Abstract
Our aim is to determine indicators of survival in children with severe hypoxic respiratory failure (HRF) after transition to high-frequency oscillatory ventilation (HFOV). Single-center retrospective examination of children with HRF transitioned to HFOV. Blood gases and ventilator settings 24 hours prior to and 48 hours after HFOV in survivors and nonsurvivors were evaluated. Sixty-two children with mean age of 7 years and mean weight of 26 kg were included with an observed mortality of 29%. Mean airway pressures (Paw), oxygenation index (OI), arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) (P/F) ratio, pH, bicarbonate, and arterial carbon dioxide partial pressure were similar prior to HFOV in survivors and nonsurvivors. During HFOV, mean OI and P/F ratio improved in both groups with an average Paw increase of ∼10 cm H2O. Survivors had lower OI than nonsurvivors (21 ± 0.9 vs. 26.5 ± 2.2; p < 0.01) beginning 24 hours after HFOV. P/F ratio appears to diverge by 36 hours, with survivors having P/F ratio >200. Survivors had higher pH than nonsurvivors at 36 hours (7.40 ± 0.01 vs. 7.32 ± 0.02; p < 0.05), higher bicarbonate levels (27.1 ± 0.7 vs. 23.9 ± 1.3 mEq/L), and similar arterial carbon dioxide partial pressure with less oscillatory support (i.e., hertz and amplitude). Inhaled nitric oxide was used in 53% of patients with improvements in oxygenation but with no effect on mortality. HFOV improves oxygenation in children with severe HRF. Nonsurvivors can be distinguished from survivors at 24 to 36 hours during HFOV by higher OI, metabolic acidosis, and higher oscillatory support. These data may assist in prognostication or timing of initiating alternative therapies, such as extracorporeal membrane oxygenation.
Keywords: high-frequency oscillatory ventilation, mechanical ventilation, pediatric critical care, hypoxic respiratory failure, oxygenation index, nitric oxide, acute respiratory distress syndrome
Introduction
The ideal strategy to manage pediatric patients, including children, with severe hypoxic respiratory failure (HRF) is still unsettled and subject to considerable debate.1 2 In 2000, the acute respiratory distress syndrome (ARDS) network published that mortality in adults with ARDS managed with conventional mechanical ventilation (CMV) could be reduced by 20% using a low tidal volume (6 mL/kg) and low-inspiratory plateau pressure (<30 cm H2O) compared with a higher tidal volume (12 mL/kg).3 The concept of reducing iatrogenic injury in patients with severe lung disease has led to the development of many ventilatory strategies to reduce volutrauma, atelectrauma, biotrauma, and hyperoxia-associated injury, collectively referred to as ventilator-associated lung injury.4
As an alternative approach to CMV, high-frequency oscillatory ventilation (HFOV) has the theoretical ability to minimize ventilator-associated lung injury,1 but data supporting outcome benefits have varied according to the patient population and operator's familiarity with the technology. Trials in pre- and near-term neonates with respiratory failure (including respiratory distress syndrome) have either shown benefit or equivalency of HFOV versus CMV.5 6 In adults, HFOV is safe as a rescue therapy in ARDS and may improve oxygenation,7 yet a recent multinational clinical trial in adults with ARDS randomized early to receive HFOV or CMV found a higher mortality in the HFOV-managed arm.8 Studies in children have not found outcome benefits of HFOV over CMV in those with HRF or ARDS, but these studies are few and underpowered.9 10 Perhaps because of familiarity with the approach from the neonatal experience, HFOV has been adopted as a rescue therapy in many pediatric intensive care units (PICU) for children who have failed CMV.11 12
Historically, survival rates in children with HRF managed with HFOV vary from 40 to 80%.13 14 15 16 To date, there is no universally accepted method to predict who may benefit from and survive this therapy, which could assist in deploying additional treatments in the attempt to improve outcomes. Extracorporeal membrane oxygenation (ECMO) is increasingly used for children with severe HRF, most frequently in those who can no longer be supported by CMV using noninjurious settings. Mortality rates for children with severe HRF, including ARDS, treated with ECMO range from 33 to 48%. This is considerably lower than the expected mortality without ECMO. This treatment modality has traditionally been used as a salvage therapy for children likely to die on CMV.17
Our center has initiated HFOV in children with sustained need of high ventilator settings during CMV (peak inspiratory pressure consistently >40 cm H2O). Although ECMO capable, in the past we have infrequently employed ECMO for severe “HFOV-resistant” HRF. We hypothesized that, by evaluating our experience, we could identify indicators that could predict outcomes in children with HRF following transition from CMV to HFOV and help make informed decisions to deploy more invasive modalities, such as ECMO, sooner.
Materials and Methods
This is a single-center retrospective review of children with HRF treated with HFOV admitted to the PICU at Riley Hospital for Children between January 2008 and August 2011. The Indiana University Institutional Review Board approved this study with waiver of informed consent. Our unit is a 36-bed tertiary care, combined medical and surgical PICU staffed by pediatric intensivists. All patients older than 1 week of age and were managed with HFOV were included in the study. Patients treated with HFOV for shorter than 12 hours or those with cyanotic heart disease, death within 24 hours of admission, and lack of an arterial catheter were criteria for exclusion. Transition to HFOV and management of children on HFOV were in accordance with our hospital respiratory care policy. Children were switched to HFOV if peak inspiratory pressure on CMV was consistently more than 40 cm H2O. Decision to switch from CMV to HFOV and inhaled nitric oxide (iNO) use were at the discretion of the care team. Generally, saturations of 86 to 90% were accepted to keep fraction of inspired oxygen (FiO2) less than 0.6. Children were switched back from HFOV to CMV when airway pressure (Paw) is less than 22 and FiO2 is less than 0.5.
