence of adverse events (at least one adverse event by 24 weeks)1 |
616 per 1000 | 718 per 1000 (664 to 767) | OR 1.59 (1.23 to 2.05) | 1396 (5 studies) |
⊕⊕⊕⊝ moderate | Adverse events significantly more frequent in the donepezil group compared with placebo group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADCS‐ADL‐sev: Alzheimer's Disease Cooperative Study‐Activities of Daily Living Scale (severe version); CI: confidence interval; CIBIC: Clinician's Interview‐Based Impression of Change; MMSE: Mini‐Mental State Examination; OR: odds ratio; SIB: Severe Impairment Battery | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1All statistics are based on the analyses of the intention to treat last observation carried forward (ITT‐LOCF) population. Although using the ITT population in the analyses for studies in degenerative conditions can be criticised as substitution of the LOCF when a patients is lost before end point may enhance the outcome, in this review the results of the analyses of the population who completed the study were similar to the ITT results and did not alter our conclusions. 2 Downgraded one level due to the risk of bias due to lack of information on allocation concealment and on the blinding of outcome assessment.
Background
Description of the condition
Dementia is a syndrome of acquired deficits in multiple domains of cognition severe enough to interfere with everyday life and not due to impaired consciousness or the effects of a systemic illness (Chertkow 2013). Memory is usually the most severely affected domain initially. Progression is evident as increasing impairment of memory, developing gradually into a global impairment of cognition, including orientation, language, judgement, perceptual ability and praxis (the ability to carry out complex actions). These cognitive impairments are accompanied by progressive deterioration in ability to carry out activities of daily living, and often by the appearance of challenging behaviours and other psychiatric features. The clinical course is associated with growing disability and dependency on carers. A characteristic feature of the disease is a widely variable rate of progression in different patients (Ritchie 2017).
Alzheimer's disease is the most common cause of dementia, and may be involved in as many as 80% of cases. It is a primary degenerative disease of the brain of unknown cause, which leads to dementia of insidious onset, most commonly in later life. The characteristic brain pathology includes progressive loss of neurons and the development in the brain of amyloid plaques and neurofibrillary tangles (Ryan 2015).
This review is an updated version of a review first published in 1998.
Description of the intervention
Donepezil (Aricept, E2020) is a second‐generation cholinesterase inhibitor (Lee 2015). The drug was developed by Eisai and received approval from the United States Food and Drug Administration (FDA) in 1996, and from the European Medicines Agency (EMA) in 1997. In most countries it is approved for the treatment of mild or moderate dementia due to Alzheimer's disease. However, in several countries, including the USA, Canada and Japan, it is also approved for use in severe dementia due to Alzheimer's disease. Donepezil is available in tablet form. Liquid and transdermal formulations have also been developed, but are not marketed in all countries. The recommended oral dose is 5 mg once a day initially, increasing to 10 mg once a day after at least one month of treatment. In 2010 the FDA approved a 23 mg, once‐a‐day tablet of donepezil. Two other cholinesterase inhibitors (rivastigmine and galantamine) are also available.
How the intervention might work
Acetylcholine is an important neurotransmitter associated with memory, and abnormalities in cholinergic neurons (including cell loss) are prominent among the pathological changes in the brains of people with Alzheimer's disease. One approach to lessening the impact of these abnormalities is to inhibit the breakdown of acetylcholine in synapses, thereby enhancing cholinergic neurotransmission. Donepezil does this by reversibly inhibiting the enzyme acetylcholinesterase (Lee 2015).
Why it is important to do this review
Large multicentre studies have been completed. Donepezil has received approval for use in more than 90 countries, including all the member states of the European Union and in the USA. It is important to assess the safety and efficacy of this intervention in a systematic review (Ryan 2015).
Objectives
To assess the clinical efficacy and safety of donepezil in people with mild, moderate or severe dementia due to Alzheimer's disease; to compare the efficacy and safety of different doses of donepezil; and to assess the effect of donepezil on healthcare resource use and costs.
Methods
Criteria for considering studies for this review
Types of studies
We included all unconfounded, randomised, double‐blind studies of people with dementia due to Alzheimer's disease in which treatment with donepezil was administered for 12 weeks or longer and compared with a placebo group, or in which two doses of donepezil were compared. We excluded studies with a withdrawal design, (i.e. studies in which participants already stable on donepezil treatment were randomised to placebo or continuing donepezil treatment), studies in which the allocation to treatment or control was not randomised, or in which treatment allocation was not concealed. This is because prior knowledge of treatment allocation may lead to biased participant allocation (Schulz 1995).
Types of participants
The participants in studies to be included were diagnosed with probable Alzheimer's disease according to internationally accepted criteria such as ICD‐10, the Diagnostic and Statistical Manual of Mental Disorders (DSM IV) (APA 1987) and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) (McKhann 1984).
Types of interventions
Donepezil of any formulation and dose given for at least 12 weeks, compared with placebo or with an alternative formulation or dose of donepezil.
Types of outcome measures
The primary outcomes of interest were as follows.
Cognitive function (as measured by psychometric tests)
Activities of daily living
Behavioural disturbance
Clinical global impression
Quality of life
Effect on carer
Dependency (such as institutionalisation)
Death
Acceptability of treatment as measured by withdrawal from trial
Safety as measured by the incidence of adverse effects (including side effects) leading to withdrawal
Safety as measured by the overall incidence of adverse effects
Direct and indirect costs
We noted physiological outcomes, such as plasma levels, changes on functional imaging or electroencephalogram (EEG) changes but did not assess them, as they are not primarily measures of efficacy.
Search methods for identification of studies
Electronic searches
We identified the studies from a search of ALOIS ‐ Cochrane Dementia and Cognitive Improvement's Specialized Register on 20 May 2017 using the search terms: donepezil, aricept, "E 2020", E‐2020 and E2020.
ALOIS is maintained by Cochrane Dementia and Cognitive Improvement's Information Specialist, and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy people. The studies in the Specialized Register are identified from:
monthly searches of a number of major healthcare databases: MEDLINE, Embase, Cinahl, Psycinfo and Lilacs;
monthly searches of a number of trials registers: the World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
quarterly searches of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL); and
six‐monthly searches of the grey literature source: ISI Web of Science Core Collection
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of studies from the healthcare databases, CENTRAL can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
The Information Specialist performed additional searches in many of the sources listed above to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. For a full list of sources searched and view the search strategies used for each source see Appendix 1.
Searching other resources
We performed an additional Internet search using Copernic 2000 on 21 and 22 June 2005 using trial names and numbers. No new studies were found other than the ones that had already been found in the update search of the CDCIG Register on 12 June 2005; we did find additional references to existing studies. We searched Eisai/Pfizer, FDA, EMEA and NICE websites.
Data collection and analysis
Selection of studies
We discarded irrelevant publications, based on the title of the publication and the abstract. In the presence of any suggestion that an article could be relevant, we retrieved it for further assessment.
We independently reviewed the studies for inclusion from the culled citation list.
Data extraction and management
One review author (JSB) extracted data from the published reports. The summary statistics required for each trial and each outcome for continuous data were the mean change from baseline, the standard deviation of the mean change, and the number of participants for each treatment group at each assessment. We defined the baseline assessment as the latest available assessment prior to randomisation, but no longer than two months before. Where changes from baseline were not reported, we extracted the mean, standard deviation and the number of participants in each treatment group at each time point if available, and we calculated the required summary statistics. In this case, we assumed a zero correlation between the measurements at baseline and assessment time. This method overestimates the standard deviation of the change from baseline, but this conservative approach is considered to be preferable in a meta‐analysis. The outcomes measured in studies of dementia and cognitive impairment often arise from ordinal rating scales. Where the rating scales used in the studies had a reasonably large number of categories (more than 10) we treated the data as continuous outcomes arising from a normal distribution. For binary data, we sought the number in each treatment group and the numbers experiencing the outcome of interest.
We sought data on every person assessed for each outcome measure. The reported analyses were performed on an intention‐to‐treat (ITT) basis, which included all participants who were randomised to treatment, assessed at baseline, received at least one dose of the study drug, and had at least one post‐baseline assessment. The ITT population consisted of those who provided complete data at endpoint regardless of compliance (the observed cases, OC) plus the LOCF population, (the last observation carried forward on double‐blind treatment ), for whom the last observation on double‐blind treatment was carried forward to endpoint. The study authors analysed these data in the endpoint analyses, which were the primary analyses and are described as ITT‐LOCF. To allow a completers' analysis, we sought the data, 'on‐treatment' or the data of those who completed the trial, and we indicated them as such.
We did not use data from 'open‐label' follow‐on phases after the randomised study to assess safety or efficacy.
Assessment of risk of bias in included studies
We conducted the 'Risk of bias' assessment using the standard recommended approach for studies included in Cochrane Reviews (Higgins 2017). The Cochrane Collaboration 'Risk of bias' tool assesses the following domains:
sequence generation
allocation concealment
blinding of participants and study personnel
blinding of outcomes assessment
incomplete outcome data
selective outcome reporting
other bias
We made a judgement about the risk of bias in each domain, assigning it to one of three categories, high, low, or unclear risk of bias, basing our assessments on the criteria for making judgements that are listed in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). The criteria focus on whether the risk is of importance (that is, whether the presence of the risk could have an important impact on the results of the trial) rather than whether a risk of bias is present or not.
If insufficient detail was reported to make a judgement, we usually considered this as an unclear risk of bias. We also used an 'unclear' judgement in situations where it was clear what happened in the trial but its likely impact on the results was not known.
Measures of treatment effect
For dichotomous outcomes the estimate of treatment effect of the intervention was the Peto odds ratio (OR) together with 95% confidence interval (CI).
For continuous data the measure of treatment effect was the mean difference (MD) if only one study was included, or the weighted mean difference (WMD) if more than one study was included with 95% CI. When the pooled studies used different rating scales to measure the same outcome, then the measure of treatment effect was the standardised mean difference, which is the absolute mean difference divided by the pooled standard deviation.
Unit of analysis issues
The review only included parallel‐group studies with individual patients randomised. There were no unit of analysis issues.
Dealing with missing data
We made no attempt at data imputation, except for the estimation of standard deviations for continuous data using the methods detailed in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Where possible we reported ITT analyses. We conducted sensitivity analyses to compare methods of dealing with missing data.
Assessment of heterogeneity
Before pooling data we assessed potential differences between the included studies in the types of participants, interventions or control used. We did not plan any subgroup analyses.
We assessed heterogeneity between the studies using the Chi2 test (with a significance level set at P < 0.10) and the I2 statistic (Higgins 2003), which calculates the percentage of variability due to heterogeneity rather than to chance, with I2 values over 50% suggesting substantial heterogeneity (Deeks 2017).
Assessment of reporting biases
We compared outcomes reported for a trial with its protocol where possible, to examine whether all of the study's pre‐specified outcomes had been reported.
Data synthesis
The duration of the studies varied. If we considered the range too great to combine all studies into one meta‐analysis, we divided the data into smaller time periods and conducted a separate meta‐analysis for each period. Studies could contribute data to more than one time period if they had made multiple assessments.
We have presented overall estimates of the treatment difference. In all cases we have presented the overall estimate from a fixed‐effect model and performed tests for heterogeneity using a standard Chi2 statistic and I2 statistic.
Subgroup analysis and investigation of heterogeneity
We examined heterogeneity both visually and using the I2 statistic (Deeks 2017).
Sensitivity analysis
This review sought to analyse data using ITT data wherever possible. Some studies reported both an ITT analysis that included all participants randomised and a per protocol analysis. The ITT analyses often involve data imputation techniques such as LOCF for participants who did not complete the study. We investigated the impact of different ways of dealing with missing data using a sensitivity analysis of ITT and per protocol analyses. We tabulated these results and discussed any important discrepancies.
Presentation of results: 'Summary of findings' table
We used the GRADE approach (Guyatt 2011) to describe our confidence in key effect estimates and presented them in 'Summary of findings' tables as recommended by Cochrane (Schünemann 2017). The GRADE approach rates the overall quality of evidence contributing to an effect estimate as high, moderate, low or very low, taking into account risk of bias in the included studies, inconsistency between studies, imprecision in the effect estimate, indirectness with respect to the review question, and possible publication bias. We produced 'Summary of findings' tables for the comparison of donepezil (10 mg/day) versus placebo, for the comparison of donepezil (10 mg/day) versus donepezil (5 mg/day), for the comparison of donepezil (10 mg/day) versus placebo for severe Alzheimer's disease, and for the comparison of donepezil (10 mg/day) versus donepezil (23 mg/day). We included the following key outcomes in the 'Summary of findings' tables: cognitive function, activities of daily living, behavioural symptoms, clinician‐rated global impression, acceptability of treatment, incidence of adverse events and quality of life.
Results
Description of studies
Results of the search
The updated searches performed in January 2015, November 2015, November 2016 and May 2017 retrieved a total of 5653 references. After de‐dulication and a first assessment based on titles and abstracts by the CDCIG information specialist, we were left with 106 references. We read the full texts of 16 references from the January and November 2015 searches. Of these, 14 references were related to two studies suitable for inclusion, and two reported studies that we excluded. From the other two searches, November 2016 and May 2017, we read the full texts of four references. Of these, two were additional references for studies already included, and two were new studies to be included, see Figure 1.
1.

Study flow diagram for searches conducted in January 2015, November 2016 and May 2017
Included studies
We have summarised the characteristics of the 30 included studies in Characteristics of included studies.
We have summarised Important details of study design (number of participants, duration of follow‐up, mean Mini‐Mental State Examination (MMSE) of participants at baseline and description of interventions) in Table 5, the outcome measures in Table 6, and the objectives of the studies in Table 7.
1. Description of included studies at baseline.
| Study | Duration (weeks) | Number of participants | Mean age (years) | % female | Mean MMSE | Dose mg/day donepezil | Phase | Country |
| Homma 1998 | 12 | 190 | 69.1 | 69 | ‐ | 3, 5 | II | Japan |
| Homma 2000 | 24 | 268 | 69.8 | 67 | 17.2 | 5 | III | Japan |
| Rogers 1996 | 12 | 161 | 71.8 | 60 | 18.6 | 1, 3, 5 | II | USA |
| Tune 2003 | 24 | 28 | 73.0 | 75 | 21.0 | 10 | ‐ | USA |
| Krishnan 2003 | 24 | 67 | 73.4 | 71.6 | 19.2 | 10 | ‐ | USA |
| Study 205 | 16 | 12 | ‐ | ‐ | 21.4 | 10 | ‐ | USA |
| Rogers 1998a | 12 | 468 | 73 | 63 | 19.5 | 5, 10 | III | USA |
| Rogers 1998b | 24 | 473 | 73.4 | 62 | 19.0 | 5, 10 | III | USA |
| Burns 1999 | 24 | 818 | 71.7 | 57 | 20.0 | 5, 10 | III | Europe |
| Study 306 | 12 | 39 | ‐ | ‐ | ‐ | 10 | III | Italy |
| Tariot 2001 | 24 | 208 | 85.7 | 82 | 14.4 | 10 | III | USA |
| Mohs 2001 | 54 | 431 | 75.3 | 62.9 | 17.1 | 10 | USA | |
| Black 2007 | 24 | 343 | 78.0 | 68 | 7.5 | 10 | Australia, Canada, France, UK, USA | |
| Seltzer 2004 | 24 | 153 | 74.0 | 53.6 | 24.1 | 10 | USA | |
| AD2000 | 60 | 566 | 75.5 | 59 | 19 | 5, 10 | UK | |
| Lebert 1999 | 12 | 318 | 72.0 | 68 | 21.6 | 10 | Unknown | |
| Farlow 2010 | 24 | 1467 | 73.9 | 10, 23 | ||||
| Feldman 2001 | 24 | 290 | 73.6 | 61.0 | 11.8 | 10 | Australia, Canada, France | |
| Hegerl 2003 | 12 | 40 | ‐ | ‐ | ‐ | 10 | III | Germany |
| Homma 2008 | 24 | 302 | 78.2 | 80 | 7.8 | 5, 10 | Japan | |
| Homma 2016 | 24 | 351 | 76.0 | 69.4 | 8.7 | 10, 23 | Japan | |
| Jia 2017 | 24 | 313 | 70.8 | 65 | 7.3 | 10 | China | |
| Howard 2007 | 12 | 159 | 84.5 | 85 | 8.2 | 10 | England, UK | |
| Maher‐Edwards 2011 | 24 | 130 | 71.2 | 67 | 10 | Austria, Bulgaria, Chile, Estonia, Germany, The Russian Federation, Slovakia, UK | ||
| Mazza 2006 | 24 | 51 | 68.5 | 54 | 18.7 | 5 | Italy | |
| Moraes 2006a | 12 | 23 | 74.7 | 65 | 10 | Brazil | ||
| Moraes 2006b | 26 | 35 | 76 | 69 | 10 | Brazil | ||
| Winblad 2001 | 52 | 286 | 72.5 | 64 | 19.3 | 10 | III | Europe |
| Schindler 2004 | 24 | 31 | ‐ | ‐ | ‐ | 10,20 | ‐ | ‐ |
| Winblad 2006 | 26 | 248 | 84.9 | 76.6 | 6.1 | 10 | ‐ | Sweden |
MMSE: Mini‐Mental State Examination
2. Outcome measures.
| Study | MMSE | ADAS‐Cog | CDR‐SB | CIBIC‐plus | QoL | Other |
| Homma 1998 | X | X | MENFIS, Crichton, FGIR, GIR, OSR, GUR | |||
| Homma 2000 | X | X | MENFIS, Japanese‐CGIC, Crichton | |||
| Rogers 1996 | X | X | X | X | X | ADL |
| Tune 2003 | X | NPI, functional brain activity | ||||
| Krishnan 2003 | X | Hippocampal volumes and brain concentrations of N‐acetylaspartate | ||||
| Study 205 | X | X | X | |||
| Rogers 1998a | X | X | X | X | X | |
| Rogers 1998b | X | X | X | X | X | |
| Burns 1999 | X | X | X | X | IDDD | |
| Study 306 | X | X | ||||
| Tariot 2001 | X | X | NPI‐NH | |||
| Mohs 2001 | X | X | ADFACS | |||
| Black 2007 | X | x | SIB, ADCS‐ADL‐sev, NPI, CBQ, RUSP | |||
| Seltzer 2004 | X | X | X | CMBT, apathy scale | ||
| AD2000 | entry to institutional care, BADLS | |||||
| Lebert 1999 | X | X | NPI, BADLS, GHQ‐30, institutionalisation | |||
| Farlow 2010 | X | X | X | SIB, ADCS‐ADL | ||
| Feldman 2001 | X | X | SIB, CIBIS, DAD, NPI, FRS, CSS, CAUST, SF‐36 | |||
| Hegerl 2003 | X | Hand‐motor function | ||||
| Homma 2008 | X | SIB, ADCS‐ADL‐sev, BEHAVE‐ADL | ||||
| Homma 2016 | X | SIB | ||||
| Jia 2017 | X | X | SIB | |||
| Howard 2007 | CMAI, NPI NPI‐D SIB | |||||
| Maher‐Edwards 2011 | X | X | DAD, NPI, ACQLI | |||
| Mazza 2006 | X | SKT, CGIC | ||||
| Moraes 2006a | X | |||||
| Moraes 2006b | X | |||||
| Winblad 2001 | X | GBS, PDS, NPI, GDS | ||||
| Schindler 2004 | TEAE | |||||
| Winblad 2006 | X | ADCS‐ADL‐sev, SIB, CGIC, NPI | ||||
| The descriptions of the scales and tests appears in Appendix 2. | ||||||
ACQLI: Alzheimer Carer's Quality of Life Instrument; ADAS‐Cog: Alzheimer's Disease Assessment Scale;ADCS‐ADL‐sev: Alzheimer's Disease Cooperative Study‐Activities of Daily Living Scale (severe version); ADFACS: AD Functional Assessment and Change Score; ADL: Activities of Daily Living; aRSS: abridged Relative's Stress Scale; BADLS: Bristol Activities of Daily Living Scale; BEHAVE‐ADL: Behavioural Pathology in Alzheimer's Disease Activities of Daily Living; CAUST: Canadian Utilization of Services Tracking; CBQ: Caregiver Burden Questionnaire; CDR‐SB: Clinical Dementia Scale, sum of boxes; CGIC: Clinician's Global Impression of Change; CIBIC+: Clinician's Interview‐Based Impression of Change;CIBIS: Clinician's Interview‐based Impression of Severity, CMAI: Cohen‐Mansfield Agitation Inventory; CSS: Caregiver Stress Scale, CMBT: Computerized Memory Battery Test, DAD: Disability Assessment for Dementia; FRS: Functional Rating Scale, GBS: Gottfries, Brane and Steen scale; GDS: Geriatric Depression Scale; GHQ‐30: General Health Questionnaire; IDDD: Interview for Deterioration in Daily living in Dementia scale; MENFIS: Mental Function Impairment Scale; MMSE: Mini Mental State Examination; NOSGER: Nurses' Observation Scale for Geriatric Patients; NPI: Neuropsychiatric Instrument; NPI‐D: Neuropsychiatric Inventory Distress scale; NPI‐NH: Neuropsychiatric Inventory Nursing Home version PDS: Progressive Deterioration Scale; QoL: Quality of Life; RUSP: Resources Utilisation for Severe Alzheimer's Disease Patients; SF‐36: Short Form ‐ 36; SKT: Syndrom Kurz Test; SIB: Severe Impairment Battery; TEAE: treatment‐emergent adverse event
3. Study objectives.
| Study | Objectives |
| AD2000 | "We aimed to determine whether donepezil produces worthwhile improvements in disability, dependency, behavioural and psychological symptoms, carers’ psychological wellbeing, or delay in institutionalisation. If so, which patients benefit, from what dose, and for how long?" |
| Black 2007 | "To evaluate the safety and efficacy of donepezil for severe Alzheimer disease (AD)." |
| Burns 1999 | "To evaluate the efficacy and safety of once‐daily administration of donepezil at doses of 5 and 10 mg versus placebo in a large, multinational cohort of patients with mild‐moderately severe Alzheimer's disease" |
| Farlow 2010 | "The objective of this study was to compare the effectiveness and safety profile of high‐dose donepezil (23 mg/day) and standard dose donepezil (10 mg/day) in patients with moderate to severe AD" |
| Feldman 2001 | "To investigate the efficacy and safety of donepezil in patients with moderate to severe AD" |
| Hegerl 2003 | "To evaluate the effects of donepezil on hand motor function in patients with mild‐moderate AD" |
| Homma 1998 | "To evaluate efficacy, safety and the optimal dose of E2020 in patients with mild to moderate Alzheimer‐type dementia" |
| Homma 2000 | "To evaluate the efficacy and safety of donepezil hydrochloride (donepezil) at 5mg/day in patients with mild to moderately Alzheimer's disease for 24 weeks" |
| Homma 2008 | "A 24‐week, randomized, parallel‐group, double‐blind placebo‐controlled study was conducted to evaluate the efficacy and tolerability of donepezil in severe Alzheimer’s disease (AD)." |
| Homma 2016 | "To demonstrate the superiority of SR 23 mg/day donepezil over IR 10 mg/day donepezil in Japanese patients with severe AD (SAD)." |
| Howard 2007 | "The primary question was whether donepezil is better than placebo in the management of agitation that is inappropriate for, or has not responded to, a psychosocial treatment." |
| Jia 2017 | "To examine the effects of donepezil on N‐acetylaspartate concentration and hippocampal volume in patients with mild‐moderate AD" |
| Krishnan 2003 | "The authors examined the effect of the acetylcholinesterase inhibitor donepezil on magnetic resonance markers of neurodegeneration in Alzheimer’s disease." |
| Lebert 1999 | AD2000 is a large, simple, ‘real‐life’ trial that aims to produce reliable evidence on the value of donepezil (Aricept®) in routine practice |
| Maher‐Edwards 2011 | "This exploratory study was designed to estimate the effects of donepezil and SB‐742457 in a current day setting and population using a study design similar to those employed in two pivotal studies with donepezil (Rogers 1998b, Burns 1999)" |
| Mazza 2006 | "To assess the efficacy of Ginkgo biloba special extract in patients with dementia of the Alzheimer type in slowing down the disease's progression and patient's cognitive impairment compared with donepezil and placebo" |
| Mohs 2001 | "To examine the effects of donepezil compared with placebo on the preservation of function in patients with AD over a 1‐year period" |
| Moraes 2006a | "This study evaluates the effects of donepezil on obstructive sleep apnea in patients with Alzheimer disease." |
| Moraes 2006b | "Examine the effects of donepezil on sleep and rapid eye movement (REM) sleep electroencephalogram (EEG) in patients with Alzheimer disease, using polysomnography" |
| Rogers 1996 | "To evaluate the efficacy and safety of donepezil in patients with mild to moderately severe Alzheimer's disease and to examine the relationships between plasma donepezil concentration, red blood cell acetylcholinesterase activity and clinical response" |
| Rogers 1998a | "The present phase I11 study was undertaken to further evaluate the efficacy and safety of donepezil at dosage levels of 5 and 10 mg/d versus placebo in patients with mild to moderate AD" |
| Rogers 1998b | "This phase 3 study was 1 of 2 pivotal trials undertaken to establish the efficacy and safety of using donepezil in patients with mild to moderately severe Alzheimer disease" |
| Schindler 2004 | "To determine the safety and tolerability of treatment with 15 or 20 mg/day donepezil in mild to moderate AD" |
| Seltzer 2004 | "To evaluate the efficacy of donepezil in patients with early‐stage Alzheimer disease" |
| Study 205 | "To evaluate the effect of donepezil on visuospatial attention in Alzheimer's disease patients" |
| Study 306 | "To evaluate the utility of APo‐E subtype in predicting response to treatment with donepezil in Alzheimer's disease patients" |
| Tariot 2001 | "To evaluate the safety and efficacy of donepezil in the management of patients with Alzheimer’s disease (AD) residing in nursing home facilities." |
| Tune 2003 | "This study evaluated the effects of donepezil on functional brain activity in patients with AD." |
| Winblad 2001 | "To evaluate the long‐term clinical efficacy and safety of donepezil versus placebo over 1 year in patients with mild to moderate AD." |
| Winblad 2006 | "Our aim was to assess the eff ect of donepezil on cognition and activities of daily living in patients with severe Alzheimer’s disease living in nursing homes ran by trained staff ." |
AD: Alzheimer's disease; SAD: severe Alzheimer's disease;; REM: rapid eye movement
Design, participants, sample sizes and interventions
Only randomised, double‐blind, placebo‐controlled studies, or studies comparing different doses of donepezil were included in this review. All included studies were described as randomised and double‐blind, but further details on the randomisation and blinding were not always reported.
All included studies have been reported since 1996.
Studies published in 2001 or earlier
Eleven studies were reported in 2001 or earlier.
Of these, six studies (Homma 1998; Rogers 1996; Rogers 1998a; Rogers 1998b; Burns 1999; Winblad 2001) were designed to evaluate the efficacy and safety of donepezil in people with mild to moderately severe dementia due to Alzheimer's disease and one study (Tariot 2001), was designed to examine efficacy, safety and tolerability in the management of very elderly residents with Alzheimer's disease in nursing homes, particularly the effect of donepezil on neuropsychiatric manifestations. Rogers 1996, Rogers 1998a, Rogers 1998b, Burns 1999 and Tariot 2001 were all supported by Eisai Inc. The Winblad 2001 was supported by Pfizer Inc. It is not clear how Homma 1998 was supported. Rogers 1996 was described as a phase II study, and Rogers 1998a, Rogers 1998b, Burns 1999 and Tariot 2001 as phase III studies.
These studies had many features in common. They were all multicentre, parallel‐group studies. Four studies were based in the USA, two in Europe and one in Japan. All studies compared donepezil with placebo.
These seven studies made a diagnosis for probable Alzheimer's disease according to NINCDS‐ADRDA criteria, with participants also fitting DSM‐III‐R illness categories 290.00 or 290.10 in six studies. Rogers 1996, Rogers 1998a, Rogers 1998b, Burns 1999 and the Winblad 2001 measured the severity of the disease using the MMSE scale, and recruited participants with mild to moderate dementia (MMSE 10‐26). Tariot 2001 recruited participants with MMSE between 5 and 26, inclusive, and consequently the mean MMSE at baseline (14.4) was lower than that in the other studies. Homma 1998 did not use the MMSE. Homma 1998, Rogers 1996, Rogers 1998a, Rogers 1998b, and Burns 1999 required a Clinical Dementia Rating (CDR) of 1 (mild) or 2 (moderate) at screening and baseline.
The list of exclusions was quite extensive and consistent across the phase II and III studies. Patients were excluded if they had insulin‐dependent diabetes mellitus or other endocrine disorder, asthma, obstructive pulmonary disease or clinically significant uncontrolled gastrointestinal hepatic or cardiovascular diseases. Patients known to be hypersensitive to cholinesterase inhibitors or who had taken tacrine or other investigational medicines within one month of baseline were excluded. Concomitant medications such as anticholinergics, anticonvulsants, antidepressants and antipsychotics were not allowed. Drugs with central nervous system (CNS) activity were prohibited or partially restricted. The participants included in Tariot 2001 were on average older than in the other studies, and were more likely to have comorbid illness. They were required to have reported at a frequency of several times a week at least one symptom from the Neuropsychiatric Inventory Nursing Home version (NPI‐NH).
The Winblad 2001 published an economic valuation of donepezil.
The doses used in the phase III studies were within the range shown to be clinically useful and reasonably well tolerated in the earlier studies. Treatment was once daily. When the dose of donepezil was 5 mg/day or less, the participants began with the full dose; with 10 mg/day the initial dose was 5 mg/day for one week followed by the full dose for Rogers 1998a, Rogers 1998b and Burns 1999. For the two later studies, Tariot 2001 and the Winblad 2001, the time on 5 mg/day was four weeks, before increasing to 10 mg/day. The forced titration schemes were blinded. Homma 1998 and Rogers 1996 were dose‐finding studies.
Four other studies were published in 2001 or before (Lebert 1999; Feldman 2001; Homma 2000; Mohs 2001).
Lebert 1999 was a large, multi‐centred trial designed to evaluate the stress on carers.
Homma 2000 was a multicentre, phase III study carried out in Japan, funded by Eisai, with a similar protocol to the other Eisai phase III studies, except that only the lower dose of donepezil, 5 mg/day was tested and participants began with a lower dose for the first week.
Mohs 2001 investigated the effect of donepezil on the preservation of function over a one‐year period. The inclusion and exclusion criteria were similar to the phase III studies, except for baseline MMSE, which was approximately 5 points lower on average, and for the requirement that participants had to be able to perform eight of 10 instrumental activities of daily living and five of six basic activities of daily living, each scored on a scale of 0 (no impairment ) to 3 (very severe impairment) to a level no greater than 2. The primary endpoint was time to clinically evident decline in function, as defined in the protocol. Participants reaching this endpoint left the trial and received open‐label donepezil treatment. It was not possible to include the results of this trial in the meta‐analyses due to the removal of participants from the study. The only outcome we could include was the number in each group reaching the primary endpoint.
Feldman 2001 recruited patients with probable or possible Alzheimer's disease of moderate to severe severity. Causes of the dementia, other than Alzheimer's disease, had to be ruled out. Patients randomised to donepezil took 5 mg/day for 4 weeks, followed by 10 mg/day for 20 weeks if the higher dose was tolerated. The trial was supported by Pfizer Inc and Eisai Co Ltd. An economic evaluation of donepezil from Feldman 2001 has been published. Data were collected at four time points, including baseline, during the randomised treatment period, on patient and carer health resource utilisation and costs. Details are described below.
Studies published after 2001
The remaining 19 included studies were reported after 2001.
Tune 2003, with only 28 participants, was primarily aimed at investigating brain glucose metabolism; Study 205, with 12 participants investigated the effect on visuospatial attention and Study 306, with 39 participants, investigated whether Apo E genotype predicted response to donepezil. Krishnan 2003, with 67 participants, was primarily to investigate brain measurements. There is very little published information on these studies.
AD2000 randomised 566 people with Alzheimer's disease, with or without vascular dementia, to 12 weeks of 5 mg/day donepezil or placebo, and then re‐randomised them to donepezil (5 mg/day or 10 mg/day) or placebo for another 48 weeks of treatment. Thus the trial was partially of a cross‐over design, some participants changed treatments, others did not. In addition, suitable participants were randomised to aspirin or aspirin avoidance. This trial was carried out independently of the pharmaceutical company. An extensive description of and the results from AD2000 have been published. The trial was designed with the intention of recruiting 3000 people, but only 566 were randomised. When this UK‐based trial started in 2000 donepezil was not available on the National Health Service (NHS), but became available in 2001. This affected not only recruitment, but also the retention of participants because participants already randomised left the trial to benefit from open‐label prescription of donepezil. Any patient referred to a memory clinic was potentially eligible if they were diagnosed (according to DSM IV) with dementia of Alzheimer type with or without a coexisting diagnosis of vascular dementia (16% of participants were also diagnosed with vascular dementia). Only 86% (486/566) of participants randomised at baseline entered the second randomisation at 12 weeks. During the next 48 weeks of treatment 40% (193) of participants were lost to follow‐up, 32 died, 42 were admitted to institutional care, 62 stopped treatment and 57 withdrew to open‐label donepezil. The trial continued with a six‐week washout before beginning a further 48 weeks of treatment (no further randomisation), but only 194 out of 293 finishing the previous phase entered. After the second 48‐week phase there was a four‐week washout, another 48 weeks of treatment, four‐week washout and 48 weeks of treatment. In theory, treatment could continue for 204 weeks, but the loss of participants continued at a substantial rate. The two primary endpoints were entry to an institution, and loss of either two of four basic, or six of 11 instrumental activities on the BADLS. Secondary outcome measures were functional ability (BADLS), behavioural symptoms as assessed by the NPI, MMSE, psychological well‐being of the carer (GHQ‐30), and death from Alzheimer's disease. In addition, AD2000 assessed costs, including NHS, social services and private, from information provided by the carer and by the family doctor, and costed using national (UK) average unit costs for each item, for example a stay in hospital.
Hegerl 2003 was a small pilot study of 40 participants diagnosed with probable Alzheimer's disease according to DSM‐IV and NINCDS‐ADRDA, designed to investigate whether donepezil is associated with Parkinsonian effects in people with Alzheimer's disease, by assessing cognition and hand‐motor function. It was supported by Pfizer Inc. and Eisai Europe.
Schindler 2004 was a small study of 31 participants with mild to moderate Alzheimer's disease, who were already taking 10 mg/day donepezil at baseline. The objective was to assess the safety and tolerability of higher doses of donepezil. In addition to the 10 mg/day that the participants were already taking, participants were randomised to either placebo or to a further dose of 5 mg /day increasing to 10 mg/day donepezil over 24 weeks. It was funded by Pfizer/Eisai.
Seltzer 2004, a 24‐week, parallel‐group study of 10 mg/day donepezil compared with placebo, evaluated the efficacy of donepezil in participants with early‐stage Alzheimer's disease. The mean MMSE at baseline was 24.
Winblad 2006, a placebo‐controlled, six‐month study conducted in Sweden and funded by Pfizer, was designed to investigate the efficacy of donepezil in people with severe Alzheimer's disease. The 241 participants were living in assisted‐care nursing homes. Their baseline MMSE was between 1 and 10 points. Participants were randomised to donepezil (5 mg/day for 30 days followed by up to 10 mg/day) or placebo. The primary outcomes were cognition, as assessed by the SIB, and activities of daily living.
Black 2007, a placebo‐controlled, 24‐week study conducted in Australia, Canada, France, the UK and the USA, and funded by Pfizer, was designed to investigate the efficacy of donepezil in people with severe Alzheimer's disease. The 343 participants were living in the community or in assisted‐care nursing homes. Their baseline MMSE was between 1 and 12 points. Participants were randomised to donepezil (5 mg/day for six weeks followed by up to 10 mg/day) or placebo. The primary outcomes were cognition as assessed by the SIB and CIBIC‐Plus.
Homma 2008, sponsored by Eisai Ltd. Japan, was a placebo‐controlled, 24‐week trial to investigate two doses of donepezil, 5 mg/day and 10 mg/day, in Japanese people with severe Alzheimer's disease.
Howard 2007, sponsored by the Medical Research Council (MRC) UK, and the Alzheimer Society, was a placebo‐controlled, 12‐week trial of 10 mg/day donepezil, for people with severe Alzheimer's disease and significant agitation, a subgroup of the population of people with severe Alzheimer's disease.
Mazza 2006 was a small, 24‐week trial, not industry‐sponsored, designed to compare Ginkgo biloba with donepezil and placebo in mild to moderate Alzheimer's disease. They reported only some of the results.
Moraes 2006a was a small, 12‐week trial of people with sleep apnoea and Alzheimer's disease, investigating the effect of donepezil on polysomnography outcomes. Cognition was a secondary outcome.
Moraes 2006b was a small, 26‐week trial studying the effect of donepezil on rapid eye movement (REM) sleep in Alzheimer's disease, with cognition as a secondary outcome.
Maher‐Edwards 2011 was a small, exploratory study (n = 130) of 24 weeks' duration with three treatment arms, donepezil (10 mg/day), SB‐742457 and placebo.
Farlow 2010 and Homma 2016 were parallel‐group studies of 24 weeks' duration designed to compare donepezil (10 mg/day) with a slow‐release formulation of donepezil (23 mg/day).