Data Collection
Demographic data included age, weight, gender, primary diagnosis, intensive care unit (ICU) length of stay, and disposition (survival to ICU discharge). Clinical and laboratory data included duration of total ventilation, CMV and HFOV ventilator settings, arterial blood gas analysis, and the concomitant use of iNO. Both clinical and laboratory data were obtained at 6-hour intervals, for 24 hours prior to transition from CMV to HFOV and for 48 hours that followed. [(OI) = Paw × (FiO2/PaO2) × 100)] and PaO2/FiO2 oxygen (P/F) ratio were calculated for each relevant time point.
Statistical Analysis
Demographics, duration of mechanical ventilation, and length of stay data were presented as means and ranges. This included p values from Student t-test or Wilcoxon rank-sum test for continuous variables, depending on the distribution, or Fisher exact tests for categorical variables. Graphical displays of OI, Paw, P/F ratio, pH, bicarbonate, and arterial carbon dioxide partial pressure (PaCO2) present as means and standard error of mean. The data distributions were checked for normality using QQ plots and Kolmogorov-Smirnov goodness-of-fit tests. To determine if there were differences between survivors and nonsurvivors, data were compared using repeated measures ANOVA models with generalized linear mixed models. Autoregressive covariance structures were used to model within subject variance and models and then adjusted for age, gender, and weight. To determine at which time points mean values were different between survivors and nonsurvivors, post hoc pairwise t-tests were performed using Bonferroni correction. Receiver-operating characteristic curves were also analyzed for OI and pH to determine if there were cutoff points that could accurately differentiate between survivors and nonsurvivors. Analyses were run using SAS v9.3 SAS Institute, Cary, North Carolina, United States).
Results
Patient Demographics and Outcomes
Eighty children received HFOV during the study period, but 18 were excluded from this analysis. Five received HFOV for less than 12 hours, two were transitioned to ECMO within the first 12 hours, seven had cardiac arrest on admission and died within 24 hours, three did not have an arterial catheter during the entire data collection period, and one had cyanotic heart disease. Data from the remaining 62 children were analyzed. Forty-four patients (71%) survived to ICU discharge. Survivors and nonsurvivors were similar in age, gender, weight, total ventilation, and time on CMV before HFOV (Table 1). The average time between initiation of CMV and transition to HFOV in survivors was 1.6 days and 3.4 days in nonsurvivors. Diagnoses are presented in Table 2.
Table 1. Demographic characteristics.
| Total (n = 62) | Survivors (n = 44) | Nonsurvivors (n = 18) | p-value | |
|---|---|---|---|---|
| Age in months | 83.5 (0.2–398) | 82.3 (0.2–398) | 86.3 (0.4–249) | 0.66 |
| Weight in kg | 25.8 (2.7–93.7) | 25.7 (3–93.7) | 26.0 (2.7–74) | 0.75 |
| Gender, male:female | 33:29 | 20:24 | 13:5 | 0.09 |
| Total ventilation (days) | 20.9 (1.9–56.9) | 22.1 (3.3–56.9) | 18.1 (1.9–53) | 0.23 |
| CMV before HFOV (hours) | 50.9 (4–610) | 38.2 (4–187) | 81.9 (14–610) | 0.65 |
| HFOV hours | 135 (16–503) | 129.2 (16–432) | 149.4 (25–503) | 0.82 |
| CMV after HFOV (days) | 13.2 (0–45) | 15.1 (2–45) | 8.4 (0–45) | <0.01 |
| PICU LOS (days) | 27.5 (2–96) | 31.3 (5–96) | 18.2 (2–58) | 0.01 |
| Ventilator-free patients Day 30 |
31(70%) |
Not applicable |
||
| Day 60 | 39(88%) | Not applicable |
Abbreviations: CMV, conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation; LOS, length of stay; PICU, pediatric intensive care unit.
Note: Data are presented as mean and range.
Table 2. Diagnoses of children treated with HFOV.
| Diagnosis | Total (n = 62) | Survivors (n = 44) | Nonsurvivors (n = 18) |
|---|---|---|---|
| Malignancy and complications | 14 | 5 | 9 |
| Head injury and trauma | 5 | 4 | 1 |
| Sepsis with ARDS | 10 | 10 | 0 |
| Pneumonia viral and bacterial | 16 | 11 | 5 |
| Postoperative ARDS | 4 | 4 | 0 |
| Aspiration-related respiratory failure | 7 | 5 | 2 |
| Toxic shock syndrome | 3 | 3 | 0 |
| Eosinophilic pneumonitis | 1 | 1 | 0 |
| Pulmonary hemorrhage (Wegener granulomatosis) | 1 | 1 | 0 |
| Liver disease | 1 | 0 | 1 |
Abbreviations: ARDS, acute respiratory distress syndrome; HFOV, high-frequency oscillatory ventilation.
Ventilatory Parameters Prior to HFOV
Survivors and nonsurvivors had similar respiratory data during CMV (Figs 1 and 2, Table 3). In all patients, OI nearly doubled (from ∼16 to ∼30) with ∼30% decrease in P/F ratios (∼120 to 80) over the 24 hours leading to transition to HFOV (Fig. 1A, B). There were no differences between survivors and nonsurvivors in the continuous values of Paw, pH, bicarbonate, PaCO2, OI, and P/F ratios in the 24-hour period during CMV prior to HFOV (Figs. 1 and 2, Table 3). At base line, 6 hours prior to transition to HFOV there were no statistically significant differences in any of the analyzed parameters.
Fig. 1.
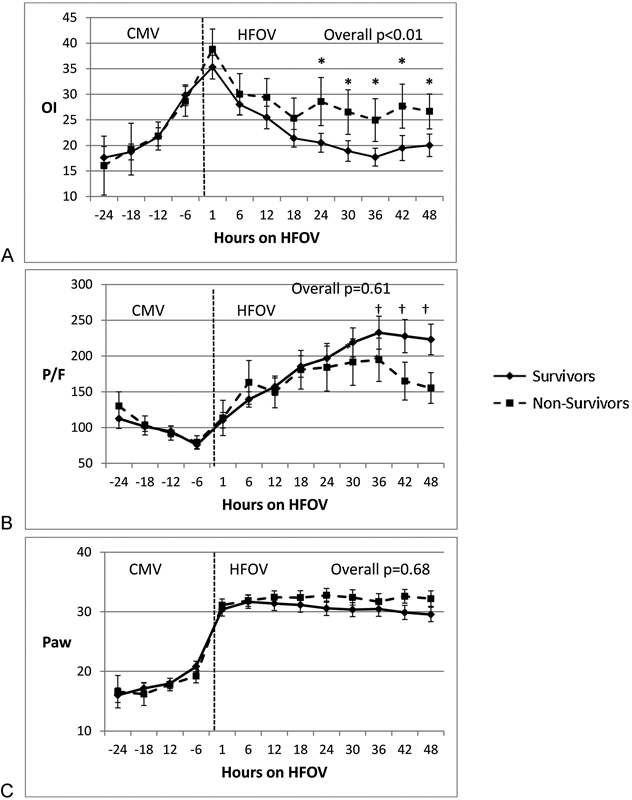
Comparison of oxygenation parameters in children with hypoxic respiratory failure between survivors and nonsurvivors before and after transitioning to HFOV. (A) Differences in OI between survivors and nonsurvivors during CMV and HFOV (*p < 0.05). (B) Differences in arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) (P/F) ratio between survivors and nonsurvivors († p < 0.05). (C) Paw settings between survivors and nonsurvivors during CMV and HFOV. Paw is not significant at all time points between survivors and nonsurvivors. HFOV, high-frequency oscillatory ventilation; OI, oxygenation index; CMV, conventional mechanical ventilation; Paw, mean airway pressure.
Fig. 2.
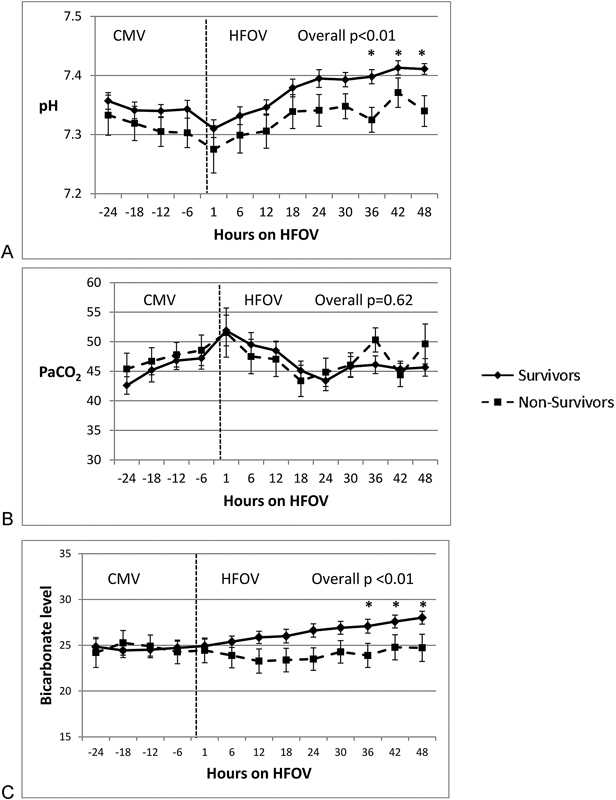
Comparison of PaCO2, pH, and bicarbonate in children with hypoxic respiratory failure between survivors and nonsurvivors before and after transitioning to HFOV. (A) Differences in pH between survivors and nonsurvivors before and after HFOV (*p < 0.05). (B) PaCO2 (Torr) trends between survivors during CMV and HFOV. There is no statistical significance at any time points. (C) Bicarbonate (mEq/L) between survivors and nonsurvivors before and after HFOV (*p < 0.05). PaCO2, arterial carbon dioxide partial pressure; HFOV, high-frequency oscillatory ventilation; CMV, conventional mechanical ventilation.
Table 3. Oxygenation index, PaO2/FiO2 ratio, and mean airway pressure during CMV 24 hours prior and 48 hours during HFOV between survivors (S) and nonsurvivors (NS).