Jia 2017, sponsored by Eisai China was a placebo‐controlled, 24‐week trial to investigate donepezil, 10 mg/day, in Chinese people with severe Alzheimer's disease.
Outcomes
The studies examined cognition, functional and behavioural symptoms, and global effects, as well as the safety and tolerability of donepezil. Apart from the outcomes related to safety or adverse effects, the included studies measured all the outcomes for the effectiveness of donepezil by questionnaires or psychometric tests and used different types of instruments to measure each outcome. We have summarised the details of the outcomes measured and reported in each trial in Table 6.
In all studies assessments were carried out at more than one time point between the base line assessment and the reported end‐point.
Details of adverse events were ascertained by the questioning of each patient at each assessment. Serious adverse events were reported immediately.
Excluded studies
These are listed in Characteristics of excluded studies. We excluded the greatest number of studies because the control was another drug and not placebo, or because the study was open label.
Risk of bias in included studies
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We considered all studies sponsored by Eisai Inc. and Pfizer Inc. to be at low risk of bias for randomisation and allocation concealment. Of the independent studies, most had a low risk of bias with clearly described procedures.
Blinding
We considered most studies to be at low risk of bias. All studies were described as double‐blinded and were either placebo‐controlled or compared different doses of donepezil. Most studies described the interventions as having identical appearance and taste. Nearly all studies described a computer‐generated randomisation process, and most studies described the placebo and interventions as having identical appearance and taste, but only six studies described the blinding of the assessors.
Incomplete outcome data
We considered most studies to be at low risk of bias.The number of dropouts was usually small, that is, less than 20%, except for Mohs 2001, and AD2000.
The primary endpoint for Mohs 2001 was time to clinically evident decline in function, as defined in the protocol. Participants reaching this endpoint left the trial and received open‐label donepezil treatment. It was not possible to include most of the results of this trial in the meta‐analyses owing to the withdrawal of participants. It was possible to consider the time to clinically evident decline in function, but other outcomes would be biased by the risk of differential dropout related to treatment allocation.
In order to compare the different methods of dealing with missing assessments, we conducted meta analyses where possible on two populations of participants, the ITT population and the completers' population in order to compare the results. The results from the analyses of the completers' population did not cause us to change our conclusions.
Selective reporting
We considered most studies to be at low risk of bias.Twenty‐three of the 30 included studies reported all outcomes according to the outcomes identified in the methods section. There was insufficient information to assess the risk in the other seven studies.
Other potential sources of bias
We considered some studies to be at high risk of bias.There are serious concerns about the methodological quality of Lebert 1999. There is limited information available as this trial has never been published except in conference proceedings. These proceedings report that five participants took the wrong treatment.
Participants were withdrawn from Mohs 2001 if they met criteria of clinically evident decline in functional status.
Participants were withdrawn from Seltzer 2004 if they could not tolerate the 10 mg dose.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
There are 30 included studies, 21 of which reported results in sufficient detail for analysis. Nine studies contributed limited data or no data. Krishnan 2003 , Study 205, Mazza 2006, Moraes 2006a, Moraes 2006b contributed a tiny amount of data or no data . Detailed results have been published from AD2000 but few are reported in this review as they are difficult to interpret due to the second randomisation three months after baseline, and the high percentage of participants leaving the trial early. Extraction for the meta‐analyses and interpretation of the published results is not straight forward due to the complex design of the study and the form in which results were reported. The results from Lebert 1999 and Hegerl 2003 were not published with sufficient detail to allow extraction of the data for the meta‐analyses. We have serious concerns about the methodological quality of Lebert 1999. Schindler 2004 published data on the number of adverse events only.
The older studies included participants with mild to moderate Alzheimer's disease, mean baseline MMSE ranging from 17 to 22, but more recent studies have been including participants with more severe Alzheimer's disease. Tariot 2001, Black 2007, Feldman 2001, Homma 2008, Howard 2007, Jia 2017 and Winblad 2006 have mean baseline MMSE ranging from 6 to 14. We have reported the results of Howard 2007 separately, as the included participants were suffering with severe agitation, and thus were a subset of the total population of people with severe Alzheimer's disease. We have combined the results of the other studies with studies of the mild to moderate group and, in addition, we reported separately the results of Black 2007, Feldman 2001, Homma 2008, Howard 2007, Jia 2017 and Winblad 2006, the six studies that included only participants with severe or moderately severe dementia.
We have reported the main objective of each study in Table 7. Nineteen studies examined the cognitive, functional and global effects of donepezil. We analysed the results for treatment groups taking 5 mg/day and 10 mg/day of donepezil separately. Phase II dose‐finding studies had also used doses less than 5 mg/day, but donepezil is not prescribed at less than 5 mg/day and therefore we did not carry out meta‐analyses for the treatment groups where dose was less than 5 mg/day.
We analysed results after treatment periods of 12, 24 to 26, and 52 weeks separately.
Where data are available we have reported meta‐analyses on the ITT population, where LOCF assessments were incorporated when assessments were missing, and on the completers' population. The results appear similar, suggesting no differential dropout between the treatment groups. Models were fitted using fixed effects. There is evidence of heterogeneity between the studies for a few meta‐analyses. We rated outcomes as moderate quality, downgraded one level due to the risk of bias due to lack of information on allocation concealment and on the blinding of outcome assessment for some of the studies. Twenty‐two of the 30 studies did not describe allocation concealment and blinding of outcome, and 17 of the 30 studies did not describe blinding of the intervention.
The rating scales and cognitive tests differ in the direction representing improvement, or fewer symptoms:
a decrease in score indicates improvement with the Alzheimer's Disease Assessment Scale‐Cognitive (ADAS‐Cog), Activities of Daily Living (ADL), Behavioural Pathology in Alzheimer's Disease Rating Scale (BEHAVE‐AD), Clinical Dementia Rating scale (CDR), Clinician's Interview‐Based Impression of Change scale (CIBIC‐Plus), Cohen‐Mansfield Agitation Inventory (CMAI), Chrichton Scale (CMCS), Gottfries, Brane and Steen scale (GBS), Neuropsychiatric Instrument (NPI), Neuropsychiatric Inventory Distress scale (NPI‐D), and Syndrom Kurz Test (SKT);
an increase in score shows improvement for the Alzheimer's Disease Cooperative Study‐Activities of Daily Living Scale (severe version) (ADCS‐ADL‐sev), Disability Assessment for Dementia (DAD), Mini Mental State Examination (MMSE), Progressive Deterioration Scale (PDS), Quality of Life scale (QoL), and Severe Impairment Battery (SIB). (See Appendix 2 for more information about tests and rating scales.)
Comparison of donepezil (10 mg/day) with placebo
Cognitive function
The meta‐analysis, using mean differences (MDs) revealed a benefit on cognitive function as measured by the ADAS‐Cog test score for donepezil compared with placebo at 24 to 26 weeks (MD ‐2.67 , 95% CI ‐3.31 to ‐2.02, P < 0.00001, 5 studies, 1130 participants, ITT analysis; Analysis 1.1).
1.1. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 1 ADAS‐Cog (change from baseline at 24‐26 weeks) ITT‐LOCF.
The MMSE showed similar results in favour of donepezil at 24 to 26 weeks compared with placebo (MD 1.05, 95% CI 0.73 to 1.37, P < 0.00001, 7 studies, 1757 participants, ITT analysis; Analysis 1.2).
1.2. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 2 MMSE (change from baseline at 24‐26 weeks) ITT‐LOCF.
The SIB showed similar results in favour of donepezil at 24 to 26 weeks compared with placebo (MD 5.92, 95% CI 4.53 to 7.31, P < 0.00001, 5 studies, 1348 participants, ITT analysis; Analysis 1.3).
1.3. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 3 SIB (change from baseline at 24‐26 weeks) ITT‐LOCF.
Activities of daily living
The meta‐analysis, using MDs revealed a benefit on activities of daily living as measured by the ADCS‐ADL‐sev test score for donepezil compared with placebo at 24 to 26 weeks (MD 1.03 , 95% CI 0.21 to 1.85, P = 0.01, 3 studies, 733 participants, ITT analysis; Analysis 1.4).
1.4. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 4 ADCS‐ADL‐severe (change from baseline at 24‐26 weeks) ITT‐LOCF.
Global assessment
We dichotomised the seven‐point CIBIC‐Plus scale, or the Clinician's Global Impression of Change (CGIC) by counting those showing no change or decline against those showing improvement. There was benefit associated with donepezil (276/834), compared with placebo (173/840), at 24 to 26 weeks (OR 1.92, 95% CI 1.54 to 2.39, P < 0.00001, 6 studies, 1674 participants, ITT analysis; Analysis 1.5).
1.5. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 5 CIBIC‐Plus or CGIC (numbers improved at 24‐26 weeks) ITT‐LOCF.
We also used mean differences to analyse the CDR sum of boxes, measuring both cognitive function and aspects of everyday functioning together in a single score. It shows a benefit for donepezil compared with placebo at 24 to 26 weeks, (MD ‐0.53, 95% CI ‐0.73 to ‐0.33, P < 0.00001, 3 studies, 1028 participants, ITT analysis; Analysis 1.6).
1.6. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 6 CDR‐SB (change from baseline at 24‐26 weeks) ITT‐LOCF.
Behavioural symptoms
Four studies (MD ‐1.62, 95% CI ‐3.43 to 0.19, P = 0.08, 4 studies, 1035 participants, ITT analysis; Analysis 1.8), assessed behavioural symptoms using the NPI, and one study (MD 0.40, 95% CI ‐1.28 to 2.08, P = 0.64, 194 participants, ITT analysis; Analysis 1.7) using the BEHAVE‐AD score. There was no difference between donepezil and placebo at 24 to 26 weeks for either score.
1.8. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 8 Behavioural disturbance (Total NPI ) (change from baseline at 24‐26 weeks) ITT‐LOCF.
1.7. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 7 BEHAVE‐AD (change from baseline at 24‐26 weeks) ITT‐LOCF.
Withdrawals before the end of treatment
The meta‐analysis of withdrawals before the end of treatment showed a benefit in favour of placebo (282/1401) compared with donepezil (348/1445) at 24 to 26 weeks (OR 1.25, 95% CI 1.05 to 1.50, P = 0.0013, 12 studies, 2846 participants, ITT analysis; Analysis 1.10).
1.10. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 10 Total number of withdrawals before end of treatment at 24‐26 weeks.
Adverse events
The meta‐analysis of numbers of participants with at least one adverse event before the end of treatment showed a benefit in favour of placebo (793/1226) compared with donepezil (913/1274) at 24 to 26 weeks (OR 1.59, 95% CI 1.31 to 1.95, P < 0.00001, 10 studies, 2500 participants, ITT analysis; Analysis 1.11).
1.11. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 11 Total number of participants who suffered from at least one adverse event by 24‐26 weeks.
Quality of life
The meta‐analysis, using MDs showed no difference for QoL between donepezil 10 mg/day compared with placebo at 24 weeks (MD ‐2.79, 95% CI ‐8.15 to 2.56, P = 0.31, 2 studies, 815 participants, ITT analysis; Analysis 1.9).
1.9. Analysis.

Comparison 1 Donepezil (10 mg/day) versus placebo, Outcome 9 QoL (participant‐rated quality of life at 24‐26 weeks) ITT‐LOCF.
Comparison of donepezil (5 mg/day and 10 mg/day) with placebo
Cognitive function
The meta‐analyses, using MDs, revealed a benefit on cognitive function as measured by ADAS‐Cog test scores for the lower‐dose and higher‐dose donepezil compared with placebo at 12, and 24 to 26 weeks.
5 mg/day donepezil at 12 weeks (MD ‐2.27, 95% CI ‐3.16 to ‐1.39, P < 0.00001, 3 studies, 488 participants, ITT analysis; Analysis 2.2)
10 mg/day donepezil at 12 weeks (MD ‐2.99, 95% CI ‐3.99 to ‐1.99, P < 0.00001, 5 studies, 459 participants, ITT analysis; Analysis 2.2)
5 mg/day donepezil at 24 weeks (MD ‐2.01, 95% CI ‐2.69 to ‐1.34, P < 0.00001, 3 studies, 1089 participants, ITT analysis; Analysis 2.2)
10 mg/day donepezil at 24 weeks (MD ‐2.67, 95% CI ‐3.31 to ‐2.02, P < 0.00001, 5 studies, 1130 participants, ITT analysis; Analysis 2.2)
2.2. Analysis.
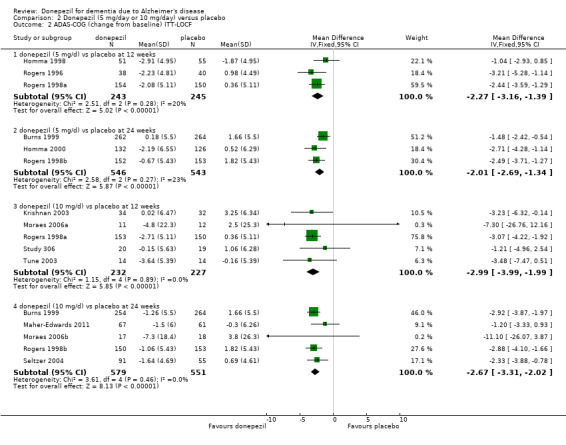
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 2 ADAS‐COG (change from baseline) ITT‐LOCF.
The MMSE showed similar results in favour of the lower‐dose and higher‐dose donepezil compared with placebo at 12, 24 to 26, and 52 weeks.
5 mg/day donepezil at 12 weeks (MD 0.92, 95% CI 0.32 to 1.53, P = 0.003, 2 studies, 382 participants, ITT analysis; Analysis 2.4 )
10 mg/day donepezil at 12 weeks (MD 1.19, 95% CI 0.61 to 1.77, P < 0.0001, 2 studies, 511 participants, ITT analysis; Analysis 2.4 )
5 mg/day donepezil at 24 weeks (MD 1.22, 95% CI 0.54 to 1.90, P = 0.0004, 2 studies, 358 participants, ITT analysis; Analysis 2.4 )
10 mg/day donepezil at 24 weeks (MD 1.05, 95% CI 0.73 to 1.37, P < 0.000001, 7 studies, 1757 participants, ITT analysis; Analysis 2.4 )
10 mg/day donepezil at 52 weeks (MD 1.70, 95% CI 0.81 to 2.59, P = 0.0002, 1 study, 272 participants, ITT analysis; Analysis 2.4 )
2.4. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 4 MMSE (change from baseline) ITT‐LOCF.
The meta‐analyses, using MDs, revealed a benefit on cognitive function as measured by SIB test scores for the lower‐dose and higher‐dose donepezil compared with placebo at 24 to 26 weeks.
5 mg/day donepezil at 24 weeks (MD 6.70, 95% CI 3.66 to 9.74, P < 0.0001, 1 study, 198 participants, ITT analysis; Analysis 2.5 )
10 mg/day donepezil at 24 weeks (MD 5.92, 95% CI 4.53 to 7.31, P < 0.000001, 5 studies, 1348 participants, ITT analysis; Analysis 2.5)
2.5. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 5 SIB (change from baseline) ITT‐LOCF.
Activities of daily living
The Winblad 2001 was the only study to assess activities of daily living using the PDS scale. 10 mg/day donepezil showed benefit compared with placebo at 52 weeks (MD 3.80, 95% CI 1.70 to 5.90, P = 0.0004, 1 study, 276 participants, ITT analysis; Analysis 2.15).
2.15. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 15 PDS ‐ progressive deterioration scale ITT‐LOCF.
Two other studies assessed activities of daily living, Feldman 2001 (using the DAD, Instrumental ADL and Physical Self‐maintenance Scale (PSMS)) and Homma 2000 (CMCS). There was evidence of benefit of donepezil at 12 and 24 weeks.
5 mg/day donepezil at 24 weeks (completers' analysis, MD ‐2.42, 95% CI ‐4.32 to ‐0.52, P = 0.01, 1 study, 228 participants, Analysis 2.12) (CMCS)
10 mg/day donepezil at 12 weeks (completers' analysis, MD 4.83, 95% CI 1.35 to 8.31, P = 0.007, 1 study, 254 participants, Analysis 2.13) (DAD)
10 mg/day donepezil at 24 weeks (completers' analysis, MD 8.00, 95% CI 3.61 to 12.39, P = 0.0004, 1 study, 247 participants, Analysis 2.13) (DAD)
10 mg/day donepezil at 12 weeks (completers' analysis, MD ‐4.31, 95% CI ‐7.72 to ‐0.90, P = 0.01, 1 study, 250 participants, Analysis 2.22 ) (IADL)
10 mg/day donepezil at 24 weeks (completers' analysis, MD ‐6.32, 95% CI ‐10.02 to ‐2.62, P = 0.0008, 1 study, 243 participants, Analysis 2.22) (IADL)
2.12. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 12 ADL and IADL (CMCS) (change from baseline) completers.
2.13. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 13 ADl and IADL (DAD) (change from baseline) completers.
2.22. Analysis.
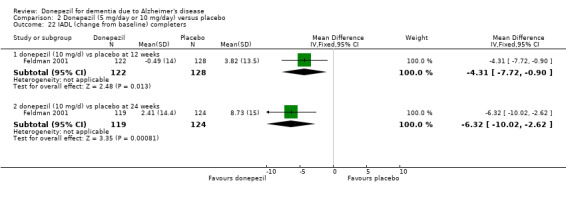
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 22 IADL (change from baseline) completers.
Winblad 2006, Homma 2008 and Black 2007 used the ADCS‐ADL‐severe scale. There was evidence of benefit of donepezil.
10 mg/day donepezil at 24 weeks (MD 1.03, 95% CI 0.21 to 1.85, P = 0.01, 3 studies, 733 participants, ITT analysis; Analysis 2.14).
2.14. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 14 ADCS‐ADL‐severe (change from baseline) ITT‐LOCF.
The primary endpoint of Mohs 2001 was time to clinically evident decline in function, as defined in the protocol. There was evidence of benefit of donepezil 10 mg/day (84/207) compared with placebo (116/206) at 54 weeks (OR 0.53, 95% CI 0.36 to 0.78, P = 0.001, 1 study, 413 participants; Analysis 2.16).
2.16. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 16 Total number meeting criterion for functional decline before end of treatment.
Global assessment
We dichotomised the seven‐point CIBIC‐Plus scale, measuring global clinical state, counting those showing no change or decline, against those showing improvement, and we analysed the results using the OR. There are benefits associated with 5 mg/day and 10 mg/day donepezil compared with placebo at 12, and 24 to 26 weeks, as shown by the ITT analyses.
5 mg/day donepezil at 12 weeks (49/153 donepezil, 27/150 placebo) (OR 2.10, 95% CI 1.25 to 3.53, P = 0.005, 1 study, 303 participants, ITT analysis; Analysis 2.7)
10 mg/day donepezil at 12 weeks (58/152 donepezil, 27/150 placebo) (OR 2.70, 95% CI 1.64 to 4.46, P = 0.0001, 1 study, 302 participants, ITT analysis; Analysis 2.7)
5 mg/day donepezil at 24 weeks (187/633 donepezil, 102/640 placebo) (OR 2.20, 95% CI 1.69 to 2.87, P < 0.00001, 4 studies, 1273 participants, ITT analysis; Analysis 2.7)
10 mg/day donepezil at 24 weeks (276/834 donepezil, 173/840 placebo) (OR 1.92, 95% CI 1.54 to 2.39, P < 0.00001, 6 studies, 1674 participants, ITT analysis; Analysis 2.7)
2.7. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 7 CIBIC‐plus or CGIC (numbers improved) ITT‐LOCF.
The GBS and Mental Function Impairment Scale (MENFIS) are both global assessment scales. Only two studies used these scales, the Winblad 2001 used the GBS and Homma 2000 used the MENFIS. There was some evidence of benefit associated with donepezil (completers).
5 mg/day donepezil at 24 weeks (completers, MD ‐2.56, 95%CI ‐4.27 to ‐0.85, P = 0.003, 1 study, 228 participants, Analysis 2.10)
10 mg/day donepezil at 52 weeks (completers, MD ‐6.01, 95%CI ‐11.93 to ‐0.09, P = 0.05, 1 study, 190 participants, Analysis 2.10 )
2.10. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 10 GBS or MENFIS ‐ global assessment completers.
We also analysed the CDR‐SB using MDs. This measures both cognitive function and aspects of everyday functioning together in a single score, and showed a benefit with 5 mg/day and 10 mg/day of donepezil compared with placebo at 12 weeks, and 10 mg/day of donepezil compared with placebo at 24 weeks, but there was no difference between donepezil 5 mg/day and placebo at 12 weeks.
5 mg/day donepezil at 12 weeks (MD ‐0.02, 95% CI ‐0.25 to 0.21, P = 0.86, 3 studies, 487 participants, ITT analysis; Analysis 2.9)
10 mg/day donepezil at 12 weeks (MD ‐0.23, 95% CI ‐0.47 to 0.00, P = 0.05, 4 studies, 559 participants, ITT analysis; Analysis 2.9)
5 mg/day donepezil at 24 weeks (MD ‐0.51, 95% CI ‐0.70 to ‐0.32, P < 0.00001, 3 studies, 1093 participants, ITT analysis; Analysis 2.9)
10 mg/day donepezil at 24 weeks (MD ‐0.53, 95% CI ‐0.73 to ‐0.33, P < 0.00001, 3 studies, 1028 participants, ITT analysis; Analysis 2.9)
2.9. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 9 CDR‐SB (change from baseline) ITT‐LOCF.
Behavioural symptoms
Feldman 2001, Tune 2003, Winblad 2006, Black 2007 and Tariot 2001 assessed behavioural disturbance (NPI‐TOTAL), and there was no evidence of benefit.
10 mg/day donepezil at 12 weeks (MD ‐1.45, 95% CI ‐4.43 to 1.53, P = 0.34, 2 studies, 279 participants, ITT analysis; Analysis 2.17)
10 mg/day donepezil at 24 weeks (MD ‐1.04, 95% CI ‐3.16 to 1.07, P = 0.33, 4 studies, 692 participants, ITT analysis; Analysis 2.17)
2.17. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 17 Behavioural disturbance (total NPI) (change from baseline) completers.
Homma 2008 assessed BEHAVE‐AD and there was no evidence of benefit of donepezil.
5 mg/day donepezil at 24 weeks (MD 0.00, 95% CI ‐1.67 to 1.67, P = 1.0, 1 study, 198 participants, ITT analysis; Analysis 2.18)
10 mg/day donepezil at 24 weeks (MD 0.40, 95% CI ‐1.28 to 2.08, P = 0.64, 1 study, 194 participants, ITT analysis; Analysis 2.18)
2.18. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 18 BEHAVE‐AD (change from baseline) ITT‐LOCF.
Withdrawals before the end of treatment
Donepezil was judged to be fairly well tolerated. The meta‐analyses of withdrawals before the end of treatment, using the OR, showed benefit in withdrawals between the 5 mg/day group and the placebo group at 12 weeks in favour of placebo but not at 24 weeks, and for the 10 mg/day group at 12 and 24 weeks in favour of placebo but not at 52 weeks.
5 mg/day donepezil at 12 weeks (70/543 donepezil, 40/536 placebo) (OR 1.81, 95% CI 1.22 to 2.68, P = 0.003, 4 studies, 1079 participants, ITT analysis; Analysis 2.26)
10 mg/day at 12 weeks (29/184 donepezil, 13/178 placebo) (OR 2.31, 95% CI 1.21 to 4.40, P = 0.01, 2 studies, 362 participants, ITT analysis; Analysis 2.26)
10 mg/day donepezil at 24 to 26 weeks (348/1445 donepezil, 282/1401 placebo) (OR 1.25, 95% CI 1.05 to 1.50, P = 0.013, 12 studies, 2846 participants, ITT analysis; Analysis 2.26).
10 mg/day donepezil at 52 week (47/142 donepezil, 47/144 placebo) (OR 1.02, 95% CI 0.62 to 1.67, P = 0.93, 1 study, 286 participants, ITT analysis; Analysis 2.26)
2.26. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 26 Total number of withdrawals before end of treatment.
Dependency
Feldman 2001 assessed the time spent each day by the carer assisting with the activities of daily living but there was no evidence of a treatment effect. 10 mg/day donepezil at 24 weeks (MD ‐52.4, 95% CI ‐118.78 to 13.98, P = 0.12, 1 study, 221 participants, ITT analysis; Analysis 2.24).
2.24. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 24 Time (mins/day) spent by carer assisting in IADL and PSMS (change from baseline) LOCF.
Withdrawals before the end of treatment due to adverse events
The meta‐analyses of withdrawals before the end of treatment due to adverse events, using the OR, showed differences in withdrawals between the 5 mg/day group and the placebo group at 12 weeks but not at 24 weeks, and for the 10 mg/day group at 12, and 24 to 26 weeks, but not at 52 weeks in favour of placebo.
5 mg/day donepezil at 12 weeks (17/260 donepezil, 7/253 placebo) (OR 2.33, 95% CI 1.02 to 5.28, P = 0.04, 3 studies, 513 participants, ITT analysis; Analysis 2.27)
5 mg/day donepezil at 24 weeks (43/662 donepezil, 55/673 placebo) (OR 0.78, 95% CI 0.52 to 1.18, P = 0.25, 4 studies, 1335 participants, ITT analysis; Analysis 2.27)
10 mg/day at 12 weeks (16/184 donepezil, 43/178 placebo) (OR 3.45, 95% CI 1.40 to 8.50, P = 0.007, 3 studies, 362 participants, ITT analysis; Analysis 2.27)
10 mg/day donepezil at 24 to 26 weeks (199/1431 donepezil, 121/1388 placebo) (OR 1.68, 95% CI 1.33 to 2.12, P < 0.00001, 11 studies, 2819 participants, ITT analysis; Analysis 2.27)
10 mg/day donepezil at 52 weeks (10/142 donepezil, 9/144 placebo) (OR 1.14, 95% CI 0.45 to 2.88, P = 0.79, 1 study, 286 participants, ITT analysis; Analysis 2.27)
2.27. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 27 Total number of participants who withdrew due to an adverse event.
Adverse events
The meta‐analyses of numbers of participants with at least one adverse event showed a difference between the 5 mg/day group and placebo in favour of placebo, at 24 to 26 weeks but not at 12 weeks, and a similar result for the 10 mg/day donepezil.
5 mg/day donepezil at 12 weeks (106/157 donepezil, 106/153 placebo) (OR 1.08, 95% CI 0.70 to 1.67, P = 0.24, 3 studies, 513 participants, ITT analysis; Analysis 2.28)
5 mg/day donepezil at 24 to 26 weeks (346/508 donepezil, 317/510 placebo) (OR 1.40, 95% CI 1.06 to 1.86, P = 0.02, 3 studies, 1018 participants, ITT analysis; Analysis 2.28)
10 mg/day donepezil at 12 weeks (124/158 donepezil, 106/153 placebo) (OR 1.55, 95% CI 0.94 to 2.55, P = 0.09, 2 studies, 323 participants, ITT analysis; Analysis 2.28)
10 mg/day at 24 to 26 weeks (913/1274 donepezil, 793/1226 placebo) (OR 1.59, 95% CI 1.30 to 1.94, P < 0.000001, 10 studies, 2500 participants, ITT analysis; Analysis 2.28)
10 mg/day donepezil at 52 weeks (116/142 donepezil, 109/144 placebo) (OR 1.43, 95% CI 0.81 to 2.51, P = 0.22, 1 study, 286 participants, ITT analysis; Analysis 2.28)
2.28. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 28 Total number of participants who suffered from at least one adverse event.
The studies reported 53 different causes of adverse events. The causes of adverse events seen more frequently in the 10 mg/day dose group than in the 5 mg/day group or placebo group included nausea, vomiting and diarrhoea. These were mostly mild and transient, but occasionally moderately severe. There were differences, in favour of placebo, compared with donepezil, usually the 10 mg/day dose, for several causes of adverse events.
Anorexia
10 mg/day donepezil 24 to 26 weeks (67/962 donepezil, 21/969 placebo) (OR 3.01 95% CI 1.96 to 4.62, P < 0.00001, 6 studies, 1931 participants, ITT analysis; Analysis 2.35)
2.35. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 35 Total number of participants who suffered from anorexia.
Diarrhoea
5 mg/day donepezil at 12 weeks (14/197 donepezil, 5/193 placebo) (OR 2.64 95% CI 1.05 to 6.63, P = 0.04, 2 studies, 390 participants, ITT analysis; Analysis 2.47)
10 mg/day donepezil at 12 weeks (21/158 donepezil, 4/153 placebo) (OR 4.22 95% CI 1.87 to 9.54, P = 0.0005, 1 study, 311 participants; Analysis 2.47)
5 mg/day donepezil at 24 to 26 weeks (53/662 donepezil, 30/672 placebo) (OR 1.85 95% CI 1.19 to 2.89, P = 0.007, 4 studies, 1334 participants, ITT analysis; Analysis 2.47)
10 mg/day donepezil at 24 to 26 weeks (166/1330 donepezil, 60/1292 placebo) (OR 2.69 95% CI 2.05 to 3.55, P < 0.00001, 9 studies, 2622 participants, ITT analysis; Analysis 2.47)
2.47. Analysis.
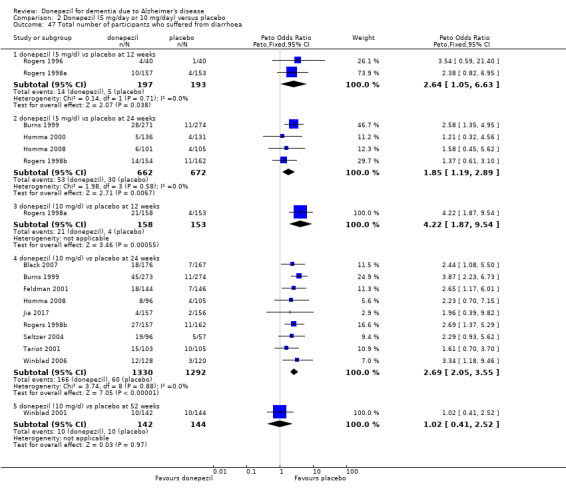
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 47 Total number of participants who suffered from diarrhoea.
Dizziness
10 mg/day donepezil at 24 to 26 weeks (68/930 donepezil, 38/900 placebo) (OR 1.77 95% CI 1.19 to 2.63, P = 0.004, 6 studies, 1830 participants, ITT analysis; Analysis 2.48)
2.48. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 48 Total number of participants who suffered from dizziness.
Fatigue
10 mg/day donepezil at 24 to 26 weeks (12/157 donepezil, 3/162 placebo) (OR 3.63, 95% CI 1.29 to 10.21, P = 0.01, 1 study, 319 participants, ITT analysis; Analysis 2.51)
2.51. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 51 Total number of participants who suffered from fatigue.
Hallucinations
10 mg/day donepezil at 24 to 26 weeks (8/128 donepezil, 1/120 placebo) (OR 4.68, 95% CI 1.24 to 17.66, P = 0.02, 1 study, 248 participants, ITT analysis; Analysis 2.56)
2.56. Analysis.
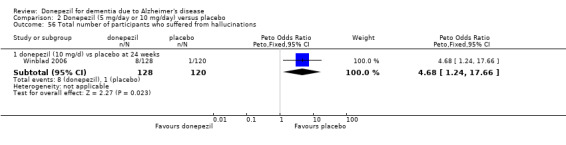
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 56 Total number of participants who suffered from hallucinations.
Insomnia
10 mg/day donepezil at 12 weeks (28/158 donepezil, 8/153 placebo) (OR 3.38, 95% CI 1.69 to 6.76, P = 0.0006, 1 study, 311 participants, ITT analysis; Analysis 2.62)
10 mg/day donepezil at 24 to 26 weeks (39/546 donepezil, 15/497 placebo) (OR 2.40, 95% CI 1.38 to 4.15, P = 0.002, 3 studies, 1043 participants, ITT analysis; Analysis 2.62)
2.62. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 62 Total number of participants who suffered from insomnia.
Muscle cramp
5 mg/day donepezil at 24 to 26 weeks (9/154 donepezil, 1/162 placebo) (OR 5.48, 95% CI 1.56 to 19.27, P = 0.008, 1 study, 316 participants, ITT analysis; Analysis 2.65)
10 mg/day donepezil at 24 to 26 weeks (12/157 donepezil, 1/162 placebo) (OR 6.00, 95% CI 1.98 to 18.18, P = 0.002, 1 study, 319 participants, ITT analysis; Analysis 2.65)
2.65. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 65 Total number of participants who suffered from muscle cramp.
Nausea
10 mg/day donepezil at 12 weeks (34/158 donepezil, 12/153 placebo) (OR 2.95, 95% CI 1.58 to 5.51, P = 0.0007, 1 study, 311 participants, ITT analysis; Analysis 2.66)
10 mg/day donepezil at 24 to 26 weeks (144/1120 donepezil, 46/1064 placebo) (OR 3.06, 95% CI 2.26 to 4.14, P < 0.00001, 8 studies, 2184 participants, ITT analysis; Analysis 2.66)
2.66. Analysis.
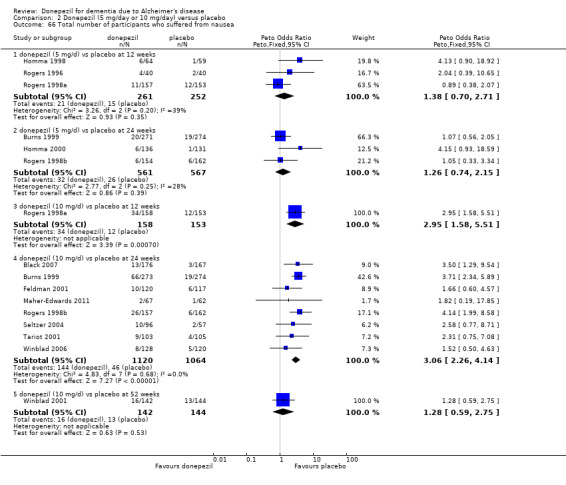
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 66 Total number of participants who suffered from nausea.
Peripheral oedema
10 mg/day donepezil at 24 to 26 weeks (25/103 donepezil, 14/105 placebo) (OR 2.04, 95% CI 1.02 to 4.09, P = 0.04, 1 study, 208 participants, ITT analysis; Analysis 2.68)
2.68. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 68 Total number of participants who suffered from peripheral oedema.
Tremor
10 mg/day donepezil at 24 to 26 weeks (8/103 donepezil, 2/105 placebo) (OR 3.58, 95% CI 1.01 to 12.71, P = 0.05, 1 study, 208 participants, ITT analysis; Analysis 2.77)
2.77. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 77 Total number of participants who suffered from tremor.
Vertigo
10 mg/day donepezil at 52 weeks (11/142 donepezil, 3/144 placebo) (OR 3.36, 95% CI 1.15 to 9.82, P = 0.03, 1 study, 286 participants, ITT analysis; Analysis 2.80 )
2.80. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 80 Total number of participants who suffered from vertigo.
Vomiting
10 mg/day donepezil at 24 to 26 weeks (109/949 donepezil, 43/959 placebo) (OR 2.65, 95% CI 1.90 to 3.70, P < 0.00001, 6 studies, 1908 participants, Analysis 2.74)
2.74. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 74 Total number of participants who suffered from vomiting.
Weight loss
10 mg/day donepezil at 24 to 26 weeks (34/404 donepezil, 19/407 placebo) (OR 1.90, 95% CI 1.08 to 3.35, P = 0.03, 3 studies, 811 participants, ITT analysis; Analysis 2.81)
2.81. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 81 Total number of participants who suffered from weight loss.
There were significant differences between numbers suffering adverse events in the donepezil group compared with the placebo group in favour of donepezil at 12 weeks but not at 24 weeks for the following causes.
Increased cough
5 mg/day donepezil at 12 weeks (2/157 donepezil, 8/153 placebo) (OR 0.28 95% CI 0.08 to 1.00, P = 0.05, 1 study, 310 participants, ITT analysis; Analysis 2.63)
2.63. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 63 Total number of participants who suffered from increased cough.
Peripheral oedema
5 mg/day donepezil at 12 weeks (1/157 donepezil, 8/153 placebo) (OR 0.20, 95% CI 0.05 to 0.74, P = 0.02, 1 study, 310 participants, ITT analysis; Analysis 2.68)
Urinary tract infection
5 mg/day donepezil at 12 weeks (10/157 donepezil, 20/153 placebo) (OR 0.47, 95% CI 0.22 to 0.99, P = 0.05, 1 study, 310 participants, ITT analysis; Analysis 2.79)
10 mg/day donepezil at 12 weeks (6/158 donepezil, 20/153 placebo) (OR 0.30, 95% CI 0.13 to 0.67, P = 0.003, 1 study, 311 participants, ITT analysis; Analysis 2.79)
2.79. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 79 Total number of participants who suffered from urinary tract infection.
The Winblad 2001 reported only causes of adverse events suffered by more than 5% of participants receiving donepezil.
Serious adverse events
The meta‐analyses of numbers of participants with at least one serious adverse event showed no difference between the 5 mg/day group and placebo, and the 10 mg/day group and placebo, at 12, and 24 to 26 weeks, and a difference at 52 weeks in favour of placebo.