| Time (hours) | OI-S | OI-NS | P/F-S | P/F-NS | Paw-S | Paw-NS |
|---|---|---|---|---|---|---|
| −24 (CMV) | 17.6 ± 1.5 | 16.1 ± 3 | 112 ± 9 | 130 ± 10 | 16.0 ± 0.8 | 16.6 ± 1.4 |
| −18 (CMV) | 18.7 ± 1.2 | 19.3 ± 3.6 | 101 ± 5 | 103 ± 9 | 17.2 ± 0.7 | 16.2 ± 1.4 |
| −12 (CMV) | 21.7 ± 1.5 | 21.8 ± 2.3 | 94 ± 6 | 92 ± 8 | 18.0 ± 0.7 | 17.8 ± 0.9 |
| −6 (CMV) | 29.8 ± 2 | 28.6 ± 2.9 | 76 ± 5 | 79 ± 9 | 20.8 ± 0.9 | 19.2 ± 1.2 |
| +1 (HFOV) | 35.3 ± 2.3 | 38.8 ± 3.9 | 110 ± 11 | 113 ± 25 | 30.4 ± 1.1 | 31.1 ± 1.0 |
| +6 (HFOV) | 28.0 ± 2.1 | 30.0 ± 4 | 140 ± 11 | 163 ± 30 | 31.7 ± 1.1 | 31.9 ± 1.0 |
| +12 (HFOV) | 25.5 ± 2.2 | 29.4 ± 3.7 | 157 ± 12 | 149 ± 22 | 31.4 ± 1.2 | 32.4 ± 1.1 |
| +18 (HFOV) | 21.4 ± 1.7 | 25.3 ± 4.0 | 185 ± 15 | 180 ± 27 | 31.1 ± 1.2 | 32.4 ± 1.2 |
| +24† (HFOV) | 20.5 ± 1.8 | 28.6 ± 4.6 | 197 ± 16 | 181 ± 32 | 30.6 ± 1.2 | 32.8 ± 1.1 |
| +30 (HFOV) | 18.9 ± 1.9 | 26.5 ± 4.2 | 220 ± 19 | 190 ± 32 | 30.4 ± 1.1 | 32.5 ± 1.3 |
| +36 (HFOV) | 17.7 ± 1.6 | 25.0 ± 4.1 | 233 ± 20 | 194 ± 30 | 30.5 ± 1.1 | 31.7 ± 1.3 |
| +42 (HFOV) | 19.5 ± 2.3 | 27.7 ± 3.9 | 227 ± 20 | 164 ± 24 | 29.9 ± 1.1 | 32.6 ± 1.1 |
| +48 (HFOV) | 20.0 ± 1.9 | 26.7 ± 3.0 | 223 ± 20 | 155 ± 19 | 29.6 ± 1.1 | 32.2 ± 1.2 |
Abbreviations: CMV, conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation.
Note: OI is statistically significant (p < 0.05) after 24 hours on during HFOV.
Ventilatory Parameters during HFOV Transition
Ventilatory parameters changed significantly in both survivors and nonsurvivors immediately after transition to HFOV compared with the CMV parameters and the magnitude of the changes were similar between survivors and nonsurvivors. Changes in pH, bicarbonate, and PaCO2 were not significant between CMV and HFOV immediately after transition.
Among survivors, OI, P/F ratio, and Paw on CMV just prior to HFOV were 30 ± 2, 75 ± 5, and 21 ± 1 cm H2O, respectively. One hour after transition to HFOV, OI, P/F ratio, and Paw in survivors were 35 ± 2.3, 110 ± 11, and 30 ± 1, respectively (p < 0.05 for all compared with CMV). Among survivors, pH, bicarbonate, and PaCO2 on CMV just prior to HFOV were 7.34 ± 0.01, 24.7 ± 0.7 mEq/L, and 47 ± 2 Torr, respectively. One hour after transition to HFOV, pH, bicarbonate, and PaCO2 in survivors were 7.31 ± 0.02, 24.9 ± 0.7 mEq/L, and 52 ± 3 Torr, respectively (p = not significant [NS] compared with CMV).
Similarly, among nonsurvivors, OI, P/F ratio, and Paw on CMV just prior to HFOV were 29 ± 3, 79 ± 9, and 19 ± 1 cm H2O, respectively. One hour after transition to HFOV, OI, P/F ratio, and Paw in nonsurvivors were 39 ± 4, 113 ± 25, and 31 ± 1, respectively (p < 0.05 for all compared with CMV). Among nonsurvivors, pH, bicarbonate, and PaCO2 on CMV just prior to HFOV were 7.3 ± 0.02, 24.2 ± 1.3 mEq/L, and 47 ± 2 Torr, respectively. One hour after transition to HFOV, pH, bicarbonate, and PaCO2 in nonsurvivors were 7.27 ± 0.04, 24.4 ± 1.3 mEq/L, and 51 ± 4 Torr, respectively (p = NS compared with CMV).
Ventilatory Parameters Following HFOV Transition
Paw in both survivors and nonsurvivors were similar during 48 hours after transition (Fig. 1C). Over this time, average OI was significantly lower in survivors compared with nonsurvivors (21 ± 0.9 vs. 26.5 ± 2.2, p < 0.01; Fig. 1A, Table 3). In point-to-point comparisons, there was a divergence in OI between survivors and nonsurvivors at 24 hours (20.5 ± 1.9 vs. 28.6 ± 4.7; p < 0.05) and OI remained different between the groups through 48 hours (Fig. 1A). Average P/F ratios increased to above 200 in survivors but remained <200 in nonsurvivors. Although P/F ratios did not achieve statistical significance, for the course during HFOV or in the point-to-point comparisons, it appeared that a divergence was beginning to become apparent at ∼36 hours (Fig. 1B).
Concerning ventilation, PaCO2 values were not different between survivor and nonsurvivors (Fig. 2B), although survivors were managed with less oscillatory support in terms of amplitude (delta pressure) throughout the 48-hour period and higher frequency (hertz) at later time points (Fig. 3). Despite similar PaCO2 values, pH was lower in nonsurvivors than in survivors during HFOV (Fig. 2A). In survivors, pHs at 36 and 48 hours on HFOV were 7.40 ± 0.01 and 7.41 ± 0.01, which were significantly higher than nonsurvivors' pHs of 7.32 ± 0.02 and 7.34 ± 0.02 at similar time points (p < 0.05). Values of PaCO2 at 36 and 48 hours in survivors were 46 ± 1.5 and 45 ± 1.4 Torr, respectively, and in nonsurvivors PaCO2 at 36 and 48 hours were 50 ± 1.5 and 49 ± 3 Torr, respectively, and were not statistically significant. Changes in serum bicarbonate levels were similar to pH changes (Fig. 2C). In survivors, bicarbonate levels at 36 and 48 hours were 27.1 ± 0.7 and 28 ± 0.7 mEq/L, respectively. Nonsurvivors' serum bicarbonate levels at 36 and 48 hours were 23.9 ± 1.3 and 24.7 ± 1.5 mEq/L, respectively, which were slightly lower compared with survivors (p < 0.05).