5 mg/day donepezil at 12 weeks (6/157 donepezil, 7/153 placebo) (OR 0.83, 95% CI 0.27 to 2.51, P = 0.74, 1 study 310 participants, Analysis 2.83)
5 mg/day donepezil at 24 weeks (35/526 donepezil, 49/541 placebo) (ITT analysis; OR 0.76, 95% CI 0.49 to 1.18, P = 0.22, 3 studies, 1067 participants, ITT analysis; Analysis 2.83 )
10 mg/day donepezil at 12 weeks (6/158 donepezil, 7/153 placebo) (OR 0.82, 95% CI 0.27 to 2.50, P = 0.73, 1 study, 311 participants, ITT analysis; Analysis 2.83 )
10 mg/day donepezil at 24 to 26 weeks (148/1301 donepezil, 161/1298 placebo) (OR 0.90, 95% CI 0.71 to 1.14, P = 0.38, 9 studies, 2599 participants, ITT analysis; Analysis 2.83)
10 mg/day donepezil at 52 weeks (35/142 donepezil, 20/144 placebo) (OR 1.99, 95% CI 1.11 to 3.59, P = 0.02, 1 study, 286 participants, ITT analysis; Analysis 2.83 )
2.83. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 83 Total number of participants who suffered from at least one serious adverse event.
Deaths
The meta‐analyses of numbers of deaths showed no difference between the 5 mg/day group and placebo, and the 10 mg/day group and placebo, at 12, 24 to 26 weeks and at 52 weeks.
5 mg/day donepezil at 12 weeks (1/260 donepezil, 1/253 placebo) (OR 0.96, 95% CI 0.06 to 15.29, P = 0.97, 3 studies, 513 participants, ITT analysis; Analysis 2.82)
5 mg/day donepezil at 24 weeks (4/662 donepezil, 4/672 placebo) (OR 1.02, 95% CI 0.25 to 4.10, P = 0.98, 4 studies, 1334 participants, ITT analysis; Analysis 2.82)
10 mg/day donepezil at 12 weeks (0/158 donepezil, 1/153 placebo) (OR 0.13, 95% CI 0.00 to 6.60, P = 0.31, 1 study, 311 participants, ITT analysis; Analysis 2.82)
10 mg/day donepezil at 24 to 26 weeks (32/1445 donepezil, 41/1402 placebo) (OR 0.74, 95% CI 0.46 to 1.19, P = 0.21, 12 studies, 2847 participants, ITT analysis; Analysis 2.82)
10 mg/day donepezil at 52 weeks (4/142 donepezil, 3/144 placebo) (OR 1.36, 95% CI 0.30 to 6.07, P = 0.69, 1 study, 286 participants, ITT analysis; Analysis 2.82)
2.82. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 82 total number of deaths before end of treatment.
Quality of life
There was no evidence of any benefit associated with donepezil in the patient‐rated Quality‐of‐Life scale at doses of either 5 mg/day or 10 mg/day compared with placebo at 12 or 24 weeks.
5 mg/day donepezil at 12 weeks (MD 1.18, 95% CI ‐3.04 to 5.40, P = 0.58, 4 studies, 1127 participants, ITT analysis; Analysis 2.20)
10 mg/day donepezil at 12 weeks (MD 1.16, 95% CI ‐3.20 to 5.52, P = 0.60, 4 studies, 1031 participants, ITT analysis; Analysis 2.20)
5 mg/day donepezil at 24 weeks (MD 2.26, 95% CI ‐3.64 to 8.16, P = 0.45, 2 studies, 681 participants, ITT analysis; Analysis 2.20)
10 mg/day donepezil at 24 weeks (MD ‐1.17, 95% CI ‐7.26 to 4.91, P = 0.71, 2 studies, 645 participants, ITT analysis; Analysis 2.20).
2.20. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 20 QoL (participant‐rated quality of life) completers.
Carer stress
Lebert 1999 assessed the stress on carers but reported the results without any measure of precision.
Comparison of donepezil (10 mg/day) with placebo (patient and carer health resource utilisation)
Feldman 2001 and Winblad 2001 assessed this outcome. We did not pool the studies as we did not consider the outcomes to be comparable across studies. Many items were assessed and reported separately, and total costs were reported. There were no significant differences between donepezil and placebo apart from total carer costs (counselling, visits to physician and medication) in favour of placebo (MD 31.00, 95% CI 7.22 to 54.78, P = 0.01, one study, 289 participants; Analysis 3.3).
3.3. Analysis.
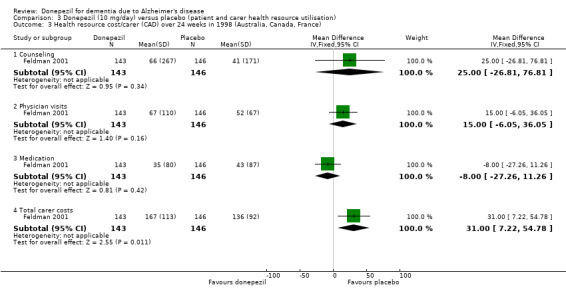
Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 3 Health resource cost/carer (CAD) over 24 weeks in 1998 (Australia, Canada, France).
Comparison of donepezil 5 mg/day with donepezil 10 mg/day
The phase III studies were designed not only to compare donepezil with placebo, but also to compare two doses, 5 mg/day and 10 mg/day. We pooled results from the three 26‐week studies, Homma 2008, Rogers 1998b and Burns 1999.
Cognitive function
There was a significant difference in favour of the 10 mg group for ADAS‐Cog (MD ‐1.05, 95% CI ‐1.80 to ‐0.30, P = 0.006, 2 studies, 818 participants, ITT analysis; Analysis 7.1), but no difference between the groups for MMSE (MD 0.15, 95% CI ‐0.55 to 0.85, P = 0.67, 1 study, 303 participants, ITT analysis; Analysis 7.2), or SIB (MD 2.20, 95% CI ‐1.00 to 5.40, P = 0.18, 1 study, 188 participants, ITT analysis; Analysis 7.3).
7.1. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 1 ADAS‐Cog (change from baseline at 24 weeks) ITT‐LOCF.
7.2. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 2 MMSE (change from baseline at 24 weeks) ITT‐LOCF.
7.3. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 3 SIB (change from baseline) at 24 weeks ITT‐LOCF.
Quality of life
There was a difference in favour of the 5 mg/day group for quality of life (MD ‐8.33, 95% CI ‐16.23 to ‐0.43, P = 0.04, 1 study, 302 participants; Analysis 7.8).
7.8. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 8 QoL (change from baseline at 24 weeks) ITT‐LOCF.
Global assessment
There was no difference between the 10 mg/day and 5 mg/day groups at 24 to 26 weeks for the global assessment using CIBIC‐plus (OR 1.26, 95% CI 0.94 to 1.67, P = 0.12, 3 studies, 981 participants, ITT analysis; Analysis 7.5), or CDR‐SB (MD ‐0.08, 95% CI ‐0.29 to 0.14, P = 0.48, 2 studies, 824 participants, ITT analysis; Analysis 7.6).
7.5. Analysis.
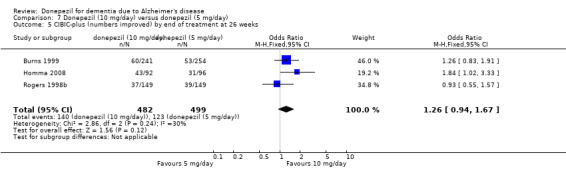
Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 5 CIBIC‐plus (numbers improved) by end of treatment at 26 weeks.
7.6. Analysis.
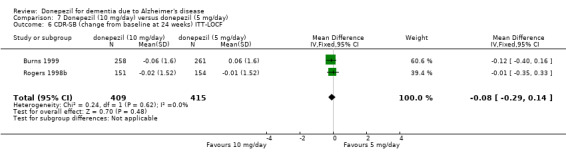
Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 6 CDR‐SB (change from baseline at 24 weeks) ITT‐LOCF.
Behavioural symptoms
There was no difference between the 10 mg/day and 5 mg/day groups at 24 to 26 weeks for the BEHAVE‐AD (MD 0.40, 95% CI ‐1.27 to 2.07, 1 study, 198 participants, ITT analysis; Analysis 7.7).
7.7. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 7 BEHAVE‐AD (change from baseline) at 24 weeks ITT‐LOCF.
Withdrawals before the end of treatment
The meta‐analyses of withdrawals before the end of treatment at 24 to 26 weeks, using the OR, showed differences in withdrawals in favour of the 5 mg/day group (143/526 10 mg/day, 96/526 5 mg/day) (OR 1.67, 95% CI 1.24 to 2.23, P = 0.0006, 3 studies, 1052 participants, ITT analysis; Analysis 7.9).
7.9. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 9 Total number of participants who withdrew before end of treatment at 26 weeks.
Withdrawals before the end of treatment due to adverse events
The meta‐analyses of withdrawals before the end of treatment at 24 to 26 weeks, due to an adverse event, using the OR, show that there was a difference in favour of the 5 mg/day group (89/526 10 mg/day, 41/526 5 mg/day) (OR 2.41 95% CI 1.63 to 3.57, P < 0.0001, 3 studies, 1052 participants, ITT analysis; Analysis 7.10).
7.10. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 10 Total number of participants who withdrew due to an adverse event before end of treatment at 26 weeks.
Adverse events
The meta‐analyses of the total number of participants who suffered at least one adverse event showed a difference in favour of the 5 mg/day group (314/369 10 mg/day, 292/372 5 mg/day) (OR 1.56, 95% CI 1.07 to 2.28, P = 0.02, 2 studies,741, ITT analysis; Analysis 7.11).
7.11. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 11 Total number of participants who suffered an adverse event before end of treatment at 26 weeks.
There were significant differences, in favour of 5 mg/day, compared with 10 mg/day, for several causes of adverse events. The adverse events seen more frequently in the 10 mg/day group than in the 5 mg/day group were anorexia, nausea, diarrhoea, rhinitis and vomiting.
Anorexia: (39/526 10 mg/day, 15/526 5 mg/day) (OR 2.72, 95% CI 1.48 to 5.00, P = 0.001, 3 studies, 1052 participants, ITT analysis; Analysis 7.12)
Diarrhoea: (80/526 10 mg/day, 48/526 5 mg/day) (OR 1.78 ,95% CI 1.22 to 2.61, P = 0.003, 3 studies, 1052 participants, ITT analysis; Analysis 7.14)
Nausea: (92/430 10 mg/day, 26/425 5 mg/day) (OR 4.22, 95% CI 2.67 to 6.70, P < 0.00001, 2 studies, 855 participants, ITT analysis; Analysis 7.20)
Rhinitis: (9/157 10 mg/day, 1/154 5 mg/day) (OR 9.30, 95% CI 1.16 to 74.35, P = 0.04, 1 study, 311 participants, ITT analysis; Analysis 7.21)
Vomiting: (73/526 10 mg/day, 24/526 5 mg/day) (OR 3.40, 95% CI 2.10 to 5.48, P < 0.00001, 3 studies, 1052 participants, ITT analysis; Analysis 7.22)
7.12. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 12 Total number of participants who suffered an adverse event of anorexia before end of treatment at 26 weeks.
7.14. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 14 Total number of participants who suffered an adverse event of diarrhoea before end of treatment at 26 weeks.
7.20. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 20 Total number of participants who suffered an adverse event of nausea before end of treatment at 26 weeks.
7.21. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 21 Total number of participants who suffered an adverse event of rhinitis before end of treatment at 26 weeks.
7.22. Analysis.
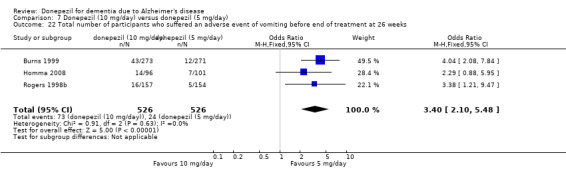
Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 22 Total number of participants who suffered an adverse event of vomiting before end of treatment at 26 weeks.
Comparison of donepezil (15‐20 mg/day) with donepezil (10 mg/day)
Schindler 2004 reported the number of adverse events in 26 weeks of treatment when participants already taking 10 mg/day of donepezil were randomised to either placebo or a further 5 mg/day or 10 mg/day of donepezil. There was no difference between treatment and placebo (that is, between 15‐20 mg/day and 10 mg/day) (6/16 15‐20 mg/day, 4/15 10 mg/day) (OR 1.65, 95% CI 0.36 to 7.60, P = 0.52, 1 study, 31 participants, ITT analysis; Analysis 5.1). Schindler 2004 did not assess cognitive function.
5.1. Analysis.

Comparison 5 Donepezil (15‐20 mg/day) versus donepezil (10 mg/day), Outcome 1 Number who suffered an adverse event before end of treatment at 26 weeks.
Comparison of donepezil (10 mg/day) with placebo at 12 weeks for participants with severe agitation
One study included participants with severe agitation, Howard 2007. There was no difference between donepezil (13/128), and placebo (19/131) for withdrawals before end of treatment (OR 0.67, 95% CI 0.31 to 1.41, P = 0.29, 1 study, 259 participants, Analysis 6.4), CMAI (MD 0.18, 95% CI ‐4.23 to 4.59, P = 0.94 , 1 study 221 participants, Analysis 6.1), NPI (MD 0.10, 95% CI ‐3.78 to 3.98, P = 0.96, 1 study, 201 participants, Analysis 6.2), and NPI‐carer distress (MD ‐0.45, 95% CI ‐2.06 to 1.16, P = 0.58, 1 study, 200 participants, Analysis 6.3). The SIB and MMSE were also outcomes but less than half of the participants were able to complete these assessments.
6.4. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 4 Total number of withdrawals before end of treatment.
6.1. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 1 CMAI (change from baseline) completers.
6.2. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 2 NPI (change from baseline) completers.
6.3. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 3 NPI caregiver distress (change from baseline).
Comparison of donepezil (23 mg/day) with donepezil (10 mg/day)
Cognitive Function
There was no significant difference between the 23 mg group and the 10 mg group for SIB (MD 1.05, 95% CI ‐0.15 to 2.25, P = 0.09, 2 studies, 1704 participants, ITT analysis; Analysis 4.1), and there was no difference between the groups for the MMSE (MD 0.20, 95% CI ‐0.33 to 0.73, P = 0.46, 1 study, 1370 participants, ITT analysis; Analysis 4.2).
4.1. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 1 SIB (change from baseline) at 24 weeks ITT‐LOCF.
4.2. Analysis.
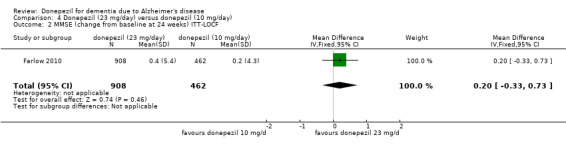
Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 2 MMSE (change from baseline at 24 weeks) ITT‐LOCF.
Activities of daily living
There was no difference between the groups for the ADCS‐ADL‐sev (MD 0.0, 95% CI ‐1.18 to 1.18, 1 study, 1369 participants, ITT analysis; Analysis 4.3).
4.3. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 3 ADCS‐ADL‐sev (change from baseline) at 24 weeks ITT‐LOCF.
Global assessment
There was no difference between the groups for the CIBIC‐plus (OR 0.99, 95% CI 0.78 to 1.26, P = 0.93, 2 studies, 1704 participants, ITT analysis; Analysis 4.4).
4.4. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 4 CIBIC‐plus (numbers improved) by end of treatment at 24 weeks.
Withdrawals before the end of treatment
The meta‐analysis of withdrawals before the end of treatment at 24 to 26 weeks, using the OR, showed a significant difference in withdrawals in favour of the 10 mg/day group (112/652), compared with the 23 mg/day group (348/1166), (OR 2.02, 95% CI 1.59 to 2.57, P < 0.00001, 2 studies, 1818 participants, ITT analysis; Analysis 4.5).
4.5. Analysis.
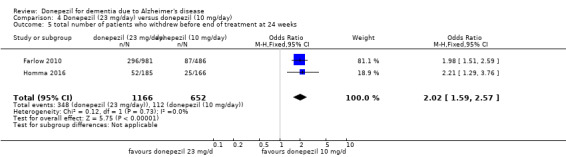
Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 5 total number of patients who withdrew before end of treatment at 24 weeks.
Withdrawals before the end of treatment due to adverse events
The meta‐analysis of withdrawals before the end of treatment due to adverse events at 24 to 26 weeks, using the OR, showed a significant difference in withdrawals in favour of the 10 mg/day group (54/652) compared with the 23 mg group (215/1166), (OR 2.51, 95% CI 1.83 to 3.45, P < 0.00001, 2 studies, 1818 participants, ITT analysis; Analysis 4.6).
4.6. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 6 total number of patients who withdrew due to an adverse event before end of treatment at 24 weeks.
Adverse events
The meta‐analyses of the total number of participants who suffered at least one adverse event showed a significant difference in favour of the 10 mg/day group (398/637), compared with the 23 mg/day group (844/1148) at 24 to 26 weeks, (OR 1.65, 95% CI 1.34 to 2.03, P < 0.0001, 1 study, 1785 participants, ITT analysis; Analysis 4.7).
4.7. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 7 total number of patients who suffered an adverse event before end of treatment at 24 weeks.
There was no difference between the groups for the numbers of participants who suffered a serious adverse event at 24 to 26 weeks (103/1148 23 mg/day, 59/637 10 mg/day) (OR 0.99, 95% CI 0.71 to 1.38, P = 0.94. 2 studies, 1785 participants, ITT analysis; Analysis 4.8).
4.8. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 8 total number of patients who suffered a serious adverse event before end of treatment at 24 weeks.
There were significant differences, in favour of 10 mg/day, compared with 23 mg/day, for several causes of adverse events. The adverse events seen more frequently in the 23 mg/day dose group than in the 105 mg/day group were asthenia, anorexia, contusion, nausea, vomiting and diarrhoea, fatigue and bradycardia.
Anorexia: (51/963 23 mg/day, 8/471 10 mg/day) (OR 3.24, 95% CI 1.52 to 6.88, P = 0.002, 1 study, 1434 participants, ITT analysis; Analysis 4.11)
Asthenia: (20/963 23 mg/day, 3/471 10 mg/day) (OR 3.31, 95% CI 0.98 to 11.19, P = 0.05, 1 study, 1434 participants, ITT analysis; Analysis 4.9)
Bradycardia: (27/963 23 mg/day, 3/471 10 mg/day) (OR 4.50, 95% CI 1.36 to 14.91, P = 0.01, 1 study, 1434 participants, ITT analysis; Analysis 4.22)
Contusion: (34/1148 23 mg/day, 5/63710 mg/day) (OR 4.9997, 95% CI 1.88 to 13.26, P = 0.001, 2 studies, 1785 participants, ITT analysis; Analysis 4.10)
Diarrhoea: (94/1148 23 mg/day, 30/637 10 mg/day) (OR 1.76, 95% CI 1.15 to 2.68, P = 0.009, 2 studies, 1785 participants, ITT analysis; Analysis 4.12)
Fatigue: (23/963 23 mg/day, 4/471 10 mg/day) (OR 2.86, 95% CI 0.98 to 8.31, P = 0.05, 1 study, 1434 participants, ITT analysis; Analysis 4.14)
Nausea: (123/1148 23 mg/day, 21/637 10 mg/day) (OR 3.36, 95% CI 2.09 to 5.42, P < 0.00001, 2 studies, 1785 participants, ITT analysis; Analysis 4.17)
Vomiting: (105/1148 23 mg/day, 16/637 10 mg/day) (OR 3.88, 95% CI 2.27 to 6.65, P < 0.00001, 2 studies, 1785 participants, ITT analysis; Analysis 4.18)
4.11. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 11 total number of patients who suffered an adverse event of anorexia before end of treatment at 24 weeks.
4.9. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 9 total number of patients who suffered an adverse event of asthenia before end of treatment at 24 weeks.
4.22. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 22 total number of patients who suffered an adverse event of bradycardia and sinus bradycardia before end of treatment at 24 weeks.
4.10. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 10 total number of patients who suffered an adverse event of contusion before end of treatment at 24 weeks.
4.12. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 12 total number of patients who suffered an adverse event of diarrhoea before end of treatment at 24 weeks.
4.14. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 14 total number of patients who suffered an adverse event of fatigue before end of treatment at 24 weeks.
4.17. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 17 total number of patients who suffered an adverse event of nausea before end of treatment at 24 weeks.
4.18. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 18 total number of patients who suffered an adverse event of vomiting before end of treatment at 24 weeks.
Deaths
There was no difference between the groups for the numbers of participants who died before end of treatment at 24 to 26 weeks (8/1148 23 mg/day, 6/637 10 mg/day) (OR 0.69, 95% CI 0.24 to 1.95, P = 0.48, 2 studies, 1785 participants, ITT analysis; Analysis 4.27).
4.27. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 27 total number of patients who died before end of treatment at 24 weeks.
Comparison of donepezil (5 mg/day and 10 mg/day) with placebo in participants with severe dementia
Cognitive function
The MMSE, using MDs, showed a benefit on cognitive function in favour of the higher‐dose donepezil compared with placebo at 24 weeks.
10 mg/day donepezil at 24 weeks (MD 0.97, 95% CI 0.56 to 1.38, P = <0.00001, 4 studies, 1102 participants, ITT analysis; Analysis 8.1)
8.1. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 1 MMSE (change from baseline) ITT‐LOCF.
The meta‐analyses, using MDs, revealed a benefit on cognitive function as measured by SIB test scores for the lower‐dose and higher‐dose donepezil compared with placebo at 24 weeks.
5 mg/day donepezil at 24 weeks (MD 6.70, 95% CI 3.66 to 9.74, P < 0.0001, 1 study, 198 participants, ITT analysis; Analysis 8.2)
10 mg/day donepezil at 24 weeks (MD 5.92, 95% CI 4.53 to 7.31, P < 0.00001, 5 studies, 1348 participants, ITT analysis; Analysis 8.2).
8.2. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 2 SIB (change from baseline) ITT‐LOCF.
Activities of daily living
Winblad 2006, Homma 2008 and Black 2007 used the ADCS‐ADL‐severe scale. There was evidence of benefit of 10 mg/day donepezil at 24 weeks (MD 1.03, 95% CI 0.21 to 1.85, P = 0.01, 3 studies, 733 participants, ITT analysis; Analysis 8.4), but not for 5 mg/day at 24 weeks (MD 1.00, 95% CI ‐0.54 to 2.54, P = 0.20, 1 study, 198 participants, ITT analysis; Analysis 8.4).
8.4. Analysis.
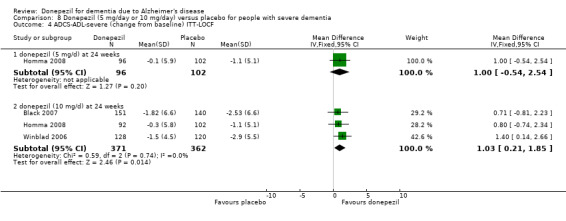
Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 4 ADCS‐ADL‐severe (change from baseline) ITT‐LOCF.
Dependency
Feldman 2001 assessed the time spent each day by the carer assisting with the activities of daily living but there was no evidence of a treatment effect. 10 mg/day donepezil at 24 weeks (MD ‐52.4, 95% CI ‐118.78 to 13.98, P = 0.12, 1 study, 221 participants, ITT analysis; Analysis 8.7).
8.7. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 7 Time (mins/day) spent by carer assisting in IADL and PSMS (change from baseline) LOCF.
Global assessment
We dichotomised the seven‐point CIBIC‐Plus scale, measuring global clinical state, counting those showing no change or decline, against those showing improvement, and analysed the results using the OR. There were benefits associated with 10 mg/day donepezil compared with placebo at 24 weeks as shown by the ITT analyses, but not with 5 mg/day.
5 mg/day donepezil at 24 weeks (31/96 donepezil, 24/102 placebo) (OR 1.54, 95% CI 0.83 to 2.87, P = 0.17, 1 study, 198 participants, ITT analysis; Analysis 8.3)
10 mg/day donepezil at 24 weeks (151/379 donepezil, 103/376 placebo) (OR 1.78, 95% CI 1.31 to 2.43, P = 0.0002, 3 studies, 755 participants, ITT analysis; Analysis 8.3)
8.3. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 3 CIBIC‐Plus or CGIC (numbers improved) ITT‐LOCF.
Behavioural symptoms
Feldman 2001, Winblad 2006, and Black 2007 assessed behavioural disturbance (NPI‐TOTAL), and there was evidence of benefit of donepezil 10 mg/day at 24 weeks.
10 mg/day donepezil at 24 weeks (MD ‐2.18, 95% CI ‐4.11 to ‐0.25, P = 0.03, 3 studies, 827 participants, ITT analysis; Analysis 8.6)
8.6. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 6 Behavioural disturbance (total NPI ) (change from baseline) ITT‐LOCF.
Homma 2008 assessed BEHAVE‐AD and there was no evidence of benefit of donepezil.
5 mg/day donepezil at 24 weeks (MD 0.00, 95% CI ‐1.67 to 1.67, P = 1.00, 1 study, 198 participants, ITT analysis; Analysis 8.5)
10 mg/day donepezil at 24 weeks (MD 0.40, 95% C I ‐1.28 to 2.08, P = 0.64, 1 study, 194 participants, ITT analysis; Analysis 8.5)
8.5. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 5 BEHAVE‐AD (change from baseline) ITT‐LOCF.
Withdrawals before the end of treatment
The meta‐analyses of withdrawals before the end of treatment, using the OR, showed significant differences in withdrawals between the 10 mg/day group and the placebo group in favour of placebo at 24 weeks, but not for the 5 mg/day group.
5 mg/day donepezil at 24 weeks (13/101 donepezil, 19/105 placebo) (OR 0.67, 95% CI 0.32 to 1.43, P = 0.30, 1 study, 206 participants, ITT analysis; Analysis 8.8).
10 mg/day donepezil at 24 weeks (164/701 donepezil, 130/695 placebo) (OR 1.32, 95% CI 1.02 to 1.71, P = 0.04, 5 studies, 1396 participants, ITT analysis; Analysis 8.8).
8.8. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 8 Total number of withdrawals before end of treatment.
Withdrawals before the end of treatment due to adverse events
The meta‐analyses of withdrawals before the end of treatment due to adverse events, using the OR, showed significant differences in withdrawals between the 10 mg/day group at 24 weeks in favour of placebo, but not for the 5 mg/day group.
5 mg/day donepezil at 24 weeks (8/101 donepezil, 11/105 placebo) (OR 0.74, 95% CI 0.29 to 1.89, P = 0.53, 1 study, 206 participants, ITT analysis; Analysis 8.9).
10 mg/day donepezil at 24 weeks (93/701 donepezil, 55/695 placebo) (OR 1.72, 95% CI 1.23 to 2.42, P = 0.002, 5 studies, 1396 participants, ITT analysis; Analysis 8.9).
8.9. Analysis.
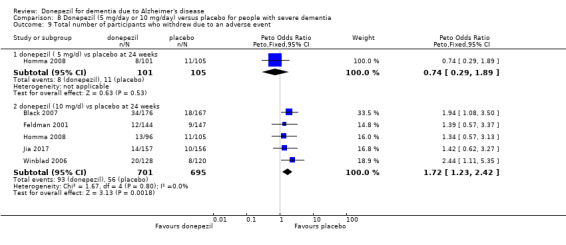
Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 9 Total number of participants who withdrew due to an adverse event.
Adverse events
The meta‐analyses of numbers of participants with at least one adverse event showed a significant difference between the 10 mg/day group and placebo in favour of placebo, at 24 weeks but not for the 5 mg/day donepezil.
5 mg/day donepezil at 24 weeks(79/101 donepezil, 77/105 placebo) (OR 1.30, 95% CI 0.69 to 2.46, P = 0.41, 1 study, 206 participants, ITT analysis; Analysis 8.10).
10 mg/day at 24 weeks (487/701 donepezil, 328/695 placebo) (OR 1.59, 95% CI 1.23 to 2.05, P = 0.0003, 5 studies, 1396 participants, ITT analysis; Analysis 8.10)
8.10. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 10 Total number of participants who suffered from at least one adverse event.
The included studies reported 32 different causes of adverse events. The causes of adverse events seen more frequently in the 10 mg/day dose group than in the 5 mg/day group or placebo group included nausea, vomiting and diarrhoea. These were mostly mild and transient, but occasionally moderately severe. There were significant differences, in favour of placebo, compared with donepezil, usually the 10 mg/day dose, for several causes of adverse events.
Anexoria: 10 mg/day donepezil 24 weeks (26/429 donepezil, 141/428 placebo) (OR 2.32 95% CI 1.20 to 4.48, P = 0.01, 3 studies, 857 participants, ITT analysis; Analysis 8.14)
Arthralgia: 10 mg/day donepezil 24 weeks (10/144 donepezil, 2/146 placebo) (OR 4.06 95% CI 1.28 to 12.86, P = 0.02, 1 study, 290 participants, ITT analysis; Analysis 8.16)
Diarrhoea: 10 mg/day donepezil at 24 weeks (60/701 donepezil, 20/694placebo) (OR 2.57 95% CI 1.65 to 4.01, P < 0.0001, 5 studies, 1395 participants, ITT analysis; Analysis 8.25)
Hallucinations: 10 mg/day donepezil at 24 weeks (8/128 donepezil, 1/120 placebo) (OR 4.68, 95% CI 1.24 to 17.66, P = 0.02, 1 study, 248 participants, ITT analysis; Analysis 8.30)
Headache: 10 mg/day donepezil at 24 weeks (17/144 donepezil, 6/146 placebo) (OR 2.86, 95% CI 1.22 to 6.69, P = 0.02, 1 study, 290 participants, ITT analysis; Analysis 8.37)
Insomnia: 10 mg/day donepezil at 24 weeks (12/176 donepezil, 4/167 placebo) (OR 2.70, 95% CI 0.99 to 7.35, P = 0.05, 1 study, 343 participants, ITT analysis; Analysis 8.33)
Nausea: 10 mg/day donepezil at 24 weeks (31/424 donepezil, 14/404 placebo) (OR 2.11, 95% CI 1.16 to 3.85, P = 0.01, 3 studies, 828 participants, ITT analysis; Analysis 8.35)
Restlessness: 5 mg/day donepezil at 24 weeks (6/101 donepezil, 1/105 placebo) (OR 4.54, 95% CI 1.01 to 20.41, P = 0.05, 1 study, 206 participants, ITT analysis; Analysis 8.36)
Vomiting: 10 mg/day donepezil at 24 weeks (35/416 donepezil, 15/418 placebo) (OR 2.42, 95% CI 1.37 to 4.31, P = 0.002, 3 studies, 834 participants, ITT analysis; Analysis 8.39)
8.14. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 14 Total number of participants who suffered from anorexia.
8.16. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 16 Total number of participants who suffered from arthralgia.
8.25. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 25 Total number of participants who suffered from diarrhoea.
8.30. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 30 Total number of participants who suffered from hallucinations.
8.37. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 37 Total number of participants who suffered from headache.
8.33. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 33 Total number of participants who suffered from insomnia.
8.35. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 35 Total number of participants who suffered from nausea.
8.36. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 36 Total number of participants who suffered from restlessness.
8.39. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 39 Total number of participants who suffered from vomiting.
Serious adverse events
The meta‐analyses of numbers of participants with at least one serious adverse event showed no difference between the 5 mg/day group and placebo, and the 10 mg/day group and placebo, at 24 weeks.
5 mg/day donepezil at 24 weeks(12/101 donepezil, 15/105 placebo) (OR 0.81, 95% CI 0.36 to 1.82, P = 0.61, 1 study, 206 participants, ITT analysis; Analysis 8.44).
10 mg/day at 24 weeks (90/701 donepezil, 107/695 placebo) (OR 0.80, 95% CI 0.59 to 1.08, P = 0.14, 5 studies, 1396 participants, ITT analysis; Analysis 8.44)
8.44. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 44 Total number of participants who suffered from at least one serious adverse event.
Deaths
The meta‐analyses of numbers of deaths before end of treatment at 24 weeks showed no difference between the 5 mg/day group and placebo, and the 10 mg/day group and placebo, at 24 weeks.
5 mg/day donepezil at 24 weeks (2/101 donepezil, 1/105 placebo) (OR 2.04, 95% CI 0.21 to 19.83, P = 0.54, 1 study, 206 participants, ITT analysis; Analysis 8.43).
10 mg/day at 24 weeks (24/701 donepezil, 31/695 placebo) (OR 0.71, 95% CI 0.41 to 1.25, P = 0.24, 5 studies, 1396 participants, ITT analysis; Analysis 8.43
8.43. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 43 Total number of deaths before end of treatment.
Discussion
Summary of main results
The main findings of the review were as follows.
The currently recommended dose of donepezil (10 mg/day) has benefits compared with placebo at 26 weeks for cognitive function, activities of daily living and the clinician‐rated global impression scales. We found no difference for behavioural symptoms or quality of life. Participants on donepezil were more likely to experience adverse events (OR of 1.6) or to withdraw from the trial before the end.
Participants on a lower dose of 5 mg/day were less likely to experience adverse events or to withdraw from the trial compared with participants on 10 mg/day. The higher dose showed some benefit for cognitive function, but not for activities of daily living and clinician‐related global impression compared with the lower dose.
A higher dose of 23 mg/day from a slow‐release formulation had no benefits for cognitive function, activities of daily living and clinician‐rated global impression scales compared with the 10 mg/day dose, but participants on the higher dose were more likely to experience adverse events or to withdraw from the trial compared with participants on the lower dose.
Four studies only included participants with severe or moderately severe dementia. The results from these four studies reported similar results to the studies that included only participants with mild to moderate dementia.
Outcomes
The included studies used three cognitive tests. The MMSE and the ADAS‐Cog assess similar domains and the results of the meta‐analyses were similar. The results from five studies showed that 10 mg daily of donepezil improved cognitive function of participants with mild to moderate probable Alzheimer's disease treated over a period of 24 to 26 weeks by 1.1 points on the MMSE (range 0 to 30) and by 2.7 points on the ADAS‐Cog (range 0 to 70), when compared with placebo. The results from five studies showed that 10 mg/day of donepezil improved cognitive function of participants with moderately severe to severe probable Alzheimer's disease treated over a period of 24 weeks by 5.9 points on the SIB (range 0 to 100) when compared with placebo.
Three studies assessed the effect of 10 mg/day donepezil on activities of daily living using the ADCS‐ADL‐sev scale (range 0 to 54). Donepezil showed a benefit of 1.0 points compared with placebo.
When we dichotomised the results of the clinician‐rated global impression measures to compare the number of participants who showed no change or whose condition had deteriorated with the number who had improved, the 10 mg/day donepezil group was significantly better than the placebo group at 24 to 26 weeks.
There was very little evidence on the effect of donepezil on behavioural problems, which only five studies assessed. In none of the studies did the participants suffer from more than mild problems at baseline. We found no evidence that donepezil affects patient‐rated quality of life measured on the scale chosen for these studies. Lebert 1999 assessed stress on carers but reported the results without any measure of precision and so we have not included them in this review.
Adverse effects
Donepezil appears to have a low incidence of serious side effects. The earlier studies (Rogers 1998a; Rogers 1998b; Burns 1999) used a short titration period of one week on 5 mg/day before proceeding to the 10 mg/day dose but later studies used a four‐week titration period, as recommended by the pharmaceutical company in the prescribing information. There were significantly more total dropouts and dropouts due to adverse events from the 10 mg/day group than from the placebo or 5 mg/day groups, and therefore side effects remain a clinical issue.
Overall completeness and applicability of evidence
We were able to include evidence from both published and unpublished studies in this systematic review. Most of the studies were sponsored by the pharmaceutical industry. The participants had mainly mild to moderate dementia due to Alzheimer's disease. In six studies participants were included with a MMSE less than 12. These participants were excluded from most of the other included studies. When these results are added to the meta‐analyses the results appear very similar to those of participants with mild to moderate dementia. Although the MMSE measurements are in a different part of the MMSE scale, the treatment effect is very similar to the other studies. In the studies of people with severe dementia, the SIB was also used to assess cognitive function, a scale designed for use with people with severe dementia. There is no evidence of a different rate of withdrawals or rates of adverse events from the studies of participants with severe dementia, compared with the studies of participants with mild to moderate dementia. The death rate is higher for participants with severe dementia compared to the participants with mild to moderate dementia, but this reflects the greater age of the people in the severe stage of dementia compared with the milder stages. There is no difference between the death rates for the treatment and placebo groups.
The main limitation in the completeness and applicability of the evidence was the lack of long‐term data beyond 26 weeks. One study reported data at 52 weeks. There were relatively few data on outcomes important to patients and carers, such as quality of life.
Quality of the evidence
The quality of the evidence at 26 weeks is moderate for most outcomes.
In terms of measuring outcomes and statistical analysis, the donepezil studies have essentially followed the relevant FDA guidance. The results reported are on an ITT basis, based on the participants' last assessments during the double‐blind phase (so‐called Last Observation Carried Forward LOCF analysis). As participants who did not complete the trial would, on average, show a further decline by the end of the double‐blind phase, this substitution of the LOCF is likely to enhance the final outcome. This effect is of significance when large numbers drop out and when there is differential dropout across the treatment groups. In these studies the latter effect applies to the 10 mg/day donepezil group, where there is a slightly greater dropout rate compared with placebo (27% compared with 21%), which is probably related to treatment. However, we have reported the results from the analysis of the ITT and completers' data, and found that the loss of participants from the studies did not alter our conclusions.
Potential biases in the review process
One review extracted the data and the same review author checked them. This was considered adequate when the previous versions of the review were written as the reviewer is a professional statistician. There have been no errors in data extraction reported.
Agreements and disagreements with other studies or reviews
The most recent systematic reviews of cholinesterase inhibitors have included all three cholinesterase inhibitors, donepezil, rivastigmine and galantamine, in the review. Tan 2014 included all three cholinesterase inhibitors and reported results for each separately. Tan 2014 included 10 studies comparing donepezil 10 mg/day with placebo and reported a treatment effect as measured on the ADAS‐Cog of MD ‐2.48 (95% CI ‐3.23 to ‐1.73, 4 studies), which is comparable to the treatment effect reported in this review.
Authors' conclusions
Implications for practice.
In people with mild, moderate or severe dementia due to Alzheimer's disease treated for periods of 12, 24 and 52 weeks, donepezil at a dose of 10 mg/day produced improvements in cognitive function, measuring ‐2.9 points as a weighted mean (95% confidence interval (CI) ‐3.6 to ‐2.2), in the midrange of the 70‐point ADAS‐Cog Scale (moderate‐quality evidence). Study clinicians, blind to other measures, rated global clinical state more positively in treated participants. Benefits of treatment were also seen on measures of activities of daily living and behaviour. Benefits on the 10 mg/day dose were only marginally larger than on the 5 mg/day dose.
Implications for research.
Important emerging issues are the economic effectiveness of the cholinesterase inhibitors. It would be helpful to see long‐term randomised studies of treatments that examine real‐world economic outcomes, such as cost of care, effects on markers of biological disease progression and the time to and need for institutionalisation. Unfortunately, given the proven clinical efficacy of donepezil it is hard to see how long‐term, placebo‐controlled randomised studies with economic primary outcomes could now be ethically undertaken.
Further important issues are duration of treatment, the severity of dementia and the effects of withdrawal at the end of the treatment period. As this review has evolved, the evidence of effectiveness of treatment has now been extended to 52 weeks, but effectiveness for some patients may not end at this point. Randomised studies of treatment involving the use of placebos over many years are unlikely to be either a practical or ethical option. Other robust trial designs will be needed to help establish the maximum duration of treatment, and the indicators that treatment is no longer beneficial. There is no evidence to suggest that the effects of donepezil are any less for those with severe dementia.
Feedback
Review does not answer carer's questions
Summary
A carer found this review did not answer many of her questions.
"As carer for someone with Alzheimers who is taking Aricept, this review did not answer many of my questions. For example, the description of participants uses language I do not understand. What do all the initials mean (ICD‐10, DSM and NINCDS‐ADRDA), and how do these criteria relate to someone with a clinical diagnosis of Alzheimers?
Top of the list of outcomes in the methods section is 'dependency (institutionalisation)'. I agree this is an important outcome, and was therefore disappointed to find no further reference to this it in the results and discussion. Also, whether a person with Alzheimers can continue to live independently will depend to a considerable extent on the level of support provided by carers. It would be good to see a range of measures of dependency, including on carers, as outcomes. This issue is closely related to quality of life, both for the patient and the carer. For example, ability to shop and cook, clean, pay bills, maintain contact with friends and family, and to get out and about.
It is also hard to understand what many of the other listed outcomes mean for someone with Alzheimers. For example, what do 'global impression', 'functional performance', 'cognitive function (as measured by psychometric tests)' mean, and how do you plan to assess quality of life for both the person and their carer?
Much of the data in the review relates to scales with unpronounceable acronyms that I do not understand. What do these scales mean in terms of the important things in day to day life? What does worsening therefore mean in terms of loss of useful function?"
Reply
The main reviewer (J. Birks) replied:
We thank you for your comment on our review and are sorry that it did not answer many of the questions you have about treatment with donepezil.
The acronyms that you refer to (ICD‐10, DSM and NINCDS‐ADRDA) are those of diagnostic criteria. DSM refers to the 'Diagnostic and Statistical Manual of Mental Disorders'. NINCDS‐ADRDA refers to the 'National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association'. ICD‐10 refers to World Health Organisation classification of mental and behavioural disorders, the clinical description and diagnostic guidelines. References to these criteria are given in the review. The use of validated criteria such as these not only increases the accuracy of diagnosis but ensures standardisation between clinicians.
We agree that dependency is an important outcome and it was included in the protocol as an outcome of interest. Unfortunately none of the clinical trials of donepezil included in the review assessed this outcome and therefore there were no results on dependency.
The outcomes of the clinical trials are measured using validated cognitive tests and rating scales. Validation refers to the process of testing and subsequent publication of the results of the testing that a scale has to undergo before it is considered suitable to use in assessment. The publication will set out the exact method of applying and scoring the scale, together with the questionnaire if applicable. References to the scales that are used in the clinical trials included in the donepezil review are given, together with a short description of the scale within the methods section of the review. Some of these scales do attempt to measure the ability to carry out everyday activities.
In future we intend to bring together information on diagnostic criteria and rating scales on our website so that it is easily accessible.
Reply: Lelia Dudley to Jacqueline Birks
I do not think the response addresses my main questions, which were how do these criteria relate to someone with a clinical diagnosis of Alzheimers? And what do the outcomes reported mean to patients and their carers? Finally, within a systematic review, if dependency was identified as one of the main outcomes at the protocol stage, the lack of data makes it even more important that this lack is highlighted and taken account off in the discussion.
Reply: Jacqueline Birks to Lelia Dudley
The diagnostic criteria for the clinical diagnosis of Alzheimer's disease (NINCDS‐ADRDA) require formal mental status testing, a medical history, physical neurological and psychiatric examination, laboratory tests and a scan. It would not be possible to include a lot of detail concerning the diagnostic criteria (DSM and NINCDS‐ADRDA) in every review, but we are intending to put this information, together with detailed description of the commonly used rating scales and cognitive tests, on our website for those who are interested. This should also help readers assess the results from the clinical trials.
Dependency was not measured in any included trial, although some aspects of dependency are covered by rating scales that assess activities of daily living. It was listed under outcomes because we would have been interested in it if it were measured. If we had some measures of dependency and some data we would then be in a better position to discuss and comment on this outcome, but at the present time we have decided that we are not in a position to comment.
Contributors
Comment: Lelia Dudley (lelia.duley@ndm.ox.ac.uk) Reply: Jacqueline Birks (jacqueline.birks@geratology.ox.ac.uk)
What's new
| Date | Event | Description |
|---|---|---|
| 20 May 2017 | New search has been performed | We carried out a top‐up search on 20 May 2017. We have included 4 new studies, and the results and conclusions have changed. |
| 20 May 2017 | New citation required and conclusions have changed | We carried out a top‐up search for this review on 20 May 2017. We included new studies, results and conclusions have changed. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 14 November 2016 | New search has been performed | Updated search carried out. |
| 9 February 2015 | New search has been performed | Updated search carried out |
| 7 June 2010 | New search has been performed | Updated 2010 to include new search of 2009. Additional data were available in a new publication of Study 315 and Winblad 2005. 5 new studies were included |
| 30 April 2009 | Amended | An update search was performed for the review on 30 April 2009 |
| 4 November 2008 | Amended | Converted to new review format. |
| 19 May 2006 | New search has been performed | May 2006: Two new trials have been included, both for severe dementia (MMSE <12) |
| 13 September 2005 | New citation required and conclusions have changed | Substantive amendment |
| 26 November 2003 | Feedback has been incorporated | Response to feedback added |
| 25 August 2003 | Feedback has been incorporated | feedback added |
Acknowledgements
The review authors acknowledge the original protocol developed by H Beppu, M Yanagi and R Hama, and the contribution to the earlier revision by D Meltzer. They also thank Dymphna Hermans and Anna Noel‐Storr for performing the search of electronic databases, and Lon Schneider, Cochrane Dementia and Cognitive Improvement contact editor, for his insightful comments and assistance.
Appendices
Appendix 1. Sources searched and search strategies used (Jan 2015, Nov 2015, Nov 2016, May 2017))
| Source | Search strategy | Hits |
| Medline (Ovid SP) [Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R) 1946 to May 19, 2017] [Date of most recent search: 20 May 2017] |
1. donepezil.mp.
2. aricept*.mp.
3. donezepil.ti,ab. 4. E2020 5. or/1‐4 6. dement*.ti,ab. 7. alzheimer*.ti,ab. 8. exp Dementia/ 9. or/6‐8 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. randomized.ab. 13. placebo.ab. 14. drug therapy.fs. 15. randomly.ab. 16. trial.ab. 17. groups.ab. 18. or/10‐17 19. 5 and 9 and 18 |
Jan 2015: 2010 Nov 2015: 192 Nov 2016: 148 May 2017: 166 |
| Embase (Ovid SP) [1974 to 2017 May 19] [Date of most recent search: 20 May 2017] |
1. donepezil.mp.
2. aricept*.mp.
3. donezepil.ti,ab. 4. E2020 5. or/1‐4 6. dement*.ti,ab. 7. alzheimer*.ti,ab. 8. exp Dementia/ 9. or/6‐8 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. randomized.ab. 13. placebo.ab. 14. drug therapy.fs. 15. randomly.ab. 16. trial.ab. 17. groups.ab. 18. or/10‐17 19. 5 and 9 and 18 |
Jan 2015: 1065 Nov 2015: 263 Nov 2016: 91 May 2017: 184 |
| Cinahl (EBSCOhost) [Date of most recent search: 20 May 2017] |
S1 (MM "Donepezil") S2 TX donepezil S3 TX donezepil S4 TX aricept S5 S1 or S2 or S3 or S4 S6 (MH "Dementia+") S7 TX dement* S8 TX alzheimer* S9 S6 or S7 or S8 S10 TX random* S11 (MH "Clinical Trials+") S12 TX placebo S13 TX trial S14 TX "control group" S15 S10 or S11 or S12 or S13 or S14 S16 S5 and S9 and S15 |
Jan 2015: 64 Nov 2015: 15 Nov 2016: 6 May 2017: 6 |
| PsycINFO (Ovid SP) [1806 to May Week 2 2017] [Date of most recent search: 20 May 2017] |
1. donepezil.mp.
2. aricept*.mp.
3. donezepil.ti,ab. 4. E2020 5. or/1‐4 6. dement*.ti,ab. 7. alzheimer*.ti,ab. 8. exp dementia/ 9. or/6‐8 10. exp Clinical Trials/ 11. random*.ti,ab. 12. randomized.ab. 13. placebo.ab. 14. trial.ab. 15. groups.ab. 16. or/10‐15 17. 5 and 9 and 16 |
Jan 2015: 375 Nov 2015: 48 Nov 2016: 0 May 2017: 7 |
| LILACs (BIREME) [Date of most recent search: 20 May 2017] |
(E2020 OR donepezil OR Aricept) AND (Alzheimer$ OR dementia OR ((cognit$ or memory$ or mental$) and (declin$ or impair$ or los$ or deteriorat$)) AND (randomized OR randomized OR double blind$ OR single blind$ OR placebo$ OR controlled) | Jan 2015: 2 Nov 2015: 9 Nov 2016: 0 May 2017: 0 |
| ALOIS (CRS Web) [Date of most recent search: 20 May 2017] |
(E2020 OR donepezil OR Aricept) | Jan 2015: 6 Nov 2015: 16 Nov 2016: 2 May 2017: 0 |
| CENTRAL, The Cochrane Library, Issue 5 [Date of most recent search: 20 May 2017] |
(E2020 OR donepezil OR Aricept) AND (Alzheimer* OR dementia OR ((cognit* or memory* or mental*) and (declin* or impair* or los* or deteriorat*)) | Jan 2015: 138 Nov 2015: 20 Nov 2016: 66 May 2017: 110 |
| Web of Science Core Collection [ISI Web of Science] [Date of most recent search: 20 May 2017] |
TOPIC: (donepezil OR aricept*) ANDTOPIC: (dementia OR alzheimer* OR "cognit* impair*") ANDTOPIC: (random* OR trial OR placebo OR "double blind*" OR "blinded" OR "single blind*" OR "control group*") | Nov 2015:252 Nov 2016: 201 May 2017: 141 |
| ICTRP (WHO portal) [Date of most recent search: 20 May 2017] |
(E2020 OR donepezil OR Aricept) AND (Alzheimer* OR dementia) | Nov 2015: 18 Nov 2016: 2 May 2017: 0 |
| ClinicalTrials.gov [Date of most recent search: 20 May 2017] |
(E2020 OR donepezil OR Aricept) AND Alzheimer* OR dmentia) | Jan 2015: 21 Nov 2015: 5 Nov 2016: 2 May 2017: 0 |
| Total before de‐duplication | Jan 2015: 3681 Nov 2015: 840 Nov 2016: 518 May 2017: 614 TOTAL: 5653 |
|
| Total after de‐duplication | Jan 2015: 3201 Nov 2015: 618 Nov 2016: 411 May 2017: 423 TOTAL: |
|
| Total after first assessment by CDCIG information specialist | Jan 2015: 44 Nov 2015: 41 Nov 2016: 14 May 2017: 6 TOTAL: 106 |
|
Appendix 2. Description of tests and rating scales
Cognitive Function
The primary cognitive test in nine studies was the cognitive part of the Alzheimer's Disease Assessment Scale (ADAS‐Cog) (Rosen 1984), modified and called the ADAS‐Jcog for Japanese patients. ADAS‐Cog comprises 11 individual tests, spoken language ability (0‐5), comprehension of spoken language (0‐5), recall of test instructions (0‐5), word finding difficulty (0‐5), following commands (0‐5), naming object (0‐5), construction drawing (0‐5), ideational praxis (0‐5), orientation (0‐8), word recall (0‐10) and word recognition (0‐12). The total score ranges from 0‐70, the high score indicating greater impairment. The ADAS‐Jcog is not quite equivalent to the ADAS‐Cog, using ideograms and ideographic memory as well as verbal memory. The scoring and subtests are different.
Mini Mental State Examination (MMSE) (Folstein 1975) evaluates cognition in five areas: orientation, immediate recall, attention and calculation, delayed recall, and language. The test takes only 15 minutes to administer and the score ranges from 0 (severe impairment) to 30 (normal).
The Quality of Life (QoL) (Blau 1977) is a self‐rated, seven‐item scale, based on a 'social indicators' approach, examining relationships, eating and sleeping, and social and leisure activity. The items are scored on an analogue scale between 0 (worst quality) and 50 (best). The Blau scale originally contained 10 items, and it is unclear both how the seven items were chosen, and whether the scale has been validated for use in people with dementia.
The Severe Impairment Battery (SIB) (Panisset 1994) is a 40‐item questionnaire designed to assess the severity of cognitive dysfunction in advanced AD and is divided into nine domains: memory, language, orientation, attention, praxis, visuospatial, construction, orientation to name, and social interaction. The score ranges from 0 (greatest impairment) to 100 (no impairment).
The Syndrom Kurz Test (SKT) (Overall 1992) is a brief psychometric test battery for the assessment of memory and attention. There are nine subtests, six speed orientated (language fluency, number fluency, attention planning and praxis, short‐term memory, attention and concentration) and three span orientated (short‐term visual memory, long‐term memory span and recognition memory span) resulting in a score 0 to 27(severe impairment).
Activities of daily living (ADLs)
The Winblad 2001 used the Progressive Deterioration Scale (PDS) (DeJong 1989), which is a disease‐specific measure of changes in 29 items of the ADLs. The interview is conducted with the caregiver. DeJong describes this scale as a measure of quality of life for Alzheimer's disease, on account of the correlation between ability to perform ADLs and quality of life.
Homma 2000 used the CMCS, which is derived from the Crichton geriatric rating scale. A nine‐point scoring system, from 0 (normal function) to 8 (maximum disturbance or presence of symptoms) measures orientation, communication, co‐operation, restlessness, ability to dress, work and social activities and leisure. The range of scores is 0 to 56. Strictly this scale is a more comprehensive scale than an ADL scale.
Feldman 2001 used the Disability Assessment for Dementia (DAD) (Gélinas 1999) a 10‐domain, 40‐item instrument that measures instrumental and basic ADLs. A higher score indicates less behavioural symptomatology.
Feldman 2001 used the Instrumental Activities of Daily Living (IADL) (Lawton 1969), modified to assess people with moderate to severe dementia. The IADL scale assesses the ability to perform eight complex daily tasks: ability to use the telephone, shopping, food preparation, household tasks, laundry, transportation, responsibility for medications and ability to manage finances. The modified version omits the laundry item and includes items from the Alzheimer's Disease Functional Assessment Change Scale (ADFACS) relating to managing household appliances, mail, hobbies and the ability to get around inland outside home.
Feldman 2001 uses the Physical Self Maintenance Scale (PSMS) (Lawton 1969), a six‐item scale that rates self‐care ability (toileting, feeding, dressing, personal hygiene, locomotion and bathing). The modified version used includes three extra items believed to be important for the provision of basic ADL in moderate to severe Alzheimer's disease (loss of recognition of carer, impaired ambulation and wandering).
AD2000 used the Bristol Activities of Daily Living Scale (BADLS) (Bucks 1996).
Winblad 2006 used the Modified Alzheimer's Disease Cooperative Study activities of daily living inventory for severe Alzheimer's disease (ADCS‐ADL‐severe) (Galasko 2000). This is a 19‐item scale for basic and complex abilities validated in people with moderate to severe dementia. Total score ranges from 0 to 54 (no impairment). Items include basic ADLs (eating, bathing and complex (operating taps, switching lights).
Global Assessment
A Clinician's Interview‐Based Impression of Change scale (CIBIC‐Plus) (Schneider 1997) was used in four studies. It provides a global rating of patient function in four areas, general, cognitive, behaviour and ADLs. All patients are scored on global severity at baseline and subsequent assessments on a scale of 1 to 7 are relative to baseline, with 1 showing marked improvement, 7 marked worsening with 4 representing no change. Information is obtained from the caregiver and patient and the clinician is blind to all other measures.
The Winblad 2001 used the Gottfries, Brane and Steen scale (GBS) (Gottfries 1982) for the global assessment. The GBS is a comprehensive scale for rating dementia syndromes, based on a semi‐structured interview with the caregiver. A seven‐point scoring system, from 0 (normal function) to 6 (maximum disturbance or presence of symptoms) measures orientation, memory and concentration (12 items), ADLs (6 items), emotional function (3 items) and pathological aspects of behaviour (6 items).
Clinical Rating Scale (CDR) (Berg 1988) is usually reported as a score, 0.5, 1, 2, 3 but these scores are derived from ratings in six domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies and personal care), each scored from 0 (normal) to 3 (severe dementia) and the sum of the ratings (0 to 18) provides the CDR‐sum of boxes (CDR‐SB).
Homma 2000 used the Mental Function Impairment Scale (MENFIS) (Homma 1991) which is a modification of the GBS. It evaluates cognitive function (7 items), motivational function (3 items) and emotional function (3 items).
Winblad 2006 used the Cinical Global Impression of Improvement scale (CGI‐C) (ECDEU 1976).
Behavioural Disturbance
The Neuropsychiatric Instrument (NPI) (Cummings 1994), a 12‐item, carer‐rated instrument, was used by Feldman 2001, and Tariot 2001 to evaluate behavioural and neuropsychiatric symptoms, including delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy, disinhibition, irritability, aberrant motor behaviour, night‐time behaviour, and appetite/eating disorder. Frequency is rated from 1 (occasional, less than once a week) to 4 (very frequent) and severity from 1 (mild) to 3 (severe). The product of frequency and severity ranges from 1 to 12, with a total score ranging from 12 to 120 for the 10 domains summed. A lower score indicates improvement.
The Behavioural Pathology in Alzheimer's Disease Rating Scale (BEHAVE‐AD) (Reisberg 1987) a secondary outcome in the Homma 2008 study, assesses paranoid and delusional ideations, hallucinations, activity disturbance, aggressiveness, diurnal rhythm disturbances, affective disturbances and anxieties and phobias; each item is scored from 0 (none) to 3 (most severe). Scores range from 0 to 78, with higher scores indicating more severe symptoms.
Cohen–Mansfield Agitation Inventory (CMAI) (Cohen‐Mansfield 1987) (on a scale of 29 to 203, with higher scores indicating more agitation) was used in the Howard 2007 trial.
Stress on carers
The abridged Relative Stress Scale (aRSS) is a self‐assessment scale for carers. They rate their stress on a scale of 0 (not at all) to 4 (extreme stress) on 10 items that cover their experience of dealing with a person with Alzheimer's disease. This outcome was assessed only in the Lebert 1999 trial.
The Neuropsychiatric Inventory Distress scale (NPI‐D) assesses the degree of distress caused to the carer by the 10 individual items (each scores 0 to 5) of the NPI.
AD2000 assessed the psychological well‐being of the carer using the General Health Questionnaire (GHQ‐30) (0‐30) (Goldberg 1988).
Black 2007 assessed the time and stress associated with assisting the patient with performance of daily tasks using the Caregiver Burden Questionnaire (CBQ).
Health resource utilisation
-
This outcome is assessed in the Feldman 2001 study.
At a time within two days of a clinic visit, carers kept records of the time per day they spent assisting with instrumental and basic ADL using a version of the IADL+ and the PSMS scale. The total time over the study period was calculated by multiplying the estimate per day by the number of days since the last clinic visit. The utilisation of health resources by patients and carers was measured using the Canadian Utilization of Services Tracking (CAUST) questionnaire and the carer time assessment. This covered many items that belonged to one of the following categories: community medical care, hospitalisations and residential care for the patient and medical care, hospitalisations and counselling for the carer.
The analysis of the CAUST data included those services that were considered to be AD‐related.
For those who left the study before the end, the use of services over the entire 24‐week period was estimated using the LOCF principle. For other missing data, where there was no previous observation, imputed values were used based on the assessment data of patients from the same country of similar MMSE.
Costs were calculated for patients and carers in each treatment group based on unit prices, based on Ontario fees schedules regardless of the country in which the patient lived for each resource. There are estimates of costs for carer time helping with ADLs, based on the average Ontario minimum wage of CAD 6.85/hour.
Although the health resource utilisation was quite different over the three countries, Australia, Canada and France, the mean for each item over all participants was used in the analyses. The group means and mean costs for each item, summaries over categories and overall totals were reported. The costs were assessed in 1998.
Winblad 2001 also reported on the direct and indirect costs of caring for a person with Alzheimer's disease, and included the informal costs for care provided by the carer, assessed using the Resource Utilization in Dementia (RUD) (Wimo 1998) questionnaire at baseline, and weeks 12, 24, 36 and 52. RUD covers the patients' study medication, use of social services and living accommodation; patients' and carers' hospitalisation, visits to healthcare professionals, concomitant medication; and carers' time caring for patients or missed work. The study included people from Finland and Sweden, with smaller numbers from Norway, Denmark and the Netherlands. The costs were reported in 1999 values, converted from Swedish Kroner to USD, using a mean conversion rate for 1999.
Black 2007 assessed the resources used by the participant, accomodation, visits to emergency department, hospitalizations, visiting nurse, home health aid, day care, respite care, meal delivery using the Resources used by Patient (RUSP) scale.
Data and analyses
Comparison 1. Donepezil (10 mg/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog (change from baseline at 24‐26 weeks) ITT‐LOCF | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1130 | Mean Difference (IV, Fixed, 95% CI) | ‐2.67 [‐3.31, ‐2.02] |
| 2 MMSE (change from baseline at 24‐26 weeks) ITT‐LOCF | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 donepezil (10 mg/d) at 24 weeks | 7 | 1757 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [0.73, 1.37] |
| 3 SIB (change from baseline at 24‐26 weeks) ITT‐LOCF | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 donepezil (10 mg/d) at 24 weeks | 5 | 1348 | Mean Difference (IV, Fixed, 95% CI) | 5.92 [4.53, 7.31] |
| 4 ADCS‐ADL‐severe (change from baseline at 24‐26 weeks) ITT‐LOCF | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 donepezil (10 mg/d) at 24 weeks | 3 | 733 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [0.21, 1.85] |
| 5 CIBIC‐Plus or CGIC (numbers improved at 24‐26 weeks) ITT‐LOCF | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 donepezil (10 mg/d) vs placebo at 24 weeks | 6 | 1674 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [1.54, 2.39] |
| 6 CDR‐SB (change from baseline at 24‐26 weeks) ITT‐LOCF | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.73, ‐0.33] |
| 7 BEHAVE‐AD (change from baseline at 24‐26 weeks) ITT‐LOCF | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 donepezil (10 mg/d) at 24 weeks | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐1.28, 2.08] |
| 8 Behavioural disturbance (Total NPI ) (change from baseline at 24‐26 weeks) ITT‐LOCF | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 donepezil (10 mg/d) at 24 weeks | 4 | 1035 | Mean Difference (IV, Fixed, 95% CI) | ‐1.62 [‐3.43, 0.19] |
| 9 QoL (participant‐rated quality of life at 24‐26 weeks) ITT‐LOCF | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 815 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐8.15, 2.56] |
| 10 Total number of withdrawals before end of treatment at 24‐26 weeks | 12 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 donepezil (10 mg/d) vs placebo at 24 weeks | 12 | 2846 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [1.05, 1.50] |
| 11 Total number of participants who suffered from at least one adverse event by 24‐26 weeks | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 donepezil (10 mg/d) vs placebo at 24 weeks | 10 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.31, 1.95] |
Comparison 2. Donepezil (5 mg/day or 10 mg/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐COG (change from baseline) completers | 10 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 donepezil (5 mg/d) vs placebo at 12 weeks | 6 | 1441 | Mean Difference (IV, Fixed, 95% CI) | ‐2.15 [‐2.69, ‐1.61] |
| 1.2 donepezil (5 mg/d) vs placebo at 24 weeks | 3 | 906 | Mean Difference (IV, Fixed, 95% CI) | ‐2.02 [‐2.77, ‐1.26] |
| 1.3 donepezil (10 mg/d) vs placebo at 12 weeks | 7 | 1245 | Mean Difference (IV, Fixed, 95% CI) | ‐2.45 [‐3.01, ‐1.89] |
| 1.4 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 848 | Mean Difference (IV, Fixed, 95% CI) | ‐2.81 [‐3.55, ‐2.06] |
| 2 ADAS‐COG (change from baseline) ITT‐LOCF | 13 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 488 | Mean Difference (IV, Fixed, 95% CI) | ‐2.27 [‐3.16, ‐1.39] |
| 2.2 donepezil (5 mg/d) vs placebo at 24 weeks | 3 | 1089 | Mean Difference (IV, Fixed, 95% CI) | ‐2.01 [‐2.69, ‐1.34] |
| 2.3 donepezil (10 mg/d) vs placebo at 12 weeks | 5 | 459 | Mean Difference (IV, Fixed, 95% CI) | ‐2.99 [‐3.99, ‐1.99] |
| 2.4 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1130 | Mean Difference (IV, Fixed, 95% CI) | ‐2.67 [‐3.31, ‐2.02] |
| 3 MMSE (change from baseline) completers | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 632 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [0.61, 1.54] |
| 3.2 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 264 | Mean Difference (IV, Fixed, 95% CI) | 1.44 [0.64, 2.24] |
| 3.3 donepezil (10 mg/d) vs placebo at 12 weeks | 6 | 1173 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [0.90, 1.62] |
| 3.4 donepezil (10 mg/d) vs placebo at 24 weeks | 6 | 1257 | Mean Difference (IV, Fixed, 95% CI) | 1.29 [0.90, 1.69] |
| 3.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 189 | Mean Difference (IV, Fixed, 95% CI) | 1.84 [0.53, 3.15] |
| 4 MMSE (change from baseline) ITT‐LOCF | 11 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 donepezil (5 mg/d) at 12 weeks | 2 | 382 | Mean Difference (IV, Fixed, 95% CI) | 0.92 [0.32, 1.53] |
| 4.2 donepezil (5 mg/d) at 24 weeks | 2 | 358 | Mean Difference (IV, Fixed, 95% CI) | 1.22 [0.54, 1.90] |
| 4.3 donepezil (10 mg/d) at 12 weeks | 2 | 511 | Mean Difference (IV, Fixed, 95% CI) | 1.19 [0.61, 1.77] |
| 4.4 donepezil (10 mg/d) at 24 weeks | 7 | 1757 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [0.73, 1.37] |
| 4.5 donepezil (10 mg/d) at 52 weeks | 1 | 272 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.81, 2.59] |
| 5 SIB (change from baseline) ITT‐LOCF | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 donepezil (5 mg/d) at 24 weeks | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 6.7 [3.66, 9.74] |
| 5.2 donepezil (10 mg/d) at 24 weeks | 5 | 1348 | Mean Difference (IV, Fixed, 95% CI) | 5.92 [4.53, 7.31] |
| 6 CIBIC‐plus or CGIC (numbers improved) completers | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 228 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.93 [2.20, 7.02] |
| 6.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 196 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.40, 4.43] |
| 7 CIBIC‐plus or CGIC (numbers improved) ITT‐LOCF | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 303 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.10 [1.25, 3.53] |
| 7.2 donepezil (5 mg/d) vs placebo at 24 weeks | 4 | 1273 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.20 [1.69, 2.87] |
| 7.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 302 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [1.64, 4.46] |
| 7.4 donepezil (10 mg/d) vs placebo at 24 weeks | 6 | 1674 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.92 [1.54, 2.39] |
| 8 CDR‐SB (change from baseline) completers | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 donepezil (5 mg/d) vs placebo at 12 weeks | 6 | 1461 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.38, ‐0.12] |
| 8.2 donepezil (5 mg/d) vs placebo at 24 weeks | 3 | 920 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.80, ‐0.36] |
| 8.3 donepezil (10 mg/d) vs placebo at 12 weeks | 5 | 1038 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.43, ‐0.12] |
| 8.4 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 656 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.83, ‐0.30] |
| 9 CDR‐SB (change from baseline) ITT‐LOCF | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 487 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.25, 0.21] |
| 9.2 donepezil (5 mg/d) vs placebo at 24 weeks | 3 | 1093 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.70, ‐0.32] |
| 9.3 donepezil (10 mg/d) vs placebo at 12 weeks | 4 | 559 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.47, 0.00] |
| 9.4 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 1028 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.73, ‐0.33] |
| 10 GBS or MENFIS ‐ global assessment completers | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐2.56 [‐4.27, ‐0.85] |
| 10.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 258 | Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐4.15, 1.99] |
| 10.3 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 243 | Mean Difference (IV, Fixed, 95% CI) | ‐3.16 [‐6.85, 0.53] |
| 10.4 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | ‐6.01 [‐11.93, ‐0.09] |
| 11 GBS ‐ global assessment ITT‐LOCF | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐3.26 [‐7.38, 0.86] |
| 12 ADL and IADL (CMCS) (change from baseline) completers | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 donepezil (5 mg/day) vs placebo at 24 weeks | 1 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐2.42 [‐4.32, ‐0.52] |
| 13 ADl and IADL (DAD) (change from baseline) completers | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 254 | Mean Difference (IV, Fixed, 95% CI) | 4.83 [1.35, 8.31] |
| 13.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 247 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [3.61, 12.39] |
| 14 ADCS‐ADL‐severe (change from baseline) ITT‐LOCF | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 donepezil (5 mg/d) at 24 weeks | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.54, 2.54] |
| 14.2 donepezil (10 mg/d) at 24 weeks | 3 | 733 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [0.21, 1.85] |
| 15 PDS ‐ progressive deterioration scale ITT‐LOCF | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 donepezil ( 10 mg/d) vs placebo at 52 weeks | 1 | 276 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [1.70, 5.90] |
| 16 Total number meeting criterion for functional decline before end of treatment | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 16.1 donepezil (10 mg/day) vs placebo | 1 | 413 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.36, 0.78] |
| 17 Behavioural disturbance (total NPI) (change from baseline) completers | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 donepezil (10 mg/d) vs placebo at 12 weeks | 2 | 279 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐4.43, 1.53] |
| 17.2 donepezil (10 mg/d) vs placebo at 24 weeks | 4 | 692 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐3.16, 1.07] |
| 18 BEHAVE‐AD (change from baseline) ITT‐LOCF | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 donepezil (5 mg/d) at 24 weeks | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.67, 1.67] |
| 18.2 donepezil (10 mg/d) at 24 weeks | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐1.28, 2.08] |
| 19 Behavioural disturbance (total NPI ) (change from baseline) ITT‐LOCF | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 donepezil (10 mg/d) at 24 weeks | 4 | 1035 | Mean Difference (IV, Fixed, 95% CI) | ‐1.62 [‐3.43, 0.19] |
| 20 QoL (participant‐rated quality of life) completers | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 donepezil (5 mg/d) vs placebo at 12 weeks | 4 | 1127 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [‐3.04, 5.40] |
| 20.2 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 681 | Mean Difference (IV, Fixed, 95% CI) | 2.26 [‐3.64, 8.16] |
| 20.3 donepezil (10 mg/d) vs placebo at 12 weeks | 4 | 1031 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [‐3.20, 5.52] |
| 20.4 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 645 | Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐7.26, 4.91] |
| 21 QoL (participant‐rated quality of life) ITT‐LOCF | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 donepezil (5 mg/d) vs placebo at 12 weeks | 2 | 377 | Mean Difference (IV, Fixed, 95% CI) | 3.07 [‐3.81, 9.95] |
| 21.2 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 827 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [‐4.60, 6.04] |
| 21.3 donepezil (10 mg/d) vs placebo at 12 weeks | 2 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐8.40 [‐15.72, ‐1.08] |
| 21.4 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 815 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐8.15, 2.56] |
| 22 IADL (change from baseline) completers | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐4.31 [‐7.72, ‐0.90] |
| 22.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 243 | Mean Difference (IV, Fixed, 95% CI) | ‐6.32 [‐10.02, ‐2.62] |
| 23 PSMS (change from baseline) completers | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.17, 0.35] |
| 23.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 244 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.77, 0.01] |
| 24 Time (mins/day) spent by carer assisting in IADL and PSMS (change from baseline) LOCF | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | ‐52.4 [‐118.78, 13.98] |
| 25 Total number who enter long‐term institutional care before end of treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 donepezil (10 mg/d) at 52 weeks | 1 | 286 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.10, 1.41] |
| 26 Total number of withdrawals before end of treatment | 21 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 26.1 donepezil (5 mg/d) vs placebo at 12 weeks | 4 | 1079 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.81 [1.22, 2.68] |
| 26.2 donepezil (5 mg/d) vs placebo at 24 weeks | 5 | 1386 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.66, 1.14] |
| 26.3 donepezil (10 mg/d) vs placebo at 12 weeks | 3 | 362 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [1.21, 4.40] |
| 26.4 donepezil (10 mg/d) vs placebo at 24 weeks | 12 | 2846 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [1.05, 1.50] |
| 26.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.62, 1.67] |
| 27 Total number of participants who withdrew due to an adverse event | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 27.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 513 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.33 [1.02, 5.28] |
| 27.2 donepezil (5 mg/d) vs placebo at 24 weeks | 4 | 1335 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.52, 1.18] |
| 27.3 donepezil (10 mg/d) vs placebo at 12 weeks | 3 | 362 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.45 [1.40, 8.50] |
| 27.4 donepezil (10 mg/d) vs placebo at 24 weeks | 11 | 2819 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.68 [1.33, 2.12] |
| 27.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.45, 2.88] |
| 28 Total number of participants who suffered from at least one adverse event | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 28.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 513 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.70, 1.67] |
| 28.2 donepezil (5 mg/d) vs placebo at 24 weeks | 3 | 1018 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [1.06, 1.86] |
| 28.3 donepezil (10 mg/d) vs placebo at 12 weeks | 2 | 323 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.94, 2.55] |
| 28.4 donepezil (10 mg/d) vs placebo at 24 weeks | 10 | 2500 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [1.30, 1.94] |
| 28.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.81, 2.51] |
| 29 Total number of participants who suffered from abdominal pain | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 29.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.53, 4.17] |
| 29.2 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.11, 3.75] |
| 29.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.31, 3.06] |
| 29.4 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 627 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.58, 2.24] |
| 29.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.12, 1.32] |
| 30 Total number of participants who suffered from abnormal gait | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 30.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.63, 3.98] |
| 31 Total number of participants who suffered from abnormal dreams | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 donepezil (10 mg/d) at 24 weeks | 1 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 12.49 [0.71, 218.72] |
| 32 Total number of participants who suffered from accidental fall | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 32.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.40, 3.76] |
| 32.2 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 449 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.58, 2.02] |
| 33 Total number of participants who suffered from accidental injury | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 33.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.32, 1.94] |
| 33.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.36, 2.11] |
| 33.3 donepezil (10 mg/d) vs placebo at 24 weeks | 4 | 899 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.87, 1.98] |
| 34 Total number of participants who suffered from agitation | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 34.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.61 [0.23, 1.57] |
| 34.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.36, 2.11] |
| 34.3 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 551 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.60, 2.22] |
| 35 Total number of participants who suffered from anorexia | 9 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 35.1 donepezil (5 mg/d) vs placebo at 12 weeks | 2 | 433 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.91 [0.61, 6.02] |
| 35.2 donepezil (5 mg/d) vs placebo at 24 weeks | 4 | 1334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.90 [0.88, 4.13] |
| 35.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.37 [0.81, 6.90] |
| 35.4 donepezil (10 mg/d) vs placebo at 24 weeks | 6 | 1931 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [1.96, 4.62] |
| 36 Total number of participants who suffered from anxiety | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 36.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.28, 1.92] |
| 36.2 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.84, 4.60] |
| 37 Total number of participants who suffered from arthralgia | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 37.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 498 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.62, 2.40] |
| 38 Total number of participants who suffered from asthenia | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 38.1 donepezil (10 mg/d) vs placebo at 24 weeks | 4 | 899 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [0.95, 2.64] |
| 38.2 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.24 [0.82, 6.13] |
| 39 Total number of participants who suffered from back pain | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 39.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 498 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.68 [0.87, 3.23] |
| 40 Total number of participants who suffered from cold syndrome | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 40.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.30, 1.99] |
| 40.2 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 473 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.86, 2.88] |
| 40.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.25, 1.77] |
| 40.4 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.54, 2.29] |
| 41 Total number of participants who suffered from confusion | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 41.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.61, 2.40] |
| 41.2 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 1045 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.57, 1.56] |
| 41.3 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.15, 1.38] |
| 42 Total number of participants who suffered from conjunctivitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 42.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [0.72, 5.20] |
| 43 Total number of participants who suffered from constipation | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 43.1 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 473 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.06 [0.73, 5.80] |
| 43.2 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 449 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.41, 2.46] |
| 43.3 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.24, 1.88] |
| 44 Total number of participants who suffered from contusion | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 44.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.41, 7.61] |
| 44.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.22, 5.57] |
| 45 Total number of participants who suffered from cystitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 45.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.50, 4.63] |
| 46 Total number of participants who suffered from depression | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 46.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [0.54, 4.99] |
| 46.2 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [0.69, 3.37] |
| 47 Total number of participants who suffered from diarrhoea | 13 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 47.1 donepezil (5 mg/d) vs placebo at 12 weeks | 2 | 390 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.64 [1.05, 6.63] |
| 47.2 donepezil (5 mg/d) vs placebo at 24 weeks | 4 | 1334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.85 [1.19, 2.89] |
| 47.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.22 [1.87, 9.54] |
| 47.4 donepezil (10 mg/d) vs placebo at 24 weeks | 9 | 2622 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.69 [2.05, 3.55] |
| 47.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.41, 2.52] |
| 48 Total number of participants who suffered from dizziness | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 48.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 512 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.55, 2.31] |
| 48.2 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 861 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.82, 2.63] |
| 48.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.60, 3.18] |
| 48.4 donepezil (10 mg/d) vs placebo at 24 weeks | 6 | 1830 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.77 [1.19, 2.63] |
| 48.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.55, 4.36] |
| 49 Total number of participants who suffered from ecchymosis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 49.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.55, 4.47] |
| 50 Total number of participants who suffered from eczema | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 50.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.23 [0.75, 70.12] |
| 51 Total number of participants who suffered from fatigue | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 51.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.20, 1.83] |
| 51.2 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.81, 8.97] |
| 51.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.60, 3.66] |
| 51.4 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.63 [1.29, 10.21] |
| 52 Total number of participants who suffered from fever | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 52.1 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 473 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.53, 7.32] |
| 52.2 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 409 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.58, 2.41] |
| 53 Total number of participants who suffered from fracture | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 53.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.05, 2.51] |
| 53.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.49, 5.52] |
| 53.3 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [0.54, 4.99] |
| 54 Total number of participants who suffered from gastroenteritis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 54.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.24, 1.51] |
| 55 Total number of participants who suffered from haemorrhage | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 55.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.35, 3.01] |
| 56 Total number of participants who suffered from hallucinations | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 56.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.68 [1.24, 17.66] |
| 57 Total number of participants who suffered from headache | 9 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 57.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 512 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.71, 2.71] |
| 57.2 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 812 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.66, 1.75] |
| 57.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.71, 3.04] |
| 57.4 donepezil (10 mg/d) vs placebo at 24 weeks | 4 | 1174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.88, 1.82] |
| 57.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.51, 3.12] |
| 58 Total number of participants who suffered from hostility | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 58.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.58, 2.99] |
| 58.2 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.16, 1.61] |
| 59 Total number of participants who suffered from loss of appetite | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 59.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.51, 14.15] |
| 59.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.40, 12.51] |
| 60 Total number of participants who suffered from infection | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 60.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 551 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.39 [0.76, 2.55] |
| 61 Total number of participants who suffered from inflammation of upper airway | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 61.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [0.25, 8.46] |
| 62 Total number of participants who suffered from insomnia | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 62.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.62 [0.67, 3.92] |
| 62.2 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.77 [0.85, 3.69] |
| 62.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.38 [1.69, 6.76] |
| 62.4 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 1043 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.40 [1.38, 4.15] |
| 62.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.63, 3.36] |
| 63 Total number of participants who suffered from increased cough | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 63.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.08, 1.00] |
| 63.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.11, 1.26] |
| 63.3 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.56, 2.51] |
| 64 Total number of participants who suffered from myasthenia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 64.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [0.54, 7.74] |
| 65 Total number of participants who suffered from muscle cramp | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 65.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.53, 4.17] |
| 65.2 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.48 [1.56, 19.27] |
| 65.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.76, 5.06] |
| 65.4 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.00 [1.98, 18.18] |
| 66 Total number of participants who suffered from nausea | 13 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 66.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 513 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.70, 2.71] |
| 66.2 donepezil (5 mg/d) vs placebo at 24 weeks | 3 | 1128 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.74, 2.15] |
| 66.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.95 [1.58, 5.51] |
| 66.4 donepezil (10 mg/d) vs placebo at 24 weeks | 8 | 2184 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.06 [2.26, 4.14] |
| 66.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.59, 2.75] |
| 67 Total number of participants who suffered from pain | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 67.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.56, 2.85] |
| 67.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [0.93, 4.01] |
| 67.3 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 551 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.64, 2.01] |
| 68 Total number of participants who suffered from peripheral oedema | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 68.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.05, 0.74] |
| 68.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.15, 1.53] |
| 68.3 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [1.02, 4.09] |
| 69 Total number of participants who suffered from pneumonia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 69.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.65, 4.19] |
| 70 Total number of participants who suffered from rash | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 70.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.37, 1.41] |
| 71 Total number of participants who suffered from restlessness | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 71.1 donepezil (5 mg/d) vs placebo at 24 weeks | 2 | 473 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.53 [0.44, 5.37] |
| 71.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.15 [0.22, 20.95] |
| 72 Total number of participants who suffered from respiratory tract infection | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 72.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.45, 3.83] |
| 72.2 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.29, 2.20] |
| 72.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.24, 2.67] |
| 72.4 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 491 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.50, 1.65] |
| 73 Total number of participants who suffered from rhinitis | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 73.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.45, 3.83] |
| 73.2 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.05, 1.82] |
| 73.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.24, 2.67] |
| 73.4 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 527 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.75, 2.56] |
| 74 Total number of participants who suffered from vomiting | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 74.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.22, 2.18] |
| 74.2 donepezil (5 mg/d) vs placebo at 24 weeks | 4 | 1334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.68, 2.17] |
| 74.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.53, 3.72] |
| 74.4 donepezil (10 mg/d) vs placebo at 24 weeks | 6 | 1908 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.65 [1.90, 3.70] |
| 75 Total number of participants who suffered from skin ulcer | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 75.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [0.56, 4.03] |
| 76 Total number of participants who suffered from syncope | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 76.1 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.27 [0.75, 6.88] |
| 77 Total number of participants who suffered from tremor | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 77.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.58 [1.01, 12.71] |
| 78 Total number of participants who suffered from urinary incontinence | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 78.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [0.79, 6.72] |
| 79 Total number of participants who suffered from urinary tract infection | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 79.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.22, 0.99] |
| 79.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.13, 0.67] |
| 79.3 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1188 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 79.4 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.31, 2.08] |
| 80 Total number of participants who suffered from vertigo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 80.1 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.36 [1.15, 9.82] |
| 81 Total number of participants who suffered from weight loss | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 81.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.19, 4.89] |
| 81.2 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.48 [0.74, 8.23] |
| 81.3 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 811 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.90 [1.08, 3.35] |
| 82 total number of deaths before end of treatment | 17 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 82.1 donepezil (5 mg/d) vs placebo at 12 weeks | 3 | 513 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.06, 15.29] |
| 82.2 donepezil (5 mg/d) vs placebo at 24 weeks | 4 | 1334 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.25, 4.10] |
| 82.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.60] |
| 82.4 donepezil (10 mg/d) vs placebo at 24 weeks | 12 | 2847 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.46, 1.19] |
| 82.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.30, 6.07] |
| 83 Total number of participants who suffered from at least one serious adverse event | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 83.1 donepezil (5 mg/d) vs placebo at 12 weeks | 1 | 310 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.27, 2.51] |
| 83.2 donepezil (5 mg/d) vs placebo at 24 weeks | 3 | 1067 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.49, 1.18] |
| 83.3 donepezil (10 mg/d) vs placebo at 12 weeks | 1 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.27, 2.50] |
| 83.4 donepezil (10 mg/d) vs placebo at 24 weeks | 9 | 2599 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.71, 1.14] |
| 83.5 donepezil (10 mg/d) vs placebo at 52 weeks | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.99 [1.11, 3.59] |
2.1. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 1 ADAS‐COG (change from baseline) completers.