Fig. 3.
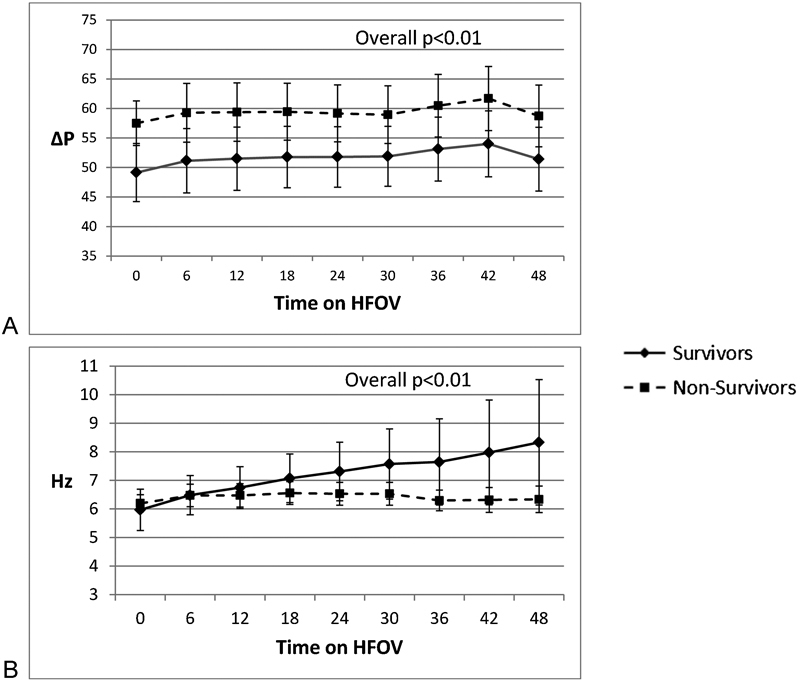
Differences in HFOV settings between survivors and nonsurvivors with hypoxic respiratory failure. (A) Difference in ∆P settings between survivors and nonsurvivors during HFOV. Overall ∆P settings were higher in nonsurvivors (p < 0.01). (B) Differences in frequency (Hz) between survivors and nonsurvivors. Hz settings were significantly lower (p < 0.01) in nonsurvivors. HFOV, high-frequency oscillatory ventilation; ∆P, delta pressure.
Outcomes
Children in this study had 29% mortality. In survivors, 31 (70%) patients were ventilator free at 30 days and 39 (88%) patients were ventilator free at 60 days. We computed receiver-operating characteristic curves for survival for OI at 24 hours and pH at 36 hours to evaluate cutoff values to discriminate survivors (Fig. 4). OI <25 at 24 hours and pH >7.34 at 36 hours had modest correlation and seems to be helpful to identify survivors during HFOV. The odds ratio for death with OI >25 at 24 hours during HFOV was 4.6 (95% confidence interval [CI]: 1.3–16.7) and odds ratio for death with a pH <7.3 at 36 hours was nine (95% CI: 2.1–39.2). Sixteen (26%) children developed a pneumothorax requiring chest tube placement: five during CMV, of which two died, and eleven during HFOV, of which one died. Out of 44 surviving children, 8 (18%) required tracheostomy and 5 were discharged home on a ventilator. Almost one-third (14) of the surviving children were transferred to an inpatient rehabilitation unit, with average rehabilitation length of stay of 28.3 days (7–62 days). During the study period, 15 children (13 from CMV and 2 from HFOV) were treated with ECMO and 8 (53%) survived.
Fig. 4.
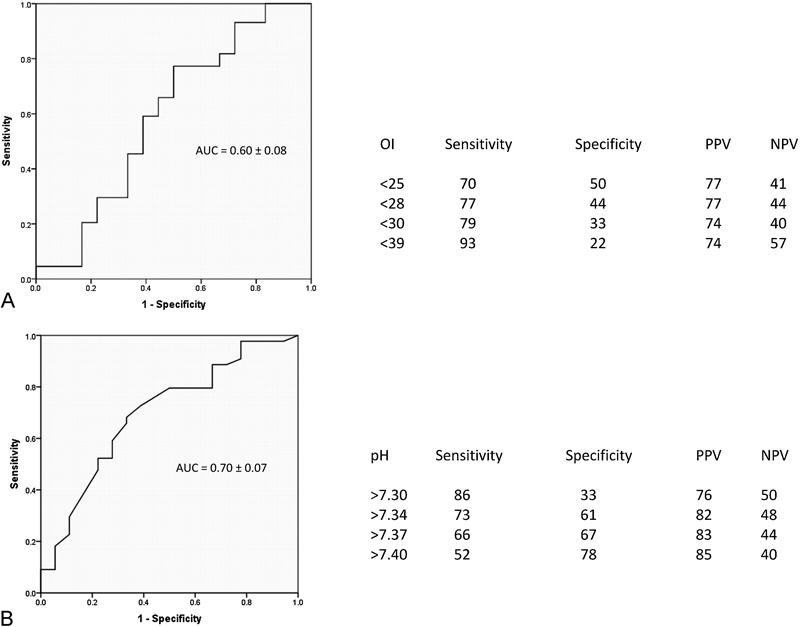
Receiver-operating characteristic (ROC) curves for oxygenation index and pH. (A) ROC curve to determine survival for oxygenation index at 24 hours during high-frequency oscillatory ventilation (B) ROC curve to evaluate survival for pH at 36 hours during high-frequency oscillatory ventilation. AUC, area under the ROC curve; PPV, positive predictive value; NPV, negative predictive value.