2.3. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 3 MMSE (change from baseline) completers.
2.6. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 6 CIBIC‐plus or CGIC (numbers improved) completers.
2.8. Analysis.
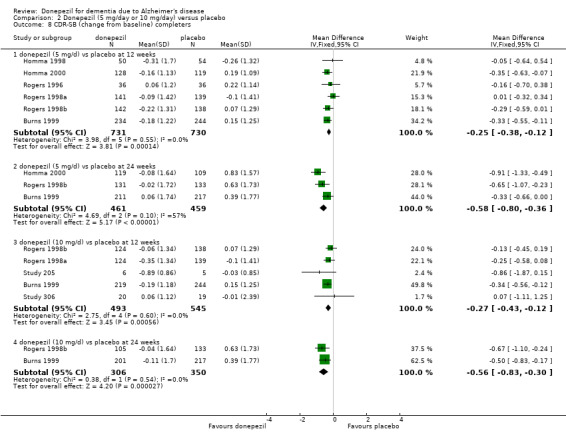
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 8 CDR‐SB (change from baseline) completers.
2.11. Analysis.
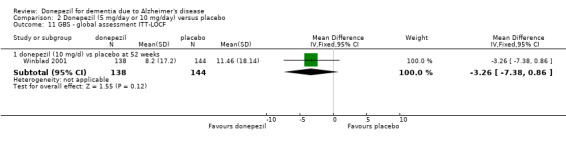
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 11 GBS ‐ global assessment ITT‐LOCF.
2.19. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 19 Behavioural disturbance (total NPI ) (change from baseline) ITT‐LOCF.
2.21. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 21 QoL (participant‐rated quality of life) ITT‐LOCF.
2.23. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 23 PSMS (change from baseline) completers.
2.25. Analysis.
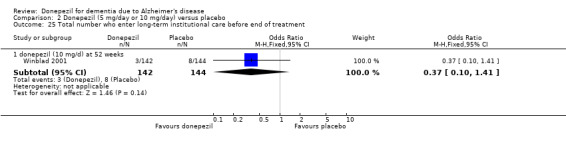
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 25 Total number who enter long‐term institutional care before end of treatment.
2.29. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 29 Total number of participants who suffered from abdominal pain.
2.30. Analysis.
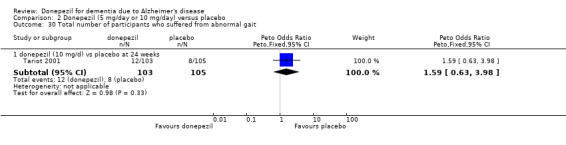
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 30 Total number of participants who suffered from abnormal gait.
2.31. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 31 Total number of participants who suffered from abnormal dreams.
2.32. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 32 Total number of participants who suffered from accidental fall.
2.33. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 33 Total number of participants who suffered from accidental injury.
2.34. Analysis.
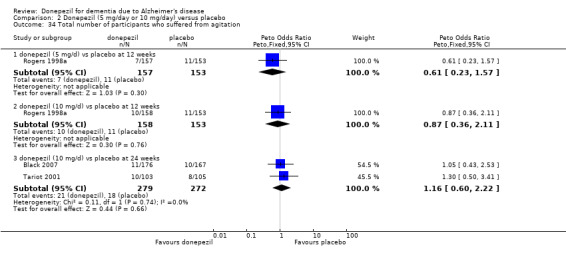
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 34 Total number of participants who suffered from agitation.
2.36. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 36 Total number of participants who suffered from anxiety.
2.37. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 37 Total number of participants who suffered from arthralgia.
2.38. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 38 Total number of participants who suffered from asthenia.
2.39. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 39 Total number of participants who suffered from back pain.
2.40. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 40 Total number of participants who suffered from cold syndrome.
2.41. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 41 Total number of participants who suffered from confusion.
2.42. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 42 Total number of participants who suffered from conjunctivitis.
2.43. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 43 Total number of participants who suffered from constipation.
2.44. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 44 Total number of participants who suffered from contusion.
2.45. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 45 Total number of participants who suffered from cystitis.
2.46. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 46 Total number of participants who suffered from depression.
2.49. Analysis.
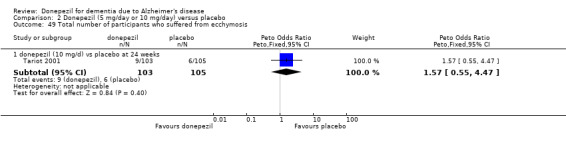
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 49 Total number of participants who suffered from ecchymosis.
2.50. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 50 Total number of participants who suffered from eczema.
2.52. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 52 Total number of participants who suffered from fever.
2.53. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 53 Total number of participants who suffered from fracture.
2.54. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 54 Total number of participants who suffered from gastroenteritis.
2.55. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 55 Total number of participants who suffered from haemorrhage.
2.57. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 57 Total number of participants who suffered from headache.
2.58. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 58 Total number of participants who suffered from hostility.
2.59. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 59 Total number of participants who suffered from loss of appetite.
2.60. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 60 Total number of participants who suffered from infection.
2.61. Analysis.
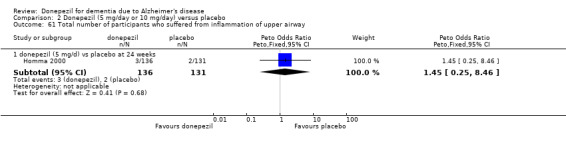
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 61 Total number of participants who suffered from inflammation of upper airway.
2.64. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 64 Total number of participants who suffered from myasthenia.
2.67. Analysis.
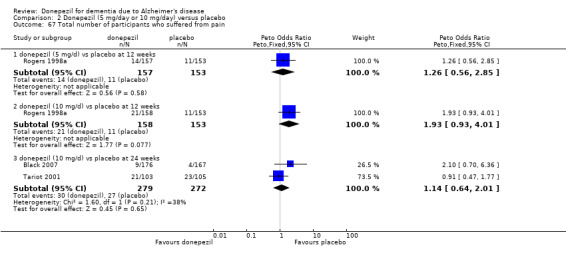
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 67 Total number of participants who suffered from pain.
2.69. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 69 Total number of participants who suffered from pneumonia.
2.70. Analysis.
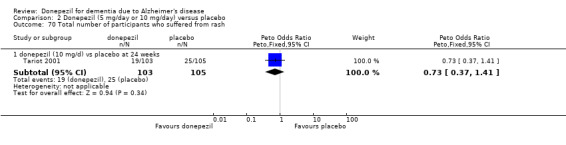
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 70 Total number of participants who suffered from rash.
2.71. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 71 Total number of participants who suffered from restlessness.
2.72. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 72 Total number of participants who suffered from respiratory tract infection.
2.73. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 73 Total number of participants who suffered from rhinitis.
2.75. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 75 Total number of participants who suffered from skin ulcer.
2.76. Analysis.

Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 76 Total number of participants who suffered from syncope.
2.78. Analysis.
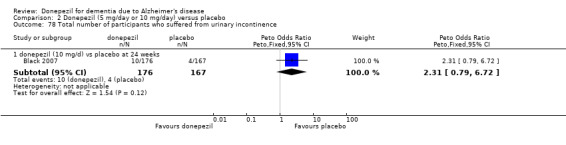
Comparison 2 Donepezil (5 mg/day or 10 mg/day) versus placebo, Outcome 78 Total number of participants who suffered from urinary incontinence.
Comparison 3. Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient and carer health resource utilisation over 24 weeks (Australia, Canada, France) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 In‐home nursing visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐2.61, 3.61] |
| 1.2 Other healthcare professional visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.98, 0.58] |
| 1.3 Day hospital visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.70, 0.70] |
| 1.4 AD‐related physician visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.71, 0.51] |
| 1.5 AD‐related medication | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.53, 0.33] |
| 1.6 Residential care days | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐6.9 [‐17.12, 3.32] |
| 1.7 Respite care days | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.9 [‐0.36, 2.16] |
| 1.8 Day centre visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐4.90, 3.90] |
| 1.9 In‐home nursing visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐2.61, 3.61] |
| 1.10 Home help visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐9.73, 14.73] |
| 1.11 Meal delivery | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐12.46, 6.66] |
| 1.12 Carer ‐ counselling visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐0.28, 1.08] |
| 1.13 Carer‐related physician visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.21, 1.41] |
| 1.14 Unpaid carer time (total hours) | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐191.45, 123.45] |
| 2 Health resource cost/participant (CAD) over 24 weeks in 1998 (Australia, Canada, France) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 In‐home nursing care | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 22.00 [‐118.50, 162.50] |
| 2.2 Other healthcare professional services | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐103.00 [‐243.15, 37.15] |
| 2.3 Day hospital use | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐74.71, 72.71] |
| 2.4 AD‐related physician services | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐15.0 [‐42.21, 12.21] |
| 2.5 AD‐related medication | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 25.0 [‐5.59, 55.59] |
| 2.6 Acute‐care hospital stays | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 22.00 [‐118.50, 162.50] |
| 2.7 In‐home nursing care | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐22.00 [‐206.26, 162.26] |
| 2.8 Residential care | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐595.0 [‐1604.31, 414.31] |
| 2.9 Respite care | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 86.0 [‐37.07, 209.07] |
| 2.10 Day centre | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐34.0 [‐311.05, 243.05] |
| 2.11 Home help | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 112.0 [‐524.20, 748.20] |
| 2.12 Meal delivery service | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐41.0 [‐169.68, 87.68] |
| 2.13 Total cost including cost of donepezil | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 34.0 [‐641.33, 709.33] |
| 3 Health resource cost/carer (CAD) over 24 weeks in 1998 (Australia, Canada, France) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Counseling | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 25.00 [‐26.81, 76.81] |
| 3.2 Physician visits | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [‐6.05, 36.05] |
| 3.3 Medication | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐27.26, 11.26] |
| 3.4 Total carer costs | 1 | 289 | Mean Difference (IV, Fixed, 95% CI) | 31.0 [7.22, 54.78] |
| 4 Unpaid carer time cost (CAD) in 1998 (Australia, Canada, France) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Total cost to society (CAD) in 1998 (Australia, Canada, France) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Health resource cost/participant (USD) over one year in 1999 (northern Europe) | 1 | MD (Fixed, 95% CI) | Subtotals only | |
| 6.1 Total participant direct costs including cost of donepezil | 1 | 286 | MD (Fixed, 95% CI) | 291.0 [‐2645.03, 3227.03] |
| 7 Health resource cost/carer (USD) over one year in 1999 (northern Europe) | 1 | MD (Fixed, 95% CI) | Subtotals only | |
| 7.1 Total carer direct medical costs | 1 | 286 | MD (Fixed, 95% CI) | 355.0 [‐84.03, 794.03] |
| 7.2 Total carer time costs | 1 | 286 | MD (Fixed, 95% CI) | 1033.0 [‐1765.83, 3831.83] |
| 8 Health resource cost/participant + carer (USD) over one year in 1999 (northern Europe) | 1 | MD (Fixed, 95% CI) | Subtotals only | |
| 8.1 Total participant and carer costs including cost of donepezil | 1 | 286 | MD (Fixed, 95% CI) | 1097.0 [‐3052.24, 5246.24] |
3.1. Analysis.

Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 1 Patient and carer health resource utilisation over 24 weeks (Australia, Canada, France).
3.2. Analysis.

Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 2 Health resource cost/participant (CAD) over 24 weeks in 1998 (Australia, Canada, France).
3.4. Analysis.

Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 4 Unpaid carer time cost (CAD) in 1998 (Australia, Canada, France).
3.5. Analysis.
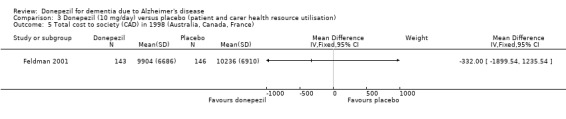
Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 5 Total cost to society (CAD) in 1998 (Australia, Canada, France).
3.6. Analysis.

Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 6 Health resource cost/participant (USD) over one year in 1999 (northern Europe).
3.7. Analysis.

Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 7 Health resource cost/carer (USD) over one year in 1999 (northern Europe).
3.8. Analysis.

Comparison 3 Donepezil (10 mg/day) versus placebo (patient and carer health resource utilisation), Outcome 8 Health resource cost/participant + carer (USD) over one year in 1999 (northern Europe).
Comparison 4. Donepezil (23 mg/day) versus donepezil (10 mg/day).
4.13. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 13 total number of patients who suffered an adverse event of dizziness before end of treatment at 24 weeks.
4.15. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 15 total number of patients who suffered an adverse event of headache before end of treatment at 24 weeks.
4.16. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 16 total number of patients who suffered an adverse event of insomnia before end of treatment at 24 weeks.
4.19. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 19 total number of patients who suffered an adverse event of weight decrease before end of treatment at 24 weeks.
4.20. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 20 total number of patients who suffered an adverse event of accidental fall before end of treatment at 24 weeks.
4.21. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 21 total number of patients who suffered an adverse event of urinary tract infection before end of treatment at 24 weeks.
4.23. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 23 total number of patients who suffered an adverse event of agitation before end of treatment at 24 weeks.
4.24. Analysis.
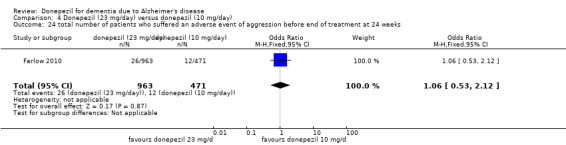
Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 24 total number of patients who suffered an adverse event of aggression before end of treatment at 24 weeks.
4.25. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 25 total number of patients who suffered an adverse event of urinary incontinence before end of treatment at 24 weeks.
4.26. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 26 total number of patients who suffered an adverse event of somnolence before end of treatment at 24 weeks.
4.28. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 28 total number of patients who suffered an adverse event of nasopharyngitis before end of treatment at 24 weeks.
4.29. Analysis.
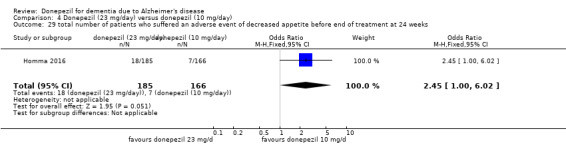
Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 29 total number of patients who suffered an adverse event of decreased appetite before end of treatment at 24 weeks.
4.30. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 30 total number of patients who suffered an adverse event of ECG QT prolonged before end of treatment at 24 weeks.
4.31. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 31 total number of patients who suffered an adverse event of anger before end of treatment at 24 weeks.
4.32. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 32 total number of patients who suffered an adverse event of constipation before end of treatment at 24 weeks end of treatment.
4.33. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 33 total number of patients who suffered an adverse event of bronchitis before end of treatment at 24 weeks.
4.34. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 34 total number of patients who suffered an adverse event of conjunctivitis before end of treatment at 24 weeks.
4.35. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 35 total number of patients who suffered an adverse event of upper respiratory tract infection before end of treatment at 24 weeks.
4.36. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 36 total number of patients who suffered an adverse event of arthralgia before end of treatment at 24 weeks.
4.37. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 37 total number of patients who suffered an adverse event of back pain before end of treatment at 24 weeks.
4.38. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 38 total number of patients who suffered an adverse event of spinal compression fracture before end of treatment at 24 weeks.
4.39. Analysis.

Comparison 4 Donepezil (23 mg/day) versus donepezil (10 mg/day), Outcome 39 total number of patients who suffered an adverse event of dermatitis contact before end of treatment at 24 weeks.
Comparison 5. Donepezil (15‐20 mg/day) versus donepezil (10 mg/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number who suffered an adverse event before end of treatment at 26 weeks | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.36, 7.60] |
Comparison 6. Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CMAI (change from baseline) completers | 1 | 221 | Mean Difference (Fixed, 95% CI) | 0.18 [‐4.23, 4.59] |
| 2 NPI (change from baseline) completers | 1 | 201 | Mean Difference (Fixed, 95% CI) | 0.1 [‐3.78, 3.98] |
| 3 NPI caregiver distress (change from baseline) | 1 | 200 | Mean Difference (Fixed, 95% CI) | ‐0.45 [‐2.06, 1.16] |
| 4 Total number of withdrawals before end of treatment | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.41] |
| 5 Total number of participants who suffered from nausea | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.32, 30.40] |
| 6 Total number of participants who suffered from diarrhoea | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.20 [0.25, 109.33] |
| 7 Total number of participants who suffered from rash | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.18, 23.04] |
| 8 Total number of participants who suffered from increased agitation | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.12] |
| 9 Total number of participants who suffered from postural hypotension | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.39] |
| 10 Total number of participants who suffered from a fall | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.14, 7.38] |
| 11 Total number of participants who suffered from femoral fracture | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.20 [0.25, 109.33] |
| 12 Total number of participants who suffered from a stroke | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.12, 76.66] |
| 13 Total number of participants who suffered from myocardial infarct | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.12, 76.66] |
| 14 Total number of participants who suffered from urinary tract infection | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.12] |
| 15 Total number of participants who suffered from chest infection | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.54] |
| 16 Total number of participants who suffered from seizure | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.11, 4.12] |
| 17 Total number of deaths | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.17, 3.47] |
6.5. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 5 Total number of participants who suffered from nausea.
6.6. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 6 Total number of participants who suffered from diarrhoea.
6.7. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 7 Total number of participants who suffered from rash.
6.8. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 8 Total number of participants who suffered from increased agitation.
6.9. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 9 Total number of participants who suffered from postural hypotension.
6.10. Analysis.
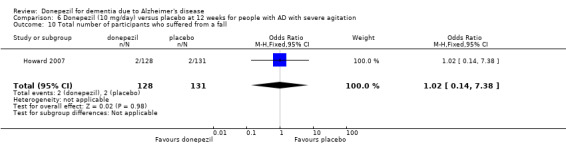
Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 10 Total number of participants who suffered from a fall.
6.11. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 11 Total number of participants who suffered from femoral fracture.
6.12. Analysis.
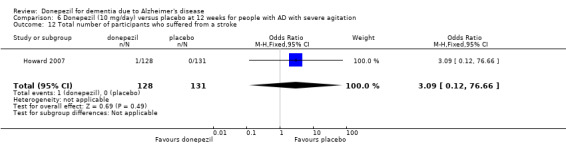
Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 12 Total number of participants who suffered from a stroke.
6.13. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 13 Total number of participants who suffered from myocardial infarct.
6.14. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 14 Total number of participants who suffered from urinary tract infection.
6.15. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 15 Total number of participants who suffered from chest infection.
6.16. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 16 Total number of participants who suffered from seizure.
6.17. Analysis.

Comparison 6 Donepezil (10 mg/day) versus placebo at 12 weeks for people with AD with severe agitation, Outcome 17 Total number of deaths.
Comparison 7. Donepezil (10 mg/day) versus donepezil (5 mg/day).
7.4. Analysis.
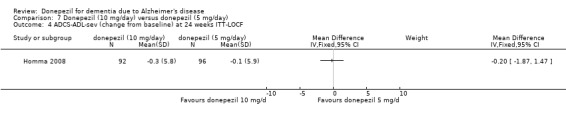
Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 4 ADCS‐ADL‐sev (change from baseline) at 24 weeks ITT‐LOCF.
7.13. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 13 Total number of participants who suffered an adverse event of confusion before end of treatment at 26 weeks.
7.15. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 15 Total number of participants who suffered an adverse event of dizziness before end of treatment at 26 weeks.
7.16. Analysis.
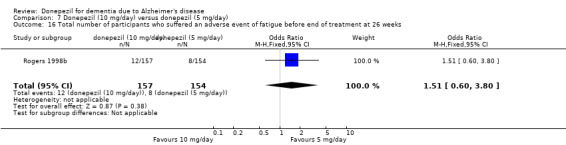
Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 16 Total number of participants who suffered an adverse event of fatigue before end of treatment at 26 weeks.
7.17. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 17 Total number of participants who suffered an adverse event of headache before end of treatment at 26 weeks.
7.18. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 18 Total number of participants who suffered an adverse event of insomnia before end of treatment at 26 weeks.
7.19. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 19 Total number of participants who suffered an adverse event of muscle cramp before end of treatment at 26 weeks.
7.23. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 23 Total number of participants who suffered an adverse event of cold syndrome before end of treatment at 26 weeks.
7.24. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 24 Total number of participants who suffered an adverse event of accidental fall before end of treatment at 26 weeks.
7.25. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 25 Total number of participants who suffered an adverse event of respiratory tract infection before end of treatment at 26 weeks.
7.26. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 26 Total number of participants who suffered an adverse event of constipation before end of treatment at 26 weeks.
7.27. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 27 Total number of participants who suffered an adverse event of fever before end of treatment at 26 weeks.
7.28. Analysis.
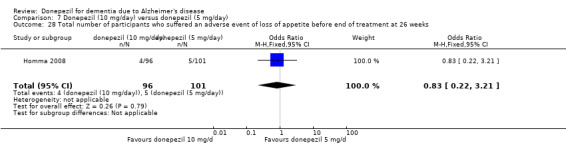
Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 28 Total number of participants who suffered an adverse event of loss of appetite before end of treatment at 26 weeks.
7.29. Analysis.

Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 29 Total number of participants who suffered an adverse event of bruising before end of treatment at 26 weeks.
7.30. Analysis.
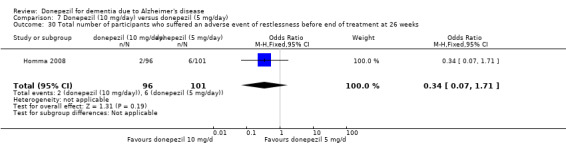
Comparison 7 Donepezil (10 mg/day) versus donepezil (5 mg/day), Outcome 30 Total number of participants who suffered an adverse event of restlessness before end of treatment at 26 weeks.
Comparison 8. Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE (change from baseline) ITT‐LOCF | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 donepezil (10 mg/d) at 24 weeks | 4 | 1102 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [0.56, 1.38] |
| 2 SIB (change from baseline) ITT‐LOCF | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 donepezil (5 mg/d) at 24 weeks | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 6.7 [3.66, 9.74] |
| 2.2 donepezil (10 mg/d) at 24 weeks | 5 | 1348 | Mean Difference (IV, Fixed, 95% CI) | 5.92 [4.53, 7.31] |
| 3 CIBIC‐Plus or CGIC (numbers improved) ITT‐LOCF | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.83, 2.87] |
| 3.2 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 755 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [1.31, 2.43] |
| 4 ADCS‐ADL‐severe (change from baseline) ITT‐LOCF | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 donepezil (5 mg/d) at 24 weeks | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.54, 2.54] |
| 4.2 donepezil (10 mg/d) at 24 weeks | 3 | 733 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [0.21, 1.85] |
| 5 BEHAVE‐AD (change from baseline) ITT‐LOCF | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 donepezil (5 mg/d) at 24 weeks | 1 | 198 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.67, 1.67] |
| 5.2 donepezil (10 mg/d) at 24 weeks | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐1.28, 2.08] |
| 6 Behavioural disturbance (total NPI ) (change from baseline) ITT‐LOCF | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 donepezil (10 mg/d) at 24 weeks | 3 | 827 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐4.11, ‐0.25] |
| 7 Time (mins/day) spent by carer assisting in IADL and PSMS (change from baseline) LOCF | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | ‐52.4 [‐118.78, 13.98] |
| 8 Total number of withdrawals before end of treatment | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 donepezil ( 5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.32, 1.43] |
| 8.2 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [1.02, 1.71] |
| 9 Total number of participants who withdrew due to an adverse event | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 donepezil ( 5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.29, 1.89] |
| 9.2 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.23, 2.42] |
| 10 Total number of participants who suffered from at least one adverse event | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.69, 2.46] |
| 10.2 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [1.23, 2.05] |
| 11 Total number of participants who suffered from abdominal pain | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.36, 2.30] |
| 12 Total number of participants who suffered from accidental fall | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 12.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.40, 3.76] |
| 12.2 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 449 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.58, 2.02] |
| 13 Total number of participants who suffered from accidental injury | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 13.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 538 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.46, 1.70] |
| 14 Total number of participants who suffered from anorexia | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 14.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.16] |
| 14.2 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 857 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.32 [1.20, 4.48] |
| 15 Total number of participants who suffered from anxiety | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 15.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.28, 1.92] |
| 16 Total number of participants who suffered from arthralgia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 16.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.06 [1.28, 12.86] |
| 17 Total number of participants who suffered from asthenia | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 17.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 538 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.59, 2.50] |
| 18 Total number of participants who suffered from back pain | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 18.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.63 [0.63, 4.22] |
| 19 Total number of participants who suffered from cold syndrome | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 19.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.55, 2.28] |
| 19.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.54, 2.29] |
| 20 Total number of participants who suffered from confusion | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 20.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.43, 3.06] |
| 21 Total number of participants who suffered from constipation | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 21.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [0.66, 6.75] |
| 21.2 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 449 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.41, 2.46] |
| 22 Total number of participants who suffered from contusion | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.41, 7.61] |
| 22.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.22, 5.57] |
| 23 Total number of participants who suffered from cystitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 23.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [0.50, 4.63] |
| 24 Total number of participants who suffered from depression | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 24.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [0.54, 4.99] |
| 25 Total number of participants who suffered from diarrhoea | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 25.1 donepezil ( 5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.58 [0.45, 5.62] |
| 25.2 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1395 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.57 [1.65, 4.01] |
| 26 Total number of participants who suffered from dizziness | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 26.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 603 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.69, 3.66] |
| 27 Total number of participants who suffered from fever | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 27.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.87 [0.40, 20.69] |
| 27.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.32 [0.85, 21.86] |
| 28 Total number of participants who suffered from fracture | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 28.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.49, 5.52] |
| 29 Total number of participants who suffered from gastroenteritis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 29.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.24, 1.51] |
| 30 Total number of participants who suffered from hallucinations | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 30.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.68 [1.24, 17.66] |
| 31 Total number of participants who suffered from pneumonia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 31.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.65, 4.19] |
| 32 Total number of participants who suffered from hostility | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 32.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.58, 2.99] |
| 33 Total number of participants who suffered from insomnia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 33.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.99, 7.35] |
| 34 Total number of participants who suffered from loss of appetite | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 34.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.51, 14.15] |
| 34.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.40, 12.51] |
| 35 Total number of participants who suffered from nausea | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 35.1 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 828 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [1.16, 3.85] |
| 36 Total number of participants who suffered from restlessness | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 36.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.54 [1.01, 20.41] |
| 36.2 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 201 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.15 [0.22, 20.95] |
| 37 Total number of participants who suffered from headache | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 37.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.86 [1.22, 6.69] |
| 38 Total number of participants who suffered from respiratory tract infection | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 38.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.29, 2.20] |
| 38.2 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 491 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.50, 1.65] |
| 39 Total number of participants who suffered from vomiting | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 39.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.35, 3.08] |
| 39.2 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 834 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.42 [1.37, 4.31] |
| 40 Total number of participants who suffered from urinary incontinence | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 40.1 donepezil (10 mg/d) vs placebo at 24 weeks | 1 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.31 [0.79, 6.72] |
| 41 Total number of participants who suffered from urinary tract infection | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 41.1 donepezil (10 mg/d) vs placebo at 24 weeks | 3 | 851 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.55, 1.48] |
| 42 Total number of participants who suffered from weight loss | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 42.1 donepezil (10 mg/d) vs placebo at 24 weeks | 2 | 603 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.59 [0.69, 3.66] |
| 43 Total number of deaths before end of treatment | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 43.1 donepezil ( 5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [0.21, 19.83] |
| 43.2 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.41, 1.25] |
| 44 Total number of participants who suffered from at least one serious adverse event | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 44.1 donepezil (5 mg/d) vs placebo at 24 weeks | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.36, 1.82] |
| 44.2 donepezil (10 mg/d) vs placebo at 24 weeks | 5 | 1396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.59, 1.08] |
8.11. Analysis.
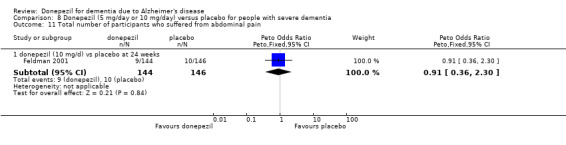
Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 11 Total number of participants who suffered from abdominal pain.
8.12. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 12 Total number of participants who suffered from accidental fall.
8.13. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 13 Total number of participants who suffered from accidental injury.
8.15. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 15 Total number of participants who suffered from anxiety.
8.17. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 17 Total number of participants who suffered from asthenia.
8.18. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 18 Total number of participants who suffered from back pain.
8.19. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 19 Total number of participants who suffered from cold syndrome.
8.20. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 20 Total number of participants who suffered from confusion.
8.21. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 21 Total number of participants who suffered from constipation.
8.22. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 22 Total number of participants who suffered from contusion.
8.23. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 23 Total number of participants who suffered from cystitis.
8.24. Analysis.
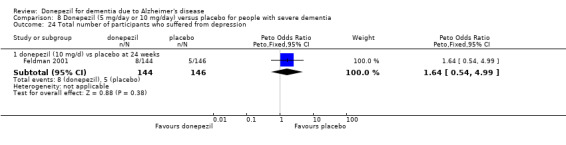
Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 24 Total number of participants who suffered from depression.
8.26. Analysis.
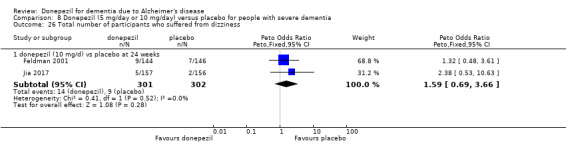
Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 26 Total number of participants who suffered from dizziness.
8.27. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 27 Total number of participants who suffered from fever.
8.28. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 28 Total number of participants who suffered from fracture.
8.29. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 29 Total number of participants who suffered from gastroenteritis.
8.31. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 31 Total number of participants who suffered from pneumonia.
8.32. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 32 Total number of participants who suffered from hostility.
8.34. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 34 Total number of participants who suffered from loss of appetite.
8.38. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 38 Total number of participants who suffered from respiratory tract infection.
8.40. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 40 Total number of participants who suffered from urinary incontinence.
8.41. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 41 Total number of participants who suffered from urinary tract infection.
8.42. Analysis.

Comparison 8 Donepezil (5 mg/day or 10 mg/day) versus placebo for people with severe dementia, Outcome 42 Total number of participants who suffered from weight loss.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AD2000.
| Methods | Double‐blinded, placebo‐controlled, part cross‐over, part parallel‐group, randomised trial 60 weeks |
|
| Participants | Setting: UK, multicentre Sample size: 566 participants (female, male) Age: Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | NHS Executive R&D (West Midlands) | |
| Declaration of interest | Reported | |
| Notes | Initially participants randomised to placebo or 5 mg/d donepezil. After 12 weeks participants randomised again to placebo, 5 mg/d or 10 mg/d. In addition suitable participants were randomised to aspirin or aspirin avoidance. After 60 weeks, following a washout period, there was an option of open‐label treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone randomisation |
| Allocation concealment (selection bias) | Low risk | Study is described as double blind. At the first randomisation there was little risk of revealing the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was described as matching |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study is described double blind but no information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | From the first phase, the 12‐week phase, 36/282 (36%) withdrew from the donepezil arm, and 18/283 (6%) from the placebo arm |
| Selective reporting (reporting bias) | Low risk | All assessed outcomes were reported |
| Other bias | High risk | The participants were randomised at baseline and again at 3 months. On average half of the participants may have changed intervention arm as though in a cross‐over trial. This may have affected the blinding. |
Black 2007.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised trial 24 weeks |
|
| Participants | Setting: Australia, Canada, France, UK, USA, multicentre (98 sites) Sample size: 343 participants (102 men and 241 women) Age: mean age 78.0 (8.2), mean MMSE 7.5 (3.5) Selection criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | Assessments were made at baseline, 16 and 24 weeks
|
|
| Source of funding | Supported by Eisai Inc. and Pfizer Inc. | |
| Declaration of interest | Reported | |
| Notes | This study investigated the potential treatment benefits of donepezil in community‐dwelling people with severe AD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation according to a computerised randomisation schedule generated by Almedica Service Corp. |
| Allocation concealment (selection bias) | Low risk | The labels on the medication kits were attached unopened to the case report form by the study personnel |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and donepezil tablets were identical |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals 59/176 in donepezil group (43 due to adverse events), 40/167 (18 due to adverse events) in placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other source of bias expected |
Burns 1999.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 24 weeks |
|
| Participants | Setting: Australia, Canada, Europe, New Zealand and South Africa, multicentre (82 sites)
Sample size: 818 participants, 348 men and 470 women Age: mean age 71.7 (8.3) MMSE: mean MMSE 20.2 (5.0) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | Assessments at weeks 3, 6, 12, 18, 24 weeks and follow up at 30 weeks
|
|
| Source of funding | Eisai Inc. | |
| Declaration of interest | None reported | |
| Notes | Participants in the 10 mg/d group received 5 mg/d for the 1st week of treatment. 6‐week placebo washout phase followed the double‐blind phase The group on 10 mg/d of donepezil was on a blinded forced titration scheme of 5 mg/d for week 1, and 10 mg/d for the remainder of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participant‐randomisation schedule was computer‐generated by Unival Europe Ltd |
| Allocation concealment (selection bias) | Low risk | Unival Europe and Eisai both maintained sealed envelopes containing the master randomisation list, which was only to be opened in a medical emergency. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical appearance of 7.2 mm, film‐coated tablets for all groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was maintained at all times, apart from one medical emergency. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23% of participants discontinued from the study, 20% in the placebo group, 22% in the 5 mg/d group, and 26% in the 10 mg/d group |
| Selective reporting (reporting bias) | Low risk | Data were available on all outcomes. |
| Other bias | Low risk | No other source of bias anticipated |
Farlow 2010.
| Methods | Double‐blinded, 2‐arm, parallel‐group randomised trial 24 weeks of treatment |
|
| Participants | Setting: 219 sites in Asia, Europe, Australia, North America, South Africa, and South America Sample size: 1467 participants (63% female, 37% male) Age: mean age 73.9 (SD = 8.5) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Participants were randomly assigned in a 1:2 ratio (10 mg to 23 mg) |
|
| Outcomes | Assessments made at baseline, 3, 6, 12, 18 and 24 weeks
|
|
| Source of funding | Eisai Inc | |
| Declaration of interest | The study authors reported receiving research funding from various pharmaceutical companies. | |
| Notes | The objective of this study was to compare the effectiveness and safety profile of high‐dose donepezil (23mh/d) and standard dose donepezil (10 mg/d) in participants with moderate‐severe AD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using computer‐generated randomisation codes |
| Allocation concealment (selection bias) | Low risk | Participants, caregivers and study personnel were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Because the treatments were not identical in appearance, a double‐dummy design was used to maintain blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No further information but likely to be adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 296/981 (30%) discontinued in the 23 mg/d arm, 87/486 (18%) in the 10 mg/d arm |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Reporting according to CONSORT statement and no other source of bias anticipated |
Feldman 2001.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 24 weeks |
|
| Participants | Setting: Australia, Canada, France, multicentre (32 sites)
Sample size: 292 participants, 115 men and 177 women Age: aged 51‐94 years Inclusion criteria:
Exclusion criteria:
Most concomitant medications were allowed except those with notable cholinomimetic or anticholinergic effects. |
|
| Interventions |
|
|
| Outcomes | Assessments were carried out at 4, 8, 12, 18 and 24 weeks
|
|
| Source of funding | Pfizer Inc. and Eisai Inc. | |
| Declaration of interest | None reported | |
| Notes | The group on donepezil took 5 mg/d for the first 4 weeks, followed by 10 mg/d for 20 weeks. The dose could be reduced to 5 mg/d at any point if necessary | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Participants, caregivers and study personnel were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical film‐coated tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blinded, no further information but probably done adequately |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers were lost to follow‐up from both groups, 20/146 withdrew from the placebo group and 23/144 from the donepezil group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Reported according to the CONSORT statement and no other source of bias anticipated |
Hegerl 2003.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 12 weeks |
|
| Participants | Setting: Germany
Sample size: 40 participants
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Pfizer Inc. and Eisai Europe | |
| Declaration of interest | None reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised, double blind, probably adequate |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Not reported according to CONSORT guidelines |
Homma 1998.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study 12 weeks |
|
| Participants | Setting: Japan, multicentre (55 sites) Sample size: 190 participants Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | No information | |
| Declaration of interest | No information | |
| Notes | The tablets were sent to the sites as units of 3 cases (3 mg, 5 mg and placebo) of concealed identity. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Homma 2000.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study 24 weeks |
|
| Participants | Setting: Japan, multicentre (54 sites) Sample size: 268 participants (67% female) Age: mean age 69.8 (8.2) Inclusion criteria:
Exclusion criteria:
Use of choline activators, anticholinergics, cerebral vasodilators, activators of cerebral metabolism, psychotropic drugs, hypnotics, antiparkinsonism agents and nonsteroidal anti‐inflammatory drugs prohibited during the trial period. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | No details | |
| Declaration of interest | None reported | |
| Notes | To avoid gastrointestinal problems the donepezil dose was 3 mg/d for the first week | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18/134 withdrew from the donepezil group and 17/129 from the placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | No information |
Homma 2008.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised trial 24 weeks |
|
| Participants | Setting: Japan (multicentre) Sample size: 302 participants (20% men) with severe AD, Age: mean 78.2 (8.0) years , mean MMSE 7.8 (3.5) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
The dose was escalated in the donepezil groups |
|
| Outcomes | Assessments were carried out at baseline, weeks 8, 16, and 24.
|
|
| Source of funding | Not reported | |
| Declaration of interest | None reported | |
| Notes | This study was conducted to assess the efficacy and safety of donepezil in Japanese patients with severe AD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised by a computerised randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Allocation carried out independently of all parties in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching donepezil and placebo tablets, identical in appearance, taste and smell |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blind, no further details were reported but methods probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The discontinuation rate due to adverse events and illness were 10.5% in the placebo group, 7.9% in the 5 mg group, and 13.5% in the 10 mg group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Does not appear to be reported according to CONSORT guidelines but no other source of bias anticipated |
Homma 2016.
| Methods | Double‐blind, parallel‐group, randomised trial 24 weeks |
|
| Participants | Setting: 69 sites in Japan Sample size: 351 participants (69% female, 31% male) Age: mean age 76.0 (SD = 8.8) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Participants were randomly assigned in a 1:2 ratio (10 mg to 23 mg) |
|
| Outcomes | Assessments made at baseline, 3, 6, 12, 18 and 24 weeks
|
|
| Source of funding | Not reported | |
| Declaration of interest | The study authors reported receiving research funding from various pharmaceutical companies. | |
| Notes | The objective of this study was to compare the effectiveness and safety profile of high‐dose donepezil (23 mg/d) and standard dose donepezil (10 mg/d) in people with moderate‐severe AD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Study described as double‐blinded, no further details given, but methods probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablets were used in both groups together with the active tablets to blind allocation. Tablets not described as identical but blinding was probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study described as double‐blinded, no further details given, but methods probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few participants were lost to follow‐up. Sensitivity analyses carried out to assess the effect of non completers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Unclear whether CONSORT guidelines followed but no other source of bias anticipated |
Howard 2007.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 12 weeks |
|
| Participants | Setting: England, UK, 8 centres Sample size: 259 participants, 15% male Age: mean age 84.5 (8.0) MMSE: mean MMSE 8.2 (6.5) Inclusion criteria:
Exclusion criteria: unstable, uncontrolled medical conditions |
|
| Interventions |
|
|
| Outcomes | Assessments at weeks 4 and 12
|
|
| Source of funding | Supported by Grants from the MRC and the Alzheimer's Society. Donepezil was provided by Eisai UK. | |
| Declaration of interest | These were reported. | |
| Notes | The trial was planned with a third group taking risperidone, but this was abandoned after 13 participants had been randomised to this arm, after warnings had been issued by the UK Committee for Safety of Medicines that risperidone and olanzapine were not be used for the treatment of behavioral symptoms in dementia. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone randomisation centrally by MRC Clinical Trials Unit |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study medication was encapsulated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians, patients, carers and assessors were all unaware of the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/128 were lost to follow‐up from the donepezil group, and 19/131 from the placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Study was reported according to CONSORT guidelines and no other sources of bias anticipated |
Jia 2017.
| Methods | Double‐blind, parallel‐group, randomised trial 24 weeks |
|
| Participants | Setting: 38 hospitals in China Sample size: 313 participants (65% female, 35% male) Age: mean age 70.8 (SD = 9.6) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Titration at 6 weeks from 5 mg/d |
|
| Outcomes | Assessments made at baseline, 6, 12, 18 and 24 weeks
|
|
| Source of funding | Donepezil was provided by Eisai China | |
| Declaration of interest | Reported | |
| Notes | No down‐titration to a previous dose was allowed for those who could not tolerate the study drug | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised by a computerised randomisation schedule generated by staff independent of study in other respects |
| Allocation concealment (selection bias) | Low risk | Allocation carried out independently of all parties in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo and donepezil tablets (5 mg and 10 mg) were identical in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants, caregivers, site investigators, and the sponsor were blind to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30/156 withdrew from the placebo arm (10 adverse events, 5 protocol violations, 6 withdrew consent, 2 lost to follow‐up, 7 others), 29/157 in the donepezil arm (14 due to adverse events, 3 protocol violations, 3 lost to follow‐up, 6 withdrew consent, and 3 others) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | There is no explicit statement that CONSORT reporting guidelines were used, but the report follows these. |
Krishnan 2003.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study 24 weeks |
|
| Participants | Setting : USA, 3 centres
Sample size: 67 participants (48 women, 19 men) Age: mean age 73.4 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Eisai Inc. and Pfizer Inc. | |
| Declaration of interest | Reported | |
| Notes | The MRI study Primary outcomes were neuronal markers and hippocampal volumes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical donepezil and placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 67 participants enrolled. In the placebo group 10/33 withdrew, and in the donepezil group 6/34 withdrew before end of treatment. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other source of bias anticipated |
Lebert 1999.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 12 weeks |
|
| Participants | Setting: unknown multicentre (53 sites) Sample size: 318 participants Age: mean age 72 years Inclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Eisai SA | |
| Declaration of interest | None reported | |
| Notes | 1/3 randomised to placebo, 2/3 to donepezil 4‐week titration on 5 mg/d before increasing to 10 mg/d. 5 participants were mis‐randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers who withdrew were not reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Information is confined to a number of conference abstracts, which showed inconsistent reporting. |
Maher‐Edwards 2011.
| Methods | Double‐blinded, placebo‐controlled, randomised trial 24 weeks |
|
| Participants | Setting: 24 centres in Austria, Bulgaria, Chile, Estonia, Germany, the Russian Federation, Slovakia, and the UK Sample size: 130 participants (33% male, 76% female) Age: mean age 71.2 (7.8) years inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Titration at 4 weeks from 5 mg/d |
|
| Outcomes | Assessments at baseline, weeks 8, 16 and 24
|
|
| Source of funding | GlaxoSmithKline | |
| Declaration of interest | None declared | |
| Notes | This was a 3‐arm trial. The third arm (n = 68) was an experimental drug SB‐742457, the results of which were not relevant for this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A permutated block randomisation schedule was generated by GlaxoSmithKline |
| Allocation concealment (selection bias) | Low risk | Study described as double‐blinded, no further details given, but methods probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules were described as maintaining the blindness of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The CIBIC+ was rated by an independent rater with no access to other study information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15/63 withdrew from the placebo arm (1 adverse event, 2 protocol violations, 8 withdrew consent, 4 other), 10/67 in the donepezil arm (4 due to adverse events,1 lost to follow‐up, 3 withdrew consent, 1 for non‐compliance and 1 other) |
| Selective reporting (reporting bias) | High risk | Descriptive results only for some outcomes, which were reported to show no treatment effect. |
| Other bias | Low risk | No CONSORT checklist but no other source of bias anticipated |
Mazza 2006.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 24 weeks |
|
| Participants | Setting: Italy Sample size: 51 participants (54 % female) Age: Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Independent, no conflict of interest declared | |
| Declaration of interest | None reported | |
| Notes | There was a 3rd arm to the trial (n = 25), testing Ginkgo biloba special extract. The main aim of the trial was to compare Ginkgo biloba with donepezil and with placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | High risk | No information on the blinding of the placebo, donepezil capsules |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This was carried out by researchers who had previously not been involved in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/25 withdrew from the donepezil group, 6/26 from the placebo group |
| Selective reporting (reporting bias) | Low risk | Results of all outcomes were reported |
| Other bias | Low risk | No CONSORT checklist but no other source of bias anticipated |
Mohs 2001.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 54 weeks |
|
| Participants | Setting: USA, multicentre (31 sites) Sample size: 431 participants, 160 men and 271 women with mild‐moderately severe AD, mean age 75.4 (8.8), mean MMSE 17.1 (3.0) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Eisai Inc. Pfizer Inc. | |
| Declaration of interest | Reported | |
| Notes | At each visit participants who had met predefined criteria for clinically evident decline in functional status were discontinued | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments were carried out by investigators who were blind to the participant's treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The difference in rates of withdrawals before the end of treatment between the groups was small |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Participants were withdrawn if they met predefined deterioration criteria |
Moraes 2006a.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 12 weeks |
|
| Participants | Setting: Brazil Sample size: 23 participants (65% female) Age: mean age 74.6 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | ADAS‐Cog | |
| Source of funding | Fundação de Amparo à Pesquisa do Estado de São Paulo and Associação Fundo de Incentivo à Psicofarmacologia | |
| Declaration of interest | There were no conflicts of interest to declare. | |
| Notes | The primary purpose of the trial was to investigate sleep apnoea, and the polysomnography formed the primary outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number list was generated using Statistica |
| Allocation concealment (selection bias) | Unclear risk | A randomised number list was used but there were no further details of the administration of the list |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were blinded to participants' conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appear to be no withdrawals from the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No CONSORT checklist but no other source of bias anticipated |
Moraes 2006b.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 26 weeks |
|
| Participants | Setting: Brazil Sample size: 35 participants (69% female) Age: mean age 75.9 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | This was not an industry‐supported study. Fundação de Amparo à Pesquisa do Estado de São Paulo and Associação Fundo de Incentivo à Psicofarmacologia | |
| Declaration of interest | There are no financial conflicts | |
| Notes | The primary purpose of the trial was to investigate rapid eye movement (REM) sleep, and the polysomnography forms the primary outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No computer‐generated randomisation code reported but probably adequate |
| Allocation concealment (selection bias) | Low risk | The allocation was carried out blinded to treatment code |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Wyeth‐Whitehall laboratories produced both the placebo and donepezil tablets in a coded form |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were blind to participants' conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals from treatment |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No CONSORT checklist but no other source of bias anticipated |
Rogers 1996.
| Methods | Double‐blind, randomised, parallel‐group, placebo‐controlled trial 12 weeks |
|
| Participants | Setting: USA (multicentre)
Sample size: 161 participants (64 men, 97 women) Age: mean 71.8 years, range 55‐85 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | Assessments at baseline, 1, 3, 6, 9, 12 and 14 weeks
|
|
| Source of funding | No information | |
| Declaration of interest | No information | |
| Notes | The double‐blind phase was followed by a 2‐week single‐blind placebo washout phase. The dose ranging study was undertaken to explore the potential efficacy and safety of donepezil in people with AD, and to examine the relationships between plasma donepezil concentration, red blood cell AChE activity and clinical response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/39 participants withdrew from the intervention arm (3 for adverse events, 1 for protocol violation and 1 by request), and 5/40 from the placebo arm (2 for adverse events, 3 for protocol violation ), before endpoint |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No additional sources of bias expected |
Rogers 1998a.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study 12 weeks |
|
| Participants | Setting: USA, multicentre (23 sites)
sample size: 468 participants, 171 men and 297 women Age: aged 50‐94 years Selection criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Eisai Inc. | |
| Declaration of interest | None reported | |
| Notes | The group on 10 mg/d of donepezil was on a blinded, forced titration scheme of 5 mg/d for week 1, and 10 mg/d for the remainder of the study. Measures of clinical outcome were assessed at baseline and at 3‐week intervals. At the end of the double‐blind treatment all participants began a 3‐week single‐blind washout period with placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 93% of participants in the placebo group completed the trial, 90% of the 5 mg/d donepezil group and 82% of the 10 mg/d donepezil group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other source of bias anticipated |
Rogers 1998b.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study 24 weeks |
|
| Participants | Setting: USA, multicentre (20 sites)
Sample size: 473 participants, 180 men and 293 women age: mean age 73.4 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Eisai Inc | |
| Declaration of interest | None reported | |
| Notes | The group on 10 mg/d of donepezil was on a blinded, forced titration scheme of 5 mg/d for week 1, and 10 mg/d for the remainder of the study. Measures of clinical outcome were assessed at baseline and at 6‐week intervals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisations schedule was used |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80% of participants in the placebo group completed the trial, 85% of the 5 mg/d donepezil group and 68% of the 10 mg/d donepezil group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other source of bias anticipated |
Schindler 2004.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 24 weeks |
|
| Participants | 31 participants with mild‐moderate AD (MMSE 10‐26) currently taking 10 mg/d donepezil | |
| Interventions |
|
|
| Outcomes | TEAEs | |
| Source of funding | No information | |
| Declaration of interest | No information | |
| Notes | Participants randomised to the treatment group took a further 5 mg/d of donepezil for 12 weeks, followed by a further 5 mg/d for weeks 12‐24. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Seltzer 2004.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised trial 24 weeks |
|
| Participants | Setting: USA, multicentre (17 sites) Sample size: 153 participants, 71 men and 82 women Age: mean age 74.0 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes | Assessments carried out at baseline, 6, 12, 18 and 24 weeks
|
|
| Source of funding | Eisai Inc. and Pfizer Inc. | |
| Declaration of interest | Not reported | |
| Notes | Participants unable to tolerate 10 mg/d were dropped from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26/96 withdrew from the donepezil group (15 due to adverse events) and 11/57 from the placebo group (5 due to adverse events) |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of some outcomes |
| Other bias | High risk | Participants who could not tolerate 10 mg donepezil were discontinued from study |
Study 205.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study 12 weeks |
|
| Participants | Setting: USA Sample size: 12 participants | |
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Not reported | |
| Declaration of interest | Not reported | |
| Notes | The visuospatial attention study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Study 306.
| Methods | 12‐week, randomised, double‐blind, parallel‐group, placebo‐controlled study | |
| Participants | Country: Italy 39 participants | |
| Interventions |
|
|
| Outcomes | ADAS‐Cog CDR‐SB | |
| Source of funding | Not reported | |
| Declaration of interest | Not reported | |
| Notes | ApoE study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Tariot 2001.
| Methods | Double‐blind, parallel group, placebo‐controlled, randomised study 24 weeks |
|
| Participants | Setting: USA, multicentre (27 sites) Sample size: 208 participants, 37 men and 171 women Age: mean age 85.7 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Eisai Inc. Pfizer Inc | |
| Declaration of interest | Reported | |
| Notes | The group on donepezil took 5 mg/d for the first 4 weeks, followed by 10 mg/d for 20 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation schedule |
| Allocation concealment (selection bias) | Low risk | No treatment codes were broken during the course of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was achieved by using identical‐appearing film‐coated tablets of donepezil and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18% of the placebo group and 11% of the donepezil withdrew before end of treatment |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other source of bias anticipated |
Tune 2003.
| Methods | Randomised, double‐blind, parallel‐group, placebo‐controlled study 24 weeks |
|
| Participants | Setting: USA
Sample size: 28 participants (7 male, 21 female) Age: mean age 72.9 years Inclusion criteria:
Exclusion criteria: |
|
| Interventions |
|
|
| Outcomes | Assessments at weeks 6, 12, 18, and 24
|
|
| Source of funding | Eisai, Inc. | |
| Declaration of interest | None reported | |
| Notes | Functional brain activity assessed by cerebral glucose metabolism | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 28 participants (14 in each group) were enrolled, and 26 participants completed. Two participants in the placebo group withdrew before end of study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No additional sources of bias expected |
Winblad 2001.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 52 weeks |
|
| Participants | Setting: northern Europe, multicentre (28 sites) Sample size: 286 participants, 102 men and 184 women Age: mean age 72.5 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Pfizer Pharmaceuticals Group, Pfizer, Inc. | |
| Declaration of interest | None reported | |
| Notes | The group on donepezil received 5 mg/d for 28 days initially, and then 10 mg/d according to the clinician's judgement for 1 year. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated randomisations list produced by Pfizer, Inc. (NY) |
| Allocation concealment (selection bias) | Low risk | No information but probably adequate described as double‐blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No information but probably adequate described as double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information but probably adequate described as double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 47/142 (33%) withdrew from the donepezil group and 47/144 (33%) from the placebo group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No CONSORT checklist but no other source of bias anticipated |
Winblad 2006.
| Methods | Double‐blind, parallel‐group, placebo‐controlled, randomised study 6 months |
|
| Participants | Setting: Sweden, multicentre (50 nursing homes) Sample size: 248 participants, 58 men and 190 women Age: mean age 84.9 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Source of funding | Pfizer Pharmaceuticals | |
| Declaration of interest | Reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule was generated centrally by the Global Clinical Data Services at Pfizer |
| Allocation concealment (selection bias) | Low risk | Described as randomised, double‐blind, probably adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as randomised, double‐blind, probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7% of the placebo group and 16% of the donepezil group withdrew before the end of study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other source of bias anticipated |
Some of the first studies published were identified by their study number, and this has been retained since the first version of this review.
AChE: acetylcholinesterase; ACQLI: Alzheimer Carer's Quality of Life Instrument; AD: Alzheimer's disease; ADAS‐Cog: Alzheimer's Disease Assessment Scale (cognitive);ADAS‐Jcog: Alzheimer's Disease Assessment Scale (Japanese cognitive);ADCS‐ADL: Alzheimer's Disease Cooperative Study‐Activities of Daily Living Scale (severe version); ADCS‐CGIC: Alzheimer's Disease Cooperative Study ‐Clinician's Interview‐Based Impression of Change; ADFACS: AD Functional Assessment and Change Score; ADL: Activities of Daily Living; ADRDA: ?; aRSS: abridged Relative's Stress Scale; BADLS: Bristol Activities of Daily Living Scale; BEHAVE‐AD: Behavioural Pathology in Alzheimer's Disease Rating Scale; CAUST: Canadian Utilization of Services Tracking; CBQ: Caregiver Burden Questionnaire; CDR‐SB: Clinical Dementia Scale, sum of boxes; CGIC: Clinician's Global Impression of Change; CIBIC+: Clinician's Interview‐Based Impression of Change;CMAI: Cohen‐Mansfield Agitation Inventory; CMCS: Crichton Scale; CONSORT: Consolidated Standards of Reporting Trials; COPD: Chronic Obstructive Pulmonary disease; CSS: Caregiver Stress Scale; CT: computed tomography; DSM: Diagnostic and Statistical Manual of Mental Disorders;DAD: Disability Assessment for Dementia; FAST: Functional Assessment Staging; GHQ‐30: General Health Questionnaire; GBS: Gottfries, Brane and Steen scale; GDS: Geriatric Depression Scale IADL: Instrumental Activities of Daily Living; MENFIS: Mental Function Impairment Scale; MMSE: Mini Mental State Examination; MRC: Medical research Council; MRI: magnetic resonance imaging; NINCDS‐ADRDA: Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association; NOSGER: Nurses' Observation Scale for Geriatric Patients; NPI: Neuropsychiatric Instrument; NPI‐D: Neuropsychiatric Inventory Distress scale; PDS: Progressive Deterioration Scale; PSMS: Physical Self Maintenance Scale; QoL: Quality of Life; RUD: Resource Utilization in Dementia; RUSP: Resources Utilisation for Severe Alzheimer's Disease Patients; SIB: Severe Impairment Battery; SD: standard deviation; SF‐36: Short Form ‐ 36; SKT: Syndrom Kurz Test; SPECT: single‐photon emission computed tomography; SR: sustained release; TEAE: treatment‐emergent adverse event; UADL: Uniform Activities of Daily Living
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ames 2001 | Open‐label study, one group all on donepezil |
| AWARE | This is a withdrawal study, the participants had all been taking Donepezil immediately before randomisation. |
| Barak 2001 | Open‐label study |
| Berger 2000 | Open‐label study, one group all on donepezil |
| Birt 2002 | Open‐label study |
| Borroni 2001 | Open‐label study, one group all on donepezil |
| Brodaty 2000 | Open‐label study, one group all on donepezil |
| Bullock 2000 | Open‐label study, one group all on donepezil |
| Bullock 2001 | Randomised study comparing donepezil with rivastigmine |
| Cameron 2000 | Open‐label study carried out in general practice, one group all on donepezil |
| Clary 2000 | Sertraline+donepezil vs donepezil. Study of sertraline not donepezil |
| Cumbo 2011 | Open‐label randomised study comparing donepezil with memantine, galantine, and rivastigmine. There was no placebo arm. |
| Cummings 2000 | Matched groups, not randomised |
| DOMINO‐AD | The participants were already stable on donepezil. On randomisation memantine was added and in some donepezil withdrawn. Withdrawal studies are not considered in this review. |
| Dong 2011 | Donepezil is compared with usual care. This is not a randomsied study. |
| Fillit 2002 | Case‐control study |
| Froelich 2000 | Open‐label, 24‐week study, 237 participants from routine clinical practice, all on donepezil (5 mg/dor 10 mg/d) |
| Fuschillo 2001 | Open‐label study |
| Geldmacher 2003 | Open‐label extension to studies 210, 301 and 302. Reports results of analysis of time to institutionalisation |
| Ghorbani 2010 | This study was not randomised, participants were allocated to study arm by consecutive non‐random sampling, and the only outcomes were measures of cerebral blood flow. |
| Greenberg 2000 | Duration of treatment in this randomised, cross‐over trial was only 6 weeks. |
| Hampel 2002 | Retrospective population study |
| Holmes 2004 | This is a withdrawal study, the participants had all been taking Donepezil immediately before randomisation. |
| Homma 1998a | Open‐label study |
| Homma 1998b | Randomisation was not mentioned. The treatment of 2 mg/d was compared with 0.1 mg/d and there was no placebo group |
| Imai 1998a | Open‐label study |
| Imai 1998b | Open study |
| Imai 1998c | Open study |
| Janssen 2005 b | Donepezil was compared with galantamine. There is no placebo arm. |
| Kauffer 1998 | Open‐label study, one group all on donepezil |
| Kemp 2003 | Randomised, placebo‐controlled, double‐blind study of 12 participants. Brain image outcomes only |
| Leube 2002 | All participants on donepezil. Outcome was change of neural activation measured by functional MRI |
| Lopez 2008 | Open‐label study of donepezil with no placebo group |
| Maltz 2002 | Non‐randomised study. Outcome was response to methacholine‐induced cutaneous vasodilation |
| Matthews 2000 | Open‐label study, one group all on donepezil |
| McRae 1999 | Open‐label study, one group all on donepezil |
| McRae 2001a | Retrieval of participants from studies 301 and 302 after treatment ended for follow‐up |
| Mega 1999 | Open‐label study, one group all on donepezil |
| Mega 2001 | Donepezil vs memantine, matched groups |
| Mega 2002 | Non‐randomised study of donepezil, metrifonate or galantamine. Outcome was response to cerebral metabolic activation |
| Modrego 2010 | Donepezil compared with memantine. There was no placebo arm. |
| NCT00423228‐BRAINz | Donepezil was compared with ZT‐1, an investigational product. There was no placebo arm. |
| Nikolova 2001 | Open‐label study, one group all on donepezil |
| Nobili 2002 | Open‐label study using retrospective control group. Brain perfusion SPECT and MMSE assessed |
| Ollat 2007 | This RCT had no placebo group. |
| Onofrj 2002 | Small, randomised study comparing donepezil with vitamin E for mild AD and for moderate‐severe AD. There was no placebo group. The latency of P300 ERP was the primary outcome, MMSE was a secondary outcome |
| Onofrj 2003 | Small, randomized, cross‐over study comparing donepezil with vitamin E. There was no placebo group. The primary outcomes were EEG abnormalities, investigated in those with fluctuating cognition |
| Parsa 2000 | Open‐label study, one group all on donepezil+quetiapine |
| Peng 2002 | Randomised, placebo‐controlled study, but only single‐blind |
| Peng 2005 | Single‐blind, randomised, placebo‐controlled study. Participants were not blinded to treatment. |
| Requena 2006 | This 4‐group study was not blinded. There was no placebo group. |
| Richarz 2011 | Donepezil was compared with galantamine. There was no placebo arm. |
| Rocca 2002 | Open‐label study, one group all on donepezil |
| Rockwood 2002 | Open‐label study, all participants on donepezil |
| Rockwood 2007 | Open‐label study, all participants on donepezil |
| Rodriguez 2002 | Open‐label study, one group all on donepezil |
| Rogers 1997 | Open‐label titration study using placebo participants from a phase III trial |
| Rogers 1997b | Non‐randomised open‐label follow‐on study |
| Rozzini 2002 | Donepezil compared with rivastigmine |
| Rozzini 2007a | Open‐label study, all participants on donepezil |
| Rozzini 2007b | Open‐label study, all participants on donepezil |
| Salloway 2002 | Donepezil was compared with galantamine. There was no placebo arm. |
| Sampson 2007 | Placebo‐controlled RCT of donepezil, but the participants did not have dementia. |
| Saumier 2007 | Open‐label study, all participants on donepezil |
| Shua‐Haim 2002a | Cross‐sectional study of rivastigmine +donepezil compared with rivastigmine |
| Shua‐Haim 2002b | Donepezil compared with rivastigmine compared with galantamine. No mention of randomisation |
| Stewart 1998 | Open‐label study, following participants in studies 301 and 302, during randomised phase and after, to examine costs of care |
| Tarraga 2006 | This was a study of cognitive stimulation. All participants were taking a cholinesterase inhibitor. |
| Teipel 2006 | This was a cross‐over study of donepezil, the primary outcome being cortical metabolic response assessed by PET. It was not a suitable design to assess cognition. |
| Tessitore 2000 | Open‐label study, one group all on donepezil |
| Tettamanti 2000 | Prospective observational study |
| Thal 2004 | RCT, parallel groups, placebo, vitamin E and placebo. Participants with mild cognitive impairment, not AD |
| Thomas 2001 | Donepezil compared with vitamin E |
| Touchon 2006 | This was a subgroup analysis of a study that compared rivastigmine with donepezil. There was no placebo group. |
| Tsolaki 2002 | Donepezil compared with rivastigmine |
| Vanmechelen 2002 | No mention of randomisation. Only AD biomarkers assessed |
| Wattmo 2008 | Open‐label, observational studies, all participants on donepezil. |
| Weiner 2000 | Open‐label study, one group all on donepezil |
| Werber 2002 | Non‐randomised study of tacrine, donepezil or rivastigmine |
| Wilcock 2003 | Single‐blind, randomised, 52‐week study comparing donepezil with galantamine. No placebo group |
| Winstein 2007 | The duration of treatment in the RCT was only 4 weeks. |
| Wyeth 2005 | Donepezil was compared with lecozatan. There was no placebo arm. |
| Zhang 2012 | Donepezil was compared with galantamine. There was no placebo arm. |
AD: Alzheimer's Disease; EEG: electro‐encephalogram; MMSE: Mini‐Mental State Exam; MRI: magnetic resonance imaging; PET: positron emission tomography; RCT: randomised controlled trial; SPECT: single‐photon emission computed tomography
Differences between protocol and review
When the protocol was written donepezil had received United States Food and Drug Administration (FDA) approval (1996) for mild and moderate dementia. Later studies were published that included participants with severe dementia and we included these studies in updated versions of the review. The FDA approved donepezil for people with severe dementia in 2006.
The methods for this current 2017 update of the review follow current Cochrane guidelines, which have changed substantially since the publication of the protocol and the first version of the review in 1998.
In the 2017 update of the review we reorganised the results to focus on the currently recommended dose. The main analysis was at 26 weeks and we prioritised seven outcomes for the meta‐analyses.
We also expanded the 'Risk of bias' assessment of individual studies for this update, carrying out additional assessments on blinding, selective reporting and other biases.
Contributions of authors
Hirukuni Beppu wrote the protocol, searched the Japanese literature, provided copies of the Japanese studies together with translations into English. Jacqueline Birks and David Melzer wrote the original review.
May 2003: Jacqueline Birks updated the review and analysed the data for the meta‐analyses, and Richard Harvey replaced David Melzer as co‐reviewer. He contributed to the background, the conclusions and discussion. Dymphna Hermans performed the update search.
October 2005: Jacqueline Birks updated the review and analysed the data for the meta‐analyses. Richard Harvey contributed to the background, the conclusions and discussion. Dymphna Hermans performed the update search.
This review was peer reviewed in November 2005.
July 2010: Jacqueline Birks updated the review and analysed data for the meta‐analyses. Anna Noel‐Storr performed the update search.
Contact editor: Lon Schneider Consumer editor: David Janes
Sources of support
Internal sources
Division of Clinical Geratology, Nuffield Department of Clinical Medicine, University of Oxford, UK.
-
The National Institute of Health Research (NIHR) Oxford Biomedical Research Centre Programme, UK.
JB receives salary support
External sources
NHS R&D, UK.
University of Melbourne, Australia.
Barwon Health, Australia.
-
NIHR, UK.
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Dementia and Cognitive Improvement. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
Jacqueline Birks: none known Richard Harvey: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
AD2000 {published data only}
- AD2000 Collaborative Group. Long‐term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double‐blind trial. Lancet 2004;363:2105‐15. [DOI] [PubMed] [Google Scholar]
- Bentham P, Gray R, Hill R, Sellwood E, Courtney C. Twelve week response to cholinesterase inhibitors dose not predict future benefit the AD2000 trial experience. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:337.