Inhaled Nitric Oxide Data
In this study, 33 (53%) children received iNO and 11 (33%) died. iNO was started in 9 children during CMV and in 24 children during HFOV. Seven of nine children in the CMV-iNO group continued to receive iNO during HFOV. P/F ratios and OI improved in both survivors and nonsurvivors in response to iNO (Fig. 5A,B). P/F ratio improvements were greater when iNO was instituted during HFOV versus CMV, for example, with P/F ratio increase of 34 during HFOV versus 13 on CMV and 116 during HFOV versus 35 on CMV at 1 and 24 hours, respectively (all p < 0.01; Fig. 5C). OI improvement following iNO was similar between CMV and HFOV (Fig. 5D). There were no differences in survival in patients who received iNO versus those who did not (67 and 76%, respectively, p = NS).
Fig. 5.
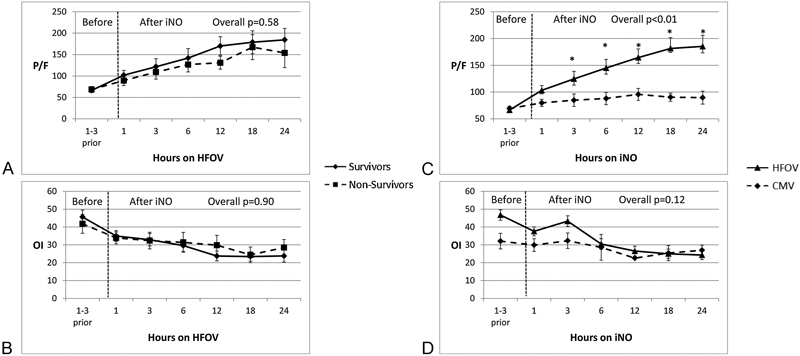
Effect of iNO in survivors and nonsurvivors and changes in PaO2/FiO2 (P/F) ratios and OI during CMV and HFOV. (A) Changes in P/F ratios in response to iNO were compared between survivors and nonsurvivors. (B) Oxygenation index (OI) changes following iNO were compared between survivors and nonsurvivors. Neither P/F ratios nor OI changes were statistically significant between survivors and nonsurvivors. (C) Changes in P/F ratio before and after iNO during HFOV versus CMV (*p < 0.05 at 3 hours and subsequent time points after starting iNO). (D) OI changes before and after iNO during HFOV versus CMV. OI changes were statistically not significant between CMV and HFOV at any time points. iNO, inhaled nitric oxide; PaO2/FiO2 (P/F), arterial oxygen partial pressure/fraction of inspired oxygen ratio; HFOV, high-frequency oscillatory ventilation; CMV, conventional mechanical ventilation; OI, oxygenation index.
Discussion
In this study, we sought to determine predictors of survival in children with severe HRF managed with HFOV. In pediatric critical care, HFOV is often used to manage children with HRF that is refractory to management with CMV. Determining a method to identify children who may not survive HFOV would assist in developing interventions to improve outcomes in children with severe HRF, such as earlier deployment of more invasive techniques (e.g., ECMO). In this cohort of children with severe HRF treated with HFOV, nonsurvivors and survivors had similar respiratory parameters during CMV, yet differences in oxygenation, ventilation, and acid–base balance became apparent within 48 hours after transition to HFOV. Although OI decreased in both survivors and nonsurvivors following HFOV, greater improvement was observed in survivors. Specifically, by 24 hours after HFOV, survivors had OIs that averaged ∼20 or less, while nonsurvivors had OIs ≥25. Of interest is that these improvements (from OI peaks of 35–40) occurred with an increase in Paw from 20 to ∼30 cm H2O, which is ∼50% increase. P/F ratios also improved in both groups following HFOV, from <100 to above 200 at 36 hours in survivors, but ranged 150 to 200 in nonsurvivors. The P/F ratio differences were not statistically significant for the whole duration of HFOV or for point-to-point comparisons. Perhaps this reflects a later divergence of the P/F values, limitations of our sample size, and/or an enhanced sensitivity of OI over P/F ratios in characterizing a patient's oxygenation status. The former takes into consideration the level of ventilatory support (Paw) employed. To detect P/F difference of 50 between survivors and nonsurvivors with 80% power (α = 0.05), we required sample size of 186; if the P/F difference was to be >100, sample size of 64 would have been sufficient. Our sample size of 62 adequately detects OI difference of 2.8 and pH difference of 0.032 with 80% power (α = 0.05) between survivors and nonsurvivors.
In addition to oxygenation parameters, our data indicate that ventilation in survivors can be more easily managed than in nonsurvivors, given the fact that both groups had similar PaCO2 despite lower amplitude throughout and higher frequency over time in survivors. Survivors also had slightly higher bicarbonate levels than nonsurvivors. Taken together, nonsurvivors had modest, but significant, metabolic acidosis compared with survivors.
There are important similarities and differences comparing our results to other studies in this field. There are some recent studies on children and adults which showed the association of improved oxygenation with survival.14 18 19 20 In pediatric studies, nonsurvivors had higher OI (close to 30 or higher) compared with OI of <25 in survivors before transition to HFOV compared with that of our study where OI was close to 30 in both survivors and nonsurvivors. In the study of Babbitt et al,14 OI improvement in survivors and nonsurvivors was similar to our study with OI of less than 20 in survivors and >25 in nonsurvivors. Yehya et al18 in their elegant studies have shown that in children with immunocompromised condition, OI does not improve in nonsurvivors, whereas in survivors OI improves ∼50% and OI improvement of <5% is 100 sensitive and 85% specific in predicting mortality in children with immunocompromised condition. In their study with mixed population of children, OI improvement in nonsurvivors was 6% compared with 30% in survivors. In our study, OI improvement in survivors during first 48 hours was ∼35% compared with ∼15% in nonsurvivors. OI improvement in our study is comparable to the above-mentioned studies, but nonsurvivors in our study showed slightly higher improvement in OI. In our study, P/F improvement was observed in both survivors and nonsurvivors. And P/F improvement was 100% or more in survivors, which is similar to other pediatric studies,14 18 19 but P/F improvement in nonsurvivors was greater compared with those studies and P/F ratio difference could not differentiate survivors and nonsurvivors in our study which contrasted to those studies. A recent study in adults with ARDS who were transitioned from CMV to HFOV found that OIs and P/F ratios only improved in survivors,20 whereas in children these indices improve in both survivors and nonsurvivors,14 19 but again with a greater improvement of OIs only in survivors. In adults, differences in OIs and P/F ratios were apparent at 3 hours during HFOV, but we found differences in OIs beginning at 24 hours after transition and what may be the start of differences in P/F ratio at 36 hours. In children, OI and P/F improvement has been observed around 4 to 6 hours after HFOV transition14 21 22 to 24 hours on HFOV.18 19 In adults, lower pH due to higher PaCO2 correlated with mortality, whereas in our report, lower pH, a consequence of metabolic acidosis, was associated with mortality. In both studies, survivors had OIs less than ∼20 and pH higher than ∼7.4. In a subgroup of children with sepsis during HFOV, metabolic acidosis of 7.25 is associated with mortality compared with 7.32 in survivors.14 In our study, survivors had P/F ratios of >200, whereas P/F ratios never improved over 200 in adult survivors, even at or after 72 hours. In other pediatric studies, P/F improvement was also noted to be <200 which is similar to the adult study.14 19
Similar to other reports in adults and children,14 19 20 Paw increased by more than 10 cm H2O with the transition from CMV to HFOV in our study, which suggests that higher positive end-expiratory pressure may be beneficial in the management of both adults and children with HRF during CMV. On HFOV, we typically gauge proper lung expansion (i.e., 8–10 ribs) by chest X-ray and traditionally advocate increasing Paw by 3 to 5, whereas patients may require >10 Paw increase while transitioning from CMV to HFOV.
OI has been suggested as a predictor of mortality with OI > 42 at 24 hours during HFOV in pediatric respiratory failure and is associated with mortality odds ratio of 21.9 Another study on children reported OI of >35 after 24 hours of HFOV being associated with 31 times higher odds of mortality,14 whereas most of our patients had OIs < 35 at 24 hours after HFOV. Our data are more similar to a report published by our group, which shows that an OI > 25 in hematopoietic stem cell transplant patients with respiratory failure at any time on HFOV is highly associated with mortality.23
Data on pH during HFOV and outcome are limited. In this study, most (90%) survivors had pH > 7.3 at 48 hours and children with pH < 7.3 had nine times higher odds of death. As stated earlier, a subgroup of pediatric sepsis patients treated with HFOV had higher mortality when pH was < 7.32.14 Our study is unique because it does show that presence of metabolic acidosis (pH < 7.34) is associated with decreased survival in children with acute HRF, whereas permissive hypercapnia and accepting respiratory acidosis (pH < 7.3) may be beneficial in some children.24 Differentiating warning signs of persistent metabolic acidosis from acceptable respiratory acidosis is important in clinical decision making.
Although no data currently suggest that iNO impacts outcome in children with HRF or ARDS, about half of our patients received iNO, consistent with a recent survey of pediatric intensivists and their management of pediatric HRF/ARDS.25 26 Like others, we show that there is “positive” physiologic response to iNO as evidenced by lower OIs and higher P/F ratios, and greater improvement of these indices while patients were treated with HFOV rather than CMV.27 28 29 Consistent with previous reports, iNO did not appear to improve survival, as the mortality rate was 33% in those who received iNO and 24% in those who did not (p = NS), although it is possible that iNO is initiated on those, at least subjectively, considered more critically ill. These findings should not negate the potential utility of iNO in severely hypoxemic patients when used for stabilization while bridging to alternative therapies.
In conclusion, our study provides additional information for the assessment and management of children with the most severe forms of hypoxemic respiratory failure. Our study suggests that an OI > 25, metabolic acidosis with pH of <7.34, and the inability to reduce ventilatory support in the first 48 hours during HFOV are associated with mortality. In real-time scenarios, these data may help caretakers to identify children at risk for death during HFOV within 2 days and may assist in prognostication and earlier consideration of therapeutic alternatives. Data from this study need confirmation in multicenter studies, yet may provide the basis for identifying children with HRF at the highest risk for death who can participate in clinical trials for alternative ventilator strategies, novel therapeutics, or ECMO to improve survival.