- Lendon CL. Determination of responses to anticholinesterase therapy in the treatment of Alzheimer's disease. 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20‐25, Stockholm, Sweden: Abstract No 1287. 2002.
- Roberts N. A reliable assessment of the efficacy and safety of donepezil and aspirin in Alzheimer's disease (AD2000). National Research Register 2001.
- Schneider LS. AD2000: donepezil in Alzheimer's disease. Lancet 2004;363(9427):2100‐1. [DOI] [PubMed] [Google Scholar]
- Waghray S. AD2000 A reliable assessment of the efficacy and safety of donepezil and aspirin in Alzheimer's disease. National Research Register 2000. [MEDLINE: ]
Black 2007 {published data only}
- Black S, Li HL, McRae T, Richardson S. Treatment of severe Alzheimer's disease with donepezil: results from a 24‐week, multinational, randomized, double‐blind, placebo‐controlled trial. Neurology 2006;66(5):A347. [Google Scholar]
- Black S, Li Honglang, McRae T, Richardson S. Donepezil treatment of severe Alzheimer's disease: results from a 24‐week, multinational, randomized, double‐blind, placebo controlled trial. Poster Presented at the Geneva Springfield Conference April 2006.
- Black SE, Doody R, Li H, McRae T, Jambor KM, Xu Y, et al. Donepezil preserves cognition and global function in patients with severe Alzheimer disease. Neurology 2007;69(5):459‐69. [DOI] [PubMed] [Google Scholar]
- Pfizer. E2020‐A001‐315: a 24 week multinational RCT evaluating the efficacy and safety of donepezil in severe AD. www.marc.soton.ac.uk/Research%20Page.htm.
- Richardson S. A 24 week, multicenter, randomized, double‐blind, placebo‐controlled evaluation of the safety and efficacy of donepezil hydrochloride (E2020) in patients with severe Alzheimer's disease followed by a 12 week open‐label extension period. www.clinicaltrials.gov/ct/show/NCT00096473 2004.
Burns 1999 {published and unpublished data}
- Bayer AJ, Rossor M, Hecker J, Gauthier S, Burns A, Petite H, et al. International Donepezil Study Group. Donepezil improves functional activity in patients with Alzheimer's disease. 21st Collegium Internationale Neuro Psychopharmacologicum; 1998 July 12‐16, Glasgow, Scotland. 1998. [MEDLINE: ]
- Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open‐label, multicentre study in patients with Alzheimer's disease. International Journal of Geriatric Psychiatry 2007;22(8):806‐12. [DOI] [PubMed] [Google Scholar]
- Burns A, Rossor M, Hecker J, Gauthier S, Petit H, Möller H‐J, et al. International Donepezil Study Group. The effects of donepezil in Alzheimer's disease ‐ results from a multinational trial. Dementia and Geriatric Cognitive Disorders 1999;10(3):237‐44. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Rosser M, Hecker J, Petite H, Rogers S, Mohr E, et al. Donepezil produces both clinical global and cognitive test improvement in patients with Alzheimer's disease. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐June 4, Toronto, Canada 1998. [MEDLINE: ]
- Gauthier S, Rossor M, Hecker J. Results from a multinational phase III clinical trial of donepezil in Alzheimer's disease. Poster presentation at 5th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy. April 15‐18 1998.
- Pratt RD, Gauthier S, Burns A, Perdomo CA. Donepezil provides long‐term clinical benefits for patients with Alzheimers disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington. 2000.
Farlow 2010 {published data only}
- Cummings JL, Geldmacher D, Farlow M, Sabbagh M, Christensen D, Betz P. High‐dose donepezil (23 mg/day) for the treatment of moderate and severe Alzheimer's disease: drug profile and clinical guidelines. CNS Neuroscience & Therapeutics 2013;19(5):294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody RS, Geldmacher DS, Farlow MR, Sun Y, Moline M, Mackell J. Efficacy and safety of donepezil 23 mg versus donepezil 10 mg for moderate‐to‐severe Alzheimer's disease: a subgroup analysis in patients already taking or not taking concomitant memantine. Dementia and Geriatric Cognitive Disorders 2012;33(2‐3):164‐73. [DOI] [PubMed] [Google Scholar]
- Doody RS, Ramos H, Faison W, Zou H. Efficacy and safety of donepezil 23 mg/d vs. donepezil 10 mg/d in patients with moderate to severe Alzheimer's disease: impact of concomitant memantine use. Journal of the American Geriatrics Society 2011 Annual Scientific meeting of the American Geriatrics Society, National Harbor, MD, USA 2011.
- Farlow M, Richardson S, Mackell J, Sun Y. Long‐term safety and tolerability of donepezil 23 MG in patients with moderate‐to‐severe Alzheimer's disease: an 18‐month analysis. Alzheimer's and Dementia 2011 Alzheimer's Association International Conference, Paris, France. 2011.
- Farlow M, Veloso F, Moline M, Yardley J, Brand‐Schieber E, Bibbiani F, et al. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer's disease. BMC Neurology 2011;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow MR, Salloway S, Tariot PN, Yardley J, Moline ML, Wang Q, et al. Effectiveness and tolerability of high‐dose (23 mg/d) versus standard‐dose (10 mg/d) donepezil in moderate to severe Alzheimer's disease: a 24‐week, randomized, double‐blind study. Clinical Therapeutics 2010;32(7):1234‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S, Cummings J, Christensen D, Doody R, Farlow M, Sabbagh M, et al. Donepezil 23 MG/D for moderate to severe Alzheimer's disease: assessing subdomains of the Severe Impairment Battery. Journal of Nutrition, Health and Aging 5th Conference Clinical Trials on Alzheimer's Disease, Monte Carlo, Monaco. 2012.
- Ferris S, Cummings J, Christensen D, Doody R, Farlow M, Sabbagh M, et al. Effects of donepezil 23 mg on Severe Impairment Battery domains in patients with moderate to severe Alzheimer's disease: evaluating the impact of baseline severity. Alzheimer's Research & Therapy 2013;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S, MacKell J, Bai Z, Sun Y. Effect of donepezil 23 mg/day on language function in patients with moderate‐to‐severe Alzheimer's disease (AD): subgroup analysis of united states (U.S.)‐based patients. Alzheimer's Association International Conference 2012 Vancouver, BC Canada. 2012.
- Ferris SH, Schmitt FA, Saxton J, Richardson S, Mackell J, Sun Y, et al. Analyzing the impact of 23 mg/day donepezil on language dysfunction in moderate to severe Alzheimer's disease. Alzheimer's Research & Therapy 2011;3(3):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S‐H, Lee J‐H, Kim SY, Park KW, Chen C, Tripathi M, et al. Donepezil 23 mg in Asian patients with moderate‐to‐severe Alzheimer's disease. Acta Neurologica Scandinavica 2017;135(2):252‐56. [DOI] [PubMed] [Google Scholar]
- Sabbagh M, Cummings J, Christensen D, Doody R, Farlow M, Liu L, et al. Evaluating the cognitive effects of donepezil 23 mg/d in moderate and severe Alzheimer's disease: analysis of effects of baseline features on treatment response. BMC Geriatrics 2013;13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh M, Cummings J, Christensen D, Doody R, Farlow M, MacKell J, et al. Evaluating the cognitive effects of donepezil 23 MG/D in moderate and severe Alzheimer's disease: a patient subgroup analysis. Journal of Nutrition, Health and Aging 5th Conference Clinical Trials on Alzheimer's Disease, Monte Carlo, Monaco. 2012.
- Sabbagh M, Han S, Kim S, Na H‐R, Lee J‐H, Kandiah N, et al. Clinical recommendations for the use of donepezil 23 mg in moderate‐to‐severe Alzheimer's disease in the Asia‐Pacific region. Dementia and Geriatric Cognitive Disorders Extra 2016;6:382‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot P, Salloway S, Yardley J, Mackell J, Moline M. Long‐term safety and tolerability of donepezil 23 mg in patients with moderate to severe Alzheimer's disease. BMC Research Notes 2012;5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot P, Yardley J, Moline M, Brand‐Schieber E, Zou H, Timothy H, et al. Long‐term safety and tolerability of high dose donepezil (23 mg/day) in moderate to severe Alzheimer's disease: a 12‐month open‐label study. Neuropsychopharmacology 49th Annual Conference of the American College of Neuropsychopharmacology, Miami Beach, FL, USA. 2010.
Feldman 2001 {published data only}
- Feldman H. Therapeutic benefits of acetylcholinesterase inhibitor therapy in the moderate to severe stage of Alzheimer's disease. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm 2000:59.
- Feldman H, Gauthier S, Hecker J, Vellas B, Emir B, Mastey V, et al. Donepezil treatment benefits caregivers of patients with moderate to severe Alzheimer's disease (AD). European Journal of Neurology 2002;9(Suppl 2):34. [Google Scholar]
- Feldman H, Gauthier S, Hecker J, Vellas B, Emir B, Mastey V, et al. and the Donepezil Study Group. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate to severe Alzheimer's disease and the effect on caregiver burden.. Journal of the American Geriatrics Society 2003;51:737‐44. [DOI] [PubMed] [Google Scholar]
- Feldman H, Gauthier S, Hecker J, Vellas B, Hux M, Emir B, et al. Improved health outcomes with donepezil in moderate to severe Alzheimer's disease are associated with economic benefits. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:285.
- Feldman H, Gauthier S, Hecker J, Vellas B, Hux M, Xu Y, et al. Donepezil MSAD Study Investigators Group. Economic evaluation of donepezil in moderate to severe Alzheimer disease. Neurology 2004;63(4):644‐50. [DOI] [PubMed] [Google Scholar]
- Feldman H, Gauthier S, Hecker J, Vellas B, Ieni J, Xu Y, et al. Treatment benefits of donepezil in patients with severe Alzheimer's disease and their caregivers. 57th Annual Meeting of the American Academy of Neurology, Miami Beach, April 2005 2005b:P02.097. 2005.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. Donepezil provides benefits in global function in moderate to severe Alzheimer's Disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E. Donepezil's benefits on cognition, global function, activities of daily living and behavior in patients with moderate to severe Alzheimer's disease. Proceedings of the Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 Apr 5‐8, Stockholm 2000:174.
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E, Donepezil MSAD Study Investigators Group. A 24‐week, randomized, double‐blind study of donepezil in moderate to severe Alzheimer's disease. Neurology 2001; Vol. 57, issue 4:613‐20. [DOI] [PubMed]
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E, Donepezil MSAD Study Group. Benefits of donepezil on global function, behavior, cognition and ADLs in patients with moderate to severe Alzheimer's disease. Neurology 2000; Vol. 54, issue Suppl 3:A469.
- Feldman H, Gauthier S, Hecker J, Vellas B, Xu Y, Ieni JR, et al. Donepezil MSAD Study Investigators Group. Efficacy and safety of donepezil in patients with more severe Alzheimer's disease: a subgroup analysis from a randomized, placebo‐controlled trial. International Journal of Geriatric Psychiatry 2005;20(6):559‐69. [DOI] [PubMed] [Google Scholar]
- Gauthier S. Efficacy of donepezil on maintenance of activities of daily living in patients with moderate‐to‐severe Alzheimer's disease, and impact on caregiver burden. Geriatrics and Aging 2004;7(5):34‐6. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Feldman H, Hecker J, Vellas B, Ames D, Subbiah P, et al. Donepezil MSAD Study Investigators Group. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer's disease. International Psychogeriatrics 2002;14(4):389‐404. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Feldman H, Hecker J, Vellas B, Emir B, McGill PS. Exploratory analysis of the effects of donepezil in moderate and severe Alzheimer's disease patients. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:277.
- Gauthier S, Feldman H, Hecker J, Vellas B, Emir B, Subbiah P, The Donepezil MSAD Study Investigators' Group. Functional, cognitive and behavioral effects of donepezil in patients with moderate Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2002;18(6):347‐54. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Feldman H, Hecker J, Vellas B, Subbiah P, Whalen E. Benefits of donepezil on performance of basic and instrumental activities of daily living in moderate to severe Alzheimer's disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000.
- Gauthier S, Feldman H, Hecker J, Vellas B, Subbiah P, Whalen E. Effects of donepezil on behaviour and other domains in moderate to severe Alzheimer's disease. Journal of the European College of Neuropsychopharmacology 2000;10(Suppl 3):S359. [MEDLINE: ] [Google Scholar]
- Gauthier S, Feldman H, Vellas B, Subbiah P. Efficacy of donepezil on functional, behavioural and cognitive symptoms in patients with moderate to severe Alzheimer's disease. Journal of the American Geriatrics Society 2000; Vol. 48, issue 8:S2.
- Hecker J, Foti D, Gauthier S, Vellas B, Subbiah P, Whalen E. Benefits of donepezil in the treatment of behavioural problems in moderate to severe Alzheimer's disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington DC 2000.
- Luckmann R. Donepezil improved the clinical state and quality of life in moderate‐to‐severe Alzheimer disease. ACP‐Journal‐Club 2002; Vol. 136, issue 2:59. [PubMed]
- Panisset M, Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, et al. Use of the severe impairment battery in a clinical trial of donepezil in moderate to severe Alzheimers disease. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington 2000.
- Shah SN, Feldman H, Gauthier S, Hecker J, Vellas B, Hux M, et al. Pfizer Inc, New York, NY, USA. Pharmacoeconomic benefits of donepezil treatment in severe Alzheimer's disease. Neurobiology of Aging 2004;25(S2):208. [Google Scholar]
- Vellas B, Feldman H, Gauthier S, Hecker J, Subbiah P, Whalen E, et al. Donepezil treatment in patients with moderate to severe Alzheimer's disease reduces caregiver stress. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington 2000.
Hegerl 2003 {published data only}
- Hegerl U, Mergl R, Henkel V, Gallinat J, Kotter G, Muller Siecheneder F, et al. Kinematic analysis of the effects of donepezil hydrochloride on hand motor function in patients with Alzheimer dementia. Journal of Clinical Psychopharmacology 2003;23(2):214‐6. [DOI] [PubMed] [Google Scholar]
Homma 1998 {published and unpublished data}
- Homma A, Imai Y, Hariguchi S, Hasegawa K, Kameyama M, Nishimura T. Late phase II clinical study of acetyl cholinesterase inhibitor E2020 in patients with Alzheimer‐type dementia. Clinical Evaluation 1998;26(2):251‐84. [Google Scholar]
Homma 2000 {published data only}
- Homma A, Takeda M, Imai Y, Udaka F, Hasegawa K, Kameyama M, et al. E2020 Study Group. Clinical efficacy and safety of donepezil on cognitive and global function in patients with Alzheimer's disease: a 24‐week, multicenter, double‐blind, placebo‐controlled study in Japan. Dementia and Geriatric Cognitive Disorders 2000; Vol. 11, issue 6:299‐313. [DOI] [PubMed]
Homma 2008 {published data only}
- Homma A, Arimoto I, Kaidoji K, Ohbayashi T, Ozawa H. Treatment of severe Alzheimer's disease with donepezil: results from a 24 week, parallel, placebo‐controlled study in Japan. 10th International Conference on Alzheimer's Disease and Related Disorders, Madrid, July 2006. 2006.
- Homma A, Imai Y, Tago H, Asada T, Shigeta M, Iwamoto T, et al. Donepezil treatment of patients with severe Alzheimer's disease in a Japanese population: results from a 24‐week, double‐blind, placebo‐controlled, randomized trial. Geriatric Cognitive Disorders 2008;25(5):399‐407. [DOI] [PubMed] [Google Scholar]
- Homma A, Imai Y, Tago H, Asada T, Shigeta M, Iwamoto T, et al. Long‐term safety and efficacy of donepezil in patients with severe Alzheimer's disease: results from a 52‐week, open‐label, multicenter, extension study in Japan. Dementia and Geritatric Congitive Disorders 2009;27(3):232‐9. [DOI] [PubMed] [Google Scholar]
Homma 2016 {published data only}
- Homma A, Atarashi H, Kubota N, Nakai K, Takase T. Efficacy and safety of sustained release donepezil high dose versus immediate release donepezil standard dose in Japanese patients with severe Alzheimer's disease: a randomized, double‐blind trial. Journal of Alzheimer's disease 2016;52(1):345‐57. [DOI] [PubMed] [Google Scholar]
Howard 2007 {published data only}
- Howard RJ, Juszczak E, Ballard CG, Bentham P Brown RG, Bullock R, et al. CALM‐AD Trial Group. Donepezil for the treatment of agitation in Alzheimer's disease. New England Journal of Medicine 2007;357(14):1382‐92. [DOI] [PubMed] [Google Scholar]
- Pelosi A. Donepezil is no more effective than placebo for agitation in people with Alzheimer's disease. Evidence‐Based Mental Health 2008;11(3):84. [DOI] [PubMed] [Google Scholar]
Jia 2017 {published data only}
- Jia JP, Wei CB, Jia LF, Tang Y, Liang JH, Zhou AH, et al. Efficacy and safety of donepezil in Chinese patients with severe Alzheimer's disease: a randomized controlled trial. Journal of Alzheimer's disease 2017;56(4):1495‐504. [DOI] [PubMed] [Google Scholar]
Krishnan 2003 {published data only}
- Anon. No title. Eisai Inc.
- Krishnan KR, Charles HC, Doraiswamy PM, Mintzer J, Weisler R, Yu X, et al. Randomized, placebo‐controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer's disease. The American Journal of Psychiatry 2003;160(11):2003‐11. [DOI] [PubMed] [Google Scholar]
Lebert 1999 {published data only}
- Robert P, Lebert F, Goni S, Touchon J, Vincent S, ARIAL Study Investigators Collaborative Group. The impact on caregiver distress of donepezil treatment of patients with mild Alzheimer's disease. Quality Research in Dementia; 19‐22 November, 2000, London. 2000.
- Robert PH, Lebert F, Goni S, Touchon J, ARIAL Study Investigators Collaborative Group. The impact of caregiver distress of donepezil treatment of patients with mild Alzheimer's disease. 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20, Washington DC. 1999. [MEDLINE: ]
Maher‐Edwards 2011 {published data only}
- Maher‐Edwards G, Dixon R, Hunter J, Gold M, Hopton G, Jacobs G, et al. SB‐742457 and donepezil in Alzheimer's disease: a randomized, placebo‐controlled study. International Journal of Geriatric Psychiatry 2011;26(5):536‐44. [DOI] [PubMed] [Google Scholar]
- Maher‐Edwards G, Zvartau‐Hind M, Davies J, Alexander K, Schronen J, Boswell D, et al. Effects of 6‐month monotherapy treatment with the 5HT6 receptor antagonist SB 742457 or donepezil in subjects with mild‐to‐moderate Alzheimer's disease. Alzheimer's Association International Conference, Paris, France. 2011.
Mazza 2006 {published data only}
- Korczyn AD. Comments on the article by Mazza et al. concerning Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo‐controlled double‐blind study. European Journal of Neurology 2007;14(9):e9. [DOI] [PubMed] [Google Scholar]
- Mazza M, Capuano A, Bria AP, Mazza S. Letter to the editor. European Journal of Neurology 2007;14(9):e10. [Google Scholar]
- Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo‐controlled double‐blind study. European Journal of Neurology 2006;13(9):981‐85. [DOI] [PubMed] [Google Scholar]
Mohs 2001 {published data only}
- Ebell M. Does donepezil help patients with moderate Alzheimer's dementia preserve their ability to function independently?. Evidence Based Practice 2002.
- Mohs R, Doody R, Morris J, Ieni J, Perdomo C, Pratt R, et al. Donepezil preserves activities of daily living in Alzheimer's disease patients: results from a one‐year placebo‐controlled functional survival study. Neurology 2000;54(Suppl 3):A415. [Google Scholar]
- Mohs R, Doody R, Morris J, Ieni J, Rogers S, Perdomo C, et al. Donepezil preserves functional status and improves cognition in Alzheimer's disease patients: results from a 1‐year prospective placebo‐controlled study. Journal of the American Geriatrics Society 2000;48(8):S46. [Google Scholar]
- Mohs R, Doody R, Morris J, Ieni JR, Rogers SL, Perdomo CA, et al. Donepezil preserves functional status in Alzheimer's disease patients: results from a 1‐year prospective placebo‐controlled attrition study. Journal of the European College of Neuropsychopharmacology 1999;9(Suppl 5):S328. [Google Scholar]
- Mohs R, Doody R, Morris J, Ieni JR, Rogers SL, Perdomo CA, et al. Donepezil preserves functional status in Alzheimer's disease patients: results from a 1‐year prospective placebo‐controlled study. Quality Research in Dementia Conference; 2000 November 19‐22, London. 2000.
- Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo C, et al. 312 Study Group. A 1‐year, placebo‐controlled preservation of function survival study of donepezil in AD patients [Erratum]. Neurology 2001; Vol. 57, issue 10:1942. [MEDLINE: ] [DOI] [PubMed]
- Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, et al. A 1‐year, placebo‐controlled preservation of function survival study of donepezil in AD patients. Neurology 2001;57(3):481‐8. [DOI] [PubMed] [Google Scholar]
- Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, et al. Donepezil preserves functional status in Alzheimer's disease. 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18, Chicago, Illinois. 2000. [MEDLINE: ]
- Pratt R, Mohs R, Doody R, Morris J, Rogers S, Ieni J, et al. Donepezil preserves functional status in Alzheimer's disease patients results from a 1‐year prospective placebo controlled functional study. Proceedings of the 6th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden 2000:178.
Moraes 2006a {published data only}
- Moraes W, Poyares D, Sukys‐Claudino L, Guilleminault C, Tufik S. Donepezil improves obstructive sleep apnoea in Alzheimer disease: a double‐blind, placebo‐controlled study. Chest 2008;133(3):677‐83. [DOI] [PubMed] [Google Scholar]
- Moraes W, Sukys‐Claudino L, Poyares D, Guilleminault C, Tufik S. Donepezil improves oxygen desaturation in patients with alzheimer's disease and obstructive sleep apnoea. Sleep Medicine 2006;7(S47):368. [Google Scholar]
Moraes 2006b {published data only}
- Moraes W dos S, Poyares DR, Guilleminault C, Ramos LR, Bertolucci PH, Tufik S. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double‐blind placebo‐controlled study. Sleep 2006;29(2):199‐205. [DOI] [PubMed] [Google Scholar]
Rogers 1996 {published and unpublished data}
- Friedhoff LT, Ieni JR, Rogers SL, Pratt RD. Donepezil provides long‐term clinical benefits for patients with Alzheimer's Disease. Proceedings of the 21st Collegium Internationale Neuro psychopharmacologicum;1998 July 12‐16, Glasgow, Scotland 1998:ABSTRACT REF: PW11017. [MEDLINE: ]
- Friedhoff LT, Rogers SL. Correlation between the clinical efficacy of donepezil HCL (E2020) and red blood cell (RBC) acetylcholinesterase (ACHE) inhibition in patients with Alzheimer's disease. Proceedings of the 98th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics; 1997 March 5‐8, San Diego. UK: Medical Education Network, 1997; Vol. 2:6‐7.
- Rogers S, Perdomo C, Friedhoff L. Clinical benefits are maintained during long‐term treatment of Alzheimer's disease with the acetylcholinesterase inhibitor, E2020. Proceedings of the 8th European College of Neuropsychopharmacology Congress; 1995 September 30‐October 4, Venice 1995a. [MEDLINE: ]
- Rogers SL, Doody RS, Pratt RD, Ieni JR. Long‐term efficacy and safety of donepezil in the treatment of Alzheimer's disease: final analysis of a US multicentre open‐label study. European Neuropsychopharmacology 2000; Vol. 10, issue 3:195‐203. [DOI] [PubMed]
- Rogers SL, Friedhoff LT. E2020 improves cognition and quality of life in patients with mild to moderate Alzheimers Disease: results of a phase II trial. Proceedings of the 46th Annual meeting of the American Academy of Neurology. 1994 May 1‐7, Washington DC 1994; Vol. 44, issue Suppl 2:A165.
- Rogers SL, Friedhoff LT and the Donepezil Study Group. The Efficacy and safety of Donepezil in patients with Alzheimer's disease: results of a US multicentre, randomised, double‐blind, placebo‐controlled trial. Dementia 1996;7:293‐303. [DOI] [PubMed] [Google Scholar]
Rogers 1998a {published and unpublished data}
- Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD, Donepezil Study Group. Open‐label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Archives of Neurology 2001;58(3):427‐33. [DOI] [PubMed] [Google Scholar]
- Pratt RD, Perdomo CA, Ieni JK. Long‐term safety and tolerability of donepezil: results from a phase iii extension trial of patients with mild to moderately severe Alzheimer's disease. European Journal of Neurology 1999;6(Suppl 3):116. [MEDLINE: ] [Google Scholar]
- Rogers SL. Donepezil new clinical trials support long term use. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 April 5‐8, Stockholm. 2000:133.
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT, the Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer disease. Archives of Internal Medicine 1998;158:1021‐31. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Mohs RC, Friedhoff LT. Donepezil (E2020) improves cognition and function in patients with mild to moderately severe Alzheimer's disease. Results from phase III trials. American Psychiatric Association 150th Annual Meeting, San Diego. 1997.
Rogers 1998b {published data only}
- Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD, Donepezil Study Group. Open‐label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Archives of Neurology 2001;58(3):427‐33. [DOI] [PubMed] [Google Scholar]
- Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E, Donepezil MSAD Study Investigators Group. Erratum: a 24‐week, randomized, double‐blind study of donepezil in moderate to severe Alzheimer's disease (Neurology (2001) 57 (613‐620)). Neurology 2001; Vol. 57, issue 11:2153. [DOI] [PubMed]
- Friedhoff LT, Rogers L. Donepezil lengthens time to loss of activities of daily living in patients with mild to moderate Alzheimer's disease ‐ results of a preliminary evaluation. Presented at the annual meeting of the American Academy of Neurology. Boston. Neurology 1997;48(3):A100. [Google Scholar]
- Friedhoff LT, Rogers SL. Donepezil lengthens time to loss of activities of daily living and cognition in patients with mild to moderate Alzheimer's disease. Proceedings of the 10th European College of Neuropsychopharmacology Congress; 1997 Sep 13‐17, Vienna, Austria 1997. [MEDLINE: ]
- Friedhoff LT, Rogers SL. Donepezil maintains activities of daily living in patients with mild to moderately severe Alzheimer's disease: results of a retrospective analysis. European Journal of Neurology 1997;4(Suppl 1):S9. [Google Scholar]
- Rogers SL. Donepezil new clinical trials support long term use. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 April 5‐8, Stockholm. 2000:133.
- Rogers SL, Doody R, Mohs R, Friedhoff LT. E2020 produces both clinical, global and cognitive test improvement in patients with mild to moderately severe Alzheimer's disease: results of a 30‐week phase III trial. Neurology 1996;46:A217 S14.001 ARI‐8. [Google Scholar]
- Rogers SL, Doody R, Mohs R, Friedhoff T, Donepezil Study Group. E2020 (Aricept TM) improves global and cognitive function in patients with Alzheimer's disease. Results of a 30‐week trial. Unpublished paper 1996b.
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT, Donepezil Study Group. A 24‐week, double‐blind, placebo‐controlled trial of donepezil in patients with Alzheimer's disease. Neurology 1998;50(1):136‐45. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Friedhoff LT, Farlow MR, Doody RS, Mohs R. Efficacy of donepezil in Alzheimer's disease: fact or artifact? [Reply]. Neurology 1999; Vol. 52, issue 1:218‐19. [DOI] [PubMed]
- Rogers SL, Mohs RC, Friedhoff LT. Donepezil (E2020) improves cognition and function in patients with mild to moderately severe Alzheimer's disease. Results from phase III trials. American Psychiatric Association 150th Annual Meeting, San Diego 1997.
Schindler 2004 {published data only}
- Schindler R, Corey‐Bloom J, Doody R, Zhang R, Ieni JR, Li H. Donepezil is safe and well tolerated in Alzheimer's disease patients, at doses of up to 20 mg/day. 8th Congress of the European Federation of the Neurological Sciences. Paris, France. September 4‐7, 2004. 2004.
- Schindler R, Zhang R, Ieni JR, Li H. Donepezil is safe and well tolerated in Alzheimer's disease patients, at doses of up to 20 mg/day. Neurobiology of Aging 2004;25(S2):195. [Google Scholar]
Seltzer 2004 {published data only}
- Seltzer B, Zolnouni P, Nunez M, Goldman R, Kumar D, Ieni J, et al. Erratum: efficacy of donepezil in early‐stage alzheimer disease: a randomized placebo‐controlled trial (Archives of Neurology (December 2004) 61 (1852‐1856)). Archives of Neurology 2005;62(5):825. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Zolnouni P, Nunez M, Goldman R, Kumar D, Ieni J, et al. Donepezil 402 Study Group. Efficacy of donepezil in early‐stage Alzheimer disease. Archives of Neurolology 2004;61:1852‐6. [DOI] [PubMed] [Google Scholar]
Study 205 {published data only}
- Anon. No title. Eisai Inc.
Study 306 {published data only}
- Anon. No title. Eisai Inc.
Tariot 2001 {published data only}
- Steinman MA, Covinsky KE, Tariot PN, Cummings JL, Katz IR, Mintzer J, et al. Donepezil for nursing home patients with dementia: a reinterpretation of the evidence. Journal of the American Geriatrics Society 2003;51(1):132‐33. [DOI] [PubMed] [Google Scholar]
- Tariot P, Cummings JL, Katz IR, Perdomo CA, Whalen E, Sovel MA, et al. Donepezil was well‐tolerated and enhanced cognition in nursing home patients with Alzheimer's disease. Journal of the American Geriatrics Society 1999;47:S3. [PubMed] [Google Scholar]
- Tariot P, Perdomo CA, Whalen E, Sovel MA, Scham EM. Age is not a barrier to donepezil treatment of Alzheimer's disease in the long‐term care setting. International Psychogeriatrics 1999;11(Supplement 1):134. [Google Scholar]
- Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, et al. A randomised, double‐blind, placebo‐controlled study of the efficacy and safety of Donepezil in patients with Alzheimer's disease in the nursing home setting. Journal of the American Geriatrics Society 2001;49(12):1590‐9. [PubMed] [Google Scholar]
- Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, et al. A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. Journal of the American Geriatrics Society 2003;51(1):133‐4. [PubMed] [Google Scholar]
Tune 2003 {published data only}
- Tune L, Tiseo P, Hoffman J, Perdomo C, Votaw J, Rogers S, et al. PET in AD: Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer's disease: results of a 24‐week study. 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 February 23‐26 San Francisco. 2001.
- Tune L, Tiseo PJ, Ieni J, Perdomo C, Pratt RD, Votaw JR, et al. Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease: results of a 24‐week, double‐blind, placebo‐controlled study. American Journal of Geriatric Psychiatry 2003;11(2):169‐77. [PubMed] [Google Scholar]
- Tune LE, Tiseo PJ, Hoffman JM, Perdomo CA, Votow JR, Rogers SL, et al. Functional brain activity in Alzheimer's disease. 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐ June 4, Toronto. 1998:NR345. [MEDLINE: ]
Winblad 2001 {published data only}
- Engedal K, Soininen H, Verhey F, Waldemar G, Winblad B, Wimo A, et al. Donepezil improved or stabilized cognition over one year in patients with mild and moderate Alzheimer's disease. Journal of the European College of Neuropsychopharmacology 2000; Vol. 10, issue Suppl 3:S368. [MEDLINE: ]
- Mastey V, Wimo A, Winblad B, Haglund A, Jacobson L, Miceli R, et al. An economic evaluation of donepezil in mild to moderate Alzheimer's disease: results of a one year, double‐blind, randomized trial. Journal of the American Geriatrics Society 2001; Vol. 49, issue 4:S131.
- Mastey V, Wimo A, Winblad B, Haglund A, Jacobson L, Miceli R, et al. Donepezil reduces the time caregivers spend providing care: results of a one‐year, double‐blind, randomized trial in patients with mild to moderate Alzheimer's disease. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco 2001.
- Soininen H, Winblad B, Engedal K, Verhey F, Waldemar G, Wimo A, et al. Long term benefits of donepezil on ADLs in AD patients. Annual Scientific Meeting of the American Geriatric Society and the American Federation for Aging Research; 2000 May 17‐21, Nashville. 2000:171.
- Soininen H, Winblad B, Engedal K, Verhey F, Waldemar G, Wimo A, et al. Donepezil Nordic Study Group. Response to donepezil is not predicted by apolipoprotein E genotype and/or gender. World Alzheimer Congress; 2000 July 9‐13, Washington. 2000.
- Waldemar G, Winblad B, Engedal K, Soininen H, Donepezil Nordic Study Group et al. Benefits of donepezil on cognition, function and/or neuropsychiatric symptoms in patients with Alzheimers disease over one year. World Alzheimer Congress; 2000 July 9‐13, Washington DC. 2000.
- Waldemar G, Winblad B, Engedal K, Soininen HS, Verhey FR, Wimo A, et al. Donepezil Nordic Study Group. Donepezil benefits patients with either mild of moderate Alzheimer's disease over one year. Neurology 2000;54(Suppl 3):A470. [Google Scholar]
- Wimo A. Erratum: an economic evaluation of donepezil in mild to moderate Alzheimer's disease: results of a 1‐year, double‐blind, randomized trial (Dementia and Geriatric Cognitive Disorders (2003) 15 (44‐54)). Dementia and Geriatric Cognitive Disorders 2003; Vol. 16, issue 2:102. [DOI] [PubMed]
- Wimo A, Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, et al. Donepezil Nordic Study Group. An economic evaluation of donepezil in mild to moderate Alzheimer's disease: results of a 1‐year, double‐blind, randomized trial. Dementia and Geriatric Cognitive Disorders 2003;15(1):44‐54. [DOI] [PubMed] [Google Scholar]
- Wimo A, Winblad B, Mastey V. An economic evaluation of donepezil in mild to moderate Alzheimers disease: results of a one‐year, double‐blind, randomized trial. World Alzheimer Congress; 2000 July 9‐13, Washington DC. 2000. [DOI] [PubMed]
- Wimo A, Winblad B, Mastey V, Haglund A, Hertzman P, Miceli R, et al. An economic evaluation of donepezil in mild to moderate alzheimer's disease patients: results of a one‐year, double‐blind, randomized trial. 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18, Chicago, Illionois. 2000. [MEDLINE: ]
- Winblad B. Long term therapeutic benefits of acetylcholinesterase inhibitor therapy in patients with Alzheimer's disease. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 2000 April 5‐8, Stockholm. 2000:164.
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P. Donepezil enhances global function, cognition and activities of daily living compared with placebo in a one‐year, double‐blind trial in patients with mild to moderate Alzheimer's disease. International Psychogeriatrics 1999;11(Supplement 1):138. [Google Scholar]
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, et al. A 1‐year, randomized, placebo‐controlled study of donepezil in patients with mild to moderate AD. Neurology 2001;57(3):489‐95. [DOI] [PubMed] [Google Scholar]
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, et al. Donepezil enhances global function and activities of daily living compared with placebo in a one‐year, double‐blind trial in patients with mild to moderate Alzheimer's disease. Quality Research in Dementia Conference; 2000 Nov 19‐22, London. 2000.
- Winblad B, Wimo A, Engedal K, Soininen H, Verhey F, Waldemar G, et al. 3‐year study of donepezil therapy in Alzheimer's disease: effects of early and continuous therapy. Dementia and Geriatric Cognitive Disorders 2006;21(5‐6):353‐63. [DOI] [PubMed] [Google Scholar]
Winblad 2006 {published data only}
- Batsman S, Minthon L, Eeriksson S, Kilander L, Jansson‐Blixt C, Wetterholm A, et al. Study design and baseline patients characteristics in a randomized controlled trial of the efficacy and tolerability of donepezil in severe Alzheimer's disease. IPA 12th International Congress, Stockholm, Sweden, 20‐24 September 2005. 2005.
- Eriksson S, Winblad B, Kilander L, Batsman S, Jansson‐Blixt C, Wetterholm A, et al. Efficacy of donepezil on secondary end points in a randomized, double‐blind placebo‐controlled study in severe Alzheimer's disease. IPA 12th International Congress, Stockholm, Sweden, 20‐24 September 2005. 2005.
- Jelic V, Haglund A, Kowalski J, Langworth S, Winblad B. Donepezil treatment of severe Alzheimer's disease in nursing home settings. Dementia and Geriatric Cognitive Disorders 2008;26(5):458‐46. [DOI] [PubMed] [Google Scholar]
- Kilander L, Winblad B, Minthon L, Batsman S, Jansson‐Blixt C, Cronlund A, et al. Donepezil is well tolerated in patients with severe Alzheimer's disease. IPA 12th International Congress, Stockholm, Sweden, 20‐24 September 2005. 2005.