References
- 1.Kneyber M C, van Heerde M, Markhorst D G. Reflections on pediatric high-frequency oscillatory ventilation from a physiologic perspective. Respir Care. 2012;57(9):1496–1504. doi: 10.4187/respcare.01571. [DOI] [PubMed] [Google Scholar]
- 2.Sud S, Sud M, Friedrich J O. et al. High-frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;2:CD004085. doi: 10.1002/14651858.CD004085.pub3. [DOI] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Pierrakos C, Karanikolas M, Scolletta S, Karamouzos V, Velissaris D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res. 2012;4(1):7–16. doi: 10.4021/jocmr761w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson-Smart D J, De Paoli A G, Clark R H, Bhuta T. High frequency oscillatory ventilation versus conventional ventilation for infants with severe pulmonary dysfunction born at or near term. Cochrane Database Syst Rev. 2009;(3):CD002974. doi: 10.1002/14651858.CD002974.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cools F Askie L M Offringa M et al. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients' data Lancet 201037597312082–2091. [Review Erratum in: Lancet 2011;377(9777):1572] [DOI] [PubMed] [Google Scholar]
- 7.Derdak S, Mehta S, Stewart T E. et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166(6):801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson N D, Cook D J, Guyatt G H. et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 9.Arnold J H, Hanson J H, Toro-Figuero L O, Gutiérrez J, Berens R J, Anglin D L. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med. 1994;22(10):1530–1539. [PubMed] [Google Scholar]
- 10.Samransamruajkit R, Prapphal N, Deelodegenavong J, Poovorawan Y. Plasma soluble intercellular adhesion molecule-1 (sICAM-1) in pediatric ARDS during high frequency oscillatory ventilation: a predictor of mortality. Asian Pac J Allergy Immunol. 2005;23(4):181–188. [PubMed] [Google Scholar]
- 11.Santschi M, Jouvet P, Leclerc F. et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. 2010;11(6):681–689. doi: 10.1097/PCC.0b013e3181d904c0. [DOI] [PubMed] [Google Scholar]
- 12.Randolph A G, Meert K L, O'Neil M E. et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167(10):1334–1340. doi: 10.1164/rccm.200210-1175OC. [DOI] [PubMed] [Google Scholar]
- 13.Fedora M, Klimovic M, Seda M, Dominik P, Nekvasil R. Effect of early intervention of high-frequency oscillatory ventilation on the outcome in pediatric acute respiratory distress syndrome. Bratisl Lek Listy (Tlacene Vyd) 2000;101(1):8–13. [PubMed] [Google Scholar]
- 14.Babbitt C J, Cooper M C, Nussbaum E, Liao E, Levine G K, Randhawa I S. High-frequency oscillatory ventilation in pediatric acute hypoxemic respiratory failure: disease-specific morbidity survival analysis. Lung. 2012;190(6):685–690. doi: 10.1007/s00408-012-9417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moniz M, Silvestre C, Nunes P. et al. High-frequency oscillatory ventilation in children: a 10-year experience. J Pediatr (Rio J) 2013;89(1):48–55. doi: 10.1016/j.jped.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Ben Jaballah N, Mnif K, Bouziri A, Kazdaghli K, Belhadj S, Zouari B. High-frequency oscillatory ventilation in paediatric patients with acute respiratory distress syndrome—early rescue use. Eur J Pediatr. 2005;164(1):17–21. doi: 10.1007/s00431-004-1544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton H, Fortenberry J D, Frenckner B, Palmer P. Ann Arbor, MI: ELSO; 2012. ECMO for pediatric respiratory failure; pp. 265–270. [Google Scholar]
- 18.Yehya N, Topjian A A, Thomas N J, Friess S H. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome*. Pediatr Crit Care Med. 2014;15(4):e147–e156. doi: 10.1097/PCC.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yehya N, Topjian A A, Lin R, Berg R A, Thomas N J, Friess S H. High frequency oscillation and airway pressure release ventilation in pediatric respiratory failure. Pediatr Pulmonol. 2014;49(7):707–715. doi: 10.1002/ppul.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camporota L, Sherry T, Smith J, Lei K, McLuckie A, Beale R. Physiological predictors of survival during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care. 2013;17(2):R40. doi: 10.1186/cc12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarnaik A P, Meert K L, Pappas M D, Simpson P M, Lieh-Lai M W, Heidemann S M. Predicting outcome in children with severe acute respiratory failure treated with high-frequency ventilation. Crit Care Med. 1996;24(8):1396–1402. doi: 10.1097/00003246-199608000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Ben Jaballah N, Khaldi A, Mnif K. et al. High-frequency oscillatory ventilation in pediatric patients with acute respiratory failure. Pediatr Crit Care Med. 2006;7(4):362–367. doi: 10.1097/01.PCC.0000227108.38119.2E. [DOI] [PubMed] [Google Scholar]
- 23.Rowan C M, Hege K M, Speicher R H. et al. Oxygenation index predicts mortality in pediatric stem cell transplant recipients requiring mechanical ventilation. Pediatr Transplant. 2012;16(6):645–650. doi: 10.1111/j.1399-3046.2012.01745.x. [DOI] [PubMed] [Google Scholar]
- 24.Rotta A T, Steinhorn D M. Is permissive hypercapnia a beneficial strategy for pediatric acute lung injury? Respir Care Clin N Am. 2006;12(3):371–387. doi: 10.1016/j.rcc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Rowan C M, Nitu M E, Rigby M R. Inconsistencies in care of the pediatric hematopoietic stem cell transplant recipient with respiratory failure: opportunity for standardization and improved outcome. Pediatr Transplant. 2014;18(2):230–235. doi: 10.1111/petr.12222. [DOI] [PubMed] [Google Scholar]
- 26.Santschi M Randolph A G Rimensberger P C Jouvet P; Pediatric Acute Lung Injury Mechanical Ventilation Investigators; Pediatric Acute Lung Injury and Sepsis Investigators Network; European Society of Pediatric and Neonatal Intensive Care. Mechanical ventilation strategies in children with acute lung injury: a survey on stated practice pattern Pediatr Crit Care Med 2013147e332–e337. [DOI] [PubMed] [Google Scholar]
- 27.Afshari A, Brok J, Møller A M, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis. Anesth Analg. 2011;112(6):1411–1421. doi: 10.1213/ANE.0b013e31820bd185. [DOI] [PubMed] [Google Scholar]
- 28.Dobyns E L, Anas N G, Fortenberry J D. et al. Interactive effects of high-frequency oscillatory ventilation and inhaled nitric oxide in acute hypoxemic respiratory failure in pediatrics. Crit Care Med. 2002;30(11):2425–2429. doi: 10.1097/00003246-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Fioretto J R, Batista K A, Carpi M F. et al. High-frequency oscillatory ventilation associated with inhaled nitric oxide compared to pressure-controlled assist/control ventilation and inhaled nitric oxide in children: randomized, non-blinded, crossover study. Pediatr Pulmonol. 2011;46(8):809–816. doi: 10.1002/ppul.21452. [DOI] [PubMed] [Google Scholar]