- Marder K. Donepezil in patients with severe Alzheimer's disease: double‐blind parallel‐group, placebo controlled study. Current Neurology and Neuroscience Reports 2006;6(5):364‐73. [DOI] [PubMed] [Google Scholar]
- Opie LH. Donepezil for severe Alzheimer's disease. Lancet 2006;368:361‐2. [DOI] [PubMed] [Google Scholar]
- Winblad B. Donepezil for severe Alzheimer's disease ‐ author's reply. Lancet 2006;368(9533):362. [DOI] [PubMed] [Google Scholar]
- Winblad B. Severe Alzheimer's disease: benefits of donepezil therapy. International Psychogeriatrics 2006;18(5):S25‐S31. [Google Scholar]
- Winblad B, Kilander L, Eriksson S, Minthon L, Båtsman S, Wetterholm A, et al. for the Severe Alzheimer’s Disease Study Group. Donepezil in patients with severe Alzheimer’s disease: double‐blind, parallel‐group, placebo‐controlled study. Lancet 2006;367(9156):1057‐65. [DOI] [PubMed] [Google Scholar]
- Winblad B, Minthon L, Eriksson S, Batsman S, Jansoon‐Blixt C, Wetterholm A, et al. Efficacy of donepezil on primary end points in a randomized, double‐blind placebo‐controlled study in severe Alzheimer's disease. IPA 12th International Congress, Stockholm, Sweden, 20‐24 September 2005. 2005.
- Winblad B, Minthon L, Eriksson S, Batsman S, Jansson‐Blixt C, Wetterholm AL, et al. Efficacy of donepezil on primary end points in a randomized, double‐blind placebo‐controlled study in severe Alzheimer's disease. International Psychogeriatrics 2005;18(S1):S25‐S31. [Google Scholar]
References to studies excluded from this review
Ames 2001 {published data only}
- Ames D, Boada M, Sakka P, Triau E, Turcani P, Vagenas V, et al. Efficacy and tolerability of donepezil in patients with Alzheimer's disease: findings from a large multinational experience study. 17th Alzheimer's Disease International Conference; 2001 October 25‐27, Christchurch, New Zealand. 2001:181.
AWARE {published data only}
- Johannsen P, Barcikowska M, Hasselbalch S, Ihl R, Karageorgiou C, Nunez M, et al. AWARE Study Group. Results from the pre‐randomization phase of the donepezil aware study further understanding the meaning of clinical benefit. 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, 2002 Apr 3‐6, Geneva. 2002:201.
- Johannsen P, Holub R, Jakab G, Jakobsen S, Kalisvaart CJ, Kozubski W, et al. Behavioral benefits with continued donepezil treatment in Alzheimer's disease patients. Neurobiology of Aging 2004;25(S2):20. [Google Scholar]
- Johannsen P, Salmon E, Hampel H, Xu Y, Richardson S, Qvitzau S, et al. AWARE Study Group. Assessing therapeutic efficacy in a progressive disease: a study of donepezil in Alzheimer's disease. CNS Drugs 2006;20(4):311‐25. [DOI] [PubMed] [Google Scholar]
- Johanssen O, Dautzenburg P, Heun R, Holub R, Jakab G, Kozubski W, et al. AWARE Study Group. Results from the pre‐randomization phase of the donepezil AWARE study: further understanding the meaning of "clinical benefit". 8th International Conference on Alzheimer's Disease and Related Disorders; 2002 July 20‐25, Stockholm, Sweden. 2002:Abstract No 305.
- Johanssen P, Hasselbalch S, Jakab G, Kalisvaart CJ, Kozubski W, Kurz A, et al. Behavioral benefits with continued donepezil treatment in Alzheimer's disease patients. 8th Congress of the European Federation of the Neurological Sciences. Paris, France. September 4‐7, 2004. 2004.
Barak 2001 {published data only}
- Barak Y, Bodner E, Zemishlani H, Mirecki I, Aizenberg D. Donepezil for the treatment of behavioral disturbances in Alzheimer's disease: a 6‐month open trial. Archives of Gerontology and Geriatrics 2001;33:237‐41. [DOI] [PubMed] [Google Scholar]
Berger 2000 {published data only}
- Berger E, Sramko CA, Frölich L, Calabrese P. Donepezil provides relevant therapeutic benefits in different domains to real world patients with Alzheimer's disease. The 12th ENCP Congress European Neuropsychopharmacology, London, 2000. 2000; Vol. 10, issue S4:369.
- Sramko CA, Berger F, Calabrese P, Frölich L. Tolerability and safety of donepezil in the treatment of Alzheimer's disease results from a post marketing surveillance study. The 12th ENCP Congress European Neuropsychopharmacology, London, 2000. 2000; Vol. 10, issue S4:368.
Birt 2002 {published data only}
- Birt AR, Fay S, Graham JE, Rockwood K. Recovery of intention as a novel effect on treating Alzheimer's disease with donepezil. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:595.
Borroni 2001 {published data only}
- Borroni B, Colciaghi F, Pastorino L, Pettenati C, Cottini E, Rozzini L, et al. Amyloid precursor protein in platelets of patients with Alzheimer disease: effect of acetylcholinesterase inhibitor treatment. Archives of Neurology 2001;58(3):442‐6. [DOI] [PubMed] [Google Scholar]
Brodaty 2000 {published data only}
- Boundy K, Brodaty H, Australian Donepezil Study Group, Barrington M, O'Leary M, Short K, et al. Efficacy of donepezil in patients with Alzheimer's disease: findings from the Australian subset of a large multinational experience study. Proceedings of the 17th Alzheimer's Disease International Conference; 2001 Oct 25‐27, Christchurch, New Zealand. 2001.
- Brodaty H, Bahara R, Zhang R, O'Leary M, Short K, Barrington M. Efficacy and safety of donepezil in patients with Alzheimer's disease preliminary findings from the Australian subset of a global clinical experience study. Journal of the European College of Neuropsychopharmacology 2000;10(Suppl 4):S367. [Google Scholar]
Bullock 2000 {published data only}
- Bullock RA, Voss SE. The clinical utility of donepezil: from randomized clinical trials to practice. World Alzheimer Congress; 2000 July 9‐13, Washington. 2000.
Bullock 2001 {published data only}
- Blesa R, Bullock R, He Y, Bergman H, Gambina G, Meyer J, et al. Effect of butyrylcholinesterase genotype on the response to rivastigmine or donepezil in younger patients with Alzheimer's disease. Pharmacogenetics and Genomics 2006;16(11):771‐4. [DOI] [PubMed] [Google Scholar]
- Bullock R, Bergman H, Touchon J, Gambina G, He Y, Nagel J, et al. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer's disease. Current Medical Research and Opinion 2006;22(3):483‐94. [DOI] [PubMed] [Google Scholar]
- Bullock R, Passmore F, Potocnik F, Hock C. The tolerability, ease of use and efficacy of donepezil and rivastigmine in Alzheimer's disease patients: a 12‐week, multinational, comparative study. Journal of the American Geriatrics Society 2001;49(4):S19. [Google Scholar]
- Bullock R, Wilkinson DG, Passmore P, Hopker SW, Smith R, Potocnik FC, et al. Caregiver and physician determination of satisfaction with and ease of use of donepezil and rivastigamine treatment in Alzheimer's disease patients. 17th Alzheimer's Disease International Conference; 2001 Oct 25‐27, Christchurch, New Zealand. 2001:39.
- Böttcher‐Buhler E. Well tolerated, effective and inexpensive therapy of Alzheimer's dementia with donepezil [Therapie der Alzheimer‐Demenz mit Donepezil: gut vertraglich, wirksam und kostengunstig]. Neurologie und Rehabilitation 2000;6(6):332‐3. [Google Scholar]
- Potocnik FC, Smith R, Passmore P, Hock C, Wilkinson D, Maud CM, et al. Tolerability, ease of use, and efficacy of donepezil and rivastigmine in Alzheimer's disease patients. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans. 2001.
- Touchon J, Bergman H, Bullock R, Rapatz G, Nagel J, Lane R. Response to rivastigmine or donepezil in Alzheimer's patients with symptoms suggestive of concomitant Lewy body pathology. Current Medical Research and Opinion 2006;22(1):49‐59. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Passmore P, Potocnik F, Maud C, Hock C. Donepezil compared to rivastigmine in Alzheimer's disease: similar efficacy but better tolerability and physician and caregiver satisfaction in a multinational randomized trial. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco 2001.
Cameron 2000 {published data only}
- Cameron I, Curran S, Newton P, Petty D, Wattis J. Use of donepezil for the treatment of mild‐moderate Alzheimer's disease: an audit of the assessment and treatment of patients in routine clinical practice. International Journal of Geriatric Psychiatry 2000;15(10):887‐91. [DOI] [PubMed] [Google Scholar]
Clary 2000 {published data only}
- Clary C, McRae T, Griesing T, Whalen E. The safety of donepezil and sertraline for the management of behavioral symptoms in patients with Alzheimer's disease. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S267. [MEDLINE: ] [Google Scholar]
- Finkel S, McRae T, Burt T. Sertraline and donepezil demonstrate greater efficacy and similar tolerability compared to donepezil alone in non‐depressed patients with Alzheimer's disease. 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 February 23‐26, San Francisco. 2001.
- McRae T, Griesing T, Whalen E. Donepezil and sertraline for the management of behavioral symptoms in patients with Alzheimer's disease. Neurology 2000;54(Suppl 3):A416‐7. [Google Scholar]
- McRae T, Griesing T, Whalen E. Effectiveness of donepezil on behavioural disturbances in mild to moderate Alzheimer's disease patients. World Alzheimer Congress; 2000 July 9‐13, Washington. 2000.
- McRae T, Griesling T, Whalen E. Managing behaviour symptoms in patients with Alzheimer's disease (AD). Annual Scientific Meeting of the American Geriatric Society and the American Federation for Aging Research; 2000 May 17‐21, Nashville. 2000:173.
Cumbo 2011 {published data only}
- Cumbo E. Improvement in behavioral and psychiatric symptoms (BPSD) in patients with moderate‐to‐severe Alzheimer's disease by current antidementia treatments. Alzheimer's Association International Conference, Paris, France. 2011.
Cummings 2000 {published data only}
- Cummings JL, Donohue JA, Brooks RL. The relationship between donepezil and behavioral disturbances in patients with Alzheimer's disease. American Journal of Geriatric Psychiatry 2000;8(2):134‐40. [PubMed] [Google Scholar]
DOMINO‐AD {published data only}
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer's Disease (DOMINO‐AD) trial: secondary and post‐hoc analyses. Lancet Neurology 2015; Vol. 14, issue 12:1171‐81. [DOI] [PubMed]
- Knapp M, King D, Romeo R, Adams J, Baldwin A, Ballard C, et al. Cost‐effectiveness of donepezil and memantine in moderate to severe Alzheimer's disease (the DOMINO‐AD trial). International Journal of Geriatric Psychiatry 2016;‐:No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dong 2011 {published data only}
- Dong GS, Li X, Jiang QH, Yang HQ. Effects of donepezil treatment on platelets alpha and beta secretase activities in Alzheimer's disease patients. Chinese Medical Journal 2011;91(47):3341‐5. [PubMed] [Google Scholar]
Fillit 2002 {published data only}
- Fillit H, Hill JW, Futtertman R, Mastery V. Sustained donepezil therapy reduces healthcare costs in Alzheimer's disease. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:192.
Froelich 2000 {published data only}
- Froelich L, Gertz HJ, Heun R, Heuser I, Jendroska K, Kornhuber J, et al. Donepezil for Alzheimer's disease ‐ the Donald Study ‐ a multicenter 24 weeks clinical trial in Germany. Journal of the European College of Neuropsychopharmacology 2000;10(Suppl 3):S360. [MEDLINE: ] [Google Scholar]
Fuschillo 2001 {published data only}
- Fuschillo C, Pia S, Campana F, Pinto A, Simone L. Cognitive deficits in Alzheimer's disease: treatment with acetylcholinesterase inhibitor agents. Archives of Gerontology and Geriatrics 2001;33(Suppl 1):151‐8. [DOI] [PubMed] [Google Scholar]
Geldmacher 2003 {published data only}
- Finucane TE. Another advertisement for donepezil. Comments to the editor; reply Geldmacher. Journal of the American Geriatrics Society 2004;52(5):843‐6. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS, Provenzano G, McRae T, Mastey V, Ieni JR. Donepezil is associated with delayed nursing home placement in patients with Alzheimer's disease. Journal of the American Geriatrics Society 2003;51(7):937‐44. [DOI] [PubMed] [Google Scholar]
- Karlawish JH. Donepezil delay to nursing home placement study is flawed. Journal of the American Geriatrics Society 2004;52(5):845; author reply 845‐6. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Qizilbash N. Delay in nursing home placement with donepezil. Journal of the American Geriatrics Society 2004;52(6):1024‐6; author reply 1026‐7. [DOI] [PubMed] [Google Scholar]
Ghorbani 2010 {published data only}
- Ghorbani A, Chitsaz A, Shishegar M, Akbari M. Evaluation of the effect of donepezil on cerebral blood flow velocity in Alzheimer's disease. Neurosciences (Riyadh, Saudi Arabia) 2010; Vol. 15, issue 3:172‐6. [PubMed]
Greenberg 2000 {published data only}
- Greenberg SM, Tennis MK, Brown LB, Gomez‐Isla T, Hayden DL, Schoenfeld‐DA, et al. Donepezil therapy in clinical practice: a randomized crossover study. Archives of Neurology 2000;57:94‐9. [DOI] [PubMed] [Google Scholar]
Hampel 2002 {published data only}
- Hampel H, Berger F, Froelich L. Switching from other antidementive therapies to donepezil (Aricept): improvement of quality of life of Alzheimer patients in routine clinical use. The International Symposium on advances in Alzheimer therapy, 2002, Geneva. 2002:198.
Holmes 2004 {published data only}
- Hepple J. A study of the effects of donepezil on non‐cognitive symptoms in patients with Alzheimer's disease (AD) and the clinical characteristics of responders. National Research Register 2003.
- Holmes C. Non‐cognitive symptoms and response to donepezil. National Research Register 2000.
- Holmes C, Wilkinson D, Dean C, Vethanayagam S, Olivieri S, Langley A, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology 2004;63(2):214‐9. [DOI] [PubMed] [Google Scholar]
- Pandita‐Gunawardena D. Non‐cognitive symptoms and response to donepezil. National Research Register 2001.
Homma 1998a {published data only}
- Homma A, Imai Y, Hariguchi S, Hasegawa K, Kameyama M, Nishimura T. Late phase II clinical study of acetylcholinesterase inhibitor E2020 in patients with Alzheimer‐type dementia. Clinical Evaluation 1998;26:185‐207. [Google Scholar]
Homma 1998b {published data only}
- Homma A, Imai Y, Hariguchi S, Hasegawa K, Kameyama M, Nishimura T. Late phase II clinical study of acetylcholinesterase inhibitor E2020 in patients with Alzheimer‐type dementia. Clinical Evaluation 1998;26:209‐31. [Google Scholar]
Imai 1998a {published data only}
- Imai Y, Homma A, Hariguchi S, Hasegawa K, Kameyama M, Nishimura T. Early phase II clinical study of acetylcholinesterase inhibitor E2020 in patients with Alzheimer‐type dementia. Clinical Evaluation 1998;26:145‐64. [Google Scholar]
Imai 1998b {published data only}
- Imai Y, Homma A, Hariguchi S, Hasegawa K. Early phase II clinical study of acetylcholinesterase inhibitor E2020 in patients with Alzheimer‐type dementia. Clinical Evaluation 1998;26:165‐83. [Google Scholar]
Imai 1998c {published data only}
- Imai Y, Homma A, Hariguchi S, Hasegawa K, Kameyama M, Nishimura T. Late phase II clinical study of acetylcholinesterase inhibitor E2020 in patients with Alzheimer‐type dementia. Clinical Evaluation 1998;26:233‐50. [Google Scholar]
Janssen 2005 b {published data only}
Kauffer 1998 {published data only}
- Kauffer D, Catt K, Pollock B, DeKosky S. Assessing the effects of donepezil in Alzheimer's patients and its impact on caregivers. Journal of the American Geriatrics Society 1998; Vol. 46:S66.
Kemp 2003 {published data only}
- Kemp PM, Holmes C, Hoffmann S, Wilkinson S, Zivanovic M, Thom J, et al. A randomised placebo controlled study to assess the effects of cholinergic treatment on muscarinic receptors in Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry 2003;74(11):1567‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leube 2002 {published data only}
- Leube D, Grodd W, Erb M, Henning W, Bartels M, Kircher T. Task related cortical areas are differentially activated in patients with Alzheimer's disease after a ten week treatment with donezepil. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:1548.
Lopez 2008 {published data only}
- Lopez OL, Mackell JA, Sun Y, Kassalow LM, Xu Y, McRae T, et al. Effectiveness and safety of donepezil in Hispanic patients with Alzheimer's disease: a 12‐week open‐label study. Journal of the National Medical Association 2008;100(11):1350‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maltz 2002 {published data only}
- Maltz J, Eberling J, Jagust W, Budinger T. Donepezil therapy enhances methacholine induced cutaneous vasodilation in Alzheimer's disease patients. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:318.
Matthews 2000 {published data only}
McRae 1999 {published data only}
- McRae T, Orazem J. A large, community‐based trial of donepezil in the treatment of Alzheimer's disease (AD). Journal of the American Geriatrics Society 1999; Vol. 47:S63.
McRae 2001a {published data only}
- McRae T, Knopman D, Duttagupta S, Ieni J, Provenzano G. Donepezil delays time to nursing home placement in patients with Alzheimer's disease. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 February 23‐26, San Francisco 2001a.
- McRae T, Knopman D, Mastey V, Ieni J, Provenzano G. Donepezil is strongly associated with delayed nursing home placement in patients with Alzheimer's disease. Journal of Neuroscience 2001; Vol. 187, issue Suppl 1:S536.
Mega 1999 {published data only}
Mega 2001 {published data only}
- Mega MS, Manese M, Felix J, Tran N, O'Connor SM, Masterman DM, et al. Laboratory of Neuroimaging and Alzheimer's disease. Anterior cingulate activation occurs across cholinesterase inhibitor therapy in Alzheimer's disease. 10th congress of the international psychogeriatric association, Nice, France, September 9‐14, 2001 2001; Vol. 13, issue Suppl 2:S108.
Mega 2002 {published data only}
- Mega M, Dinov I, Manese M, Felix J, O'Connor S, Toga A, et al. Cerebral metabolic activation with cholinesterase inhibitor therapy in Alzheimer's disease. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:430.
Modrego 2010 {published data only}
- Modrego PJ, Fayed N, Errea JM, Rios C, Pina MA, Sarasa M. Memantine versus donepezil in mild to moderate Alzheimer's disease: a randomized trial with magnetic resonance spectroscopy. European Journal of Neurology 2010;17(3):405‐12. [DOI] [PubMed] [Google Scholar]
NCT00423228‐BRAINz {published data only}
- NCT00423228‐BRAINz. A randomised, double‐blind, double‐dummy, oral donepezil controlled study on the safety and efficacy of repeated monthly subcutaneous injections of a sustained‐release implant of ZT 1 in patients with moderate Alzheimer's disease. ClinicalTrials.gov 2007.
Nikolova 2001 {published data only}
- Nikolova G, Traykov L. Efficacy of donepezil in patients with Alzheimer's disease ‐ results of 12‐week open clinical trial. Acta Medica Bulgarica 2001; Vol. 28:70‐5.
Nobili 2002 {published data only}
- Nobili F, Vitali P, Canfora M, Girtler N, Leo C, Mariani G, et al. Effects of long‐term donepezil therapy on rCBF of Alzheimer's patients. Clinical Neurophysiology 2002;113(8):1241‐8. [DOI] [PubMed] [Google Scholar]
Ollat 2007 {published data only}
- Ollat H, Laurent B, Bakchine S, Michel BF, Touchon J, Dubois B. Effects of the association of sulbutiamine with an acetylcholinesterase inhibitor in early stage and moderate Alzheimer disease [Effets de l'association de la sulbutiamine à un inhibiteur de l'acétylcholinestérase dans les formes légères à modérées de la maladie d'Alzheimer]. L'Encéphale 2007;33(2):211‐5. [DOI] [PubMed] [Google Scholar]
Onofrj 2002 {published data only}
- Onofrj M, Thomas A, Luciano AL, Iacono D, Rollo A, D'Andreamatteo G, et al. Donepezil versus vitamin E in Alzheimer's disease: part 2: mild versus moderate‐severe Alzheimer's disease. Clinical Neuropharmacology 2002;25(4):207‐15. [DOI] [PubMed] [Google Scholar]
Onofrj 2003 {published data only}
- Onofrj M, Thomas A, Iacono D, Luciano AL, Iorio A. The effects of a cholinesterase inhibitor are prominent in patients with fluctuating cognition: a part 3 study of the main mechanism of cholinesterase inhibitors in dementia. Clinical Neuropharmacology 2003;26(5):239‐51. [DOI] [PubMed] [Google Scholar]
Parsa 2000 {published data only}
- Parsa MA, Poggi E, Barte L. Treatment of dementia patients with psychotic and behavioural symptoms with quetiapine and donepezil. Journal of the European College of Neuropsychopharmacology 2000; Vol. 10, issue Suppl 3:S302. [MEDLINE: ]
Peng 2002 {published data only}
- Peng D, Xu X, Hou Q. The safety and efficacy of Aricept in patients with Alzheimer disease. Chinese Journal of Neurology 2002; Vol. 35, issue 1:19‐21.
Peng 2005 {published data only}
- Peng DT, Xu XH, Wang LN. Efficiency and safety assessment of donepezil for treating mild and moderate Alzheimer disease. Chinese Journal of Clinical Rehabilitation 2005;9(13):170‐2. [Google Scholar]
Requena 2006 {published data only}
- Requena C, Maestu F, Campo P, Fernandez A, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia: 2‐year follow‐up. Dementia and Geriatric Cognitive Disorders 2006;22(4):339‐45. [DOI] [PubMed] [Google Scholar]
Richarz 2011 {published data only}
- Richarz U, Gaudig M, Schaeuble B, Zhang Z. Cognitive outcomes of patients with Alzheimer's disease treated with galantamine or donepezil: a randomized, double‐blind study. European Journal of Neurology 15th Congress of the EFNS, Budapest, Hungary. 2011.
Rocca 2002 {published data only}
Rockwood 2002 {published data only}
- Rockwood K, Fay S, Gorman M, Carver D, Graham JE. The Clinical Meaningfulness of Adas‐Cog Changes in Alzheimer's Disease Patients Treated With Donepezil in an Open‐Label Trial. BMC Neurology 2007;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Graham J, Fay S. Translating from regulatory measures to patients' daily lives an analysis of Alzheimer's disease treatment with donepezil. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:2034.
Rockwood 2007 {published data only}
- Rockwood K, Black S, Bedard MA, Tran T, Lussier I, TOPS Study Investigators. Specific symptomatic changes following donepezil treatment of Alzheimer's disease: a multi‐centre, primary care, open‐label study. International Journal of Geriatric Psychiatry 2007;22(4):312‐19. [DOI] [PubMed] [Google Scholar]
Rodriguez 2002 {published data only}
- Rodriguez G, Vitali P, Leo C, Cadi F, Girtler N, Nobili F. Quantitative EEG changes in Alzheimer patients during long‐term donepezil therapy. International Symposium on advances in Alzheimer therapy, 2002, Geneva 2002:239.
Rogers 1997 {published data only}
- Rogers SL, Friedhoff LT. Donepezil is well tolerated at clinically effective doses for the treatment of Alzheimer's disease (AD). Proceedings of the 10th European College of Neuropsychopharmacology Congress; 1997 September 13‐17, Vienna, Austria 1997. [MEDLINE: ]
Rogers 1997b {published data only}
- Friedhoff LT, Jeni R, Rogers SL, Pratt RD. Donepezil provides long term benefits for patients with Alzheimer's disease. International Journal of Psychopharmacology 1999;2(Suppl 1):5175(PW11017). [Google Scholar]
- Rogers SL, Friedhoff LT. Donepezil provides long‐term clinical benefits for patients with Alzheimer's disease (AD). International Journal of Neurological Sciences 1997;150:296. [Google Scholar]
- Rogers SL, Friedhoff LT. Long‐term efficacy and safety of donepezil in the treatment of Alzheimer's disease: an interim analysis of the results of a US multicentre open label extension study. European Neuropsychopharmacology 1998;8:67‐75. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Perdomo C, Friedhoff LT. Clinical benefits are maintained during long‐term treatment of Alzheimer's disease with the acetylcholinesterase inhibitor E2020. European Journal of Neuropsychopharmacology 1995;5(3):386. [Google Scholar]
Rozzini 2002 {published data only}
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzani S, Leonardi R, et al. Acetylcholinesterase inhibitors are effective in real world patients with mild to moderate Alzheimer disease evidence from a large population treated with rivastigmine or donepezil. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:329.
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzoni S, Leonardi R, et al. Comparison of efficacy and safety of rivastigmine and donepezil in patients with mild to moderate Alzheimer disease: results from a multicentre randomised trial. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:240.
Rozzini 2007a {published data only}
- Rozzini L, Chilovi BV, Bertoletti E, Ghianda D, Conti M, Trabucchi M, et al. Serum albumin level interferes with the effect of donepezil in Alzheimer's disease. Aging Clinical Experimental Research 2008;20(6):509‐12. [DOI] [PubMed] [Google Scholar]
Rozzini 2007b {published data only}
- Rozzini L, Chilovi BV, Bertoletti E, Trabucchi M, Padovani, A. Acetylcholinesterase inhibitors and depressive symptoms in patients with mild to moderate Alzheimer's disease. Clinical and Experimental Research 2007;19(3):220‐3. [DOI] [PubMed] [Google Scholar]
Salloway 2002 {published data only}
- Salloway S. A double blind randomized pilot study to evaluate the effects of galantamine and donepezil on sleep and attention and gastrointestinal GI tolerance in patients with mild to moderate Alzheimer's disease AD [Effects on sleep and attention of two currently marketed drugs for Alzheimer's disease]. Clinical Trials.gov 2002:1‐2.
Sampson 2007 {published data only}
- Sampson EL, Raven PR, Ndhlovu PN, Vallance A, Garlick N, Watts J, et al. A randomized, double‐blind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. International Journal of Geriatric Psychiatry 2007;22(4):343‐9. [DOI] [PubMed] [Google Scholar]
Saumier 2007 {published data only}
- Saumier D, Murtha S, Bergman H, Phillips N, Whitehead V, Chertkow H. Cognitive predictors of donepezil therapy response in Alzheimer disease. Dementia and Geriatric Cognitive Disorders 2007;24(1):28‐35. [DOI] [PubMed] [Google Scholar]
Shua‐Haim 2002a {published data only}
- Shua‐Haim J, Smith J, Amin S, Shua‐Haim V. Comparison of combination therapy with rivastigmine Exelon and donepezil Aricept versus rivastigmine alone for treatment of Alzheimer's disease safety tolerability and clinical experience after one year of treatment a cross section study. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:292.
Shua‐Haim 2002b {published data only}
- Shua‐Haim J, Smith J, Potel S. A head to study of donepezil Aricept rivastigmine Exlon and galantamine Reminyl for the treatment of Alzheimer's disease safety tolerability clinical and caregiver impression after 4‐5 months of treatment a prospective study. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:286.
Stewart 1998 {published data only}
Tarraga 2006 {published data only}
- Tarraga L, Boada M, Modinos G, Espinosa A, Diego S, Morera A, et al. A randomised pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry 2006;77(10):1116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teipel 2006 {published data only}
- Teipel SJ, Drzezga A, Bartenstein P, Moller HJ, Schwaiger M, Hampel H. Effects of donepezil on cortical metabolic response to activation during (18)FDG‐PET in Alzheimer's disease: a double‐blind cross‐over trial. Psychopharmacology 2006;187(1):86‐94. [DOI] [PubMed] [Google Scholar]
Tessitore 2000 {published data only}
- Tessitore A, Iavarone A, Tessitore A. Donepezil in the treatment of mild to moderate Alzheimer's disease: follow‐up at 12 months in 40 treated patients. Nuova Rivista di Neurologia 2000; Vol. 10, issue 5:183‐6.
Tettamanti 2000 {published data only}
- Tettamanti M, Casilli D, Baldinetti F, Apollonio I, Ruffo P, Nobili A, et al. Donepezil Italian Global Impact Study (DIGIS). Proceedings of the World Alzheimer Congress; 2000 July 9‐13, Washington DC 2000.
Thal 2004 {published data only}
- Galasko DR, Gauthier S, Bennett D, Sano M, Kaye J, Marson D, et al. Impairment of activities of daily living in patients with amnestic mild cognitive impairment in an ADCS randomized clinical trial. 57th Annual Meeting of the American Academy of Neurology, Miami Beach, April 2005. 2005:S15.001.
- Petersen R, Grundman R, Thomas R, Thal L. Donepezil and vitamin E as treatments for mild cognitive impairment. Neurobiology of Aging 2004;25(S2):20. [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine 2005;352(23):2379‐88. [DOI] [PubMed] [Google Scholar]
Thomas 2001 {published data only}
- Thomas A, Iacono D, Bonanni L, D' Andreamatteo G, Onofrj M. Donepezil, rivastigmine,and vitamin E in Alzheimer disease: a combined P300 event‐related potentials/neuropsychologic evaluation over 6 months. Clinical Neuropharmacology. US: Lippincott Williams and Wilkins Inc., 2001; Vol. 24, issue 1:31‐42. [DOI] [PubMed]
Touchon 2006 {published data only}
- Touchon J, Bergman H, Bullock R, Rapatz G, Nagel J, Lane R. Response to rivastigmine or donepezil in Alzheimer's patients with symptoms suggestive of concomitant Lewy body pathology. Current Medical Research and Opinion 2006;22(1):49‐59. [PUBMED: 16393430] [DOI] [PubMed] [Google Scholar]
Tsolaki 2002 {published data only}
- Tsolaki M, Gerothanassis D, Aristotle CP. Efficacy and safety of cholinesterase inhibitors a longitudinal comparative study between donepezil and rivastigmine. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:2038.
Vanmechelen 2002 {published data only}
- Vanmechelen E, Andreasen N, Minthon L, Davidsson P, Amici S, Gallai V, et al. Effects of cholinesterase inhibitors on alzheimer disease biomarkers. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, 2002 Apr 3‐6, Geneva 2002:252.
Wattmo 2008 {published data only}
- Wattmo C, Hansson O, Wallin AK, Londos E, Minthon, L. Predicting long‐term cognitive outcome with new regression models in donepezil‐treated Alzheimer patients in a naturalistic setting.. Dementia and Geriatric Cognitive Disorders 2008;26(3):203‐11. [DOI] [PubMed] [Google Scholar]
Weiner 2000 {published data only}
Werber 2002 {published data only}
- Werber EA, Klein C, Rabey MJ. Evaluation of cholinergic treatment in demented by p300 evoked related potentials. 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden 2002:442.
Wilcock 2003 {published data only}
- Haworth J. Comparison of Aricept and galantamine (Reminyl) in Alzheimer's disease. National Research Register 2000.
- O'Brien A. A pilot study comparing the effect of galantamine (Reminyl) with donepezil in patients with Alzheimer's Disease. National Research Register 2000.
- Wilcock G, Howe I, Coles H, Lilienfeld S, Truyen L, Young Z, et al. and members of the GAL‐GBR‐2 Study Group. A long‐term comparison of galantamine and donepezil in the treatment of Alzheimer's disease. Drugs & Aging 2003;20(10):777‐89. [DOI] [PubMed] [Google Scholar]
Winstein 2007 {published data only}
- Winstein CJ, Bentzen KR, Boyd L, Schneider LS. Does the cholinesterase inhibitor, donepezil, benefit both declarative and non‐declarative processes in mild to moderate Alzheimer's disease?. Current Alzheimer Research 2007;4(3):273‐76. [DOI] [PubMed] [Google Scholar]
Wyeth 2005 {published data only}
- Wyeth Research. A 3‐month, randomized, double‐blind, placebo‐controlled, multicenter, safety, tolerability, and efficacy study of 3 doses of lecozotan (SRA‐333) SR in outpatients with mild to moderate Alzheimer's disease with donepezil as active control. ClinicalTrials.gov 2005.
Zhang 2012 {published data only}
- Zhang Z, Yu L, Gaudig M, Schauble B, Richarz U. Galantamine versus donepezil in Chinese patients with Alzheimer's disease: results from a randomized, double‐blind study. Neuropsychiatric Disease and Treatment 2012; Vol. 8:571‐7. [DOI] [PMC free article] [PubMed]
Additional references
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington DC: APA, 1987:American Psychiatric Association. [Google Scholar]
Berg 1988
- Berg L. Clinical Dementia Rating (CDR). Psychopharm Bull 1988;24:637‐9. [PubMed] [Google Scholar]
Blau 1977
- Blau TH. Quality of life, social indicators and criteria of change. Professional Psychology 1977;8:464‐73. [Google Scholar]
Bucks 1996
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age and Ageing 1996;25:113‐20. [DOI] [PubMed] [Google Scholar]
Chertkow 2013
- Chertkow H, Feldman HF, Jacova C, Massoud F. Definitions of dementia and predementia states in Alzheimer's disease and vascular cognitive impairment: consensus from the Canadian conference on diagnosis of dementia. Alzheimer's Research & Therapy 2013;5(Supplement 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Mansfield 1987
- Cohen‐Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursinghome. Journal of Gerontology 1989;44:M77‐M84. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenburg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐13. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf ofthe Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DeJong 1989
- DeJong R, Osterlund OW, Roy GW. Measurement of quality‐of‐life changes in patients with Alzheimer's disease. Clinical Therapeutics 1989;11(4):545‐54. [PubMed] [Google Scholar]
ECDEU 1976
- CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Publication ADM 76‐338. Rockville: US Dept of Health, Education and Welfare, 1976.
Folstein 1975
- Folstein NF, Folstein SE, McHugh PR. Mini‐Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Galasko 2000
- Galasko D, Schmitt FA, Jin S. Detailed assessment of cognition and activities of daily living in moderate to severe Alzheimer's disease. Neurobiology of Aging 2000;21(Suppl 1):S168. [DOI] [PubMed] [Google Scholar]
Goldberg 1988
- Goldberg DP, Williams P. A User's Guide to General Health Questionnaire. Windsor: NFER‐Nelson, 1988. [Google Scholar]
Gottfries 1982
- Gottfries CG, Brane G, Gullberg B, Steen G. A new rating scale for dementia syndromes. Archives of Gerontology and Geriatrics 1982;1:311‐30. [DOI] [PubMed] [Google Scholar]
Gélinas 1999
- Gélinas I, Gauthier L, McIntyre M. Development of a functional measure for persons with Alzheimer's disease: the Disability Assessment for Dementia. American Journal of Occupational Therapy 1999;53:471‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Homma 1991
- Homma A, Niina R, Ishii T, Hasegawa K. Development of a new rating scale for dementia in the elderly: Mental Function Impairment Scale (MENFIS). Journal of Geriatric Psychiatry 1991;2:1217‐22. [Google Scholar]
ICD‐10
- World Health Organization. The ICD‐10 classification of mental and behavioural disorders: clinical description and diagnostic guidelines. Geneva: World Health Organisation, Division of Mental Health, 1992. [Google Scholar]
Lawton 1969
- Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9:179‐86. [PubMed] [Google Scholar]
Lee 2015
- Lee JH, Jeong SK, Kim BC, Park KW, Dash A. Donepezil across the spectrum of Alzheimer's disease: dose optimization and clinical relevance.. Acta Neurologica Scandinavica 2015;131(5):259‐67. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;4:939‐44. [DOI] [PubMed] [Google Scholar]
Overall 1992
- Overall JE, Schaltenbrand R. The SKT neuropsychological test battery. Journal of Geriatric Psychiatry and Neurology 1992;5:220‐7. [DOI] [PubMed] [Google Scholar]
Panisset 1994
- Panisset M, Roudier M, Saxton J, Boller F. Severe Impairment Battery: a neurological test for severely demented patients. Archives of Neurology 1994;51:41‐5. [DOI] [PubMed] [Google Scholar]
Reisberg 1987
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. Journal of Clinical Psychiatry 1987;Suppl 48:9‐17. [PubMed] [Google Scholar]
Ritchie 2017
- Ritchie CW, Russ TC, Banerjee S, Barber B, Boaden A, Fox NC, Holmes C, Isaacs JD, Leroi I, Lovestone S, Norton M, O'Brien J, Pearson J, Perry R, Pickett J, Waldman AD, Wong WL, Rossor MN, Burns A. The Edinburgh Consensus: preparing for the advent of disease‐modifying therapies for Alzheimer's disease.. Alzheimers Research & Therapy 2017;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis K. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:1356‐64. [DOI] [PubMed] [Google Scholar]
Ryan 2015
- Ryan NS, Rossor MN, Fox NC. Alzheimer's disease in the 100 years since Alzheimer's death.. Brain 2015;138(Pt 12):3816‐21. [DOI] [PubMed] [Google Scholar]
Schneider 1997
- Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study ‐ Clinical Global Impression of Change. Alzheimer Disease and Associated Disorders 1997;11(Suppl 2):S22‐32. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R,Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Tan 2014
- Tan C‐C, Yu J‐T, Wang H‐F, Tan M‐S, Meng X‐F, Wang C, et al. Efficacy and safety of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease: a systematic review and meta‐analysis. Journal of Alzheimer's Disease 2014;41:615‐31. [DOI: 10.3233/JAD-132690] [DOI] [PubMed] [Google Scholar]
Wimo 1998
- Wimo A, Wetterholm AL, Mastey V, Winblad B. Evaluation of the healthcare resource utilization and caregiver time in anti‐dementia drug trials. In: Wimo A, Jönsson B, Karlsson G, Winblad B editor(s). Health Economics in Dementia. Chichester: Wiley, 1998:465‐77. [Google Scholar]


